Abstract
In a study of 121 hospitals from 38 US states, 44% had access to an allergist for inpatient consultations and 39% had access to inpatient penicillin skin testing, indicating that the majority of US hospitals lack sufficient resources to address inpatient penicillin allergies.
Keywords: beta-lactam, hypersensitivity, testing, workforce, skin test
Approximately 15% of hospitalized patients in the United States report an allergy to penicillin antibiotics [1]. A reported penicillin allergy is associated with delays in antibiotic treatment, less-effective treatment, and an increased risk of adverse events [1]. A reported penicillin allergy rarely represents true immunologically mediated drug allergy; allergy assessment disproves penicillin allergy in approximately 95% of tested patients [1].
In 2017, the Joint Commission required hospitals to formalize their antibiotic stewardship practice [2]. In 2018, antibiotic stewardship guidelines encouraged penicillin allergy assessments [3]. With just 5000 practicing allergists in the United States [4], we hypothesized that there was insufficient penicillin allergy testing access in US hospitals.
We performed a national survey study using Acute Care Hospital Groups within Vizient, Inc. Vizient is the largest member-driven, healthcare-performance-improvement company in the United States, with over 3200 acute care hospitals and more than 95% of all US academic medical centers (AMCs) representing all US states [5].
Investigators developed a questionnaire to assess institutionally available allergy resources. After revision based on cumulative feedback from the Vizient Antimicrobial Stewardship Committee, the questionnaire was distributed to a listserv of 147 actively engaged Vizient pharmacy network members with completion by a clinical pharmacy or antibiotic stewardship representative. This study was deemed exempt by the Partners Human Subject Research Committee.
Responses from AMCs and community hospitals were included. We assessed hospital demographics (region, geographic classification, size) using Definitive Healthcare data [6]. Bed-count grouping was used to distinguish small hospitals (<100 beds), medium hospitals (between 100 and 399 beds), and large hospitals (>400 beds) [7]. We considered 2 main outcomes of interest related to penicillin allergy assessment: (1) access to an inpatient allergy specialist and (2) access to inpatient penicillin skin testing.
We report numbers with frequencies, using chi-square tests with 2-sided P values, and P < .05 considered statistically significant. We used log-binomial regression models to assess the relation of hospital demographics to allergy specialist and penicillin skin testing access, reporting adjusted prevalence ratios (aPRs) with 95% confidence intervals (CIs) [8]. Analyses were performed using the SAS version 9.4 (SAS Institute).
Of 147 Vizient members receiving study invitations, 129 (88%) completed questionnaires; 121 (94%) were AMCs or community hospitals (Supplementary Table 1). All 4 US Census geographic regions were represented (Supplementary Figure 1). Most hospitals were urban (88%) and either large (44%) or medium (49%) in size.
Of the 121 respondents, 53 (44%) had access to inpatient allergy specialist consultations and 47 (39%) had access to inpatient penicillin skin testing (Table 1). Thirty-nine hospitals (32%) had both inpatient allergy consultations and penicillin skin testing access; 60 hospitals (50%) had neither.
Table 1.
Hospital Access to Penicillin Allergy Assessment Resources
| All (N = 121) | Academic Medical Centers (n = 55) | Community Hospitals (n = 66) | |
|---|---|---|---|
| Allergy specialist access | |||
| Allergy specialist available for inpatient consultation | 53 (44) | 44 (80)a | 9 (14)a |
| Allergist on antibiotic stewardship team | 4 (3) | 3 (5) | 1 (2) |
| Time to see allergist for inpatientsb | |||
| Within a few hours | 5 (9) | 5 (11) | 0 (0) |
| More than a few hours but within the day | 22 (42) | 20 (45) | 2 (22) |
| More than 1 day but within a few days | 22 (42) | 15 (34) | 7 (78) |
| Penicillin skin testing access | |||
| Inpatient skin testing available | 47 (39) | 35 (63)a | 12 (18)a |
| Other skin testing locations | |||
| Outpatient | 38 (81) | 31 (89)c | 7 (58)c |
| Emergency | 3 (6) | 2 (6) | 1 (8) |
| Preoperative area | 1 (2) | 1 (3) | 0 (0) |
| Skin testing performers | |||
| Allergy/immunology medical doctors | 33 (70) | 27 (77) | 6 (50) |
| Infectious diseases medical doctorsb | 7 (15) | 6 (17) | 1 (8) |
| Pharmacistc | 6 (13) | 5 (14) | 1 (8) |
| Nurse | 4 (9) | 2 (6) | 2 (17) |
| Pediatrics | 1 (2) | 1 (3) | 0 (0) |
| Otherd | 4 (9) | 2 (6) | 2 (17) |
| Skin testing reagents used | |||
| Benzyl penicilloyl-polylysine (major determinant) | 31 (66) | 23 (66) | 8 (67) |
| Penicillin G | 29 (62) | 20 (57) | 9 (75) |
| Cephalosporins | 9 (19) | 8 (23) | 1 (8) |
| Ampicillin | 8 (17) | 5 (14) | 3 (25) |
| Amoxicillin | 3 (6) | 3 (9) | 0 (0) |
| Minor determinant(s) | 2 (4) | 1 (3) | 1 (8) |
| Unknown | 7 (15) | 6 (17) | 1 (8) |
| Other penicillin allergy assessment resources | |||
| Drug challenge available | 62 (51) | 41 (75)a | 21 (32)a |
| Antibiotic desensitization available | 100 (83) | 54 (98)a | 46 (70)a |
| Guideline for prescribing antibiotics to patients reporting penicillin or B-lactam allergies (approved or in development) | 57 (47) | 31 (56)e | 26 (39)e |
Data are presented as n (%).
