Abstract
Exposed surfaces of mammals are colonized with 100 trillion indigenous bacteria, fungi, and viruses, creating a diverse ecosystem known as the human microbiome. The gut microbiome is the richest microbiome and is now known to regulate postnatal skeletal development and the activity of the major endocrine regulators of bone. Parathyroid hormone (PTH) is one of the bone-regulating hormone that requires elements of the gut microbiome to exert both its bone catabolic and its bone anabolic effects. How the gut microbiome regulates the skeletal response to PTH is object of intense research. Involved mechanisms include absorption and diffusion of bacterial metabolites, such as short-chain fatty acids, and trafficking of immune cells from the gut to the bone marrow. This review will focus on how the gut microbiome communicates and regulates bone marrow cells in order to modulate the skeletal effects of PTH.
Keywords: microbiome, bone, PTH, SCFA, Th17 cells
The term “microbiome” refers to the collection of microorganisms, their genomes, and their interactions in a given environment. Exposed surfaces of mammals are colonized with 100 trillion indigenous bacteria, fungi, and viruses, creating a diverse ecosystem known as the human microbiome (1, 2). The microbiome of humans and mice is quantitatively different, but they share a large qualitatively similar core (3). Because of these qualitative similarities, mice are extensively used to investigate the role of the microbiota in health and disease (3).
The gastrointestinal tract harbors the greatest numbers of these microorganisms, which regulate human nutrition, metabolism, and immune system function. Microbial colonization of the gastrointestinal tract starts at birth, eventuating in a taxonomically diverse community by early adulthood (4). Intestinal microbes flourish in an environment that is rich in nutrients, with specific taxa recognized as causative of conferring beneficial effects on the host, such as improved energy extraction from food, exclusion of pathogenic bacteria, and stimulation of tissue development (5, 6). Gut luminal bacteria also beneficially influence tissue homeostasis in the intestine by enhancing epithelial cell proliferation and survival and strengthening barrier function (7-12). Indeed, mice raised in germ-free conditions exhibit many functional weaknesses (13) and have impaired homeostasis (14). These observations show that there is an active and dynamic association between microbes that reside within the gut and host cells.
Maternal transmission is the key determinant of the composition of the gut microbiota in newborns (15). In children, the gut microbiota reaches an adult-like composition by ~3 years of age, through maternal transmission and close cohabitation (15). Therefore, sibling and house pets shape the composition of the microbiota in children. This phenomenon is known as microbial inheritance (16). By age ~3, the gut microbiota becomes resistant to colonization by new organisms (17). Indeed, once acquired, the majority of strains are retained in an individual for decades (17). Thus, early gut colonizers, once established, have the potential to exert their biological effects on our health for most and perhaps all of our adult lives (15). The effect of a strain’s residency may take decades to manifest itself (16). In adults, long-lasting modifications of the microbiota require permanent, significant dietary changes, major changes in the health status of the host, or extensive manipulations, such as long-term antibiotic treatment (18) or stool transplantation (19).
Microbial inheritance occurs preferentially between family members and much less between unrelated individuals (16). Attesting to the relevance of microbiota inheritance, the relative risk of Crohn’s disease (20), rheumatoid arthritis (21), or multiple sclerosis (22) is highly increased when a sibling is affected, even after accounting for the genetic predisposition. By contrast, cohabitation is not sufficient to modify the gut microbiome in older individuals. This explains why transmission of diseases linked to the microbiome is unusual between spouses. The community composition of the microbiota has profound effects on the immune status of the host and impacts the development and/or progression of inflammatory diseases. Accordingly, numerous studies have demonstrated differences in the microbiota of patients with or without a given inflammatory condition (23), although it remains unclear whether the observed dysbiosis is a cause or consequence of the underlying disease process (23).
Gut microbes modulate systemic immune responses (23). For instance, gut-resident microbes have a robust influence on the emergence and/or maintenance of CD4+ T-cell subsets. Examples include the effects of specific bacteria on the emergence of Th17 cells (24) and the impact of Bacteroides fragilis in Treg differentiation (25). Indeed, abnormalities in gut microbial diversity (“dysbiosis”) have been suggested to be sufficient to aggravate intestinal pathologies related to the immune system, such as in inflammatory bowel disease (26).
Another key activity of the gut microbiota is the production of metabolites that are capable of traveling to distant organs and exerting important biological effects. Indole derivatives were among the first bacterial metabolites to be described to influence intestinal immunity (27, 28). For example, 4-ethylphenol sulfate, a metabolite produced by intestinal saprophytes was shown to regulate human behavior and has been implicated in autism (29). Insulin-like growth factor 1 (IGF-1), produced predominantly in the liver in response to food intake and regulated by microbes and microbial products, was the first metabolite identified as a linker in the gut-bone axis (30, 31). Another bone-regulating molecule is hydrogen sulfide (H2S), a gasotransmitter generated by gastrointestinal cells and by bacteria residing within the gut (32, 33). The microbiota accounts for a substantial portion of the overall blood levels of H2S, as germ-free mice have low serum and gastrointestinal tissues levels of H2S (33). In turn, H2S can modify the composition of the microbiota (32, 33). H2S has been implicated in inflammatory bowel disease and other gastrointestinal pathologic conditions. Importantly, H2S stimulates bone formation and postnatal skeletal development (34). In addition, the H2S-donating compound GYY4137 increases bone formation by activating Wnt signaling, via increased Wnt10b production (35), and prevents the loss of trabecular bone induced by ovariectomy (35). However, the family of metabolites produced by intestinal bacteria that has received the greatest attention for their capacity to diffuse to distant organs and induce potent regulatory effects are short-chain fatty acids (SCFAs). These metabolites initially emerged as powerful immune cell controllers, and more recently have been recognized as pivotal regulators of bone resorption and bone formation.
