Abstract
Context
Follicle-stimulating hormone (FSH) concentrations increase during the perimenopausal transition and remain high after menopause. Loss of bone mineral density (BMD) and gain of bone marrow adiposity (BMA) and body fat mass also occur during this time. In mice, blocking the action of FSH increases bone mass and decreases fat mass.
Objective
To investigate the associations between endogenous FSH levels and BMD, BMA, and body composition in older adults, independent of estradiol and testosterone levels.
Design, Setting, and Participants
Older adults from the AGES-Reykjavik Study, an observational cohort study.
Main Outcome Measures
Areal BMD, total body fat, and lean mass were measured with dual-energy x-ray absorptiometry. Lumbar vertebral BMA was measured by 1H-magnetic resonance spectroscopy. Volumetric BMD and visceral and subcutaneous adipose tissue (VAT, SAT) areas were measured with quantitative computed tomography. The least squares means procedure was used to determine sex hormone–adjusted associations between quartiles of serum FSH and BMD, BMA, and body composition.
Results
In women (N = 238, mean age 81 years), those in the highest FSH quartile, compared with the lowest quartile, had lower adjusted mean spine integral BMD (−8.6%), lower spine compressive strength index (−34.8%), higher BMA (+8.4%), lower weight (−8.4%), lower VAT (−17.6%), lower lean mass (−6.1%), and lower fat mass (−11.9%) (all P < 0.05). In men, FSH level was not associated with any outcome.
Conclusions
Older postmenopausal women with higher FSH levels have higher BMA, but lower BMD and lower fat and lean mass, independent of estradiol and testosterone levels. Longitudinal studies are needed to better understand the underlying mechanisms.
Keywords: follicle-stimulating hormone (FSH), bone, bone marrow adiposity, adiposity, body composition, aging
The cessation of procreation is marked by the irreversible loss of ovarian functions, including ovulation and estradiol production, initially across several years of perimenopause. The most rapid rates of bone loss, characterized by increased bone turnover, occur during late perimenopause (1, 2); this is followed by a slower decline in bone mass solely as a function of age (3). In fact, at advanced ages, the rates of mainly low-turnover bone loss are similar in women and men (3). The perimenopausal transition also witnesses the onset and progression of visceral obesity, dysregulated energy homeostasis, and reduced physical activity (4). Moreover, recent studies document a marked increase in bone marrow adiposity (BMA) with age that is accelerated during menopause (5, 6).
A progressive decline in ovarian reserve triggers an increased production from the pituitary of follicle-stimulating hormone (FSH), to compensate for the declining estradiol levels. In peri- and postmenopausal women, low estradiol levels are associated with variable elevations in FSH. While the effects of low estradiol have been widely studied, less is known about the distinct contributions of high FSH to the physiological changes in bone mass and body composition. The Study of Women’s Health Across the Nation (SWAN) has characterized the effects of FSH on bone and body composition during the menopausal transition (1, 2, 7, 8). Late perimenopausal bone loss and the onset of weight gain track with elevations in FSH in the face of minimal reductions in serum estradiol (1, 2, 4, 7, 8). Notably, cross-sectional analyses from SWAN show that high FSH levels strongly correlate with low bone mineral density (BMD) and high bone turnover, independent of serum estradiol levels (1, 2, 9, 10). Likewise, women gained body fat and lost lean mass during the menopausal transition when FSH was high (4). These lines of evidence from SWAN support other cross-sectional and longitudinal studies documenting increased rates of bone loss at the beginning of the menopausal transition, a time when FSH levels rise sharply (1, 2, 9, 11-18).
Recent animal studies suggest that FSH may play a direct role in regulating bone and body composition. Notably, in mature mice, targeted FSH-blocking antibodies or anti-FSH vaccines have been shown to increase bone mass, reduce body fat including BMA, increase energy expenditure, and reduce serum cholesterol in various models of osteoporosis and obesity (19-27). This has led to the premise that an anti-FSH agent could have potential clinical utility during and after menopause. However, any clinical intervention focused on blocking FSH requires a thorough understanding of the function of FSH in both younger and older adults. In this cross-sectional study in the well-characterized AGES-Reykjavik cohort of older adults living in Reykjavik, Iceland (28), we assessed the associations between serum FSH levels and BMD, BMA, lean mass, and fat mass, independent of serum estradiol and testosterone levels.
Subjects and Methods
Study population
The AGES-Reykjavik Study is a longitudinal, observational study of community-dwelling older adults in Iceland, which was designed to examine genetic susceptibility and gene-environment interactions that contribute to phenotypes of old age (28). The baseline AGES-Reykjavik visit, conducted from 2002 to 2006, included 5764 participants between the ages of 67 and 93 years. Between 2007 and 2011, 3411 participants attended a second visit.
Two subgroups of participants attending this second visit were enrolled in the Bone Marrow Adiposity (BMA) Ancillary Study. Eligibility criteria included completion of quantitative computed tomographic (QCT) scans at the second visit and having no restriction for magnetic resonance imaging (MRI). AGES-BMA substudy participants were brought in as 2 cohorts, with 303 participants in 2010-2011 (subgroup A) and 241 participants in 2014-2015 (subgroup B). BMA measurements were obtained for both subgroups. Of the total 544 participants from both subgroups, 3 were excluded due to missing or inadequate BMA measurements, 3 were excluded for missing FSH measurements, and 54 were excluded for using medications known to affect BMA and/or FSH, namely hormone replacement therapy (estradiol or testosterone), selective estrogen receptor modulators (SERMs), glucocorticoids, anti-estrogens, aromatase inhibitors, gonadotropin-releasing hormone (GnRH) analogs, or anti-androgens. In addition, 1 female participant with high estradiol and low FSH levels was excluded due to suspected exogenous estradiol exposure, leaving 483 participants in the analytic sample. The ancillary study was approved by the National Bioethics Committee in Iceland (VSN: 14-001-V3 and VSN: 07-062-V9), the National Institute on Aging, and the University of California, San Francisco Institutional Review Board. All participants provided written informed consent.
Biochemical assays
Samples were taken after overnight fasting within 2 weeks of BMA measurements. Serum was stored at −80 ºC. FSH levels were measured on archived serum in June 2017 as a single batch using an ELISA (ALPCO, Salem, USA). The assay had a sensitivity of 1 IU/L, an intra-assay coefficient of variation (CV) of 3.0%, and an inter-assay CV of 4.5%. All samples were measured in duplicate.
Sex hormones were also measured on the archived serum in January 2016 as a single batch (Endoceutics Clinique, Quebec, Canada). Total estradiol and testosterone were analyzed using gas chromatography/mass spectrometry (Shimadzu Nexera/Qtrap 6500, Shimatdzu, Kyoto, Japan). Lower limits of quantitation (LLOQ) were 1 and 50 pg/mL for estradiol and testosterone, respectively. The inter-assay CVs at the LLOQ were 4.7% and 3.7% for estradiol and testosterone, respectively, and values were extrapolated below the LLOQ using Analyst software (AB Sciex, Concord, Canada).