aChi-square, P < .001.
bFour academic medical centers responded “unknown”.
cChi-square, P = .035.
d“Other” includes critical care providers, intensive care units, infectious disease pharmacy residents, and trained nurse practitioners and physician assistants.
eChi-square, P = .07.
Eight hospitals (7%) performed penicillin skin testing without allergist access. In these hospitals, skin testing was performed by infectious disease specialists, critical care specialists, advance practice providers, pharmacists, or nurses.
Inpatient allergy consultation was more frequent at AMCs (44/55, 80%) than at community hospitals (9/66, 14%). Four hospitals (3%) reported that their antibiotic stewardship program had an allergy specialist member. Waiting more than a few hours to see an inpatient allergist was common (84%). Penicillin skin testing was more commonly available at AMCs (63% vs 18%). Other allergy assessment resources included drug challenges (51%), antibiotic desensitization (83%), and hospital guidelines (47%).
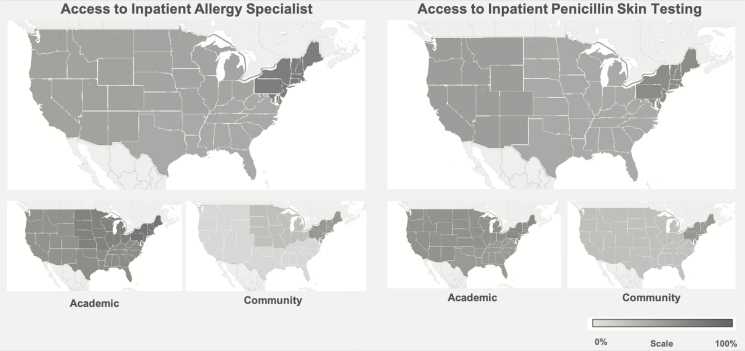
Access varied by region and by hospital type (Figure 1). In the multivariable model adjusted for hospital type, region, and geographic classification, AMCs had a higher prevalence of allergy specialist access (aPR, 1.53; 95% CI, 1.24–1.88) and penicillin skin testing access (aPR, 2.64; 95% CI, 1.41–4.97) than community hospitals. Region, geographic classification, and hospital size were not significant factors in the multivariable model.
Figure 1.
Geographic distribution demonstrating access to allergy specialists and penicillin skin testing considering the hospital respondent sample of 121 academic medical centers and community hospitals, the hospital respondent sample of 55 academic medical centers, and the hospital respondent sample of 66 community hospitals. The shades of gray from light to dark indicate percentages from 0 to 100, as shown in the key.
In this national study of 121 hospitals from 38 states and all 4 US census regions, 50% of hospitals did not have access to any resources for inpatient penicillin allergy evaluations, 44% of hospitals had access to an allergist for inpatient consultations, and 39% had access to penicillin skin testing. Although penicillin allergy evaluation is a proven method for improving inpatient antibiotic choice [9] and promoting antibiotic stewardship [1, 3], these data indicate that the majority of US hospitals lack sufficient resources to address inpatient penicillin allergies.
Academic medical centers had improved access with a 50% increased prevalence of inpatient allergy specialist access and an over 2.5-fold increased prevalence of inpatient penicillin skin testing access. This is attributable to academic allergy programs, which exist at 106 US hospitals (S. Heitzig, American Academy of Allergy Asthma and Immunology, personal communication, 7 January 2020). Access was somewhat improved in the northeastern United States where prior allergist workforce studies demonstrated the highest allergist prevalence: New England (1.95 per 100 000 population) and Middle Atlantic (1.75 per 100 000 population) [4].