Human and animal studies have implicated the gut microbiome as a regulator of bone mineral density in health and disease. For example, a human study has described the first evidence of a correlation between microbiome diversity and osteoporosis (36), while another has shown that subjects with high bone mineral density have fewer Bacteroidetes and more abundant Firmicutes (37). Animal studies have disclosed that bone mass in mice raised in a germ-free condition is different from controls raised in conventional housing (30, 31, 38, 39). The microbiome is also relevant for the skeletal response to pathological conditions. For example, the presence of the microbiota is required for estrogen deficiency and glucocorticoids to cause bone loss (40). It is also known that antibiotic treatment increases bone density in mice (30), while bacterial recolonization after antibiotic treatment causes bone loss (41). The mechanism by which antibiotics increase bone density include a decrease in serum IGF-1 that blunts bone formation (30) and an increase in bone resorption due to activation of intestinal immune cells by the gut microbiota, and their subsequent migration to the bone marrow.
In addition, antibiotics prevent ovariectomy-induced bone loss (42). Intriguingly, low-dose antibiotics are commonly fed to chickens, cows, and pigs to increase size and body weight, and it would be interesting to determine how much of the increased weight gain is due to increases in bone density (43).
Skeletal Effects of PTH
PTH plays a key regulatory role in calcium metabolism, defending the body against hypocalcemia. PTH acts to replenish serum calcium through mobilization of skeletal calcium stores by stimulating the differentiation of osteoclasts and thus promoting bone resorption. PTH further enhances the tubular reabsorption of calcium and stimulates the kidneys to produce 1,25-dihydroxy vitamin D3. Chronic excessive PTH production is a cause of skeletal and extraskeletal disease. Secondary hyperparathyroidism has been implicated in the pathogenesis of age-related teriparatide osteoporosis (44), while primary hyperparathyroidism (PHPT), is associated with accelerated bone loss (45), osteopenia (46-48), and increased bone turnover (47), an independent risk factor for fractures. Furthermore, PHPT is a cause of extraskeletal manifestations stemming from increased bone resorption such as hypercalcemia, recurrent nephrolithiasis, renal failure, peptic ulcers, and mental changes (46). However, PHPT is a heterogeneous disease as some patients develop bone loss while others do not (49, 50). Hypercalcemia is a frequent complication of PHPT, although a normocalcemic variant of PHPT has been described (49, 50). The presence or absence of hypercalcemia does not define or predict who will experience skeletal manifestations of PHPT (49, 50). Primary and secondary hyperparathyroidism are mimicked by continuous PTH (cPTH) infusion. By contrast, when injected daily, a regimen known as intermittent PTH (iPTH) treatment, the hormone markedly stimulates trabecular and cortical bone formation. Although this bone forming activity is antagonized, in part, by a stimulation of bone resorption, the net effect of iPTH is an improvement in bone microarchitecture and increased strength (51-53). As a result, daily injections of an active fragment of human PTH, known as teriparatide, decreases the risk of fractures in humans, and is an FDA-approved treatment modality for postmenopausal osteoporosis (54, 55).
PTH acts by binding to the PTH-PTHrP receptor (PPR), which is expressed in stromal cells, osteoblasts, and osteocytes (56-58), as well as in T cells (59). A key mechanism whereby PTH induces bone resorption is the induction of RANKL production by osteocytes (58, 60-62). PTH promotes bone formation by increasing the number of osteoblasts (63, 64). This is achieved through activation of quiescent osteoblastic lining cells (65), an increase in proliferation (66) and differentiation (67) of osteoblasts, attenuation of osteoblasts apoptosis (68, 69), and by increasing signaling in osteocytes (70). The relative contributions of each of these mechanisms to the overall anabolic activity of PTH remains controversial.
Osteoimmunological Effects of Primary Hyperparathyroidism and Continuous PTH Treatment
In recent years, an unexpected connection has emerged between the immune system and the skeletal activity of PTH (59, 71-78). This line of research was opened by the report that cPTH fails to elicit bone waste in T-cell null mice, and in mice with conditional deletion of the PTH receptor PPR in T cells (71, 72), thus revealing that T cells are required for the mechanism of action of cPTH in bone. Mechanistic studies disclosed that cPTH increases the level of tumor necrosis factor (TNF) in the bone marrow (BM) by expanding CD4+ and CD8+ TNF+ T cells (72, 75). In addition, cPTH treatment does not result in bone loss in mice specifically lacking TNF production by T cells (72, 75), demonstrating a requirement for T-cell–produced TNF for the bone catabolic activity of PTH. Additional studies revealed that PHPT in humans and cPTH infusion in mice promotes the differentiation of naïve CD4+ T cells into Th17 cells via a TNF-dependent pathway, thus expanding the size of the Th17 cell pool in the BM (75). Th17 cells are a pro-osteoclastogenic lineage of CD4+ cells defined by their capacity to produce interleukin (IL)-17 (79-81). Th17 cells potently induce osteoclastogenesis by secreting IL-17, RANKL, TNF, IL-1, and IL-6, along with low levels of interferon-γ (82-84). IL-17 binds to a heterodimeric IL-17 receptor (IL-17R) (85). IL-17R signaling stimulates the release of RANKL osteoblasts and osteocytes (82, 86). In vivo neutralization of IL-17 or global deletion of IL-17R prevent the bone loss induced by cPTH in mice (75). These findings demonstrate the critical role of IL-17 for PTH-induced bone loss. Important mechanistic studies revealed that cPTH fails to induce bone loss and fails to stimulate RANKL production by osteocytes in mice specifically lacking osteocytic expression of IL-17R (86). These reports led to the conclusion that IL-17A is an upstream mediator of cPTH-induced bone loss that acts by enabling osteocytes to release RANKL when stimulated by PTH. This hypothesis is in full agreement with reports indicating that direct targeting of osteocytes by cPTH is required for cPTH to induce RANKL production and bone loss (60, 61).
