Abstract
Chronic kidney disease (CKD) is associated with a substantial increased risk of cardiovascular disease. There is growing evidence that uremic metabolites, which accumulate in the blood with CKD, have detrimental impacts on endothelial cell health and function. However, the molecular mechanisms by which uremic metabolites negatively impact endothelial cell biology are not fully understood. In this study, activation of the aryl hydrocarbon receptor (AHR) via indoxyl sulfate, a known uremic metabolite, was found to impair endothelial cell tube formation and proliferation but not migratory function. Moreover, aortic ring cultures treated with indoxyl sulfate also exhibited decreased sprouting and high AHR activation. Next, genetic knockdown of the AHR using shRNA was found to rescue endothelial cell tube formation, proliferation, and aortic ring sprouting. Similarly, pharmacological AHR antagonism using resveratrol and CH223191 were also found to rescue angiogenesis in cell and aortic ring cultures. Finally, a constitutively active AHR (CAAHR) vector was generated and used to confirm AHR-specific effects. Expression of the CAAHR recapitulated the impaired tube formation and proliferation in cultured endothelial cells and decreased sprouting in aortic ring cultures. Taken together, these data define the impact of AHR activation on angiogenesis and highlight the potential for therapeutic AHR antagonists, which may improve angiogenesis in the context of CKD and cardiovascular disease.
Keywords: cardiovascular, chronic kidney disease, endothelial cell, neovascularization, uremia
INTRODUCTION
Chronic kidney disease (CKD) is defined as any condition that causes chronic damage to the kidney that results in a reduction in glomerular filtration rate (GFR) below 60 mL/min/1.73 m2 for 3 mo or longer and affects an estimated 8%–16% of the global population (1). Patients with CKD are at a substantially higher risk for developing cardiovascular disease, which leads to high rates of cardiovascular disease mortality in this population (2–4). For example, patients with peripheral arterial disease (PAD) along with CKD have two to four times higher risk for mortality than patients with PAD but without CKD (5–9). CKD also increases the likelihood that patients with PAD will present with ischemic ulceration or gangrene, which substantially increases the risk of limb amputation (6, 10, 11). Moreover, studies have reported that CKD accelerates development of atherosclerosis (12, 13) and impairs angiogenesis (14, 15) in rodent models. Although it is clear that CKD negatively impacts vascular tissues, the underlying molecular mechanisms are incompletely understood and present a significant gap in knowledge that compromises development of effective treatments to improve health outcomes in CKD.
Angiogenesis is a process that involves growth of new blood vessels through either sprouting of new vessels or splitting of existing vessels. This physiological process is especially crucial for ischemic diseases where recovery of tissue perfusion is necessary for survival. Endothelial cells play key regulatory roles in sprouting angiogenesis that involve both proliferative and migratory actions, which are governed by established angiogenic and metabolic signaling pathways (16). Previous studies have demonstrated that rodents with CKD exhibit impaired perfusion recovery and angiogenesis following surgically induced ischemia (12, 17). Despite these observations, mechanistic data aiming to understand the molecular mechanisms linking CKD to impaired angiogenesis are scarce.
Patients with CKD have impaired filtration of endogenous and exogenous waste products (normally filtered by healthy kidneys) that accumulate within the blood, a condition defined as uremia (18). There is growing evidence supporting a hypothesis by which uremic metabolites, or toxins, may be key factors linking kidney function to the systemic disease manifestation (hypertension, obesity, muscle wasting, and cardiovascular disease) often present in the patient with CKD. Interestingly, some uremic metabolites have been shown to reduce nitric oxide availability within the endothelium of cultured endothelial cells (4, 19, 20). Notably, indoxyl sulfate (IS) has gained popularity in the literature as a driver for cardiovascular disease risk (21). Recent work has established IS as a potent endogenous ligand of the aryl hydrocarbon receptor (AHR), a powerful transcription factor involved in xenobiotic metabolism (22–24). Activation of the AHR results in nuclear translocation and subsequent transcription of downstream cytochrome P450 enzymes (CYP1A1 and CYP1B1), which normally metabolize xenobiotics/drugs as well as endogenous metabolites. It has been shown that chronic activation of the AHR caused by exposure to tobacco smoke (or dioxins) can reduce angiogenesis (25) and increase atherosclerosis (26–28). However, the role of the AHR in IS-mediated impairment in angiogenesis has not been established.
Thus, this study sought to examine the role of the AHR activation via IS exposure on angiogenic processes central to endothelial cell biology. It was hypothesized that chronic AHR activation mediates IS-associated impairment in angiogenesis and that the AHR could be therapeutically targeted to prevent the negative impact of IS. To test this hypothesis, a comprehensive assessment of key biological processes involved in angiogenesis was performed, including proliferation, migration, tube formation, sprouting using both in vitro (cultured endothelial cells) and in situ (aortic rings) models. To manipulate the AHR pathway, genetic and pharmacologic interventions were utilized.
MATERIALS AND METHODS
All source data herein have been deposited to Figshare and can be accessed using the following link: https://doi.org/10.6084/m9.figshare.12417542.
