Abstract
Context
Results of previous studies demonstrated clear racial differences in the prevalence of somatic mutations among patients with aldosterone-producing adenoma (APA). For instance, those in East Asian countries have a high prevalence of somatic mutations in KCNJ5, whereas somatic mutations in other aldosterone-driving genes are rare.
Objectives
To determine somatic mutation prevalence in Japanese APA patients using an aldosterone synthase (CYP11B2) immunohistochemistry (IHC)-guided sequencing approach.
Method
Patients with a unilateral form of primary aldosteronism who underwent adrenalectomy at the Tohoku University Hospital were studied. Based on CYP11B2 immunolocalization of resected adrenals, genomic DNA was isolated from the relevant positive area of 10% formalin-fixed, paraffin-embedded tissue of the APAs. Somatic mutations in aldosterone-driving genes were studied in APAs by direct Sanger sequencing and targeted next-generation sequencing.
Results
CYP11B2 IHC-guided sequencing determined APA-related somatic mutations in 102 out of 106 APAs (96%). Somatic KCNJ5 mutation was the most frequent genetic alteration (73%) in this cohort of Japanese patients. Somatic mutations in other aldosterone-driving genes were also identified: CACNA1D (14%), ATP1A1 (5%), ATP2B3 (4%), and CACNA1H (1%), including 2 previously unreported mutations. KCNJ5 mutations were more often detected in APAs from female patients compared with those from male patients [95% (36/38) vs 60% (41/68); P < 0.0001].
Conclusion
IHC-guided sequencing defined somatic mutations in over 95% of Japanese APAs. While the dominance of KCNJ5 mutations in this particular cohort was confirmed, a significantly higher KCNJ5 prevalence was detected in female patients. This study provides a better understanding of genetic spectrum of Japanese APA patients.
Keywords: primary aldosteronism, aldosterone-producing adenoma, CYP11B2, somatic mutation, Asian
In the past 9 years, significant progress has been made in the determination of genetic causes of primary aldosteronism (PA). Aldosterone-producing adenoma (APA) is a major subtype of PA. The application of next-generation sequencing (NGS) resulted in the identification of somatic gene mutations in APAs including KCNJ5 (1), ATP1A1 (2), ATP2B3 (2), and CACNA1D (3, 4). More recently, somatic mutations in CACNA1H (5) and CLCN2 (6) were also identified in small subsets of APAs. The great majority of these mutations cause increased intracellular calcium levels, leading to enhanced aldosterone synthase (CYP11B2) expression and subsequent aldosterone production. Prevalence studies have suggested that there are racial differences in the prevalence of APA-related somatic mutations (aldosterone-driver mutations). In Europeans and Caucasian Americans, KCNJ5 mutation was the most common genetic cause of APA (7, 8), whereas in African Americans, CACNA1D mutation dominated (9). In APAs from East Asians, KCNJ5 mutation is the most frequent genetic alteration with an even higher prevalence compared with that in Europeans and Caucasian Americans (7, 8, 10-14). However, there still appears to be a significant number (up to 40%) of APAs with unknown genetic mutations in East Asian patients (10, 11, 13, 15).
APAs are also characterized by the expression of CYP11B2 in tumor cells (16, 17), which is required for aldosterone biosynthesis. We recently developed a CYP11B2 immunohistochemistry (IHC)-guided NGS approach, which could provide a higher detection rate of somatic mutations compared with conventional Sanger sequencing of the whole tumor materials (8). Using the approach above, APA-related somatic mutations could be detected in approximately 90% of APAs (8, 9, 18, 19). In this study, we evaluated the prevalence of somatic mutations in APAs in a Japanese cohort using our CYP11B2 IHC-guided NGS approach.
Methods
Patients
A total of 131 Japanese patients with unilateral PA who underwent unilateral adrenalectomy at the Tohoku University Hospital (Sendai, Japan) from 2012 to 2017 were studied. Of them, 16 were excluded from this study due to unavailability of formalin-fixed, paraffin-embedded (FFPE) tissue blocks. The diagnosis of PA was made as previously reported (20-22). Briefly, the patients were considered as having PA when they met the following criteria: a plasma aldosterone concentration (PAC) >12 ng/dL and an aldosterone-to-renin ratio (ARR) >20 (ng/dL per ng/mL/h) at baseline; an ARR >20 after the administration of captopril (50 mg). All the patients underwent computed tomography (CT) and adrenal venous sampling (AVS) with cosyntropin stimulation for subtype classification. A lateralized index of 2.6 was used as a cutoff value for AVS lateralization (23), while in 9 cases, the lateralization was confirmed based on the results of segmental AVS due to anatomical reasons (24). All the patients provided written informed consent. The study was approved by the institutional review boards of Tohoku University (2019-1-995) and the University of Michigan (HUM00106809).