Volumetric bone mineral density by quantitative computed tomography
QCT scans of the spine and hip were obtained using a 4-detector system (Sensation; Siemens Medical Systems, Erlangen, Germany), as described (29). QCT images were transferred to a network of computer workstations and processed to extract measures of volumetric bone mineral density (vBMD) using analysis techniques as described (30). There was an algorithm change in 2014, which was necessary owing to an update in the operating system. Baseline QCT scans for subgroup A were performed using the original algorithm, while baseline scans for subgroup B were analyzed using the revised algorithm. Participants from subgroup A who attended a follow-up visit had their baseline QCT scans re-analyzed using the revised algorithm, creating a subset of 172 participants in whom we had baseline QCT measurements obtained with both algorithms. Linear regression analyses of QCT bone parameters obtained with both algorithms were used to derive a regression formula for each bone parameter, in order to estimate QCT bone parameters using the revised algorithm for the 131 participants in subgroup A who attended the baseline visit only. Bone parameters derived from the revised algorithm were used for the QCT analyses of all participants.
Areal bone mineral density by dual-energy x-ray absorptiometry
Dual-energy x-ray absorptiometry (DXA) scanning of the hip, anteroposterior (AP) spine, and lateral spine was performed using a GE Lunar iDXA scanner (GE Healthcare, Madison, WI, USA; software version 11.4) as described (6). Vertebral fractures were assessed from lateral spine images using the quantitative morphometry method. By evaluating the extent of anterior or middle vertebral body height reduction in comparison to posterior height, vertebrae were classified as (0) normal (<20% reduction) or fractured (wedge, biconcave or crush), and graded as (1) mild (20%-25% reduction), (2) moderate (25%-40% reduction) or (3) severe (>40% reduction) according to Genant’s criteria (31). For these analyses, a grade of (2) moderate or (3) severe was considered evidence of a prevalent vertebral fracture (32).
Body composition measurements
Visceral adipose tissue (VAT) area (cm2) and subcutaneous adipose tissue (SAT) area (cm2) were obtained by QCT (Sensation; Siemens Medical Systems) using a 10 mm cross-section through the L4-L5 intervertebral space at 140 kVp, 330 mAs. The VAT compartment was first outlined manually. Analysis of abdominal images was carried out using a program adapted to characterize the VAT compartment as described previously (33). As with the bone parameters (above), VAT and SAT values used the revised algorithm and were estimated by linear regression formulas for the 131 participants in subgroup A who attended the baseline visit only. Total body fat mass (kg), total body lean mass (kg), and appendicular lean mass (kg) were measured with total body DXA (GE Healthcare Lunar iDXA scanner, software version 11.4). Appendicular lean mass index (ALMI) was calculated as appendicular lean mass/height2 (kg/m2).
Bone marrow adiposity
BMA was measured with a 1.5-T magnetic resonance (MR) scanner (GE Healthcare, Milwaukee, Wisconsin) with an eight-channel cervical-thoracic-lumbar spine coil (using the 3 lower elements; GE Healthcare). Single voxel proton MR spectroscopy (1H-MRS) was acquired in vertebral bodies from L1-L4 using single voxel proton MR spectroscopy based on point resolved spectroscopy (PRESS) sequence as previously described (34). The PRESS box was positioned in the middle of the vertebral body and the PRESS box size was kept the same for each vertebral level for all subjects.
The spectral data were analyzed with an in-house software using a Lorentzian model fitting in time domain. A water peak at 4.67 ppm and a lipid peak (bulk CH2 methylene protons) at 1.3 ppm were identified, and the area under each peak was calculated. BMA was then calculated as the ratio of fat to water plus fat (%). The mean (L1-L4) BMA was used in this analysis.
Daily quality assurance testing was performed at the AGES-Reykjavik imaging center, in addition to weekly stability and calibration testing. Two events were noted: a software upgrade in 2012 and a hardware failure in 2014. Both events occurred after subgroup A completed the baseline visit and before the baseline visit for subgroup B. To evaluate the effects of these events on BMA measurements, we compared the mean baseline BMA, adjusted for age and gender, between subgroups A and B. The difference between mean BMA in subgroup A vs B was 0.25% (95% CI: −1.54, +2.04; P = 0.79). This difference was small and not statistically significant, suggesting no systematic bias in the BMA measurement between the baseline visits. In addition, all adjusted models included a variable for “subgroup” to adjust for any systematic differences.
Other measurements
Height and weight were measured by study personnel at the AGES-BMA study visit. An interviewer administered a questionnaire, which included demographics. Participants were asked to bring in all medications and supplements used in the previous 2 weeks, which were recorded and coded according to the Anatomical Therapeutic Chemical Classification System. Diabetes was defined by self-report, diabetes medication use, and/or fasting glucose ≥7 mM at the study visit.
Statistical analyses
Baseline characteristics of participants were summarized using means and SDs for continuous measures and counts and percentages for categorical measures. The distributions for serum FSH levels and all outcomes were sufficiently normal, except for spine compressive strength index which required log transformation. The least squares means procedure was used to determine the association between quartiles of serum FSH and BMD, BMA, and body composition for men and women separately, with results presented as adjusted means and 95% CI. The adjusted mean for each serum FSH quartile was compared to the adjusted mean for those in the highest FSH quartile, with statistical significance set at P < 0.05. Logistic regression models were used to evaluate the likelihood of prevalent vertebral fracture for each serum FSH quartile compared to those in the highest quartile, separately for men and women. A test for trend across the quartiles was performed for each association. All models included age, subgroup (A or B), estradiol, and testosterone. Diabetes was also added to these models to determine if it was a confounder of the associations between FSH and our outcomes. All analyses were performed with SAS software (version 9.4, SAS Institute Inc., Cary, NC).
Results
Baseline parameters
Baseline characteristics of the cohort of 238 women and 245 men are presented in Table 1. The women were of mean age 80.8 (SD 4.2) years. Women had mean serum FSH levels of 71.6 (SD 23.2) IU/L and mean estradiol levels of 5.1 (SD 4.2) pg/mL. The women had mean body mass index (BMI) of 27.4 (SD 4.1) kg/m2 and mean BMA of 55.2% (SD 8.3%). A total of 52 women (22.0%) had prevalent vertebral fractures. The men, who were of mean age 82.6 (SD 4.1) years, had mean serum FSH levels of 19.0 (SD 16.9) IU/L, mean estradiol levels of 19.8 (SD 7.0) pg/mL, and mean testosterone levels of 388.6 (SD 166.9) ng/dL. Men had mean BMI of 26.7 (SD 3.6) kg/m2 and mean BMA of 54.1% (SD 8.8%). A total of 53 men (21.7%) had prevalent vertebral fractures.
Table 1.