Animals
Aortic rings were dissected from 12-wk-old C57BL6J male mice (Stock No. 000664) obtained from The Jackson Laboratory (N = 20 total mice). Mice were euthanized by thoracotomy under a surgical plan of anesthesia via inhaled isoflurane. All animal experiments adhered to the Guide for the Care and Use of Laboratory Animals from the Institute for Laboratory Animal Research, National Research Council, Washington, D.C., National Academy Press, 1996, and any updates. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Florida.
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were obtained from ATCC (CRL-1730). All experiments were performed in three biologically independent cell samples. HUVECs were grown to ∼90% confluency on flasks coated with 0.25% gelatin (Sciencell Cat. No. 0423) in endothelial cell medium (ECM; Sciencell Cat. No. 1001) supplemented with 5% fetal bovine serum (Sciencell Cat. No. 0025), 1% endothelial cell growth supplement (Sciencell Cat. No. 1502), and 1% penicillin/streptomycin solution (Sciencell Cat. No. 0503) using standard culture conditions (37°C with 5% CO2).
Measurement of Cell Viability
HUVECs were plated in gelatin-coated 24-well dishes and grown to ∼70% confluency. Medium was then replaced with fresh ECM supplemented with 500 μM indoxyl sulfate (Millipore-Sigma; Cat. No. I3875) or vehicle (DMSO; ThermoFisher Scientific; Cat. No. BP231) for 18 h at 37°C with 5% CO2. This concentration of indoxyl sulfate was chosen because of data demonstrating this to be the average concentration found in blood of patients with CKD (18, 29). After treatment, cells were washed with PBS and incubated with 10 µM ethidium homodimer-1 (EtHD-1; Millipore-Sigma; Cat. No. 46043) and 1 µM Calcein-AM (Enzo Life Sciences; Cat. No. 148504-34-1) in warm Hanks’ balance salt solution (HBSS; ThermoFisher Scientific; Cat. No. 24020) for 15 min at 37°C in 5% CO2. After incubating cells, the media was aspirated and fresh HBSS was added to each well. EtHD-1 is a cell-impermeable fluorescent probe that binds to DNA in cells that have been permeabilized and thus undergoing cell death. Live cells were counterstained with Calcien-AM to provide contrast with dead cells. Five ×10 images were taken of each well, and EtHD-1-positive cells were measured using a custom routine in CellProfiler. A positive control group was permeabilized with 0.25% Triton X-100 for 10 min before staining.
Western Blotting
HUVECs were grown to 90% confluency, transfected with respective plasmids as described below, and lysed 48 h after transfection in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor. Samples underwent four freeze/thaw cycles and were then centrifuged at 10,000 g for 10 min at 4°C. Protein content was determined using Pierce BCA protein assay (ThermoFisher Scientific; Cat. No. P23225). Proteins were then boiled for 5 min and separated using an SDS-PAGE gel (BioRad; Cat. No. 456-1044). Proteins were transferred to PVDF membranes using Bio-Rad Turbo Transfer system and subsequently imaged for total protein content. PVDF membranes were blocked in Li-Cor Odessey blocking buffer for 60 min at room temperature. Following blocking, membranes were incubated overnight at 4°C in 1 µg/mL anti-AHR (NSJ Reagents, Cat. No. R30877). Following incubation with primary antibodies, membranes were washed with tris buffered saline with 0.1% Tween and incubated with Li-Cor 800 nm secondary antibody (1:15,000) for 1 h at room temperature. Blots were visualized using a Li-Cor Odyssey CLx infrared imaging system (Li-Cor; Cat. No. 9410).
RNA Isolation and qRT-PCR
Total RNA was extracted from treated HUVECs and aortic rings using Trizol-Phenol Reagent (Invitrogen; Cat. No. 15596026) as described by manufacturer’s instructions. RNA quantity and quality was assessed using UV spectroscopy (ThermoFisher Scientific; Nanodrop 2000). cDNA was generated from 500 ng RNA using Superscript IV (ThermoFisher; Cat. No. 18091200) according to manufacturer’s directions. Real-time PCR (RT-PCR) was performed on a Quantstudio 3 (ThermoFisher Scientific) using Taqman Fast Advanced Master mix (ThermoFisher Scientific; Cat. No. 4444963) and Taqman FAM-labeled probes for AHR (ThermoFisher Scientific; Hs00169233_m1, Mm00478932_m1), CYP1A1 (ThermoFisher Scientific; Hs01054797_g1, Mm00487218_m1), CYP1B1 (ThermoFisher Scientific; Hs00164383_m1) multiplexed with VIC-labeled probe for 18S (ThermoFisher Scientific; Hs03003631_g1) or β-actin (ThermoFisher Scientific; Hs03023943_g1, Mm002619580_g1). Relative gene expression was calculated using 2−ΔΔCT from the corresponding control group within each experiment.
Endothelial Cell Proliferation
To measure proliferation of HUVECs, cells were plated into gelatin-coated 96-well plates at a density of 2,000 cells/well and allowed to adhere. Cell proliferation was inferred using a simple cell-counting method performed every 24 h for a 3-day period following plating. To image cell numbers, HUVECs were then fixed with a 1:1 solution of methanol acetone at −20°C for 10 min. Cell nuclei were labeled with a DAPI stain (ThermoFisher Scientific; Cat. No. R37606) added to 1× PBS and incubated at room temperature for 30 min. The well plates were subsequently imaged using an automated imaging routine on an Evos FL2 Auto inverted fluorescent microscope (ThermoFisher Scientific), which captured the entire well using a stitching feature. Total cell number was determined by process images through custom written routines in Cell Profiler (The Broad Institute).
