Abstract
Context
Severe hypothyroidism has profound effects on lipoprotein metabolism including high-density lipoprotein (HDL) cholesterol elevations but effects on HDL function metrics are unknown.
Objective
To determine the impact of severe short-term hypothyroidism on HDL particle characteristics, HDL cholesterol efflux capacity (CEC), and HDL antioxidative capacity.
Design
Observational study with variables measured during severe short-term hypothyroidism (median TSH 81 mU/L) and after 20 weeks of thyroid hormone supplementation (median TSH 0.03 mU/L) (Netherlands Trial Registry ID 7228).
Setting
University hospital setting in The Netherlands.
Patients
Seventeen patients who had undergone a total thyroidectomy for differentiated thyroid carcinoma.
Main outcome measures
HDL particle characteristics (nuclear magnetic resonance spectrometry), CEC (human THP-1-derived macrophage foam cells and apolipoprotein B-depleted plasma), and HDL anti-oxidative capacity (inhibition of low-density lipoprotein oxidation).
Results
During hypothyroidism plasma total cholesterol, HDL cholesterol and apolipoprotein A-I were increased (P ≤ 0.001). HDL particle concentration was unchanged, but there was a shift in HDL subclasses toward larger HDL particles (P < 0.001). CEC was decreased (P = 0.035), also when corrected for HDL cholesterol (P < 0.001) or HDL particle concentration (P = 0.011). HDL antioxidative capacity did not change.
Conclusion
During severe short-term hypothyroidism CEC, an important antiatherogenic metric of HDL function, is impaired. HDL cholesterol and larger HDL particles are increased but HDL particle concentration is unchanged. Combined, these findings suggest that HDL quality and quantity are not improved, reflecting dysfunctional HDL in hypothyroidism.
Keywords: cholesterol efflux capacity, HDL ant-oxidative capacity, HDL cholesterol, HDL subfractions, hypothyroidism, nuclear magnetic resonance
Overt hypothyroidism is a well-established risk factor for atherosclerotic cardiovascular disease (ASCVD) (1, 2), which is in part attributable to profound effects on lipoprotein metabolism (3, 4). Hypothyroidism results in hypercholesterolemia, which is mainly due to an increase in low-density lipoprotein (LDL) cholesterol, coinciding with plasma lipoprotein(a) (Lp(a)) and proprotein convertase subtilisin/kexin type 9 elevations (1, 5, 6). Remarkably, high-density lipoprotein (HDL) cholesterol has been repeatedly demonstrated to be elevated in severe hypothyroidism as well, even though triglycerides are higher (7-10). In view of the inverse association of HDL cholesterol with cardiovascular morbidity (11-13), this raises the question whether the high HDL cholesterol levels could oppose the deleterious effect of LDL cholesterol elevations on ASCVD risk in hypothyroidism.
During the past few years, the causal role of HDL cholesterol as a cardioprotective lipid biomarker has been questioned, leading to the concept that assessing the functional metrics of HDL may provide more insight into the antiatherogenic properties of this lipoprotein fraction than measurement of HDL cholesterol per se (14-16). HDL is able to promote cellular cholesterol efflux, an early step in the atheroprotective reverse cholesterol transport pathway, whereby cholesterol is transported from macrophage foam cells in the vascular wall to the liver for metabolism and excretion into the bile (14, 16). Indeed, several population-based studies have shown that the so-called cholesterol efflux capacity (CEC) predicts incident ASCVD, even independent and irrespective of the plasma HDL cholesterol concentration (17-19). Cross-sectionally, CEC is impaired in metabolic syndrome (20), whereas hyperglycemia, particularly in the context of low-normal free T4 (fT4) levels, may confer a lower antioxidative capacity of HDL, another important metric of HDL function (21, 22).
HDL comprises a heterogenous group of particles of different sizes, structures, and compositions (23, 24). A novel high throughput nuclear magnetic resonance (NMR) spectrometry-based technique has enabled the determination of various plasma HDL subfractions and the HDL particle concentration with good precision and accuracy (25). Using such an NMR spectrometry-based technique, a recent meta-analysis involving individual level participant data in a pooled cohort of 4 population studies showed that the HDL particle concentration was inversely associated with incident myocardial infarction and ischemic stroke even after adjustment for HDL cholesterol (26). This association was similar in women and men (26). Moreover, it has been found that HDL particle concentration is a better predictor of ASCVD than HDL cholesterol in the context of statin therapy (13, 27).
A study in hypothyroid rats has shown that ATP-binding cassette transporter A1 (ABCA1)-mediated CEC is improved in response to liothyronine administration (28). No such effect was found for scavenger receptor class B type I (SR-BI) or ATP-binding cassette transporter G1 (ABCG1)-mediated CEC (28, 29). In addition, 1 study in humans showed that CEC, assayed using THP-1 macrophages, is reversibly decreased in hypothyroidism (30), but there are no data on the effect of overt hypothyroidism on NMR-based HDL particle characteristics.
