Abstract
Background
A vaccine would be an ideal tool for reducing malaria’s impact. PfSPZ Vaccine (radiation attenuated, aseptic, purified, cryopreserved Plasmodium falciparum [Pf] sporozoites [SPZ]) has been well tolerated and safe in >1526 malaria-naive and experienced 6-month to 65-year-olds in the United States, Europe, and Africa. When vaccine efficacy (VE) of 5 doses of 2.7 × 105 PfSPZ of PfSPZ Vaccine was assessed in adults against controlled human malaria infection (CHMI) in the United States and Tanzania and intense field transmission of heterogeneous Pf in Mali, Tanzanians had the lowest VE (20%).
Methods
To increase VE in Tanzania, we increased PfSPZ/dose (9 × 105 or 1.8 × 106) and decreased numbers of doses to 3 at 8-week intervals in a double blind, placebo-controlled trial.
Results
All 22 CHMIs in controls resulted in parasitemia by quantitative polymerase chain reaction. For the 9 × 105 PfSPZ group, VE was 100% (5/5) at 3 or 11 weeks (P < .000l, Barnard test, 2-tailed). For 1.8 × 106 PfSPZ, VE was 33% (2/6) at 7.5 weeks (P = .028). VE of dosage groups (100% vs 33%) was significantly different (P = .022). Volunteers underwent repeat CHMI at 37–40 weeks after last dose. 6/6 and 5/6 volunteers developed parasitemia, but time to first parasitemia was significantly longer than controls in the 9 × 105 PfSPZ group (10.89 vs 7.80 days) (P = .039), indicating a significant reduction in parasites in the liver. Antibody and T-cell responses were higher in the 1.8 × 106 PfSPZ group.
Conclusions
In Tanzania, increasing the dose from 2.7 × 105 to 9 × 105 PfSPZ increased VE from 20% to 100%, but increasing to 1.8 × 106 PfSPZ significantly reduced VE.
Clinical Trials Registration
Keywords: malaria, Plasmodium falciparum, PfSPZ, vaccine efficacy, controlled human malaria infection
In Tanzania, increasing the total dosage of PfSPZ Vaccine from 1.35 × 106 to 2.7 × 106 PfSPZ, increased vaccine efficacy from 20% to 100%, but increasing the dosage further to 5.4 × 106 PfSPZ was associated with reduction of vaccine efficacy to 33%.
In 2015–2017 there were 429 000–730 500 deaths caused by malaria annually, most by Plasmodium falciparum (Pf) [1–3]. Our goal is to deploy a vaccine that prevents infection with Pf and thereby prevents all manifestations of malaria and halts transmission [4]. Sanaria® PfSPZ Vaccine, composed of radiation-attenuated, aseptic, purified, cryopreserved Pf sporozoites (SPZ), has been well tolerated, safe, and efficacious [5–10].
We are using small trials including controlled human malaria infection (CHMI) to assess vaccine efficacy (VE) to optimize dosage regimens. When the same dosage regimen was assessed against homologous (same parasites in vaccine and challenge) CHMI in adults in the United States and Tanzania, VEs at 3 and 24 weeks against homologous CHMI were 93% and 65% in the United States and 20% and 20% in Tanzania [8, 11]. Protection against heterologous (different strain of Pf in vaccine and challenge) CHMI in the United States was 80% and 8% at 3 and 24 weeks [8]. This same PfSPZ Vaccine regimen gave a VE of 52% by time to event and 29% by proportional analysis over 24 weeks against intense transmission of Pf in Mali [10], suggesting homologous CHMI in Tanzanians at 3 weeks and heterologous CHMI in Americans at 24 weeks are more rigorous tests of VE than natural exposure.
A US study demonstrated 54% VE against heterologous CHMI at 33 weeks after 3 doses at 8-week intervals of 9 × 105 PfSPZ [9]. To improve VE in Tanzania, we assessed 3 doses of 9 × 105 or 1.8 × 106 PfSPZ.
METHODS
Study Design
A double-blind, randomized, placebo-controlled trial was conducted in Bagamoyo, Tanzania (December 2015 to March 2017). It had an age de-escalation, dose escalation component to assess safety, and immunogenicity of PfSPZ Vaccine (part A) [12], and a controlled human malaria infection (CHMI) component to assess VE (part B), described herein. For part B, 30 healthy males and females aged 18–45 years were recruited from the Bagamoyo region as described [12]. Eligibility criteria are at https://clinicaltrials.gov/show/NCT02613520.
Investigational Products (IP)
Sanaria® PfSPZ Vaccine [5–10, 13], PfSPZ Challenge [14–19], or normal saline (NS) was prepared in 0.5 mL (supervised by study pharmacist). All were administered by direct venous inoculation (DVI) through a 25-gauge needle.