Immunohistochemistry
10% FFPE tissue sections were cut to a thickness of 5 µm. Heat-induced epitope retrieval was performed with antigen unmasking solution (Vector) for 15 minutes. After peroxidase blocking, the primary antibody was applied. The primary antibodies against CYP11B2 and CYP17A1 used in IHC were as follows: mouse monoclonal antibody against CYP11B2 (clone 41-17B, kindly provided by Dr. Celso E. Gomez-Sanchez, RRID: AB_2650562, 1:100, overnight at 4 °C) (17, 25, 26) and a rabbit polyclonal antibody against CYP17A1 (LSBio, LS-B14227, RRID: AB_2088387, 1:1000, 1 hour at room temperature). Polink-2 Plus HRP with DAB kit (GBI Labs) was used for detection. The reacted slides were counterstained with Harris hematoxylin and then dehydrated and coverslipped.
Sequencing analysis
Genomic DNA (gDNA) was selectively isolated from APAs using AllPrep DNA/RNA FFPE kit (QIAGEN) as described previously (8). Sanger sequencing for the mutation hotspot of the KCNJ5 gene was performed using the following PCR primers: forward CGACCAAGAGTGGATTCCTT, reverse AGGGTCTCCGCTCTCTTCTT. For APA samples without KCNJ5 mutation by Sanger sequencing, targeted NGS was performed to determine somatic mutations using Ion Torrent Ampliseq sequencing (Thermo Fisher Scientific) as described previously (8). The panel for library preparation contained amplicons targeting the full coding regions of aldosterone-driver genes (KCNJ5, ATP1A1, ATP2B3, CACNA1D, CACNA1H, and CLCN2), PRKACA, which is associated with cortisol-producing adenoma, and hotspots in the oncogenes GNAS and CTNNB1 (β-catenin). The variant identification was performed using validated pipeline as described previously (8). The NGS-identified previously unreported variants were further confirmed by direct Sanger sequencing using the following PCR primers: ATP1A1 (exon 21), forward CATAAAGATGTTGATCTGCC, reverse CCTAAGAGCAACACCCATTC; CACNA1D (exon 22), forward GAGCCCAGGTTTCTTTTCCTTTGG, reverse CCCTTACATAAACCATTCAGCCC.
Statistical analysis
Clinical characteristics were presented as medians with interquartile ranges or counts with frequencies. The Mann-Whitney U test and Fisher exact test were used for the comparison of 2 groups using GraphPad Prism ver. 8.0.0. Results were considered significantly different when the P value was less than 0.05.
Results
Somatic mutation prevalence in APAs from Japanese patients
CYP11B2 IHC was performed in relevant FFPE sections of 115 resected adrenals from 115 patients with PA. Among those 115 adrenals, APAs were histopathologically identified in 105 adrenals. The remaining 10 adrenals were classified as diffuse hyperplasia or multiple adrenocortical micronodules (26) following careful histopathological evaluation and excluded from the sequencing analysis. In 1 adrenal, there were 2 small APAs within the same adrenal gland. A total of 106 APAs were captured based on the results of CYP11B2 IHC. APA gDNA samples were sequenced to determine the prevalence of somatic mutations. Direct Sanger sequence for the mutation hotspots of the KCNJ5 gene and targeted NGS for specimens mutation-negative by Sanger sequencing identified aldosterone-driver somatic gene mutations in 102 out of 106 APAs, resulting in a mutation detection rate of 96%, which was higher than those previously reported in East Asian APA patients (10-14). KCNJ5 mutation was the most common genetic cause of APA (77/106, 73%), followed by CACNA1D (15/106, 14%), ATP1A1 (5/106, 5%), and ATP2B3 (4/106, 4%) (Table 1). One APA had a somatic CACNA1H mutation (p.I1430T) which was recently reported as an aldosterone-driver somatic mutation in an American APA cohort (5). In an adrenal harboring 2 APAs, 2 different CACNA1D mutations were identified (p.F747V and p.R990H). No CLCN2, CTNNB1, or GNAS mutations were detected in our APA cohort. The KCNJ5 mutations were more frequently detected in APAs from female patients compared with those from male patients (95% of APAs from female vs 60% of APAs from male; P < 0.0001).