Microbiome and PTH-Induced Bone Loss
In the mouse, Th17 cells are mostly produced in the intestinal lamina propria, where their development is heavily influenced by the presence of segmented filamentous bacteria (SFB) (24), which are spore-forming, Gram-positive commensal bacteria that potently induce differentiation of Th17 cells (87, 88). Whereas in healthy mice, SFB is the only known taxa of the microbiota that expands Th17 cells, several pathogens that cause infection, such as Candida albicans and Citrobacter rodentium, are also known to expand gut Th17 cells in mice. In healthy mice, which have saprophytes but lack a significant load of pathogens, SFB are required for the expansion of Th17 cells. In humans ~20 species of “SFB-like” saprophytic bacteria are known to cause the expansion of intestinal Th17 cells. Among them the most are Bifidobacterium adolescentis, Staphylococcus saprophyticus, Klebsiella, Enterococcus faecalis, and Acinetobacter baumanii (89, 90). A complete list of Th17 cell–inducing bacteria is reported in (89, 90).
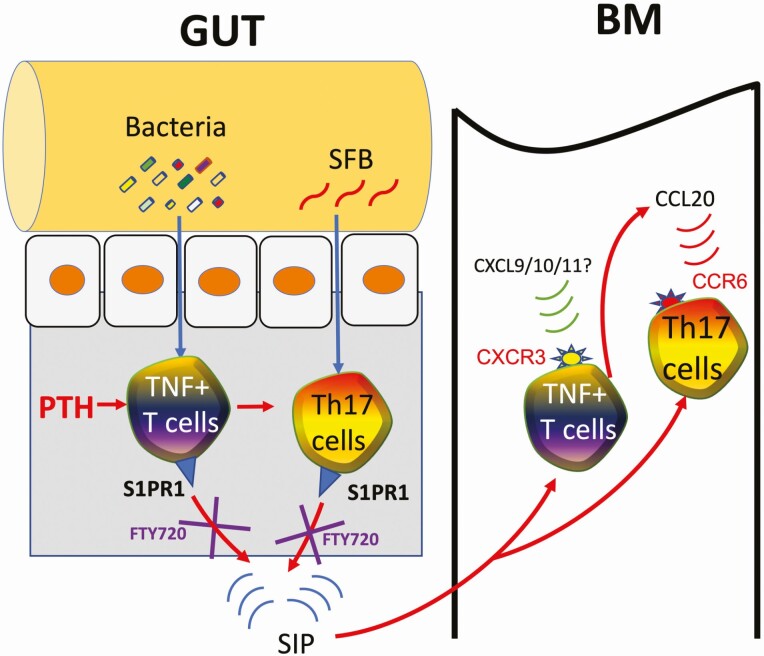
Surprisingly, PTH has been shown to cause the expansion of Th17 cell in the small intestine (78). Stimulation of intestinal Th17 cell differentiation by PTH is dependent on the presence of SFB. However, SFB requires the concomitant stimulation from additional elements of the microbiota. A key mechanism whereby PTH expands intestinal Th17 cells is linked to its capacity to stimulate the production of transforming growth factor-β (TGFβ) and to expand TNF-producing T cells (TNF+ T cells) via a mechanism mediated by bacterial components such as lipopolysaccharide (LPS) and flagellin. Th17 cells express the sphingosine 1 phosphate (S1P) receptor S1PR1, and egress from intestinal lymphoid tissues when attracted to circulating S1P (91). Once in the circulation, Th17 cells home to distant peripheral sites of inflammation (92). For example, studies in autoimmune disorders have shown that SFB-induced Th17 cells migrate from the gut to the lungs, kidneys, and joints (92-94). cPTH leads to the migration of intestinal Th17 cells to the BM, where these cells release IL-17 and cause bone loss (78). The mechanisms responsible for the homing of Th17 cells to the BM are complex and involve TNF. The first critical step is the capacity of cPTH to expand TNF+ T cells. Like Th17 cells, TNF+ T cells residing in intestinal lymphoid tissues express S1PR1 and enter the systemic circulation attracted by circulating S1P. TNF+ T cells also express the chemokine receptor CXCR3 that binds to CXCL9, CXCL10, and CXCL11, which are ligands expressed by several BM cells, including stromal cells and monocytes. Once in the systemic circulation, TNF+ T cells of intestinal origin reach the BM driven by the sensing of the CXCR3/CXCL9/10/11 chemokine gradient (95), causing the expansion of the pool of BM TNF+ T cells and an increase in the BM levels of TNF. Thereafter, TNF upregulates the expression of CCL20 by BM stromal cells (78). CCL20 is the ligand for the chemokine receptor CCR6, which is expressed on Th17 cells (96, 97). Thus, the TNF-driven upregulation of CCL20 in the BM causes the influx of Th17 cells into the BM (Fig. 1).