Endothelial Cell Migration Assay (Scratch Assay)
Endothelial cell migration was measured using an in vitro scratch-wound assay as previously described (30, 31). HUVECs were plated into gelatin-coated 48-well plates at a density of 5.0 × 104 cells/well in ECM media and allowed to adhere. Cells were then treated with either 500 µM indoxyl sulfate or an equal volume of DMSO for 18 h. After the treatment period, cells were washed with 1× PBS and new ECM media supplemented with 5 μg/mL of mitomycin C (Millipore Sigma; Cat. No. M4287) was added for 2 h to inhibit cell proliferation as previously described (32). The cell monolayer was then scratched with a p200 pipet tip in a vertical line and then washed with 1× PBS before replacing medium with fresh ECM. Phase-contrast images were taken of each well at ×4 magnification with an Evos FL2 Auto inverted fluorescent microscope (ThermoFisher Scientific) immediately after the scratch and every 4 h over a 24-h time course. Images were analyzed using ImageJ software to measure the length of the scratch, and a calculation of percent closure of the scratch was made by a blinded investigator.
Endothelial Cell Tube Formation Assay
Tube formation assays were performed as previously described (33). Fifty microliters of Matrigel (Corning; Cat. No. 354234) was added into individual wells and incubated for 30 min at 37°C with 5% CO2. HUVECs were pretreated with indoxyl sulfate or DMSO (500 µM) for 12 h and then seeded into Matrigel-coated 48-well plates at 2.5 × 104 cells/well. HUVECs were then placed in ECM supplemented with either indoxyl sulfate or DMSO and returned to the incubator for 6 h at 37°C with 5% CO2 to allow for the formation of tube-like structures. For experiments involving the chemical AHR antagonist’s resveratrol and CH223191, antagonists were provided throughout all exposures to indoxyl sulfate. After the 6-h incubation, plates were gently washed with 1× phosphate-buffered saline, fixed with prewarmed 2% paraformaldehyde (SantaCruz; Cat. No. 30525-89-4) for 5 min, and subsequently permeabilized with 0.25% Triton X-100 (Millipore-Sigma; Cat. No. 9002931) for 15 min. Next, cells were labeled with Alexa Fluor-594 conjugated phalloidin (ThermoFisher Scientific; Cat. No. A12381) in PBS. Tube formation was imaged at ×10 using an Evos FL2 auto microscope, and the number of completed tubes were analyzed using ImageJ by a blinded investigator.
Aortic Ring Sprouting Assay
Aortas were isolated from mice as previously described (34). Briefly, aortas were dissected and placed into a 35-mm culture dish containing Dulbecco’s modified Eagle medium (ThermoFisher Scientific; Cat. No. 10569010) supplemented with 1% penicillin/streptomycin (ThermoFisher Scientific; Cat. No. 15140122) but without serum. Upon isolation, aortas were carefully trimmed using a scalpel to remove any connective or adipose tissue under a dissecting microscope (AmScope). The aortas were cut into 0.5-mm rings and placed into fresh 35-mm dishes containing DMEM +P/S for overnight serum starvation. Serum starvations allows for equilibration of growth factors, creating uniform baseline levels. After overnight starvation, 50 μL of Matrigel was added into 96-well plates and incubated for 30 min at 37°C for gel formation. Aortic rings were placed horizontally into the center of the well, and an additional 50 μL of Matrigel was added and incubated for 30 min. Aorta were cultured in ECM media supplemented with indoxyl sulfate or DMSO (0.5 mM) accordingly and allowed to sprout for 8 days (media with treatment was changed every 24 h). Z-stack bright-field images were taken (Evos FL2 auto microscope) of each ring and analyzed in ImageJ for total sprouts, sprout length, and connected sprouts by a blinded investigator.
Plasmid Construction and AAV Delivery
To genetically manipulate AHR expression and signaling, custom plasmids and adeno-associated viruses (AAV) were developed. First, the AAV-CMV-GFP plasmid was developed by inserting a cytomegalovirus (CMV) promoter and GFP (ZsGreen 1) into a promoterless AAV vector (Cell BioLabs; Cat. No. VPK-411-DJ) using in-fusion cloning (Takare Bio; Cat. No. 638911). To generate a constitutively active AHR vector, the human AHR coding sequence was PCR amplified from HUVEC cells such that the ligand-binding domain was deleted as previously described for the murine AHR (35) and subsequently cloned into the pAAV-CMV vector using in-fusion cloning.
AAVs were produced using triple transfection of HEK293T cells with the DJ serotype packaging plasmids from Cell Biolabs (Cell BioLabs; Cat. No. VPK-411-DJ). AAV purification was performed ∼72 h after triple transfection using purification kits from Takara Bio (Takare Bio; Cat. No. 6666) according to manufacturer instructions. Purified AAVs were titered using a qPCR-based kit (Takara Bio; Cat. No. 6233). HUVECs and aortic rings were treated with AAVs at a multiplicity of infection (MOI) of 1,000. For shRNA knockdown of the AHR, we utilized established siRNA sequences (36) (AHR siRNA: 5′- AAG UCG GUC UCU AUG CCG CTT -3′; or scramble control 5′-GCG CGC UUU GUA GGA UUC GTT-3′) that were packaged at shRNAs and inserted into a custom AAV plasmid containing a U6 promoter for shRNA expression. Knockdown was confirmed by qPCR.