Immunization
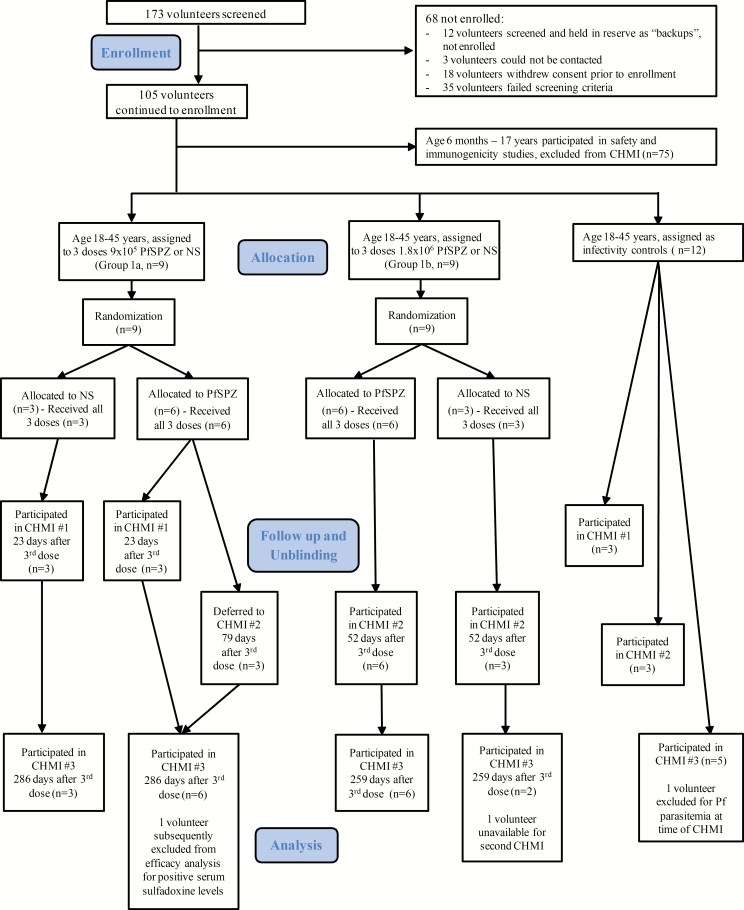
Enrolled participants were sequentially entered into the randomization table by the unblinded study pharmacist. Nine volunteers were allocated to group 1a (9 × 105 PfSPZ) and 9 to group 1b (1.8 × 106 PfSPZ) (Figure 1). Within each group volunteers were randomized to receive PfSPZ Vaccine (n = 6) or NS (n = 3) on days 1, 57, and 113.
Figure 1.
Volunteer participation (CONSORT 2010 Diagram). Abbreviations: CONSORT, Consolidated Standards of Reporting Trials; CHMI, controlled human malaria infection; NS, normal saline; PfSPZ, Plasmodium falciparum sporozoite.
Vaccine Efficacy
CHMI
VE was assessed by CHMI by DVI of 3.2 × 103 PfSPZ of PfSPZ Challenge [11]. CHMIs were planned for 3 and 24 weeks after last vaccine dose; timing was changed for most. Three additional volunteers were enrolled as infectivity controls for each of the first 2 CHMIs and 6 for the third. Volunteers were observed as inpatients beginning on day 9 after CHMI until diagnosed and treated for malaria or until day 21; daily outpatient monitoring for thick blood smear (TBS) negative volunteers continued until day 28. TBSs were made every 12 hours (days 8–14), then daily until positive or until day 28. Each TBS was paired with a sample for quantitative polymerase chain reaction (qPCR); qPCRs were performed retrospectively except when a TBS was positive, in which case qPCR was performed immediately. TBSs could be performed more frequently, if clinically indicated. Posttreatment, TBSs were assessed until 2 consecutive daily TBSs were negative and on day 28.
Adverse Events (AEs) After CHMI
AEs after CHMI were recorded for 5 days (Table S1) [12]. Volunteers were observed as inpatients for 48 hours after administration of PfSPZ Challenge, discharged with AE diaries and thermometers, and followed up with daily telephone calls. Hematological and biochemical parameters were assessed. AEs were assessed for grade and relatedness to IP (Table S5) through prespecified (Table S1) and open-ended questioning. They were considered related to Pf infection if the event was within 3 days prior to and 7 days after TBS was first positive.
Treatment
Malaria diagnosed prior to CHMI was treated with artesunate-amodiaquine (AS-AQ) or artemether-lumefantrine (AL). Volunteers with positive TBSs confirmed by qPCR in the 28-day interval following CHMI were treated with AL. Volunteers who were TBS negative throughout were treated at day 28.
Detection of Pf Parasites and Parasite DNA
TBSs were prepared and read as described [17]. The theoretical lower limits of detection were 2 and 0.5 parasites/µL blood for asymptomatic and symptomatic subjects respectively. qPCR detecting Plasmodium spp. 18S genes had a sensitivity of 0.1 parasites/µL [20]. A second qPCR assay (sensitivity of 0.05 parasites/µL), targeting the Pf specific telomere-associated repetitive element 2 [21] was used to reanalyze samples negative by 18S based qPCR. The time to first positivity by qPCR was the prepatent period.
Drug Levels
Amodiaquine levels were assessed for volunteers who received AS-AQ. Sulfadoxine and lumefantrine levels were assessed for all volunteers in CHMIs no. 1 and no. 2. Plasma samples (−80°C) were shipped to Swiss BioQuant, AG, Reinach, Switzerland for analysis by high performance liquid chromatography coupled to mass spectrometry.
Antibody and T-cell Assays
Methods were previously described [12].
Statistical Analysis
Six vaccinees per dosage group and a minimum of 6 controls for each CHMI was able to show with a power of 80% that a 17% (1/6) Pf infection frequency in vaccinees was different (α = 0.05, 2-tailed) than a 100% (6/6) Pf infection frequency in controls. Categorical variables were summarized using absolute (n) and relative (%) frequencies. Continuous variables were summarized using mean, standard deviation, median, and range. Comparisons of categorical variables were analyzed using Barnard’s 2-sided exact test, and continuous variables by the Mann-Whitney U Test, 2-sided. No corrections were made for multiple comparisons due to the early phase nature of this trial. P < .05 was considered significant.
Study Approval
The protocol was approved by institutional review boards (IRBs) of the Ifakara Health Institute (IHI/IRB/ No: 32–2015), the National Institute for Medical Research Tanzania (NIMR/HQ/R.8a/Vol.IX/2049), and the Ethikkommission Nordwest- und Zentralschweiz (EKNZ), Basel, Switzerland (15/104). The protocol was approved by the Tanzania Food and Drug Authority (TFDA) (TZ15CT013), registered at Clinical Trials.gov (NCT02613520) and conducted under a US Food and Drug Administration (FDA) investigative new drug application. All subjects provided written informed consent.
RESULTS
Twelve vaccinees, 6 NS controls, and 12 added infectivity controls underwent CHMI (Table 1).
Table 1.
