Abstract
Context
Functional hypothalamic amenorrhea (HA) is a common, acquired form of hypogonadotropic hypogonadism that occurs in the setting of energy deficits and/or stress. Variability in individual susceptibility to these stressors, HA heritability, and previous identification of several rare sequence variants (RSVs) in genes associated with the rare disorder, isolated hypogonadotropic hypogonadism (IHH), in individuals with HA suggest a possible genetic contribution to HA susceptibility.
Objective
We sought to determine whether the burden of RSVs in IHH-related genes is greater in women with HA than controls.
Design
We compared patients with HA to control women.
Setting
The study was conducted at secondary referral centers.
Patients and Other Participants
Women with HA (n = 106) and control women (ClinSeq study; n = 468).
Interventions
We performed exome sequencing in all patients and controls.
Main Outcome Measure(s)
The frequency of RSVs in 53 IHH-associated genes was determined using rare variant burden and association tests.
Results
RSVs were overrepresented in women with HA compared with controls (P = .007). Seventy-eight heterozygous RSVs in 33 genes were identified in 58 women with HA (36.8% of alleles) compared to 255 RSVs in 41 genes among 200 control women (27.2%).
Conclusions
Women with HA are enriched for RSVs in genes that cause IHH, suggesting that variation in genes associated with gonadotropin-releasing hormone neuronal ontogeny and function may be a major determinant of individual susceptibility to developing HA in the face of diet, exercise, and/or stress.
Keywords: female reproduction, genetics, hypogonadotropic hypogonadism, GnRH deficiency, exome sequencing
Functional hypothalamic amenorrhea (HA) is a reversible form of hypogonadotropic hypogonadism (HH) that is defined by 3 to 6 months of amenorrhea in the absence of pregnancy, androgen excess, hyperprolactinemia, or other endocrine dysfunction. Women with HA demonstrate variable patterns of deficient pulsatile luteinizing hormone secretion, from apulsatile secretion to sleep augmentation (1-4), indicating that HA represents a spectrum of gonadotropin-releasing hormone (GnRH) secretory dysfunction. Epidemiologic studies that define HA as 3 months of amenorrhea in women with no history of oligomenorrhea (an irregular bleeding pattern that would be more consistent with the diagnosis of polycystic ovarian syndrome) suggest a population prevalence of approximately 4.5% (5).
The prevalence of HA is significantly greater in individuals who exercise intensely, have clinical, or even subclinical, eating disorders (6, 7), and/or have faced stressful life situations, such as abuse, divorce, the unexpected death of a relative, and related trauma (8, 9). A number of studies have shown that caloric restriction, decreased body fat, and vigorous exercise training are all associated with a higher incidence of HA (6). Thus, a negative energy balance (ie, more energy expended than is taken in), however achieved (10, 11), appears to be a primary mediator of neuroreproductive compromise in women. Depression, dysfunctional attitudes (12), stress (13), and/or activation of the hypothalamic-pituitary-adrenal axis (14-18) also appear to play a role in the development of HA. Importantly, we have previously shown that HA is reversible in 83% of cases, with a mean duration of HA of 7.5 years (range, 2.5-17 years), particularly in the context of subclinical eating disorder or stress-induced weight loss (4). Women with typical HA, associated with a clear risk factor, had an increased likelihood of recovery of menstrual function than women with idiopathic HA (71% vs 29%, respectively). By contrast, recovery has been described in approximately 15% of cases of congenital isolated hypogonadotropic hypogonadism (IHH), though few such cases have been identified in women (19). Taken together, these studies suggest that a combination of physiological stressors (excessive exercise, undernutrition, and increased stress) play a causative role in functional HA.
What is less recognized is the marked interindividual variability of menstrual cycle and/or hormonal responses to apparently similar stressors. For example, in early studies that related menstrual cycle characteristics to body fat as determined by total water/body weight percentage, in a girl of 165 cm with secondary amenorrhea, the 95% CI associated with resumption of menses ranged from 43 to 60 kg (20). Likewise, in studies of middle-distance runners, amenorrhea was positively associated with miles run per week, but even at 80 miles per week, only 50% of athletes were amenorrheic (6). While differences in the relationship between caloric intake and energy expenditure may account for at least some of the interindividual variability in these observational studies, a similar heterogeneity in reproductive axis response has been demonstrated in precisely controlled interventional studies in which a negative energy balance was achieved through alterations in diet and/or exercise (21). We therefore hypothesized that some women with HA may have a genetic susceptibility to reproductive-axis dysfunction that is manifest only in the setting of energy deficit or significant stress. Heritability studies, including twin studies, of HA have not been published. However, the idea of heritability of this phenotype has been bolstered by studies in a female macaque model of HA that indicate that there are stress-sensitive and stress-resilient monkeys and that stress-sensitive monkeys have a significantly higher number of GnRH neurons but a lower density of GnRH fibers in the median eminence compared with highly stress-resilient monkeys (22) Taken together, these studies suggest that genetic differences in genes that control the development and/or function of GnRH neurons contribute to variability in reproductive responses to physiologic stressors.
Pathogenic variants in genes that control GnRH neuronal migration, secretion, or action at the pituitary have been discovered in individuals with a congenital form of IHH who fail to enter puberty. IHH is subdivided into normosmic IHH (nIHH) when olfaction is intact and Kallmann syndrome (KS) when olfaction is deficient or absent. After identifying individuals with HA in some multiplex families with KS/nIHH, we previously screened 55 patients with HA for mutations in 7 GnRH-related genes. Although we identified 6 heterozygous rare sequence variants (RSVs) in 7 of the HA women that were not present in 422 healthy control women (23), this study had important limitations that have recently come to light because of major advances in our understanding of human genetics. In particular, our previous approach did not test whether control women may have harbored additional deleterious variants in these GnRH-related genes beyond the specific RSVs identified in the HA cases. In addition, the study was likely underpowered for detecting significant differences between HA and control women using rare-variant association testing (24). Thus, our previous study did not determine whether there was an overrepresentation, that is, an increased burden, of KS/nIHH-associated RSVs in patients with HA. We have addressed this question in the present study after assembling a larger cohort of individuals with HA and by using a greatly expanded KS/nIHH-associated gene panel.