Statistical Analysis
Data are presented as mean ± SD. Normality of data was tested with the Shapiro–Wilk test. Data were analyzed with either a two-tailed student’s t test (two groups) or two-way ANOVA with Tukey’s post hoc multiple comparisons (more than two groups). All statistical analysis was performed in GraphPad Prism (v. 8.0). P < 0.05 was considered statistically significant.
RESULTS
Indoxyl Sulfate Impairs Endothelial Cell Proliferation, Tube Formation, and Sprouting but Not Migration
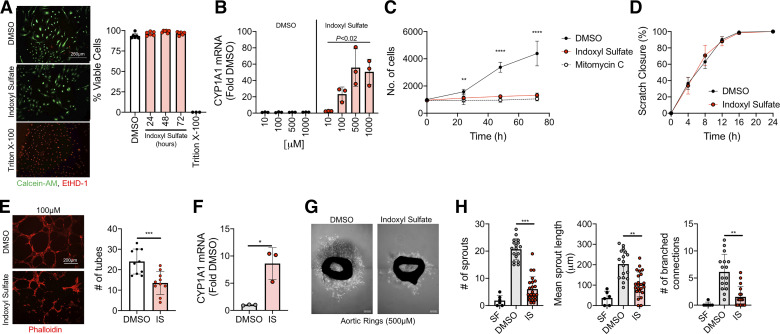
Indoxyl sulfate, a uremic metabolite derived from tryptophan metabolism, is a known AHR agonist and is present in concentrations of ∼500 µM among patients with CKD (18). First, treatment of HUVECs with 500 µM indoxyl sulfate was confirmed to not cause measureable cell death (Fig. 1A). Consistent with knowledge that indoxyl sulfate is an AHR ligand, indoxyl sulfate resulted in significantly dose-dependent elevation in mRNA levels of CYP1A1 in HUVECs (Fig. 1B), confirming robust AHR activation. Indoxyl sulfate-mediated AHR activation resulted in significant decreases in endothelial cell proliferation at 24, 48, and 72 h (Fig. 1C), which were not in part from decreased cell viability during treatment (Fig. 1A). The impairments in cell proliferation that was observed are comparative to the proliferation inhibitor mitomycin c. To determine the impact on cell migration, a 2-D scratch wound assay was used. Interestingly, scratch wound closure (a measure of endothelial cell migration) was not altered by IS treatment (Fig. 1D). Next, a Matrigel-based tube formation assay demonstrated that IS treatment resulted in a ∼55% reduction in number of tubes formed (P < 0.05) compared with vehicle control-treated cells (Fig. 1E). Since impairments on several angiogenic processes were found in vitro, an in situ approach using cultured murine aortic rings was used in follow-up experiments. This assay allows for a comprehensive look at angiogenesis by embedding small rings of a mouse aorta into a 3-D matrix that allows for vascular sprouting to occur (Fig. 1G). Aortic rings were allowed to sprout under chronic treatment of either IS (500 µM) or DMSO. Similar in observations in HUVECs, aortic rings displayed robust AHR activation with IS treatment, evidenced by a ∼8-fold increase in CYP1A1 mRNA levels (P < 0.01; Fig. 1F). This increased activation of AHR led to a ∼70% reduction in total number of sprouts (P < 0.01), a ∼50% reduction in sprout length, and 75% reduction in connecting branches compared with vehicle-treated rings (Fig. 1, G and H). Taken together, these findings demonstrate direct impairments in angiogenesis with IS treatment.
Figure 1.
Indoxyl sulfate impairs angiogenesis. A: endothelial cell viability was first tested by incubation with ethidium homodimer-1 (EtHD-1) and Calcein-AM (n = 6/group). Quantification demonstrated that 500 µM treatment of indoxyl sulfate (IS) did not result in measurable cell death. Scale bar = 250 µm. B: qPCR was used to quantify activation of the AHR through expression of downstream CYP1A1 mRNA levels (n = 3/group). C: quantification of endothelial cell proliferation under control (DMSO) and IS-treated conditions (n = 6/group). Mitomycin c, a known inhibitor of cell proliferation, was used as a negative control. D: scratch wound closure quantification was performed using time-lapse imaging (n = 8/group). E: endothelial cell tube formation was quantified by culturing cells on Matrigel (n = 10/group). Scale bar = 200 µm. F: CYP1A1 mRNA levels (index of AHR activation) in cultured murine aortic rings (n = 3/group). G: representative images of aortic rings with DMSO and indoxyl sulfate treatment. Scale bar = 0.01 inches. H: quantification of aortic ring sprouts, sprout length, and branching (n = 6–23/group). As a negative control, quantification is also shown for aortic rings cultured in serum-free (SF) conditions. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. DMSO control. Error bars = SD. AHR, aryl hydrocarbon receptor.