Volunteer Characteristics
| Group 1 (18–45 years) | |||||
|---|---|---|---|---|---|
| 9 × 105 (N = 6) | 1.8 × 106 (N = 6) | Placebo (N = 6) | CHMI Controls (N = 12) | ||
| Age (years) | Mean (SD) | 23.5 (5.7) | 24.2 (5.3) | 28.7 (7.9) | 23.9 (4.8) |
| Median | 22 | 24 | 30 | 23 | |
| (Min, Max) | (20,35) | (18,33) | (19,38) | (18,36) | |
| Sex | Male | 4 (66.7%) | 4 (66.7%) | 5 (83.3%) | 7 (58.3%) |
| Female | 2 (33.3%) | 2 (33.3%) | 1 (16.7%) | 5 (41.7%) | |
| Race | African | 6 (100%) | 6 (100%) | 6 (100%) | 12 (100%) |
| Height (cm) | Mean (SD) | 163.2 (5.0) | 166.5 (10.4) | 166.3 (8.0) | 157.1 (9.0) |
| Median | 164.0 | 166.0 | 166.0 | 158.3 | |
| (Min, Max) | (154,168) | (149,178) | (153,175) | (136,167) | |
| Weight (kg) | Mean (SD) | 62.3 (8.1) | 65.8 (11.2) | 64.3 (3.3) | 58.0 (8.4) |
| Median | 62.5 | 67.0 | 65.0 | 57.5 | |
| (Min, Max) | (53,70) | (51,80) | (60,68) | (44,75) | |
| BMI | Mean (SD) | 23.6 (4.1) | 23.7 (2.9) | 23.4 (2.6) | 23.6 (3.8) |
| Median | 23.5 | 23.6 | 23.1 | 23.4 | |
| (Min, Max) | (19,30) | (20,28) | (20,27) | (19,31) |
Abbreviations: BMI, body mass index; CHMI, controlled human malaria infection; SD, standard deviation.
Vaccine Efficacy (VE)
Infections by qPCR in Controls
There were 22 injections of PfSPZ Challenge for CHMI in controls in CHMIs no. 1 (6), no. 2 (6), no. 3 (10) (Table 2). All controls developed parasitemia; the median prepatent periods (time to first parasitemia) of controls were similar (CHMIs no. 1, no. 2, no. 3: 7.99, 7.90, 7.79 days). Thus, for assessing VE statistical significance all 22 infections in controls were used (median: 7.90 days).
Table 2.
Vaccine Efficacy
| CHMI no.1 | CHMI no. 2 | CHMI no. 3 | ||||
|---|---|---|---|---|---|---|
| 9.0 × 105 PfSPZ (n = 3) | 9.0 × 105 PfSPZ (n = 2) | 1.8 × 106 PfSPZ (n = 6) | 9.0 × 105 PfSPZ (n = 6) | 1.8 × 106 PfSPZ (n = 6) | All Controlsa (n = 22) | |
| Which CHMI | First | First | First | Second | Second | Pooled |
| Time from last dose of vaccine to CHMI (days) | 23 | 79 | 52 | 286 | 259 | … |
| qPCR + (n) | 0 | 0 | 4 | 6 | 5 | 22 |
| VE by proportional analysis (P value based on all 22 control infections)b | 100% (0.0001) | 100% (0.001) | 33% (0.028) | 0 | 16.7% (0.119) | … |
| 95% confidence interval for VE by proportional analysis | [38.3, 100] | [29.0, 100] | [9.3, 70.4] | … | [1.1, 58.2] | … |
| Prepatent period by qPCR | ||||||
| median | … | … | 8.43 | 10.89c | 7.78 | 7.90 |
| min, max | … | … | 7.89, 12.18 | 7.79, 17.58 | 7.73, 27.78 | 7.71, 19.59 |
| TBS+ (n) | 0 | 0 | 3 | 5 | 4 | 17 |
| Prepatent period by TBS | … | … | ||||
| median | … | … | 13.92 | 18.59c | 14.30 | 13.64 |
| min, max | … | … | 13.78, 19.71 | 14.09, 22.78 | 12.92, 27.77 | 12.03, 17.80 |
Volunteers were continuously monitored by qPCR until malaria treatment based on TBS positivity. The WHO International Standard for Pf DNA Nucleic Acid Amplification Techniques (NIBSC, Hertfordshire, UK) was used as standard for calculation of parasite densities. DNA was extracted from 100 µL whole blood and eluted with 50 µL Elution Buffer using Quick-gDNA Blood MicroPrep Kit (Zymo Research, Irvine, USA). All TBS negative blood samples were analyzed retrospectively by qPCR after storing at −80°C after the conclusion of CHMIs. To exclude field strain infections, parasite genotyping was performed on samples randomly chosen as described [22]. In all cases in which TBS was negative and qPCR was considered positive, 2 consecutive samples were positive by qPCR.
Abbreviations: CHMI, controlled human malaria infection; PfSPZ, Plasmodium falciparum sporozoite; qPCR, quantitative polymerase chain reaction; TBS, thick blood smear; VE, vaccine efficacy.
a11 normal saline (NS) and 11 infectivity controls.
b P value calculated by Barnard’s test, 2-tailed.
c P = .021 and .03, respectively, Mann-Whitney U test, compared with prepatent periods of pooled controls.
CHMI#1
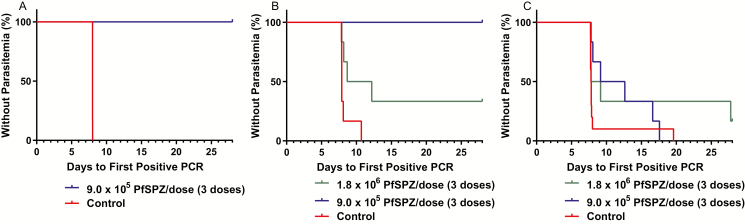
Parasitemia was detected by qPCR 7.98–8.00 days after injection of PfSPZ Challenge in controls (Figure 2A). TBSs were positive in all 6; median time to TBS positivity was 16.79 days (range 12.03–17.80 days). Three volunteers immunized with 9 × 105 PfSPZ were negative for parasitemia by TBS and qPCR 18 days after last dose of PfSPZ Vaccine, and underwent CHMI at 23 days (3.3 weeks) (Table 2, Figure 2A). None developed parasitemia. VE was 100% (Table 2) (P = .0001, Barnard’s exact test, 2-sided).