Table 1.
Somatic Mutation Spectrum in Japanese APA
| APA from male (n = 68) | APA from female (n = 38) | Total (n = 106) | |
|---|---|---|---|
| KCNJ5 mutations | 41 (60%) | 36 (95%) | 77 (73%) |
| p.E145Q | 1 | 1 | 2 |
| p.G151R | 25 | 21 | 46 |
| p.T158A | 1 | 1 | 2 |
| p.L168R | 14 | 13 | 27 |
| ATP1A1 mutations | 5 (7%) | 0 | 5 (5%) |
| p.L104R | 3 | 0 | 3 |
| p.F959_E961delinsL | 1 | 0 | 1 |
| p.E960_A965delinsALVa | 1 | 0 | 1 |
| ATP2B3 mutations | 4 (6%) | 0 | 4 (4%) |
| p.V424_L425del | 3 | 0 | 3 |
| p.L425_V426del | 1 | 0 | 1 |
| CACNA1D mutations | 14 (21%) | 1 (3%) | 15 (14%) |
| p.G403R | 3 | 0 | 3 |
| p.S652L | 1 | 0 | 1 |
| p.F747V | 3 | 1 | 4 |
| p.S969La | 1 | 0 | 1 |
| p.R990H | 1 | 0 | 1 |
| p.A998V | 2 | 0 | 2 |
| p.A998I | 1 | 0 | 1 |
| p.I1015T | 1 | 0 | 1 |
| p.V1338M | 1 | 0 | 1 |
| CACNA1H mutation | 1 (1%) | 0 | 1 (1%) |
| p.I1430T | 1 | 0 | 1 |
| Mutation-negative | 3 (4%) | 1 (3%) | 4 (4%) |
Two APAs were identified in the resected adrenal from one of the studied patients. One of the APAs from the patient had a CACNA1D p.F747V mutation and the other had a CACNA1D p.R990H mutation.
Following reference sequences were used to determine amino acid changes: NM_000890 (KCNJ5), NM_000701 (ATP1A1), NM_021949 (ATP2B3), NM_1128839 (CACNA1D), NM_021098 (CACNA1H).
a previously unreported variant
Clinical characteristics of Japanese patients with KCNJ5-mutated APA
Since somatic KCNJ5 mutation was the most frequent genetic alteration in Japanese APAs, we compared the clinical characteristics between APA patients with and without KCNJ5 mutation. As summarized in Table 2, proportion of female patients, systolic blood pressure, prevalence of hypokalemia, urinary sodium excretion, PAC, and prevalence of adrenal tumor on CT were all significantly higher in the patients with KCNJ5-mutated APA compared with those with non-KCNJ5-mutated APA. Tumor size on CT was significantly larger in KCNJ5-mutated than that of non-KCNJ5-mutated APA. Of particular note, in order to avoid potential bias on APA clinical phenotype, the patient with 2 distinct CACNA1D-mutated APAs (p.F747V and p.R990H) above was excluded from the statistical analysis. The patient was a 50-year-old male with a longstanding history of hypertension, hypokalemia, and elevated ARR (PAC 25.9 ng/dL, plasma renin activity 0.8 ng/mL/h). No obvious tumor was discernible by CT. AVS successfully lateralized the disease in this particular patient. After unilateral adrenalectomy, normalization of serum potassium and improvement of blood pressure were clinically detected.
Table 2.