Genetic Knockdown of the AHR Prevents IS-Mediated Impairment in Angiogenesis
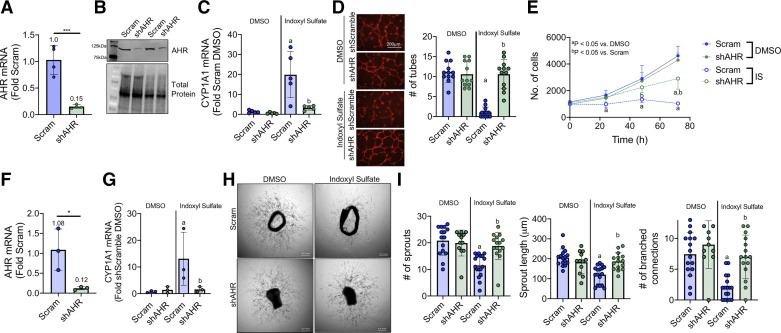
To examine the role of the AHR in IS-mediated impairments in angiogenesis processes, plasmids containing short hairpin targeting the AHR was developed to knockdown the AHR. Validation of short hairpin sequence was confirmed by an 85% reduction in AHR mRNA levels (Fig. 2A) and subsequent decrease in AHR protein abundance (Fig. 2B) in HUVECS transfected with the shAHR plasmid compared with a scramble control. shRNA-mediated knockdown of AHR abolished the IS-mediated increase in CYP1A1 mRNA (Fig. 2C). Remarkably, knockdown of AHR rescued endothelial cell tube formation (Fig. 2D) and partially rescued endothelial cell proliferation (Fig. 2E) with IS treatment. Similar to HUVECs, cultured aortic rings transfected with the shAHR exhibited a ∼88% reduction in AHR mRNA levels (Fig. 2F) and ∼89% reduction in CYP1A1 mRNA levels following IS treatment (Fig. 2G), confirming robust knockdown in this tissue culture model. Genetic knockdown of the AHR in aortic rings abolished IS-mediated impairments in aortic ring sprouting (Fig. 2, H and I), confirming a direct role for the AHR in mediating IS-associated impairments in angiogenesis.
Figure 2.
Genetic knockdown of AHR rescues IS impairment in angiogenesis. A: mRNA validation of AHR knockdown in HUVECs (n = 4/group). B: representative Western blot demonstrating AHR knockdown at the protein level. C: mRNA quantification of CYP1A1 activation in HUVECs following DMSO or IS treatment (n = 5/group). D: representative images and quantification endothelial cell tube formation on Matrigel-coated dishes (n = 12/group). Scale bar = 200 µm. E: endothelial cell proliferation at 0, 24, 48, 72 h with IS and DMSO treatment (n = 8/group). F: quantification of AHR knockdown in aortic rings (n = 3/group). G: mRNA quantification of CYP1A1 in aortic rings following DMSO or IS treatment (n = 3/group). H: representative phase-contrast images of aortic rings transfected with AHR knockdown and treated with IS. Scale bar = 0.01 inches. I: quantification of total sprouts, mean sprout length (µm), and number of branched connections in treated aortic rings (n = 12–17/group). aP < 0.05 vs. DMSO, bP < 0.05 vs. Scramble (within treatment) using two-way ANOVA with Tukey’s post hoc testing. Error bars = SD. AHR, aryl hydrocarbon receptor; IS, indoxyl sulfate; HUVECs, human umbilical vein endothelial cells.
Pharmacological AHR Antagonism Rescues IS-Mediated Impairment in Endothelial Cell Proliferation and Tube Formation
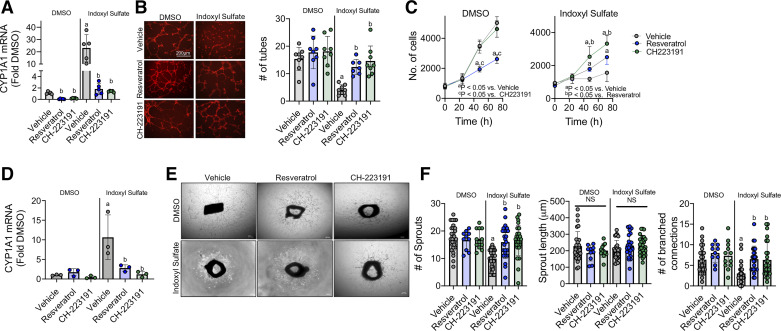
Next, we sought to examine the impact of two established pharmacologic AHR antagonists, resveratrol (37) and CH223191 (38) on IS-treated HUVECs and aortic rings. Treatment of HUVECs with either 25 μM resveratrol or 1 μM CH223191 markedly reduced indoxyl sulfate-mediated increases in CYP1A1 mRNA (Fig. 3A). Similar to AHR knockdown, both antagonists rescued the impairments in endothelial cell tube formation with IS treatment (Fig. 3B). Interestingly, both CH223191 and resveratrol improved endothelial cell proliferation (Fig. 3C) with IS exposure, although CH223191 clearly had a stronger benefit compared with resveratrol. Notably, resveratrol significantly impaired proliferation in DMSO treatment (Fig. 3C). Nonetheless, resveratrol treatment still resulted a modest, but statistically significant, increase in endothelial cell proliferation compared with vehicle-treated cells exposed to IS. In tissue culture, aortic rings treated with resveratrol or CH223191 extinguished the indoxyl sulfate-mediated increase in CYP1A1 mRNA levels (Fig. 3D). Pharmacologic AHR antagonism significantly improved aortic ring sprouting (Fig. 3, E and F). Altogether, these data highlight the therapeutic potential of both resveratrol and CH223191 in preventing IS impairments on angiogenesis in cell and tissue culture models.