Figure 2.
Kaplan-Meier survival curves in immunized volunteers vs controls as assessed by qPCR. Kaplan-Meier curves in volunteers undergoing: A, CHMI 23 days after the last of 3 doses with 9.0 × 105 PfSPZ (n = 3) vs NS and infectivity controls (n = 6); B, CHMI 79 days after the last of 3 doses with 9.0 × 105 PfSPZ (n = 2) vs CHMI 52 days after the last of 3 doses of 1.8 × 106 PfSPZ (n = 6) vs NS and infectivity controls (n = 6); C, volunteers undergoing a second CHMI 259 to 286 days after the last dose of 9.0 × 105 PfSPZ (n = 6) or 1.8 × 106 PfSPZ (n = 6) vs NS and infectivity controls (n = 10). Abbreviations: CHMI, controlled human malaria infection; NS, normal saline; PCR, polymerase chain reaction; PfSPZ, Plasmodium falciparum sporozoite.
Three volunteers immunized with 9 × 105 PfSPZ were asymptomatic but positive for Pf (N = 1) or P. malariae (Pm) (N = 2) by qPCR 18 days after last vaccine dose. Retrospective qPCR demonstrated Pm in the volunteers at enrollment 4 months previously (Table S2). The volunteer with Pf was qPCR negative at enrollment but positive (35.3 parasites/μL) prior to second immunization (asymptomatic and TBS negative). Four weeks previously she reported fever for 2 days, was diagnosed with gastroenteritis, and treated with ciprofloxacin and metronidazole. The Pf was genotypically distinct from the Pf in PfSPZ Vaccine (Table S3). To provide time for treatment with AS-AQ (confirmed successful by negative qPCR), CHMI for the 3 was deferred to CHMI no. 2.
CHMI no. 2
Parasitemia was detected by qPCR 7.85–10.69 days after injection of PfSPZ Challenge in controls (Figure 2B). TBSs were positive in 5 of 6 controls a median of 14.12 (range 13.67–17.69) days after injection of PfSPZ Challenge.
Six volunteers immunized with 1.8 × 106 PfSPZ (group 1b) were confirmed negative for parasitemia by TBS and qPCR and underwent CHMI at 52 days after 3rd immunization (Table 2, Figure 2B). Four of 6 group 1b volunteers were positive by qPCR after a median of 8.43 (range 7.89–12.18) days. Three of these 4 positive vaccinees were also positive by TBS after a median of 13.92 (range 13.78–19.71) days. VE based on qPCR positivity at this day 52 CHMI (7.4 weeks) was 33% (Table 2) (P = .028).
CHMI no. 2 included 3 of the 9 × 105 PfSPZ volunteers who had been positive for Pf or Pm by qPCR at time of first CHMI and treated with AS-AQ. None of the 3 volunteers had detectable AQ (amodiaquine) 58 days after treatment was completed on day 8 of CHMI no. 2. This first CHMI in these 3 group 1a volunteers was 79 days after last vaccine dose (Table 2, Figure 2B). No vaccinee developed parasitemia detectable by TBS or qPCR, but 1 volunteer was excluded from VE analysis because the volunteer had positive serum levels of sulfadoxine on day 8 of CHMI. VE at this CHMI on day 79 (11.3 weeks) was 100% (Table 2) (P = .001).
CHMI no. 3
Five of the 6 NS controls who had previously undergone CHMI, and 6 new infectivity controls underwent CHMI at 259 to 286 days after last NS dose; 1 infectivity control was identified as qPCR positive for Pf at time of CHMI and excluded from analysis. By qPCR, parasitemia was detected 7.79–19.59 days for the 5 NS controls and 7.71–7.99 days for the 5 infectivity controls. By TBS, parasitemia was detected in 1/5 NS controls (13.64 days) and in 5/5 infectivity controls after a median of 13.82 (range 12.03–14.57) days. In the 2 control groups (NS and infectivity) (N = 10), parasitemia was detected by qPCR after a median of 7.79 (range 7.71–19.59) days. By TBS parasitemia was detected in 6/10 with a median prepatent period of 13.73 (range 12.03–14.57) days.
The 6 volunteers immunized with 9 × 105 PfSPZ underwent second CHMI at 286 days (40.9 weeks) after last immunization. (Table 2, Figure 2C). All developed parasitemia by qPCR a median of 10.89 (range 7.79–17.58) days after CHMI, and 5/6 developed parasitemia by TBS a median of 18.59 (range 14.09–22.78) days.
The 6 volunteers immunized with 1.8 × 106 PfSPZ underwent second CHMI at 259 days (37 weeks) after 3rd immunization (Table 2, Figure 2C). Five of the 6 developed parasitemia by qPCR a median of 7.78 (range 7.74–27.78) days after CHMI (P = .119). The 1 vaccinee who did not develop parasitemia did not develop parasitemia after first CHMI. Four of 6 developed parasitemia by TBS after a median of 14.3 (range 12.92–27.77) days.
Prepatent periods
The median prepatent period by qPCR for control volunteers in all 3 CHMIs was 7.90 days (Table 2). The median prepatent period for volunteers immunized with 1.8 × 106 PfSPZ was 8.43 days for their first CHMI (CHMI no. 2), and 7.78 days for the second CHMI (CHMI no. 3). In CHMI no. 3 the median prepatent period for the 9 × 105 PfSPZ group was significantly longer (10.89 vs 7.90 days, P = .021, Mann-Whitney U test) than in the NS and infectivity controls. In CHMI no. 3 the prepatent period of 10.89 days for the 9 × 105 PfSPZ group was nonsignificantly longer than the 7.78 days for the 1.8 × 106 PfSPZ group (P = .27).