Figure 1.
Diagrammatic representation of the mechanism of action of cPTH in bone. Bacterial products such as lipopolysaccharide and flagellin translocate into the intestinal wall where, in concert with PTH, they induce the differentiation of Cd4+ T cells into TNF+ T cells. Intestinal TNF, together with stimuli provided by segmented filamentous bacteria (SFB) induce the expansion of intestinal Th17 cells. TNF+ T cells and Th17 cells, which express the receptor S1PR1, egress from the intestine and enter the blood vessels attracted by the S1PR1 ligand S1P. TNF+ T cells express the chemokine CXCR3 and are attracted to the bone marrow (BM) by CXCL9/10/11 produced by stromal cells. Once in the BM, TNF+ T cells release TNF, which upregulate CCL20, the ligand for the Th17 cell receptor CCR6, causing the homing of circulating Th17 cells into the BM.Th17 cells release Il-17, which induce the production of RANKL by osteoblasts and osteocytes, causing bone loss. Reproduced with Permission from Springer Nature from: Yu M, Malik Tyagi A, Li JY, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat Commun. 2020;11(1):468.
Osteoimmunological Effects of Intermittent PTH Treatment
The bone anabolic activity of teriparatide is modeled in mice by daily administration of PTH, a regimen known as intermittent PTH (iPTH) treatment. iPTH markedly increases bone volume and strength due to a stimulation of bone formation tempered by a more moderate increase in resorption (98, 99).
Studies conducted in T-cell–deficient mice revealed that mice lacking T cells or the expression of the PTH receptor PPR in T cells, exhibit a blunted increase in bone formation and trabecular bone volume in response to iPTH (59, 73). Furthermore, adoptive transfer of T cells into T-cell–deficient mice restored a normal response to iPTH. Mechanistic studies revealed that in the absence of T cells, iPTH is unable to increase the commitment of stromal cells to the osteoblastic lineage, induce osteoblast proliferation and differentiation, and mitigate osteoblast apoptosis. All of these actions of PTH were found to center on the capacity of T cells to activate Wnt signaling in osteoblastic cells (59).
iPTH activates Wnt signaling through multiple mechanisms, including blunted osteocytic production of the Wnt inhibitor sclerostin (100-102) and decreased production by osteoblasts of the Wnt inhibitor Dkk1 (103). In addition, teriparatide and iPTH treatment activate Wnt signaling by inducing the production of Wnt10b, an osteogenic Wnt ligand secreted by T cells (59, 73, 74, 104). Animal studies further show that the relevant pool of Wnt10b derives from BM CD8+ T cells (59, 73, 74). Accordingly, global or T-cell–specific silencing of Wnt10b production blocks the iPTH-induced bone anabolism (59, 73, 74). By contrast, the anabolic effects of iPTH in cortical bone are completely T-cell–independent (59, 73, 74), likely due to the fact that T cells have no contacts with periosteal surfaces and have limited capacity to communicate with osteocytes.
iPTH causes a ~2-3-fold increase in the number of BM regulatory T cells (Tregs) in humans and mice, which is required for iPTH to exert its bone anabolic activity (77). Tregs are suppressive helper CD4+ T cells that play an essential role in dampening inflammation and maintaining immune tolerance (105). Mechanistically, Treg repression of CD8+ T cells promotes binding of NFAT1/2 and SMAD3 complexes to a NFAT/SMAD-activated Wnt10b promoter region that stimulates Wnt10b gene expression in CD8+ T cells. A regulatory effect of iPTH on the production of Wnt10b has been confirmed in humans (104).