Materials and Methods
Study participants
All research protocols were approved by institutional review boards at the National Institutes of Health Clinical Center (National Institute of Child Health and Human Development, National Human Genome Research Institute) or Massachusetts General Hospital/Partners Healthcare. Participants provided written informed consent for participation.
Participants with functional hypothalamic amenorrhea
Women with HA (n = 106) were referred to either the National Institutes of Health Clinical Center or the Massachusetts General Hospital for participation in the research study. A small number of individuals participated remotely (14 from Chile, 8 from the UK). Participants were diagnosed by an endocrinologist based on a history of secondary amenorrhea of at least 6 months’ duration; or primary amenorrhea with a history of spontaneous thelarche and clear risk factors for HA during ages 11 to 16 years. Risk factors for HA were defined as more than 5 hours per week of exercise or more than 20 miles/week of running, caloric restriction (self-report of dieting/restricting food intake, weight loss at the time of diagnosis, and/or loss of > 15% body weight) (23), and/or self-report of significant psychological stress (eg, death of a close family member, parental divorce, unexpected unemployment). None of the women were pregnant, had a history of oligoamenorrhea (defined as irregular cycles) or clinical evidence of hyperandrogenism, or other endocrine or chronic disorder that could cause menstrual cycle abnormalities, and none had a family history of IHH. Phenotype and partial genotype data from a subset of the HA patients described herein have been included in previous publications from our group (n = 48 in Caronia et al [23]; n = 49 in Perkins et al [3, 4]; and n = 2 in Shaw et al [25]).
Healthy control women
Controls consisted of a sample of 477 women between ages 45 and 65 years at enrollment who were selected for a range of atherosclerosis phenotypes as part of the ClinSeq Project (26). An abbreviated health history was collected, and the initial clinical assessment included height, weight, head and waist circumferences, blood pressure, and other measures of cardiovascular health, as well as a broad panel of blood and urine tests. Reproductive history was not collected in this cohort at the time of enrollment. However, because informed consent for future clinical evaluation had been obtained as part of enrollment, we attempted to recontact all participants to complete a brief survey of their reproductive health history. A history of spontaneous menarche was considered suggestive of a history of HA. Of the 125 women who completed the survey, 9 had a history suggestive of HA and were excluded from analysis. None of these 9 women were obese, and the 2 who reported some acne and body hair in androgen-sensitive areas had clear HA risk factors (intense military training; subclinical eating disorder and emotional stress).
Genetic studies
Peripheral blood samples were collected from all participants to extract genomic DNA. Exome sequencing (ES) was performed on HA participants at the Broad Institute (Cambridge, Massachusetts, USA; n = 100) or the Yale Center for Mendelian Genomics (Orange, Connecticut, USA; n = 6). Controls were sequenced at the NIH Intramural Sequencing Center (Bethesda, Maryland, USA), and analyzed as described (27). Alignment of the ES data against the reference genome (hg19), initial quality control, and variant calling algorithms were applied using GATK best practices (Broad Institute), as previously described (28). Briefly, reads were aligned using bwa aln 0.7.15, duplicate fragments were flagged using Picard Tools MarkDuplicates 2.9.2, and base quality score recalibration and local realignment were performed using GATK 3.7. Variant calling and joint genotyping were performed using GATK components HaplotypeCaller and GenotypeGVCFs. VCF files were annotated using SnpSift 4.3k and Ensembl VEP release 93. To fully explore the role of GnRH-associated genes, the analysis was based on a comprehensive list of genes that have been associated with nIHH and/or KS with or without other syndromic features: ANOS1 (KAL1), AXL, CCDC141, CHD7, DCC, DLX1, DMXL2, DUSP6, FEZF1, FGF17, FGF8, FGFR1, FLRT3, GLCE, GNRH1, GNRHR, HESX1, HS6ST1, IL17RD, KISS1, KISSR1, KL, KLB, LEP, LEPR, LHX3, NR0B1 (DAX1), NR5A1, NSMF (NELF), NTN1, OTUD4, PCSK1, PNPLA6, POLR3A, POLR3B, PROK2, PROKR2, PROP1, RAB3GAP1, RAB3GAP2, RNF216, SEMA3A, SEMA3E, SMCHD1, SOX10, SOX2, SPRY4, SRA1, STUB1, TAC3, TACR3, TUBB3, and WDR11 (29).
Rare sequence variants in these 53 genes were initially selected based on the following criteria: variants on canonical transcripts that passed all GATK filters; variants with minor allele frequency (MAF) of less than 1% in all populations included in the gnomAD browser, to limit the inclusion of ethnicity-specific common polymorphisms (30, 31); missense, nonsense, frameshift, canonical splice (± 1-2 base pair) variants, and in-frame indels; and variants with common capture intervals across all sequencing platforms. Regions with median coverage of less than 10× were identified for exclusion from analysis, but no variants from either cases or controls were in these regions.
The Human Gene Mutation Database (HGMD) (32), ClinVar (33), and PubMed were reviewed for each RSV to determine whether there was a prior association with IHH. If the specific variant was identified in an individual with IHH, it was considered associated with IHH, regardless of functional data supporting the association, for the purposes of this analysis.
Statistical analyses
The total number of alternate and reference nucleotides across the coding regions of all 53 genes were aggregated into a single alternate allele count (AAC) and reference allele count (RAC) per group. The AACs and RACs were then used in a single rare variant burden test between the HA participants and control women (Fisher exact test). Because the deleteriousness of these RSVs is largely unconfirmed, we performed additional region-based rare variant association tests, including the C-α test (34), the sequence kernel association test (SKAT) (35), and UNIQ (36) using PLINK/SEQ version 0.10 (http://atgu.mgh.harvard.edu/plinkseq/). These tests are more powerful in the setting of many noncausal variants or if the causal variants have different directions of effect [37]. Comparisons between categorical phenotypic variables were performed using Fisher exact test and continuous phenotypic variables were compared using t test or Mann-Whitney rank-sum test, as appropriate. Results are presented as mean ± SD, unless otherwise indicated.