Antimalarial Drug Levels
In the 3 group 1a subjects treated with AS-AQ for parasitemia, 58 days after treatment completion (8 days after initiation of CHMI no. 2 and 1 day after parasites would have emerged from the liver), no amodiaquine (AQ) was detectable. One volunteer from group 1a (9.0 × 105 PfSPZ) with a low, but detectable, sulfadoxine level of 781 ng/mL, did not develop parasitemia after CHMI and was excluded from the analysis (Table S4).
Safety of CHMI
There were no local or systemic solicited AEs during the 5 days postadministration of PfSPZ Challenge. One unsolicited AE, an upper respiratory infection, occurred in an infectivity control at 14 days. Symptoms or signs of malaria were recorded in 14/29 subjects with parasitemia detected by TBS and qPCR (Table S5). All 8 infections negative by TBS but positive by qPCR were asymptomatic. Symptom onset was on the same day as the positive TBS in 11 of 14 TBS positive infections with symptoms in the other 3 volunteers preceding the TBS positivity by 1, 2, and 4 days. The qPCR was positive a median of 6 days prior to symptom onset (range 3–14 days). All symptoms were 1–3 days in duration and mild to moderate in severity with headache present in all symptomatic volunteers. Four volunteers had elevated axillary temperatures (all ≤ 39.0°C). There were no unexpected or clinically significant laboratory abnormalities.
Antibodies
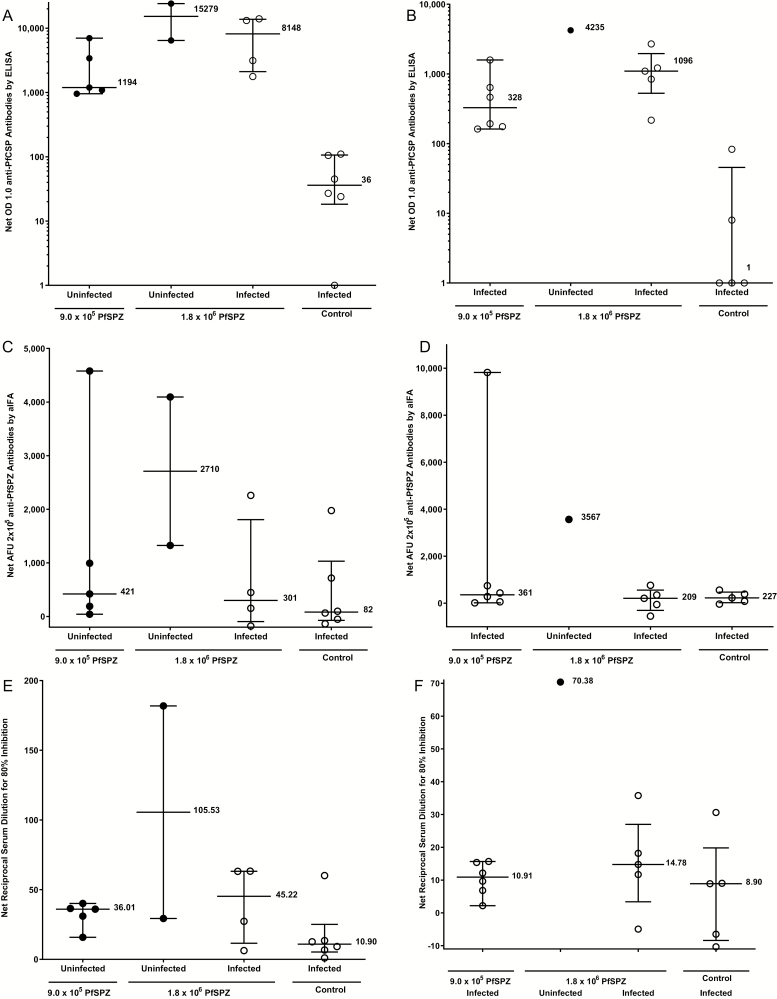
For the Pf circumsporozoite protein (PfCSP) enzyme-linked immunosorbent assay (ELISA) (Figure 3A, 3B, Table S6, Figure S1), the serum dilution at which the optical density was 1.0 was determined, and the net optical density (OD) 1.0 and OD 1.0 ratio, calculated, respectively, by subtracting or dividing the OD 1.0 by the prevaccination OD 1.0. At time of the first CHMI the 5 uninfected subjects in the 9 × 105 PfSPZ group had a nonsignificantly lower median net OD 1.0 (1194) than did the 2 uninfected (15 279) and 4 infected (8148) subjects in the 1.8 × 106 group (P = .19 in each case, Wilcoxon-Mann-Whitney test, 2-tailed). At time of second CHMI the 6 infected subjects in the 9 × 105 PfSPZ group had a nonsignificantly lower median net OD 1.0 (328) than did the 1 uninfected (4235) and 5 infected (1096) subjects in the 1.8 × 106 group (P = .29 and .13).
Figure 3.