Baseline Characteristics
| Women (n = 238) | Men (n = 245) | |
|---|---|---|
| Age, years, mean ± SD | 80.8 ± 4.2 | 82.6 ± 4.1 |
| FSH, IU/L, mean ± SD | 71.6 ± 23.2 | 19.0 ± 16.9 |
| Total estradiol, pg/mL, mean ± SD | 5.1 ± 4.2 | 19.8 ± 7.0 |
| Total testosterone, ng/dL, mean ± SD | 24.2 ± 16.4 | 388.6 ± 166.9 |
| Spine trabecular vBMD, g/cm3, mean ± SD | 0.064 ± 0.029 | 0.075 ± 0.031 |
| Spine integral vBMD, g/cm3, mean ± SD | 0.178 ± 0.036 | 0.192 ± 0.037 |
| Spine compressive strength index, g2/cm4, mean ± SD | 0.117 ± 0.084 | 0.202 ± 0.147 |
| Total hip trabecular vBMD, g/cm3, mean ± SD | 0.047 ± 0.029 | 0.066 ± 0.033 |
| Total hip cortical vBMD, g/cm3, mean ± SD | 0.506 ± 0.035 | 0.527 ± 0.035 |
| Total hip integral vBMD, g/cm3, mean ± SD | 0.206 ± 0.036 | 0.225 ± 0.039 |
| Femoral neck trabecular vBMD, g/cm3, mean ± SD | 0.025 ± 0.034 | 0.039 ± 0.040 |
| Femoral neck cortical vBMD, g/cm3, mean ± SD | 0.523 ± 0.040 | 0.538 ± 0.041 |
| Femoral neck integral vBMD, g/cm3, mean ± SD | 0.217 ± 0.037 | 0.228 ± 0.041 |
| Lumbar spine areal BMD, g/cm2, mean ± SD | 1.063 ± 0.173 | 1.240 ± 0.213 |
| Total hip areal BMD, g/cm2, mean ± SD | 0.824 ± 0.117 | 0.969 ± 0.144 |
| Femoral neck areal BMD, g/cm2, mean ± SD | 0.782 ± 0.110 | 0.898 ± 0.135 |
| Prevalent vertebral fracture, n (%) | 52 (22.0) | 53 (21.7) |
| (L1-L4) Bone marrow adipose tissue, %, mean ± SD | 55.2 ± 8.3 | 54.1 ± 8.8 |
| Visceral fat area, cm2, mean ± SD | 168.7 ± 68.6 | 220.0 ± 88.2 |
| Subcutaneous fat area, cm2, mean ± SD | 291.7 ± 97.9 | 208.7 ± 78.3 |
| Total body lean mass, kg, mean ± SD | 39.2 ± 4.4 | 52.2 ± 5.5 |
| Total body fat mass, kg, mean ± SD | 29.7 ± 8.2 | 27.4 ± 8.4 |
| Appendicular lean mass index, kg/m2, mean ± SD | 6.3 ± 0.8 | 7.4 ± 0.8 |
| Weight, kg, mean ± SD | 71.0 ± 11.6 | 82.4 ± 12.0 |
| Body mass index, kg/m2, mean ± SD | 27.4 ± 4.1 | 26.7 ± 3.6 |
| Visceral fat as percent of total abdominal fat, %, mean ± SD | 32.7 ± 7.9 | 45.8 ± 8.1 |
| Lean mass as percent of total body mass, %, mean ± SD | 56.0 ± 5.7 | 63.8 ± 5.8 |
| Fat mass as percent of total body mass, %, mean ± SD | 41.2 ± 5.8 | 32.6 ± 6.1 |
| Prevalent diabetes, n (%) | 15 (6.3) | 33 (13.5) |
| Prevalent osteoporosis (aBMD FN T-score ≤ −2.5), n (%) | 2 (0.8) | 1 (0.4) |
| Prevalent bisphosphonates use, n (%) | 13 (5.5) | 1 (0.4) |
| Fair or poor self-reported health, n (%) | 62 (26.0) | 59 (24.4) |
Abbreviations: aBMD, areal bone mineral density; BMD, bone mineral density; FSH, follicle-stimulating hormone; vBMD, volumetric bone mineral density.
Serum FSH, BMD, and BMA
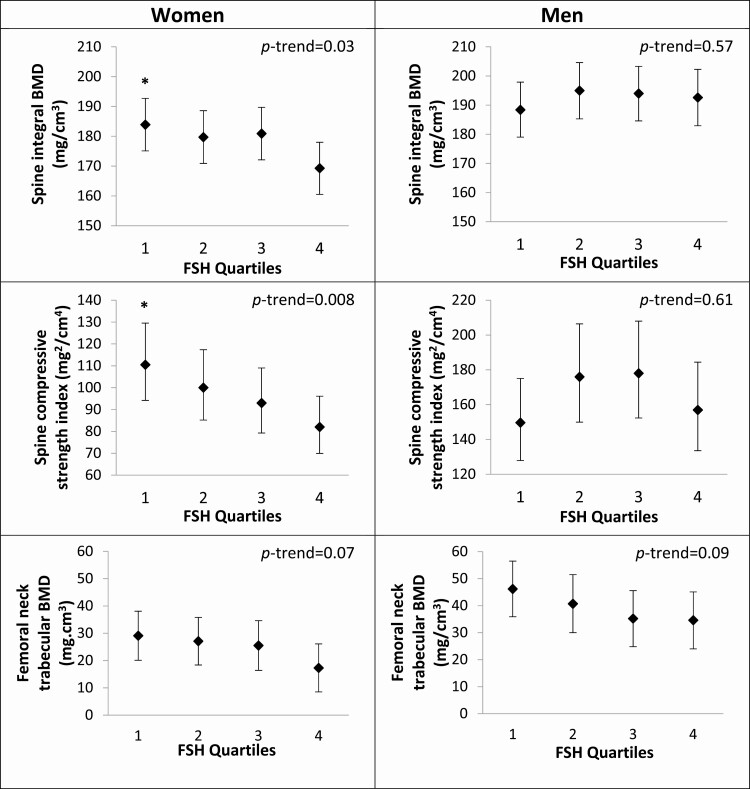
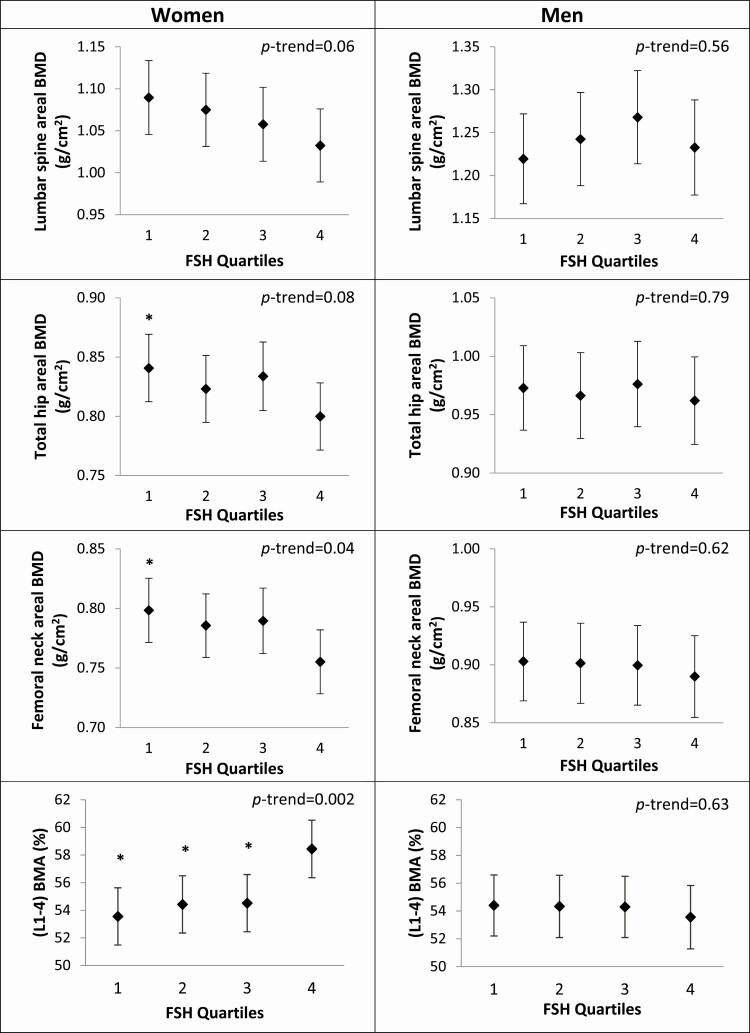
Among women, statistically significant differences in bone parameters and BMA were observed across quartiles of FSH in models adjusted for age, subgroup, total testosterone, and total estradiol (Table 2 and Figs 1A and 2A). Higher serum FSH levels were associated with lower adjusted mean spine integral vBMD (P trend = 0.03), lower mean spine compressive strength index (P trend = 0.008), lower mean femoral neck trabecular vBMD (P trend = 0.07), lower mean lumbar spine areal BMD (aBMD; P trend = 0.06), lower mean total hip aBMD (P trend = 0.08), lower mean femoral neck aBMD (P trend = 0.04), and greater mean BMA (P trend = 0.002). Compared with women in the lowest quartile (Q1) of FSH (Q1 17.9-55.2 IU/L), women in the highest quartile of FSH (Q4 85.2-180.0 IU/L) had lower spine integral vBMD (−8.6%), lower spine compressive strength index (−34.8%), lower total hip aBMD (−5.1%), and lower femoral neck aBMD (−5.7%) (all P < 0.05). Other bone density measures were similar across FSH quartiles in women.
