Abstract
Context/Objective
Increases of thyroid cancer (TC) incidence emerged in the past several decades in several countries. This study aimed to estimate time trends of TC incidence in India and the proportion of TC cases potentially attributable to overdiagnosis by sex, age, and area.
Design
TC cases aged 0 to 74 years reported to Indian cancer registries during 2006 through 2014 were included. Age-standardized incidence rates (ASR) and TC overdiagnosis were estimated by sex, period, age, and area.
Results
Between 2006-2008 and 2012-2014, the ASRs for TC in India increased from 2.5 to 3.5/100,000 women (+37%) and from 1.0 to 1.3/100,000 men (+27%). However, up to a 10-fold difference was found among regions in both sexes. Highest ASRs emerged in Thiruvananthapuram (14.6/100,000 women and 4.1/100,000 men in 2012-2014), with 93% increase in women and 64% in men compared with 2006-2008. No evidence of overdiagnosis was found in Indian men. Conversely, overdiagnosis accounted for 51% of TC in Indian women: 74% in those aged < 35 years, 50% at ages 35 to 54 years, and 30% at ages 55 to 64 years. In particular, 80% of TC overdiagnosis in women emerged in Thiruvananthapuram, whereas none or limited evidence of overdiagnosis emerged in Kamrup, Dibrugarh, Bhopal, or Sikkim.
Conclusions
Relatively high and increasing TC ASRs emerged in Indian regions where better access to health care was reported. In India, as elsewhere, new strategies are needed to discourage opportunistic screening practice, particularly in young women, and to avoid unnecessary and expensive treatments. Present results may serve as a warning also for other transitioning countries.
Keywords: Thyroid cancer, overdiagnosis, incidence, India, time trends
Thyroid cancer (TC) is the most common endocrine malignancy and, in 2018, it globally constituted 3.3% of all neoplasms (1). Incidence rates of TC have been increasing in the past several decades worldwide, both in high-resource (2) and in low- or middle-income countries (3). However, heterogeneity exists not only across countries (3, 4), but also among regions within the same country (5-10). Although India currently ranks among the countries with the lowest TC incidence average, it is affected by a huge regional variation, with a 10-fold difference between lowest and highest incidence areas covered by cancer registries (CRs) (10, 11). There is wide variation in access to health care and quality within the various regions and states of India (12). In addition, although TC incidence rates have been estimated to be below the world average (5.4/100,000 in India vs 6.7/100,000 worldwide), TC is currently the fifth most commonly diagnosed cancer among Indian women (1).
Possible reasons able to explain the heterogeneity among areas and the increasing trends worldwide have been previously extensively discussed (13, 14). Among them, overdiagnosis, defined as the detection of tumors that would be unlikely to progress to symptoms or death even if left untreated (4, 15), seems to account for 70% to 90% of TC cases in high-income countries (4), causing potential harms to patients and relevant unnecessary costs on the health system (16). However, the impact of TC overdiagnosis has never been studied in low- to middle-income countries, where resources for cancer control and oncology care are limited.
The aim of this study was to describe TC incidence trends in India, in 2006 through 2014, using the most recent data from 14 Indian population-based CRs and to estimate the corresponding fraction of TC incidence potentially attributable to overdiagnosis by sex and region.
Materials and Methods
Data from 14 Indian population-based CRs were analyzed in this study (Table 1), including 65 million people, 5.1% of the Indian population, largely living in urban areas (17). Eleven of 14 Indian CRs (i.e., all but Dibrugarh, Kolkata, and Manipur) were included in Cancer Incidence in Five Continents publications (18, 19).
Table 1.