Microbiome and PTH-Induced Bone Formation
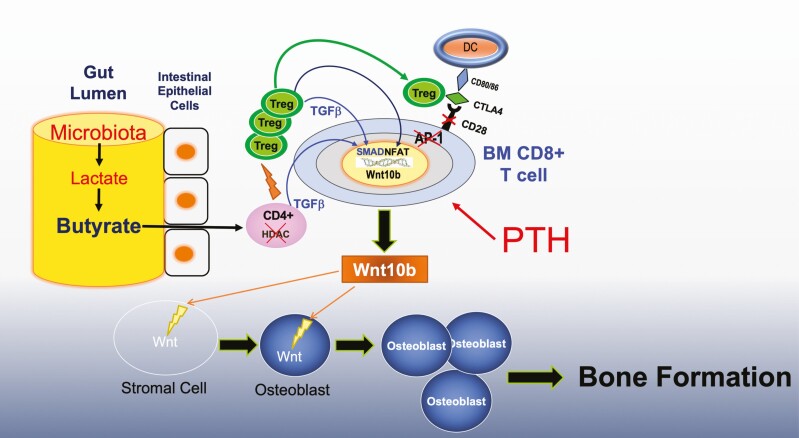
Recently, it has become evident that Tregs are important regulators of bone remodeling. Tregs reside preferentially on the endosteal surfaces of bone (106), where they regulate osteoclast formation (107-109), blunt bone resorption (108, 110), and prevent ovariectomy-induced bone loss (111). In addition, Tregs are recognized as mediators of the bone anabolic activity of the probiotic Lactobacillus rhamnosus GG (LGG) (112, 113). Supporting a role for Tregs in the skeletal activity of iPTH are reports that blockade of Treg expansion silences the bone anabolic activity of PTH (77). The differentiation of naïve CD4+ T cells into Tregs is potently induced by SCFAs (114-116). Many bacterial species contained in the gut microbiota of animals and humans can digest carbohydrates to generate the SCFAs butyrate, propionate, and acetate (117). This suggests that microbiota-induced generation of SCFAs in the gut may promote Treg differentiation in BM, which is pivotal for the bone anabolic activity of iPTH. This hypothesis was validated in a recent report that showed that the production of permissive amounts of butyrate from the gut microbiota was required for iPTH to induce bone anabolism in mice (118). This conclusion was obtained injecting iPTH in conventionally raised mice treated with antibiotics or in germ-free mice. These experiments clearly demonstrate that iPTH does not induce bone anabolism in the absence of the gut microbiome. Antibiotic treatment decreases the circulating levels of butyrate by ~50 % and blocks the expansion of intestinal and BM Tregs induced by iPTH. However, oral supplementation with doses of butyrate, reinstating physiologic circulating levels of butyrate, restored Treg expansion and the bone anabolic activity of iPTH. Butyrate stimulates Treg differentiation via multiple mechanisms, including receptor independent effects on naïve T cells and receptor mediated activation of dendritic cells. The effects of iPTH on Treg differentiation, upregulation of Wnt10b production, and bone anabolism were blocked by deletion of the butyrate receptor GPR43. Thus GPR43 plays a pivotal role in the mechanism by which butyrate potentiates the activity of iPTH (Fig. 2). It should be noted that when butyrate circulates at concentrations 2 to 3 times above physiologic levels, as in the case of mice treated with probiotics, this SCFA can directly upregulate Treg differentiation. However, when butyrate circulates at physiologic doses, additional stimuli, such as treatment with iPTH, are required to induce Treg expansion. Thus, physiologic levels of butyrate exert the permissive function to enable PTH to induce Treg expansion. While butyrate induces Treg differentiation via multiple mechanisms, the permissive activity of butyrate in iPTH-treated mice involve activation of the SCFA receptor GPR43 in dendritic cells and GPR43 independent targeting of T cells (118).
Figure 2.
Diagrammatic representation of the mechanism of action of iPTH in bone. The intestinal microbiota produces butyrate via fermentation of complex carbohydrates. Intestinal butyrate diffuses through the intestinal wall into the systemic circulation, which carries it to distant organs. In the bone marrow (BM), butyrate enhances the differentiation of naïve helper CD4+ cells into regulatory T cells (Tregs) in concert with PTH. The expansion of BM Tregs makes possible for PTH to induce the production of wnt10b by BM CD8+ T cells. Wnt10b activates Wnt signaling in BM stromal cells, causing their proliferation and differentiation into osteoblasts. The expansion of the osteoblastic population results in increased bone formation and improved bone structure.
Conclusions
One of the most intriguing aspect of PTH biology is the capacity of the hormone to induce opposite skeletal responses depending on whether the PTH administration regimen is continuous or intermittent. Among the hypotheses put forward to explain this phenomenon is a differential effect of cPTH and iPTH on osteoblast life span, which is based on the observation that iPTH attenuates osteoblast apoptosis (68, 69, 119, 120), while cPTH does not (69). However, this explanation does not account for the differences in bone resorption elicited by iPTH and cPTH. In vitro studies have led to the alternative hypothesis that the bone response to PTH reflects the intermittent or continuous activation of PPR in bone cells (121, 122). However, this theory does not explain why transgenic mice expressing a constitutively active PPR in osteoblasts or osteocytes exhibit a dramatic increase in trabecular bone formation (56, 70) that resembles that induced by iPTH. Reports of differential osteoimmunological effects of cPTH and iPTH T cells introduced an additional layer of complexity and possibilities. The recent discovery that the microbiota plays a pivotal role in the skeletal activities of PTH provide an example of unsuspected integration and complexity.
PTH is a calciotrophic hormone critical for skeletal development. Similar to butyrate, PTH stimulates bone formation and induces bone anabolism via the Treg/Wnt10b/Wnt signaling pathway (52, 99, 123). BM CD8+ T cells respond to PTH and butyrate by releasing Wnt10b, while silencing of Wnt10b expression by CD8+ T cells blocks the capacity of PTH and butyrate to stimulate bone formation and increase bone volume (59, 73, 74, 112). Moreover, PTH and butyrate increase the production of Wnt10b by CD8+ T cells by expanding Tregs (77, 112).