Table 2.
Bone Density, Strength, and BMA in Women and Men by Quartiles of FSH
| Women | Q1 17.9-55.2 IU/L (n = 59) | Q2 55.2-71.3 IU/L (n = 60) | Q3 71.3-85.2 IU/L (n = 59) | Q4 85.2-180.0 IU/L (n = 60) | P trend |
|---|---|---|---|---|---|
| Spine trabecular BMD (mg/cm3) | 65.7 (58.6-72.7) | 66.6 (59.5-73.7) | 63.8 (56.8-70.8) | 58.3 (51.3-65.3) | 0.12 |
| Spine integral BMD (mg/cm3) | 183.9 (175.1-192.7)* | 179.7 (170.8-188.5) | 180.9 (172.1-189.7) | 169.3 (160.6-178.1) | 0.03 |
| Spine compressive strength index (mg2/cm4) | 110.5 (94.2-129.5)* | 100.0 (85.2-117.4) | 93.0 (79.3-109.0) | 82.0 (70.0-96.1) | 0.008 |
| Total hip trabecular BMD (mg/cm3) | 51.1 (43.5-58.7) | 47.6 (40.2-55.0) | 47.4 (39.7-55.0) | 43.1 (35.7-50.5) | 0.16 |
| Total hip cortical BMD (mg/cm3) | 504.1 (495.0-513.3) | 504.8 (495.9-513.7) | 514.0 (504.8-523.2) | 501.4 (492.5-510.3) | 0.99 |
| Total hip integral BMD (mg/cm3) | 208.5 (199.5-217.5) | 205.8 (197.1-214.6) | 211.5 (202.4-220.6) | 199.5 (190.7-208.3) | 0.28 |
| Femoral neck trabecular BMD (mg/cm3) | 29.1 (20.1-38.1) | 27.1 (18.4-35.8) | 25.5 (16.4-34.6) | 17.3 (8.5-26.1) | 0.07 |
| Femoral neck cortical BMD (mg/cm3) | 522.2 (511.3-533.0) | 520.7 (510.2-531.2) | 527.2 (516.3-538.1) | 521.5 (510.9-532.1) | 0.86 |
| Femoral neck integral BMD (mg/cm3) | 219.8 (210.4-229.3) | 216.1 (206.9-225.2) | 221.5 (212.0-231.0) | 210.4 (201.2-219.6) | 0.27 |
| Lumbar spine areal BMD (g/cm2) | 1.090 (1.046-1.134) | 1.075 (1.031-1.119) | 1.058 (1.014-1.102) | 1.033 (0.989-1.076) | 0.06 |
| Total hip areal BMD (g/cm2) | 0.841 (0.812-0.869)* | 0.823 (0.795-0.851) | 0.834 (0.805-0.863) | 0.800 (0.772-0.828) | 0.08 |
| Femoral neck areal BMD (g/cm2) | 0.798 (0.771-0.825)* | 0.786 (0.759-0.812) | 0.790 (0.762-0.817) | 0.755 (0.728-0.782) | 0.04 |
| (L1-L4) BMA (%) | 53.5 (51.5-55.6)* | 54.4 (52.3-56.5)* | 54.5 (52.4-56.6)* | 58.4 (56.4-60.5) | 0.002 |
| Men | Q1 2.6-7.6 IU/L (n = 61) | Q2 7.6-12.9 IU/L (n = 61) | Q3 12.9-24.5 IU/L (n = 61) | Q4 24.5-99.8 IU/L (n = 62) | P trend |
| Spine trabecular BMD (mg/cm3) | 75.3 (67.4-83.1) | 79.5 (71.5-87.5) | 71.4 (63.6-79.2) | 75.7 (67.6-83.8) | 0.75 |
| Spine integral BMD (mg/cm3) | 188.4 (179.0-197.9) | 195.0 (185.3-204.6) | 194.0 (184.6-203.3) | 192.6 (182.9-202.3) | 0.57 |
| Spine compressive strength index (mg2/cm4) | 149.6 (127.9-175.0) | 175.9 (149.9-206.4) | 178.0 (152.3-208.0) | 156.9 (133.5-184.4) | 0.61 |
| Total hip trabecular BMD (mg/cm3) | 70.6 (62.1-79.0) | 66.9 (58.1-75.7) | 63.2 (57.4-71.7) | 64.2 (55.6-72.9) | 0.25 |
| Total hip cortical BMD (mg/cm3) | 524.2 (515.0-533.4) | 526.8 (517.1-536.4) | 529.0 (519.7-538.3) | 529.9 (520.4-539.4) | 0.37 |
| Total hip integral BMD (mg/cm3) | 227.7 (217.6-237.9) | 221.8 (2111.1-232.4) | 223.7 (213.4-234.0) | 226.0 (215.5-236.4) | 0.85 |
| Femoral neck trabecular BMD (mg/cm3) | 46.2 (35.9-56.5) | 40.7 (30.0-51.5) | 35.2 (24.8-45.6) | 34.6 (24.0-45.1) | 0.09 |
| Femoral neck cortical BMD (mg/cm3) | 535.3 (524.7-545.9) | 538.9 (527.8-550.1) | 535.4 (524.6-546.2) | 541.9 (531.0-552.9) | 0.51 |
| Femoral neck integral BMD (mg/cm3) | 232.5 (221.9-243.0) | 227.9 (216.8-238.9) | 223.9 (213.2-234.6) | 228.4 (217.6-239.3) | 0.50 |
| Lumbar spine areal BMD (g/cm2) | 1.220 (1.167-1.272) | 1.243 (1.188-1.297) | 1.268 (1.214-1.322) | 1.233 (1.177-1.288) | 0.56 |
| Total hip areal BMD (g/cm2) | 0.973 (0.937-1.009) | 0.966 (0.930-1.003) | 0.976 (0.940-1.013) | 0.962 (0.925-1.000) | 0.79 |
| Femoral neck areal BMD (g/cm2) | 0.903 (0.869-0.937) | 0.901 (0.867-0.936) | 0.900 (0.865-0.934) | 0.890 (0.855-0.925) | 0.62 |
| (L1-L4) BMA (%) | 54.4 (52.2-56.6) | 54.3 (52.1-56.6) | 54.3 (52.1-56.5) | 53.6 (51.3-55.8) | 0.63 |
Values are adjusted for age, subgroup (A or B), estradiol, and testosterone. Values are means (95% CIs).
Abbreviations: BMA, bone marrow adiposity; BMD, bone mineral density.
Significantly different from quartile 4: *P < 0.05.
Figure 1.
Estimated mean QCT bone density and strength and 95% CIs by quartiles of FSH in women (a) and men (b). Values are adjusted for age, subgroup (A or B), estradiol, and testosterone. Significantly different from quartile 4: *P < 0.05.
Figure 2.
Estimated mean DXA bone density and BMA and 95% CIs by quartiles of FSH in women (a) and men (b). Values are adjusted for age, subgroup (A or B), estradiol, and testosterone. Significantly different from quartile 4: *P < 0.05.
Compared with women in the lowest FSH quartile, mean BMA was 8.4% higher for women in the highest FSH quartile (P < 0.05).
There were no statistically significant associations between serum FSH level and BMD or BMA parameters in men (Table 2 and Figs 1B and 2B).
When we included diabetes in the models, the results were not meaningfully changed (results not shown).
Serum FSH and body composition
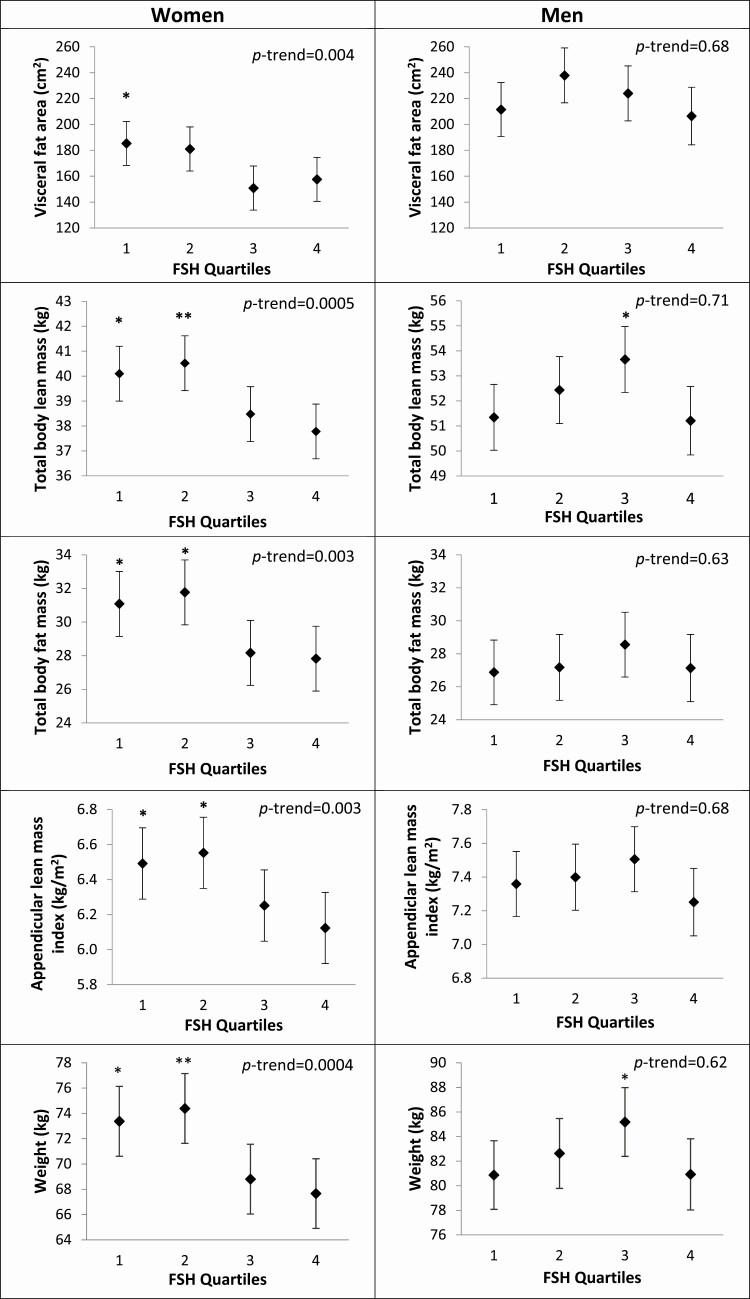
Among women, adjusted mean weight, mean visceral fat area, mean total body lean mass, mean total body fat mass, and mean ALMI were similar within the bottom 2 quartiles of FSH and similar within the top 2 quartiles of serum FSH, with women in the bottom 2 quartiles having higher means than those in the upper quartiles (Table 3 and Fig. 3A). Compared to women in the lowest FSH quartile, women in the top quartile had −8.4% lower weight, −17.6% lower VAT area, −6.1% lower lean mass, −11.9% lower fat mass, and −6.6% lower ALMI (all P < 0.05). The pattern for differences in fat mass as a percentage of total mass was similar to the pattern for differences in absolute levels of fat mass, with higher FSH associated with lower percentage fat mass. However, while absolute levels of lean mass were inversely associated with serum FSH level, this association was lost when lean mass was considered as a percentage of total mass.
Table 3.
Body Composition in Women and Men by Quartiles of FSH
| Women | Q1 17.9-55.2 IU/L (n = 59) | Q2 55.2-71.3 IU/L (n = 60) | Q3 71.3-85.2 IU/L (n = 59) | Q4 85.2-180.0 IU/L (n = 60) | P trend |
|---|---|---|---|---|---|
| Visceral fat area (cm2) | 185.4 (168.3-202.4)* | 181.1 (164.0-198.1) | 150.8 (133.8-167.9) | 157.6 (140.6-174.6) | 0.004 |
| Subcutaneous fat area (cm2) | 293.5 (269.7-317.2) | 313.8 (290.1-337.5)* | 284.7 (261.0-308.5) | 274.7 (251.0-298.3) | 0.12 |
| Total body lean mass (kg) | 40.1 (39.0-41.2)* | 40.5 (39.4-41.6)** | 38.5 (37.4-39.6) | 37.8 (36.7-38.9) | 0.0005 |
| Total body fat mass (kg) | 31.1 (29.1-33.0)* | 31.8 (29.8-33.7)* | 28.2 (26.2-30.1) | 27.8 (25.9-29.7) | 0.003 |
| Appendicular lean mass index (kg/m2) | 6.5 (6.3-6.7)* | 6.5 (6.3-6.8)* | 6.2 (6.0-6.5) | 6.1 (5.9-6.3) | 0.003 |
| Weight (kg) | 73.4 (70.6-76.1)* | 74.4 (71.6-77.1)** | 68.8 (66.0-71.6) | 67.7 (64.9-70.4) | 0.0004 |
| BMI (kg/m2) | 28.1 (27.2-29.1)* | 28.3 (27.3-29.3)* | 26.8 (25.8-27.8) | 26.4 (25.4-27.3) | 0.003 |
| Visceral fat as percent of total abdominal fat (%) | 34.9 (32.9-36.9) | 33.0 (31.0-35.0) | 31.0 (29.0-33.0) | 32.2 (30.2-34.2) | 0.03 |
| Lean mass as percent of total body mass (%) | 55.3 (53.9-56.6) | 55.4 (54.0-56.7) | 56.9 (55.5-58.3) | 56.4 (55.0-57.7) | 0.12 |
| Fat mass as percent of total body mass (%) | 42.0 (40.6-43.4) | 41.9 (40.5-43.3) | 40.3 (38.9-41.7) | 40.8 (39.4-42.2) | 0.10 |
| Men | Q1 2.6-7.6 IU/L (n = 61) | Q2 7.6-12.9 IU/L (n = 61) | Q3 12.9-24.5 IU/L (n = 61) | Q4 24.5-99.8 IU/L (n = 62) | P trend |
| Visceral fat area (cm2) | 211.5 (190.6-232.4) | 237.9 (216.7-259.1) | 224.0 (202.7-245.2) | 206.5 (184.3-228.7) | 0.68 |
| Subcutaneous fat area (cm2) | 200.7 (182.4-219.0) | 208.8 (190.1-227.4) | 217.7 (199.0-236.3) | 207.8 (188.3-227.3) | 0.46 |
| Total body lean mass (kg) | 51.3 (50.0-52.7) | 52.4 (51.1-53.8) | 53.6 (52.3-55.0)* | 51.2 (49.8-52.6) | 0.71 |
| Total body fat mass (kg) | 26.9 (24.9-28.8) | 27.2 (25.2-29.2) | 28.5 (26.6-30.5) | 27.1 (25.1-29.2) | 0.63 |
| Appendicular lean mass index (kg/m2) | 7.4 (7.2-7.5) | 7.4 (7.2-7.6) | 7.5 (7.3-7.7) | 7.2 (7.0-7.4) | 0.68 |
| Weight (kg) | 80.9 (78.1-83.7) | 82.6 (79.8-85.5) | 85.2 (82.4-88.0)* | 80.9 (78.0-83.8) | 0.62 |
| BMI (kg/m2) | 26.4 (25.6-27.3) | 26.6 (25.7-27.4) | 27.3 (26.4-28.1) | 26.4 (25.6-27.3) | 0.72 |
| Visceral fat as percent of total abdominal fat (%) | 45.2 (43.1-47.2) | 47.9 (45.8-50.0) | 44.9 (42.9-47.0) | 45.2 (43.0-47.4) | 0.67 |
| Lean mass as percent of total body mass (%) | 63.8 (62.5-65.2) | 64.2 (62.8-65.6) | 63.5 (62.1-64.8) | 63.6 (62.2-65.1) | 0.72 |
| Fat mass as percent of total body mass (%) | 32.5 (31.1-34.0) | 32.2 (30.7-33.6) | 33.0 (31.6-34.4) | 32.7 (31.2-34.2) | 0.71 |
Values are adjusted for age, subgroup (A or B), estradiol, and testosterone. Values are means (95% CIs).
Abbreviations: BMI, body mass index.
Significantly different from quartile 4: *P < 0.05, **P < 0.001.
Figure 3.
Estimated means and 95% CIs for body composition parameters by quartiles of FSH in women (a) and men (b). Values are adjusted for age, subgroup (A or B), estradiol, and testosterone. Significantly different from quartile 4: *P < 0.05, **P < 0.001.
We found little evidence of associations between FSH level and body composition in men (Table 3 and Fig. 3B).
When we included diabetes in the models, the results were not meaningfully changed (results not shown).
FSH and prevalent vertebral fractures
Among women, those in the highest FSH quartile were more likely to have prevalent vertebral fractures than those in the lower FSH quartiles, but none of the comparisons were statistically significant (Table 4). FSH level did not appear to be associated with prevalent vertebral fracture in men.
Table 4.
Prevalent Vertebral Fracture in Women and Men by Quartiles of FSH
| Women | ||||
|---|---|---|---|---|
| Q1 (n = 59) | Q2 (n = 60) | Q3 (n = 59) | Q4 (n = 58) | P trend |
| 0.72 (0.31, 1.67) | 0.48 (0.20, 1.16) | 0.45 (0.18, 1.11) | 1.00 (referent) | 0.48 |
| Men | ||||
| Q1 (n = 61) | Q2 (n = 61) | Q3 (n = 60) | Q4 (n = 62) | P trend |
| 0.70 (0.27, 1.77) | 1.04 (0.42, 2.57) | 0.86 (0.36, 2.08) | 1.00 (referent) | 0.54 |
Values are adjusted for age, subgroup (A or B), estradiol, and testosterone.
Values are odds ratios (95% CIs).
Other associations with FSH
In women, serum FSH was not correlated with estradiol (r = −0.02, P = 0.72) or testosterone (r = 0.07, P = 0.31). In men, serum FSH was inversely correlated both with estradiol (r = −0.27, P < 0.0001) and testosterone (r = −0.35, P < 0.0001), and estradiol and testosterone were highly correlated (r = 0.66, P < 0.0001). Finally, in both women and men, serum FSH level was positively correlated with age (r = 0.11, P = 0.08 and r = 0.20, P < 0.01, respectively).
Discussion
Here, we report that high serum FSH levels are associated with lower BMD and bone strength, greater vertebral BMA, and lower lean mass and total and visceral fat mass in older postmenopausal women in the AGES-Reykjavik Study. These cross-sectional findings provide insights into the biology of FSH in older adults, particularly when interpreted in the context of preclinical observations. Importantly, this study was conducted rigorously under the aegis of the Icelandic Heart Association, using state-of-the-art, carefully calibrated and validated methodologies.
With this study, we show for the first time that in older postmenopausal women, high FSH is associated with greater BMA independent of estradiol. This finding is consistent with the reduction in BMA seen with FSH receptor (FSHR) blocking antibody treatment in mice (23). We demonstrated previously in this cohort that estradiol and testosterone are negatively associated with BMA (35), consistent with the BMA-lowering effects of estradiol administration in postmenopausal women (36, 37). However, to our knowledge, there are no previously published data on FSH and BMA in humans. In addition, we know that bone marrow adiposity in older adults is associated with lower bone density and vertebral fractures (6, 38). Remarkably, we found that the difference in vertebral BMA between women in the lowest vs highest FSH quartile was 4.9%, which is similar to the 3.5% BMA difference between women with and without vertebral fractures in this cohort (34) and comparable to the ~5% BMA difference between healthy and osteoporotic patients in another cohort (39). In addition, with aging, vertebral BMA increases approximately 4.1% per decade (40). Thus, the magnitude of difference in BMA between low and high FSH levels is in a range of clinical relevance.
The negative correlation between serum FSH and bone mineral density in older postmenopausal women in the AGES-Reykjavik cohort is consistent with the findings of SWAN (1, 2, 17, 41) and other studies (41), in which high FSH levels in perimenopause are associated with and track over time with low BMD. We also show an association between serum FSH and bone strength, measured as the mean spine compressive strength index, although we did not find an association with prevalent vertebral fractures. There are no other published studies of FSH and fracture in women; results in men suggest at most a modest association with incident fracture (42). Our data are also consistent with multiple mouse studies wherein it is clear that FSH exacerbates bone loss (43, 44) and that inhibiting FSH is osteoprotective (22, 23, 26, 45). An association of low bone mass with activating polymorphisms of the FSHR gene further emphasizes the importance of a putative contributory role for FSH in skeletal regulation in postmenopausal women (46).
In the older women in the AGES-Reykjavik cohort, there is a correlation between high serum FSH level and low—rather than high—fat mass. This is consistent with previous studies reporting negative associations between FSH and total fat mass measured by DXA as well as visceral fat mass measured by MRI (10, 47). In contrast, the SWAN Study found a positive correlation between FSH and visceral fat, using waist circumference as a surrogate (4, 48). The latter is consistent with the mouse studies reporting reduced fat mass in response to FSH blockade (23). Three biological explanations may account for differences in the relationship between FSH and body fat in the AGES-Reykjavik women and the relatively younger SWAN cohort. One possibility is that the older Icelandic women were exposed to high circulating concentrations of FSH over decades, which could conceivably result in FSHR downregulation. A second possibility, albeit speculative, may arise from nuances in appetite between quartiles due to putative effects of FSH on food intake, as noted in mice (23); any differences in food intake will be most reflected in visceral fat accumulation. Yet another possibility is an issue of reverse causality, namely that fat mass and/or body weight affect FSH levels, in addition to any effect of FSH on fat. In 2 human studies, weight loss predicted increase in FSH level (49, 50). Thus, our cross-sectional observation of a negative correlation between FSH and fat may be driven by an effect of fat mass on FSH, particularly as adipocytes do express aromatase and can synthesize estrogen. Due to the cross-sectional nature of our analyses, we cannot determine the temporal relationship between FSH and body weight or fat mass.
With that said, and consistent with the SWAN perimenopausal cohort, we found that the other body composition parameter—lean mass—negatively correlates with FSH level (10, 48). However, this association is not observed with lean mass as a percentage of total weight. In mice, blocking FSH action increased the percentage lean mass, but not the absolute amount of lean mass (51). However, the mechanism of a putative effect of FSH on lean (mainly muscle) mass remains unclear and may involve cross-signaling with myostatin (52).
There is no effect of serum FSH on any parameter in men, at least in this cross-sectional analysis. This overall finding aligns with and supports the hypothesis that the bone loss and body fat accrual in normal aging men may not be FSH-driven, particularly as serum FSH levels rise slowly at a rate of 1% to 3% a year. Also, older men retain significantly higher estradiol levels (mean estradiol 20 pg/mL in our cohort), 4 times greater than in women, due to aromatization of testosterone. The pituitary response is therefore also different. Mean FSH level is 71 U/L in women in our cohort; the mean FSH in men is several times lower, 19 IU/L. Hypothetically, there could be a threshold level of FSH above which an effect on bone and fat mass may be observed; FSH may only influence body composition when estradiol levels are in the hypogonadal range, as they are in the postmenopausal women in this cohort but not in the men. However, longitudinal studies, such as the CHAMP study of older Australian men (70 years old and older) in Australia found that serum FSH correlated longitudinally with bone loss, although the models were not adjusted for estradiol (42). In the same study, FSH was not significantly associated with hip fracture in models adjusted for age, BMI, smoking status, physical activity, and comorbidities (HR: 0.93; 95% CI, 0.73-1.18) (51). A cross-sectional association between higher FSH and lower BMD has also been reported among men with type 2 diabetes (53).
Furthermore, and in the translational context, fat mass in men has been shown to be responsive to a drop in serum FSH, particularly when the fall was precipitous. An interventional clinical trial in patients with hormone-naive prostate cancer compared the effects on body composition of subcapsular orchidectomy, where FSH rises sharply, vs the GnRH agonist triptorelin where FSH is suppressed. In both cases, testosterone levels fell to near zero (54). It was notable that suppression of serum FSH was associated with significantly reduced body weight, total body fat, and subcutaneous fat as well as a trend to reduced visceral fat (54). In contrast, in patients with Klinefelter syndrome, a high serum FSH level is accompanied by increased fat mass, which is independent of testosterone in young patients (55). Thus, small increases in serum FSH over time may not drive bone loss or obesity in older men, but suppression of serum FSH may change body composition in younger men, and elevated FSH during early adolescence might increase adiposity.
Strengths of this study include a well-characterized cohort, high-quality measurement of marrow fat, body composition, and bone, and the ability to adjust for serum estradiol and testosterone levels. An important limitation is the cross-sectional design, which precludes determination of temporal relationships. Since we adjusted our analyses for estradiol and testosterone, we have evidence that the associations we report with FSH and our outcomes are independent of estradiol and testosterone levels, but we did not investigate the relative contribution of these 3 hormones. This is an important topic for further exploration. Although we could control for estradiol and testosterone levels, there may be other confounders that we did not identify which might account for the observed associations. Therefore, we cannot conclude from this study that FSH mediates biological effects, and future studies to elucidate the mechanisms behind our findings are warranted. Finally, the cohort we studied was limited to older men and women in Iceland, and our results may not apply to other populations.
In conclusion, our cross-sectional study of older healthy adults from Iceland reveals that elevated serum FSH is associated with lower bone mass, elevated bone marrow adiposity, and lower fat and lean mass in women. Longitudinal studies are needed to better understand the mechanisms that underlie these relationships.
Acknowledgments
Financial Support: This ancillary study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR0577819, R01AR065645, P30AR075055) and National Institute on Aging (NIA, U19AG060917). The AGES-Reykjavik Study is supported by funding from the National Institutes of Health (NIH) (Contract N01-AG-12100), the NIA Intramural Research program, Hjartavernd (the Icelandic Heart Association) and the Althingi (Icelandic Parliament).
A.G.V. received funding from the European Society for Endocrinology (Short-Term Fellowship 2017 and International Endocrine Scholars Programme 2017) and the Catharine van Tussenbroek Fund.
Glossary
Abbreviations
- 1H-MRS
proton magnetic resonance spectroscopy
- aBMD
areal bone mineral density
- ALMI
appendicular lean mass index
- BMA
bone marrow adiposity
- BMD
bone mineral density
- BMI
body mass index
- CV
coefficient of variation
- DXA
dual-energy x-ray absorptiometry
- FSH
follicle-stimulating hormone
- LLOQ
lower limit of quantitation
- MR
magnetic resonance
- QCT
quantitative computed tomography
- SWAN
Study of Women’s Health Across the Nation
- VAT
visceral adipose tissue
- vBMD
volumetric bone mineral density
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191-197. [DOI] [PubMed] [Google Scholar]
- 2. Sowers MR, Finkelstein JS, Ettinger B, et al. ; Study of Women’s Health Across the Nation . The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos Int. 2003;14(1):44-52. [DOI] [PubMed] [Google Scholar]
- 3. Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763-773. [DOI] [PubMed] [Google Scholar]
- 4. Thurston RC, Sowers MR, Sternfeld B, et al. Gains in body fat and vasomotor symptom reporting over the menopausal transition: the study of women’s health across the nation. Am J Epidemiol. 2009;170(6):766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Woods GN, Ewing SK, Sigurdsson S, et al. Greater bone marrow adiposity predicts bone loss in older women. J Bone Miner Res. 2020;35(2):326-332. [DOI] [PubMed] [Google Scholar]
- 7. Santoro N, Randolph JF Jr. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am. 2011;38(3):455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sowers MR, Zheng H, McConnell D, Nan B, Harlow S, Randolph JF Jr. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93(10):3958-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ebeling PR, Atley LM, Guthrie JR, et al. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81(9):3366-3371. [DOI] [PubMed] [Google Scholar]
- 10. Gourlay ML, Specker BL, Li C, Hammett-Stabler CA, Renner JB, Rubin JE. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone. 2012;50(1):311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chapurlat RD, Garnero P, Sornay-Rendu E, Arlot ME, Claustrat B, Delmas PD. Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int. 2000;11(6):493-498. [DOI] [PubMed] [Google Scholar]
- 12. Ito M, Nakamura T, Tsurusaki K, Uetani M, Hayashi K. Effects of menopause on age-dependent bone loss in the axial and appendicular skeletons in healthy Japanese women. Osteoporos Int. 1999;10(5):377-383. [DOI] [PubMed] [Google Scholar]
- 13. Perrone G, Galoppi P, Capri O, Anelli G, Borrello M, Zichella L. Lumbar and femoral bone density in perimenopausal women with irregular cycles. Int J Fertil Menopausal Stud. 1995;40(3):120-125. [PubMed] [Google Scholar]
- 14. Recker R, Lappe J, Davies K, Heaney R. Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res. 2000;15(10):1965-1973. [DOI] [PubMed] [Google Scholar]
- 15. Seifert-Klauss V, Fillenberg S, Schneider H, Luppa P, Mueller D, Kiechle M. Bone loss in premenopausal, perimenopausal and postmenopausal women: results of a prospective observational study over 9 years. Climacteric. 2012;15(5):433-440. [DOI] [PubMed] [Google Scholar]
- 16. Seifert-Klauss V, Link T, Heumann C, et al. Influence of pattern of menopausal transition on the amount of trabecular bone loss. Results from a 6-year prospective longitudinal study. Maturitas. 2006;55(4):317-324. [DOI] [PubMed] [Google Scholar]
- 17. Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261-1267. [DOI] [PubMed] [Google Scholar]
- 18. Steinberg KK, Freni-Titulaer LW, DePuey EG, et al. Sex steroids and bone density in premenopausal and perimenopausal women. J Clin Endocrinol Metab. 1989;69(3):533-539. [DOI] [PubMed] [Google Scholar]
- 19. Geng W, Yan X, Du H, Cui J, Li L, Chen F. Immunization with FSHβ fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem Biophys Res Commun. 2013;434(2):280-286. [DOI] [PubMed] [Google Scholar]
- 20. Guo Y, Zhao M, Bo T, et al. Blocking FSH inhibits hepatic cholesterol biosynthesis and reduces serum cholesterol. Cell Res. 2019;29(2):151-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han X, Guan Z, Xu M, et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology. 2020;148:103-111. [DOI] [PubMed] [Google Scholar]
- 22. Ji Y, Liu P, Yuen T, et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc Natl Acad Sci U S A. 2018;115(9):2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca(2+)/CREB pathway. Aging Cell. 2015;14(3):409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaidi M, Lizneva D, Kim SM, et al. FSH, bone mass, body fat, and biological aging. Endocrinology. 2018;159(10):3503-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu LL, Blair H, Cao J, et al. Blocking antibody to the β-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc Natl Acad Sci U S A. 2012;109(36):14574-14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu LL, Tourkova I, Yuen T, et al. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012;422(1):54-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris TB, Launer LJ, Eiriksdottir G, et al. Age, gene/environment susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165(9):1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gudmundsdottir SL, Indridason OS, Franzson L, Sigurdsson G. Age-related decline in bone mass measured by dual-energy X-ray absorptiometry and quantitative ultrasound in a population-based sample of both sexes: identification of useful ultrasound thresholds for osteoporosis screening. J Clin Densitom. 2005;8(1):80-86. [DOI] [PubMed] [Google Scholar]
- 30. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006-1012. [DOI] [PubMed] [Google Scholar]
- 31. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137-1148. [DOI] [PubMed] [Google Scholar]
- 32. Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14(5):821-828. [DOI] [PubMed] [Google Scholar]
- 33. Koster A, Murphy RA, Eiriksdottir G, et al. Fat distribution and mortality: the AGES-Reykjavik Study. Obesity (Silver Spring). 2015;23(4):893-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mistry SD, Woods GN, Sigurdsson S, et al. Sex hormones are negatively associated with vertebral bone marrow fat. Bone. 2018;108:20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19(9):1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Limonard EJ, Veldhuis-Vlug AG, van Dussen L, et al. Short-term effect of estrogen on human bone marrow fat. J Bone Miner Res. 2015;30(11):2058-2066. [DOI] [PubMed] [Google Scholar]
- 38. Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279-285. [DOI] [PubMed] [Google Scholar]
- 40. Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217(2):527-538. [DOI] [PubMed] [Google Scholar]
- 41. Shieh A, Greendale GA, Cauley JA, Karvonen-Gutierrez C, Crandall CJ, Karlamangla AS. Estradiol and follicle-stimulating hormone as predictors of onset of menopause transition-related bone loss in pre- and perimenopausal women. J Bone Miner Res. 2019;34(12):2246-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hsu B, Cumming RG, Seibel MJ, et al. Reproductive hormones and longitudinal change in bone mineral density and incident fracture risk in older men: the concord health and aging in men project. J Bone Miner Res. 2015;30(9):1701-1708. [DOI] [PubMed] [Google Scholar]
- 43. Liu S, Cheng Y, Fan M, Chen D, Bian Z. FSH aggravates periodontitis-related bone loss in ovariectomized rats. J Dent Res. 2010;89(4):366-371. [DOI] [PubMed] [Google Scholar]
- 44. Qian H, Guan X, Bian Z. FSH aggravates bone loss in ovariectomised rats with experimental periapical periodontitis. Mol Med Rep. 2016;14(4):2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun L, Peng Y, Sharrow AC, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247-260. [DOI] [PubMed] [Google Scholar]
- 46. Rendina D, Gianfrancesco F, De Filippo G, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163(1):165-172. [DOI] [PubMed] [Google Scholar]
- 47. Senapati S, Gracia CR, Freeman EW, et al. Hormone variations associated with quantitative fat measures in the menopausal transition. Climacteric. 2014;17(2):183-190. [DOI] [PubMed] [Google Scholar]
- 48. Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women’s Health Across the Nation (SWAN). Menopause. 2008;15(3):414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim C, Randolph JF, Golden SH, et al. Weight loss increases follicle stimulating hormone in overweight postmenopausal women [corrected]. Obesity (Silver Spring). 2015;23(1):228-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin Dand Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy of Sciences; 2011. [Google Scholar]
- 52. Camporez JP, Petersen MC, Abudukadier A, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci U S A. 2016;113(8):2212-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jing Y, Wang X, Yu J, et al. Follicle-stimulating hormone and estradiol are associated with bone mineral density and risk of fractures in men with type 2 diabetes mellitus. J Diabetes. 2020;12(6):426-437. [DOI] [PubMed] [Google Scholar]
- 54. Østergren PB, Kistorp C, Fode M, et al. Luteinizing hormone-releasing hormone agonists are superior to subcapsular orchiectomy in lowering testosterone levels of men with prostate cancer: results from a randomized clinical trial. J Urol. 2017;197(6):1441-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wong SC, Scott D, Lim A, Tandon S, Ebeling PR, Zacharin M. Mild deficits of cortical bone in young adults with klinefelter syndrome or anorchia treated with testosterone. J Clin Endocrinol Metab. 2015;100(9):3581-3589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.