Comparison of Clinical Characteristics Between APA Patients With and Without KCNJ5 Mutation
| KCNJ5-mutated APA (n = 77) | Non-KCNJ5-mutated APA (n = 27) | P value | |
|---|---|---|---|
| Age, yrs | 53 (46-62) | 55 (51-65) | 0.1410 |
| Sex (female), N / % | 36 / 47% | 2 / 7% | 0.0002 |
| BMI, kg/m2 | 24 (21-26) | 27 (23-26) | 0.2532 |
| Number of anti-hypertensive medications | 2 (1-3) | 2 (1-3) | 0.6632 |
| Systolic blood pressure, mmHg | 153 (139-167) | 144 (132-154) | 0.0434 |
| Diastolic blood pressure, mmHg | 97 (87-107) | 96 (83-105) | 0.4031 |
| Prevalence of hypokalemia, N / % | 60 / 78% | 14 / 52% | 0.0140 |
| Urinary sodium excretion, mmol/gCr | 108 (83-136) | 78 (57-99) | 0.0009 |
| PAC, ng/dL | 44.4 (29.6-61.7) | 33.2 (21.8-41.9) | 0.0153 |
| PRA, ng/mL/h | 0.2 (0.1-0.4) | 0.2 (0.1-0.3) | 0.2238 |
| ARR, ng/dL per ng/mL/h | 178.8 (80.7-408.3) | 174.0 (82.0-326.0) | 0.8178 |
| ARR post CCT, ng/dL per ng/mL/h | 157.5 (76.0-295.3) | 123.0 (62.5-206.0) | 0.1559 |
| Prevalence of adrenal tumor on CT, N / % | 73 / 95% | 18 / 67% | 0.0006 |
| Largest tumor size on CT, mm | 16.0 (12.0-20.0)a | 11.5 (8.0-15.6)b | 0.0051 |
One patient with 2 distinct APAs was excluded from this analysis. The patient was considered as having hypokalemia when a serum potassium level was lower than 3.5 mM or potassium supplementation was administered. Urinary sodium excretion was measured from spot urine samples.
Abbreviations: ARR, aldosterone-to-renin ratio; BMI, body mass index; CCT, captopril challenge test; CT, computed tomography;PAC, plasma aldosterone concentration; PRA, plasma renin activity.
a N = 73; b N = 18
Newly identified somatic mutations in APAs
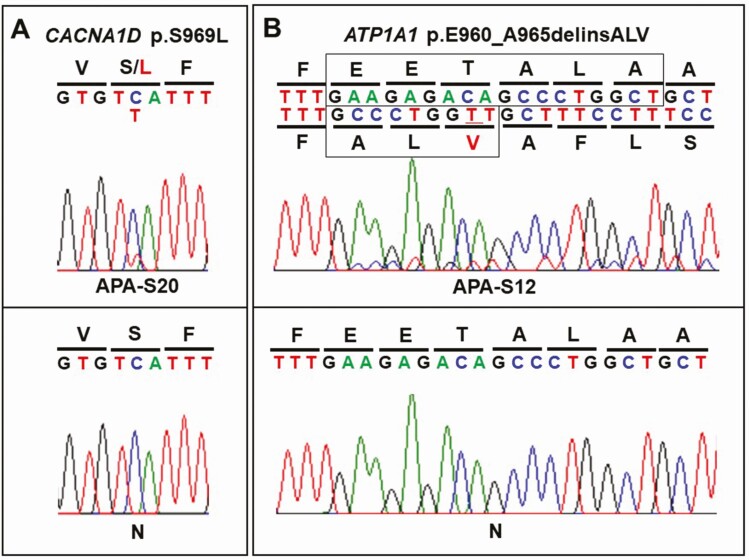
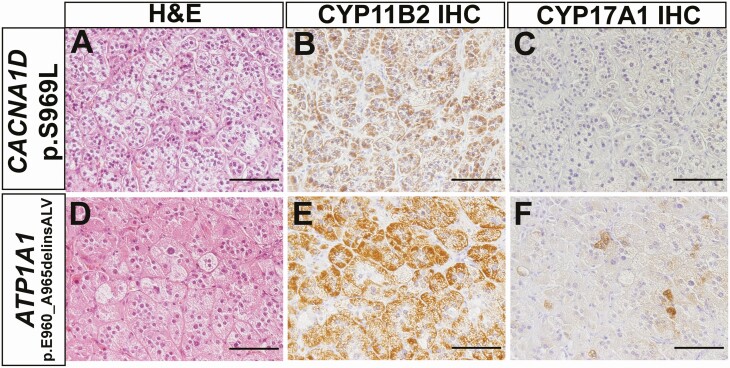
To the best of our knowledge, there were 2 previously unreported somatic mutations in our cohort of APA patients, including one in CACNA1D (c.C2906T, p.S969L) and the other in ATP1A1 (c.2878_2895delinsGCCCTGGTT, p.E960_A965delinsALV). These mutations were identified in male APA patients. Results of NGS regarding novel mutations in aldosterone-driver genes are summarized in Table 3. Of particular note, there was no evidence of these variants in DNA extracted from adjacent nonpathological adrenal tissue, consistent with somatic origin. Direct Sanger sequencing further confirmed the NGS findings (Fig. 1). The histologic characteristics of APA carrying novel somatic mutations are illustrated in Fig. 2. All APAs had abundant immunoreactivity of CYP11B2, whereas CYP17A1, required for cortisol but not aldosterone biosynthesis, was mostly negative or only sporadically positive in tumors with novel mutations.
Table 3.
NGS Results of Previously Unreported Somatic Mutations in APA
| Age, years | Sex | NGS ID | Gene | Reference allele | Variant allele | Amino acid change | FDP | VAF (%) | Reference sequence |
|---|---|---|---|---|---|---|---|---|---|
| 45 | Male | APA_S20 | CACNA1D | C | T | S969L | 978 | 21 | NM_1128839 |
| 71 | Male | APA_S12 | ATP1A1 | GAAGAGACAGCCCTGGCT | GCCCTGGTT | E960_A965delinsALV | 1900 | 12 | NM_000701 |
Abbreviations: FDP, flow-corrected read depth; VAF, variant allele frequency.
Figure 1.
Sanger sequencing results of APA with previously unreported somatic mutations. NGS-identified previously unreported variants were confirmed by Sanger sequencing. There was no evidence of these variants in matched adjacent normal adrenals. Abbreviation: N, adjacent normal adrenal.
Figure 2.
Histopathologic findings of APA carrying novel somatic mutations. A, D. Hematoxylin and eosin staining. B, E. CYP11B2 IHC. C, F. CYP17A1 IHC. Abbreviation: IHC, immunohistochemistry. Scale bars, 100 µm.
Discussion
Racial differences in the prevalence of somatic mutations in APA have been previously reported. Somatic mutations in KCNJ5 were reported to be much more frequent in East Asian APA patients compared with those in other races, including Caucasian and African Americans. However, the accurate somatic mutation spectrum in East Asian APA patients has remained unknown because past prevalence studies defined mutations based on the results of conventional Sanger sequencing without the assessment of tumor CYP11B2 expression prior to the mutational analysis. In this study, we determined the prevalence of somatic mutations in APA using a newly developed CYP11B2 IHC-guided NGS approach.
Aldosterone-driver somatic mutations were identified in the vast majority of Japanese APA patients (96%) using this CYP11B2 IHC-guided sequencing approach. In agreement with results of previously reported studies from Asian countries (10-14, 27), KCNJ5 mutation was a common genetic cause of Japanese APA patients with a prevalence of 73% of APAs examined, which was significantly higher than other races studied, including Caucasians (43%, P < 0.0001) (8) and African Americans (34%, P < 0.0001) (9). A previously reported meta-analysis of 13 studies focusing on the prevalence of somatic KCNJ5 mutations in APAs demonstrated positive correlation between the rate of KCNJ5 mutation and the mean daily urinary sodium excretion (28). Of particular note, the urinary sodium excretion data was referred from epidemiological surveys in the regions where the studies performed, because urinary sodium excretion data was not available in the APA studies (28). In our present study, significantly higher urinary sodium excretion in KCNJ5-mutated APA patients compared with that in non-KCNJ5 mutated APA patients was detected (Table 2). However, the causal relationship between high sodium intake and KCNJ5 mutation has remained unknown at this juncture. Therefore, further investigations are required to clarify the effects of sodium intake amount and incidence of aldosterone-driver mutations.
In contrast to results of some of the previously reported studies in East Asian APA patients, including Japan (11-14), a female-predominant prevalence of KCNJ5 mutations was detected in our present study. Of particular interest, similar female predominance of the prevalence of KCNJ5 mutations has been reported in large multicentric studies mainly conducted in Western European countries (7, 29, 30). The high prevalence of KCNJ5 mutations in female patients has also been documented in APAs from African American (9) as well as Caucasian American (8) patients in the studies using the CYP11B2 IHC-guided NGS method, although the molecular mechanisms underlying the gender differences in the KCNJ5 mutation prevalence remain unknown at this juncture. In accordance with results of aforementioned meta-analysis (28), the patients with KCNJ5-mutated APA had higher plasma aldosterone concentrations and larger adrenal tumors than those with non-KCNJ5-mutated APA.