The evolutionary advantage of the mechanistic convergence between the skeletal effects of SCFAs and those of PTH remains unknown, but it is tempting to speculate that it may be related to energy balance during health and sickness. Immune cells depend on calcium for their activation (124). A highly activated immune system is accompanied by sickness behavior and anorexia, which renders the immune system dependent on calcium released by bone resorption rather than the calcium absorbed in the gut. A consequence of starvation is hypocalcemia that in turn leads to continuous production of PTH, which stimulates bone resorption causing calcium release, which then becomes available for immune cell activation (125). Food ingestion interrupts PTH secretion, causing the pattern of PTH release to change from continuous to intermittent. It is only when intermittently produced that PTH exerts a net bone anabolic activity. This activity hinges on a mechanism involving Tregs and Wnt10b. One goal of this response might be to induce calcium deposition in the skeleton, so as to create a calcium reserve for the immune system. Generation of SCFAs is an event linked to food intake. Thus, SCFAs generation may signal the presence of normal state of health, thereby activating a pathway that replenishes calcium reserve in the skeleton. Thus, SCFAs may act in concert with intermittent PTH release to expand Tregs and stimulate bone formation. Further studies will be required to define the relationship between SCFAs and PTH, to demonstrate a role of butyrate in the bone anabolic activity of PTH in humans, and to develop bone anabolic strategies based on the use of nutritional supplementation with SCFAs.
Acknowledgments
Financial Support: This study was supported by grants from the National Institutes of Health (DK112946, DK108842, and RR028009).
Conflict of interest: The author has no conflict of interest exists.
Glossary
Abbreviations
- BM
bone marrow
- cPTH
continuous PTH infusion
- IGF
insulin-like growth factor
- IL-
interleukin
- iPTH
intermittent PTH infusion
- PHPT
primary hyperparathyroidism
- PPR
PTH-PTHrP receptor
- PTH
parathyroid hormone
- S1P
sphingosine 1 phosphate
- S1PR1
sphingosine 1 phosphate receptor
- SCFA
short-chain fatty acid
- SFB
segmented filamentous bacteria
- TNF
tumor necrosis factor
- Treg
regulatory T cell
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Sommer F, Bäckhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227-238. [DOI] [PubMed] [Google Scholar]
- 2. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242-249. [DOI] [PubMed] [Google Scholar]
- 3. Krych L, Hansen CH, Hansen AK, van den Berg FW, Nielsen DS. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. Plos One. 2013;8(5):e62578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915-1920. [DOI] [PubMed] [Google Scholar]
- 6. Li JY, Chassaing B, Tyagi AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ismail AS, Hooper LV. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;289(5):G779-G784. [DOI] [PubMed] [Google Scholar]
- 8. Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121(3):580-591. [DOI] [PubMed] [Google Scholar]
- 9. Alam A, Leoni G, Quiros M, et al. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alam A, Leoni G, Wentworth CC, et al. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7(3):645-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jones RM, Luo L, Ardita CS, et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. Embo J. 2013;32(23):3017-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wentworth CC, Alam A, Jones RM, Nusrat A, Neish AS. Enteric commensal bacteria induce extracellular signal-regulated kinase pathway signaling via formyl peptide receptor-dependent redox modulation of dual specific phosphatase 3. J Biol Chem. 2011;286(44):38448-38455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99(24):15451-15455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102(1):99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faith JJ, Guruge JL, Charbonneau M, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faith JJ, Colombel JF, Gordon JI. Identifying strains that contribute to complex diseases through the study of microbial inheritance. Proc Natl Acad Sci U S A. 2015;112(3):633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vindigni SM, Surawicz CM. Fecal Microbiota Transplantation. Gastroenterol Clin North Am. 2017;46(1):171-185. [DOI] [PubMed] [Google Scholar]
- 20. Hemminki K, Li X, Sundquist K, Sundquist J. Familial association of inflammatory bowel diseases with other autoimmune and related diseases. Am J Gastroenterol. 2010;105(1):139-147. [DOI] [PubMed] [Google Scholar]
- 21. Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661-668. [DOI] [PubMed] [Google Scholar]
- 22. Westerlind H, Ramanujam R, Uvehag D, et al. Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain. 2014;137(Pt 3):770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Surana NK, Kasper DL. Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest. 2014;124(10):4197-4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazmanian SK, Kasper DL. The love-hate relationship between bacterial polysaccharides and the host immune system. Nat Rev Immunol. 2006;6(11):849-858. [DOI] [PubMed] [Google Scholar]
- 26. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372-385. [DOI] [PubMed] [Google Scholar]
- 28. Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542(7640):242-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yan J, Herzog JW, Tsang K, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554-E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Novince CM, Whittow CR, Aartun JD, et al. Commensal gut microbiota immunomodulatory actions in bone marrow and liver have catabolic effects on skeletal homeostasis in health. Sci Rep. 2017;7(1):5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linden DR. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal. 2014;20(5):818-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med. 2013;60:195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y, Yang R, Liu X, et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell. 2014;15(1):66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grassi F, Tyagi AM, Calvert JW, et al. Hydrogen sulfide is a novel regulator of bone formation implicated in the bone loss induced by estrogen deficiency. J Bone Miner Res. 2016;31(5):949-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Wang Y, Gao W, et al. Diversity analysis of gut microbiota in osteoporosis and osteopenia patients. Peerj. 2017;5:e3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li C, Huang Q, Yang R, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporos Int. 2019;30(5):1003-1013. [DOI] [PubMed] [Google Scholar]