Results
Participant characteristics
HA cases and controls were predominantly White (n = 97 [92%] and 419 [88%], respectively), non-Hispanic/Latina women (67 [63%] and 459 [96%], respectively) based on self-report by all individuals. There were more Black (28 [6%]) and Asian (22 [5%]) participants in the control group (both vs 0 in HA cases; Table 1), and more Hispanic or Latinas (16 [15%)] in the HA group (controls, 15 [3%]; P < .001). The race and ethnicity distribution of controls was consistent with the community from which they were recruited for participation in the ClinSeq study. These differences do not contribute in a significant way to interpretation of our results because variants were excluded based on MAF in all racial/ethnic populations available in the gnomAD database, which greatly reduces the possibility of including population-specific common polymorphisms. The mean body mass index (20.2 ± 3 kg/m2) in the HA group was lower than in controls (26.5 ± 5.6, P < .001) as might be expected; however, body mass index data for controls were obtained at the time of enrollment, when they were older than 45 years, limiting the significance of this finding. Additional clinical data were not available for controls, but they were recontacted for additional reproductive health history.
Table 1.
Ethnicity of participants with functional hypothalamic amenorrhea and control women
| HA | Controls | |
|---|---|---|
| No. | 106 | 477 |
| Race, self-reported; n (%) | ||
| White | 97 (91.5) | 419 (87.8) |
| Blacka | 0 | 28 (5.9) |
| Asian | 0 | 22 (4.6) |
| Mixed | 1 (0.9) | 2 (0.4) |
| Other/Unknowna | 8 (7.5) | 6 (1.3) |
| Ethnicity, self-reported; n (%) | ||
| Hispanicb | 16 (15.1) | 15 (3.1) |
| Not Hispanicb | 67 (63.2) | 459 (96.2) |
| Not reportedb | 23 (24.5) | 3 (0.6) |
| BMI at diagnosis, kg/ m2; mean (SD)b | 20.2 (3.0) n = 97 | 26.5 (5.6) n = 457 |
Abbreviations: BMI, body mass index; HA, hypothalamic amenorrhea.
a P less than .01. bP less than .001.
Ninety-two percent of women with HA had a history of secondary amenorrhea beginning at an average age of 20.9 ± 6.2 years. Among these women, 28% reported delayed menarche (age > 15 years; Table 2). Primary amenorrhea was seen in 7.5%. All HA participants had one or more of the following risk factors for HA: nutritional risk factors (weight loss or caloric restriction; n = 52), intensive exercise (n = 57), or significant psychological stress (n = 59). None of the 73 women with family histories available had a history of IHH in the family; however, 19 women reported a family history of HA and 25 had a family history of delayed puberty. Detailed family history of reproductive disorders was not available in control women.
Table 2.
Clinical characteristics of participants with hypothalamic amenorrhea
| Clinical characteristics | HA participants |
|---|---|
| Age at menarche, mean (SD), y | 13.6 (1.9) |
| Delayed menarche, age > 15 y; n (%) | 30 (28.3) |
| Age at amenorrhea, mean (SD), y | 20.9 (6.2) |
| Primary amenorrhea, n (%) | 8 (7.5) |
| Family history of HA, n (%)a | 19 (26.0) |
| Family history of delayed puberty, n (%)a | 25 (23.6) |
| Risk factors | |
| Weight loss/caloric restriction, n (%) | 52 (49.1) |
| Intensive exercise, n (%) | 57 (53.8) |
| Psychosocial stress, n (%) | 59 (55.7) |
Abbreviations: HA, hypothalamic amenorrhea.
a Percentage of those with family histories (n = 73).
Women with hypothalamic amenorrhea are enriched with rare sequence variants in isolated hypogonadotropic hypogonadism genes
We identified 78 heterozygous variants in 58 of 106 women with HA (Table 3, Supplemental Table [38]), including 68 unique missense, 1 nonsense, and 2 frameshift variants. We confirmed 3 RSVs that were identified in the original, smaller HA cohort (23) and identified 75 novel variants, including 32 that were identified in participants who were previously reported as being negative for mutations in 7 IHH genes, and 2 that were identified in a participant previously reported to have a heterozygous missense RSV in ANOS1 (23). Two hundred control women harbored 255 RSVs, including 206 unique missense variants, 3 nonsense, 4 frameshift, 2 canonical splice, and 2 in-frame insertions (Supplemental Table [38]). All RSVs in control women were heterozygous, except for one homozygous frameshift variant in CCDC141.
Table 3.
Rare sequence variants in gonadotropin-releasing hormone–associated genes identified in women with hypothalamic amenorrhea
| Gene | Chrom | Position | Ref | Alt | RefSeq ID | Protein consequence | No. of HA participants with RSV | No. of control participants with RSV |
|---|---|---|---|---|---|---|---|---|
| RAB3GAP2 | 1 | 220327339 | C | A | NM_012414.4 | Asp1206Tyr | 1 | 0 |
| RAB3GAP2 | 1 | 220363770 | G | A | NM_012414.4 | Pro527Leu | 1 | 0 |
| RAB3GAP2 | 1 | 220366594 | G | A | NM_012414.4 | Arg420Cys | 1c | 0 |