Figure 3.
Pharmacological antagonism of AHR attenuates IS impairments in angiogenesis. A: mRNA quantification of CYP1A1 levels (index of AHR activation) with 25 µM resveratrol or 1 µM CH223191 in HUVECs (n = 5/group). B: representative images and quantification of endothelial cells tube formation with either IS or DMSO with or without the AHR antagonists (n = 7–8/group). Scale bar = 200 µm. C: quantification of endothelial cell proliferation following treatment with IS or DMSO coincident with either resveratrol or CH223191 (n = 12/group). D: quantification of CYP1A1 mRNA levels in aortic rings treated with either DMSO or indoxyl sulfate with or without AHR antagonism (n = 3/group). E: representative phase-contrast images of aortic rings treated with AHR antagonists alongside with DMSO or IS. Scale bar = 0.01 inches. F: quantification of total sprouts, mean sprout length (µm), and number of branch connections from aortic ring experiments (n = 10–17/group). aP < 0.05 vs. DMSO (within treatment), bP < 0.05 vs. Vehicle (within group—DMSO or IS) using two-way ANOVA with Tukey’s post hoc testing. Error bars = SD. AHR, aryl hydrocarbon receptor; IS, indoxyl sulfate; HUVECs, human umbilical vein endothelial cells.
Expression of a Constitutively Active AHR Decreases Endothelial Cell Proliferation and Impairs Tube Formation
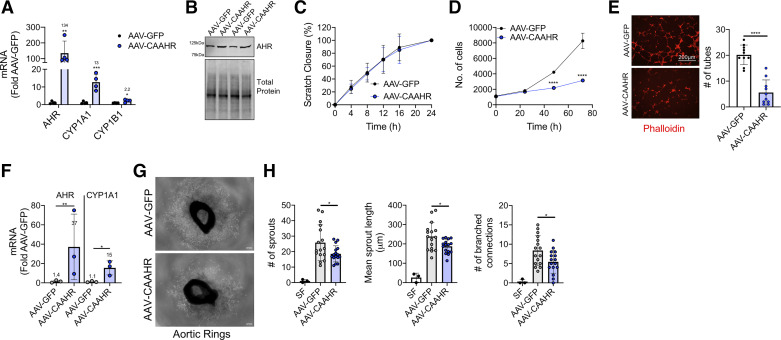
It is plausible that indoxyl sulfate may have non-AHR-mediated effects on endothelial cell biology that negatively impact angiogenic processes. Under normal circumstances, the AHR is sequestered in the cytosol until ligand binding, which triggers conformation changes that facilitate nuclear translocation. Deletion of the ligand-binding domain within the AHR results in constitutive transcriptional activity (35). We generated an adeno-associated virus encoding a constitutively active AHR (AAV-CAAHR) using this approach. Expression of the AAV-CAAHR in HUVECs significantly increased in AHR mRNA (Fig. 4A) and elevated protein abundance (Fig. 4B). Crucially, expression of the CAAHR plasmid resulted in substantial increases in both CYP1A1 and CYP1B1 mRNA expression (Fig. 4A), confirming constitutive transcriptional activity of the CAAHR. AAV-CAAHR expression did not alter endothelial cell migration (Fig. 4C) but significantly impaired both endothelial cell proliferation (Fig. 4D) and tube formation (Fig. 4D). In the aortic ring model, infection with AAV-CAAHR resulted in significant increases in mRNA levels for AHR and CYP1A1 (Fig. 4F), which was found to cause decreased sprouting and branching (Fig. 4, G and H) Taken together, these findings demonstrate that AHR activation plays a direct role in indoxyl sulfate mediated impairments in angiogenesis.
Figure 4.
Expression of a constitutively active AHR impairs endothelial cell proliferation and angiogenesis. A: AHR, CYP1A1, and CYP1B1 mRNA levels in HUVECs transfected with AAV-GFP or the constitutively active AHR (AAV-CAAHR; n = 4/group). B: representative western blot image AHR/CAAHR protein abundance in HUVECs. C: quantification of endothelial cell migration at 0, 4, 8, 12, 16, and 24 h treated with AAV-GFP or AAV-CAAHR (n = 5–7/group). D: endothelial cell proliferation transfected with AAV-CAAHR or AAV-GFP (n = 16/group). E: representative images (scale bar = 200 µm) and quantification endothelial cell tube formation on Matrigel with HUVECs transfected with AAV-CAAHR or AAV-GFP (n = 10/group). F: mRNA expression of AHR and CYP1A1 in murine aortic rings infected with AAV-GFP or AAV-CAAHR (n = 3/group). G: representative phase-contrast images of aortic rings infected with AAV-CAAHR or AAV-GFP. Scale bar = 0.01 inches. H: quantification of total sprouts, mean sprout length (µm), and number of branch connections in aortic rings infected with AAV-GFP or AAV-CAAHR (n = 18/group; n = 3 for serum free). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 using two-tailed Student’s t test. Error bars = SD. AHR, aryl hydrocarbon receptor; HUVECs, human umbilical vein endothelial cells.