Age-adjusted Incidence Rates (ASR)a,b for Thyroid Cancer Computed by Sex in 14 Indian Cancer Registries (CRs), 2006-2014
| WOMEN | MEN | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASR | ASR | |||||||||
| CR | Years Included | Population in 2012 (million) | 2006- 2008 | 2009- 2011 | 2012- 2014 | Δ%c | 2006- 2008 | 2009- 2011 | 2012- 2014 | Δ%c |
| Ahmedabad (urban) | 2006-2010, 2012-2013 | 5.8 | 0.9 | 1.1 | 1.2 | 34 | 0.4 | 0.7 | 0.9 | 101 |
| Bangalore | 2006-2009, 2012 | 8.7 | 4.2 | 3.7 | 4.6 | 9 | 1.4 | 1.7 | 1.8 | 31 |
| Barshi | 2006-2010, 2012-2014 | 0.5 | 1.2 | 1.7 | 1.4 | 15 | 0.2 | 0.2 | 0.5 | 127 |
| Bhophal | 2006-2010, 2012-2013 | 1.3 | 1.9 | 1.5 | 1.6 | -12 | 0.8 | 0.5 | 0.5 | -40 |
| Chennai | 2006-2009, 2012-2013 | 4.6 | 2.5 | 4.2 | 2.7 | 11 | 1.1 | 1.1 | 1.1 | 4 |
| Delhi | 2006-2009, 2012 | 16.7 | 2.8 | 2.7 | 2.3 | -19 | 1.4 | 1.1 | 1.2 | -17 |
| Dibrugarh | 2006-2014 | 1.3 | 1.1 | 0.8 | 1.7 | 55 | 0.2 | 0.5 | 0.3 | 66 |
| Kamrup district | 2006-2014 | 1.3 | 1.9 | 2.1 | 3.3 | 77 | 0.8 | 1.1 | 1.3 | 59 |
| Kolkata | 2006-2009, 2012 | 4.4 | 1.6 | 1.8 | 2.4 | 44 | 0.8 | 0.8 | 1.3 | 71 |
| Manipur | 2006-2010, 2012-2014 | 3.0 | 4.0 | 4.5 | 5.4 | 34 | 0.9 | 1.1 | 1.1 | 26 |
| Mizoram | 2006-2010, 2012-2014 | 1.1 | 2.9 | 2.0 | 3.3 | 14 | 1.3 | 2.1 | 1.0 | -21 |
| Mumbai | 2006-2010, 2012 | 12.2 | 2.0 | 2.2 | 2.9 | 46 | 2.2 | 1.1 | 1.1 | -49 |
| Sikkim | 2006-2014 | 0.6 | 3.1 | 2.4 | 2.1 | -33 | 1.4 | 1.1 | 1.0 | -25 |
| Thiruvananthapuram | 2006-2014 | 3.2 | 7.6 | 11.3 | 14.6 | 93 | 2.5 | 3.5 | 4.1 | 64 |
| Pool of Indian CRs | 64.9 | 2.5 | 2.8 | 3.5 | 37 | 1.0 | 1.1 | 1.3 | 27 | |
aAge 0-74 years.
bAge-standardized (on World Population) incidence rates per 100,000.
cASR in 2012-2014 versus 2006-2008.
Patients aged 0 to 74 years diagnosed with a TC (International Classification of Disease and Related Health Problems, 10th revision, code C73) between 2006 and 2014 were analyzed. The 75-year age restriction was selected for 2 reasons. First, people aged 75 years or more in India represented less than 1% on the overall population. Second, the dataset used provided only an open category (≥ 75 years), so we could not distinguish older ages for incidence rates calculation.
The data collection process in the CRs in India involved the retrieval of information from several Indian hospitals. Once cancer patients were identified and verified for residence in areas covered by a CR, the additional detailed data (i.e., data on diagnosis, tumor site, and extent of disease) were collected from 3 major sources (hospitals, diagnostic departments, and death certificates) (20). Each Indian CR publishes periodic reports, accessible online (17) with different cancer registration periods (Table 1).
Age-specific incidence rates were calculated for each 10-year age group, with the exception of the first group: 0 to 14, 15 to 24, 25 to 34, ..., 65 to 74 years) by sex and period of diagnosis (2006-2008, 2009-2011, 2012-2014). Age-standardized incidence rates (ASR) per 100,000 person-years were standardized to the world standard population (in 5-year age groups), calculated by CR, sex, and period of diagnosis.