| RAB3GAP1 | 2 | 135887597 | C | T | NM_001172435.2 | Arg336Cys | 3c | 0 |
| RAB3GAP1 | 2 | 135926245 | G | A | NM_001172435.2 | Arg954His | 1c | 0 |
| HESX1 | 3 | 57232493 | C | T | NM_003865.3 | Val129Ile | 1c | 0 |
| PROK2 | 3 | 71830659 | T | C | NM_001126128.2 | Met61Val | 1 | 0 |
| SOX2 | 3 | 181430212 | G | A | NM_003106.4 | Gly22Ser | 1c | 0 |
| KLB | 4 | 39409074 | G | A | NM_175737.4 | Ala169Thr | 1 | 0 |
| KLB | 4 | 39449069 | G | T | NM_175737.4 | Gly908Val | 1c | 0 |
| GNRHR b | 4 | 68606400 | C | T | NM_000406.3 | Arg262Gln | 1 | 0 |
| GNRHR | 4 | 68619550 | A | T | NM_000406.3 | Ser168Agr | 1c | 0 |
| TACR3 | 4 | 104577496 | T | C | NM_001059.3 | His248Arg | 1c | 0 |
| OTUD4 | 4 | 146058934 | G | C | NM_001102653.1 | Pro933Arg | 1 | 0 |
| SRA1 | 5 | 139931626 | TCA | T | NM_001035235.3 | 110AspfsTer25 | 1 | 0 |
| SPRY4 | 5 | 141694021 | G | T | NM_030964.4 | Ser241Tyr | 2 | 0 |
| SPRY4 | 5 | 141694468 | C | A | NM_030964.4 | Gly92Val | 1 | 0 |
| PROP1 | 5 | 177419966 | G | A | NM_006261.4 | Ala142Val | 1c | 0 |
| SEMA3E | 7 | 83047745 | G | A | NM_012431.3 | Pro171Ser | 1c | 0 |
| SEMA3A | 7 | 83689870 | T | C | NM_006080.3 | Asn153Ser | 1c | 0 |
| FEZF1 | 7 | 121942136 | T | G | NM_001024613.4 | Gln448Pro | 1 | 0 |
| GNRH1 | 8 | 25279183 | A | C | NM_000825.3 | Ile48Arg | 1 | 0 |
| FGFR1 | 8 | 38282184 | C | T | NM_001174067.1 | Gly291Glu | 1 | 0 |
| CHD7 | 8 | 61654723 | C | G | NM_017780.4 | Ser244Arg | 1 | 0 |
| CHD7 | 8 | 61655366 | C | T | NM_017780.4 | Arg459Cys | 1c | 0 |
| CHD7 | 8 | 61707630 | G | C | NM_017780.4 | Asp728His | 1c | 0 |
| CHD7 | 8 | 61757872 | C | A | NM_017780.4 | Pro1705Gln | 1c | 0 |
| CHD7 | 8 | 61764736 | C | T | NM_017780.4 | Arg1942Trp | 1d | 0 |
| CHD7 | 8 | 61769418 | A | C | NM_017780.4 | Met2527Leu | 1c | 0 |
| LHX3 | 9 | 139089431 | C | T | NM_014564.5 | Gly317Ser | 1 | 0 |
| WDR11 | 10 | 122610948 | G | A | NM_018117.12 | Val6Met | 1 | 0 |
| POLR3B | 12 | 106821117 | T | C | NM_018082.6 | Met415Thr | 1c | 0 |
| POLR3B | 12 | 106848357 | A | T | NM_018082.6 | Lys721e | 1 | 0 |
| POLR3B | 12 | 106890644 | C | T | NM_018082.6 | Arg978Cys | 1d | 0 |
| KL | 13 | 33635467 | A | G | NM_004795.4 | Arg751Gly | 1c | 0 |
| KL | 13 | 33635750 | T | G | NM_004795.4 | Val845Gly | 1c | 0 |
| DMXL2 | 15 | 51828990 | T | C | NM_001174116.2 | Met563Val | 1 | 0 |
| DMXL2 | 15 | 51829875 | G | C | NM_001174116.2 | Thr476Ser | 1 | 0 |
| DCC | 18 | 50683873 | G | A | NM_005215.4 | Gly470Asp | 2c | 0 |
| DCC | 18 | 50912508 | G | A | NM_005215.4 | Asp819Asn | 1 | 0 |
| DCC | 18 | 50918216 | G | A | NM_005215.4 | Val883Ile | 1c | 0 |
| PNPLA6 | 19 | 7626135 | G | A | NM_001166111.2 | Gly1329Arg | 1 | 0 |
| AXL | 19 | 41743932 | G | GC | NM_021913.5 | His292ProfsTer47 | 1 | 0 |
| AXL | 19 | 41754430 | G | A | NM_021913.5 | Gly517Ser | 1c | 0 |
| PROKR2 | 20 | 5282822 | G | C | NM_144773.3 | Thr340Ser | 1 | 0 |
| PROKR2 | 20 | 5294684 | A | C | NM_144773.3 | Met111Arg | 1 | 0 |
| PROKR2 b | 20 | 5294762 | C | T | NM_144773.3 | Arg85His | 1 | 0 |
| FLRT3 | 20 | 14306951 | T | A | NM_013281.3 | Gln401Leu | 1c | 0 |
| ANOS1 | X | 8501064 | T | C | NM_000216.4 | His672Arg | 1 | 0 |
| ANOS1 | X | 8503715 | C | A | NM_000216.4 | Val587Leu | 2 | 0 |
| ANOS1 b | X | 8536369 | C | T | NM_000216.4 | Val371Ile | 1 | 0 |
| NR0B1 | X | 30322875 | T | C | NM_000475.5 | Ser412Gly | 1c | 0 |
| RAB3GAP2 | 1 | 220324983 | G | T | NM_012414.4 | Leu1331Ile | 1 | 1 |
| SEMA3E | 7 | 83014747 | C | T | NM_012431.3 | Asp580Asn | 1c | 1 |
| CHD7 | 8 | 61655179 | G | T | NM_017780.4 | Met396Ile | 1c | 1 |
| CHD7 | 8 | 61655388 | C | T | NM_017780.4 | Ser466Leu | 1c | 1 |
| CHD7 | 8 | 61778448 | C | T | NM_017780.4 | Leu2984Phe | 2 | 1 |
| LHX3 | 9 | 139089436 | C | G | NM_014564.5 | Arg315Pro | 1c | 1 |
| DMXL2 | 15 | 51756963 | T | C | NM_001174116.2 | Ile2573Val | 1 | 1 |
| DMXL2 | 15 | 51791472 | T | C | NM_001174116.2 | Ile1317Val | 1c | 1 |
| AXL | 19 | 41743930 | G | A | NM_021913.5 | Val289Met | 1c | 1 |
| ANOS1 | X | 8504901 | G | T | NM_000216.4 | Ser511Tyr | 1 | 1 |
| LEPR | 1 | 66083694 | G | A | NM_002303.5 | Val754Met | 1 | 2 |
| CCDC141 | 2 | 179730592 | C | T | NM_173648.4 | Glu876Lys | 1 | 2 |
| KLB | 4 | 39448789 | A | G | NM_175737.4 | Lys815Glu | 1 | 2 |
| KLB | 4 | 39450295 | G | A | NM_175737.4 | Val1042Ile | 1c | 2 |
| GNRHR | 4 | 68619737 | T | C | NM_000406.3 | Gln106Arg | 2c | 2 |
| PCSK1 | 5 | 95729049 | T | C | NM_000439.5 | Thr640Ala | 1c | 2 |
| DCC | 18 | 50741960 | A | G | NM_005215.4 | Asn635Ser | 1c | 2 |
| SPRY4 | 5 | 141694117 | C | T | NM_030964.4 | Cys209Tyr | 1 | 3 |
| CHD7 | 8 | 61655009 | A | G | NM_017780.4 | Met340Val | 1 | 3 |
Abbreviations: Alt, alternate allele; Chrom, chromosome; HA, hypothalamic amenorrhea; ID, identification; Low conf, low confidence; Ref, reference allele; RSV, rare sequence variant.