DISCUSSION
Atherosclerotic cardiovascular diseases including peripheral arterial disease, coronary heart disease, and cerebrovascular disease are rampant in patients in CKD (39–41). In these conditions, adaptive angiogenesis is required for maintaining adequate balance of oxygen delivery to match tissue/organ demands. It has been previously reported that rodents with chronic kidney disease display impaired angiogenic abilities (12, 15, 17); however, the molecular mechanisms underlying this phenotype remain unknown. In this study, mechanistic cell and tissue culture experiments establish that indoxyl sulfate, a known uremic toxin associated with cardiovascular disease risk (42), impairs endothelial cell proliferation, tube formation, and sprouting. Mechanistically, genetic knockdown and pharmacologic antagonism of the AHR prevented the negative impact of indoxyl sulfate in these endothelial cell and tissue culture models. Finally, expression of a constitutively active AHR alone was sufficient to impair endothelial cell proliferation, tube formation, and sprouting confirming a direct role of the AHR in impaired angiogenic abilities.
Indoxyl sulfate is produced from tryptophan metabolism by bacteria present in the gastrointestinal tract which is normally filtered by the kidneys and excreted in the urine. Indoxyl sulfate is present at concentrations of ∼0.5–1 mM in the blood of patients with CKD (18, 29, 43) and is poorly filtered by dialysis membranes because it is protein bound (44). Notably, indoxyl sulfate has been reported to negatively impact multiple aspects of endothelial cell biology. Recently studies have demonstrated that treatment of endothelial cells and/or isolated blood vessels resulted in impaired nitric oxide signaling (45), cell senescence (46, 47), and increased apoptosis (48). Consistent with data reported herein, previous studies in endothelial cell culture systems have reported impaired tube formation (14, 49) and cell proliferation (49–51). These biological effects of indoxyl sulfate have been shown to manifest as impaired neovascularization and perfusion recovery in a rodent model of limb ischemia (14). The ability for endothelial cells to adequately proliferate is essential for the development of new blood vessels. Mechanistically, ligand activation of the AHR has been implicated in blocking entry into the S phase of the cell cycle. The AHR interacts with the RB/E2F axis leading to decreased phosphorylation of cyclin-dependent kinase 2 (CDK2) resulting in decreased CDK2 activity and the inability for cells to complete DNA synthesis [reviewed by (52)]. In fact, decreased DNA synthesis in HUVECs has been demonstrated following treatment with the AHR agonist 3-methylcholanthrene (53). Recent studies have reported that indoxyl sulfate impaired endothelial cell migration (14, 49, 54). These findings contrast the data herein, as well as other published studies (55), which observed no impairment in endothelial cell migration. The reasons for these conflicting results are difficult to decipher but could be related to slight differences in endothelial cell types and matrix used, as well as the inclusion of proliferation inhibitors (mitomycin c) during migration assays.
It has been established that indoxyl sulfate is an endogenous ligand of the AHR (21, 24, 56, 57). The AHR is a cytosolic, ligand-mediated transcription factor best known for its role in xenobiotic metabolism of exogenous and endogenous toxins. Accordingly, there is growing interest in understanding the role of the AHR in mediating the cardiovascular effects on CKD. The AHR is best studied in the context of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure, a halogenated aromatic hydrocarbon commonly found in tobacco smoke and air pollutants. Consistent with findings in the current study with indoxyl sulfate-mediated AHR activation, treatment of HUVECs with TCDD decreases endothelial cell viability and proliferation (58). Moreover, 3-methylcholanthrene (a polycystic aromatic hydrocarbon) known to activate the AHR was also found to impair proliferation in HUVECs (53). In agreement with these cell culture studies, AHR activation by benzo[a]pyrene was found to impair postischemia angiogenesis in mice, whereas postischemic angiogenesis was normal in AHR-null mice (59). To isolate the role of the AHR activation, this is the first study to demonstrate that expression of a constitutively active AHR (via deletion of the ligand binding domain) alone is sufficient to impair endothelial cell proliferation and angiogenesis, confirming a pivotal role of the AHR in endothelial cell biology.
In the current study, indoxyl sulfate was found to cause robust AHR activation, evidenced by substantial increases in CYP1A1 gene expression. Genetic knockdown and pharmacological blockade of the AHR both improved proliferation and rescued angiogenesis with indoxyl sulfate treatment. These findings are consistent with results obtained for other AHR ligands such as 3-methylcholanthrene and TCDD (53). Further to this, treatment of CKD mice with AST-120, an oral adsorbent of uremic toxins, was also found to improve postischemic angiogenesis (14). Hence, these consistent observations across numerous studies highlight the therapeutic potential of targeting AHR antagonists and/or depletion of uremic toxins for improving endothelial cell health and function in the context of CKD. It is important to note that many genes in addition to CYP1A1 have dioxin response elements within their promoter regions and could play a role in the AHR-mediated impairment in angiogenesis. Future work is needed to dissect the downstream molecular mechanisms by which AHR activations impairs angiogenesis.