TC overdiagnosis was estimated using previously described method and assumptions (2, 4). In brief, they were based on several well-reported features of the TC epidemic, including that: (1) a vast reservoir of thyroid disease existed in the general population; (2) the mortality trends remained stable (21); (3) incidence increased substantially but with a large variability among and within countries; and (4) the increase involved mostly small papillary subtypes and young or middle-age adults (i.e., when surveillance is particularly higher). Age-specific curves of TC incidence have changed dramatically since the 1980s, albeit with large differences across several high-income countries (2, 4). In countries where longstanding high-quality cancer data was available (i.e., the Nordic countries, Israel, Canada, and Connecticut in the United States), the incidence rates of TC in periods before the advent of ultrasonography increased approximately proportionally to the second power of age, in both sexes.
Using the Nordic countries as reference population, we have deduced the shape of age-specific rates of thyroid cancer incidence in the 1960s (i.e., before the introduction of ultrasonography in the late 1970s). The shape of the “historical” age curves was remarkably similar across all studied populations, showing exponential growth of rates with age, consistently with the behavior of most epithelial carcinomas and according to the multistage model of carcinogenicity described by Armitage and Doll (22). Furthermore, even in countries for which shorter duration of cancer registration data were available, the gradual distortion of the age-specific curves over periods of time allowed us to deduce a similar pattern.
According to the multistage model of carcinogenesis, the incidence rate of cancer is proportional to agek, where the exponent k could be estimated for individual cancer types. The relationship between the logarithm of incidence rate and the logarithm of age is linear according to the following formula: log(rate) = c + k • log(age), where c is a constant value. This relationship appears in a straight line when plotting incidence rates against age on a log–log scale, with a slope approximately equal to 2, in both sexes (2).
In the past few decades, the incidence of TC increased remarkably over time among middle-aged women in several populations but with little variation at older ages, thus, roughly altering the age curves from an exponential-growth shape to an inverted-U shape. This modification in the shape of the age-specific curves was mainly attributed to the introduction of modern diagnostic techniques, like ultrasonography and ultrasound-guided fine-needle biopsy, and to a progressively more intense search in young and middle-age individuals for thyroid nodules that very seldom lead to death. As a consequence of these observations, the expected incidence rates of thyroid cancer for 5-year age groups from 2006-2008 and 2012-2014 in India were obtained by hypothesizing that the age curve of the disease would have retained the historical shape (not the absolute level) observed before the 1970s in the Nordic countries. A key observation is that, within all the studied countries, incidence rates at older ages were of approximately equal magnitude across time periods and birth cohorts, in contrast with the large rate increases seen in younger age groups. This reasonably allowed the possibility to add a constraint that set the expected incidence rates to be equal to those observed within the older age group. The expected rates for all other age groups were extrapolated according to the multistage model, assuming linearity on a log–log scale, with the same slope as that estimated for the reference countries and restricted to pass by the mid-point of the age group of 65 to 74 years.
For the estimation of TC overdiagnosis, we have hypothesized that the progressive departure from linearity on the log–log scale (multistage model) of the observed rates was attributable to the increased detection of asymptomatic, nonlethal disease. The number and the fraction of cases attributable to overdiagnosis were estimated, based on the difference between the observed and expected age-specific rates.
Studies on cancer overdiagnosis are strongly dependent on the assumptions used; therefore, the proportion of TC cases attributable to diagnostic changes should be interpreted with caution. As a consequence, we also chose not to report uncertainty intervals because these may lead to a false impression of precision of our estimates. We relied on the Nordic countries to establish the historical age curve of TC incidence before diagnostic changes (2). However, consistent estimates were obtained using other long-term duration registries (2, 9). Gaps in nationwide information on the TC incidence are another possible limitation of the present study and, therefore, our findings may not be fully representative of India (coverage of 4% population) given the possible internal TC variability.
Results
The ASRs for TC in India (Table 1) increased from 2.5/100,000 women in 2006-2008 to 3.5/100,000 women in 2012-2014 (+37%), whereas a less marked increase (+27%) emerged in men (from 1.0 to 1.3/100,000 men, respectively), in the same period.