The present study was initiated to determine the effect of overt hypothyroidism and the response to thyroid hormone supplementation on CEC, the HDL antioxidative capacity and NMR-measured HDL particle characteristics in patients who had undergone a total thyroidectomy for differentiated thyroid carcinoma (DTC).
Patients and Methods
The present observational study was performed among patients with DTC. Our treatment protocol followed Dutch guidelines of DTC treatment (31). Newly diagnosed DTC patients (18 to 75 years old) were eligible to participate in the study. Pregnant women and patients with cerebrovascular/coronary events, atrial fibrillation, or heart failure were not allowed to participate. No subjects with suspected distant metastases were included. The study was approved by the Medical Ethics Committee of the University Medical Center Groningen (registration number 2015/116). This study was registered at the Netherlands Trial Register (NTR ID 7228). All participants gave written informed consent. Inclusion of participants was from October 2016 until August 2018.
This study comprised 2 outpatient study visits. The first visit took place under circumstances of profound short-term hypothyroidism (i.e., 4 to 6 weeks after total or completion thyroidectomy, the latter when a hemithyroidectomy was performed initially) at the day before ablative radioactive iodine treatment. This procedure was performed at high endogenous TSH levels to enhance uptake of radioactive iodine in any remaining thyroid tissue. Shortly after radioactive iodine treatment, liothyronine (n = 13; 75 µg daily) or levothyroxine (n = 4; 150-200 µg daily) supplementation was started, aimed to achieve TSH suppression. All study measurements were repeated after 20 weeks of thyroid hormone supplementation. Patients were advised by a dietician not to change the diet cholesterol content starting 5 days before each study visit. At both study visits, patients were studied after an overnight fast. Height (m), weight (kg), blood pressure (mm Hg), and pulse rate (beats per minute [bpm]) were measured. Body mass index was calculated as weight divided by height squared (kg/m2).
Laboratory methods
Venous blood was drawn after an overnight fast. Plasma and serum samples were obtained by centrifugation at 1400g for 15 minutes at 4°C. EDTA-anticoagulated venous blood samples were kept frozen at –80°C until analysis.
TSH and fT4 were assayed in serum using the Roche Modular E170 Analyzer (Roche Diagnostics, Mannheim, Germany).
EDTA plasma samples were sent frozen to LabCorp for testing. Lipoprotein parameters were measured by 1H-NMR spectroscopy using a Vantera NMR Clinical Analyzer (LabCorp, Morrisville, PA) (25, 32). Plasma total cholesterol, HDL cholesterol, and triglycerides were also measured on the NMR platform. Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL cholesterol. HDL particles and HDL subclasses (small, medium, and large) were quantified using the amplitudes of their spectroscopically distinct lipid methyl group NMR signals (25). HDL size was calculated using the weighted averages derived from the sum of the diameters of each subclass multiplied by its relative mass percentage. Total HDL particle numbers were calculated by the sums of the concentrations of the respective subclasses. Estimated ranges of particle diameter for the subclasses were as follows: large HDL, 10.3 to 12.0 nm; medium HDL, 8.7 to 9.5 nm; and small HDL, 7.4 to 7.8 nm. All lipoprotein parameters were measured using an optimized version (LP4 algorithm) of NMR LipoProfile Test (33). Estimated diameters for the HDL subspecies were as follows: H7P, 12.0 nm; H6P, 10.8 nm; H5P, 10.3 nm; H4P, 9.5 nm; H3P, 8.7 nm; H2P, 7.8 nm; and H1P, 7.4 nm. Linear regression of the HDL subclass signal areas against serum apolipoprotein A-1 (apoA-1) levels measured chemically in a large reference range study population (n = 698) provided the conversion factor to generate NMR-derived concentrations of apoA-1. NMR-derived concentrations of apoA-1 are highly correlated (r ≥ 0.95) with values reported using a standard chemistry method.