- 38. Sjögren K, Engdahl C, Henning P, et al. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwarzer M, Makki K, Storelli G, et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science. 2016;351(6275):854-857. [DOI] [PubMed] [Google Scholar]
- 40. Li JY, Chassaing B, Tyagi AM, et al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schepper JD, Collins FL, Rios-Arce ND, et al. Probiotic lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J Bone Miner Res. 2019;34(4):681-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pytlik M, Folwarczna J, Janiec W. Effects of doxycycline on mechanical properties of bones in rats with ovariectomy-induced osteopenia. Calcif Tissue Int. 2004;75(3):225-230. [DOI] [PubMed] [Google Scholar]
- 43. Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13(1):7-27. [DOI] [PubMed] [Google Scholar]
- 44. Riggs BL, Melton LJ. Medical progress: involutional osteoporosis. N Eng J Med. 1986;314:1676-1684. [DOI] [PubMed] [Google Scholar]
- 45. Grey AB, Stapleton JP, Evans MC, Reid IR. Accelerated bone loss in post-menopausal women with mild primary hyperparathyroidism. Clin Endocrinol (Oxf). 1996;44(6):697-702. [DOI] [PubMed] [Google Scholar]
- 46. Potts J. Primary hyperparathyroidism. In: Avioli LV, Krane S, eds. Metabolic Bone Diseases. vol 1. 3rd ed. San Diego: Academic Press; 1998:411-442. [Google Scholar]
- 47. Parisien M, Dempster DW, Shane E, Bilezikian JP. Histomorphometric Analysis of Bone in Primary Hyperparathyroidism. The Parathyroids. Basic and Clinical Concepts. San Diego: Academic Press; 2001:423-436. [Google Scholar]
- 48. Silverberg SJ, Shane E, de la Cruz L, et al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4(3):283-291. [DOI] [PubMed] [Google Scholar]
- 49. Bilezikian JP. Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2018;103(11):3993-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pierreux J, Bravenboer B. Normocalcemic primary hyperparathyroidism: a comparison with the hypercalcemic form in a tertiary referral population. Horm Metab Res. 2018;50(11):797-802. [DOI] [PubMed] [Google Scholar]
- 51. Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14(6):690-709. [DOI] [PubMed] [Google Scholar]
- 52. Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15(2):60-65. [DOI] [PubMed] [Google Scholar]
- 53. Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791-801. [DOI] [PubMed] [Google Scholar]
- 54. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434-1441. [DOI] [PubMed] [Google Scholar]
- 55. Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357(9):905-916. [DOI] [PubMed] [Google Scholar]
- 56. Calvi LM, Sims NA, Hunzelman JL, et al. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lanske B, Amling M, Neff L, Guiducci J, Baron R, Kronenberg HM. Ablation of the PTHrP gene or the PTH/PTHrP receptor gene leads to distinct abnormalities in bone development. J Clin Invest. 1999;104(4):399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Powell WF Jr, Barry KJ, Tulum I, et al. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol. 2011;209(1):21-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Terauchi M, Li JY, Bedi B, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10(3):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saini V, Marengi DA, Barry KJ, et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J Biol Chem. 2013;288(28):20122-20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xiong J, Piemontese M, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone. 2014;66:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ben-awadh AN, Delgado-Calle J, Tu X, et al. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology. 2014;155(8):2797-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21(3):366-373. [DOI] [PubMed] [Google Scholar]
- 64. Ma YL, Zeng Q, Donley DW, et al. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(6):855-864. [DOI] [PubMed] [Google Scholar]
- 65. Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136(8):3632-3638. [DOI] [PubMed] [Google Scholar]
- 66. Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42(4):806-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meng XW, Liang XG, Birchman R, et al. Temporal expression of the anabolic action of PTH in cancellous bone of ovariectomized rats. J Bone Miner Res. 1996;11(4):421-429. [DOI] [PubMed] [Google Scholar]
- 68. Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104(4):439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278(50):50259-50272. [DOI] [PubMed] [Google Scholar]
- 70. O’Brien CA, Plotkin LI, Galli C, et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. Plos One. 2008;3(8):e2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gao Y, Wu X, Terauchi M, et al. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8(2):132-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tawfeek H, Bedi B, Li JY, et al. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. Plos One. 2010;5(8):e12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bedi B, Li JY, Tawfeek H, et al. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci U S A. 2012;109(12):E725-E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li JY, Walker LD, Tyagi AM, Adams J, Weitzmann MN, Pacifici R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J Bone Miner Res. 2014;29(1):43-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li JY, D’Amelio P, Robinson J, et al. IL-17A is increased in humans with primary hyperparathyroidism and mediates PTH-induced bone loss in mice. Cell Metab. 2015;22(5):799-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Robinson JW, Li JY, Walker LD, et al. T cell-expressed CD40L potentiates the bone anabolic activity of intermittent PTH treatment. J Bone Miner Res. 2015;30(4):695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu M, D’Amelio P, Tyagi AM, et al. Regulatory T cells are expanded by Teriparatide treatment in humans and mediate intermittent PTH-induced bone anabolism in mice. EMBO Rep. 2018;19(1):156-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yu M, Malik Tyagi A, Li JY, et al. PTH induces bone loss via microbial-dependent expansion of intestinal TNF+ T cells and Th17 cells. Nat Commun. 2020;11(1):468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203(12):2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361(9):888-898. [DOI] [PubMed] [Google Scholar]