a See Supplemental References.
b Participant and mutation previously reported.
c Variant identified in a participant previously reported as mutation negative.
d Variant identified in a participant previously reported with a heterozygous ANOS1 RSV (23).
eDenotes stop codon.
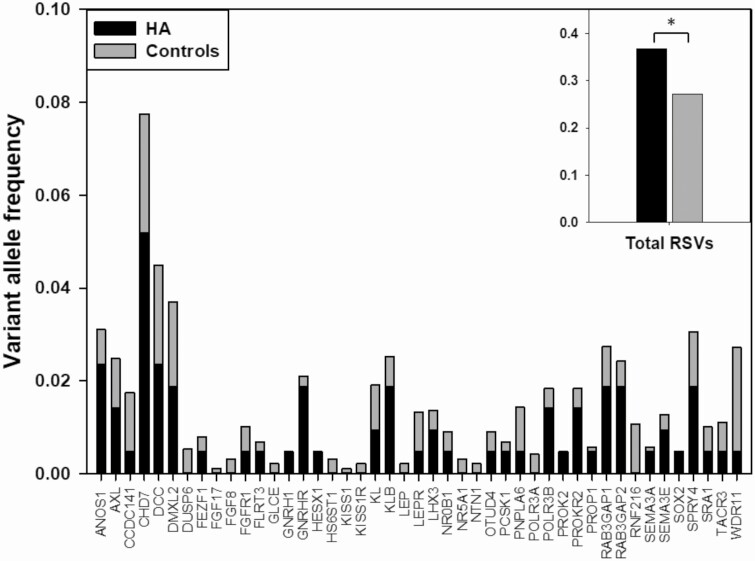
The frequency of RSVs in the 53-gene panel was significantly higher in HA women (0.37) than in controls (0.27, P = .007, burden test; Fig. 1). Statistical significance was validated using variance-component tests, including C-α (P = .022) and SKAT (P = .023), which test for association by comparing the distribution of genetic effects for a group of variants, as well as with UNIQ, which evaluates the number of case-only variants in cases vs controls (P = .015). One of the exome capture kits used in sequencing the control women (SureSelect Human All Exon, v2, Agilent Technologies, Inc) did not include CHD7, a well-established causative gene for IHH (39), but the burden test remained significant when excluding this gene from the analysis (P = .046).
Figure 1.
Rare sequence variants (RSVs) in women with HA and controls. RSVs in each gene expressed as frequency of variant alleles per gene in HA (black bars) vs control (gray bars) participants. The inset indicates the aggregate total frequency of variant alleles among all genes. No variants were found in either group in the following genes, which are excluded from the figure: DLX1, NSMF, SOX10, STUB1, and TAC3. Excluded from analysis because of being absent from at least one capture probe design: IL17RD, SMCHD1, and TUBB3. *P equal to .007.
Of the RSVs identified in women with HA, 35% have been previously reported to be associated with IHH in the literature compared with only 19% in the control group (P = .006; Table 3, Supplemental Table, Supplemental Refs [38]). This finding is particularly relevant because other tests that are designed to predict functional effects of RSVs failed to demonstrate a difference between HA and control women. Median CADD scores (40), a proxy for deleteriousness, were similar between groups (HA: 21.4; control: 21.9), and the proportion of RSVs considered to be possibly damaging/deleterious by other in silico prediction programs such as PolyPhen (41), SIFT (42), and MutationTaster (43) also did not differ between groups (33% vs 29%, respectively). Nineteen of the RSVs were present in both groups, 52 were unique to HA, and 198 were unique to controls. Among the RSVs present in both groups, the proportion that were predicted to be deleterious was not statistically different from the proportion of RSVs found only in the HA group that were predicted to be deleterious.
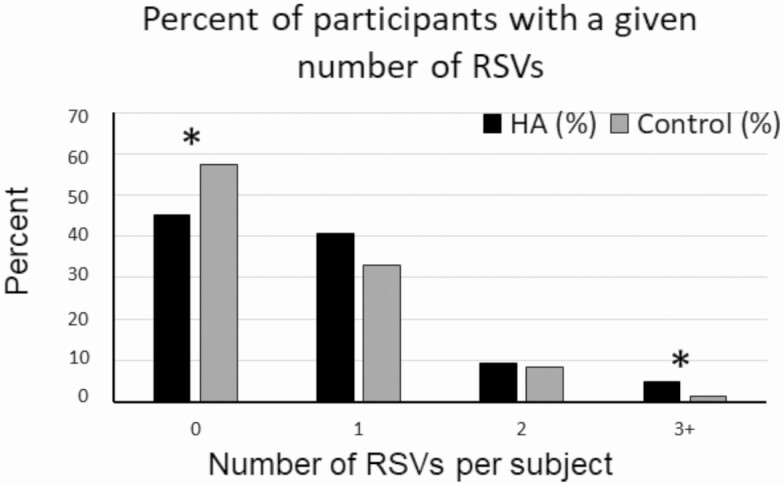
HA participants were more likely to harbor 3 or more RSVs compared with controls (4.7% vs 1.2%; P = .035), whereas a higher percentage of controls had no RSVs compared with HA participants (57% vs 45%, P = .03, Fig. 2). Among HA participants, RSV frequency was not associated with the number of risk factors (nutritional, exercise, or psychological stress) nor with family history of HA (variant frequency 0.45 in those with vs 0.34 in those without family history, respectively), although with family history data available for only 73 participants, this comparison may be underpowered.