CEC was measured as described previously (19, 20, 34). Briefly, THP-1 human monocytes (ATTC via LCG Promochem, Teddington, UK) were differentiated into macrophages and loaded with 50 μg of acetylated LDL per milliliter and 1 μCi/mL 3H-cholesterol (PerkinElmer, Boston, MA) for 24 hours. Following overnight equilibration in Roswell Park Memorial Institute 1640 Glutamax medium (penicillin 100 U/mL/streptomycin 100 μg/mL) containing 2% BSA (Sigma-Aldrich) cells were thoroughly washed with PBS and 2% apoB-depleted individual patient plasma was added in Roswell Park Memorial Institute 1640 Glutamax medium containing penicillin/streptomycin. ApoB-depleted plasma was obtained by precipitation with polyethylene glycol-6000 17-19). After 5 hours, the medium was collected and centrifuged in a table-top centrifuge for 5 minutes at 10 000rpm to pellet cellular debris. Effluxed cholesterol label was determined in an aliquot of medium by liquid scintillation counting (Packard 1600CA Tri-Carb, Packard, Meriden, CT). Meanwhile, the cells were incubated for at least 30 minutes with 0.1 M NaOH at room temperature, whereupon the radioactivity remaining within the cells was measured. Efflux per well is expressed as the percentage of counts (desintegrations per minute) released into the medium related to the total dose of radioactivity initially present (counts recovered within the medium added to the counts recovered from the cells). Values obtained from control cells without added apoB-depleted patient plasma were subtracted to correct for unspecific efflux. CEC measurements were carried out in all respective patient samples in duplicate at the same time to limit potential variation due to different assay conditions. To correct for potential plate-to-plate variation, pooled apoB-depleted control plasma was included on each plate at 4 different concentrations. Presented data were further normalized to the mean values for efflux obtained with control apoB-depleted plasma. CEC was expressed in arbitrary units (AU).
HDL antioxidative function was determined as previously published (33). For the antioxidation assay, 2% of individual HDL preparations isolated as detailed previously were added to native, unoxidized LDL particles (100 mg/dL final protein concentration), after which oxidation was induced by 2.5 mM 2,20-azobis [2-amidinopropane] dihydrochloride followed by incubation at 37°C for 10 hours. Thereafter, thiobarbituric acid reactive substances were determined as a measure for the degree of LDL oxidation as detailed previously (35). The HDL antioxidative capacity was calculated as the percent reduction in thiobarbituric acid reactive substance formation obtained with an individual HDL sample relative to a reaction to which only LDL and 2,20-azobis [2-amidinopropane] dihydrochloride but no HDL had been added (35). Lower values thus indicate poorer protection against LDL oxidation. The antioxidation assay for all samples was carried out at the same time and with the same badges of reagents to limit potential variation due to different assay conditions.
Statistical analysis
SPSS 23 (version 23.0, IBM Corp., Armonk, NY) was used for data analysis. Data were presented as the median (interquartile range [IQR]), and categorical data in numbers and percentages. Changes in variables between hypothyroidism and thyroid hormone supplementation were determined by paired samples Wilcoxon signed-rank tests. Two-sided P values < 0.05 were considered significant.
Results
Seventeen participants were included in the present study. Their median age was 46 (IQR 36, 50) years. Sixteen participants were women (94%). Fourteen participants (82%) were diagnosed with papillary thyroid carcinoma, of which 7 had lymph node metastases; the other 3 (18%) were diagnosed with follicular thyroid carcinoma. None of the participants had distant metastases. The most important medication prescription included insulin for type 1 diabetes in one participant, prednisolone 5 mg daily in another participant, previously diagnosed with polymyalgia rheumatica (dose unchanged during follow-up), and hormonal contraceptives in 8 women. One subject used a statin that was continued during the study. None of the participants were current smokers. Nine participants (53%) consumed alcohol; the average weekly intake of alcohol varied between 0.25 and 8 drinks and was unchanged during the follow-up period.
Median TSH was 81.0 (IQR 67.0, 120.5) mU/L and fT4 was 2.3 (IQR 1.7, 3.5) pmol/L in the hypothyroid condition. TSH had decreased to 0.03 (IQR 0.01, 0.14) mU/L during thyroid hormone supplementation (P < 0.001). Pulse rate was 66 (IQR 60, 70) bpm during hypothyroidism and increased to 76 (IQR 71, 80) bpm during thyroid hormone treatment (P = 0.016), whereas body mass index was decreased from 26.5 (IQR 24.8, 29.1) kg/m2 during hypothyroidism to 25.8 (IQR 24.1, 28.5) kg/m2 during thyroid hormone supplementation (P = 0.014). As shown in Table 1, thyroid hormone supplementation resulted in considerable decreases in plasma total cholesterol, non-HDL cholesterol, LDL cholesterol, and triglycerides compared with the values during hypothyroidism. HDL cholesterol and apoA-1 were decreased during thyroid hormone supplementation, but the total HDL particle concentration remained unchanged. There was a size shift in HDL from larger toward more medium sized HDL particles in response to thyroid hormone supplementation. These changes were mirrored by a decrease in average HDL size during thyroid hormone supplementation.
Table 1.
Plasma Lipids, HDL Parameters, CEC, Anti-Inflammatory Function, and Antioxidant Function During Hypothyroidism and During TH Supplementation in 17 Patients with Differentiated Thyroid Carcinoma
| Parameter | Hypothyroidism | Thyroid Hormone Supplementation | P Value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 248.0 (216.5, 288.5) | 152.0 (140, 167.5) | <0.001 |
| Non-HDL cholesterol (mg/dL) | 180.0 (148.5, 218.0) | 99.0 (83.0, 109.5) | <0.001 |
| LDL cholesterol (mg/dL) | 138.0 (120.5, 169.5) | 79 (64.5, 92.0) | <0.001 |
| HDL cholesterol (mg/dL) | 68.0 (57.5, 85.5) | 57.0 (45.0, 66.0) | 0.001 |
| Triglyceride (mg/dL) | 130.0 (99.5, 175.0) | 83.0 (73.5, 116.0) | <0.001 |
| ApoA-1 (mg/dL) | 156.0 (136.0, 181.0) | 144.0 (133.0, 161.0) | 0.007 |
| Total HDLP (µmol/L) | 23.3 (21.4, 25.5) | 23.2 (21.7, 24.8) | 0.948 |
| Large HDLP (µmol/L) | 4.2 (2.6, 6.4) | 1.0 (1.6 ,2.9) | <0.001 |
| Medium HDLP (µmol/L) | 5.1 (3.3, 5.5) | 6.6 (5.5, 8.8) | <0.001 |
| Small HDLP (µmol/L) | 14.4 (13.3, 15.9) | 13.2 (12.3, 16.3) | 0.463 |
| H7P (µmol/L) | 1.0 (0.5, 1.5) | 0.5 (0.2, 0.8) | 0.001 |
| H6P (µmol/L) | 2.1 (0.8, 4.1) | 0.6 (0.3, 1.2) | 0.001 |
| H5P (µmol/L) | 0.8 (0.2, 1.0) | 0.4 (0.2, 0.8) | 0.255 |
| H4P (µmol/L) | 1.1 (0.9, 1.7) | 2.3 (1.3, 3.3) | 0.008 |
| H3P (µmol/L) | 3.2 (2.3, 4.5) | 5.6 (3.1, 6.3) | 0.002 |
| H2P (µmol/L) | 11.1 (8.8, 12.4) | 10.8 (8.7, 12.7) | 0.554 |
| H1P (µmol/L) | 3.2 (3.1, 5.1) | 3.3 (1.8, 5.1) | 0.079 |
| HDL size (nm) | 9.6 (9.2, 9.9) | 9.0 (9.3, 9.8) | <0.001 |
| CEC (AU) | 0.90 (0.83, 1.01) | 1.01 (0.93, 1.13) | 0.035 |
| Anti-oxidative capacity (%) | 60.1 (58.3, 63.1) | 61.6 (58.6, 64.1) | 0.332 |
Data in median (interquartile range). Statistical comparisons were done by paired samples Wilcoxon signed-rank tests. Significant changes are indicated in bold.
Abbreviations: ApoA-1, apolipoprotein A-1; CEC: cholesterol efflux capacity; HDL, high-density lipoproteins; HDLP, HDL particles; H7P to H1P, larger to smaller HDL particles (see Methods); LDL, low-density lipoproteins.
In marked contrast to the decrease in HDL cholesterol and apoA-1, CEC was increased during thyroid hormone supplementation (Table 1). Notably, this increase was also evident after correction for either HDL cholesterol (0.0165 [0.0146, 0.0219] vs. 0.0133 [0.0108, 0.0156] AU/[mmol/L], P < 0.001) or the HDL particle concentration (0.0416 [0.0375, 0.0506] vs. 0.0380 [0.0350, 0.0447] AU/[µmol/L], P = 0.011). CEC values during hypothyroidism and in response to thyroid hormone supplementation are shown in Fig. 1. The antioxidant function of HDL did not change in response to thyroid hormone supplementation (Table 1).
Figure 1.
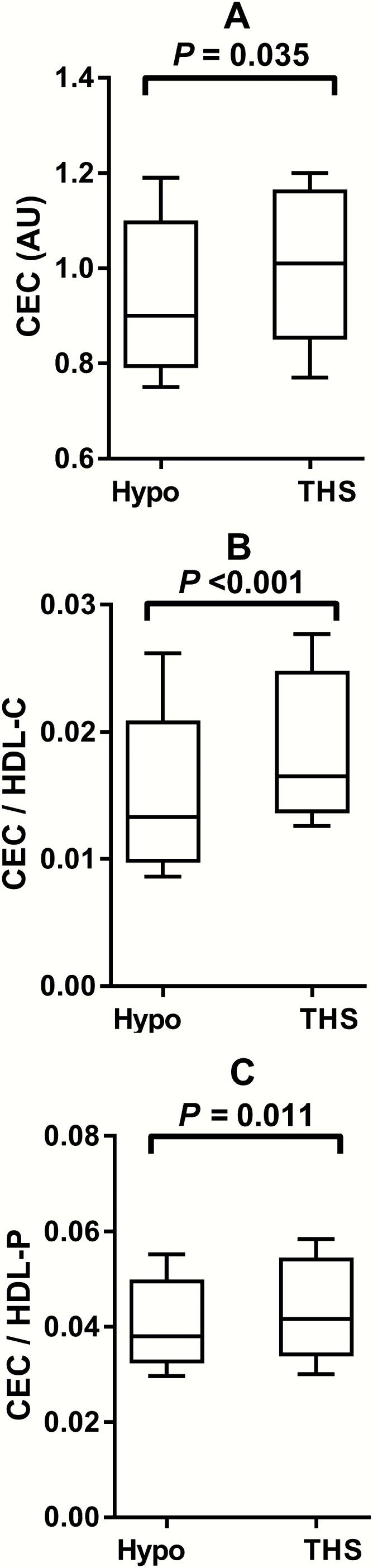
Cholesterol efflux capacity (CEC) during hypothyroidism (Hypo) and during thyroid hormone supplementation (THS) in 17 patients with differentiated thyroid carcinoma. The median, interquartile range, and total range are shown as boxplots. (A) CEC (AU), (B) CEC after correction for high density lipoprotein cholesterol (HDL-C), (C) CEC after correction for HDL particle (HDL-P) concentration.
No significant differences in changes in HDL cholesterol (P = 0.60), the HDL particle concentration (P = 0.88), CEC (P = 0.80), and the HDL antioxidative capacity (P = 0.92) were found between women using hormonal contraceptives (n = 8) and those who did not (n = 8) (data not shown).
Discussion
The present study demonstrates in a limited number of patients that short-term severe hypothyroidism leads to an impaired CEC, an important metric of HDL function. Of note, this decrease in CEC was also observed after correction for either the HDL cholesterol or HDL particle concentration. Furthermore, large HDL particles were increased, whereas the HDL particle concentration, measured by NMR spectroscopy, remained unchanged. Combined, these data suggest that HDL quality and quantity are not improved in hypothyroidism. Our findings therefore reflect that HDL likely becomes dysfunctional in the hypothyroid state, suggesting that the higher HDL cholesterol levels observed as such may not translate into an amelioration of the increased ASCVD risk conferred by the elevations of apoB-containing lipoproteins. The magnitude of the observed changes in CEC may be clinically relevant, particularly in view of our recent longitudinal findings showing that CEC averaged 0.93 AU in subjects who developed ASCVD compared with 1.01 AU in subjects who remained free of clinically manifest ASCVD during 12 years of follow-up (19).
It is commonly appreciated that the ability of cells to promote cholesterol efflux to extracellular acceptors is driven by ABCA1-, ABCG1-, and SR-BI-mediated pathways as well as by aqueous diffusion (14, 16, 23, 36). However, there is no consensus about which cell system to use, or with respect to the use of plasma or serum to determine CEC in individual patient samples. In the current study, we used THP-1-derived macrophage foam cells, generated by loading them with modified LDL. In this cell system, ABCA1, ABCG1, and SR-BI are all functional (19, 20). As in our previous studies, we used EDTA plasma samples obtained in the fasting state (19, 20, 34). The advantage of EDTA plasma over serum is that for serum preparation incubation at room temperature is required subsequent to blood drawing. This likely results in remodeling of HDL, particularly of lipid poor pre β-HDL particles, which interact with macrophages via the ABCA1 pathway (16). Moreover, we used apoB-depleted plasma, a widely accepted HDL preparation method for efflux assays (13, 17, 18, 34), in which CEC depends to >90% on the presence of HDL (19). The use of a standardized amount of patient plasma allowed us to calculate HDL cholesterol- and HDL particle-adjusted CEC values to better delineate functional HDL abnormalities in the setting of hypothyroidism. Hence, it is relevant that the decrease in CEC during hypothyroidism remained significant after adjustment for HDL cholesterol and HDL particle concentration. Notably, CEC has been observed to be inversely associated with ASCVD in several prospective and cross-sectional studies (17-20). Therefore, it seems plausible that impaired CEC in overt hypothyroidism, could translate into elevated ASCVD risk. It should be noted, however, that CEC may not relate to (subclinical) atherosclerosis in specific patient categories such as those with (pre)diabetes, renal transplant recipients and diabetic patients on hemodialysis (35-37). Therefore, the impact of decreased CEC on ASCVD in overt hypothyroidism needs to be tested in longitudinal studies.
The present study was not designed to extensively explore mechanisms responsible for the impaired CEC during hypothyroidism. The ABCA1 pathway is important to explain hypothyroidism-induced alterations in HDL function as reported in rat studies (29). If this also holds true for the human hypothyroid condition, then it seems plausible that changes in pre β-HDL, a lipid-poor nascent HDL particle that interacts with the ABCA1 receptor, play a role in hypothyroidism-induced CEC impairment. In this regard, it is noteworthy that pre-β-HDL formation correlates positively with high-normal fT4 levels in humans (40). Furthermore, we observed a shift toward larger, more cholesterol-containing HDL particles during hypothyroidism as expected (7, 8). In fact, HDL cholesterol was increased during hypothyroidism without a change in the HDL particle concentration. Such cholesterol-rich HDL particles in hypothyroidism are likely the result of lowering of cholesteryl ester transfer protein activity (9, 41) and could conceivably negatively impact on CEC (42). Notably, low-grade chronic inflammation also exerts a negative effect on CEC (20, 24). In this regard, it is relevant that overt hypothyroidism may confer higher levels of C-reactive protein and other inflammatory biomarkers (1). Finally, recent findings suggest that ABCA1-mediated CEC is impaired by Lp(a), which is reversibly elevated in overt hypothyroidism (5, 43). Because we used apoB-depleted plasma to determine CEC, it seems unlikely that Lp(a) effects could to an important extent explain impaired CEC during hypothyroidism.
Another not-yet-reported finding is that the antioxidative function of HDL remained unchanged during overt hypothyroidism, although this metric of HDL function correlates inversely with fT4 among euthyroid subjects (22). Paraoxonase-1 (PON-1) is an important anti-oxidative enzyme that resides on HDL, and is associated inversely with incident ASCVD (44). Using the same assay to measure PON-1 activity, we have documented that serum PON-1 activity is a determinant of the HDL antioxidative function (21). Recent reports showed that PON-1 activity remains unaltered during overt hypothyroidism (10), but was decreased when expressed as the PON-1/apoA-I ratio (30). Moreover, in euthyroid subjects, PON-1 is more closely related to HDL particle concentration than to HDL cholesterol (45). Altogether, the lack of effect of overt hypothyroidism on PON-1 activity (10), as well as on the HDL particle number (present study), may help to explain why the HDL antioxidative function was unaffected in the hypothyroid state.
Several other methodological aspects and limitations of our study need to be ascertained. First, this study was carried out in the context of Dutch guidelines to manage and treat DTC patients (31). We took advantage of postthyroidectomy status without thyroid hormone replacement to assess the effects of short-term profound hypothyroidism on the HDL biomarkers reported here. As a consequence, it was not possible to include a control group of DTC patients or of other hypothyroid patients observed during a prolonged period of overt untreated hypothyroidism. Furthermore, it should be appreciated that TSH levels were low during thyroid hormone supplementation, indicating (mild) overreplacement. Whether this could to some extent have affected the magnitude of changes in CEC is unknown. In this context, it may be relevant that CEC did not fully recover after reversal of overt hypothyroidism in another study (30), and that the anti-inflammatory function of HDL is positively correlated with fT4 levels within the euthyroid reference range (46). The use of hormonal contraceptives apparently did not affect the effects of hypothyroidism on HDL cholesterol, the HDL particle concentration, CEC, and the antioxidative capacity of HDL. Because most patients participating in the present study were women, our findings do not necessarily hold true for men. Although our conclusions with respect to HDL quality and quantity appear to be robust, our study was carried out in a rather small number of DTC patients as in other reports (9, 10).
In conclusion, using total thyroidectomy as a condition of severe short-term hypothyroidism in humans, it was observed that HDL function, in particular CEC, is impaired. The plasma HDL particle concentration remained unaltered despite HDL cholesterol elevations during hypothyroidism. In view of the inverse associations of CEC, as well as of the HDL particle concentration with ASCVD, we surmise that the increase in HDL cholesterol does not sufficiently counteract the deleterious effects of LDL cholesterol elevations and other pro-atherogenic biomarkers on overt hypothyroidism-related ASCVD risk.
Acknowledgments
Lipids and lipoproteins were measured at LabCorp, Morrisville USA at no costs. The methodological support of Prof. F. Kuipers, Department of Pediatrics and Department of Laboratory Medicine, University Medical Center Groningen, University of Groningen, is highly appreciated.
Financial Support: U.J.F.T. received funding from The Netherlands Organization for Scientific Research (NWO, VIDI grant 917-56-358, to U.J.F.T.). C.J. was supported by a fellowship from the Chinese Scholarship Council.
Clinical Trial Information: NTR ID 7228, 20-08-2018.
Author Contributions : Conceptualization, R.P.F.D; data curation, T.P.L, J.D.L, C.J.; formal analysis, T.v.d.B., R.P.F.D.; funding acquisition, T.P.L., M.A.C., U.J.F.T.; resources, M.A.C., U.F.J.T; supervision, R.P.F.D., T.P.L., U.F.F.T; writing original draft, R.P.F.D.; writing, reviewing, and editing, T.v.d.B., R.P.F.D., U.J.F.T., T.P.L., J.D.L. M.A.C., C.J. All authors read and approved the final version of the manuscript.
