Abstract
Background
Vascular endothelial growth factor (VEGF)-induced angiogenesis is a critical compensatory response to microvascular rarefaction in the diabetic retina that contributes to proliferative diabetic retinopathy (PDR). In this study, we sought to determine the role of specific micro ribonucleic acids (RNAs) (miRs) associated with VEGF in patients with PDR pathology.
Methods
RNA sequencing was employed to detect differentially circulating miR associated with VEGF in patients with diabetes mellitus (DM), nonproliferative diabetic retinopathy (NPDR) and PDR. Quantitative real-time polymerase chain reaction was performed to measure the concentration of miR-15b in the serum of patients with DM (n = 115), NPDR (n = 47), or PDR (n = 76). The effects of miR-15b on DR and regulation of VEGF and endothelial cell function were also characterized.
Results
We demonstrated that circulating miR-15b was directly associated with VEGF compared with other miRs in patients with PDR. We found a significant inverse relationship between low levels of miR-15b and high levels of VEGF in patients with PDR when compared with the DM or NPDR groups. We found that miR-15b regulates the expression of VEGF by targeting the 3'-untranslated regions to inhibit its transcription. Similarly, overexpression of miR-15b suppressed vascular abnormalities in vivo in diabetic GK rats, inhibiting endothelial tube formation and VEGF expression.
Conclusion
Circulating miR-15b is associated with PDR and may be targeted to regulate VEGF expression and angiogenesis.
Keywords: diabetic retinopathy, microRNA-15b, proliferative diabetic retinopathy, diabetes, vascular endothelial growth factor, angiogenesis
Proliferative diabetic retinopathy (PDR) is a microvascular complication that contributes to blindness in patients with diabetes (1). Increased vascular endothelial growth factor (VEGF) has been found to induce retinal endothelial permeability and proliferation during PDR pathogenies (2, 3), resulting in retinal angiogenesis (4, 5). In PDR, hyperglycemia alters the retinal microenvironment, which leads to the aberrant endothelial cell function caused by hypoxia and increased VEGF expression (6-8). There is a vast knowledge gap and growing interest in the mechanisms governing the expression of proteins and metabolites involved in the angiogenic pathways responsible for the PDR pathogenesis (9).
Recent reports have suggested a significant role for micro ribonucleic acids (RNAs) (miRs) as a therapeutic strategy to regulate the diabetic retina (10, 11). MiRs are endogenously expressing, noncoding RNA molecules comprising approximately of 20 to 24 nucleotides found in the plasma, serum, and other body fluids (12, 13). However, the relationship between circulating miRs and PDR has been meager. Recent data highlight miRs to control VEGF production by targeting the 3′ untranslated region (UTR) (14, 15), a region known to regulate protein expression. However, whether the miRs related to VEGF are released into serum and participate in regulating retinal endothelial cell function remains unknown. In this study, we sought to determine the specific miR(s) involved in the patients with PDR and their role in retinal endothelial angiogenesis.
Methods
Human participants
A cohort of 238 patients with type 2 diabetes (DM) were recruited from the Yunnan Province Second Hospital, China, between January 2014 and January 2015, according to the guidelines described by the American Diabetes Association (16). The study protocol was approved by the Ethics Committee of the Yunnan Province Second Hospital, and written informed consent was obtained from all the patients before their participation in the study. All patients underwent a complete ophthalmological examination, including corrected slit-lamp microscopic examination (with and without preset lens), fundoscopic examination, and fluorescence angiography. They were broadly classified into DM without diabetic retinopathy (DR, n = 115) and DM with DR (n = 123). The patients with DR included nonproliferative DR (NPDR, n = 47) and proliferative DR (PDR, n = 76). Detailed medical and family history and fasting blood samples were collected for the measurement of circulating microRNAs. The hypertension (HTN) (17) was defined according to the appropriate guidelines. Patients with a history of rheumatic disease and inflammatory disease were excluded from the study. The serum levels of VEGF were detected by enzyme-linked immunosorbent assay (Invitrogen, Carlsbad, CA).
Circulating miRNA sequencing
Total RNA was extracted from the serum of patients with DM (n = 3) and PDR (n = 3) by Trizol reagent (Invitrogen) separately. The RNA had been stored at –80°C, and the RNA quality was confirmed by Bioanalyzer 2200 (Agilent) to measure the RNA Integrity Number (RIN). The RIN of RNA > 8.0 was used for miRNA purification. miRNA was purified using the miRNeasy Mini Kit (Qiagen) and validated by gel electrophoresis. The complementary DNA (cDNA) libraries for single-end sequencing were prepared using Ion Total RNA-Seq Kit v2.0 (Life Technologies) according to the manufacturer’s instructions. The cDNA library was size selected by polyacrylamide gel electrophoresis for miRNA sequencing. The cDNA libraries were then processed for the proton sequencing process following the commercially available protocols. The samples of cDNA were diluted, and the mixture was processed on a OneTouch 2 instrument (Life Technologies) and enriched on a OneTouch 2 ES station (Life Technologies) to prepare the template-positive Ion PI Ion Sphere Particles (Life Technologies) using Ion PI Template OT2 200 Kit v2.0 (Life Technologies). After enrichment, the mixed template-positive Ion PI Ion Sphere Particles of samples were loaded onto 1 P1v2 Proton Chip (Life Technologies) and sequenced on Proton Sequencers according to Ion PI Sequencing 200 Kit v2.0 (Life Technologies) by NovelBio Corporation Laboratory, Shanghai, China.
Bioinformatics of circulating miRNA targeting VEGF
The detected signals were analyzed by gene hierarchical clustering of logarithmic values of each time point and then were displayed in a heat map. Clustering was performed using Cluster 3.0, and the patterns were created and viewed using Java TreeView 1.0.13 software. We applied a degree sequence algorithm to filter the differentially expressed circulating miRNAs after statistical and false discovery rate (FDR) analysis under the following criteria: (a) fold change >1.5 or <0.667 and (b) FDR < 0.01, which was showed with a volcano plot. Moreover, the signaling pathway of predicted miRNAs targeting the gene was analyzed by NovelBio Corporation Laboratory, Shanghai, China. To identify the differentially circulating miRNAs related to VEGF, a microRNA support vector regression (mirSVR) score (18) was used. The intersection of VEGF predicting microRNAs and differentially circulating microRNAs was calculated and described with a Venn figure.
Circulating miR-15b assay
The circulating miRNAs were isolated from the patient’s serum as described above (DM, n = 115; NPDR, n = 47; PDR, n = 76). The concentration of miR-15b was verified by sequence-specific primers using TaqMan real-time polymerase chain reaction (Applied Biosystems, CA). A TaqMan miRNA assay kit using a stem-looped primer for reverse transcription was used to accurately detect mature miR-15b expression. Caenorhabditis elegans miRNA-39 was used as a spiked-in control to normalize the results.