Of particular interest, the second most frequent genetic alterations in our Japanese cohort were somatic CACNA1D mutations, which has been rarely reported in previous studies from East Asian countries. The combination of CYP11B2 IHC-guided DNA capture and gene-targeted NGS has therefore the advantage in identifying somatic mutations, especially in the CACNA1D gene. In contrast to KCNJ5 mutations, somatic mutations in CACNA1D occur throughout the gene (31) and conventional selected exon-based sequencing approach could possibly underestimate the prevalence of CACNA1D mutations among PA patients. Recent studies also confirmed the importance of CYP11B2 IHC-guided NGS for accurate identification of somatic mutations (18, 19) and this approach should provide us better understanding on genotype-phenotype correlation in future studies.
Two novel somatic mutations were identified in our cohort, including CACNA1D p.S969L and ATP1A1 p.E960_A965delinsALV. The ATP1A1 deletion mutation was located in transmembrane segment 9 (TM9) of Na+/K+ pump. In TM9, several mutations have been reported, including p.I955_E960delinsK (9), p.F956_E961delinsW (32), p.F959_E961delinsL (32), p.E960_A963delinsS (3), p.E960_L964delinsV (32), and p.E960_L964delinsAV (8). A recent study using Xenopus oocytes demonstrated that deletion mutations in TM9 caused the loss of pump function, subsequently resulting in abnormal inward currents which presumably induced excess aldosterone production (33). The CACNA1D p.S969L mutation was located in transmembrane S3 segment of repeat III. Although the identical mutation was observed in one of the aldosterone-producing cell clusters in an adrenal from a patient with idiopathic hyperaldosteronism (34), further functional studies are required to define the potential pathologic roles of these newly identified mutations.
The major findings of our present study are summarized as follows: (i) high detection rate of somatic mutations using a newly developed CYP11B2 IHC-guided NGS approach (96% of APA had one of the aldosterone-driver gene mutations); (ii) first detailed determination of mutation spectrum of Japanese APA patients, including the demonstration of significant differences in mutation prevalence between Japanese male and female patients; and (iii) the identification of previously unreported somatic mutations in genes that are associated with PA. Expanded CYP11B2 IHC-guided analyses of APA DNA from other Asian countries should help determine whether APA mutations differ between distinct Asian populations.
Acknowledgments
Financial Support: This work was supported by grants from the American Heart Association (17SDG33660447 to K. Nanba), National Institute of Diabetes and Digestive and Kidney Diseases (DK106618 to W.E. Rainey) and JSPS KAKENHI (JP18K08500, Health Labor Sciences Research Grant Number H29-Nanji-Ippan-046 to F. Satoh).
Glossary
Abbreviations
- APA
aldosterone-producing adenoma
- ARR
aldosterone-to-renin ratio
- AVS
adrenal vein sampling
- CT
computed tomography
- FFPE
formalin-fixed, paraffin-embedded
- IHC
immunohistochemistry
- NGS
next-generation sequencing
- PA
primary aldosteronism
- PAC
plasma aldosterone concentration
Additional Information
Disclosure Summary: S.A. Tomlins is a previous consultant for, cofounder of, equity holder in, and current employee of Strata Oncology.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331(6018):768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45(4):440-4, 444e1. [DOI] [PubMed] [Google Scholar]
- 3. Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45(9):1055-1060. [DOI] [PubMed] [Google Scholar]
- 4. Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45(9):1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanba K, Blinder AR, Rege J, et al. Somatic CACNA1H mutation as a cause of aldosterone-producing adenoma. Hypertension. 2020:HYPERTENSIONAHA11914349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dutta RK, Arnesen T, Heie A, et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur J Endocrinol. 2019;181(5):K37-K41. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes-Rosa FL, Williams TA, Riester A, et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64(2):354-361. [DOI] [PubMed] [Google Scholar]
- 8. Nanba K, Omata K, Else T, et al. Targeted molecular characterization of aldosterone-producing adenomas in White Americans. J Clin Endocrinol Metab. 2018;103(10):3869-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nanba K, Omata K, Gomez-Sanchez CE, et al. Genetic characteristics of aldosterone-producing adenomas in Blacks. Hypertension. 2019;73(4):885-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zheng FF, Zhu LM, Nie AF, et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension. 2015;65(3):622-628. [DOI] [PubMed] [Google Scholar]
- 11. Wang B, Li X, Zhang X, et al. Prevalence and characterization of somatic mutations in Chinese aldosterone-producing adenoma patients. Medicine (Baltimore). 2015;94(16):e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitamoto T, Suematsu S, Matsuzawa Y, Saito J, Omura M, Nishikawa T. Comparison of cardiovascular complications in patients with and without KCNJ5 gene mutations harboring aldosterone-producing adenomas. J Atheroscler Thromb. 2015;22(2):191-200. [DOI] [PubMed] [Google Scholar]
- 13. Wu VC, Wang SM, Chueh SJ, et al. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7:39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okamura T, Nakajima Y, Katano-Toki A, et al. Characteristics of Japanese aldosterone-producing adenomas with KCNJ5 mutations. Endocr J. 2017;64(1):39-47. [DOI] [PubMed] [Google Scholar]
- 15. Kitamoto T, Suematsu S, Yamazaki Y, et al. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J Clin Endocrinol Metab. 2016;101(2):494-503. [DOI] [PubMed] [Google Scholar]
- 16. Nanba K, Tsuiki M, Sawai K, et al. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab. 2013;98(4):1567-1574. [DOI] [PubMed] [Google Scholar]
- 17. Gomez-Sanchez CE, Qi X, Velarde-Miranda C, et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383(1-2):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Sousa K, Boulkroun S, Baron S, et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension. 2020;75(4):1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Z, Nanba K, Udager A, et al. Biochemical, histopathological and genetic characterization of posture responsive and unresponsive APAs. J Clin Endocrinol Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwakura Y, Morimoto R, Kudo M, et al. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99(5):1593-1598. [DOI] [PubMed] [Google Scholar]
- 21. Satoh F, Morimoto R, Ono Y, et al. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65(5):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tezuka Y, Yamazaki Y, Kitada M, et al. 18-oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension. 2019;73(6):1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Satoh F, Abe T, Tanemoto M, et al. Localization of aldosterone-producing adrenocortical adenomas: significance of adrenal venous sampling. Hypertens Res. 2007;30(11):1083-1095. [DOI] [PubMed] [Google Scholar]
- 24. Satoh F, Morimoto R, Seiji K, et al. Is there a role for segmental adrenal venous sampling and adrenal sparing surgery in patients with primary aldosteronism? Eur J Endocrinol. 2015;173(4):465-477. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura Y, Maekawa T, Felizola SJ, et al. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392(1-2):73-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamazaki Y, Nakamura Y, Omata K, et al. Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4): 1182-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warachit W, Atikankul T, Houngngam N, Sunthornyothin S. Prevalence of somatic KCNJ5 mutations in Thai patients with aldosterone-producing adrenal adenomas. J Endocr Soc. 2018;2(10):1137-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lenzini L, Rossitto G, Maiolino G, Letizia C, Funder JW, Rossi GP. A meta-analysis of somatic KCNJ5 K(+) channel mutations in 1636 patients with an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2015;100(8):E1089-E1095. [DOI] [PubMed] [Google Scholar]
- 29. Åkerström T, Crona J, Delgado Verdugo A, et al. Comprehensive re-sequencing of adrenal aldosterone producing lesions reveal three somatic mutations near the KCNJ5 potassium channel selectivity filter. PLoS One. 2012;7(7):e41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boulkroun S, Beuschlein F, Rossi GP, et al. Prevalence, clinical, and molecular correlates of KCNJ5 mutations in primary aldosteronism. Hypertension. 2012;59(3):592-598. [DOI] [PubMed] [Google Scholar]
- 31. Azizan EA, Brown MJ. Novel genetic determinants of adrenal aldosterone regulation. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):209-217. [DOI] [PubMed] [Google Scholar]
- 32. Åkerström T, Willenberg HS, Cupisti K, et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr Relat Cancer. 2015;22(5):735-744. [DOI] [PubMed] [Google Scholar]
- 33. Meyer DJ, Gatto C, Artigas P. Na/K pump mutations associated with primary hyperaldosteronism cause loss of function. Biochemistry. 2019;58(13):1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Omata K, Satoh F, Morimoto R, et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension. 2018;72(4):874-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.