Abstract
The metabolic syndrome (MetS) comprises cardiometabolic risk factors frequently found in individuals with obesity. Guidelines to prevent or reverse MetS suggest limiting fat intake, however, lowering carbohydrate intake has gained attention too. The aim for this review was to determine to what extent either weight loss, reduction in caloric intake, or changes in macronutrient intake contribute to improvement in markers of MetS in persons with obesity without cardiometabolic disease. A meta-analysis was performed across a spectrum of studies applying low-carbohydrate (LC) and low-fat (LF) diets. PubMed searches yielded 17 articles describing 12 separate intervention studies assessing changes in MetS markers of persons with obesity assigned to LC (<40% energy from carbohydrates) or LF (<30% energy from fat) diets. Both diets could lead to weight loss and improve markers of MetS. Meta-regression revealed that weight loss most efficaciously reduced fasting glucose levels independent of macronutrient intake at the end of the study. Actual carbohydrate intake and actual fat intake at the end of the study, but not the percent changes in intake of these macronutrients, improved diastolic blood pressure and circulating triglyceride levels, without an effect of weight loss. The homeostatic model assessment of insulin resistance improved with both diets, whereas high-density lipoprotein cholesterol only improved in the LC diet, both irrespective of aforementioned factors. Remarkably, changes in caloric intake did not play a primary role in altering MetS markers. Taken together, these data suggest that, beyond the general effects of the LC and LF diet categories to improve MetS markers, there are also specific roles for weight loss, LC and HF intake, but not reduced caloric intake, that improve markers of MetS irrespective of diet categorization. On the basis of the results from this meta-analysis, guidelines to prevent MetS may need to be re-evaluated.
Keywords: low-fat diet, low-carbohydrate diet, macronutrients, metabolic syndrome, weight loss
INTRODUCTION
The metabolic syndrome (MetS) is a cluster of cardiometabolic risk factors found in individuals with obesity and that frequently escalate in cardiometabolic diseases like diabetes and cardiovascular disease.1–5 MetS is defined by the International Diabetes Federation4 as “a cluster of the most dangerous heart attack risk factors: diabetes and prediabetes, abdominal obesity, high cholesterol and high blood pressure.” There are additional metabolic and endocrine criteria that may aggravate the condition of MetS; insulin resistance is one of the most important causative factors.4
To prevent progression toward cardiovascular disease and/or diabetes, individuals with MetS are recommended to lose weight and limit caloric intake.4 However, dietary guidelines are confusing because reductions of saturated fat intake6,7 and carbohydrate intake8 have been suggested. Several thorough reviews have been published on the short- and long-term health consequences of low-carbohydrate (LC) and low-fat (LF) diets. In reviews in which studies included patients with diabetes, results were positive for both diets.9–14 However, differential dietary benefits on weight loss and markers of MetS in persons with obesity without a cardiometabolic diagnosis (eg, type 2 diabetes, myocardial infarction, stroke) are less consistent and, therefore, it is difficult to determine whether an LC diet can prevent or reverse cardiometabolic derangements in MetS as well as an LF diet can.15 For that reason, Mansoor et al16 performed a meta-analysis on the long-term effects of LC and LF diets in individuals with obesity without cardiometabolic disease and concluded that they experienced greater weight loss and lower plasma triglyceride (or triacylgyceride [TAG]) levels on an LC diet than on an LF diet, with variable effects on plasma levels of high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol. Although their meta-analysis was very insightful, Mansoor et al16 stated that dietary intake diverted from the prescribed diet and there was a wide range of macronutrient intakes. These findings raise the question of whether these results can be attributed to type of diet or if actual dietary macronutrient composition is a better explanation for changes in body weight and markers of MetS. Another question is how much of the changes in markers of MetS were due to weight loss or change in caloric intake.
To answer these questions, the variations in the Mansoor et al16 study characteristics were used, and a meta-analysis on the existing LC and LF studies that provided the actual macronutrient compositions as well as caloric intake was performed, and their efficacy to induce weight loss and changes in markers of MetS in persons with obesity without cardiometabolic disease was assessed. Then meta-regression analyses were performed to assess whether the actual macronutrient composition, caloric intake, and/or weight loss could explain changes in markers of MetS.
METHODS
Search strategy
A search of PubMed was conducted in December 2018 and updated in April 2020. The search was limited to articles published in English about studies performed in humans, without restriction of publication date, using the following search terms: “low carbohydrate diet” insulin cardiovascular human; Atkins insulin cardiovascular human; “low carbohydrate” diet insulin cardiovascular human; “low fat diet” insulin cardiovascular human; and “low fat” diet insulin cardiovascular human. Relevant articles emerging from the search were studied and reference lists were screened for articles that met the inclusion criteria.
Inclusion and exclusion criteria
Studies had to meet the following criteria to be included: (1) the design of the study was a randomized intervention; (2) study designs according to national or international guidelines and practices were used; (3) study participants were adults, defined as at least 18 years old; (4) average body mass index (BMI) of the included participants was greater than 30 kg/m2; (5) study participants had neither diabetes nor cardiovascular disease; (6) the study investigated both an LC and an LF dietary intervention; (7) minimum duration of the dietary intervention was 6 months, including follow-up; (8) study reported body weight loss and at least 2 markers of MetS at 6 and/or 12 months; (9) studies reported data on caloric intake and carbohydrate and fat intake at 6 and/or 12 months; and (10) studies were excluded when pharmaceutical intervention or specific testing of supplements was noted. An LC diet was defined as prescribed intake of carbohydrates less than 40% (200 g carbohydrates per 2000 kcal) of the total energy intake to include a range of LC diets, and an LF diet was defined as prescribed fat intake less than 30% of total energy intake (67 g fat per 2000 kcal). The criteria for the diagnosis of MetS, according to the International Diabetes Federation, are central obesity (assessed by waist circumference > 94 cm for European men and >80 cm European women; circumference may vary with different ethnicities) and at least 2 of the following 4 factors:(1) elevated TAG level (> 150 mg/dL or > 1.7 mmol/L); (2) reduced HDL cholesterol level (men: < 40 mg/dL or <1.03 mmol/L; women: <50 mg/dL or < 1.29 mmol/L); (3) increased blood pressure (systolic blood pressure [SBP] > 130 mmHg or diastolic blood pressure [DBP] >85 mmHg), and (4) increased fasting plasma glucose level (> 100 mg/dL or > 5.6 mmol/L).4 Energy-restricted and ad libitum diets were included. Several popular weight loss diets also could be included in this review if they met the aforementioned criteria for macronutrient composition. For example, in the Atkins diet, carbohydrate intake is reduced to < 20 g/d for the first 8 weeks, after which carbohydrate intake is gradually increased until a balance between carbohydrate intake and weight maintenance is found.17 A Weight Watchers diet is a low-calorie, LF diet; and the Ornish diet is an LF diet (fat intake < 10%).18,19
Studies were initially screened on the basis of title and abstract content. Relevant studies were assessed in full text and included if they fulfilled the inclusion criteria. For the articles that passed full-text screening, data were extracted for one LC diet and one LF diet per study. One investigator performed the searches and the screening and extracted the data. Another investigator checked whether the selected articles fulfilled criteria and if the data were extracted correctly.
Data extraction
From the included articles, information about study design was extracted, including participant characteristics, sex, age, BMI, number of participants per intervention, duration and location of the intervention, and dietary intake assessment. For each dietary intervention, changes from baseline were extracted for body weight, SBP and DBP, and plasma levels of TAGs, HDL cholesterol, and fasting glucose for all time points exceeding 6 months. Homeostatic model assessment of insulin resistance (HOMA-IR) values were calculated for all studies that reported fasting glucose and fasting insulin values using the HOMA calculator, version 2. All values were converted to SI units, except HOMA-IR, which is an arbitrary unit. Where results are presented graphically, data were extracted as precisely as possible. When available, only data from participants who completed the study were extracted; otherwise, data from the intention-to-treat principle were extracted.
Food intake data were extracted as energy percentage (en%) for carbohydrate, protein, fat, and saturated fat (SFA) intakes, as kilocalories for caloric intake, and as grams per day for fiber intake. Data were extracted at baseline and at 6 and 12 months, as actual intake and as change from baseline intake. This allowed us to analyze the change of, as well as the final macronutrient intake of, a given participant. No requests for additional data were made of authors of studies included in this review.