- 81. Basu R, Hatton RD, Weaver CT. The Th17 family: flexibility follows function. Immunol Rev. 2013;252(1):89-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Komatsu N, Takayanagi H. Autoimmune arthritis: the interface between the immune system and joints. Adv Immunol. 2012;115:45-71. [DOI] [PubMed] [Google Scholar]
- 83. Waisman A. T helper cell populations: as flexible as the skin? Eur J Immunol. 2011;41(9):2539-2543. [DOI] [PubMed] [Google Scholar]
- 84. Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160(7):3513-3521. [PubMed] [Google Scholar]
- 85. Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149-162. [DOI] [PubMed] [Google Scholar]
- 86. Li JY, Yu M, Tyagi AM, et al. IL-17 receptor signaling in osteoblasts/osteocytes mediates PTH-induced bone loss and enhances osteocytic RANKL production. J Bone Miner Res. 2018. [DOI] [PubMed] [Google Scholar]
- 87. Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677-689. [DOI] [PubMed] [Google Scholar]
- 89. Atarashi K, Tanoue T, Ando M, et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell. 2015;163(2):367-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tan TG, Sefik E, Geva-Zatorsky N, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci U S A. 2016;113(50):E8141-E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baeyens A, Fang V, Chen C, Schwab SR. Exit strategies: S1P signaling and T cell migration. Trends Immunol. 2015;36(12):778-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Krebs CF, Paust HJ, Krohn S, et al. Autoimmune renal disease is exacerbated by S1P-receptor-1-dependent intestinal Th17 cell migration to the kidney. Immunity. 2016;45(5):1078-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wu HJ, Ivanov II, Darce J, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bradley CP, Teng F, Felix KM, et al. Segmented filamentous bacteria provoke lung autoimmunity by inducing gut-lung axis Th17 cells expressing dual TCRs. Cell Host Microbe. 2017;22(5):697-704.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214(2):179-189. [DOI] [PubMed] [Google Scholar]
- 96. Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8(6):639-646. [DOI] [PubMed] [Google Scholar]
- 97. Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409-426. [DOI] [PubMed] [Google Scholar]
- 98. Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone. 1995;16(4): 477-484. [DOI] [PubMed] [Google Scholar]
- 99. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148-158. [DOI] [PubMed] [Google Scholar]
- 101. Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577-4583. [DOI] [PubMed] [Google Scholar]
- 102. Silvestrini G, Ballanti P, Leopizzi M, et al. Effects of intermittent parathyroid hormone (PTH) administration on SOST mRNA and protein in rat bone. J Mol Histol. 2007;38(4): 261-269. [DOI] [PubMed] [Google Scholar]
- 103. Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab. 2010;11(2):161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. D’Amelio P, Sassi F, Buondonno I, et al. Treatment with intermittent PTH increases Wnt10b production by T cells in osteoporotic patients. Osteoporos Int. 2015;26(12): 2785-2791. [DOI] [PubMed] [Google Scholar]
- 105. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30(5):636-645. [DOI] [PubMed] [Google Scholar]
- 106. Fujisaki J, Wu J, Carlson AL, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zaiss MM, Axmann R, Zwerina J, et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56(12):4104-4112. [DOI] [PubMed] [Google Scholar]
- 108. Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2007;357(4):1046-1052. [DOI] [PubMed] [Google Scholar]
- 109. Kelchtermans H, Geboes L, Mitera T, Huskens D, Leclercq G, Matthys P. Activated CD4+CD25+ regulatory T cells inhibit osteoclastogenesis and collagen-induced arthritis. Ann Rheum Dis. 2009;68(5):744-750. [DOI] [PubMed] [Google Scholar]
- 110. Yuan FL, Li X, Lu WG, et al. Regulatory T cells as a potent target for controlling bone loss. Biochem Biophys Res Commun. 2010;402(2):173-176. [DOI] [PubMed] [Google Scholar]
- 111. Zaiss MM, Sarter K, Hess A, et al. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum. 2010;62(8):2328-2338. [DOI] [PubMed] [Google Scholar]
- 112. Tyagi AM, Yu M, Darby TM, et al. The microbial metabolite butyrate stimulates bone formation via T regulatory cell-mediated regulation of WNT10B expression. Immunity. 2018;49(6):1116-1131.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zaiss MM, Jones RM, Schett G, Pacifici R. The gut-bone axis: how bacterial metabolites bridge the distance. J Clin Invest. 2019;129(8):3018-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446-450. [DOI] [PubMed] [Google Scholar]
- 116. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bach Knudsen KE. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr. 2015;6(2):206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Li JY, Yu M, Pal S, et al. Microbiota dependent production of butyrate is required for the bone anabolic activity of PTH. J Clin Invest. 2020. [Google Scholar]
- 119. Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by beta-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280(50):41342-41351. [DOI] [PubMed] [Google Scholar]
- 120. Tobimatsu T, Kaji H, Sowa H, et al. Parathyroid hormone increases beta-catenin levels through Smad3 in mouse osteoblastic cells. Endocrinology. 2006;147(5):2583-2590. [DOI] [PubMed] [Google Scholar]
- 121. Ishizuya T, Yokose S, Hori M, et al. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99(12):2961-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Locklin RM, Khosla S, Turner RT, Riggs BL. Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem. 2003;89(1):180-190. [DOI] [PubMed] [Google Scholar]
- 123. Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res. 2010;25(2):178-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7(9):690-702. [DOI] [PubMed] [Google Scholar]
- 125. Straub RH, Cutolo M, Pacifici R. Evolutionary medicine and bone loss in chronic inflammatory diseases–A theory of inflammation-related osteopenia. Semin Arthritis Rheum. 2015;45(2):220-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.