Glossary
Abbreviations
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- apoA-1
apolipoprotein A-1
- ASCVD
atherosclerotic cardiovascular disease
- AU
arbitrary unit
- bpm
beats per minute
- CEC
cholesterol efflux capacity
- DTC
differentiated thyroid carcinoma
- fT4
free T4
- HDL
high-density lipoprotein
- IQR
interquartile range
- LDL
low-density lipoprotein
- Lp(a)
lipoprotein(a)
- NMR
nuclear magnetic resonance
- PON-1
paraoxonase-1
- SR-BI
scavenger receptor class B type I
Additional Information
Disclosure Summary: M.A.C. is an employee of LabCorp, Morrisville USA. The other authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88(6):2438-2444. [DOI] [PubMed] [Google Scholar]
- 2. Biondi B, Klein I. Hypothyroidism as a risk factor for cardiovascular disease. Endocrine. 2004;24(1):1-13. [DOI] [PubMed] [Google Scholar]
- 3. Heimberg M, Olubadewo JO, Wilcox HG. Plasma lipoproteins and regulation of hepatic metabolism of fatty acids in altered thyroid states. Endocr Rev. 1985;6(4):590-607. [DOI] [PubMed] [Google Scholar]
- 4. Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am. 2012;96(2):269-281. [DOI] [PubMed] [Google Scholar]
- 5. Dullaart RPF. Lipoprotein(a): the renaissance of an enigmatic lipoprotein. J Clin Endocrinol Meta. 2020; 105(3):896-898. [DOI] [PubMed] [Google Scholar]
- 6. Gong Y, Ma Y, Ye Z, et al. Thyroid stimulating hormone exhibits the impact on LDLR/LDL-c via up-regulating hepatic PCSK9 expression. Metabolism. 2017;76:32-41. [DOI] [PubMed] [Google Scholar]
- 7. Muls E, Rosseneu M, Blaton V, Lesaffre E, Lamberigts G, de Moor P. Serum lipids and apolipoproteins A-I, A-II and B in primary hypothyroidism before and during treatment. Eur J Clin Invest. 1984;14(1):12-15. [DOI] [PubMed] [Google Scholar]
- 8. Muls E, Rosseneu M, Lamberigts G, de Moor P. Changes in distribution and composition of high-density lipoproteins in primary hypothyroidism. Metabolism. 1985;34(4):345-353. [DOI] [PubMed] [Google Scholar]
- 9. Dullaart RP, Hoogenberg K, Groener JE, Dikkeschei LD, Erkelens DW, Doorenbos H. The activity of cholesteryl ester transfer protein is decreased in hypothyroidism: a possible contribution to alterations in high-density lipoproteins. Eur J Clin Invest. 1990;20(6):581-587. [DOI] [PubMed] [Google Scholar]
- 10. Sigal GA, Tavoni TM, Silva BMO, Kalil Filho R, Brandão LG, Maranhão RC. Effects of short-term hypothyroidism on the lipid transfer to high-density lipoprotein and other parameters related to lipoprotein metabolism in patients submitted to thyroidectomy for thyroid cancer. Thyroid. 2019;29(1):53-58. [DOI] [PubMed] [Google Scholar]
- 11. The Emerging Risk Factors Collaboration. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappelle PJ, Gansevoort RT, Hillege JL, Wolffenbuttel BH, Dullaart RP; PREVEND study group . Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J Intern Med. 2011;269(2):232-242. [DOI] [PubMed] [Google Scholar]
- 13. Khera AV, Demler OV, Adelman SJ, et al. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin). Circulation. 2017;135(25):2494-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenson RS, Brewer HB Jr, Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15):1905-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenson RS, Brewer HB Jr, Barter PJ, et al. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat Rev Cardiol. 2018;15(1):9-19. [DOI] [PubMed] [Google Scholar]
- 16. Triolo M, Annema W, Dullaart RP, Tietge UJ. Assessing the functional properties of high-density lipoproteins: an emerging concept in cardiovascular research. Biomark Med. 2013;7(3):457-472. [DOI] [PubMed] [Google Scholar]
- 17. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saleheen D, Scott R, Javad S, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. HDL (high-density lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. 2019;39(9):1874-1883. [DOI] [PubMed] [Google Scholar]
- 20. Annema W, Dikkers A, de Boer JF, et al. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: the CODAM study. Sci Rep. 2016;6:27367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kappelle PJ, de Boer JF, Perton FG, et al. Increased LCAT activity and hyperglycaemia decrease the antioxidative functionality of HDL. Eur J Clin Invest. 2012;42(5):487-495. [DOI] [PubMed] [Google Scholar]
- 22. Triolo M, de Boer JF, Annema W, Kwakernaak AJ, Tietge UJ, Dullaart RP. Low normal free T4 confers decreased high-density lipoprotein antioxidative functionality in the context of hyperglycaemia. Clin Endocrinol (Oxf). 2013;79(3):416-423. [DOI] [PubMed] [Google Scholar]
- 23. Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21(3):229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ronsein GE, Vaisar T. Inflammation, remodeling, and other factors affecting HDL cholesterol efflux. Curr Opin Lipidol. 2017;28(1):52-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matyus SP, Braun PJ, Wolak-Dinsmore J, et al. HDL particle number measured on the Vantera®, the first clinical NMR analyzer. Clin Biochem. 2015;48(3):148-155. [DOI] [PubMed] [Google Scholar]
- 26. Singh K, Chandra A, Sperry T, et al. Associations between HDL particles and ischemic events by vascular domain gender, and ethnicity: a pooled cohort analysis. Circulation. 2020. doi: 10.1161/CIRCULATIONAHA.120.045713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128(11):1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boone LR, Lagor WR, Moya Mde L, Niesen MI, Rothblat GH, Ness GC. Thyroid hormone enhances the ability of serum to accept cellular cholesterol via the ABCA1 transporter. Atherosclerosis. 2011;218(1):77-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Franco M, Castro G, Romero L, et al. Decreased activity of lecithin:cholesterol acyltransferase and hepatic lipase in chronic hypothyroid rats: implications for reverse cholesterol transport. Mol Cell Biochem. 2003;246(1-2):51-56. [PubMed] [Google Scholar]
- 30. Jung KY, Ahn HY, Han SK, Park YJ, Cho BY, Moon MK. Association between thyroid function and lipid profiles, apolipoproteins, and high-density lipoprotein function. J Clin Lipidol. 2017;11(6):1347-1353. [DOI] [PubMed] [Google Scholar]
- 31. Schildkliercarcinoom W richtlijn. National guideline thyroid carcinoma. Oncoline. 2015. Available at: http://oncoline.nl/schildkliercarcinoom. Accessed June1, 2020.
- 32. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26(4):847-870. [DOI] [PubMed] [Google Scholar]
- 33. Berends AMA, Buitenwerf E, Gruppen EG, et al. Primary aldosteronism is associated with decreased low-density and high-density lipoprotein particle concentrations and increased GlycA, a pro-inflammatory glycoprotein biomarker. Clin Endocrinol (Oxf). 2019;90(1):79-87. [DOI] [PubMed] [Google Scholar]
- 34. Annema W, Willemsen HM, de Boer JF, et al. HDL function is impaired in acute myocardial infarction independent of plasma HDL cholesterol levels. J Clin Lipidol. 2016;10(6): 1318-1328. [DOI] [PubMed] [Google Scholar]
- 35. Leberkühne LJ, Ebtehaj S, Dimova LG, et al. The predictive value of the antioxidative function of HDL for cardiovascular disease and graft failure in renal transplant recipients. Atherosclerosis. 2016;249:181-185. [DOI] [PubMed] [Google Scholar]
- 36. Talbot CPJ, Plat J, Ritsch A, Mensink RP. Determinants of cholesterol efflux capacity in humans. Prog Lipid Res. 2018;69:21-32. [DOI] [PubMed] [Google Scholar]
- 37. Josefs T, Wouters K, Tietge UJF, et al. High-density lipoprotein cholesterol efflux capacity is not associated with atherosclerosis and prevalence of cardiovascular outcome: the CODAM study. J Clin Lipidol. 2020;14(1):122-132.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Annema W, Dikkers A, de Boer JF, et al. HDL cholesterol efflux predicts graft failure in renal transplant recipients. J Am Soc Nephrol. 2016;27(2):595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kopecky C, Ebtehaj S, Genser B, et al. HDL cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. J Am Soc Nephrol. 2017;28(3):769-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Tienhoven-Wind LJ, Perton FG, Dullaart RP. Pre-β-HDL formation relates to high-normal free thyroxine in type 2 diabetes mellitus. Clin Biochem. 2016;49(1-2):41-46. [DOI] [PubMed] [Google Scholar]
- 41. Tan KC, Shiu SW, Kung AW. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1998;83(1):140-143. [DOI] [PubMed] [Google Scholar]
- 42. Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116(7):1133-1142. [DOI] [PubMed] [Google Scholar]
- 43. Tavori H, Fenton AM, Plubell DL, et al. Elevated lipoprotein(a) levels lower ABCA1 cholesterol efflux capacity. J Clin Endocrinol Metab. 2019;104(10):4793-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kunutsor SK, Bakker SJ, James RW, Dullaart RP. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: the PREVEND study and meta-analysis of prospective population studies. Atherosclerosis. 2016;245:143-154. [DOI] [PubMed] [Google Scholar]
- 45. Dullaart RP, Otvos JD, James RW. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in type 2 diabetic and non-diabetic subjects. Clin Biochem. 2014;47(12):1022-1027. [DOI] [PubMed] [Google Scholar]
- 46. van Tienhoven-Wind LJN, Tietge UJF, Dullaart RPF. The HDL anti-inflammatory function is impaired in the context of low-normal free thyroxine in diabetic and nondiabetic individuals. Clin Endocrinol (Oxf). 2018;88(5):752-754. [DOI] [PubMed] [Google Scholar]