Quality assessment
Methodological quality was evaluated using the Cochrane Risk of bias assessment.20 The following bias categories were assessed: (1) selection bias (random sequence generation); (2) allocation concealment; (3) performance bias (blinding of the outcome assessments); (4) attrition bias (incomplete outcome data); and (5) reporting bias (selective outcome reporting). The blinding of participants and personnel was not assessed, because blinding is not possible in these dietary interventions. Publication bias was evaluated using funnel plots and the Egger regression test for body weight loss in both diets after 6 and 12 months.21
Statistical analysis
Mean difference from baseline was computed for every marker for each diet. Meta-analysis was performed for all markers. Summary weighted mean difference from baseline and 95%CIs were calculated using the random effects model, unless variance between studies was less than zero, in which case the fixed-effects model was used. Meta-regression analyses were conducted with body weight loss and change in caloric intake as moderators. The effect of these moderators was investigated on all markers of MetS, separately for each diet, after both 6 and 12 months. Meta-regression analyses, irrespective of diet type, also were conducted on the relation between body weight change and changes in caloric intake, changes in en% of macronutrient and SFA intakes, and changes (grams per day) of fiber intake; and between body weight change and actual caloric intake, actual en% of macronutrient and SFA intakes, and actual fiber intake (grams per day) after 6 and 12 months. Significance for these meta-regression analyses was detected using the false-discovery rate for multiple comparisons as described by Benjamini and Hochberg.22 The false-discovery rate was set at 5%. The meta-analysis and meta-regression analysis were conducted using the Meta-Essentials tool developed by Suurmond et al.23
Linear stepwise meta-regression modeling (stepwise criteria: probability of F: entry <0.05, removal >0.10) was used to explore which independent variables best predicted changes in markers of MetS (all corrected for weight of the study). For analysis of actual intakes, the independent variables were body weight change, change of caloric intake, diet type (categorical variable, LC [defined as 0] vs LF [defined as 1]), and one of the following combinations, measured as en%: actual carbohydrate intake and actual fat intake; actual carbohydrate intake and actual protein intake; and actual fat intake and actual protein intake. For analysis of changes in intake from baseline, the independent variables were body weight change, change of caloric intake, and one of the following combinations, measured as en%: change from baseline in carbohydrate intake and change from baseline in fat intake; change from baseline in carbohydrate intake and change from baseline in protein intake; and change from baseline in fat intake and change from baseline in protein intake.
When carbohydrate was significantly related to one of the outcomes, a post hoc regression analysis was performed with fiber intake (grams per day) and the ratio of fiber intake (grams per day) to total carbohydrate intake (grams per day) as independent variables to determine whether one of these outcomes was related to the change in metabolic marker. When fat was significantly related to one of the outcomes, a post hoc regression analysis was performed with SFA intake (en%) and the ratio of SFA intake (en%) to total fat intake (en%) as independent variables to determine whether one of these outcomes was related to the change in metabolic marker. These post hoc analyses were performed for the absolute intakes and the changes in intakes. A false-discovery rate of 5% was used for detection of statistical significance. Linear regression modeling was performed in SPSS, version 25.
RESULTS
Studies
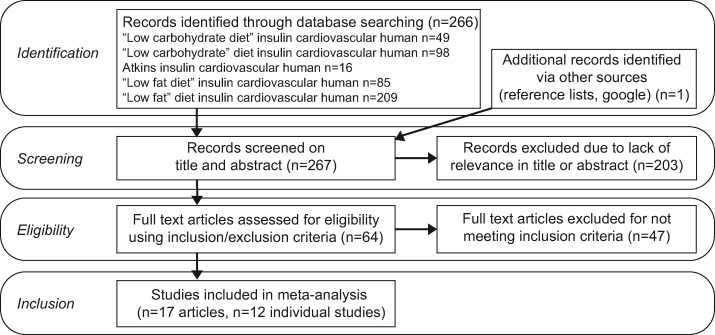
On the basis of the aforementioned criteria, 17 articles were included for the analyses, out of a search result of 267 articles (Figure 1). These articles included five follow-up studies that reported on already included cohorts, leaving a total of 12 separate studies. These studies used LC and Atkins diets and compared them with LF, Ornish, and Weight Watchers diets. The duration of the studies varied between 6 and 24 months. Because only one study had data at 18 months and one had data at 24 months, these time points were not taken into consideration for the meta-analysis; only the 6- and 12-month time points were used. Seven studies used food records from multiple days, four studies used 24-hour dietary recalls, and one study used a food frequency questionnaire. Seven of these studies applied an energy-reduced regimen rather than an ad libitum regimen. A summary of the experimental details of the studies is provided in Table 118,19,24–38 and a summary of the diets used is listed in Table 2.18,19,24–38 Table 318,19,24–38 lists the changes in cardiometabolic and endocrine markers of participants compared with their baseline levels. In particular, reported macronutrient intake varied markedly between studies (Table 2). Despite the LC diet being prescribed as < 40 en% from carbohydrates, actual carbohydrate intakes ranged from 8 to 45 at 6 months and from 9 to 43 en% at 12 months. Similarly, despite the LF diet being described as < 30 en% fat intake, actual fat intakes ranged from 24 to 36 en% at 6 months and from 24 to 31 en% at 12 months.
Figure 1.
Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram of study selection process.
Table 1.
Study and participant characteristics of each study
| Reference | Participants | Age, mean (SD) (years), by diet type | BMI, mean (SD) (kg/m2), by diet type | Durationa (mo) | Location | Food intake assessment | Type of data extracted |
|---|---|---|---|---|---|---|---|
| OB |
|
|
12 | United States | 2-d, 24-h dietary recalls, (1 weekday and 1 weekend day) | Imputed missing data with Markov Chain Monte Carlo | |
| Brehm et al (2003)33 | OB |
|
|
6 | United States | Weekly food records | Completers |
| Dansinger et al (2005)34 | OB, >1MF |
|
|
12 | United States | 3-d food records | Completers |
| Ebbeling et al (2007)35 | OB |
|
>30 | 18 | United States | 3-d, 24-h dietary recalls (2 weekdays and 1 weekend day) | Intention to treat |
| Frisch et al (2009)36 | OB |
|
|
12 | Germany | 3-Day food records | Intention to treat |
| Gardner et al (2007)19 | OB |
|
|
12 | United States | 3-d, 24-h dietary recalls (2 weekdays and 1 weekend day) | Completers |
| Haufe et al (2011)37 | OB |
|
|
6 | Germany | 7-d food records | Completers |
| OB |
|
|
12 | New Zealand | 3-d food records | Intention to treat | |
| Sacks et al (2009)25 | OB |
|
|
24 | United States | 5-d food record at baseline and 24-h recall on 3 nonconsecutive days at 6 mo | Intention to treat |
| OB, >1MF |
|
|
12 | Australia | 3-d food record (2 weekdays and 1 weekend day) | Intention to treat | |
| Thomson et al (2010)29 | OB, C | All: 56.2 (9.4) | All: 31.8 (4.3) | 6 | United States | Arizona Food Frequency Questionnaire | Intention to treat |
| OB |
|
|
6 | England | 7-d food record | Intention to treat |
Duration of the total study including follow-up.
Abbreviations: >1MF, participants with > 1 metabolic syndrome risk factor; BMI, body mass index; C, cancer survivor; LC, low cholesterol; LF, low fat; OB, participants with obesity.
Table 2.