Only 44% of hospitals had access to allergy specialist consultations and the time to see an allergist was within the day in just half of these hospitals. With a short length of hospital stay for many common inpatient infections, such as pneumonia, even hospitals with allergist access have insufficient access [10, 11]. In one prior study, one-third of penicillin skin test–eligible patients were not tested because of the requisite coordination time [12]. Additionally, allergy specialist membership in antibiotic stewardship programs was uncommon; just 4 hospitals (3 AMCs and 1 community hospital) had an identified allergy specialist member.
There were 39% of hospitals that had access to penicillin skin testing for their inpatients. Inpatient penicillin allergy assessments are safe, and lead to increased use of B-lactams following negative testing [9]. In one prior study, skin-tested patients had an increased odds of B-lactam use during their hospitalization (570%) and at discharge (250%) [12]. However, we identified only 8 hospitals (7%) that performed penicillin skin testing without an allergist available. With emerging data related to the safety and effectiveness of inpatient penicillin skin testing, even when performed by nonallergists [9], US hospitals might consider training a specialized workforce for inpatient penicillin allergy assessments.
This survey was distributed to Vizient member hospitals actively engaged in antibiotic stewardship, with a bias towards AMCs; as such, we would expect to find more limited penicillin allergy testing access in a nationally representative US hospital sample. Although almost 90% of invited hospitals participated, we do not know how responders differed from nonresponders. While outcomes were self-reported, respondents had the relevant qualifications and institutional knowledge to answer survey questions.
In summary, in a survey of over 100 US hospitals, we found that a majority of hospitals do not have the necessary resources to address the burden of inpatient penicillin allergies. Although AMCs have improved access, novel approaches are needed to improve antibiotic prescribing in hospitalized patients with reported penicillin allergies in the United States.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Academy of Allergy Asthma and Immunology Foundation, or the Massachusetts General Hospital.
Financial support. K. G. B. receives career development support from the National Institutes of Health (grant number K01AI125631), the American Academy of Allergy Asthma and Immunology Foundation, and the Massachusetts General Hospital Claflin Distinguished Scholar Award. R. P. W. is supported by the Steven and Deborah Gorlin MGH Research Scholar Award.
Potential conflicts of interest. K. G. B. reports a clinical decision support tool used institutionally for B-lactam allergy at Partners HealthCare System, which is licensed to Persistent Systems. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA 2019; 321:188–99. [DOI] [PubMed] [Google Scholar]
- 2. Joint Commission on Hospital Accreditation. Approved: new antimicrobial stewardship standard. Jt Comm Perspect 2016; 36:1, 3–4, 8. [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Antibiotic use in the United States, 2018 update: progress and opportunities. Atlanta, GA: US Department of Health and Human Services, CDC, 2019. Available at: https://www.cdc.gov/antibiotic-use/stewardship-report/pdf/stewardship-report-2018–508.pdf. Accessed 30 January 2020. [Google Scholar]
- 4. Moore J. American Academy of Allergy Asthma and Immunology report on the allergy and immunology physician workforce, 1999–2009/10. Rensselaer, NY: The Center for Health Workforce Studies, 2012. Available at: https://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Practice%20and%20Parameters/2012-AI-Physician-Workforce-Report.pdf. Accessed 30 January 2020. [Google Scholar]
- 5. Vizient. About us. Irving, TX: Vizient, 2020. Available at https://www.vizientinc.com/about-us. Accessed 30 January 2020. [Google Scholar]
- 6. Definitive Healthcare. Healthcare data & analytics that accelerates your growth. Definitive Healthcare, 2020. Available at: https://www.definitivehc.com/. Accessed 30 January 2020. [Google Scholar]
- 7. Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. N Engl J Med 2008; 359:1921–31. [DOI] [PubMed] [Google Scholar]
- 8. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 162:199–200. [DOI] [PubMed] [Google Scholar]
- 9. Blumenthal KG, Shenoy ES, Wolfson AR, et al. Addressing inpatient beta-lactam allergies: a multihospital implementation. J Allergy Clin Immunol Pract 2017; 5:616–625.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Storms AD, Chen J, Jackson LA, et al. Rates and risk factors associated with hospitalization for pneumonia with ICU admission among adults. BMC Pulm Med 2017; 17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Havers F, Bramley AM, Finelli L, et al. Statin Use and hospital length of stay among adults hospitalized with community-acquired pneumonia. Clin Infect Dis 2016; 62:1471–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017; 140:154–161.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.