Figure 2.
Percentage of participants with a given number of rare sequence variants (RSVs). Women with HA (black bars) were compared with control women (gray bars). *P less than .05.
Discussion
We have shown that, compared with healthy female controls, women with HA have a greater burden of heterozygous RSVs in a comprehensive panel of 53 genes implicated in IHH. In contrast to our earlier study, exon sequencing in HA and control women allowed us to calculate variant frequencies based on the total aggregate number of RSVs across all genes in both groups to factor in baseline variation in genes in the general population, in line with current best practices. In silico prediction programs did not demonstrate differential deleteriousness in RSVs found in HA women vs controls. Importantly, however, a greater number of RSVs that have previously been identified in individuals with severe IHH phenotypes were present in HA women than in controls, suggesting that these variants have clinical consequences, whereas RSVs in controls may be benign or require a physiologic insult for their clinical expression. Taken together, these findings indicate that the presence of heterozygous RSVs in IHH genes may contribute to the development of HA in the setting of physiologic risk factors.
Our findings significantly extend those of Caronia et al (23), which demonstrated that specific RSVs in a small number of IHH genes were present in HA, but not in control women. It is important to note that in this previous paper, the coding regions of the 7 genes in the control group were screened only for the specific variants identified in the HA women. It is estimated that an average of 1 variant is present for every 8 bases in more than 60 000 exomes, 99% of which have an MAF of less than 1% (30). It is therefore likely that additional unidentified and potentially deleterious RSVs were present in the control women in our earlier study. To address the overall burden of potentially deleterious RSVs in HA compared to control women, we screened the entire coding region for each of 53 IHH genes using ES to identify all RSVs in patients and controls. This allowed us to calculate an aggregate AAC in each group and compare the burden of variation. Although functional data were not available for all RSVs identified here, the greater proportion of IHH disease-associated variants found in the HA group, compared with the proportion seen in controls, suggests that the variants that are present in the HA group are more likely to contribute to reproductive dysfunction.
Results from the present study support the hypothesis that genetic variation plays a role in the variable reproductive axis response to known risk factors for HA. In the present study only KAL1, FGFR1, and CHD7 are inherited in an autosomal dominant pattern, whereas the majority are thought to require biallelic or digenic/oligogenic loss of function to cause IHH. We hypothesize that in some cases monoallelic RSVs result in a greater predisposition to developing a relative GnRH deficiency that is manifested only in the setting of physiologic stressors. As a corollary, the same variant would not be expected to result in HA in the absence of these stressors. Our data suggest that in such cases, the genetic architecture of GnRH deficiency may be described as semidominant—in which heterozygotes have a milder phenotype that occurs only when other factors that inhibit GnRH secretion are present, whereas those with biallelic (homozygous, compound heterozygous or digenic/oligogenic) pathogenic variants have a more severe form of GnRH deficiency that presents as nIHH or KS. Although it is intriguing to speculate about the potential role for variants in specific genes or groups of genes in contributing to the HA phenotype while others more definitively contribute to the KS/nIHH phenotype, our study was not powered to address this, and no such patterns emerged with review of the data.
Our findings provide further evidence for the importance of HA in the broader genetic spectrum of KS/nIHH, which has now been shown to include constitutional delay of puberty (CDP) (44). We have previously noted the presence of HA in some multiplex families with KS/nIHH (2). More than a quarter of our cohort had a history of delayed puberty, and HA has also been observed in families with CDP due to mutations in IGSF10 (45), suggesting that girls with CDP should be followed at least into early adulthood for the possible development of HA in association with known risk factors. Oligogenicity has been suggested as one explanation for the phenotypic variability of IHH, with a higher number of variant alleles being associated with more severe IHH, as opposed to milder or partial phenotypes (46, 47). Interestingly, having a higher number of RSVs in the present study was also associated with having HA, whereas a substantially greater proportion of control women had no RSVs. Sufficiently detailed history was not available to assess whether the number of RSVs was associated with the severity of the HA phenotype. A family history of HA is not uncommonly present among IHH probands. We found no family history of IHH in our HA cohort, but there was a family history of HA in 26%. Although the sample size did not allow for rigorous statistical comparison between groups, women with a family history of HA had a nonsignificantly increased frequency of RSVs in IHH genes compared with those without a family history.
Large genomic sequencing databases are publicly available; however, data on individual participants are not readily accessible to allow us to limit our analysis to females or, for example, to determine how many RSVs in this group of genes are present in any one participant. We therefore chose to use female controls from the ClinSeq study. Despite a range of cardiovascular risks, participants in the ClinSeq study are more highly selected to include healthy participants compared with participants in publicly available data sources. Most important, the availability of individual genomic data allowed us to use variance-component tests, which have more power than burden tests in the presence of benign or bidirectional variants (24), and confirmed the statistical significance of the enrichment of RSVs in the HA women.
A potential limitation of this analysis is the small sample size. HA is a relatively common disorder and likely to be a complex trait. As such larger, genome-wide association study (GWAS)-scale samples will be required to determine more broadly the genetic basis of HA, and indeed our results are important for justifying such studies. We were, however, able to address the more limited hypothesis that there would be a significant difference in RSV frequency among IHH-associated candidate genes. Data on risk factors and reproductive history were self-reported, and in most cases historical, and were therefore subject to recall bias. This was true both in the HA group and the control women. Psychological stress as a risk factor is particularly subjective, and because the clinical data were retrospective, there was no objective measure used to quantify stress in this study. As a result, stress was included only in cases where it was clearly significant, likely representing a conservative estimate. In addition, our controls were not selected for exposure to known HA risk factors. Future studies should be designed to not only evaluate the controls for absence of symptoms but also to match them to cases based on exposure to risk factors.
To address potential population stratification as a result of differences in race and ethnicity between groups, we limited our analysis to variants with an MAF of less than 1% in all race/ethnic groups listed in the gnomAD database, decreasing the likelihood of including ethnicity-specific common polymorphisms. To reduce bias potentially introduced because of differences among genetic sequencing platforms, we limited the genomic regions being compared to those regions that were common to all platforms used in the study, in terms of capture probes and depth of coverage. Despite these efforts, we cannot exclude the possibility that some RSVs were not detected by our next-generation sequencing methods. We did not limit the variants included in the analysis based on quality by depth, which is the quality score normalized by allele depth, or similar quality metrics, because in our experience these are not ideal measures of whether the variants are real when verified by Sanger sequencing. Instead, we relied only on whether they were “PASS” variants by GATK best practice standards, and therefore some of the variants identified may be false calls. Given the scope of the analysis, it was not practical to confirm all variants by Sanger sequencing or by manual review of BAM files.
The presence of a homozygous variant in CCDC141 (48, 49) in the control group was unexpected. It is possible that this participant has an undisclosed IHH phenotype, as this information is not readily available in the primary ClinSeq data set and this participant did not respond to our request to complete the reproductive health questionnaire. Although the quality by depth is in a range in which the variant call is more likely to be a real variant, we cannot exclude the possibility that this is a false-positive call. Confirming all variants by Sanger is not practical and it is not appropriate to treat this variant differently, so further confirmation was not performed. Additional screening of IHH cohorts for variants in this gene and/or functional testing to determine the pathogenicity of homozygous variants are necessary to address this finding because the possibility that a homozygous loss-of-function variant in this gene can be found in healthy controls would suggest that not all loss-of-function variants in CCDC141 variants cause the IHH phenotype.
Our analyses did not address potential variants in gene regulatory regions or epigenetic modifications of these genes. Epigenetic modification may be particularly relevant for this disorder given evidence in rodents that Kiss1 expression is under the epigenetic control of SIRT1 at the time of puberty and is responsive to nutritional changes (50), and studies implicating distinct epigenetic modifications in girls with normal and precocious puberty (51). Similarly, epigenetic changes may represent the mechanism through which physiologic risk factors cause HA in women with a susceptibility genotype.
We have now shown that women with HA are enriched with RSVs in well-established IHH genes, compared with healthy control women. This finding confirms that HA is on the genetic spectrum that includes IHH and supports the hypothesis of a genetic susceptibility to the development of amenorrhea in the setting of known stressors including diet, exercise, and stress. As more direct evidence of this hypothesis, further investigation is needed to test the functional consequences of introducing these environmental stressors to experimental cellular systems or animal (or human) models expressing some of the more commonly identified variants found in HA women. In addition, it will be important to define the role of candidate genes in metabolism and stress pathways known to affect GnRH secretion, to use unbiased GWAS approaches to discover new loci that may play a role in the relative GnRH deficiency seen in HA, and to determine the contribution of epigenetic modification of these genes in the pathogenesis of HA. This finding has important implications for the clinical management of patients with HA, providing an explanation for the apparent greater sensitivity to reproductive-axis disruption in association with the classic risk factors for HA in some patients, while cautioning the physician that lifestyle modifications may not be equally effective in normalizing reproductive function in all patients with HA. Future studies will be needed to define the utility of genetic screening for diagnosis and management of patients presenting with HA.
Acknowledgments
We thank Anthony Musolf, PhD, for computational and statistical genomics support, and the research participants and staff at our respective institutions.
Financial Support: This work was supported by the intramural programs of the National Institute of Environmental Health Sciences (NIEHS; ZID ES103323-01) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, ZIAHD008919); as well as the Harvard Reproductive Endocrine Sciences Center (P50 HD028138), (K24 HD01290 to J.E.H.), (R01 HD043341 to S.B.S.); and used the computational resources of the National Institutes of Health (NIH) High-Performance Computing Helix system (http://hpc.nih.gov). L.G.B., K.L.L., and ClinSeq were supported by the Intramural Research Program of the National Human Genome Research Institute (NHGRI). The Yale Center for Mendelian Genomics (NIH M#UM1HG006504-05) is funded by the NHGRI and the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Clinical Trial Information: ClinicalTrials.gov registration Nos.: NCT01500447 (registered December 28, 2011) and NCT00494169 (registered June 29, 2007).
Glossary
Abbreviations
- AAC
alternate allele count
- CDP
constitutional delay of puberty
- ES
exome sequencing
- GnRH
gonadotropin-releasing hormone
- GWAS
genome-wide association study
- HA
hypothalamic amenorrhea
- HH
hypogonadotropic hypogonadism
- IHH
isolated hypogonadotropic hypogonadism
- KS
Kallmann syndrome
- MAF
minor allele frequency
- nIHH
normosmic isolated hypogonadotropic hypogonadism
- RAC
reference allele count
- RSV
rare sequence variant
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Reame NE, Sauder SE, Case GD, Kelch RP, Marshall JC. Pulsatile gonadotropin secretion in women with hypothalamic amenorrhea: evidence that reduced frequency of gonadotropin-releasing hormone secretion is the mechanism of persistent anovulation. J Clin Endocrinol Metab. 1985;61(5):851-858. [DOI] [PubMed] [Google Scholar]
- 2. Crowley WF Jr, Filicori M, Spratt DI, Santoro NF. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473-531. [DOI] [PubMed] [Google Scholar]
- 3. Perkins RB, Hall JE, Martin KA. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab. 1999;84(6):1905-1911. [DOI] [PubMed] [Google Scholar]
- 4. Perkins RB, Hall JE, Martin KA. Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum Reprod. 2001;16(10):2198-2205. [DOI] [PubMed] [Google Scholar]
- 5. Münster K, Helm P, Schmidt L. Secondary amenorrhoea: prevalence and medical contact—a cross-sectional study from a Danish county. Br J Obstet Gynaecol. 1992;99(5):430-433. [DOI] [PubMed] [Google Scholar]
- 6. Feicht CB, Johnson TS, Martin BJ, Sparkes KE, Wagner WW Jr. Secondary amenorrhoea in athletes. Lancet. 1978;2(8100):1145-1146. [DOI] [PubMed] [Google Scholar]
- 7. Kreipe RE, Strauss J, Hodgman CH, Ryan RM. Menstrual cycle abnormalities and subclinical eating disorders: a preliminary report. Psychosom Med. 1989;51(1):81-86. [DOI] [PubMed] [Google Scholar]