In this study, in vitro cell and tissue culture systems were used to evaluate the impact of indoxyl sulfate and AHR activation on angiogenic processes. Genetic knockdown and AHR antagonism resulted in an incomplete rescue (albeit markedly improved from IS-treated controls) of cell proliferation, but a near-completed rescue of tube formation and sprouting. This observation can also be found while examining the effects of resveratrol treatment which negatively impacted proliferation (Fig. 3C), consistent with previous findings (60, 61). Despite this impact on proliferation, tube formation in resveratrol-treated HUVECs was normal under DMSO conditions and significantly improved with IS treatment. It is important to consider that many different biological processes are required for the tube formation assay. For example, the tube formation assay with HUVECs involves seeding the cells onto Matrigel (basement membrane extract) where they then undergo proliferation, migration, and differentiation to form lumen-like structures that require cellular junctions (62–64). Beyond proliferation and migration, a number of cell processes including gene expression, protein synthesis, collagen remodeling, and remodeling of the extracellular matrix and cytoskeletal structure are required for proper tube formation (65–70). We did not assess these various cellular processes that could have been impacted by AHR activation and are possible explanation for the complete rescue of tube formation despite only partial rescue in proliferative ability.
In vivo angiogenesis often involves microvascular sprouting and paracrine interactions with nonendothelial cells such as pericytes and fibroblasts. These factors cannot be recapitulated using endothelial cell culture models. Thus, we used intact vascular explants (aortic rings) to more accurately model this complex environment (34, 71). Using aortic ring explants, chronic AHR activation was found to impair sprouting, which could be rescued by genetic AHR knockdown or chemical AHR antagonism (Figs. 2 and 3). A limitation of the current study is that endothelial specific labeling was not performed for sprouting assays, but instead analysis was performed using phase-contrast images as previously described (34, 71). We performed blinded image analysis by trained personnel who distinguished sprouting microvessels from fibroblasts on the basis of their larger thickness and cohesive pattern of growth. Nonetheless, we cannot fully exclude the potential for nonendothelial cell structures impacting these results. Due to these limitations, future studies employing preclinical in vivo models of angiogenesis are warranted to confirm results herein.
In summary, the current study demonstrates that a physiologically relevant concentration of IS impairs endothelial cell proliferation and angiogenesis via AHR-dependent mechanisms. These impairments are recapitulated by expression of a constitutively active AHR, demonstrating sufficiency of AHR activation for impairing angiogenesis. On the basis that atherosclerotic diseases that disrupt oxygen delivery are highly prevalent in patients with CKD, therapies that inhibit AHR signaling may prove beneficial to patients. These future preclinical and translational studies are needed to confirm therapeutic efficacy of AHR antagonism for improving cardiovascular outcomes in CKD.
GRANTS
This study was funded in part by grants from the American Heart Association (18CDA34110044), NIH/National Heart, Blood, and Lung Institute (R01-HL149704), and a seed grant from the University of Florida Office of Research awarded to T. E. Ryan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.R.S. and T.E.R. conceived and designed research; Z.R.S., M.C., N.P.B., and T.E.R. performed experiments; Z.R.S., M.C., N.P.B., and T.E.R. analyzed data; Z.R.S. and T.E.R. interpreted results of experiments; Z.R.S. and T.E.R. prepared figures; Z.R.S. and T.E.R. drafted manuscript; Z.R.S., M.C., N.P.B., and T.E.R. edited and revised manuscript; Z.R.S., M.C., N.P.B., and T.E.R. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jeremy Lalla for assistance with some blinded image analysis.
REFERENCES
- 1.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet 382: 260–272, 2013. [Erratum in Lancet 382: 208, 2013]. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CE, Hamm LL, Batuman G, Kumbala DR, Chen CS, Kallu SG, Siriki R, Gadde S, Kleinpeter MA, Krane NK, Simon EE, He J, Chen J. The association of angiogenic factors and chronic kidney disease. BMC Nephrol 19: 117, 2018. doi: 10.1186/s12882-018-0909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David S, John SG, Jefferies HJ, Sigrist MK, Kümpers P, Kielstein JT, Haller H, McIntyre CW. Angiopoietin-2 levels predict mortality in CKD patients. Nephrol Dial Transplant 27: 1867–1872, 2012. doi: 10.1093/ndt/gfr551. [DOI] [PubMed] [Google Scholar]
- 4.Martens CR, Edwards DG. Peripheral vascular dysfunction in chronic kidney disease. Cardiol Res Pract 2011: 267257, 2011. doi: 10.4061/2011/267257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrero A, Montes R, Munoz-Terol J, Gil-Peralta A, Toro J, Naranjo M, Gonzalez-Perez P, Martin-Herrera C, Ruiz-Fernandez A. Peripheral arterial disease in patients with stages IV and V chronic renal failure. Nephrol Dial Transplant 21: 3525–3531, 2006. doi: 10.1093/ndt/gfl470. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix P, Aboyans V, Desormais I, Kowalsky T, Cambou JP, Constans J, Bura Riviere A, Investigators COPART. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J Vasc Surg 58: 966–971, 2013. doi: 10.1016/j.jvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Liew YP, Bartholomew JR, Demirjian S, Michaels J, Schreiber MJ Jr. Combined effect of chronic kidney disease and peripheral arterial disease on all-cause mortality in a high-risk population. Clin J Am Soc Nephrol 3: 1084–1089, 2008. doi: 10.2215/CJN.04411007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Hare AM, Bertenthal D, Shlipak MG, Sen S, Chren M-M. Impact of renal insufficiency on mortality in advanced lower extremity peripheral arterial disease. J Am Soc Nephrol 16: 514–519, 2005. doi: 10.1681/ASN.2004050409. [DOI] [PubMed] [Google Scholar]
- 9.Pasqualini L, Schillaci G, