Abstract
Context
Contrary to nutritional guidelines, accumulating evidence shows that pregnant women’s energy intakes remain stable throughout trimesters. Although pregnant women may eat below their needs or underreport their energy intakes, it is also relevant to question how energy requirements – estimated through measurements of energy expenditure (EE) – change throughout pregnancy.
Objective
This review examined prospective studies that measured EE during pregnancy, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Data sources
PubMed/MEDLINE, Web of Science, Embase, and CINAHL databases were searched to identify relevant publications up to November 14, 2019.
Study selection
All studies that measured EE prospectively and objectively during pregnancy were included in this systematic review. Two authors independently screened 4852 references. A total of 32 studies were included in the final analysis.
Data extraction
One author extracted data and assessed the risk of bias and a second author did so for a random sample of studies (n = 7; 22%).
Data analysis
Increases in resting EE ranged from 0.5% to 18.3% (8–239 kcal), from 3.0% to 24.1% (45–327 kcal), and from 6.4% to 29.6% (93–416 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. Increases in total EE ranged from 4.0% to 17.7% (84–363 kcal), from 0.2% to 30.2% (5–694 kcal), and from 7.9% to 33.2% (179–682 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. Participants were mainly of normal weight, although many studies did not report important covariates such as prepregnancy body mass index and gestational weight gain adequacy.
Conclusions
Additional high-quality longitudinal studies (ie, with multiple objective measurements of EE in all periods of pregnancy while considering important confounding variables, like gestational weight gain) are required.
Keywords: energy expenditure, energy intake, energy needs, energy requirements, pregnancy
INTRODUCTION
Pregnancy represents a crucial period in a woman’s life owing to, among other things, the numerous physiological changes the mother undergoes to ensure optimal fetal growth and development.1,2 In fact, to account for the increased maternal energy expenditure (EE) due to the mother’s weight gain and the development of maternal and fetal tissues, the dietary reference intakes recommend a daily increase of 340 and 452 kcal in energy intake (EI) in the first and third trimesters, respectively.3 These additional EIs should support the mother’s increasing EE and allow the accumulation of energy stores during pregnancy.3,4 Nevertheless, it should be noted that the dietary reference intake recommendations are based on a limited number of studies that all took place before 20005–19 and on a theoretical model developed by Hytten and Chamberlain in 1991.20 It is therefore possible that the resulting EI recommendations do not reflect the needs of pregnant women nowadays, who are generally older,21 have more sedentary behaviors, and have different body compositions than the populations studied at the time these recommendations were being developed.22–24 The EI recommendations have not been revised since then, even though the guidelines for gestational weight gain (GWG) were updated in 2009 to better represent the needs of women from all prepregnancy weight categories.25
Furthermore, other authors have observed, through longitudinal studies, little to no augmentation in EI across trimesters of pregnancy, which is in contradiction to current recommendations.26–29 One systematic review evaluating studies that measured EI throughout pregnancy also found no prospective increase in EI even though women gained a significant amount of weight during their pregnancy.30 In fact, excessive gestational weight gain (GWG) is now highly prevalent, affecting 1 in 2 women during pregnancy31,32 – a finding that is in contradiction with the lack of increase in EI observed by other authors. Indeed, since EI correlates with GWG,33 it is contradictory to observe reports of stable EI and excessive GWG at the same time. Furthermore, although numerous systematic reviews and meta-analysis have investigated the complex associations between EI and GWG,30,33–36 very few studies have investigated the other component of energy balance during pregnancy: energy expenditure. Thus, because contradictory results are reported in regards to EI and GWG, it is relevant to question the extent to which resting energy expenditure (REE) and total, 24-hour energy expenditure (TEE) increase during pregnancy. In fact, existing reviews on the topic have either examined the same studies or data on which the EI recommendations are based,37,38 or are focused solely on basal metabolism39 or respiratory quotient.40 Moreover, there are various methods for measuring EE, all with different limitations, thus making it difficult to compare studies that measure EE during pregnancy. Therefore, the present systematic review aimed to examine the variability in energy expenditure during pregnancy. Observational and intervention studies that prospectively measured REE and/or TEE in pregnant women were reviewed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Appendix S1; please see the Supporting Information online).41
METHODOLOGY
The present systematic review was achieved based on a predefined protocol (not registered). The database search strategy was designed by an experienced scientific librarian and the final search – of the MEDLINE, EMBASE, CINAHL and Web of Science databases – was conducted on November 14, 2019. There was no publication year limit, but only articles published in English and French were reviewed. The complete search strategy for all databases is provided in Appendix S2 (please see the Supporting Information online).
Titles and abstracts were first reviewed by 2 independent reviewers. The study selection process was performed using Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). All records presenting studies among pregnant women (or women in the preconception period) that reported or mentioned objective measurements of REE or TEE were selected for full-text review. The selected full-text records were reviewed by the same 2 independent reviewers. Full-text articles were included if the selection criteria matched (see below). For titles and abstracts as well as full-text screening, a third reviewer was contacted in cases of disagreement between the 2 independent reviewers. The PICOS (population, intervention or exposure, comparison, outcome, and study design) selection criteria are presented in Table 1. The comparison criteria were not applicable to the present systematic review.
Table 1.
PICOS criteria for inclusion of studies
| Parameter | Criterion |
|---|---|
| Population | Women with singleton pregnancies |
| Intervention/exposure | Time (pregnancy) |
| Comparison | Not applicable |
| Outcome | Energy expenditure variability |
| Study design |
Cohort studies Case-control studies Intervention studies |
Abbreviation: PICOS, population, intervention or exposure, comparison, outcome, and study design
Systematic reviews, conference abstracts, books, guidelines, case reports, and case studies were excluded from this review. Randomized controlled trials and intervention studies were also excluded, on the basis that any intervention could impact a pregnant woman’s EE. However, those studies were selected for qualitative analysis if they included a control group (no intervention) for which detailed information on EE measurement was provided. Moreover, after full-text screening, studies restricted to nonpregnant women, adolescents, and/or participants with diagnosed medical conditions were excluded. Other exclusion criteria were REE or TEE that was (1) not measured, (2) measured only once, (3) estimated using questionnaires or diaries, and (4) estimated with nonvalidated accelerometers or other devices.
Data were extracted by one reviewer using a predesigned template, and the following information was extracted: general study characteristics (author[s], journal, year of publication, study design, geographic setting, and sample size); EE measurement method(s); timing of EE assessment(s); summary results (participant characteristics, mean values of REE/TEE, mean GWG and adherence to GWG guidelines, etc.); measurement of dietary information; control of participants’ diet; and adjustment(s) of EE for covariates, and if so, for which covariates. A second reviewer extracted data from a randomly selected sample (n = 7, 22%) of included studies to ensure the adequacy of the data extraction. Quality assessment of the included studies was performed simultaneously with data extraction, using the Effective Public Health Practice Project’s quality assessment tool for quantitative studies.42 That tool is considered to be adequate for use in systematic reviews of observational studies and its quality assessment is based on (1) representativeness of study population, (2) study design, (3) inclusion of relevant confounders, (4) blinding of personnel/participants, (5) validity of measurement methods, and (6) report of dropouts/withdrawals.42,43 In brief, one reviewer assigned scores to the 6 subcategories of the quality assessment tool for all articles, and a different reviewer did the same with a random sample of selected articles (n = 7; 22%). There was 86% concordance in the scores assigned by both reviewers. A study could be classified as strong (no subcategories had been assigned the “weak” score), moderate (one subcategory had been assigned the “weak” score), or weak (2 or more subcategories had been assigned the “weak” score).
RESULTS
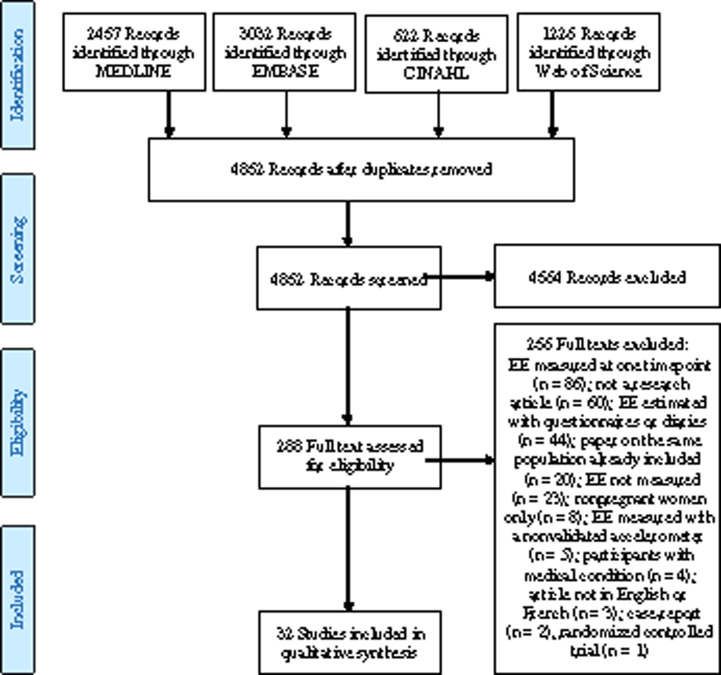
Of the 4852 records identified, 288 full-text articles were assessed for eligibility, of which 32 studies were included for qualitative analysis (Figure 1). Summaries of the included studies that measured REE and TEE are presented in Table 28,11,12,14,15,17,44–66 and Table 3,4,6,8,11,12,26,47,62 respectively. Mean values of REE were not reported in one of the studies (results presented in graphs only) and the sole author of the study was not reachable (deceased).52 Nevertheless, that study was included because it met all the inclusion criteria. Timing of EE measurement is presented as pregnancy periods (early, mid- and late pregnancy) rather than pregnancy trimesters because many studies either (1) did not have the same classification criteria for trimesters of pregnancy or (2) did not take into consideration the fact that for the same measurement period, participants were in 2 different trimesters of pregnancy. For example, one study reporting on mean REE included women that were in both the first and second trimesters (range: 0–20 wk).56 That measurement was therefore classified as being in “early pregnancy” rather than in the first trimester.