- 8. Prokai D, Berga SL. Neuroprotection via reduction in stress: altered menstrual patterns as a marker for stress and implications for long-term neurologic health in women. Int J Mol Sci. 2016;17(12):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warren MP. The effects of exercise on pubertal progression and reproductive function in girls. J Clin Endocrinol Metab. 1980;51(5):1150-1157. [DOI] [PubMed] [Google Scholar]
- 11. Bullen BA, Skrinar GS, Beitins IZ, von Mering G, Turnbull BA, McArthur JW. Induction of menstrual disorders by strenuous exercise in untrained women. N Engl J Med. 1985;312(21):1349-1353. [DOI] [PubMed] [Google Scholar]
- 12. Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76(2):310-316. [DOI] [PubMed] [Google Scholar]
- 13. Schliep KC, Mumford SL, Vladutiu CJ, et al. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. 2015;26(2):177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bruni V, Dei M, Morelli C, Schettino MT, Balzi D, Nuvolone D. Body composition variables and leptin levels in functional hypothalamic amenorrhea and amenorrhea related to eating disorders. J Pediatr Adolesc Gynecol. 2011;24(6): 347-352. [DOI] [PubMed] [Google Scholar]
- 15. Falsetti L, Gambera A, Barbetti L, Specchia C. Long-term follow-up of functional hypothalamic amenorrhea and prognostic factors. J Clin Endocrinol Metab. 2002;87(2): 500-505. [DOI] [PubMed] [Google Scholar]
- 16. Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine-metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83(1):25-32. [DOI] [PubMed] [Google Scholar]
- 17. Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91(4):1561-1565. [DOI] [PubMed] [Google Scholar]
- 18. Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011;300(1):E19-E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sidhoum VF, Chan YM, Lippincott MF, et al. Reversal and relapse of hypogonadotropic hypogonadism: resilience and fragility of the reproductive neuroendocrine system. J Clin Endocrinol Metab. 2014;99(3):861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185(4155):949-951. [DOI] [PubMed] [Google Scholar]
- 21. Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297-311. [DOI] [PubMed] [Google Scholar]
- 22. Centeno ML, Sanchez RL, Cameron JL, Bethea CL. Hypothalamic gonadotrophin-releasing hormone expression in female monkeys with different sensitivity to stress. J Neuroendocrinol. 2007;19(8):594-604. [DOI] [PubMed] [Google Scholar]
- 23. Caronia LM, Martin C, Welt CK, et al. A genetic basis for functional hypothalamic amenorrhea. N Engl J Med. 2011;364(3):215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee S, Abecasis GR, Boehnke M, Lin X. Rare-variant association analysis: study designs and statistical tests. Am J Hum Genet. 2014;95(1):5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shaw ND, Seminara SB, Welt CK, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96(3):E566-E576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biesecker LG, Mullikin JC, Facio FM, et al. ; NISC Comparative Sequencing Program . The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009;19(9):1665-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ng D, Johnston JJ, Teer JK, et al. ; NIH Intramural Sequencing Center (NISC) Comparative Sequencing Program . Interpreting secondary cardiac disease variants in an exome cohort. Circ Cardiovasc Genet. 2013;6(4):337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnston JJ, Lewis KL, Ng D, et al. Individualized iterative phenotyping for genome-wide analysis of loss-of-function mutations. Am J Hum Genet. 2015;96(6):913-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stamou MI, Cox KH, Crowley WF Jr. Discovering genes essential to the hypothalamic regulation of human reproduction using a human disease model: adjusting to life in the “-omics” era. Endocr Rev. 2016;2016(1):4-22. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Lek M, Karczewski K, Minikel E, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karczewski KJ, Francioli LC, Tiao G, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. [Published online ahead of print January 30, 2019.] bioRxiv. Doi: 10.1101/531210 [DOI] [Google Scholar]
- 32. Stenson PD, Mort M, Ball EV, et al. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet. 2017;136(6):665-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Landrum MJ, Lee JM, Benson M, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46(D1):D1062-D1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neale BM, Rivas MA, Voight BF, et al. Testing for an unusual distribution of rare variants. PloS Genet. 2011;7(3): e1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu MC, Lee S, Cai T, Li Y, Boehnke M, Lin X. Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet. 2011;89(1):82-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. PLINK/SEQ: A library for the analysis of genetic variation data website. http://atgu.mgh.harvard.edu/plinkseq/assoc.shtml. Accessed March 22, 2019. [Google Scholar]
- 37. Moutsianas L, Agarwala V, Fuchsberger C, et al. The power of gene-based rare variant methods to detect disease-associated variation and test hypotheses about complex disease. PLoS Genet. 2015;11(4):e1005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delaney A, Burkholder AB, Lavender CA, et al. Burden of Rare Variants in Hypothalamic Amenorrhea-Supplemental data. figshare data repository. Deposited 16 August 2020. 10.6084/m9.figshare.12730694.v3. https://figshare.com/articles/dataset/Burden_of_Rare_Variants_in_Hypothalamic_Amenorrhea-Supplemental_data/12730694. [DOI] [Google Scholar]
- 39. Kim HG, Kurth I, Lan F, et al. Mutations in CHD7, encoding a chromatin-remodeling protein, cause idiopathic hypogonadotropic hypogonadism and Kallmann syndrome. Am J Hum Genet. 2008;83(4):511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11(5):863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575-576. [DOI] [PubMed] [Google Scholar]
- 44. Zhu J, Choa RE, Guo MH, et al. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2015;100(4):E646-E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howard SR, Guasti L, Ruiz-Babot G, et al. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016;8(6):626-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sykiotis GP, Plummer L, Hughes VA, et al. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A. 2010;107(34):15140-15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pitteloud N, Quinton R, Pearce S, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117(2):457-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hutchins BI, Kotan LD, Taylor-Burds C, et al. CCDC141 mutation identified in anosmic hypogonadotropic hypogonadism (Kallmann syndrome) alters GnRH neuronal migration. Endocrinology. 2016;157(5):1956-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turan I, Hutchins BI, Hacihamdioglu B, et al. CCDC141 mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2017;102(6):1816-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vazquez MJ, Toro CA, Castellano JM, et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat Commun. 2018;9(1):4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bessa DS, Maschietto M, Aylwin CF, et al. Methylome profiling of healthy and central precocious puberty girls. Clin Epigenetics. 2018;10(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.