IgG antibodies to PfCSP by ELISA at time of first CHMI (CHMIs no. 1 and no. 2). A, IgG antibodies to PfSPZ by aIFA (C) and automated inhibition of PfSPZ invasion of hepatoma cells (aISI) (E) and at time of second CHMI (CHMI no.3) by ELISA (B), aIFA (D) and ISI (F) in subjects who received 9 × 105 PfSPZ or 1.8 × 106 PfSPZ doses of PfSPZ Vaccine. Filled circles (●) represent volunteers remaining uninfected after CHMI; open circles (○) represent volunteers infected after CHMI. For the PfCSP ELISA vaccinees were considered to have a positive antibody response if their net OD 1.0 and OD 1.0 ratio, calculated, respectively, by subtracting or dividing the OD 1.0 by the prevaccination OD 1.0, were ≥ 50 and ≥ 3.0. By these criteria, in the 9.0 × 105 PfSPZ group, 5/6, 6/6, and 1/6 were positive 2 weeks after third dose, and before their first and second CHMIs. In the 1.8 × 106 PfSPZ group 6/6, 6/6, and 5/6 were positive 2 weeks after third dose, and before their first and second CHMIs. No control volunteers were positive at any time point. For preimmunization anti-PfCSP levels, there were no significant differences between infected and noninfected vaccinees (Table S6). In the aIFA, volunteers with a net arbitrary fluorescence units (AFU) 2 × 105 of ≥150 and a ratio of post to pre AFU 2 × 105 of ≥ 3.0 were considered positive (Table S5). By these criteria, in the 9.0 × 105 PfSPZ group, 5/6, 5/6, and 4/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). In the 1.8 × 106 PfSPZ group, 5/6, 5/6, and 4/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). Antibodies to PfSPZ at time of first and second CHMIs by aIFA are shown in Figure 3C and 3DTable S5. At the time of the first CHMI the 5 uninfected (protected) subjects in the 9 × 105 PfSPZ group had a lower median net AFU 2 × 105 (421) than did the 2 uninfected (2710) subjects in the 1.8 × 106 group and higher than the 4 infected (301) subjects in the 1.8 × 106 group, but the differences were not significant (P = .38 and .73). At the time of the second CHMI the 6 infected subjects in the 9 × 105 PfSPZ group had a lower median net AFU 2 × 105 (361) than did the one uninfected (3567) subject in the 1.8 × 106 group and higher than the 5 infected (209) subjects in the 1.8 × 106 group (P = .57 and .43). For the aISI, volunteers with a net ISI reciprocal serum dilution for 80% inhibition of ≥10 and ratio of post to preimmune ISI reciprocal serum dilution for 80% inhibition of ≥ 3.0 were considered positive. By these criteria, in the 9.0 × 105 PfSPZ group, 0/6, 2/6, and 2/6 were positive 2 weeks after the third dose and before their first and second CHMIs (Table S5). In the 1.8 × 106 PfSPZ group, 2/6, 4/6, and 3/6 were positive 2 weeks after the third dose, and before their first and second CHMIs (Table S5). Antibodies to PfSPZ at time of first and second CHMIs by ISI are shown in Figure 3E and 3F and Table S5. At time of the first CHMI the 5 uninfected (protected) subjects in the 9 × 105 PfSPZ group had a nonsignificant lower median net reciprocal serum dilution for 80% inhibition (22.57) than did the 2 uninfected (95.19) and 4 infected (34.12) subjects in the 1.8 × 106 group, but the differences did not reach the level of statistical significance (P = .095 and .90). At the time of the second CHMI the 6 infected subjects in the 9 × 105 PfSPZ group had a nonsignificant lower median net reciprocal serum dilution for 80% inhibition (10.91) than did the one uninfected (70.38) subject in the 1.8 × 106 group and the 5 infected (14.78) subjects in the 1.8 × 106 group (P = .29 and .54). Abbreviations: aIFA, automated immunofluorescence assay; CHMI, controlled human malaria infection; ELISA, enzyme-linked immunosorbent assay; IgG, immunoglobulin G; ISI, inhibition of sporozoite invasion assay; PfCSP, Pf circumsporozoite protein; PfSPZ, Plasmodium falciparum sporozoite.
The results of the automated immunofluorescence assay (aIFA) and the automated inhibition of sporozoite invasion assay (aISI) are shown in Figure 3 and Table S6. The numbers of seroconverters as compared to ELISA were similar for the aIFA but lower for the aISI.
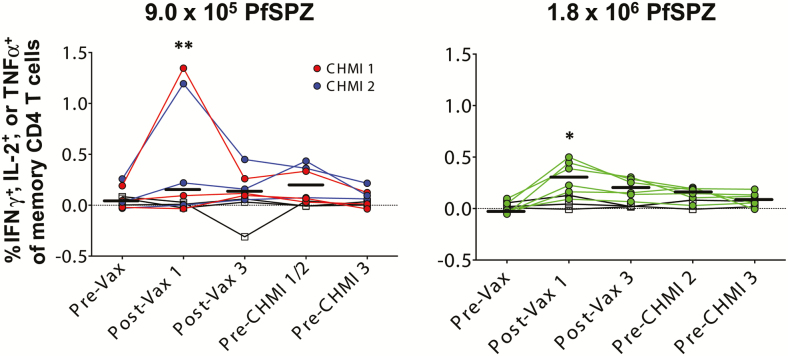
T Cells
There was a significant increase in PfSPZ-specific CD4+ responses at the group level only after the first dose in both dosage groups (Figure 4, Table S7). There were no significant increases in PfSPZ-specific CD8+ T-cell responses at any time point (data not shown). To provide further assessment of PfSPZ-specific CD4+ T-cell responses, we calculated the net percent PfSPZ-specific CD4+ T cell response (% response at a specific time point minus the % response prior to immunization) and the ratio of PfSPZ-specific CD4+ T-cell responses (% response at each time point divided by the % response prior to immunization). For these calculations any negative value was assigned a value 0.001 (Table S8).
Figure 4.