Regional incidence rates in 2012-2014 varied up to 10-fold, in both women and men (Table 1). Low ASRs (i.e., < 2/100,000 women and < 1/100,000 men) were seen in Ahmedabad, Barshi, Bhopal, and Dibrugarh. Conversely, high ASRs emerged in both sexes in Thiruvananthapuram (14.6/100,000 women and 4.1/100,000 men in 2012-2014) (Table 1), corresponding to a 93% increase in women, and 64% in men, when compared with 2006-2008. TC ASRs in women increased by > 40% from 2006-2008 and 2012-2014 in Dibrugarh, Kamrup, Kolkata, and Mumbai.
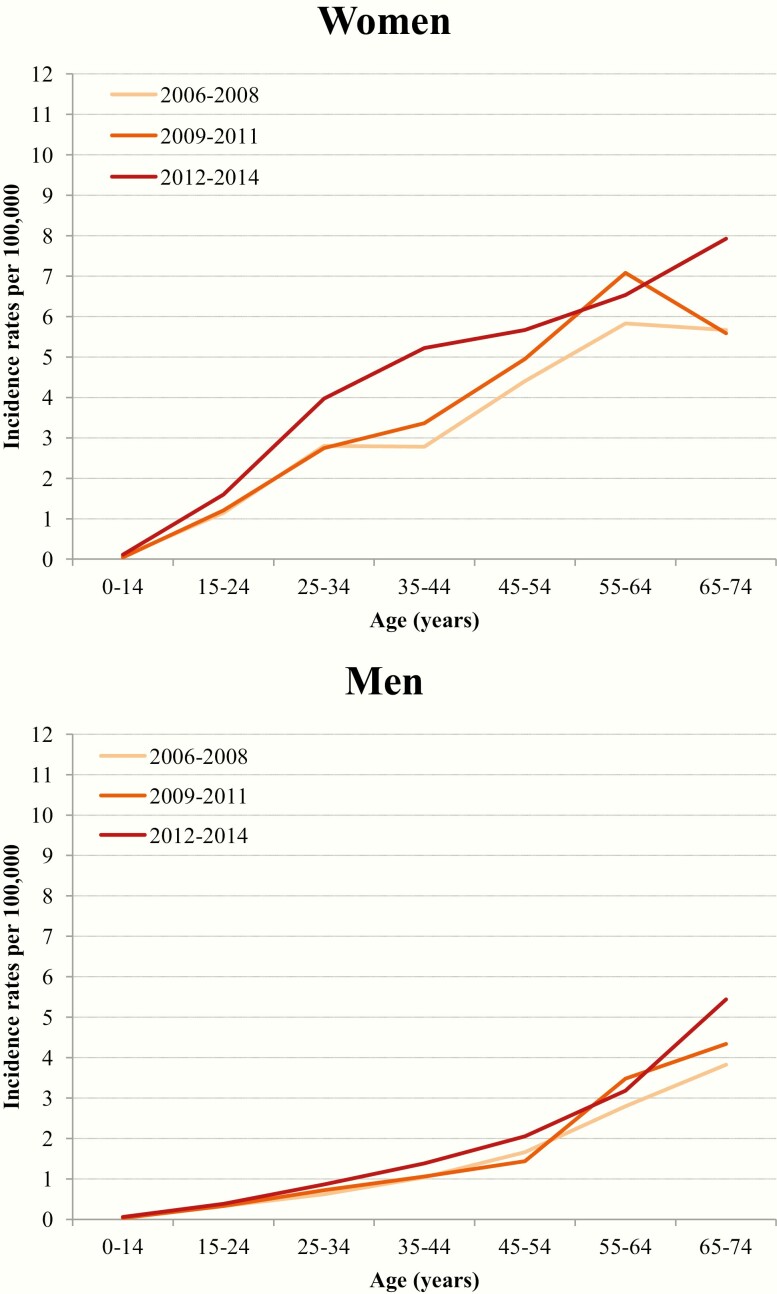
In men, ASRs remained very low (i.e., ≤ 2.2/100,000) everywhere and throughout the study period, with the exception of Thiruvananthapuram. In men, the age-specific rates showed an exponential growth consistent with the multistage model of carcinogenesis in all the 3 periods examined, suggesting an absence, or a negligible impact, of overdiagnosis (Fig. 1). Conversely, age-specific incidence rates in women increased progressively from 2006-2008 and 2012-2014, with a peak in middle-aged women and a consequent departure from the historical pattern of exponential growth shape (Fig. 1).