Human retinal microvascular endothelial cells and HeLa cell cultures
The primary human retinal microvascular endothelial cells (HRMECs) were obtained from Cell Systems (Kirkland, WA) and cultured in M131 medium with microvascular growth supplement, 100 U/mL of penicillin, 100 µg/mL of streptomycin (Gibco, NY) (in high glucose). HeLa cells (purchased from ATCC, Gaithersburg, MD, USA) were seeded into 6-well plates at 1.0 × 106 cells per well in Dulbecco's Modified Eagle Medium (Gibco) medium containing 10% fetal bovine serum (Gibco), 100 IU/mL of penicillin, and 100 μg/mL of streptomycin. All the cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Quantitative real-time polymerase chain reaction
HRMECs were cultured and transfected with a 100 nM miRNA mimic of hsa-miR-15b-5p, hsa-miR-16-5p, hsa-miR-374b-5p, hsa-miR-29a-5p, hsa-miR-200c-3p, hsa-miR-361-5p, hsa-miR-424-3p, hsa-miR-381-3p, hsa-miR-429, hsa-miR-374a-5p, hsa-miR-590-3p, hsa-miR-205-5p, and a negative control (NC) label with Cy5 for 24 hours by Lipofectamine 2000, and the transfection efficiency was measured with the cyanine (Cy) 5 fluorescence. The messenger RNA levels of VEGF expression were measured using a SYBR-Green reagent kit (Takara, Dalian, China) (19).
In vitro angiogenesis assay
The angiogenesis capacity of HRMECs was investigated using Matrigel. The miR-15b mimic and NC microRNA were synthesized and labeled with Cy3 from Dharmacon (Lafayette, CO). HRMECs (1 × 104 cells per well) were harvested and plated onto a 96-well glass slide precoated with Matrigel (BD Bioscience, San Jose, CA). Next, HRMECs were incubated with the mimic of miR-15b-Cy3 and NC-Cy3 at different doses (0, 25, 50, and 100 pM) for 4 hours. Tube formation was examined and quantified for the sprouting of new capillary tubes. Experiments were performed to examine 3 randomly selected fields. All pictures were captured under a bright light or Cy3 channel with Olympus Microsystems (Tokyo, Japan)
Western blotting
Proteins were isolated and quantified by the bicinchoninic acid method (Pierce, Rockford, IL). For each sample, 30 µg of protein was loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel. After electrophoresis, the proteins were transferred onto a polyvinylidene fluoride membrane (Millipore, Bedford, MA). The membrane was probed with VEGF (1:1000, Cell Signaling, MA) and β-actin (1:1000, Cell Signaling) overnight at 4°C followed by the secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000, Cell Signaling, MA ) for 2 hours at room temperature. After washing, the membrane was processed using the Immobilon Western Chemiluminescent HRP Substrate (Millipore, Bedford, MA). β-Actin was used as a loading control.
Prediction of miR-15b target sites and luciferase assay
A consensus approach employing miRanda, RNAhybrid, and TargetScan was applied for target prediction. According to the prediction of miR-15b and target sites of VEGF, the wild-type and mutant target site of VEGF for miR-15b were synthesized and inserted into a pMIR-REPORT miRNA Expression Reporter Vector System (Ambion, Austin, TX). The pMIR-REPORT β-galactosidase control vector (Ambion, Austin, TX) was used as a reference. For the luciferase assay, HeLa cells were cotransfected with wild-type (wt) (3'UTR-VEGF) plasmid with miR-15b or NC and treated mutant (mut) (3'UTR-VEGF) plasmid with miR-15b or NC. Luciferase activity was measured 48 hours after transfection using a dual-luciferase assay kit (Promega, Madison, WI) (15).
Animals
The animals used in this study were treated according to the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research. The male Goto-Kakizaki (GK, male, 6 weeks old) rats, each weighing approximately 200 g, were used as a type 2 DM animal model (20-22) and were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). Intraperitoneal glucose tolerance (IPGTT) and intraperitoneal insulin tolerance testing(IPITT) was conducted. The animals were fed for 20 weeks and kept on a 12-hour light/12-hour dark cycle. Intravitreal injection of miR-15b overexpression (recombinant lentivirus [Let]-miR-15b, n = 16) and NC microRNA (Let-NC, n = 12) was performed at 1 × 106 TU/total (2 µL/eye). After 20 weeks of recombinant lentivirus injection, the GK rats were sacrificed, and the retinas were obtained for biochemical and cellular assays.
In situ hybridization of miR-15b
The retinas of GK rats injected with Let-miR-15b or Let-NC were used for immunochemical analysis. The samples were fixed in 4% paraformaldehyde overnight, embedded with optimal cutting temperature compound, and cut into cryosections at 12-μm thickness. The in situ miR-15b hybridization probe was purchased from EXIQON and was labeled with digoxigenin. The sections were hybridized with probes (2 µL) mixed with hybridization buffer (50% deionized formamide; 0.3 M NaCl; 20 mM Tris hydrochloric acid, pH 8.0; 5 mM ethylenediaminetetraacetic acid; 10 mM sodium phosphate, pH 8.0; 10% dextran sulfate; 1 × Denhardt’s solution; 0.5 mg/mL yeast RNA), followed by washing the slides with 1 × saline sodium citrate 3 times. After incubation with the antibody of HRP-conjugated anti-digoxigenin (1:1500), the sections were incubated with 3, 3'-diaminobenzidine. All sections were observed and photographed microscopically (Olympus Microsystems), and the positive density of miR-15b expression was measured with ImageJ 1.50b software.
Retinal flat mount immunofluorescence
The retinas were obtained from GK rats injected with Let-miR-15b or Let-NC, followed by fixation with 4% paraformaldehyde for 4 hours. Next, the retinas were incubated with 0.5% Triton X-100 at 4°C overnight. The retinal tissues were then washed with phosphate-buffered solution 3 times and labeled with Griffonia simplicifolia-IB4 conjugated with Alexa Fluor 488 at room temperature overnight. After labeling with Griffonia simplicifolia-isolectin B4 (IB4), the retinas were cut into 4 fragments and were observed and photographed (Olympus Microsystems). The density of microvascular was measured with VesselJ software as previously described (23).
Statistical analysis
The normal distribution of data was tested by the Shapiro-Wilk test. Percentages were used for categorical variables, and means with standard deviation or medians with interquartile range were calculated for continuous variables as appropriate. Categorical variables were compared with an χ 2 test, and continuous variables were compared with a t test or the Mann-Whitney U test as appropriate. The serum level of microRNA-15b was log-transformed for analysis. A Spearman partial correlation, with adjustments for age and sex, was calculated to measure the strength of the association between the levels of microRNA-15b and VEGF. Binary and multinomial logistic regression analyses were performed to calculate the odds ratios and 95% confidence intervals for specific microRNA-15b levels associated with the risks of NPDR and PDR, respectively. Covariates were selected and adjusted because they had the potential to confound the association between the microRNA-15b levels and DR risk. The covariates included in the final model were as follows: age, sex, oral drug, diabetes duration, HTN duration, systolic blood pressure, apoprotein A, apoprotein B, and hemoglobin A1c. Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Identification of circulating miR-15b levels in patients with DR
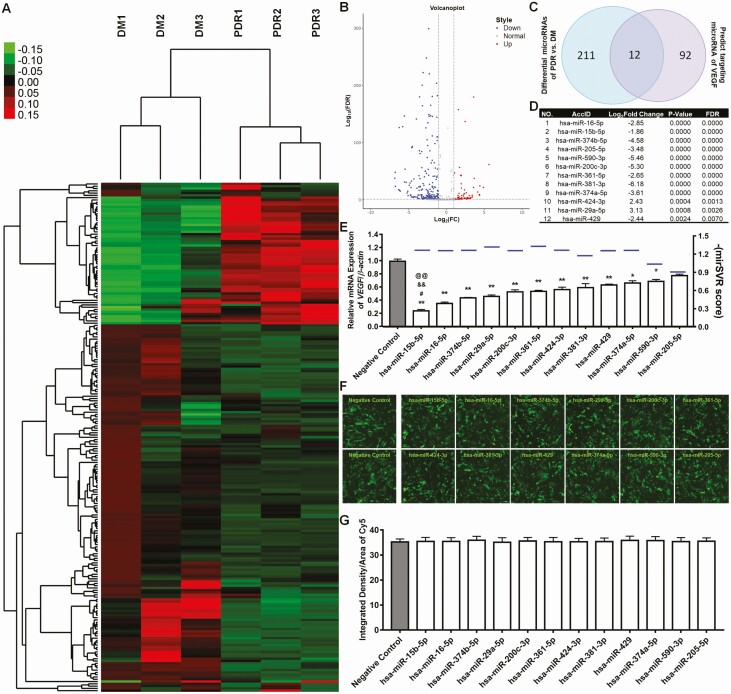
The demographic and clinical characteristics of participants with DM, NPDR, and PDR are summarized in Table 1. Individuals with DR were more likely to have longer diabetes duration and insulin treatment, higher hemoglobin A1c levels, and higher systolic and diastolic blood pressure compared with individuals with NDR. Those with PDR were more likely to be female, display HTN, and greater insulin use compared with their counterparts with NPDR. miRNA sequencing and bioinformatics were then executed to explore the circulating miRNA expression pattern of VEGF-related circulating microRNAs. For circulating miRNA sequencing, patients with DM were compared with patients with PDR, and the results are shown as a heat map and volcano plot in Fig. 1A and B. There were 223 miRNAs that exhibited differences in expression between PDR and DM, with 2 standards: (1) The relative of miRNAs expression PDR/DM, which showed the fold change >1.5 or <0.667; (2) Compared with group of PDR and DM, the value of false discovery rate (FDR) was <0.01.
Table 1.
Clinical characteristics of patients with DM, NPDR, and PDR
| Characteristicsa | DM (n = 115) | NPDR (n = 47) | PDR (n = 76) | P |
|---|---|---|---|---|
| MicroRNA-15b (log-transformed) | 2.90 ± 1.16 | 2.20 ± 1.29 | 2.20 ± 1.31 | <0.001 |
| Age (years) | 54.35 ± 11.54 | 59.13 ± 9.56 | 52.47 ± 10.38 | 0.004 |
| Male/female (n/n) | 70/49 | 30/17 | 32/44 | 0.027 |
| Body mass index (kg/m2) | 22.30 ± 7.48 | 23.92 ± 3.08 | 24.28 ± 3.06 | 0.042 |
| Waist-hip ratio | 1.01 ± 0.90 | 0.91 ± 0.07 | 0.93 ± 0.07 | 0.576 |
| Diabetes durations (years) | 5.0 (2.0-9.0) | 10.0 (6.0-15.0) | 10.0 (4.0-15.0) | <0.001 |
| Hypertension, n (%) | 58 (48.74) | 28 (59.57) | 52 (68.42) | 0.024 |
| Hypertension durations (years) | 0 (0.00-7.5) | 0.3 (0-3.0) | 0.75 (0-4.0) | 0.781 |
| Family history of diabetes, n (%) | 45 (37.8) | 15 (31.9) | 27 (35.5) | 0.772 |
| Family history of hypertension, n (%) | 29 (24.4) | 15 (31.9) | 16 (21.1) | 0.395 |
| SBP (mmHg) | 127.83 ± 16.18 | 138.00 ± 15.91 | 136.71 ± 21.00 | <0.001 |
| DBP (mmHg) | 81.01 ± 12.18 | 85.32 ± 14.14 | 85.00 ± 13.55 | 0.050 |
| Insulin usage, n (%) | 84 (70.59) | 44 (93.62) | 70 (92.11) | <0.001 |
| Oral drug usage, n (%) | 80 (67.23) | 22 (46.81) | 31 (40.79) | <0.001 |
| Fasting glucose (mmol/L) | 9.21 (6.43-12.21) | 8.67 (6.89-11.50) | 9.37 (7.51-11.69) | 0.459 |
| HbA1c | 8.60 (7.05-11.20) | 9.80 (8.00-11.40) | 9.40 (8.28-11.70) | 0.051 |
| TC (mmol/L) | 4.87 (4.19-5.35) | 4.96 (3.84-6.01) | 5.05 (4.37-6.07) | 0.209 |
| TG (mmol/L) | 1.97 (1.34-3.33) | 1.67 (1.06-2.63) | 2.25 (1.31-3.56) | 0.085 |
| HDL (mmol/L) | 1.08 (0.89-1.21) | 1.03 (0.84-1.25) | 1.02 (0.88-1.14) | 0.365 |
| LDL (mmol/L) | 2.85 (2.23-3.29) | 2.75 (2.21-3.43) | 2.83 (2.21-3.34) | 0.977 |
| Apoa (g/L) | 1.16 (1.10-1.33) | 1.11 (1.02-1.30) | 1.17 (1.05-1.27) | 0.202 |
| Apob (g/L) | 0.89 (0.75-1.01) | 0.86 (0.70-1.04) | 0.96 (0.79-1.12) | 0.045 |
| BUN (mmol/L) | 4.77 (3.90-5.88) | 5.75 (4.41-6.54) | 5.65 (4.22-7.12) | 0.003 |
| UA (μmol/L) | 323.0 (280.5-399.0) | 328.0 (263.0-378.5) | 358.0 (296.0-410.5) | 0.151 |
| Cr (μmol/L) | 64.0 (56.0-74.5) | 68.0(52.0-91.0) | 71.0 (54.0-99.0) | 0.074 |
| VEGF level (pg/mL) | 47.00 (46.70-47.45) | 65.61 (58.70-71.52) | 68.15 (58.51-72.97) | <0.001 |
Abbreviations: Apoa, apolipoprotein A; Apob, apolipoprotein B; BUN, blood urea nitrogen; Cr, creatinine; DBP, diastolic blood pressure; DM, diabetes mellitus; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic neuropathy; RNA, ribonucleic acid; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; VEGF, vascular endothelial growth factor.
a Continuous variables were expressed as the means ± standard deviation or medians (interquartile range) as appropriate.
Figure 1.
Circulating micro ribonucleic acid (RNA) (miR) sequencing and vascular endothelial growth factor (VEGF)-related circulating miR analysis. (A) Analysis of the gene expression clustering obtained from RNA sequencing of the miR isolated from the serum of participants with diabetes with no diabetic retinopathy (DM), nonproliferative diabetic retinopathy (NPDR), and with proliferative diabetic retinopathy (PDR) using Cluster 3.0. (n = 3). (B) Volcano plot analysis of the miRNAs. (C) Venn diagram depicting the intersection between the circulating miRNA and predictor targeting miRNA of VEGF. (D) The characteristics of 12 microRNAs in intersection part included AccID, log2 fold change, P value, and false discovery rate (FDR). (E) The mirSVR score of each miRNA targeting VEGF is shown with blue line, which equals –(mirSVR score). The 12 miRNAs were found in the study. miRNA mimics were transfected into the human retinal microvascular endothelial cells, and the mRNA levels of VEGF were quantified with real-time polymerase chain reaction. The negative control miRNA was used as a control, and relative levels of VEGF were quantified by 3 independent experiments in comparison with B-actin (mean ± SD, n = 3; *P < 0.05, **P < 0.01 vs negative control; #P < 0.05 miR-15b vs miR-16; &&P < 0.01 miR-15b vs miR-374b; @@P < 0.01 miR-15b vs miR-29a). The expression of miR-15b was compared with other top 3 decreased microRNAs. (F) Fluorescent microscopy shows miRNAs labeled with cyanine (Cy) 5 and transfected into endothelial cells with 100 nM for 24 hours. (G) ImageJ 1.50B software shows the intensity of Cy5 as measured with 3 different 3 areas. SD, standard deviation.
Using predict targeting sites of VEGF-related circulating miRNAs, there were 104 candidate miRNAs that targeted VEGF using mirSVR scoring. Compared with VEGF-related predicting method, the Venn analysis further narrowed that 104 into 12 candidate miRNAs (Fig. 1C). We then sorted the 12 miRNAs where miR-16 and 15b were first and second, respectively, from the cohort (Fig. 1D). However, to further dissect which miR contributed to VEGF expression, we transfected each of these miRs into endothelial cells and observed that miR-15b decreased VEGF to a greater extent than miR-16 and others obtained on mirSVR scoring (Fig. 1E). The transfection efficiency of miRNAs was measured with the Cy5 fluorescence, and the transfection efficiency of miRNAs was similar as measured with the Cy5 fluorescence (Fig. 1F and G).
Association of miR-15b levels with VEGF in PDR
Understanding that miR-15b had a very close relationship with VEGF, we found that patients with PDR had lower circulating serum levels of miR-15b and higher levels of VEGF expression (Table 1). Moreover, there existed a significant inverse correlation between levels of miR-15b and VEGF among all participants after adjustment for age and sex (r = –0.13; P < 0.05). Further analysis indicates a predominant inverse relationship between increasing levels of miR-15b and PDR (P trend < 0.001; Table 2). For each 2-fold increase in miR-15b, there was a 48% decrease in PDR.
Table 2.
Association of different levels of microRNA-15b with DR risk, stratified by disease stage
| Levels of microRNA-15b | NPDR | PDR | ||||||
|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | ORb | 95% CI | ORa | 95% CI | ORb | 95% CI | |
| 1 st quartile | 1 | reference | 1 | reference | 1 | reference | 1 | reference |
| 2 nd quartile | 0.26 | (0.09-0.76) | 0.20 | (0.06-0.68) | 0.63 | (0.27-1.46) | 0.45 | (0.16-1.25) |
| 3 rd quartile | 0.47 | (0.18-1.19) | 0.49 | (0.16-1.46) | 0.31 | (0.12-0.77) | 0.28 | (0.09-0.82) |
| 4 th quartile | 0.09 | (0.03-0.28) | 0.06 | (0.02-0.23) | 0.24 | (0.10-0.59) | 0.16 | (0.06-0.48) |
| For each 2-fold increase | 0.58 | (0.43-0.78) | 0.54 | (0.39-0.76) | 0.64 | (0.49-0.83) | 0.58 | (0.43-0.79) |
Abbreviations: Apoa, apolipoprotein A; Apob, apolipoprotein B; CI, confidence interval; HbA1c, hemoglobin A1c; NPDR, nonproliferative diabetic retinopathy; OR, odds ratio; PDR, proliferative diabetic neuropathy; RNA, ribonucleic acid; SBP, systolic blood pressure.
a Adjusted for age and sex.
b Adjusted for age, sex, oral drug, diabetes duration, hypertension duration, SBP, Apoa, Apob, and HbA1c.
MiR-15b inhibits angiogenesis in vitro
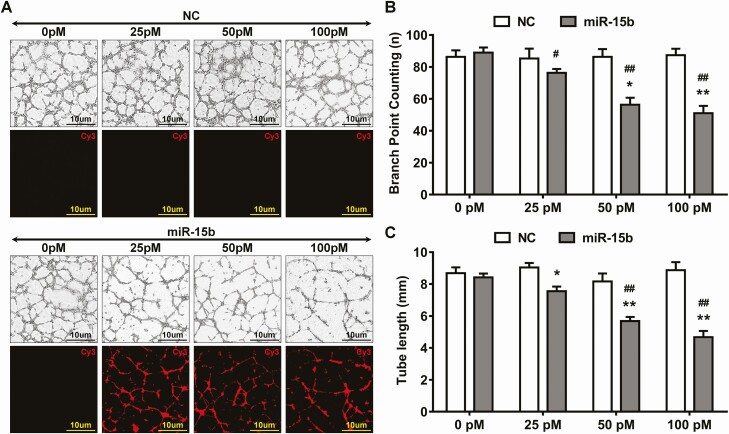
To explore whether reductions in miR-15b impacted angiogenesis in PDR, we utilized HRMECs to conduct dose-dependent response on angiogenesis. We utilized an miR-15b mimic or NC in conjunction with Cy3, added to HRMECs in 0, 25, 50, and 100 pM concentrations. After 4 hours, tube formation was reduced in Cy3-labeled miR-15b (Fig. 2A), and miR-15b significantly attenuated the number of branch points in a dose-dependent manner (Fig. 2B and C) when compared with the NC group.
Figure 2.
Micro ribonucleic acid (miR)-15 reduced angiogenesis in the retinal endothelial cells. (A) The mimic of miR-15b or negative control (NC) was labeled with cyanine (Cy) 3 and added at different doses (0, 25, 50, and 100 pM) into the culture medium of human retinal microvascular endothelial cells. The tube formation assay was used to detect the effect of miR-15b on angiogenesis. The images were taken by fluorescent microscope with bright light or Cy3 channel. (B and C) The branch points and tube lengths were quantified. (mean ± SD, n = 3; *P < 0.05, **P < 0.01, vs 0 pM treatment group; #P < 0.05, ##P < 0.001 vs NC in the respective group). SD, standard deviation.
miR-15b directly targets VEGF
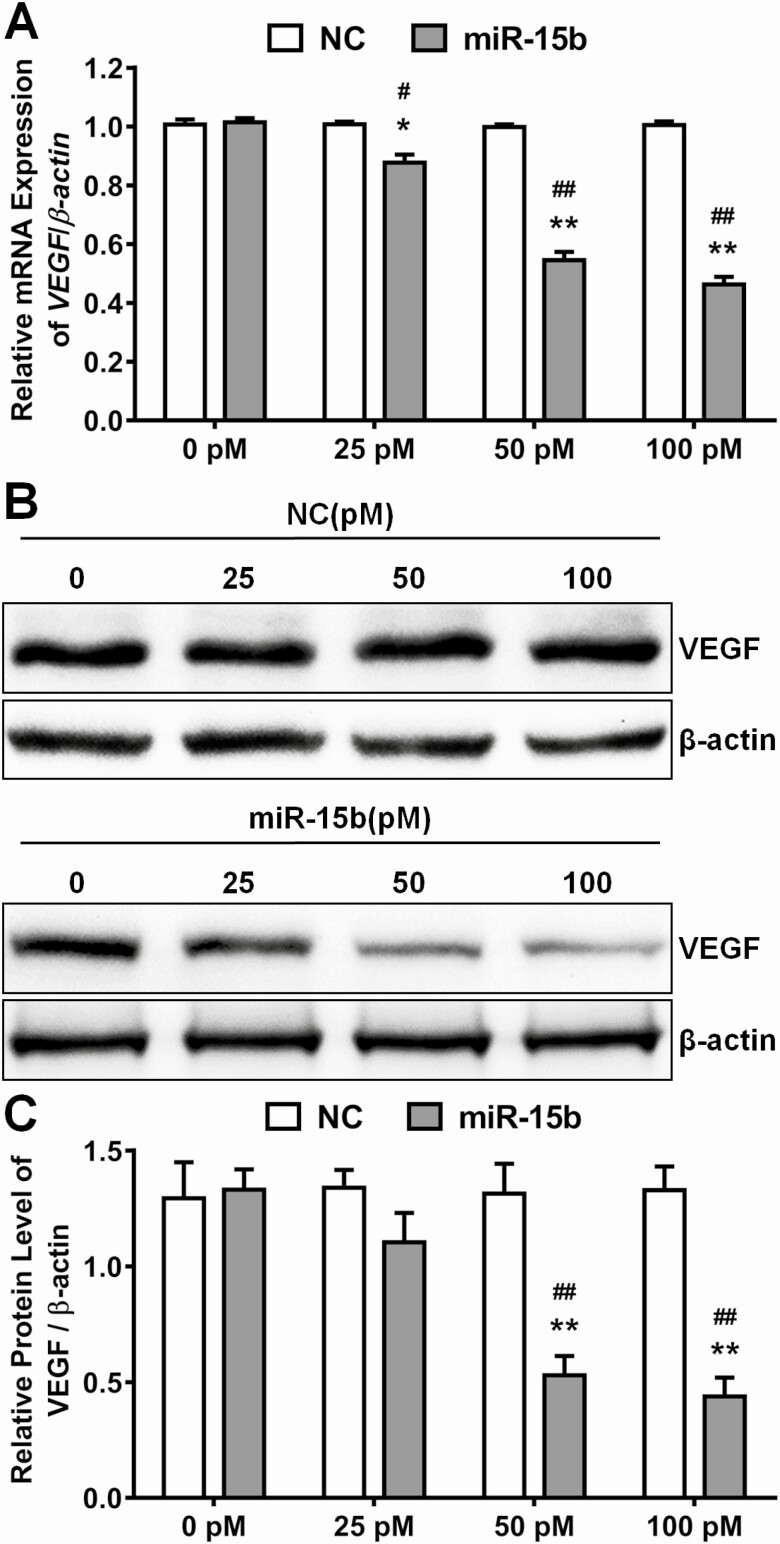
Since circulating miR-15b was strongly correlated to VEGF levels in our cohort and miR-15b inhibited angiogenesis in HRMECs, we tested whether miR-15b regulated VEGF expression. After incubation with different doses of miR-15b and NC mimics, the messenger RNA expression and protein level of VEGF were measured. We found that miR-15b significantly downregulated the expression of VEGF in a dose-dependent manner (Fig. 3A-C) compared with the same concentrations of NC treatment (Fig. 3A-C).
Figure 3.
Micro ribonucleic acid (RNA) (miR)-15b decreased messenger RNA (mRNA) and protein expression of vascular endothelial growth factor (VEGF) in the retinal endothelial cells. The mimic of miR-15b or negative control (NC) added at different doses (0, 25, 50, and 100pM) into the culture medium of human retinal microvascular endothelial cells. (A) After treatment with miR-15b and NC for 48 hours, the mRNA levels of VEGF were detected by real time polymerase chain reaction, while β-actin acted as an internal control (mean ± SD, n = 3; *P < 0.05, **P < 0.01, v. 0 pM treatment group; #P < 0.05, ##P < 0.001 vs NC in the respective group). (B and C) After treatment with miR-15b and NC for 48 hours, the protein levels of VEGF were detected by Western blot. Relative levels of VEGF were quantified by densitometry in 3 independent experiments in comparison with β-actin (mean ± SD, n = 3; **P < 0.01, vs 0 pM treatment group; ##P < 0.001 vs NC in the respective group). SD, standard deviation.
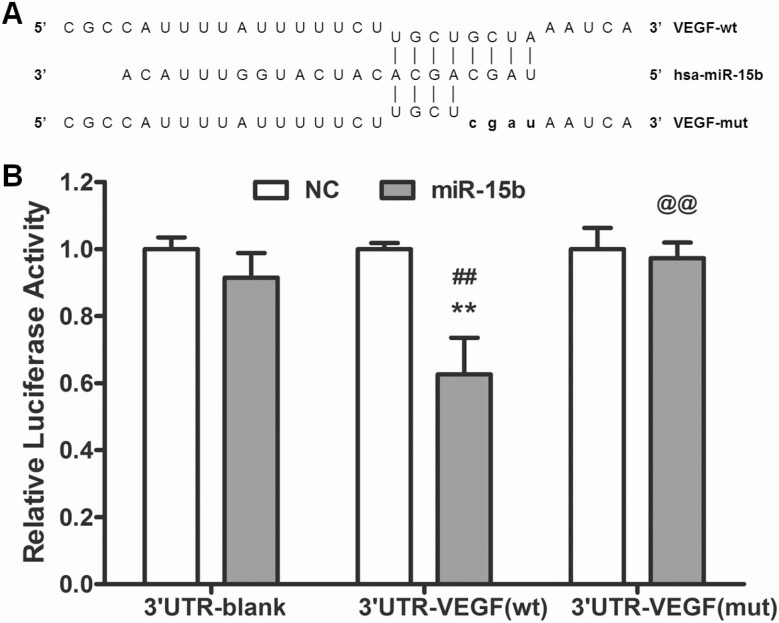
To identify VEGF as the miR-15b target gene, wt 3'UTR-VEGF and mut 3'UTR-VEGF constructs of the VEGF 3'-UTR region were synthesized and cloned into a plasmid (Fig.4A). Cotransfecting miR-15b and wt 3'UTR-VEGF vectors significantly decreased luciferase activity (P < 0.01), but there was no effect after cotransfection of miR-15b with mut 3'UTR-VEGF or blank vector in HeLa cells (Fig. 4B). Cotransfection of NC microRNA with blank, wt 3'UTR-VEGF, or mut 3'UTR-VEGF served as the NC (Fig. 4B). Thus, miR-15b directly targeted the 3'-UTR region of the VEGF transcript.
Figure 4.
Micro ribonucleic acid (RNA) (miR)-15b targeted 3' untranslated regions (UTRs) of the vascular endothelial growth factor (VEGF) gene. (A) Sequence alignment of miR-15b and its targets in wild type (wt) or mutant (mut) 3' UTRs of VEGF. (B) Changes in the luciferase activity obtained by addition of miR-15b mimic in 3'-UTR of VEGF. (n = 3; **P < 0.01 vs NC; ##P < 0.01 vs 3'UTR-blank group; @@P < 0.01 vs 3UTR-VEGF (wt) group). mut, mutant; NC, negative control; wt, wild-type.
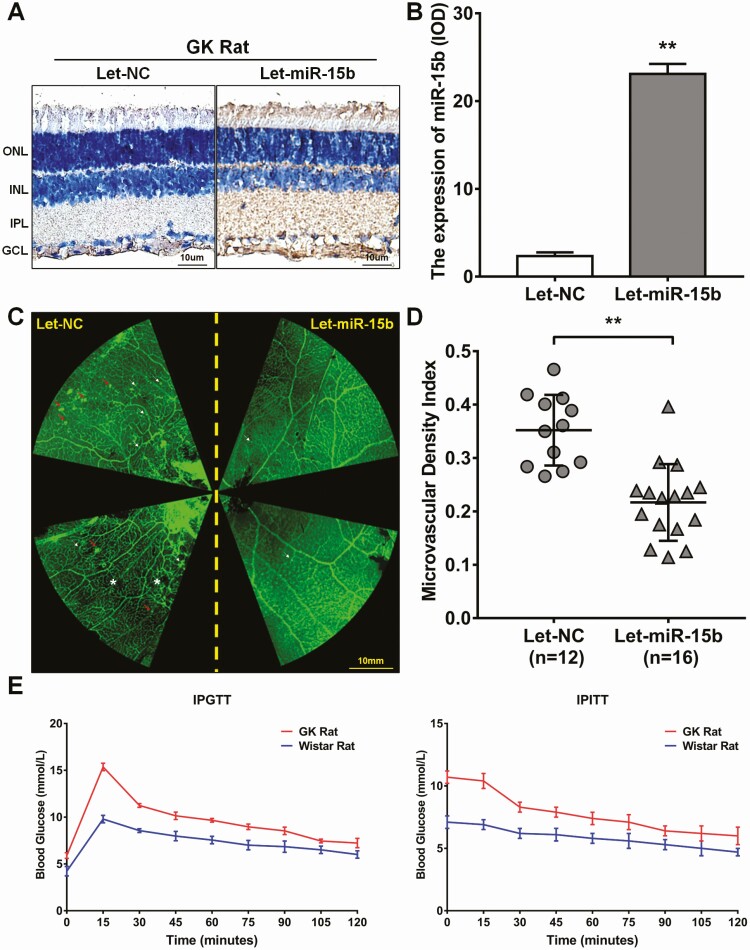
Overexpression of miR-15b reverses angiogenesis in vivo
To verify the impact of miR-15b on in vivo angiogenesis, we injected intravitreally recombinant miR-15b-overexpressing lentivirus into GK rat eyes. After 20 weeks, retinas were collected and then analyzed by in situ hybridization of miR-15b and by immunostaining (Fig. 5). Intravitreal injection of Let-miR-15b increased miR-15b expression in the retina compared with controls (Fig. 5A and B). In addition, overexpression of miR-15b decreased vessel tortuosity, microaneurysms, capillary nonperfusion and fluorescein leakage compared with the Let-NC injection group in GK rats (Fig. 5C). There were further reductions in microvascular density of Let-miR15b compared with the Let-NC controls (P < 0.01; Fig. 5C and D). The levels of IPGTT and IPITT were measured and demonstrated that GK rats were hyperglycemic and insulin resistant relative to Wistar rat controls (Fig. 5E).
Figure 5.
Micro ribonucleic acid (RNA) (miR)-15b inhibited proliferative diabetic retinopathy (PDR) in the Goto-Kakizaki (GK) rats. (A) The GK rat eye was injected with miR-15b lentivirus (Let-miR-15b) or negative control lentivirus (Let-NC), and the expression efficiency of miR-15b was detected by in situ hybridization. The brown staining was positive expression of miR-15b. GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer. (B) The concentration of miR-15b was detected by ImageJ 1.50b. The results are shown as the integrated optical density (IOD)/area. The Let-NC and Let-miR-15b are representative of 3 independent experiments and 5 different fields in each section (**P < 0.01 compared with Let-NC). (C) The retinas were obtained from the GK rat injected with Let-miR-15b or Let-NC and detected with flat mount immunofluorescence staining. White arrows: microaneurysms; red arrows: fluorescein leakage; white asterisk: capillary nonperfusion. (D) The density of microvascular was measured with VesselJ software. The Let-NC (n = 12) and Let-miR-15b (n = 16) are representative of 5 different fields in each section (**P < 0.01 compared with Let-NC). (E) Intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test (IPITT) results after feeding the GK and Wistar rats for 8 weeks.
Discussion
The mechanisms that drive PDR progression as a microvascular complication of diabetes has been a subject of intense interest in recent years. Although there are multiple factors involved in the pathology of PDR, antagonizing the activation of VEGF has been shown to be an effective intervention in patients. However, studies on the regulatory pattern of VEGF expression in PDR is not known. In this study, we identified that miR-15b was decreased in patients with PDR and inversely associated with the increased VEGF levels, suggesting miR-15b as a potential negative regulator of VEGF transcriptional regulation. Further, we found that miR-15b directly targeted VEGF expression and suppressed angiogenesis in vitro and several pathological hallmarks of vascular abnormalities in GK rats in vivo.
Previous studies support that hypoxia and inflammatory responses are strongly associated with increased VEGF expression in PDR (24, 25). Also, our previous work suggests that the VEGF concentrations were substantially elevated and play a predominant role in PDR pathogenesis (26). Currently, there are several anti-VEGF therapies used for the treatment for PDR; however, the residual risk of PDR progression still occurs in these patients. Thereby, understanding the expression pattern of VEGF including pre- and posttranscriptional regulation is a critical consideration for interrupting the progression of PDR. Therefore, in recent years, the role of miRs have been emphasized to participate in the progression of PDR by targeting and regulating VEGF (27). Meanwhile, miRNAs found in the cells and secreted into serum as circulating microRNAs could potentially serve as biomarkers for PDR. In this context, data from clinical trials including PROTECT-1 and PREVENT-1 suggest that miR-27b and miR-320a are associated with risk of DR; however, the target of miR-27b and miR-320a is thrombospondin-1 and not VEGF (28).
Despite the prominent mechanism of VEGF activation in PDR, there is no data available that identify and understand the role of circulating miRs on VEGF expression in PDR. The present miRNA sequencing study found 12 different circulating miRs that target VEGF in patients with PDR. Among these 12 microRNAs, miR-15b had the lowest mirSVR score but was significantly associated with increased VEGF expression. To our knowledge, this is the first data set showing the association between the miR-15b and VEGF expression in patients with PDR. Recent work suggests that miR-15b is an antiangiogenic factor in several diseases including nonalcoholic fatty liver disease (29), myocardial ischemia reperfusion injury (30), glioma (31), and endometrioid endometrial cancer (32). Our bioinformatic analyses support VEGF as a target gene of miR-15b, suggesting that miR-15b downregulated the expression of VEGF. Transfection of HRMECs with miR-15b mimics (and not other miRs) and further confirmed that miR-15b regulates VEGF transcription via directly targeting the 3'-UTR region of VEGF. On the contrary, overexpression of miR-15b in the culture inhibited VEGF transcript and protein expression in a dose-dependent manner in the endothelial cells. Previous data also found that hypoxia treatment reduced the expression of miR-15b but caused increased VEGF expression in human nasopharyngeal carcinoma cells and promoted the expression of miR-15b to inhibit neovascularization in glioma, corroborating our results of high miR-15b levels negatively related to VEGF in DR (33). Collectively, these data suggest that a diabetes milieu creates a microenvironment that decreases the levels of circulating miR-15b associated with increases in VEGF and PDR progression. Regarding the effects of circulating miR on cell function, previous work supports that some miRs are duplicated and packed into exosomes, intracellularly exhibiting autocrine/paracrine effect (34). Interestingly, we observed that the exogenous addition of an miR-15b mimic directly into the medium inhibited VEGF expression and tube formation. Previous work supports that the length of matured miRs were 21 to 23 nucleotides, and circulating miRNAs had a long life span of approximately more than 2 weeks (35). Therefore, there were relatively stable levels of circulating miRNAs in the serum. Besides, our in vitro data also support the miR-15b ability to directly target VEGF gene expression and effector cell function. Together, these data indicate a paracrine effect to maintain routine physiological activity in the retinal microenvironment. We further confirmed our findings with an in vivo study. Intravitreal injection of miR-15b into the GK rat eyes showed improved pathological markers of DR such as density of the microvascular, vessel tortuosity, microaneurysms, capillary nonperfusion, and fluorescein leakage.
In summary, miR-15b is an antiangiogenesis factor negatively associated with PDR and that directly targets and regulates VEGF expression in the retina. While our study suggests many important advances in the DR field, there are some limitations as with any study. Notably, this was a case-control cohort in which the relationship between circulating miR-15b and DR was preestablished. Using this study design may introduce selection bias and inverse causal bias that may distort the observed association between the circulating miR-15b levels and any population attributable risk for PDR. Prospective cohort studies are needed to illustrate the effects of variations in circulating miR-15b levels on PDR. Further, we utilized GK rats as a PDR animal model, which may not recreate the human conditions related to PDR. Currently, this is a limitation of animal models available to investigate a true PDR pathology.
Acknowledgments
Financial Support: This work was supported by grants from the Natural Science Foundation of China (81760734, 81770384, and 31660313), the Natural Science Foundation of Yunnan Province (No. 2017FA048), the fund of Diabetic Innovation Team (2019HC002), and the Endocrine Clinical Medical Center of Yunnan Province (No. ZX2019-02-02). The fund of medical leader is Yunnan Province (No. L-201609). We also thank Novel Bioinformatics Ltd for the support of bioinformatics analysis with their NovelBrain Cloud Analysis Platform (www.novelbrain.com), and Professor Habibi Javad supported the IHC image analysis (Division of Endocrinology and Metabolism, Department of Medicine, University of Missouri). A.W.C. receives support from the Deparment of Veterans Affairs Merit system (BX003391). S.S.C. is supported by the NIH RO1EY029795.
Author Contributions: All authors contributed to the conception and design, acquisition of data or analysis and interpretation of data, drafting of the article or revising it critically for important intellectual content, and gave final approval of the version to be published. Conceptualization, Adam Whaley-Connell and Ke Yang; data curation, Ying Yang, Yan Liu, Yiping Li, Zhongli Chen, and Wenyu Tao; formal analysis, Yan Liu, Yiping Li, and Zhongli Chen; investigation, Ying Yang, Yan Liu, Yiping Li, Zhongli Chen, and Fan Xu; methodology, Zhongli Chen; project administration, Adam Whaley-Connell and Ke Yang; resources, Taicheng Zhou; software, Taicheng Zhou; validation, Yixin Xiong and Hanling Yang; visualization, Yixin Xiong; writing—original draft, Adam Whaley-Connell and Ke Yang; writing—review and editing, Shyam S. Chaurasia and Seppo Ylä-Herttuala.
Glossary
Abbreviations
- cDNA
complementary deoxyribonucleic acid
- Cy
cyanine
- DM
diabetes mellitus
- DR
diabetic retinopathy
- FDR
false discovery rate
- GK
Goto-Kakizaki
- HRP
horseradish peroxidase
- HRMEC
human retinal microvascular endothelial cells
- HTN
hypertension
- IPGTT
intraperitoneal glucose tolerance testing
- IPITT
intraperitoneal insulin tolerance testing
- Let
recombinant lentivirus
- miR
microRNA
- NC
negative control
- NPDR
nonproliferative diabetic retinopathy
- PDR
proliferative diabetic retinopathy
- RNA
ribonucleic acid
- UTR
untranslated region
- VEGF
vascular endothelial growth factor
- wt
wild-type
Additional Information
Duality of Interest: The authors declare that there is no duality of interest associated with this manuscript.
Data Availability
The data are available on request from the authors.
References
- 1. Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14(4):179-183. [DOI] [PubMed] [Google Scholar]
- 2. Osaadon P, Fagan XJ, Lifshitz T, Levy J. A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond). 2014;28(5):510-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chun MY, Hwang HS, Cho HY, et al. Association of vascular endothelial growth factor polymorphisms with nonproliferative and proliferative diabetic retinopathy. J Clin Endocrinol Metab. 2010;95(7):3547-3551. [DOI] [PubMed] [Google Scholar]
- 4. Tremolada G, Del Turco C, Lattanzio R, et al. The role of angiogenesis in the development of proliferative diabetic retinopathy: impact of intravitreal anti-VEGF treatment. Exp Diabetes Res. 2012;2012:728325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016;17(9):1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Engerman RL, Kern TS. Hyperglycemia as a cause of diabetic retinopathy. Metabolism. 1986;35(4 Suppl 1):20-23. [DOI] [PubMed] [Google Scholar]
- 7. Nyengaard JR, Ido Y, Kilo C, Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53(11):2931-2938. [DOI] [PubMed] [Google Scholar]
- 8. Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18(2):258-268. [DOI] [PubMed] [Google Scholar]
- 9. Wan TT, Li XF, Sun YM, Li YB, Su Y. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. Biomed Pharmacother. 2015;74:145-147. [DOI] [PubMed] [Google Scholar]
- 10. Gong Q, Xie J, Liu Y, Li Y, Su G. Differentially expressed microRNAs in the development of early diabetic retinopathy. J Diabetes Res. 2017;2017:4727942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao D, He S, Ye Z, et al. Identification of potential molecular targets associated with proliferative diabetic retinopathy. BMC Ophthalmol. 2020;20(1):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaikkonen MU, Halonen P, Liu OH, et al. Genome-wide dynamics of nascent noncoding RNA transcription in porcine heart after myocardial infarction. Circ Cardiovasc Genet. 2017;10(3):e001702. [DOI] [PubMed] [Google Scholar]
- 14. Haque R, Hur EH, Farrell AN, Iuvone PM, Howell JC. MicroRNA-152 represses VEGF and TGFβ1 expressions through post-transcriptional inhibition of (pro)renin receptor in human retinal endothelial cells. Mol Vis. 2015;21: 224-235. [PMC free article] [PubMed] [Google Scholar]
- 15. Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the expert committee on the diagnosis and classification of diabetes mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria. World Health Organization. Diabetes Res Clin Pract. 1999;44(1):21-26. [DOI] [PubMed] [Google Scholar]
- 17. Chobanian AV, Bakris GL, Black HR, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee . Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252. [DOI] [PubMed] [Google Scholar]
- 18. Jaswani P, Prakash S, Agrawal S, Prasad N, Sharma RK. Predicting miRNA association with corresponding target genes and single nucleotide polymorphisms in altered renal pathophysiology. MicroRNA. 2017;6(3):213-221. [DOI] [PubMed] [Google Scholar]
- 19. Chan LS, Yue PY, Wong YY, Wong RN. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem Pharmacol. 2013;86(3):392-400. [DOI] [PubMed] [Google Scholar]
- 20. Gong CY, Lu B, Sheng YC, Yu ZY, Zhou JY, Ji LL. The development of diabetic retinopathy in Goto-Kakizaki rat and the expression of angiogenesis-related signals. Chin J Physiol. 2016;59(2):100-108. [DOI] [PubMed] [Google Scholar]
- 21. Allen RS, Feola A, Motz CT, et al. Retinal deficits precede cognitive and motor deficits in a rat model of type II diabetes. Invest Ophthalmol Vis Sci. 2019;60(1):123-133. [DOI] [PubMed] [Google Scholar]
- 22. Guest PC. Characterization of the Goto-Kakizaki (GK) rat model of type 2 diabetes. Methods Mol Biol. 2019;1916:203-211. [DOI] [PubMed] [Google Scholar]
- 23. Rabiolo A, Bignami F, Rama P, Ferrari G. VesselJ: a new tool for semiautomatic measurement of corneal neovascularization. Invest Ophthalmol Vis Sci. 2015;56(13):8199-8206. [DOI] [PubMed] [Google Scholar]
- 24. Valiatti FB, Crispim D, Benfica C, Valiatti BB, Kramer CK, Canani LH. [The role of vascular endothelial growth factor in angiogenesis and diabetic retinopathy]. Arq Bras Endocrinol Metabol. 2011;55(2):106-113. [DOI] [PubMed] [Google Scholar]
- 25. Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang Y, Andresen BT, Yang K, et al. Association of vascular endothelial growth factor -634C/G polymorphism and diabetic retinopathy in type 2 diabetic Han Chinese. Exp Biol Med (Maywood). 2010;235(10):1204-1211. [DOI] [PubMed] [Google Scholar]
- 27. Li EH, Huang QZ, Li GC, Xiang ZY, Zhang X. Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci Rep. 2017;37(2):BSR20160572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zampetaki A, Willeit P, Burr S, et al. Angiogenic microRNAs linked to incidence and progression of diabetic retinopathy in type 1 diabetes. Diabetes. 2016;65(1):216-227. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Cheng X, Lu Z, et al. Upregulation of miR-15b in NAFLD models and in the serum of patients with fatty liver disease. Diabetes Res Clin Pract. 2013;99(3):327-334. [DOI] [PubMed] [Google Scholar]
- 30. Liu LF, Liang Z, Lv ZR, et al. MicroRNA-15a/b are up-regulated in response to myocardial ischemia/reperfusion injury. J Geriatr Cardiol. 2012;9(1):28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramón LA, Braza-Boïls A, Gilabert J, et al. microRNAs related to angiogenesis are dysregulated in endometrioid endometrial cancer. Hum Reprod. 2012;27(10):3036-3045. [DOI] [PubMed] [Google Scholar]
- 33. Zhou XM, Sun R, Luo DH, et al. Upregulated TRIM29 promotes proliferation and metastasis of nasopharyngeal carcinoma via PTEN/AKT/mTOR signal pathway. Oncotarget. 2016;7(12):13634-13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joglekar MV, Januszewski AS, Jenkins AJ, Hardikar AA. Circulating microRNA biomarkers of diabetic retinopathy. Diabetes. 2016;65(1):22-24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available on request from the authors.