Figure 1.
Flow diagram of the literature search process.
Table 2.
Summary of studies that measured resting energy expenditure
| Authors, year | Country | Sample characteristicsa | When was REE measured? | How was REE measured? | Change in REEb |
|---|---|---|---|---|---|
| Banerjee et al (1971)44 | Singapore |
Sample size: 11 Age: not reported Prepregnancy BMI: not reported Ethnicity: all participants were Asian |
Two timepoints: second and third trimester (weeks not specified) | Portable respirometer |
Median REE (kcal/d) Mid-pregnancy: 1310 Late pregnancy: 1440 Change (%) 9.9; P not reported |
| Berggren et al (2015)45 | USA |
Sample size: 11 Age (median): 29.0 y (range 27.0–36.0) Prepregnancy BMI (median): 23.8 kg/m2 (range 19.2–31.4) Ethnicity: 91% Caucasian and 9% other |
Two timepoints: pre and late (34–36 wk) pregnancy | Ventilated open-circuit system |
Median REE (kcal/d) Pre: 1457 34–36 wk: 1743 Change (%) 19.6; P =0.003 |
| Bugatto et al (2017)46 | Spain |
Sample size: 35 (21 NW and 14 OW) Age (mean): NW, 29.3 y (SD 4.6); OW, 31.4 y (SD 3.1) Prepregnancy BMI (mean): NW, 21.4 kg/m2 (SD 1.5); OW, 32.9 kg/m2 (SD 5.9) Ethnicity: all women were Caucasian |
Seven timepoints: early (12 and 16 wk), mid- (20, 24, and 28 wk) and late (32 and 36 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) NW–OW 12 wk: 1378–1729 24 wk: 1390–1938 36 wk: 1654–2111 Change (%)NW–OW 12–24 wk: 0.9–12.1; P not reported 12–36 wk: 20.0; P < 0.001–22.1; P =0.03 24–36 wk: 18.9–8.9; P not reported |
| Butte et al (2004)47 | USA |
Sample size: 63 (17 UW, 34 NW, and 12 OW) Age (mean) UW, 30.8 y (SD 3.9); NW, 30.3 y (SD 4.3); OW, 31.2 y (SD 4.5) Prepregnancy BMI (mean) UW, 18.9 kg/m2 (SD 0.8); NW, 22.1 kg/m2 (SD 1.5); OW, 28.8 kg/m2 (SD 2.6) Ethnicity: 78% Caucasian, 9.5% African American, 9.5% Hispanic, and 3% Asian |
Five timepoints: pre, early (9 wk), mid- (22 wk), and late (36 wk) pregnancy and postpartum (27 wk after delivery) | Metabolic chamber |
Mean REE (kcal/d) UW–NW–OW Pre: 1201–1323–1505 9 wk: 1234–1350–1600 22 wk: 1330–1413–1693 36 wk: 1573–1673–2016 Change in REE (%) UW–NW–OW Pre to 9 wk: 2.7–2.0–6.3 Pre to 22 wk: 10.7–6.8–12.5 Pre to 36 wk: 31.0–26.5–34.0 9–22 wk: 7.8–4.7–5.8 9–36 wk: 27.5–23.9–26.0 22–36 wk: 18.2–18.4–19.1; P not reported |
| Catalano et al (1998)48 | USA |
Sample size: 5 Age (median): 31.8 y (SD 5.5) Prepregnancy BMI (median): 20.8 kg/m2 (SD 2.3) Ethnicity: not reported |
Three timepoints: pre, early (12–14 wk) and late (34–36 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1402 12–14 wk: 1513 34–36 wk: 1886 Change (%) Pre to 12–14 wk: 7.9; NS Pre to 34–36 wk: 34.5; P =0.0001 12–14 to 34–36 wk: 24.7; P =0.0001 |
| Cikrikci et al (1999)49 | Turkey |
Sample size: 24 Age (mean): 28.8 y (SD 4.8) Prepregnancy BMI: 24.0 kg/m2 (SD 1.9) Ethnicity: all women were Caucasian |
Three timepoints: first, second, and third trimesters (weeks not specified) | Ventilated open-circuit system |
Mean REE (kcal/d) First trimester: 1245 Second trimester: 1382 Third trimester: 1524 Change (%) 1st to 2nd: 11.0; P < 0.01 1st to 3rd: 22.4; P < 0.01 2nd to 3rd: 10.3; P < 0.001 |
| Damjanovic et al (2009)50 | Serbia |
Sample size: 31 Age (mean): 24.8 y (SD 5.7) Prepregnancy BMI: not reported Ethnicity: all women were Caucasian |
Three timepoints: early (12 wk), mid- (26 wk), and late (36 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 12 wk: 1404 26 wk: 1479 36 wk: 1564 Change (%) 12–26 wk: 5.3; P < 0.001 12–36 wk: 11.4; P < 0.001 26–36 wk: 5.7;P not reported |
| Denize et al (2019)51 | Canada |
Sample size: 10 Age (mean): 31.9 y (SD 3.7) Prepregnancy BMI (mean): 22.1 kg/m2 (SD 1.9) Ethnicity: not reported |
Three timepoints: early (15.4 ± 0.9 wk), mid- (26.4 ± 1.1 wk), and late (35.3 ± 1.1 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 15 wk: 2124 26 wk: 2304 35 wk: 2506 Change (%) 15–26 wk: 8.5;P not reported 15–35 wk: 18.0; P =0.06 26–35 wk: 8.9; P not reported |
| Durnin et al (1991)52 | Scotland |
Sample size: 162 Age (mean): 28.0 y (range 20.0–30.0) Prepregnancy BMI (mean): not reported Ethnicity: not reported |
Eleven timepoints: pre, early (between 1 and 20 wk), mid- (between 21 and 28 wk), and late (between 29 and 40 wk) pregnancy | Douglas bags | No increase from pre to 16 wk of pregnancy, then a steady increase, up to +400 kcal/d close to term. (No mean or P reported, sole author impossible to contact) |
| Emerson et al (1972)53 | USA |
Sample size: 10 Age (mean): 22.8 y (range 19.0–31.0) Prepregnancy BMI: not reported Ethnicity: not reported |
Seven timepoints: pre, early (0–20 wk), mid- (24 and 28 wk), and late (32, 36, and 38–41 wk) pregnancy | Douglas bags |
Mean REE (kcal/d) Pre: 1470 0–20 wk: 1488 28 wk: 1590 38–41 wk: 1753 Change (%) Pre to 0–20 wk: 1.2 Pre to 28 wk: 8.2 Pre to 38–41 wk: 19.3 0–20 to 28 wk: 6.9 0–20 to 38–41 wk: 17.8 28 to 38–41 wk: 10.3; P not reported |
| Eto et al (2018)54 | Japan |
Sample size: 103 Age (mean): 33.7 y (SD 5.7) Prepregnancy BMI (mean): 21.8 kg/m2 (SD 3.4) Ethnicity: all women were Asian |
Four timepoints: early (up to 15 wk), mid- (16–27 wk), and late (28 wk to delivery) pregnancy and postpartum (4–5 wk after delivery) | Portable respirometer |
Mean REE (kcal/d) 0–15 wk: 1461 16–27 wk: 1491 ≥ 28 wk: 1644 Change (%) 0–15 to 16–27 wk: 2.0; NS 0–15 to ≥ 28 wk: 12.1; P < 0.05 16–27 to ≥ 28 wk: 10.3; P < 0.05 |
| Forsum et al (1992)8 | Sweden |
Sample size: 22 Age (mean): 29.0 y (SD 4.0) Prepregnancy BMI (mean): 22.3 kg/m2 (SD 3.1) Ethnicity: not reported |
Three timepoints: pre, early (16–18 wk), and late (30 wk) pregnancy | Douglas bags |
Mean REE (kcal/d) Pre: 1340 16–18 wk: 1435 30 wk: 1651 Change in REE (%) Pre to 16–18 wk: 7.1; NS Pre to 30 wk: 23.2; P < 0.001 16–18 to 30 wk: 15.1; P not reported |
| Goldberg et al (1993)11 | England |
Sample size: 12 Age (mean): 28.8 y (SD 3.3) Prepregnancy BMI (mean): 23.0 kg/m2 (SD 3.3) Ethnicity: all women were Caucasian |
Four timepoints: early (12 wk), mid- (18 and 24 wk), and late (30 wk) pregnancyc | Metabolic chamber |
Mean REE (kcal/d) 12 wk: 1490 24 wk: 1582 30 wk: 1652 Change in REE (%) 12–24 wk: 6.2 12–30 wk: 4.4 24–30 wk: 10.9; P not reported |
| Hagobian et al (2015)55 | USA |
Sample size: 16 Age (mean): 30.3 y (SD 3.8) Prepregnancy BMI (mean): 25.2 kg/m2 (SD 3.6) Ethnicity: 81% Caucasian and 19% Hispanic |
Three timepoints: early (12–16 wk), mid- (24–26 wk), and late (32–34 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 12–16 wk: 1458 24–26 wk: 1580 32–34 wk: 1830 Change (%) 12–16 to 24–26 wk: 8.4; P < 0.05 12–16 to 32–34 wk: 25.5; P < 0.05 24–26 to 32–34 wk: 15.8; P < 0.05 |