Figure 1.
Observed changes in age-specific incidence rates of thyroid cancer per 100,000 by sex and period in India, 2006-2014.
TC rates and consequently overdiagnosis in women showed remarkable variations across CRs (Fig. 2). The highest proportion of cases attributable to overdiagnosis was found in Thiruvananthapuram (80%). In most of the other Indian CRs, the corresponding fraction was intermediate (33%-67%), whereas little or no evidence of departure from the expected age-specific rates emerged in Bhopal, Dibrugarh, Kamrup, and Sikkim (Fig. 2).
Figure 2.
Observed versus expecteda age-specific incidence rates of thyroid cancer per 100,000 women by registry in India, 2006-2014. aEstimated using the slope calculated in the historical period 1958–1967, see Methods
Overall, 51% of women diagnosed in all Indian CRs between 2006 and 2014 were likely the result of overdiagnosis (Table 2). In women, the fraction of overdiagnosed TC was particularly high (74%) below age 35 years, 50% at age 35 to 54 years, and 30% at 55 to 64 years. Estimates of overdiagnosis showed a small variation across the study period (i.e., from 49% in 2006-2008 and 2012-2014 to 54% in 2009-2011).
Table 2.
Estimated proportion of Cases Overdiagnoseda for Thyroid Cancer in Indian Women, by Age and Period (2006-2014)
| Female Population in 2012 (million)a | Attributable to overdiagnosisb | |
|---|---|---|
| Age (years) | ||
| 0–34 | 19.4 | 74% |
| 35–54 | 8.1 | 50% |
| 55–64 | 2.2 | 30% |
| 65-74 c | 1.2 | |
| Period | ||
| 2006–2008d | 27.8 | 49% |
| 2009–2011d | 29.4 | 54% |
| 2012–2014d | 30.9 | 49% |
| Pool of Indian CRs | 30.9 | 51% |
a Truncated 0-74 years.
b Estimated using the slope calculated in the historical period 1958-1967, see Methods.
c Overdiagnosis was set at 0% at age 65-74 years, see Methods.
d Average population in the period.
A sensitivity analysis for TC incidence was conducted by calculating the ASRs using only the 11 CRs included in Cancer Incidence in Five continents (18), obtaining estimates that were consistent with the overall study findings.
Discussion
Two original scientific issues are presented here. First, the study confirmed that the assumption of a “natural” (i.e., an historical/expected exponential increase with age) shape of the age-specific curve for thyroid cancer incidence is not a feature of the past only in certain high-income countries, but that it is still observable in current periods in Indian men and, in some areas, in Indian women. This supported the possibility to measure “overdiagnosis” of TC in different countries when a distortion from this “natural” pattern occurred. Second, the study showed for the first time that this distortion of the age-specific curves has already occurred in some areas of India, especially in women, and that therefore the spreading of diagnostic pressure (ultrasound examinations, in particular) and of overdiagnosis is not only a concern for high income countries, but should also be discouraged in transitioning countries, like India, where the wastage of limited funds and health care professionals’ time for unnecessary costs and treatments can be huge. Most important, it will help us avoid unnecessary harms to patients in relation to treatment of thyroid cancer.