Pf-specific memory CD4 T-cell responses following different PfSPZ Vaccine doses. T-cell responses were assessed by flow cytometry 2 weeks after first and third doses of vaccine, and prior to the first and second CHMIs. We incubated PBMCs from vaccinees and controls with radiation attenuated (150 Gy) aseptic, purified, cryopreserved PfSPZ for ~18 hours and then assessed the percent of T cells that specifically responded to the PfSPZ. The percent of memory CD4 T cells in the blood expressing IFNγ, IL-2, or TNFα preimmunization, 2 weeks after the first and third doses of 9.0 × 105 PfSPZ Vaccine (left) or 1.8 × 106 PfSPZ Vaccine (right), and before each CHMI time point is shown. Results are the percentage of cytokine-producing cells after incubation with PfSPZ minus the percentage of cytokine-producing cells after incubation with vaccine diluent (medium with 1% human serum albumin). Colored symbols indicate vaccine groups, whereas open symbols represent placebo controls. Red symbols represent individuals challenged at the first CHMI time point, whereas blue and green symbols indicate individuals challenged at the second CHMI time point. Bars indicate median values within each group. Differences within each age group between pre- and post-vaccination groups were assessed by 2-way ANOVA with Dunnett’s correction for multiple comparisons. *P < .05, **P < .01. For preimmunization T-cell responses, there were no significant differences between infected and noninfected vaccinees (Table S7). Abbreviations: ANOVA, analysis of variance; CHMI, controlled human malaria infection; IFNγ, interferon γ; IL, interleukin; PBMC, peripheral blood mononuclear cell; Pf, Plasmodium falciparum; PfSPZ, Plasmodium falciparum sporozoite; TNFα, tumor necrosis factor α.
The 2 highest net PfSPZ-specific CD4+ T-cell responses (1.155% and 0.936%) were after the first vaccine dose in the 9.0 × 105 PfSPZ group. Both vaccinees were protected against first CHMI and had prolonged prepatent periods by qPCR after the second CHMI (12.62 and 17.58 days, respectively, as compared to median of controls of 7.99 and 7.79 days). However, the 1.8 × 106 PfSPZ group as compared to the 9.0 × 105 PfSPZ group had higher net % specific CD4+ T-cell responses at 3 of the 4 time points and higher ratios of PfSPZ-specific CD4+ T-cell responses at all 4 time points.
Gamma delta (γδ) T cells, especially V delta 2 (Vδ2) cells, have been associated with protection [7, 23]. There were no significant differences in Vδ2 cell frequencies between vaccine groups and no significant changes of Vδ2 frequencies within vaccine groups following each vaccination (data not shown).
DISCUSSION
Previous PfSPZ Vaccine studies indicated increasing numbers of PfSPZ per dose would increase VE (VE) [6–9]. In a study in Bagamoyo, 5 doses of 2.7 × 105 PfSPZ of PfSPZ Vaccine gave 20% VE against homologous CHMI at 3 weeks after last dose. In the present study, a higher dose, 3 doses of 9.0 × 105 PfSPZ had 100% VE against homologous CHMI at 3 or 11 weeks after last dose (P = .0006).
In this study, increasing from 9.0 × 105 to 1.8 × 106 PfSPZ was associated with reduction of VE from 100% at 23 or 79 days after last vaccine dose to 33% at 52 days after last vaccine dose (P = .0224). The results of the second CHMI were consistent with this observation; only the 9.0 × 105 PfSPZ group had a significant delay in prepatent period. Increasing the dose of irradiated P. yoelii (Py) SPZ 5-fold decreased VE in mice 4-fold [24]. A similar phenomenon has been described for postexposure immunization against murine tuberculosis [25]. The phenomenon of high-dose tolerance/suppression has been described for T cells but not B cells. Supra-optimal engagement of the T-cell receptor induces checkpoint blockade resulting in reduced proliferation, or ability to secrete tumor necrosis factor α (TNFα) or interferon γ (IFNγ) or cause apoptosis of activated cells [26–31].
In mice and nonhuman primates (NHPs) protection by immunization with radiation attenuated SPZ is eliminated by CD8+ T-cell depletion [5, 32–35]. We think CD8+ T cells against parasite-infected hepatocytes are responsible for the protection induced by radiation attenuated SPZ [33, 36, 37]. Changing administration of PfSPZ Vaccine from intradermal or subcutaneous (SC) [5] to DVI injection [6], was based on demonstration that 4 months after last dose of PfSPZ Vaccine by DVI to NHPs, ~3% of CD8+ T cells in the livers produced IFN γ in response to stimulation with PfSPZ. In contrast, there were minimal PfSPZ-specific CD8+ T cells expressing IFNγ in NHPs immunized SC [5]. Some subsequent clinical trials have shown associations between antibody and T-cell responses and protection [7, 9, 10]. We concluded that it will be difficult to identify immune response signatures that predict VE unless we can assess responses in the liver [4, 38]. This study supports this perspective. We did not identify PfSPZ-specific CD8+ T-cell responses, and antibody and CD4+ T-cell responses were generally higher in nonprotected vaccinees immunized with 1.8 × 106 PfSPZ than in protected vaccinees immunized with 9.0 × 105 PfSPZ.
No vaccines to prevent human parasitic diseases or composed of eukaryotic cells have received marketing authorization (licensure) by the FDA or the European Medicines Agency. We have pursued an empiric development process in which we have altered PfSPZ/dose, and dose numbers and intervals to identify an optimal immunization regimen. We are currently concentrating on 9.0 × 105 PfSPZ/dose and have found that shortening the interval between doses is associated with increased VE against heterologous CHMI (Mordmüller unpublished).
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to express their gratitude to the study participants. They also thank the members of the Safety Monitoring Committee (Anna Durbin—chair, James Campbell, Karim Manji) for their thoughtful oversight, the entire study team at the Bagamoyo branch of the Ifakara Health Institute, and the teams at Sanaria and Protein Potential for manufacture and shipping of investigational products and diluents, regulatory, quality, and clinical site activities, and legal and administrative support.
Financial Support. This work supported by a public-private partnership, the Equatorial Guinea Malaria Vaccine Initiative (EGMVI), made up of the Government of Equatorial Guinea (EG) Ministries of Mines and Hydrocarbons, and Health and Social Welfare, Marathon EG Production Limited, Noble Energy, Atlantic Methanol Production Company, and EG Liquefied Natural Gas.
Potential conflicts of interest. Sanaria Inc. manufactured PfSPZ Vaccine and Protein Potential LLC is affiliated with Sanaria. Sanaria was the Sponsor of the clinical trial. L. W. P. C., S. C., A. J. R., E. R. J., Y. A., N. K. C., E. S., P. F. B., B. K. L. S., T. L. R., and S. L. H. are salaried, full-time employees of Sanaria Inc., the developer and sponsor of Sanaria PfSPZ Vaccine. Thus, all authors associated with Sanaria or Protein Potential have potential conflicts of interest. D. S. reports that The Emmes Company received payments for support of this and other Sanaria-sponsored studies. S. L. H. and B. K. L. S. are named inventors on patents related to PfSPZ Vaccine. M. T. reports contribution to travel costs from Sanaria Corp, board membership from Optimus Foundation, Novartis Institute for Tropical Disease, Botnar Foundation Basel, and University Hospital Basel. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Malaria Programme. World malaria report 2015. Geneva: World Health Organization, 2015. [Google Scholar]
- 2. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. World malaria report 2018. Geneva: World Health Organization, 2018. [Google Scholar]
- 4. Richie TL, Billingsley PF, Sim BK, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015; 33:7452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+T cell immunity. Science 2011; 334:475–80. [DOI] [PubMed] [Google Scholar]
- 6. Seder RA, Chang LJ, Enama ME, et al. ; VRC 312 Study Team . Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341:1359–65. [DOI] [PubMed] [Google Scholar]
- 7. Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22:614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017; 2:e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 2017; 17:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jongo SA, Shekalaghe SA, Church LWP, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg 2018; 99:338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jongo SA, Church LWP, Mtoro AT, et al. Safety and differential antibody and T-cell responses to the Plasmodium falciparum sporozoite malaria vaccine, PfSPZ Vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am J Trop Med Hyg 2019; 100:1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman SL, Billingsley PF, James E, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 2010; 6:97–106. [DOI] [PubMed] [Google Scholar]
- 14. Roestenberg M, Bijker EM, Sim BKL, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 2013; 88:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheehy SH, Spencer AJ, Douglas AD, et al. Optimising controlled human malaria infection studies using cryopreserved P. falciparum parasites administered by needle and syringe. PLoS One 2013; 8:e65960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hodgson SH, Juma E, Salim A, et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol 2014; 5:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shekalaghe S, Rutaihwa M, Billingsley PF, et al. Controlled human malaria infection of Tanzanians by intradermal injection of aseptic, purified, cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg 2014; 91:471–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gómez-Pérez GP, Legarda A, Muñoz J, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naïve volunteers: effect of injection volume and dose on infectivity rates. Malar J 2015; 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mordmüller B, Supan C, Sim KL, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J 2015; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamau E, Alemayehu S, Feghali KC, Saunders D, Ockenhouse CF. Multiplex qPCR for detection and absolute quantification of malaria. PLoS One 2013; 8:e71539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med 2015; 12:e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson TJ, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology 1999; 119 (Pt 2):113–25. [DOI] [PubMed] [Google Scholar]
- 23. Zaidi I, Diallo H, Conteh S, et al. γδ T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J Immunol 2017; 199:3781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedegah M. Immunization against murine malaria by sporozoites and by pre-erythrocytic stages [Ph.D. diss.]. London: University of London, 1988. [Google Scholar]
- 25. Billeskov R, Lindenstrøm T, Woodworth J, et al. High antigen dose is detrimental to post-exposure vaccine protection against tuberculosis. Front Immunol 2017; 8:1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Critchfield JM, Zúñiga-Pflücker JC, Lenardo MJ. Parameters controlling the programmed death of mature mouse T lymphocytes in high-dose suppression. Cell Immunol 1995; 160:71–8. [DOI] [PubMed] [Google Scholar]
- 27. Haneda K, Sano K, Tamura G, et al. Transforming growth factor-beta secreted from CD4+ T cells ameliorates antigen-induced eosinophilic inflammation: a novel high-dose tolerance in the trachea. Am J Respir Cell Mol Biol 1999; 21:268–74. [DOI] [PubMed] [Google Scholar]
- 28. Michallet MC, Saltel F, Flacher M, Revillard JP, Genestier L. Cathepsin-dependent apoptosis triggered by supraoptimal activation of T lymphocytes: a possible mechanism of high dose tolerance. J Immunol 2004; 172:5405–14. [DOI] [PubMed] [Google Scholar]
- 29. Han S, Asoyan A, Rabenstein H, Nakano N, Obst R. Role of antigen persistence and dose for CD4+ T-cell exhaustion and recovery. Proc Natl Acad Sci U S A 2010; 107:20453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornberg M, Kenney LL, Chen AT, et al. Clonal exhaustion as a mechanism to protect against severe immunopathology and death from an overwhelming CD8 T cell response. Front Immunol 2013; 4:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wagle MV, Marchingo JM, Howitt J, Tan SS, Goodnow CC, Parish IA. The ubiquitin ligase adaptor NDFIP1 selectively enforces a CD8+ T cell tolerance checkpoint to high-dose antigen. Cell Rep 2018; 24:577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schofield L, Villaquiran J, Ferreira A, Schellekens H, Nussenzweig R, Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature 1987; 330:664–6. [DOI] [PubMed] [Google Scholar]
- 33. Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol 2000; 165:1453–62. [DOI] [PubMed] [Google Scholar]
- 34. Sedegah M, Weiss WW, Hoffman SL. Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites. Parasite Immunol 2007; 29:559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weiss WR, Jiang CG. Protective CD8+ T lymphocytes in primates immunized with malaria sporozoites. PLoS One 2012; 7:e31247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffman SL, Isenbarger D, Long GW, et al. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science 1989; 244:1078–81. [DOI] [PubMed] [Google Scholar]
- 37. Hoffman SL, Rogers WO, Carucci DJ, Venter JC. From genomics to vaccines: malaria as a model system. Nat Med 1998; 4:1351–3. [DOI] [PubMed] [Google Scholar]
- 38. Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine 2015; 33(Suppl 4):D13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.