| Hronek et al (2011)56 | Czech Republic |
Sample size: 31 Age (mean): 29.2 y (SD 3.6) Prepregnancy BMI (mean): 21.2 kg/m2 (SD 3.0) Ethnicity: not reported |
Four timepoints: early (0–20 wk), mid- (21–29 wk), and late (30–36 and 37–39 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 0–20 wk: 1407 21–29 wk: 1493 37–39 wk: 1655 Change (%) 0–20 to 21–29 wk: 6.1 0–20 to 37–39 wk: 17.6 21–29 to 37–39 wk: 10.9; overall P < 0.0001 |
| Illingworth et al (1987)57 | Scotland |
Sample size: 7 Age (mean): 28.2 y (SD 2.9) Prepregnancy BMI: not reported Ethnicity: not reported |
Four timepoints: early (12–15 wk), mid- (25–28 wk), and late (34–36 wk) pregnancy and after cessation of lactation | Ventilated open-circuit system |
Mean REE (kcal/d) 12–15 wk: 1457 25–28 wk: 1506 34–36 wk: 1550 Change (%) 12–15 to 25–28 wk: 3.4 12–15 to 34–36 wk: 6.4 25–28 to 34–36 wk: 2.9; P not reported |
| Kopp-Hoolihan et al (1999)12 | USA |
Sample size: 10 Age (mean): 29.1 y (SD 5.0, range) Prepregnancy BMI (mean): 23.1 kg/m2 (SD 2.1) Ethnicity: all women were Caucasian |
Five timepoints: pre, early (8–10 wk), mid- (24–26 wk), and late (34–36 wk) pregnancy and 4–6 wk postpartum | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1315 8–10 wk: 1306 24–26 wk: 1545 34–36 wk: 1693 Change in REE (%) Pre to 8–10 wk: -0.7 Pre to 24–26 wk: 17.5 Pre to 34–36 wk: 28.7 8–10 to 24–26 wk: 18.3 8–10 to 34–36 wk: 29.6 24–26 to 34–36 wk: 9.6; P not reported |
| Lof et al (2005)58 | Sweden |
Sample size: 22 Age (mean): 29.0 y (SD 3.0) Prepregnancy BMI (mean): 23.0 kg/m2 (SD 3.0) Ethnicity: not reported |
Six timepoints: pre, early (8, 14 wk), mid- (20 wk), and late (32, 35 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1299 8 wk: 1325 20 wk: 1373 35 wk: 1718 Change (%) Pre to 8 wk: 2.0; NS Pre to 20 wk: 5.7; NS Pre to 35 wk: 32.3; P < 0.001 8–20 wk: 3.6;P not reported 8–35 wk: 29.7; P not reported 20–35 wk: 25.1;P not reported |
| Martin et al (2001)59 | Australia |
Sample size: 8 Age (mean): 32.0 y (SD 1.0) Prepregnancy BMI: not reported Ethnicity: not reported |
Three timepoints: mid- (19 ± 1 wk) and late (36 ± 1 wk) pregnancy and postpartum (16 wk after delivery) | Ventilated open-circuit system |
Mean REE (kcal/d) 19 wk: 1416 36 wk: 1590 Change (%) 12.3; P < 0.05 |
| Nagy et al (1983)60 | USA |
Sample size: 5 Age (mean): 25.0 y (SD 3.0) Prepregnancy BMI: not reported Ethnicity: not reported |
Four timepoints: mid- (15–25 wk) and late (25–30, 30–35, 35–40 wk) pregnancy | Metabolic chamber |
Mean REE (kcal/d) 15–25 wk: 1454 25–30 wk: 1483 30–35 wk: 1613 35–40 wk: 1656 Change (%) From 2.0 to 13.9; P < 0.05 between 15–25 and 35–40 wk |
| Okereke et al (2004)61 | USA |
Sample size: 8 Age (mean): 31.6 y (SD 3.4) Prepregnancy BMI (mean): 26.2 kg/m2 (SD 4.5) Ethnicity: not reported |
Three timepoints: pre, early (12–14 wk), and late (34–36 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1488 12–14 wk: 1600 34–36: 1897 Change (%) Pre to 12–14 wk: 7.5; NS Pre to 34–36 wk: 27.5;P =0.0001 12–14 to 34–36 wk: 18.6; NS |
| Piers et al (1995)14 | India |
Sample size: 18 Age (mean): 29.6 y (SD 5.2) Prepregnancy BMI: not reported Ethnicity: all participants were Asian (Indian) |
Three timepoints: early (12 wk), mid- (24 wk), and late (34 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 12 wk: 1226 24 wk: 1347 34 wk: 1478 Change (%) 12–24 wk: 9.9; P < 0.05 12–34 wk: 20.6; P < 0.05 24–34 wk: 9.7; P < 0.05 |
| Poppitt et al (1993)62 | Gambia |
Sample size: 9 Age (mean): 26.2 y (SD 7.1) Prepregnancy BMI (mean): 21.4 kg/m2 (SD 2.1) Ethnicity: all women were African |
Five timepoints: pre, mid- (18, 24 wk), and late (30, 36 wk) pregnancyd | Metabolic chamber |
Mean REE (kcal/d)d Pre: 1244 18 wk: 1224 30 wk: 1306 Change in REE (%) Pre to 18 wk: -1.6 Pre to 30 wk: 5.0 18–30 wk: 6.7; P not reported |
| Prentice et al (1989)15 | England |
Sample size: 8 Age (mean): 29.3 y (SD 4.5) Prepregnancy BMI (mean): 23.1 kg/m2 (SD 3.3) Ethnicity: not reported |
Three timepoints: mid- (18, 24 wk) and late (30 wk) pregnancye | Metabolic chamber |
Mean REE (kcal/d) 18 wk: 1466 24 wk: 1529 30 wk: 1647 Change (%) 18–24 wk: 4.3 18–30 wk: 12.3 24–30 wk: 7.7; P not reported |
| Spaaij et al (1994)63 | Netherlands |
Sample size: 26 Age (mean): 30.0 y (SD 3.9) Prepregnancy BMI (mean): 21.8 kg/m2 (SD 2.4) Ethnicity: not reported |
Four timepoints: pre, early (13 wk), mid- (24 wk), and late (35 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1323 13 wk: 1381 24 wk: 1499 35 wk: 1619 Change (%) Pre to 13 wk: 4.4; NS Pre to 24 wk: 13.3; P < 0.05 Pre to 35 wk: 22.4; P < 0.05 13–24 wk: 8.5; P < 0.05 13–35 wk: 17.2; P < 0.05 24–35 wk: 8.0; P < 0.05 |
| Spaanderman et al (2000)64 | Netherlands |
Sample size: 12 Age (mean): 29.0 y (SD 3.0) Prepregnancy BMI: 23.0 kg/m2 (SD 3.0) Ethnicity: not reported |
Five timepoints: pre and early (6, 8, 10, and 12 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) Pre: 1496 6 wk: 1536 8 wk: 1451 10 wk: 1486 12 wk: 1426 Change (%) From −4.7 to 2.6%; NS |
| Tuazon et al (1987)65 | Philippines |
Sample size: 40 Age (mean): 23.8 y (SD 3.4) Prepregnancy BMI: not reported Ethnicity: all participants were Asian |
Seven timepoints: early (11–16 wk), mid- (17–22, 23–28 wk), and late (29–34, 35–40 wk) pregnancy and postpartum (6, 12 wk after delivery) | Douglas bags |
Mean REE (kcal/d) 11–16 wk: 1196 23–28 wk: 1292 35–40 wk: 1411 Change (%) 11–16 to 23–28 wk: 8.0 11–16 to 35–40 wk: 18.0 23–28 to 35–40 wk: 9.2; P not reported |
| van Raaij et al (1989)17 | Netherlands |
Sample size: 23 Age: not reported (only for total sample, not the 23 pregnant women) Prepregnancy BMI: not reported Ethnicity: all participants were Caucasian |
Five timepoints: pre, early (4–8, 10–14 wk), mid- (22–26 wk), and late (34–38 wk) pregnancy | Douglas bags |
Mean REE (kcal/d) Pre: 1452 10–14 wk: 1503 22–26 wk: 1541 34–38 wk: 1742 Change (%) Pre to 10–14 wk: 3.5; NS Pre to 22–26 wk: 6.1; NS Pre to 34–38 wk: 20.0; P < 0.001 10–14 to 22–26 wk: 2.5; NS 10–14 to 34–38 wk: 15.9; NS 22–26 to 34–38 wk: 13.0; NS |
| Willommet et al (1992)66 | Gambia |
Sample size: 9 Age (mean): 23.0 (SD 3.0) Prepregnancy BMI: not reported Ethnicity: all women were African |
Three timepoints: early (11 wk), mid- (23 wk), and late (33 wk) pregnancy | Ventilated open-circuit system |
Mean REE (kcal/d) 11 wk: 1253 23 wk: 1325 33 wk: 1426 Change (%) 11–23 wk: 5.7; P < 0.05 11–33 wk: 13.8; P < 0.01 23–33 wk: 7.6; P < 0.05 |
Sample size and characteristics refer to the pregnant women (with no medical condition) for which all data was available.