This population-based study provided an estimation of the impact of overdiagnosis on the recent increase in TC incidence in India, highlighting a relevant regional heterogeneity across regions and sexes. Relatively low increases of ASRs emerged in men between 2006 and 2014, with age-specific curves following an exponential growth with age (22), consistent with patterns reported for both sexes before the 1970s in North-European CRs and in other high income countries (2). For the most recent periods, an increase with age has also been observed in men in the nearby Sri Lankan national CR (i.e., ASR = 2.2/100,000 men in 2010) (23), as well as in women in few Indian regions (i.e., Bhopal, Dibrugarh, Kamrup, and Sikkim). Conversely, in most metropolitan Indian cities (including Delhi, Mumbai, and Bangalore), TC incidence in women increased, progressively departing from an exponential growth with age to resemble an inverse U-shaped function, with the largest departure among young or middle-aged women. This pattern is an indication of overdiagnosis, which, according to our estimation, might have affected approximately one-half (51%) of TC cases for Indian women. This phenomenon was particularly intense in younger (< 35 years) women, accounting for 3 of 4 TC cases. If age-specific incidence rates were to be applied to the Indian population, it would result in approximately 7,000 of 14,000, TC diagnoses attributable to overdiagnosis in women each year during the study period from 2006 to 2014.
Overdiagnosis (i.e., the detection of tumors that, if left untreated, would unlikely progress to symptoms or death) is the most likely explanation for the worldwide increase of TC incidence (4). Even though, in India, the incidence rates were below the worldwide average, a noticeable rise in incidence of TC has occurred (+37% among women between 2006-2008 and 2012-2014). Although differences in exposure to known (e.g., radiation, iodine deficiency) (24) or unknown risk factors could contribute to the depicted increase and geographical variations of TC (13, 14, 24), they unlikely explained the observed general pattern of TC incidence. In high-income countries, geographic variation of TC incidence rates was associated with characteristics of the health care system, with high rates in urban areas where thyroid-gland examination were widely available (8, 25-28).
The issue of overdiagnosis was stronger than elsewhere in Thiruvananthapuram district, the capital city of the Kerala state, where the highest increases in TC ASRs emerged, both in men and women (4.1 and 14.6 per 100,000, respectively) (11). The state of Kerala, located in South India, has attracted public interest for many years because of its high-quality health system (28-30), which is comparable with some upper-middle/high-income countries. The high rate of TC overdiagnosis in Kerala possibly reflects better health consciousness in the population and better access to medical care. Kerala is ahead of other states in the field of health care, with an exceptionally advanced public and private health care system in the Country (31). Notably, the TC ASRs in this region (15/100,000 women in 2012-2014) were similar to those documented in some high-income countries (e.g., Germany, Japan) where the ASRs were 14/100,000 women in 2008-2012 (10). In addition, we estimated that 80% of TC in Thiruvananthapuram were overdiagnosed in 2006 to 2014, a percentage consistent with those estimated in the United State, Italy, France, and Australia (i.e., 70%-80%) in women (4). High proportions of women with TC cancers that can be attributed to overdiagnosis (51%) have also been found in 1 of the smallest states in India, Mizoram (North East India), which despite the geographical distance, shares high health care and literacy indices with Kerala (32).
One of the major strengths of the study was the population-based design and the size of population coverage (65 million of people), which provided reliable estimates of TC incidence over a 9-year period. Certainly, this study lacks some details of the disease, such as histology or stage of the diagnosis, which are important factors in analyzing trends in TC incidence and overdiagnosis. Our findings might have been affected by some limitation of CR data, such as possible incompleteness of past or present detection and reporting of TC cases, as well as, accuracy (i.e., quality of histopathologic diagnoses) (20) and this might have also affected estimates of overdiagnosis. In particular, it is not clear whether CRs with very low TC incidence, and only modest increases, may be affected by TC underdetection. However, the completeness and quality of most of the studied Indian CRs (i.e., Ahmedabad, Bangalore, Barshi, Bhopal, Chennai, Delhi, Kamrup, Mizoram, Mumbai, Sikkim, Thiruvananthapuram) have been shown to be satisfactory, in comparison with international standards (19).
Even if the location of the CRs included in the study were well distributed across India (20), the majority of the Indian CRs included in the study are located in urban areas, where nearly one-third of the total Indian population reside, and may not be representative of the whole country, particularly on account on the large variability in TC incidence. Because of this and of the large geographical heterogeneity, the interpretation of overdiagnosis found in the entire Country needs to be interpreted cautiously (33).
In conclusion, half of recent TC incidence among Indian women can be attributed to overdiagnosis, whereas no evidence of overdiagnosis emerged in men. There are also huge variations within the country, with the southwestern state of Kerala showing an overdiagnosis in women comparable to that seen in high-income populations. although further studies are needed to investigate the reasons for local variations and the increase of TC incidence, strategies are needed to discourage opportunistic screening practice, in particular in younger Indian women (34). Overdiagnosed TC patients show no excess risk of death in comparison with the general population, but almost all will undergo total thyroidectomy with a nonnegligible proportion of complications (35). The evolution of TC incidence in India may serve as a warning for other transitioning countries as well (36). As a society, we must do what it takes to minimize harms to patients and to the already over-stretched healthcare systems of these countries (29, 37).
Acknowledgments
The authors thank Mrs. Luigina Mei for editorial assistance.
Financial Support: This work was supported by the Italian Association for Cancer Research (AIRC) (grant no. 16921), by the Italian Ministry of Health (5X1000, year 2013 to CRO Aviano), by the Markey Cancer Center Support Grant (NCI P30 CA177558). The funding source had no involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Glossary
Abbreviations
- ASR
age-standardized incidence rates
- CR
cancer registry
- TC
thyroid cancer
Additional Information
Disclosure Summary: The authors declare no conflicts of interests.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon: IARC, 2018. Available from: https://gco.iarc.fr/today. Accessed December 2, 2019. [Google Scholar]
- 2. Vaccarella S, Dal Maso L, Laversanne M, Bray F, Plummer M, Franceschi S. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource Countries. Thyroid. 2015;25(10):1127-1136. [DOI] [PubMed] [Google Scholar]
- 3. James BC, Mitchell JM, Jeon HD, Vasilottos N, Grogan RH, Aschebrook-Kilfoy B. An update in international trends in incidence rates of thyroid cancer, 1973-2007. Cancer Causes Control. 2018;29(4-5):465-473. [DOI] [PubMed] [Google Scholar]
- 4. Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid cancer epidemic? The increasing impact of overdiagnosis. New Engl J Med. 2016;375:614-617. [DOI] [PubMed] [Google Scholar]
- 5. Cordioli MICV, Canalli MHBS, Coral MHC. Increase incidence of thyroid cancer in Florianopolis, Brazil: comparative study of diagnosed cases in 2000 and 2005. Arq Bras Endocrinol Metabol. 2009;53:453-460. [DOI] [PubMed] [Google Scholar]
- 6. Van den Bruel A, Francart J, Dubois C, et al. Regional variation in thyroid cancer incidence in Belgium is associated with variation in thyroid imaging and thyroid disease management. J Clin Endocrinol Metab. 2013;98(10):4063-4071. [DOI] [PubMed] [Google Scholar]
- 7. Colonna M, Uhry Z, Guizard AV, et al. ; FRANCIM network . Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol. 2015;39(4):511-518. [DOI] [PubMed] [Google Scholar]
- 8. Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016;26(11):1535-1540. [DOI] [PubMed] [Google Scholar]
- 9. Dal Maso L, Panato C, Franceschi S, et al. The impact of overdiagnosis on thyroid cancer epidemic in Italy,1998–2012. Eur J Cancer. 2018;94:6-15. [DOI] [PubMed] [Google Scholar]
- 10. Lortet-Tieulent J, Franceschi S, Dal Maso L, Vaccarella S. Thyroid cancer “epidemic” also occurs in low- and middle-income countries. Int J Cancer. 2019;144(9):2082-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mathew IE, Mathew A. Rising thyroid cancer incidence in Southern India: an epidemic of overdiagnosis? J Endocr Soc. 2017;1(5):480-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. GBD 2016 Healthcare Access and Quality Collaborators. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet 2018;391:2236-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanabria A, Kowalski LP, Shah JP, et al. Growing incidence of thyroid carcinoma in recent years: factors underlying overdiagnosis. Head Neck. 2018;40(4):855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davies L. Overdiagnosis of thyroid cancer. Bmj. 2016; 355:i6312. [DOI] [PubMed] [Google Scholar]
- 16. Lubitz CC, Kong CY, McMahon PM, et al. Annual financial impact of well-differentiated thyroid cancer care in the United States. Cancer. 2014;120(9):1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Centre for Disease Informatics & Research. National Cancer Registry Programme: NCPR Annual Reports. Available at: http://ncdirindia.org/NCRP/Annual_Reports.aspx. Accessed March 30, 2020.
- 18. Parkin DM, Ferlay J, Curado MP, et al. (Eds). Cancer Incidence in Five Continents (CI5) Volumes I to X. IARC CancerBase No. 12. 2014. Avaliable at: http://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Incidence-In-Five-Continents-Ci5-Volumes-I-To-X-2014. Accessed March 30, 2020.
- 19. Bray F, Colombet M, Mery L, et al. (eds). Cancer Incidence in Five Continents, Vol. XI (electronic version). Lyon: IARC; 2017. Available from: http://ci5.iarc.fr. Accessed March 30, 2020. [Google Scholar]
- 20. Behera P, Patro BK. Population Based Cancer Registry of India – the challenges and opportunities. Asian Pac J Cancer Prev. 2018;19(10):2885-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Brito JP, Vaccarella S. Long term declines of thyroid cancer mortality: an international age-period-cohort analysis. Thyroid. 2020; doi: 10.1089/thy.2019.0684 [DOI] [PubMed] [Google Scholar]
- 22. Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer. 1954;8(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jayarajah U, Fernando A, Prabashani S, Fernando EA, Seneviratne SA. Incidence and histological patterns of thyroid cancer in Sri Lanka 2001-2010: an analysis of national cancer registry data. BMC Cancer. 2018;18(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadeghi H, Rafei M, Bahrami M, Haghdoost A, Shabani Y. Attributable risk fraction of four lifestyle risk factors of thyroid cancer: a meta-analysis. J Public Health (Oxf). 2018;40(2):e91-e98. [DOI] [PubMed] [Google Scholar]
- 25. Lee TJ, Kim S, Cho HJ, Lee JH. The incidence of thyroid cancer is affected by the characteristics of a healthcare system. J Korean Med Sci. 2012;27(12):1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanley JP, Jackson E, Morrissey LA, et al. Geospatial and temporal analysis of thyroid cancer incidence in a rural population. Thyroid. 2015;25(7):812-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park S, Oh CM, Cho H, et al. Association between screening and the thyroid cancer “epidemic” in South Korea: evidence from a nationwide study. BMJ. 2016;355:i5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. [DOI] [PubMed] [Google Scholar]
- 29. Nath I, Reddy KS, Dinshaw KA, et al. Country profile: India. Lancet. 1998;351(9111):1265-1275. [DOI] [PubMed] [Google Scholar]
- 30. Smith RD, Mallath MK. History of the growing burden of cancer in India: from antiquity to the 21st century. J Glob Oncol. 2019;5:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jana A, Basu R. Examining the changing health care seeking behavior in the era of health sector reforms in India: evidences from the National Sample Surveys 2004 & 2014. Glob Health Res Policy. 2017;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. International Institute for Population Sciences: National Family Health Survey India 4. http://rchiips.org/nfhs/nfhs4.shtml. Accessed March 30, 2020.
- 33. Etzioni R, Gulati R. Recognizing the limitations of cancer overdiagnosis studies: a first step towards overcoming them. J Natl Cancer Inst 2015;108:pii:djv345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin JS, Bowles EJA, Williams SB, Morrison CC. Screening for thyroid cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(18):1888-1903. [DOI] [PubMed] [Google Scholar]
- 35. Deshmukh A, Gangiti K, Pantvaidya G, et al. Surgical outcomes of thyroid cancer patients in a tertiary cancer center in India. Indian J Cancer. 2018;55(1):23-32. [DOI] [PubMed] [Google Scholar]
- 36. Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020; In press (PII: S2213-8587(20)30115–7). [DOI] [PubMed] [Google Scholar]
- 37. Furuya-Kanamori L, Sedrakyan A, Onitilo AA, Bagheri N, Glasziou P, Doi SAR. Differentiated thyroid cancer: millions spent with no tangible gain? Endocr Relat Cancer. 2018;25(1):51-57. [DOI] [PubMed] [Google Scholar]