Dietary characteristics of each study at baseline and after 6 and 12 months
| Reference | Diet | Energy intake | No. | Time (mo) | Energy intake (kcal) | Carbohydrate intake (en%) | Fat intake (en%) | Protein intake (en%) | Saturated fatty acids (en%) | Fiber intake (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| LC | ad lib | 70 | BL | 1998 (740) | 48.1 (8.8) | 32.5 (7.2) | 17.3 (5.0) | 10.5 (3.4) | 18.5 (8.7) | |
| 54 | 6 | 1324 (537) | 27.5 (12.1) | 43.4 (11.8) | 26.3 (5.6) | 13.4 (4.5) | 15.1 (7.5) | |||
| 54 | 12 | 1448 (610) | 34 (13.9) | 40.7 (10.6) | 23.6 (7.4) | 13.4 (4.8) | 15.1 (8.7) | |||
| LF | ad lib | 69 | BL | 2034 (702) | 46 (7.8) | 34.7 (6.6) | 17.6 (5.2) | 11.6 (2.9) | 16.7 (6.6) | |
| 50 | 6 | 1481 (483) | 52.4 (8.9) | 27.9 (7.3) | 18.3 (5.0) | 8.4 (2.9) | 16.4 (8.1) | |||
| 49 | 12 | 1527 (522) | 54 (9.6) | 29.8 (8.8) | 18.6 (5.8) | 9.0 (3.2) | 15.6 (7.7) | |||
| Brehm et al (2003)33 | LC | ad lib | 22 | BL | 1608 (577) | 47 | 37 | 16 | 12.4 | 12.03 |
| 22 | 6 | 1302 | 30 | 46 | 23 | 17.4 | 8.4 | |||
| LF | ER | 20 | BL | 1707 (465) | 47 | 38 | 15 | 12.3 | 12.48 | |
| 20 | 6 | 1247 | 53 | 29 | 18 | 11.1 | 12.35 | |||
| Dansinger et al (2005)34 | LC | ad lib | 39 | BL | 1898 | 51.6 | 36.2 | 18.5 | 12.3 | 16.0 |
| 15 | 6 | 1846 | 42.1 | 38.3 | 18.2 | 12.4 | 13.0 | |||
| 17 | 12 | 1886 | 41.3 | 37.6 | 18.7 | 13.0 | 15.0 | |||
| LF | ad lib | 39 | BL | 1947 | 49.7 | 34.1 | 18.3 | 11.8 | 14.0 | |
| 15 | 6 | 1711 | 56.8 | 28.0 | 17.7 | 9.7 | 14.5 | |||
| 17 | 12 | 1819 | 49.1 | 31.0 | 17.2 | 10.1 | 15.0 | |||
| Ebbeling et al (2007)35 a | LC | ad lib | 36 | BL | 2050 | 48 | 34 | 18 | 11.4 | 16.6 |
| 32 | 6 | 1630 | 39 | 39 | 22 | 11.3 | 21.4 | |||
| 29 | 12 | 1680 | 41 | 38 | 21 | 12.6 | 18.6 | |||
| LF | ad lib | 37 | BL | 2050 | 46 | 35 | 19 | 12.5 | 15.2 | |
| 34 | 6 | 1580 | 55 | 24 | 21 | 8.0 | 17.9 | |||
| 26 | 12 | 1550 | 54.5 | 24 | 21.5 | 7.8 | 10.9 | |||
| Frisch et al (2009)36 | LC | ER | 100 | BL | 2140 (696) | 44.8 (8.6) | 35.2 (8.1) | 16.8 (3.6) | ||
| 100 | 6 | 1742 (624) | 40.9 (10.1) | 36.5 (9.5) | 19.3 (4.7) | |||||
| 100 | 12 | 1866 (710) | 43.5 (9.9) | 34.2(8.7) | 18.9 (4.4) | |||||
| LF | ER | 100 | BL | 2192 (668) | 47.1 (7.9) | 33.7 (6.9) | 16.0 (3.9) | |||
| 100 | 6 | 1783 (597) | 49.5 (7.6) | 29.7 (6.5) | 17.7 (4.0) | |||||
| 100 | 12 | 1854 (624) | 50.1 (8.2) | 30.2 (7.0) | 16.7 (3.1) | |||||
| Gardner et al (2007)19 | LC | ad lib | 77 | BL | 1888 (512) | 45.6 (10.5) | 36.2 (7.8) | 16.6 (4.1) | 12.6 (5.3) | 17.4 (6.6) |
| 71 | 6 | 1538 (401) | 29.5 (14.5) | 47 (11.9) | 22.4 (6.3) | 16.4 (6.5) | 14.0 (6.3) | |||
| 68 | 12 | 1599 (494) | 34.5 (14.4) | 44.3 (12.5) | 20.6 (5.3) | 15.3 (7.5) | 15.2 (6.6) | |||
| LF | ad lib | 76 | BL | 1850 (541) | 47.9 (8.6) | 35.1 (7) | 16.3 (3.1) | 12.1 (5.0) | 16.6 (6.6) | |
| 67 | 6 | 1553 (530) | 53.4 (13.4) | 28.3 (10.7) | 18.1 (4.8) | 9.4 (5.9) | 19.3 (11.1) | |||
| 56 | 12 | 1505 (437) | 52.4 (12.3) | 29.8 (10.5) | 18.3 (4.0) | 10.1 (7.5) | 19.3 (9.4) | |||
| Haufe et al (2011)37 a | LC | ER | 80 | BL | 2180 | 45.1 | 34.3 | 20.6b | 14.0 | |
| 52 | 6 | 1580 | 29.8 | 43.4 | 26.7b | 11.1 | ||||
| LF | ER | 83 | BL | 2190 | 44.9 | 38.2 | 16.9b | 14.4 | ||
| 50 | 6 | 1750 | 51.5 | 27.7 | 20.8b | 5.9 | ||||
| LC | ad lib | 31 | BL | 2006 (448) | 44 (6) | 34 (6) | 18 (4) | 14 (3) | 10 (3) | |
| 31 | 6 | 1623 (434) | 26 (11) | 47 (8) | 24 (6) | 19 (4) | 9 (3) | |||
| 22 | 12 | 1781 (473) | 33 (11) | 41 (8) | 21 (6) | 16 (4) | 18 (6) | |||
| LF | ad lib | 32 | BL | 1812 (406) | 45 (7) | 31 (6) | 18 (3) | 12 (3) | 11 (3) | |
| 32 | 6 | 1460 (294) | 45 (7) | 28 (7) | 21 (3) | 10 (4) | 13 (3) | |||
| 21 | 12 | 1474 (301) | 45 (9) | 29 (9) | 22 (4) | 11 (3) | 18 (6) | |||
| Sacks et al (2009)25 | LC | ER | 201 | BL | 1979 (599) | 44 (7) | 38 (6) | 18 (3) | 12 (2) | |
| 6 | 1624 (484) | 43 (6.7) | 34.3 (7.8) | 22.6 (4.4) | 9.0 (2.6) | |||||
| LF | ER | 204 | BL | 2015 (505) | 44 (8) | 38 (6) | 18 (4) | 12 (3) | ||
| 6 | 1636 (484) | 57.5 (11.1) | 26.2 (8) | 17.6 (3.4) | 7.5 (3.2) | |||||
| LC | ER | 33 | 6 | 1603 (1046) | 7.6 (2.9) | 55.9 (3.4) | 33.3 (0.5) | 20.4 (0.5) | ||
| 33 | 12 | 1643 (213) | 8.9 (4.6) | 54.9 (4.6) | 32.3 (0.4) | 20.4 (0.5) | ||||
| LF | ER | 36 | 6 | 1529 (1044) | 44.9 (3.6) | 26.8 (4.2) | 22.9 (0.3) | 6.1 (0.2) | ||
| 36 | 12 | 1624 (300) | 46.4 (3.6) | 26.4 (4.2) | 21.8 (0.4) | 6.2 (0.2) | ||||
| Thomson et al (2010)29 | LC | ER | 19 | BL | 2043 (1036) | 55.1 (11.3) | 25.3 (15.5) | 16.8 (6.4) | ||
| 13 | 6 | 1523 (705) | 45.4 (21.8) | 33.6 (14.3) | 22.2 (10.7) | |||||
| LF | ER | 21 | BL | 1826 (462) | 57.6 (33.5) | 32.2 (22.1) | 18.6 (11.2) | |||
| 19 | 6 | 1517 (678) | 60.5 (25.0) | 24.9 (17.4) | 17.8 (9.1) | |||||
| LC | ad lib | 44 | BL | 2281 (641) | 40 | 38 | 16 | |||
| 8 | 6 | 1630 (364) | 18 | 51 | 26 | |||||
| LF | ER | 53 | BL | 2318 (743) | 43 | 37 | 16 | |||
| 16 | 6 | 1650 (499) | 40 | 36 | 20 |
Data are reported as mean (SD) unless otherwise indicated.
Estimation of values.
When protein intake was not specified it was calculated by deducting carbohydrate and fat intake from 100%. Alcohol intake was not specified in this study.
Abbreviations: ad lib, ad libitum energy intake; BL, baseline; en%, percentage of energy; ER, restricted energy intake; LC, low-carbohydrate diet; LF, low-fat diet.
Table 3.
Study outcomes after 6 and 12 months
| Reference | Diet | Time (mo) | No. | Body weight (kg) | SBP (mmHg) | DBP (mmHg) | TAG (mmol/L) | HDL (mmol/L) | Glucose (mmol/L) | HOMA-IR |
|---|---|---|---|---|---|---|---|---|---|---|
| LC | 6 | 75 | −5.6 (4.0) | −2.9 (7.1) | −1.7 (4.9) | −0.22 (0.40) | 0.10 (0.22) | 0.03 (0.71) | −0.39 (0.18) | |
| 12 | 75 | −5.3 (6.6) | −0.2 (10.6) | −0.5 (7.5) | −0.23 (0.49) | 0.24 (0.31) | 0.02 (0.57) | −0.25 (0.22) | ||
| LF | 6 | 73 | −2.3 (3.5) | −2.2 (7.0) | −0.5 (5.2) | −0.01 (0.39) | 0.0 (0.22) | −0.10 (0.44) | −0.39 (0.18) | |
| 12 | 73 | −1.8 (6.5) | −1.3 (10.0) | 0.2 (7.4) | −0.07 (0.48) | 0.06 (0.31) | −0.10 (0.52) | −0.45 (0.22) | ||
| Brehm et al (2003)33 | LC | 6 | 22 | −8.5 (1.0) | −2.0 (4.3) | −5 (3.5) | −0.39 (2.29) | 0.18 (0.10) | −0.50 (0.19) | −0.36 (0.12) |
| LF | 6 | 20 | −3.9 (1.0) | −2.0 (3.5) | −1 (2.6) | 0.02 (1.76) | 0.11 (0.09) | 0.20 (0.16) | −0.65 (0.15) | |
| Dansinger et al (2005)34 | LC | 6 | 22 | −5.8 (5.3) | −6.7 (12.0) | −7.3 (7.4) | −0.21 (0.60) | 0.18 (0.19) | −0.78 (1.89) | −0.61 (0.83) |
| 12 | 21 | −3.9 (6.0) | 0.3 (17.0) | −2.6 (10.3) | −0.02 (1.32) | 0.17 (0.23) | 0.14 (2.33) | −0.30 (0.52) | ||
| LF | 6 | 21 | −6.7 (8.0) | −1.2 (12.0) | −0.5 (8.6) | −0.35 (1.12) | −0.08 (0.25) | −0.53 (1.89) | −0.17 (1.35) | |
| 12 | 20 | −6.6 (9.3) | 0.9 (11.0) | 0.4 (6.6) | 0.12 (0.60) | −0.03 (0.24) | −0.46 (2.39) | −0.81 (0.47) | ||
| Ebbeling et al (2007)35 | LC | 6 | 32 | −4.5 (6.1)a | −5.1 (2.3) | −2.4 (1.7) | −21.2% | 0.04 (0.04) | 0.09 (0.07) | −0.36 (0.15) |
| 12 | 29 | −2.9 (5.5)a | ||||||||
| LF | 6 | 34 | −3.7 (5.9)a | −4.8 (2.3) | −2.0 (1.7) | −4.0% | −0.11 (0.03) | −0.02 (0.07) | −0.11 (0.03) | |
| 12 | 26 | −2.5 (5.2)a | ||||||||
| Frisch et al (2009)36 | LC | 6 | 100 | −7.2 (5.4) | −6 (16.0) | −3 (8.0) | −0.18 (0.40) | −0.02 (0.20) | −0.26 (0.76) | |
| 12 | 100 | −5.8 (6.1) | −5 (14.0) | −3 (9.0) | −0.10 (0.47) | −0.02 (0.21) | −0.25 (0.75) | |||
| LF | 6 | 100 | −6.2 (4.8) | −4 (15.0) | −3 (9.0) | −0.03 (0.55) | −0.09 (0.19) | −0.28 (0.59) | ||
| 12 | 100 | −4.3 (5.1) | −1 (15.0) | −2 (8.0) | −0.04 (0.50) | −0.03 (0.17) | −0.14 (0.46) | |||
| Gardner et al (2007)19 | LC | 6 | 77 | −5.8 (2.7) | −6.4 (9.5) | −3.3 (6.9) | −0.40 (0.72) | 0.13 (0.25) | 0.01 (0.42) | −0.36 (0.15) |
| 12 | 77 | −4.7 (7.2) | −7.6 (11.0) | −4.4 (8.4) | −0.33 (0.67) | 0.13 (0.24) | −0.10 (0.74) | −0.24 (0.14) | ||
| LF | 6 | 76 | −2.4 (2.2) | −1.7 (7.0) | −1.0 (5.6) | −0.09 (0.61) | 0.0 (0.24) | −0.03 (0.41) | −0.02 (0.13) | |
| 12 | 76 | −2.6 (5.3) | −1.9 (7.7) | −0.7 (6.0) | −0.17 (0.52) | 0.0 (0.16) | −0.04 (0.44) | −0.03 (0.11) | ||
| Haufe et al (2011)37 | LC | 6 | 52 | −7.5 (4.3) | −0.19 (0.43) | −0.09 (0.72) | −0.34 (0.52) | −0.61 (0.18) | ||
| LF | 6 | 50 | −6.5 (4.9) | −0.14 (0.57) | −0.10 (0.49) | −0.29 (0.67) | −0.43 (0.11) | |||
| LC | 6 | 28 | −7.1 (14.8) | −4 (19.3) | −2 (12.4) | −0.71 (0.83) | 0.9 (0.42) | −0.30 (0.83) | −0.54 (0.31) | |
| 12 | 24 | −5.4 (15.4) | −4 (20.5) | −4 (14.1) | −0.47 (1.24) | 0.12 (0.48) | −0.20 (0.92) | −0.41 (0.31) | ||
| LF | 6 | 30 | −4.7 (20.6) | −2 (15.3) | 1 (13.9) | −0.22 (0.89) | −0.04 (0.35) | −0.30 (0.71) | −0.48 (0.18) | |
| 12 | 24 | −4.4 (22.3) | −6 (17.0) | −3 (14.9) | −0.31 (0.97) | −0.02 (0.48) | −0.10 (0.78) | −0.58 (0.33) | ||
| Sacks et al (2009)25 | LC | 6 | 201 | −5.8 (11.3) | −1.7 (19.2) | −1.8 (13.5) | −0.22 (1.38) | 0.10 (0.53) | −0.07 (1.95) | |
| 12 | 201 | −5.3 (14.2) | ||||||||
| LF | 6 | 201 | −5.7 (11.4) | −1.2 (17.7) | −1.4 (24.8) | −0.16 (1.24) | −0.01 (0.51) | −0.17 (0.90) | ||
| 12 | 201 | −5.2 (14.3) | ||||||||
| LC | 6 | 45 | −11.9 (6.3) | −12.3 (14.1) | −4.58 (9.78) | −0.64 (0.62) | 0.25 (0.28) | −0.18 (0.40) | −0.40 (0.02) | |
| 12 | 26 | −14.5 (1.7) | −13.8 (14.4) | −6.3 (9.2) | −0.58 (0.63) | 0.30 (0.40) | −0.30 (0.06) | −0.08 (0.02) | ||
| LF | 6 | 43 | −10.1 (5.7) | −10.8 (13.2) | −5.50 (8.6) | −0.35 (0.49) | 0.08 (0.17) | −0.21 (0.40) | −0.08 (0.01) | |
| 12 | 23 | −11.5 (1.2) | −14.6 (12.0) | −7.9 (9.6) | −0.22 (0.66) | 0.07 (0.36) | −0.30 (0.60) | −0.08 (0.02) | ||
| Thomson et al (2010)29 | LC | 6 | 21 | −5.9 (4.1) | −0.8 (14.1) | 1.6 (11.0) | −0.35 (0.41) | 0.06 (0.21) | −0.06 (0.48) | −0.34 (0.27) |
| LF | 6 | 19 | −6.3 (5.6) | −8.6 (16.3) | 0.9 (6.9) | 0.08 (0.76) | 0.01 (0.21) | −0.07 (0.45) | −0.58 (0.33) | |
| LC | 6 | 57 | −6.0 (6.4) | −7.2 (11.6) | −4.9 (8.1) | −0.64 (0.77) | −0.08 (0.39) | −0.19 (0.50) | −0.36 (0.31) | |
| LF | 6 | 58 | −6.5 (5.4) | −4.1 (11.7) | −4.4 (8.6) | −0.35 (0.90) | −0.18 (0.28) | −0.46 (0.60) | −0.20 (0.33) |
Outcomes are depicted as mean (SD) change from baseline. When no absolute values were available, percentage change is shown, if available. Not all standard deviations could be calculated.
Estimation of value.
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein cholesterol; LC, low-carbohydrate diet; LF, low-fat diet; ND, no data available; SBP, systolic blood pressure; TAG, plasma triglyceride.
Risk of bias of included studies
All studies had a low risk of bias. One bias was unclear, but it is not expected that this bias influenced the outcome of the interventions (Table 4).
Table 4.
Risk of bias assessed using the Cochrane Risk of Bias tool
| Reference | Selection bias |
Attrition bias |
Reporting bias |
Overall | ||
|---|---|---|---|---|---|---|
| Randomization | Allocation | Blinding outcome assessment | Incomplete outcome data | Selective reporting | ||
| Low | Low | Low | Low | Low | Low | |
| Brehm et al (2003)33 | Low | Low | Low | Low | Low | Low |
| Dansinger et al (2005)34 | Low | Low | Low | Low | Low | Low |
| Ebbeling et al (2007)35 | Low | Low | Low | Low | Low | Low |
| Frisch et al (2009)36 | Low | Low | Low | Low | Low | Low |
| Gardner et al (2007)19 | Low | Low | Low | Low | Low | Low |
| Haufe et al (2011)37 # | Low | Unclear | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low | |
| Sacks et al (2009)25 | Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low | |
| Thomson et al (2010)29 | Low | Low | Low | Low | Low | Low |
| Low | Low | Low | Low | Low | Low | |
Risk of publication bias
No evidence for publication bias was found for body weight change for either diet at 6 or 12 months (Figure S1 in the Supporting Information online).
Body weight, caloric and macronutrient intake, and markers of MetS per diet type
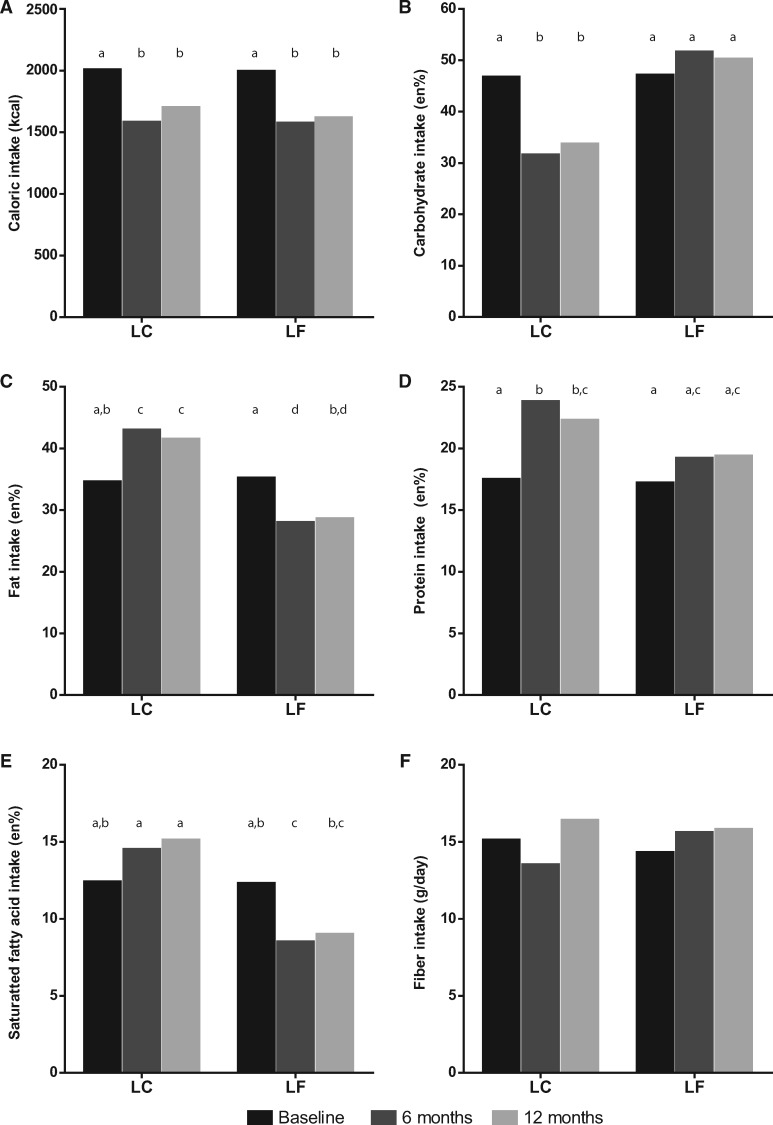
Data from 732 participants were available for LC diets after 6 months and from 553 participants after 12 months. For LF diets, data from 725 and 543 participants were available after 6 and 12 months, respectively. In all studies the mean BMI was >30, showing that, on average, the participants had obesity. Eleven studies reported baseline dietary intake, all 12 studies reported dietary intake after 6 months, and 7 of these 12 studies also reported dietary intake after 12 months. Baseline caloric and macronutrient intakes were equal in both diet types (Figure 2).
Figure 2.
Intake in the LC and LF groups at different time points. (A) Caloric intake (kcal); (B) carbohydrate intake (en%); (C) fat intake (en%); (D) protein intake (en%); (E) saturated fatty acid intake (en%); and (F) fiber intake (grams per day). Different letters (a, b, c, and d) show significant differences among diets and time points. Abbreviations: en%, energy percentage; LC, low-carbohydrate diet; LF, low-fat diet.
Both the LC and LF diet types significantly reduced caloric intake at 6 months (LC: P < 0.001; LF: P < 0.001) and 12 months (LC: P < 0.01; LF: P < 0.001). In addition, comparable significant reductions in body weight were found in either diet at 6 (LC: P < 0.001; LF: P < 0.001) and 12 months (LC: P < 0.001; LF: P < 0.001; Table S1 in the Supporting Information online). LC diets, at 6 and 12 months, significantly increased en% fat intake (P < 0.01 and P = 0.01, respectively) and en% protein intake (P < 0.001 and P < 0.01, respectively), significantly reduced en% carbohydrate intake (P < 0.001 and P < 0.01, respectively), and significantly increased en% SFA intake (P = 0.03), but only after 12 months. There was no change in fiber intake at either 6 or 12 months.
LF diet types, at 6 and 12 months, significantly reduced en% fat intake (P < 0.001 and P < 0.001, respectively) and significantly increased en% carbohydrate intake after 6 months (P = 0.04); en% protein intake was increased (P < 0.01 and P = 0.02, respectively), and en% SFA intake was significantly decreased (P < 0.001 and P < 0.001, respectively). Fiber intake was not changed after 6 or 12 months (Figure 2). Results of the meta-analysis of dietary effects on the various markers of MetS are listed in Table S1 in the Supporting Information online.
Meta-regression per diet type revealed that changes in caloric intake were not correlated with any changes in markers of MetS in either diet type at 6 and 12 months. In the LC diet type, body weight loss was positively correlated with reductions in glucose levels (P = 0.03) at 6 months and with reductions in SBP (P = 0.03) and TAG (P = 0.04) at 12 months. In the LF diet type, body weight loss was positively correlated with reductions in SBP (P = 0.01), DBP (P < 0.01), TAG (P < 0.01), and glucose levels (P = 0.04) at 6 months, and with reductions in SBP (P < 0.01), DBP (P < 0.01), and glucose levels (P = 0.02) at 12 months (Table S2 in the Supporting Information online).
Focus on actual macronutrient and caloric intake vs body weight loss irrespective of diet type
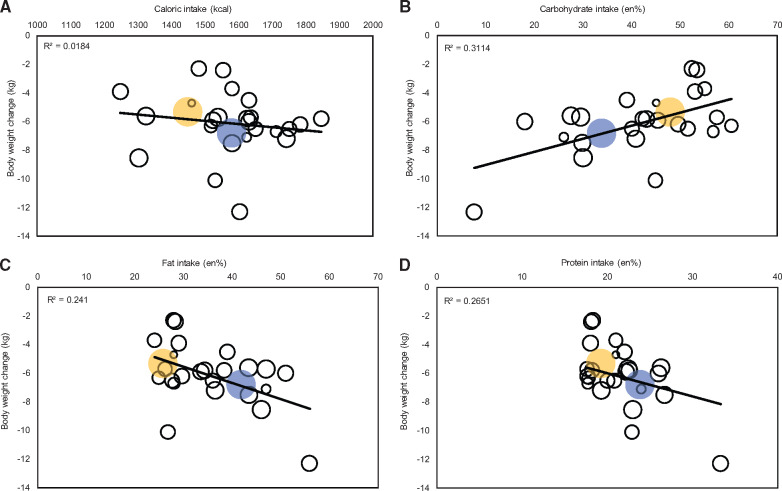
Instead of treating the LC and LF diets separately, the variation in caloric and macronutrient intakes could be viewed as a continuum. In a linear regression analysis across all studies irrespective of diet type, no correlation of change in body weight was found with change in caloric intake or actual caloric intake at 6 (Figure 3A) or 12 months. Actual carbohydrate intake had negative correlations with change in body weight (6 months: B = 0.093, R2 = 0.321; 12 months: B = 0.156, R2 = 0.485), with a 1 kg lowering of body weight related to a 10.8 en% decrease in carbohydrate intake (P < 0.01; Figure 3B) at 6 months and a 6.4 en% decrease at 12 months (P < 0.01). Actual fat intake had positive correlations with change in body weight (6 months: B = –0.118, R2 = 0.237; 12 months: B = –0.162, R2 = 0.238), with a 1 kg lowering of body weight related with an 8.5 en% increase in fat intake (P < 0.01; Figure 3C) at 6 months and a 6.2 en% increase at 12 months (P < 0.01). Actual protein intake also had positive correlations with change in body weight (6 months: B = –0.345, R2 = 0.368; 12 months: B = –0.441, R2 = 0.390), with a 2.9 en% increase in protein intake (P < 0.01; Figure 3D) at 6 months and a 2.3 en% increase at 12 months (P < 0.001) related with a 1 kg lowering of body weight. Similar to actual fat intake, actual SFA intake was also positively correlated with change in body weight (6 months: B = –0.262, R2 = 0.187, P = 0.02; 12 months: B = –0.33914, R2 = 0.197, P < 0.01). Actual fiber intake was not correlated with change in body weight. Neither percent changes in macronutrient and SFA intake from baseline nor the absolute change in fiber intake were correlated with body weight loss at 6 and 12 months.
Figure 3.
Bubble plot of body weight change moderator analysis. The size of the bubbles represents the precision of the effect size; larger bubbles indicate greater precision. Blue bubbles represent the meta-analysis outcome for body weight vs actual intake in the LC group. Yellow bubbles represent the meta-analysis outcome for body weight vs actual intake in the LF group. The regression line fitted to the raw data shows the slope of the relation between the body weight change and (A) caloric intake (kcal) after 6 months; (B) actual carbohydrate intake after 6 months (en%); (C) actual fat intake after 6 months (en%); and (D) actual protein intake after 6 months (en%). Abbreviations: en%, energy percentage; LC, low-carbohydrate diet; LF, low-fat diet.
Meta-regression of markers of MetS across all studies irrespective of diet type
Meta-regression of markers of MetS was performed with available data from the 6 month time point, because the 12-month time point did not contain enough data. Actual fat intake (B = –0.135; R2 = 0.341) and actual carbohydrate intake (B = 0.093; R2 = 0.316), respectively, had negative and positive correlations with change in DBP, with every 1 mmHg lowering in DBP related to a 7.4% increase in fat intake (P < 0.01) and with 10.8% reduction in carbohydrate intake (P < 0.01; Table 5). Additional analysis showed no relation between the ratio of SFA or fiber intake and DBP. Actual fat intake (B = –0.018; R2 = 0.590) and actual carbohydrate intake (B = 0.012; R2 = 0.582), respectively, had negative and positive correlations with change in TAG, albeit small, with every 1 mmol/L lowering in TAG levels related with 55.6% increase in actual fat intake (P < 0.001) and with 83.3% reduction in actual carbohydrate intake (P < 0.001). SFA (P < 0.01) and fiber (P = 0.02) intakes were related to TAG, in line with fat and carbohydrate intake. Studies reporting fiber intake (n = 5) no longer showed a significant decrease in TAG levels in the LF diet, whereas there was a decrease in TAG levels in the meta-analysis of all studies. In the studies reporting SFA intake (n = 8), there was a similar decrease in TAG levels compared with the meta-analysis results from all studies (data not shown).
Table 5.
Stepwise linear meta-regression modeling with actual macronutrient intakes at 6 months
| Model | Individual variable | B | SE | r 2 | df | F | P |
|---|---|---|---|---|---|---|---|
| DBP | |||||||
| 1a,c | Constant | 2.389 | 1.426 | 0.341 | 18 | 10.833 | 0.004 |
| Actual fat intake | −0.135 | 0.041 | |||||
| 2b | Constant | −6.322 | 1.367 | 0.316 | 18 | 9.768 | 0.006 |
| Actual carbohydrate intake | 0.093 | 0.030 | |||||
| Triglyceride | |||||||
| 1a,c | Constant | 0.427 | 0.118 | 0.590 | 18 | 28.342 | 0.000 |
| Actual fat intake | −0.018 | 0.003 | |||||
| 2b | Constant | −0.736 | 0.109 | 0.582 | 18 | 27.411 | 0.000 |
| Actual carbohydrate intake | 0.012 | 0.002 | |||||
| 3e | Constant | 0.093 | 0.094 | 0.441 | 16 | 12.853 | 0.003 |
| Actual SFA intake | −0.030 | 0.008 | |||||
| 4f | Constant | −0.922 | 0.247 | 0.481 | 10 | 9.355 | 0.016 |
| Actual fiber intake | 0.049 | 0.016 | |||||
| HDL cholesterol | |||||||
| 1a,b,c | Constant | 0.076 | 0.023 | 0.317 | 20 | 10.751 | 0.004 |
| LF diet (with LC as referent) | −0.118 | 0.036 | |||||
| 2a,b,c | Constant | 0.191 | 0.049 | 0.472 | 19 | 10.386 | 0.001 |
| LF diet (with LC as referent) | −0.116 | 0.032 | |||||
| Δ Caloric intake | 0.000 | 0.000 | |||||
| 3a,c,d | Constant | −0.068 | 0.086 | 0.397 | 19 | 7.914 | 0.003 |
| Δ Caloric intake | 0.000 | 0.000 | |||||
| Actual fat intake | 0.007 | 0.002 | |||||
| 4b,d | Constant | −0.197 | 0.133 | 0.362 | 19 | 6.956 | 0.005 |
| Actual protein intake | −0.020 | 0.007 | |||||
| Δ Caloric intake | 0.000 | 0.000 | |||||
| 5e | Constant | −0.146 | 0.052 | 0.512 | 18 | 18.804 | 0.001 |
| Actual SFA intake | 0.018 | 0.004 | |||||
| Glucose level | |||||||
| 1a,b,c | Constant | 0.168 | 0.099 | 0.360 | 20 | 12.796 | 0.002 |
| Δ Body weight | 0.065 | 0.018 | |||||
Stepwise regression analysis reveals at each model the strongest correlate (independent variable) to the dependent variable of interest (eg, DBP).
Entered independent variables: diet type (LF diet with LC diet as referent), Δ body weight, Δ caloric intake, actual carbohydrate intake, and actual fat intake.
Entered independent variables: diet type (LF diet with LC diet as referent), Δ body weight, Δ caloric intake, actual carbohydrate intake, and actual protein intake.
Entered independent variables: diet type (LF diet with LC diet as referent), Δ body weight, Δ caloric intake, actual fat intake, and actual protein intake.
Diet type was excluded as an individual variable.
Entered independent variables: actual ratio of SFA to total fat, and actual SFA intake.
Entered independent variables: actual ratio of fiber to carbohydrate intake, and actual fiber intake.
Abbreviations: Δ, change in; DBP, diastolic blood pressure; HDL, high-density lipoprotein; LC, low-cholesterol diet; LF, low-fat diet; SFA, saturated fat
For HDL cholesterol, actual intake of macronutrients appeared not to be of importance. Instead, type of diet was the most important factor, with the LF diet category associated with significantly lower HDL cholesterol levels compared with the LC diet category (a difference of 0.116 – 0.118 mmol/L; 0.004 < P < 0.001, depending on the model used). Exclusion of diet type resulted in models in which change in caloric intake in conjunction with actual fat intake or actual carbohydrate only marginally explained difference in HDL cholesterol (Table 5). Additional analysis also showed a correlation of SFA with HDL cholesterol, albeit small. There was no difference in meta-analysis outcome between the studies that reported SFA and all studies combined (data not shown).
Finally, change in body weight had a positive correlation with blood glucose levels (B = 0.065; R2 = 0.360), meaning that every 1 mmol/L lowering of blood glucose was related to a 15.4 kg reduction in body weight. Analyses with changes in macronutrient intakes instead of actual intake yielded similar results, as mentioned (Table S3 in the Supporting Information online).
DISCUSSION
The aim of this meta-analysis was to unravel the potential roles of changes in caloric and macronutrient intake across the spectrum of studies using LC and LF diets to explain alterations in weight loss and changes in markers of MetS in participants with obesity without cardiometabolic disease. The most important outcome of this analysis was that final, but not changes in, macronutrient intake explained reductions in markers of MetS much better than did LC vs LF dietary characterization per se, and these improvements did not necessarily depend on weight loss. Furthermore, the effect of carbohydrate intake on markers of MetS generally mirrored those observed for effects of fat and protein intake, irrespective of diet type. However, an increase in protein intake appeared to be most relevant for reducing body weight, whereas increased fat intake and reduced carbohydrate intake appeared to be most relevant for improving markers of MetS. Interestingly, caloric intake was not related to changes in body weight or markers of MetS.
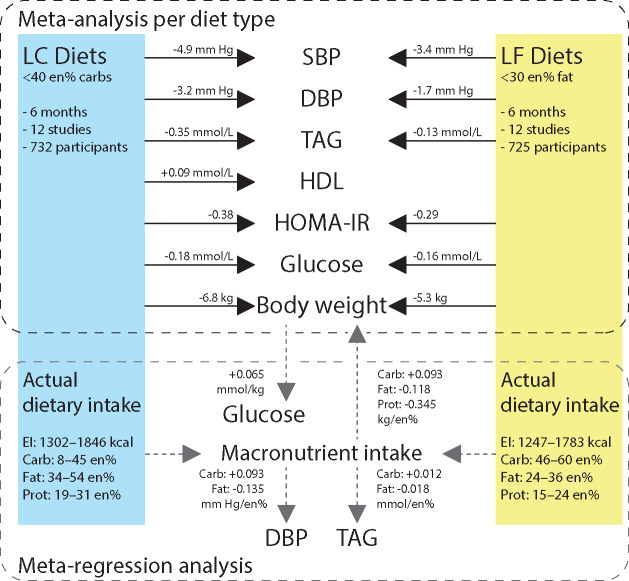
An issue that became clear from this analysis is that the different MetS indices were not uniformly altered by the various changes in dietary components and/or weight loss (Figure 4). For instance, glucose was only lowered by body weight loss. DBP was affected by body weight loss and by macronutrient composition. Reduction in SBP, however, was only correlated with body weight loss when both diets types were analyzed separately. TAG levels also appeared to be influenced differentially by the two diet types (ie, body weight loss in the LF diet category appeared to improve TAG levels, whereas in the LC diet category, both body weight loss and macronutrient composition were correlated with improvement of TAG levels). Mechanistically, such an effect may be explained by reduced hepatic TAG production in response to body weight loss, which is then accentuated by decreased carbohydrate substrate delivery.39–41
Figure 4.
Graphical summary of the meta-analysis and meta-regression at 6 months. In the blocks on the side, the requirements for each diet are seen in the upper half; actual intakes at 6 months are shown in the lower half. In the upper half of the figure is shown that both diets have significant effects on the markers of MetS. The numbers represent the outcomes of the meta-analysis per diet. In the lower half of the figure, the effect of actual macronutrient intake on markers of MetS and the effect of body weight on glucose levels are shown. The numbers represent the change in marker, per en% intake of macronutrient or, in the case of glucose, per kilogram of body weight. For instance, a diet consisting of 50 en% carbohydrates, 30 en% fat, and 20 en% protein results in a body weight change after 6 months of (0.093 × 50 – 0.118 × 30 – 0.345 × 20) – 5.8 kg. Abbreviations: carb, carbohydrate intake; DBP, diastolic blood pressure; EI, energy intake; en%, percentage of energy; glucose, fasting glucose levels; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; LC, low-carbohydrate diet; LF, low-fat diet; prot, protein intake; SBP, systolic blood pressure; TAG, plasma triglyceride levels.
Another example refers to the influences on HDL, which was selectively increased by the LC diet category, but there also was a relation between HDL cholesterol increase and lower carbohydrate and higher fat intake irrespective of diet type. Increases in HDL cholesterol levels may be explained by reduced TAG production and by the actions of cholesterol ester transferase.1,39,40,42 Downregulation of hepatic scavenger receptor B1 may play a role because this receptor binds HDL cholesterol and facilitates cholesterol transport to the liver, which may be modulated by dietary fats.39,40 In their study, Foster et al,43 indeed, found an increase in HDL cholesterol after TAG levels returned to baseline. In turn, increased HDL cholesterol levels may defend against cardiac events and insulin resistance.44,45 HOMA-IR (a proxy for insulin resistance46), however, was improved by LC and by LF diet types, apparently without any additional role for body weight and/or macronutrient composition, in the present meta-analysis.
Thus, these results can be summarized (Figure 4) as indicating that (1) both LF and LC diets are capable of inducing weight loss and improving MetS markers, (2) the resulting weight loss contributes to a reduction in glucose, and (3) lower carbohydrate intake and higher fat and protein intake reduce markers of MetS independent of weight loss. It can then be concluded that combined weight loss and changed macronutrient composition are most efficacious in reducing markers of MetS. Important for consideration of these results is that the actual macronutrient compositions were generally better at explaining improvements and variations in markers of MetS, than the changes in dietary macronutrient composition from baseline to end point.
The mechanisms by which the two main diet types reduce body weight and markers of MetS remain a matter of debate. Changes in body weight and changes in MetS indices were not explained by caloric intake. It is uncertain whether body weight loss is due to an increase in energy expenditure; only six studies measured physical activity, of which one showed an increase of activity over time that was similar in both diet groups. Alterations in macronutrient intake can also influence satiety. The increased fat intake of an LC diet can lead to stimulation of secretion of postprandial peptide YY, which is also known to reduce appetite and/or increase satiety.29,47 Increased protein intake is associated with greater weight loss,48 possibly due to the increased satiety effect of protein, among others, as a consequence of increased amino acid sensing by specific receptors in the gastrointestinal tract.49,50 In this meta-analysis, there was an increase in protein intake in both diets, albeit smaller in the LF diets compared with the LC diets, but this apparently did not contribute to alterations in caloric intake that could explain alterations in markers of MetS.
A correlation between weight loss and reduction of markers of MetS is in line with a plethora of weight loss studies indicating improvement of MetS indices by body-fat reduction, a factor contributing mostly to weight loss. Favorable alterations of adipokines (eg, leptin, adiponectin, resistin) have been mentioned as directly affecting autonomic, metabolic, and inflammatory pathways known to underlie reduced MetS indices.51 Furthermore, a reduction in visceral fat content often leads to reduced ectopic fat accumulation in organs like heart, pancreas, and skeletal muscle, which improves cardiometabolic functioning, as well.52,53
One of the findings of this meta-analysis is that a higher intake of SFA was related to body weight loss and beneficial changes in markers of MetS, whereas the ratio of SFA to total fat intake was not. This is counterintuitive because most studies recommended limiting SFA intake to <10 en%, as has been done since the publication of the Seven Nations Study, in which a higher SFA intake was related to a higher risk for cardiovascular disease.54 However, since then, several studies have shown that lower SFA intake is not related to a lower risk of cardiovascular disease.55,56 This is in accordance with the proposed mechanism that the presence of hypermetabolism (eg, in people with obesity) promoting fat oxidation (ie, fasting or increasing fat intake at the expense of carbohydrate intake) may improve cardiovascular function.57 No relation among fiber intake, body weight, and markers of MetS was found.
Limitations
Although this meta-analysis revealed several interesting insights, there are also several limitations. One of the factors that was not investigated in this meta-analysis but that could be of influence on changes in MetS markers is waist circumference, indicative of the amount of visceral fat. Because visceral fat is important for the development of MetS, as shown by the first criterion of having central obesity,4 it is recommended that future studies also look at changes in central obesity. Unfortunately, in this meta-analysis, only half of the studies specified change in waist circumference and even fewer specified change in fat percentage, making it difficult to draw conclusions.
Another concern in this meta-analysis is that different methods of food intake assessment were used. Because all but one study used intake from several days to assess average food intake, either by 24-hour recall or food records, it is unlikely that one study will influence the food intake data substantially, or that one method influenced the data substantially. In addition, the one study that used the food frequency questionnaire reported very similar intakes as the other studies, making it unlikely that this study had a large influence on the data analysis. All in all, due to the similar methodology used, it is believed that the method of food intake assessment did not substantially influence the data.
Not all studies reported SFA intake, and few reported unsaturated or polyunsaturated fatty acid intake or n-3 vs n-6 ratios. These could be additional mechanisms that affect several metabolic pathways, including cholesterol metabolism,58 intracellular lipid fat trafficking,59 and cardiovascular functioning.60 Types and sources of carbohydrates (simple or complex carbohydrates, carbohydrates from fruits and vegetables vs grains) also may differentially influence markers of MetS.61 Only half of the studies reported fiber intake and no relation to weight loss or markers of MetS was found, which could also be due to the little variation in intake, because there was no change in intake.
Another limitation is that few data have been published on trials lasting longer than 12 months. One indication of a habitual, and thus chronic, dietary effect was shown in a Korean study in which individuals with a high fat intake or an LC intake had lower occurrence of markers of MetS.58,62,63 Unfortunately, there are relatively few longitudinal data (beyond the time frames that were studied) supporting these claims. This may be due to loss of adherence to diets.18 Last, only one search engine was used to find articles. However, in this analysis, many of the same articles were found as in previously published reviews with a similar subject.14,16 Also, no additional request for data was made to the authors of included studies.
Conclusions and recommendations
Study results suggest reducing carbohydrate intake and increasing fat and protein intake (at least in the range found in the studies analyzed in this review) are beneficial for improving markers of MetS in obese persons without cardiovascular disease and type 2 diabetes. On the basis of the results of this meta-analysis, guidelines to prevent MetS may need to be reevaluated. It is clear that more long-term studies are needed with high levels of dietary adherence focused on the effects of macronutrient intake on weight loss and improvement of markers of MetS. Dietary adherence may depend on diet type, which could influence study outcomes considerably with respect to improvements of markers of MetS. To increase dietary adherence or prevent uneven drop-out, carbohydrate or fat preference of participants can be taken into account and evenly distributed among groups.
It may also be of interest to assess mood effects during dietary interventions, because changes in mood may interfere with dietary adherence in the setting of these studies.27 Also, the age of the participants may be of interest because the geometric framework hypothesis suggests that at different ages, different nutrients are required.64 Besides fat quality, the future studies may focus on the role of dietary fiber, which has been generally underappreciated in the comparison between LF and LC diets. The rapidly expanding microbiome field will undoubtedly shed light on the roles of consumption of fiber type65 and fat quality66 but also protein intake67 in cardiometabolic health of the host in relation to the efficacy of LF and LC diet types.
Supplementary Material
Acknowledgments
Author contributions. A.E.W. designed and conducted the research and analyzed data; extracted data were checked by G.v.D; A.E.W., G.v.D., M.S-J., A.P.v.B., and E.N. wrote the manuscript; A.E.W. and G.v.D. had primary responsibility for final content. All authors read and approved the final manuscript.
Funding. There are no funding sources to declare.
Declaration of interest. None of the authors have a conflict of interest.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Figure S1 Funnel plots with 95%CIs for publication bias of body weight change, separately for each diet at 6 and 12 months. The plots are weighted mean differences from individual studies (horizontal axis) against the standard error of the weighted mean difference (vertical axis). The solid vertical line represents the summary estimate of the weighted mean difference from baseline, derived using random-effects meta-analysis. (A) Body weight change in the LC group at 6 months; (B) body weight change in the LF group at 6 months; (C) body weight change in the LC group at 12 months; (D) body weight change in the LF group at 12 months. LC, low-carbohydrate diet; LF, low-fat diet.
Table S1 Meta-analysis outcomes in LC and LF diets after 6 and 12 months
Table S2 Meta-regression analysis with change in body weight or change in caloric intake as moderator, per diet
Table S3 Stepwise linear meta-regression modeling with change in macronutrient intakes
References
- 1. Seshadri P, Iqbal N, Stern L, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. [DOI] [PubMed] [Google Scholar]
- 2. Festa A, D'Agostino R, Mykkanen L, et al. LDL particle size in relation to insulin, proinsulin, and insulin sensitivity. The Insulin Resistance Atherosclerosis Study. Diabetes Care. 1999;22:1688–1693. [DOI] [PubMed] [Google Scholar]
- 3. Kang H-S, Gutin B, Barbeau P, et al. Low-density lipoprotein particle size, central obesity, cardiovascular fitness, and insulin resistance syndrome markers in obese youths. Int J Obes. 2002;26:1030–1035. [DOI] [PubMed] [Google Scholar]
- 4.International Diabetes Federation. The IDF consensus worldwide definition of the metabolic syndrome Part 1: Worldwide definition for use in clinical practice. Available at: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html. Accessed September 9, 2018.
- 5. Prasad H, Ryan DA, Celzo MF, et al. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. 2012;124:21–30. [DOI] [PubMed] [Google Scholar]
- 6. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 7. Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. [DOI] [PubMed] [Google Scholar]
- 8. Summary of revisions for the 2010 clinical practice recommendations. Diabetes Care. 2010;33:S3–S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feinman RD. Fad diets in the treatment of diabetes. Curr Diab Rep. 2011;11:128–135. [DOI] [PubMed] [Google Scholar]
- 10. Acheson KJ. Diets for body weight control and health: the potential of changing the macronutrient composition. Eur J Clin Nutr. 2013;67:462–466. [DOI] [PubMed] [Google Scholar]
- 11. Alexandraki I, Palacio C, Mooradian AD. Relative merits of low-carbohydrate versus low-fat diet in managing obesity. South Med J. 2015;108:401–416. [DOI] [PubMed] [Google Scholar]
- 12. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PLoS One. 2015;10:e0139817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Zuuren EJ, Fedorowicz Z, Kuijpers T, et al. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr. 2018;108:300–331. [DOI] [PubMed] [Google Scholar]
- 14. Hu T, Mills KT, Yao L, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol. 2012;176:S44–S54 doi: 10.1093/aje/kws264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clifton P. Assessing the evidence for weight loss strategies in people with and without type 2 diabetes. World J Diabetes. 2017;8:440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansoor N, Vinknes KJ, Veierød MB, et al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr. 2016;115:466–479. [DOI] [PubMed] [Google Scholar]
- 17. Atkins R. Dr Atkins New Diet Revolution. New York: Random House; 2009. [Google Scholar]
- 18. Morgan LM, Griffin BA, Millward DJ, et al. Comparison of the effects of four commercially available weight-loss programmes on lipid-based cardiovascular risk factors. Public Health Nutr. 2009;12:799–807. [DOI] [PubMed] [Google Scholar]
- 19. Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women. JAMA. 2007;297:969. [DOI] [PubMed] [Google Scholar]
- 20. Higgins J, Altman D, Sterne J. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Churchill R, Chandler J, Cumpston M, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017). Chichester, UK: Cochrane; 2017. [Google Scholar]
- 21. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 23. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of Meta-Essentials: a free and simple tool for meta-analysis. Res Syn Meth. 2017;8:537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAuley KA, Smith KJ, Taylor RW, et al. Long-term effects of popular dietary approaches on weight loss and features of insulin resistance. Int J Obes. 2006;30:342–349. [DOI] [PubMed] [Google Scholar]
- 25. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tay J, Brinkworth GD, Noakes M, et al. Metabolic effects of weight loss on a very-low-carbohydrate diet compared with an isocaloric high-carbohydrate diet in abdominally obese subjects. J Am Coll Cardiol. 2008;51:59–67. [DOI] [PubMed] [Google Scholar]
- 27. Brinkworth GD, Noakes M, Buckley JD, et al. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90:23–32. [DOI] [PubMed] [Google Scholar]
- 28. Wycherley TP, Brinkworth GD, Keogh JB, et al. Long-term effects of weight loss with a very low carbohydrate and low fat diet on vascular function in overweight and obese patients. J Intern Med. 2010;267:452–461. [DOI] [PubMed] [Google Scholar]
- 29. Thomson CA, Stopeck AT, Bea JW, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;62:1142–1152. [DOI] [PubMed] [Google Scholar]
- 30. Truby H, Baic S, DeLooy A, et al. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC “diet trials.” BMJ. 2006;332:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bazzano LA, Hu T, Reynolds K, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial. Ann Intern Med. 2014;161:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu T, Yao L, Reynolds K, et al. The effects of a low-carbohydrate diet vs. a low-fat diet on novel cardiovascular risk factors: a randomized controlled trial. Nutrients. 2015;7:7978–7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brehm BJ, Seeley RJ, Daniels SR, et al. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. 2003;88:1617–1623. [DOI] [PubMed] [Google Scholar]
- 34. Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease reduction. J Cardiopulm Rehabil. 2005;25:184–185. [DOI] [PubMed] [Google Scholar]
- 35. Ebbeling CB, Leidig MM, Feldman HA, et al. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. [DOI] [PubMed] [Google Scholar]
- 36. Frisch S, Zittermann A, Berthold HK, et al. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc Diabetol. 2009;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haufe S, Engeli S, Kast P, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. [DOI] [PubMed] [Google Scholar]
- 38. McAuley KA, Hopkins CM, Smith KJ, et al. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48:8–16. [DOI] [PubMed] [Google Scholar]
- 39. Stem L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial. ACC Curr J Rev. 2004;13:18. [DOI] [PubMed] [Google Scholar]
- 40. Hatahet W, Cole L, Kudchodkar BJ, et al. Dietary fats differentially modulate the expression of lecithin: cholesterol acyltransferase, apoprotein-A1 and scavenger receptor b1 in rats. J Nutr. 2003;133:689–694. [DOI] [PubMed] [Google Scholar]
- 41. Feinman RD, Fine EJ. Nonequilibrium thermodynamics and energy efficiency in weight loss diets. Theor Biol Med Model. 2007;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gotto A. Contemporary Diagnosis and Management of Lipid Disorders. Newtown, PA: Handbooks in Health Care Company; 2001. [Google Scholar]
- 43. Foster G, Wyatt H, Hill J, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Ann Intern Med. 2010;153:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaziano JM, Hennekens CH, O’Donnell CJ, et al. Fasting triglycerides, high-density lipoprotein, and risk of myocardial infarction. Circulation. 1997;96:2520–2525. [DOI] [PubMed] [Google Scholar]
- 45. McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. [DOI] [PubMed] [Google Scholar]
- 46. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 47. Essah PA, Levy JR, Sistrun SN, et al. Effect of macronutrient composition on postprandial peptide YY levels. J Clin Endocrinol Metab. 2007;92:4052–4055. [DOI] [PubMed] [Google Scholar]
- 48. Lim SS, Noakes M, Keogh JB, et al. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr Metab Cardiovasc Dis. 2010;20:599–607. [DOI] [PubMed] [Google Scholar]
- 49. Latner J, Schwartz M. The effects of a high-carbohydrate, high-protein or balanced lunch upon later food intake and hunger ratings. Appetite. 1999;33:119–128. [DOI] [PubMed] [Google Scholar]
- 50. Ojha U. Protein-induced satiation and the calcium-sensing receptor. Dmso. 2018;11:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Supriya R, Tam BT, Yu AP, et al. Adipokines demonstrate the interacting influence of central obesity with other cardiometabolic risk factors of metabolic syndrome in Hong Kong Chinese adults. PLoS One. 2018;13: e0201585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Piché M-E, Poirier P. Obesity, ectopic fat and cardiac metabolism. Expert Rev Endocrinol Metab. 2018;13:213–221. [DOI] [PubMed] [Google Scholar]
- 53. Gastaldelli A, Gaggini M. Ectopic fat: a target for cardiometabolic risk management. Expert Rev Cardiovasc Ther. 2016;14:1301–1303. [DOI] [PubMed] [Google Scholar]
- 54. Kromhout D, Menotti A, Bloemberg B, et al. Dietary saturated and transfatty acids and cholesterol and 25-year mortality from coronary heart disease: the Seven Countries Study. Prev Med (Baltim). 1995;24:308–315. [DOI] [PubMed] [Google Scholar]
- 55. Ramsden CE, Zamora D, Majchrzak-Hong S, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ. 2016;353:i1246 doi: 10.1136/bmj.i1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978 doi: 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liao W-H, Suendermann C, Steuer AE, et al. Aldosterone deficiency in mice burdens respiration and accentuates diet-induced hyperinsulinemia and obesity. JCI Insight. 2018;3:e99015 doi: 10.1172/jci.insight.99015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park S, Ahn J, Kim NS, et al. High carbohydrate diets are positively associated with the risk of metabolic syndrome irrespective to fatty acid composition in women: the KNHANES 2007–2014. Int J Food Sci Nutr. 2017;68:479–487. [DOI] [PubMed] [Google Scholar]
- 59. Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003;24:639–645. [DOI] [PubMed] [Google Scholar]
- 60. Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. [DOI] [PubMed] [Google Scholar]
- 61. Hashimoto Y, Tanaka M, Miki A, et al. Intake of carbohydrate to fiber ratio is a useful marker for metabolic syndrome in patients with type 2 diabetes: a cross-sectional study. Ann Nutr Metab. 2018;72:329–335. [DOI] [PubMed] [Google Scholar]
- 62. Park S, Ahn J, Lee B-K. Very-low-fat diets may be associated with increased risk of metabolic syndrome in the adult population. Clin Nutr. 2016;35:1159–1167. [DOI] [PubMed] [Google Scholar]
- 63. Ha K, Kim K, Chun OK, et al. Differential association of dietary carbohydrate intake with metabolic syndrome in the US and Korean adults: data from the 2007–2012 NHANES and KNHANES. Eur J Clin Nutr. 2018;72:848–860. [DOI] [PubMed] [Google Scholar]
- 64. Raubenheimer D, Simpson SJ. Nutritional ecology and human health. Annu Rev Nutr. 2016;36:603–626. [DOI] [PubMed] [Google Scholar]
- 65. Vinke PC, El Aidy S, van Dijk G. The role of supplemental complex dietary carbohydrates and gut microbiota in promoting cardiometabolic and immunological health in obesity: lessons from healthy non-obese individuals. Front Nutr. 2017;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Costantini L, Molinari R, Farinon B, et al. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18:2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma N, Tian Y, Wu Y, et al. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci. 2017;18: 348–407 doi: 10.2174/1389203718666170216153505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.