For studies with more than one REE/TEE measurement by pregnancy or prenatal period, only one measurement by period is presented, in order to lighten the table.
Only the presented timepoints included all 12 pregnant women.11
Only the 3 presented timepoints included all 9 pregnant women.62
Only the presented timepoints included all 8 pregnant women.15
Abbreviations: NS, not significant; NW, normal weight; OW, overweight; pre, preconception; REE, resting energy expenditure; SD, standard deviation; TEE, total energy expenditure; UW, underweight.
Table 3.
Summary of studies that measured total energy expenditure
| Authors, year | Country | Sample characteristicsa | When was TEE measured? | How was TEE measured? | Change in TEEb |
|---|---|---|---|---|---|
| Abeysekara et al (2016)26 | Australia |
Sample size: 26 Age (mean): 29.9 y (SD 4.0) Prepregnancy BMI (mean): 25.4 kg/m2 (SD 4.3) Ethnicity: 58% Caucasian, 31% Asian, 12% other |
Three timepoints: early (12–14 wk), mid- (24–26 wk), and late (34–36 wk) pregnancy | Accelerometer (Sensewear Armband) worn for 24 h |
Mean TEE (kcal/d) 12–14 wk: 2276 24–26 wk: 2451 34–36 wk: 2455 Change (%) 12–14 to 24–26 wk: 7.6; NS 12–14 to 34–36 wk: 7.9; P =0.003 24–26 to 34–36 wk: 0.2; NS |
| Butte et al (2004)47 | USA |
Sample size: 63 (17 UW, 34 NW, and 12 OW) Age (mean) UW, 30.8 y (SD 3.9); NW, 30.3 y (SD 4.3); OW, 31.2 y (SD 4.5) Prepregnancy BMI (mean) UW, 18.9 kg/m2 (SD 0.8); NW, 22.1 kg/m2 (SD 1.5); OW, 28.8 kg/m2 (SD 2.6) Ethnicity: 78% Caucasian, 9.5% African American, 9.5% Hispanic, 3% Asian |
Five timepoints: pre, early (9 wk), mid- (22 wk), and late (36 wk)pregnancy and postpartum (27 wk after delivery) | REE with metabolic chamber and TEE with doubly labeled water |
Mean TEE (kcal/d) UW – NW – OW Pre: 2348 – 2434 – 2940 22 wk: 2272 – 2520 – 2887 36 wk: 2439 – 2693 – 3020 Change in TEE (%) UW – NW – OW Pre to 22 wk: −3.2 to 3.5 to −1.8 Pre to 36 wk: 3.9 – 10.6 – 2.7 22–36 wk: 7.4 – 6.9 – 4.6; overall P =0.02 |
| de Groot et al (1994)6 | Netherlands |
Sample size: 10 Age (mean): 28.4 y (SD 2.5) Prepregnancy BMI (mean): 21.3 kg/m2 (SD 3.0) Ethnicity: all women were Caucasian |
Four timepoints: pre, early (12 wk), mid- (23 wk), and late (34 wk) pregnancy | Metabolic chamber |
Mean TEE (kcal/d) Pre: 2065 12 wk: 2089 23 wk: 2172 34 wk: 2378 Change (%) Pre to 12 wk: 1.2; NS Pre to 23 wk: 5.2; P < 0.05 Pre to 34 wk: 15.2; P < 0.05 12–23 wk: 4.0; NS 12–34 wk: 13.8; P < 0.05 23–34 wk: 9.5; P < 0.05 |
| Forsum et al (1992)8 | Sweden |
Sample size: 22 Age (mean): 29.0 y (SD 4.0) Prepregnancy BMI (mean): 22.3 kg/m2 (SD 3.1) Ethnicity: not reported |
Three timepoints: pre, early (16–18 wk), and late (30 wk) pregnancy | Doubly labeled water |
Mean TEE (kcal/d) Pre: 2488 16–18 wk: 2297 30 wk: 2990 Change in TEE (%) Pre to 16–18 wk: −7.7; NS Pre to 30 wk: 20.2; P < 0.05 16–18 to 30 wk: 30.2; P not reported |
| Goldberg et al (1993)11 | England |
Sample size: 12 Age (mean): 28.8 y (SD 3.3) Prepregnancy BMI (mean): 23.0 kg/m2 (SD 3.3) Ethnicity: all women were Caucasian |
Four timepoints: early (12 wk), mid- (18 and 24 wk), and late (30 wk) pregnancyc | Doubly labeled water |
Mean TEE (kcal/d) 12 wk: 2430 24 wk: 2625 30 wk: 2679 Change in TEE (%) 12–24 wk: 8.0;P not reported 12–30 wk: 10.2; P not reported 24–30 wk: 2.1; P not reported |
| Kopp-Hoolihan et al (1999)12 | USA |
Sample size: 10 Age (mean): 29.1 y (SD 5.0, range) Prepregnancy BMI (mean): 23.1 kg/m2 (SD 2.1) Ethnicity: all women were Caucasian |
Five timepoints: pre, early (8–10 wk), mid- (24–26 wk), and late (34–36 wk) pregnancy and 4–6 wk postpartum | Doubly labeled water |
Mean TEE (kcal/d) Pre: 2208 8–10 wk: 2050 24–26 wk: 2414 34–36 wk: 2732 Change in TEE (%) Pre to 8–10 wk: −7.2 Pre to 24–26 wk: 9.3 Pre to 34–36 wk: 23.7 8–10 to 24–26 wk: 17.8 8–10 to 34–36 wk: 33.3 24–26 to 34–36 wk: 13.2; P not reported |
| Most et al (2019)4 | USA |
Sample size: 54 (10 INA, 8 REC, and 36 EXS) Age (mean) INA, 29.2 y (SD 1.3); REC, 25.0 y (SD 1.7); EXS, 27.7 y (SD 0.8) Prepregnancy BMI: all women were obese at 15 wk Ethnicity: 52% Caucasian, 41% African American, 7% other |
Two timepoints: early (13–16 wk) and late (35–37 wk) pregnancy | Doubly labeled water |
Mean TEE (kcal/d) INA – REC – EXS 13–16 wk: 2719 – 2664 – 2563 35–37 wk: 2966 – 2984 – 2882 Change in TEE (%) INA – REC – EXS 9.1 – 12.0 – 12.4; P not reported |
| Poppitt et al (1993)62 | Gambia |
Sample size: 9 Age (mean): 26.2 y (SD 7.1) Prepregnancy BMI (mean): 21.4 kg/m2 (SD 2.1) Ethnicity: all women were African |
Five timepoints: pre, mid- (18, 24 wk), and late (30, 36 wk) pregnancyd | Metabolic chamber |
Mean TEE (kcal/d) Pre: 1533 36 wk: 1612 (Other TEE timepoints not reported) Change in TEE (%) Pre to 36 wk: 5.2; P =0.64 |
Sample size and characteristics refer to the pregnant women (with no medical condition) for which all data was available.
For studies with more than one REE/TEE measurement by pregnancy or prenatal period, only one measurement by period is presented, in order to lighten the table.
Only the presented timepoints included all 12 pregnant women.11
Only the preconception and 36-week value were presented in the paper and included all women.62
Abbreviations: EXS, excessive gestational weight gain; INA, inadequate gestational weight gain; NS, not significant; NW, normal weight; OW, overweight; pre, preconception; REC, recommended gestational weight gain; REE, resting energy expenditure; SD, standard deviation; TEE, total energy expenditure; UW, underweight.
Characteristics of the studies
All included studies used a prospective design. Twenty-four studies measured REE14,15,17,44–46,48–61,63–66 and the other 8 either measured TEE only4,6,26 or a combination of REE and TEE.8,11,12,47,62 The majority of studies were published before 2000 (n = 18, 56%), were from Europe and North America (n = 24, 75%), and had ≤20 participants (n = 18, 56%).
Timing and methods of measurement
On average, studies reported 4 measurements (range: 2–11 measurements). Nine studies measured EE during preconception and early, mid-, and late pregnancy,6,12,17,47,52,53,58,62,63 similarly to 14 other studies that did the same but with no preconception measurement.11,14,15,26,46,49–51,54–57,65,66 Durnin52 measured REE in 162 women at 10 different timepoints throughout pregnancy and once in the preconception period, but no mean REE values were reported by the authors (results presented in graphs only). The ventilated open-circuit system and doubly labeled water were found to be the most frequently used methods for measuring REE (59%)12,14,45,46,48–51,55–59,61,63,64,66 and TEE (63%),4,8,11,12,47 respectively.
Resting energy expenditure
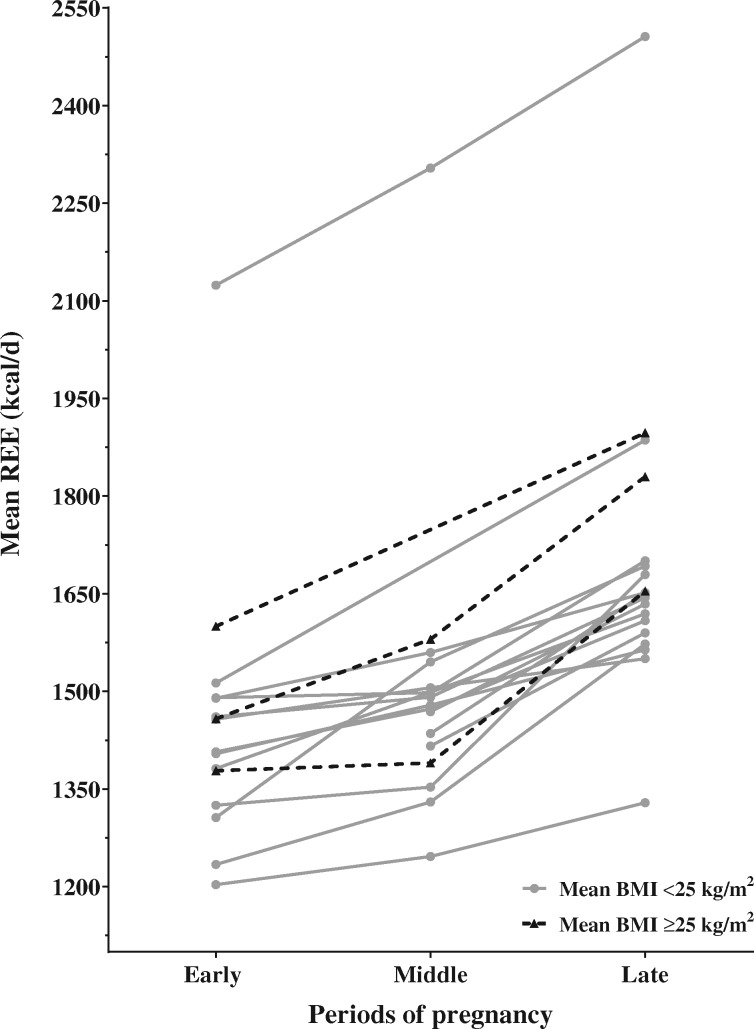
Figure 2 shows the changes in REE throughout pregnancy for the included studies. Increases in REE ranged from 0.5% to 18.3% (8–239 kcal) between early and mid-pregnancy, from 3.0% to 24.1% (45–327 kcal) between mid- and late pregnancy, and from 6.4% to 29.6% (93–416 kcal) between early and late pregnancy. The median increases in REE were 5.3% (72 kcal), 9.9% (153 kcal), and 18.0% (252 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. The greatest differences in REE were observed by Kopp-Hoolihan et al,12 who reported increases of 18.3% (239 kcal), 9.5% (147 kcal), and 29.6% (387 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively, after measuring REE at five different timepoints in 10 American pregnant women. Inversely, Illingworth et al,57 who measured REE at four different timepoints in 7 Scottish women, observed the smallest increase in REE throughout pregnancy: 3.3% (48 kcal), 3.0% (45 kcal), and 6.4% (93 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. More recently, Bugatto et al46 measured REE in 21 normal and 14 overweight Spanish pregnant women and observed increases of 0.9% (12 kcal), 19.0% (264 kcal), and 20.0% (276 kcal) in normal-weight women and increases of 12.1% (209 kcal), 8.9% (173 kcal), and 22.1% (382 kcal) in overweight women between early and mid-, mid- and late, and early and late pregnancy, respectively. All 3 studies used a ventilated open-circuit system to measure REE.12,46,57
Figure 2.
Change in resting energy expenditure throughout pregnancy for the included studies. Each data point represents the mean value of energy expenditure reported by studies that measured resting energy expenditure. Means from the same study are linked by the same line. Solid gray lines and dotted black lines represent the studies for which the participants’ mean prepregnancy BMI was <25 kg/m2 and ≥25 kg/m2, respectively. Abbreviation: REE, resting energy expenditure
Total energy expenditure
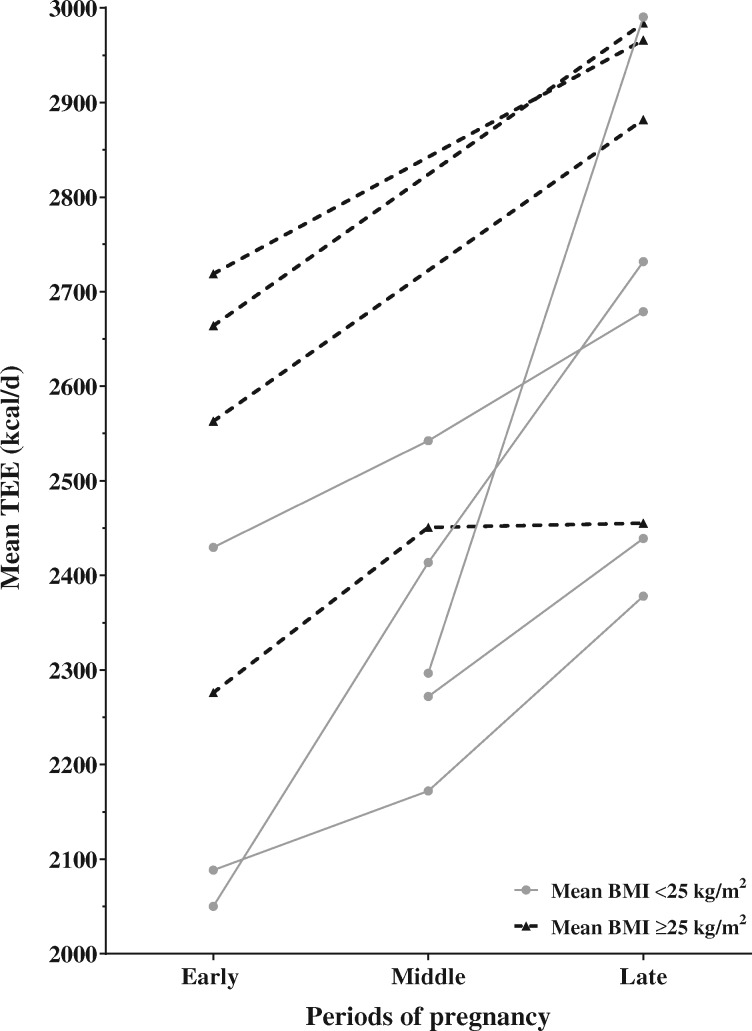
Changes in TEE throughout pregnancy, for the included studies, are presented in Figure 3. Increases in TEE ranged from 4.0% to 17.7% (84–363 kcal) between early and mid-pregnancy, from 0.2% to 30.2% (5–694 kcal) between mid- and late pregnancy, and from 7.9% to 33.2% (179–682 kcal) between early and late pregnancy, respectively. The median increases in TEE were 6.2% (144 kcal), 7.1% (170 kcal), and 12.0% (290 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. Similarly to what they observed for REE, Kopp-Hoolihan et al12 reported the greatest increases in TEE throughout pregnancy: 17.7% (239 kcal), 13.2% (147 kcal), and 33.2% (387 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. The smallest increases in TEE were reported by Goldberg et al,11 who measured TEE 4 times and observed differences of 4.6% (70 kcal), 5.4% (92 kcal), and 10.3% (162 kcal) between early and mid-, mid- and late, and early and late pregnancy, respectively. In a recent study by Most et al,4 TEE was measured in early and late pregnancy in 54 obese pregnant American women and was found to increase by 9.1% (247 kcal), 12.0%, (320 kcal) and 12.4% (319 kcal) in women with inadequate, recommended, and excessive GWG, respectively. Kopp-Hoolihan et al,12 Goldberg et al,11 and Most et al all4 measured TEE using the doubly labeled water method.
Figure 3.
Change in total energy expenditure throughout pregnancy for the included studies. Each data point represents the mean value of energy expenditure reported by studies that measured total energy expenditure. Means from the same study are linked by the same line. Solid gray lines and dotted black lines represent the studies for which the participants’ mean prepregnancy BMI was <25 kg/m2 and ≥25 kg/m2, respectively. Abbreviation: TEE, total energy expenditure
Prepregnancy energy expenditure
In general, studies observed small increases, and even decreases, in REE and TEE between the preconception and early-pregnancy periods (range: −3.3% to 7.9% or −41 to 112 kcal for REE, and −7.1% to 1.2% or −158 to 24 kcal for TEE). For example, Spaanderman et al64 measured REE in 12 women, using a ventilated open-circuit, in preconception and at 4 early-pregnancy timepoints and observed changes in REE ranging from −4.7% to 2.6% (−70 to 40 kcal) that were all not statistically significant. Similar findings were observed by de Groot et al,6 who measured TEE in 10 women in preconception and in each trimester using a metabolic chamber and found an increase of 1.2% (24 kcal) between preconception and early pregnancy.
Quality assessment of studies
The majority of studies (59.4%) were classified as being of moderate quality8,11,14,15,17,26,45,48–52,57–59,61,62,64,66 and 5 and 8 studies were classified as being of strong and weak quality, respectively. Women included in the 32 studies were predominantly Caucasian: seven studies comprised Caucasians only,6,11,17,46,49,50,63 5 studies reported a proportion of Caucasian women varying between 50% and 91%, and 6 studies had no Caucasian participant.14,44,54,62,65,66 The 14 remaining studies were conducted in Europe, North America, and Oceania and did not explicitly specify the ethnicity of their participants.8,12,15,48,51–53,56–61,64 Among the 23 studies that reported the mean prepregnancy BMI of their sample, 5 studies4,26,46,55,61 reported a mean prepregnancy BMI above the “normal weight” threshold of 24.9 kg/m2 and the other 18 studies6,8,11,12,15,47,48,50,51,54,56–60,62–64 reported mean prepregnancy BMIs that ranged from 20.8 to 24.1 kg/m2. The study by Most et al4 was the only one that included solely obese pregnant women (n = 54), and the studies by Bugatto et al46 and Butte et al47 were the only 2 studies that categorized their participants based on their prepregnancy BMI. Four studies provided their participants’ diet for a set number of days (range: 7–14 d) in order to minimize the impact of EI and macronutrient distribution on the measurement of EE.6,48,53,61 Moreover, 10 studies assessed their participants’ usual dietary habits8,11,12,14,26,45,52,55,59,65 and 6 studies assessed usual physical activity level.45,51,52,55,61,65 Almost all studies (87.5%) reported the mean or median GWG of their participants, but only 4 mentioned the proportion of their participants whose GWG fell under, within, or above the recommended guidelines. Among those 4 studies, the proportion of women whose GWG fell above the recommended guidelines ranged from 10% to 67%.4,47,51,55 Furthermore, 12 studies (37.5%) observed that the increases in REE/TEE were not statistically significant after adjustment for a weight variable (ie, prepregnancy BMI, GWG, fat mass, or fat-free mass).8,11,14,15,44,45,47,49,56,57,60,66
DISCUSSION
This systematic review aimed to examine observational studies with prospective and objective measurements of EE during pregnancy. In the present review, the majority of studies were published before 2000 and included mainly Caucasian and normal-weight pregnant women. This review showed that REE and TEE increase during pregnancy, mainly from early to late and from mid- to late pregnancy. Smaller increases were observed between pre- and early pregnancy as well as between early and mid-pregnancy. There is, however, a great variability in the extent to which REE and TEE increase throughout pregnancy. Moreover, inconsistencies were observed in the measurement and reporting of important covariates, such as prepregnancy BMI, GWG, usual dietary intakes, and physical activity level. The present review thus highlights the need for additional prospective studies of high quality – that is, with multiple measurements of REE or TEE in all periods of pregnancy (early, mid-, and late), using validated and objective measurement methods (doubly labeled water for TEE and ventilated open-circuit for REE), among a diverse population of pregnant women, while considering important confounding variables such as prepregnancy BMI and GWG.
The studies reviewed demonstrated that REE increased by 8–239 kcal (median 72 kcal) and by 93–416 kcal (median 252 kcal) between early and mid-, and between early and late, pregnancy, respectively. It is complex to compare those increases with the current EI recommendations for pregnant women because (1) the increases reported by the reviewed studies represent REE measured at specific timepoints only, whereas the recommendations for EI represent whole trimesters, and (2) those recommendations are supposed to reflect increases in TEE (ie, REE in combination with thermogenesis and physical activity).3 In studies that measured TEE, TEE increased by 84–363 kcal (median 144 kcal) and by 179–682 kcal (median 290 kcal), between early and mid-, and between early and late, pregnancy, respectively. Based on the median increases in TEE (144 and 290 kcal), half of the studies that measured TEE reported increases that were below the EI recommendations of an additional 340 and 452 kcal/d in the second and third trimesters, respectively.3 However, it should be mentioned that a comparison of measurements of TEE with EI recommendations needs to be interpreted with caution, as pregnant women are not supposed to be in energy balance, since energy is required for, and spent on, fat storage, placenta development, and changes in blood constituents and volume as well as for supporting fetal development. Nevertheless, since prospective measurements of REE and TEE do take into account pregnancy weight gain, questioning the justification behind the EI recommendations remains relevant.3,20
As recently mentioned by Most et al,67 the studies that form the foundation for the EI recommendations estimated the energy cost of GWG based on all participants, without considering each woman's adherence to GWG guidelines. Therefore, the inclusion of participants with excessive GWG, for example, has likely caused overestimation of the EI requirements, simply because such participants deposited more energy than they needed to, and, overall, larger body size leads to greater EE.67 In the present review, although most studies measured and reported their participants’ mean GWG, only 4 studies reported the proportion who adhered to or exceeded the GWG guidelines.4,47,51,55 This could be explained by the fact that most of the reviewed studies were published before the publication of the new GWG guidelines in 2009.25 Still, studies published before 2009 could have compared participants’ GWG with the previous guidelines,68 but most did not. The 4 studies that reported their participants’ adherence to GWG guidelines reported proportions of excessive GWG that ranged from 10% to 67%. Even though the other studies did not report their participants’ adherence to GWG guidelines, it can be hypothesized that the prevalence of excessive GWG was probably variable from one study to another. This could, in part, explain the large variability observed in the extent of the increases in REE and TEE, since body weight is associated with EE. In summary, since approximately half of the energy cost of pregnancy is associated with the development of maternal tissues (fat mass, breast tissues, uterus, and placenta),20 it is crucial that EI recommendations be based on measurements recorded among women with adequate GWG. Future studies should consider adherence to GWG guidelines when measuring TEE and REE during pregnancy, in order to avoid the overestimation of energy requirements associated with the development of maternal tissues.
The proportion of women entering pregnancy with overweight/obesity has increased since 2006, when EI recommendations were published. In fact, among American women of childbearing age, the overweight and obesity prevalence increased from 22.8% in 1976 to 53.5% in 2014,69 which may explain why most studies included in the present review – mainly published before 2000 – reported a mean prepregnancy BMI corresponding to a normal weight. Moreover, although it has been said that EI recommendations should be population-specific and based on observations made in healthy, normal-weight women,37 it could be argued that for a measure with such high variability as EE, women from all weight categories should not be studied as if they were in one single group. In fact, as observed by Bugatto et al,46 who measured REE in normal (n = 21) and overweight women (n = 14) throughout pregnancy, the increase in REE from mid- to late pregnancy was twice as high in normal vs. overweight women (19.0 vs 8.9%). Bugatto et al46 explained their results, in part, by the higher lipid oxidation observed in overweight vs. normal-weight women, which is in accordance with the findings of other reports on lipolysis during pregnancy.70 It could thus be hypothesized that, owing to the fact that overweight and obese women have more adipose tissue to oxidize, their energy metabolism, and therefore energy requirements, may differ from that of normal-weight women who rely more on carbohydrate oxidation.46 In fact, by wanting to recognize the different energy requirements of overweight and obese women, the American College of Obstetricians and Gynecologists acknowledged that overweight and obese pregnant women may not require as many additional calories as pregnant women of normal weight.71 However, no specific EIs were explicitly recommended.71 Additional studies are necessary to better understand the mechanisms governing energy metabolism, and requirements, of overweight and obese pregnant women.
Another similar systematic review by de Oliveira Fonseca Sally et al39 was published in Portuguese in 2013, and their database search was conducted in 2010. Their objective was to review fluctuations in basal metabolic rate during pregnancy and they found, based on the 37 studies they reviewed, that increases in basal metabolic rate ranged between 8% and 35%.39 However, that particular review included studies that were cross-sectional (one measurement of basal metabolic rate only) and studies that used physical activity questionnaires to measure the basal metabolic rate. In addition to having included articles published after 2010 (n = 8), the present review differs from the one conducted by Sally Ede et al39 firstly by the inclusion of studies that measured TEE as well as REE, secondly by including only cohort studies in which participants were compared to themselves, and thirdly by adding an inclusion criterion about the objective measurement of REE and TEE, which excluded certain studies (EE estimated with questionnaires and accelerometers) but probably increased, ultimately, the accuracy of the observations. Nevertheless, this review has some limitations, the main one being that no meta-analysis was carried out, because of the heterogeneity in the studied populations, timing of measurements, and measurement methods. Thus, the present review could not quantify the increase in REE and TEE during pregnancy. Another limitation is that only articles written in French and English were included, which could have limited the generalizability of the results, since high-quality studies from developing countries, or countries where women are of smaller stature (eg, Japan), for example, may have been excluded. Moreover, since studies among pregnant women with a serious medical condition were excluded from the present review, the observations made cannot be generalized to pregnant women with, for example, gestational diabetes mellitus. Further studies are necessary to assess changes in EE among populations of pregnant women with altered metabolic profiles.
CONCLUSION
It is clear that there is an increase in REE and TEE throughout pregnancy and particularly toward the end of pregnancy. However, the extent to which REE and TEE are increased is highly variable, and the majority of studies reported increases in TEE that were below the EI recommendations for pregnant women. Increases in REE and TEE also appear to be associated with prepregnancy weight status as well as with GWG. Future studies investigating EE during pregnancy should therefore do so in relation to the participants’ adherence to GWG guidelines. Because of the heterogeneity of the reviewed studies, it is difficult to obtain a precise overview of the situation in all pregnant women. Therefore, it is not possible to conclude to what extent EIs should be increased during pregnancy, even though this was not the purpose of this review. Nevertheless, the results of this review highlight the need to revise the current recommendations in EI during pregnancy, in order to make them more appropriate for overweight and obese women, since these are the individuals who are more at risk of excessive GWG.
Author contributions. All authors made substantial contributions to the conception and design of the manuscript, and all critically revised a first draft of the manuscript for important intellectual content. All authors contributed to the elaboration of the review protocol. C.S. and A.L. were the 2 independent reviewers in charge of titles and abstracts as well as full-text review. A.-S.M. acted as the third reviewer. Data extraction and quality assessment of the included studies was done by C.S. (all studies) and A.L. (22% of studies), under the supervision of A.-S.M. C.S. conducted primary statistical analyses of the data with the help of A.-S.M., A.L., S.O., B.F.-B., and F.H. All authors participated in the interpretation of data. All authors gave their approval of the manuscript’s final version to be published and therefore take public responsibility for the content of the manuscript. Finally, all authors agreed to be accountable for all aspects of the work.
Funding. C.S. received a graduate student award from the Canadian Institutes of Health Research (FRN: GSD-167043). The funders had no role in the design, analysis or writing of this article. No funding was obtained for the present article’s research, preparation, and publication.
Declaration of interest. All authors declare that they have no relevant interest(s) to declare.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Appendix S1 PRISMA 2009 checklist
Appendix S2 Search strategies used in the systematic review
Supplementary Material
Acknowledgments
We would like to acknowledge the valuable collaboration of Mme Carole Brault, scientific librarian at the CHU of Québec-Université Laval Research Center.
References
- 1. Symonds ME, Ramsay MM. Maternal-Fetal Nutrition during Pregnancy and Lactation. Cambridge, United Kingdom: Cambridge University Press; 2010. [Google Scholar]
- 2. Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best Pract Res Clin Obstet Gynaecol. 2013;27:791–802. [DOI] [PubMed] [Google Scholar]
- 3. Otten J, Hellwig J, Meyers L. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: U.S. National Academies Press; 2006. [Google Scholar]
- 4. Most J, Amant MS, Hsia DS, et al. Evidence-based recommendations for energy intake in pregnant women with obesity. J Clin Investig. 2019;129:4682–4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butte NF, Hopkinson JM, Mehta N, et al. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. [DOI] [PubMed] [Google Scholar]
- 6. de Groot LC, Boekholt HA, Spaaij CK, et al. Energy balances of healthy Dutch women before and during pregnancy: limited scope for metabolic adaptations in pregnancy. Am J Clin Nutr. 1994;59:827–832. [DOI] [PubMed] [Google Scholar]
- 7. Durnin JV, McKillop FM, Grant S, et al. Energy requirements of pregnancy in Scotland. Lancet. 1987;330:897–900. [DOI] [PubMed] [Google Scholar]
- 8. Forsum E, Kabir N, Sadurskis A, et al. Total energy expenditure of healthy Swedish women during pregnancy and lactation. Am J Clin Nutr. 1992;56:334–342. [DOI] [PubMed] [Google Scholar]
- 9. Forsum E, Sadurskis A, Wager J. Resting metabolic rate and body composition of healthy Swedish women during pregnancy. Am J Clin Nutr. 1988;47:942–947. [DOI] [PubMed] [Google Scholar]
- 10. Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of the components of energy balance in well-nourished lactating women. Am J Clin Nutr. 1991;54:788–798. [DOI] [PubMed] [Google Scholar]
- 11. Goldberg GR, Prentice AM, Coward WA, et al. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am J Clin Nutr. 1993;57:494–505. [DOI] [PubMed] [Google Scholar]
- 12. Kopp-Hoolihan LE, van Loan MD, Wong WW, et al. Longitudinal assessment of energy balance in well-nourished, pregnant women. Am J Clin Nutr. 1999;69:697–704. [DOI] [PubMed] [Google Scholar]
- 13. Nagy LE, King JC. Postprandial energy expenditure and respiratory quotient during early and late pregnancy. Am J Clin Nutr. 1984;40:1258–1263. [DOI] [PubMed] [Google Scholar]
- 14. Piers LS, Diggavi SN, Thangam S, et al. Changes in energy expenditure, anthropometry, and energy intake during the course of pregnancy and lactation in well-nourished Indian women. Am J Clin Nutr. 1995;61:501–513. [DOI] [PubMed] [Google Scholar]
- 15. Prentice AM, Goldberg GR, Davies HL, et al. Energy-sparing adaptations in human pregnancy assessed by whole-body calorimetry. Br J Nutr. 1989;62:5–22. [DOI] [PubMed] [Google Scholar]
- 16. Spaaij CJ, van Raaij JM, Van der Heijden LJ, et al. No substantial reduction of the thermic effect of a meal during pregnancy in well-nourished Dutch women. Br J Nutr. 1994;71:335–344. [DOI] [PubMed] [Google Scholar]
- 17. van Raaij JM, Schonk CM, Vermaat-Miedema SH, et al. Body fat mass and basal metabolic rate in Dutch women before, during, and after pregnancy: a reappraisal of energy cost of pregnancy. Am J Clin Nutr. 1989;49:765–772. [DOI] [PubMed] [Google Scholar]
- 18. van Raaij JM, Vermaat-Miedema SH, Schonk CM, et al. Energy requirements of pregnancy in The Netherlands. Lancet. 1987;330:953–955. [DOI] [PubMed] [Google Scholar]
- 19. Knuttgen HG, Emerson K Jr. Physiological response to pregnancy at rest and during exercise. J Appl Physiol. 1974;36:549–553. [DOI] [PubMed] [Google Scholar]
- 20. Hytten F, Chamberlain G. Clinical Physiology in Obstetrics. Oxford, England: Blackwell Scientific Publications; 1991. [Google Scholar]
- 21.Statistics Canada. Fertility: fewer children, older moms. Available at: https://www150.statcan.gc.ca/n1/pub/11-630-x/11-630-x2014002-eng.htm. Accessed June 8, 2020.
- 22. Church T, Martin CK. The obesity epidemic: a consequence of reduced energy expenditure and the uncoupling of energy intake? Obesity (Silver Spring). 2018;26:14–16. [DOI] [PubMed] [Google Scholar]
- 23. Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6:e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33:673–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasmussen K, Yaktine A; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 26. Abeysekera MV, Morris JA, Davis GK, et al. Alterations in energy homeostasis to favour adipose tissue gain: a longitudinal study in healthy pregnant women. Aust N Z J Obstet Gynaecol. 2016;56:42–48. [DOI] [PubMed] [Google Scholar]
- 27. Savard C, Lemieux S, Weisnagel SJ, et al. Trimester-specific dietary intakes in a sample of French-Canadian pregnant women in comparison with national nutritional guidelines. Nutrients. 2018;10:768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talai Rad N, Ritterath C, Siegmund T, et al. Longitudinal analysis of changes in energy intake and macronutrient composition during pregnancy and 6 weeks post-partum. Arch Gynecol Obstet. 2011;283:185–190. [DOI] [PubMed] [Google Scholar]
- 29. Vioque J, Navarrete-Munoz EM, Gimenez-Monzo D, et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jebeile H, Mijatovic J, Louie JC, et al. A systematic review and metaanalysis of energy intake and weight gain in pregnancy. Am J Obstet Gynecol. 2016;214:465–483. [DOI] [PubMed] [Google Scholar]
- 31. Deputy NP, Sharma AJ, Kim SY. Gestational weight gain—United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep. 2015;64:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morisset AS, Dubois L, Colapinto CK, et al. Prepregnancy body mass index as a significant predictor of total gestational weight gain and birth weight. Can J Diet Pract Res. 2017;78:66–73. [DOI] [PubMed] [Google Scholar]
- 33. Tielemans MJ, Garcia AH, Peralta Santos A, et al. Macronutrient composition and gestational weight gain: a systematic review. Am J Clin Nutr. 2016;103:83–99. [DOI] [PubMed] [Google Scholar]
- 34. Craemer KA, Sampene E, Safdar N, et al. Nutrition and exercise strategies to prevent excessive pregnancy weight gain: a meta-analysis. AJP Rep. 2019;9:e92–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shieh C, Cullen DL, Pike C, et al. Intervention strategies for preventing excessive gestational weight gain: systematic review and meta-analysis. Obes Rev. 2018;19:1093–1109. [DOI] [PubMed] [Google Scholar]
- 36. Streuling I, Beyerlein A, Rosenfeld E, et al. Weight gain and dietary intake during pregnancy in industrialized countries – a systematic review of observational studies. J Perinat Med. 2011;39:123–129. [DOI] [PubMed] [Google Scholar]
- 37. Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8:1010–1027. [DOI] [PubMed] [Google Scholar]
- 38. Catalano PM, Hollenbeck C. Energy requirements in pregnancy: a review. Obstet Gynecol Surv. 1992;47:368–372. [DOI] [PubMed] [Google Scholar]
- 39. de Oliveira Fonseca Sally E, dos Anjos LA, Wahrlich V. Basal metabolism during pregnancy: a systematic review [in Portuguese]. Ciênc Saúde Coletiva. 2013;18:413–430. [DOI] [PubMed] [Google Scholar]
- 40. Melzer K, Kayser B, Schutz Y. Respiratory quotient evolution during normal pregnancy: what nutritional or clinical information can we get out of it? Eur J Obstet Gynecol Reprod Biol. 2014;176:5–9. [DOI] [PubMed] [Google Scholar]
- 41. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thomas BH, Ciliska D, Dobbins M, et al. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1:176–184. [DOI] [PubMed] [Google Scholar]
- 43. Deeks JJ, Dinnes J, D'Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x, 1–173. [DOI] [PubMed] [Google Scholar]
- 44. Banerjee B, Khew KS, Saha N. A comparative study of energy expenditure in some common daily activities of non-pregnant and pregnant Chinese, Malay and Indian women. J Obstet Gynecol Br Commonw. 1971;78:113–116. [DOI] [PubMed] [Google Scholar]
- 45. Berggren EK, Presley L, Amini SB, et al. Are the metabolic changes of pregnancy reversible in the first year postpartum? Diabetologia. 2015;58:1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bugatto F, Quintero-Prado R, Vilar-Sanchez JM, et al. Prepregnancy body mass index influences lipid oxidation rate during pregnancy. Acta Obstet Gynecol Scand. 2017;96:207–215. [DOI] [PubMed] [Google Scholar]
- 47. Butte NF, Wong WW, Treuth MS, et al. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. 2004;79:1078–1087. [DOI] [PubMed] [Google Scholar]
- 48. Catalano PM, Roman-Drago NM, Amini SB, et al. Longitudinal changes in body composition and energy balance in lean women with normal and abnormal glucose tolerance during pregnancy. Am J Obstet Gynecol. 1998;179:156–165. [DOI] [PubMed] [Google Scholar]
- 49. Cikrikci E, Gokbel H, Bediz CS. Basal metabolic rates of Turkish women during pregnancy. Ann Nutr Metab. 1999;43:80–85. [DOI] [PubMed] [Google Scholar]
- 50. Damjanovic SS, Stojic RV, Lalic NM, et al. Relationship between basal metabolic rate and cortisol secretion throughout pregnancy. Endocrine. 2009;35:262–268. [DOI] [PubMed] [Google Scholar]
- 51. Denize KM, Akbari P, da Silva DF, et al. Greater energy demand of exercise during pregnancy does not impact mechanical efficiency. Appl Physiol Nutr Metab. 2020;45:493–499. [DOI] [PubMed] [Google Scholar]
- 52. Durnin JV. Energy requirements of pregnancy. Diabetes. 1991;40:152–156. [DOI] [PubMed] [Google Scholar]
- 53. Emerson K Jr, Saxena BN, Poindexter EL. Caloric cost of normal pregnancy. Obstet Gynecol. 1972;40:786–794. [PubMed] [Google Scholar]
- 54. Eto E, Maki J, Tamada S, et al. Assessment of resting energy expenditure and body composition in Japanese pregnant women with diabetes. J Diabetes Investig. 2018;9:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hagobian T, D’Amico A, Vranna C, et al. Prospective changes in energy intake, physical activity, and resting energy expenditure during pregnancy. Calif J Health Promot. 2015;13:66–71. [Google Scholar]
- 56. Hronek M, Klemera P, Tosner J, et al. Anthropometric measured fat-free mass as essential determinant of resting energy expenditure for pregnant and non-pregnant women. Nutrition. 2011;27:885–890. [DOI] [PubMed] [Google Scholar]
- 57. Illingworth PJ, Jung RT, Howie PW, et al. Reduction in postprandial energy expenditure during pregnancy. Br Med J Clin Res. 1987;294:1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lof M, Olausson H, Bostrom K, et al. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am J Clin Nutr. 2005;81:678–685. [DOI] [PubMed] [Google Scholar]
- 59. Martin A, Brown MA, O'Sullivan AJ. Body composition and energy metabolism in pregnancy. Aust N Z J Obstet Gynaecol. 2001;41:217–223. [DOI] [PubMed] [Google Scholar]
- 60. Nagy LE, King JC. Energy expenditure of pregnant women at rest or walking self-paced. Am J Clin Nutr. 1983;38:369–376. [DOI] [PubMed] [Google Scholar]
- 61. Okereke NC, Huston-Presley L, Amini SB, et al. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2004;287:E472–E479. [DOI] [PubMed] [Google Scholar]
- 62. Poppitt SD, Prentice AM, Jequier E, et al. Evidence of energy sparing in Gambian women during pregnancy – a longitudinal study using whole-body calorimetry. Am J Clin Nutr. 1993;57:353–364. [DOI] [PubMed] [Google Scholar]
- 63. Spaaij CJ, van Raaij JM, de Groot LC, et al. No changes during pregnancy in the net cost of cycling exercise. Eur J Clin Nutr. 1994;48:513–521. [PubMed] [Google Scholar]
- 64. Spaanderman ME, Meertens M, van Bussel M, et al. Cardiac output increases independently of basal metabolic rate in early human pregnancy. Am J Physiol Heart Circ Physiol. 2000;278:H1585–H1588. [DOI] [PubMed] [Google Scholar]
- 65. Tuazon MA, van Raaij JM, Hautvast JG, et al. Energy requirements of pregnancy in the Philippines. Lancet. 1987;330:1129–1131. [DOI] [PubMed] [Google Scholar]
- 66. Willommet L, Schutz Y, Whitehead R, et al. Whole body protein metabolism and resting energy expenditure in pregnant Gambian women. Am J Physiol. 1992;263:E624–E631. [DOI] [PubMed] [Google Scholar]
- 67. Most J, Dervis S, Haman F, et al. Energy intake requirements in pregnancy. Nutrients. 2019;11:1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Institute of Medicine. Part I. Nutritional status and weight gain. In: Nutrition During Pregnancy. Washington, DC: National Academies Press; 1990:25–233. [Google Scholar]
- 69. Singh GK, DiBari JN. Marked disparities in pre-pregnancy obesity and overweight prevalence among US women by race/ethnicity, nativity/immigrant status, and sociodemographic characteristics, 2012-2014. J Obes. 2019;2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50:938–948. [DOI] [PubMed] [Google Scholar]
- 71.American College of Obstetricians and Gynecologists (ACOG). Nutrition in pregnancy. In: Your Pregnancy and Childbirth: Month to Month. 6th ed. Washington, DC: ACOG; 2016:313–327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.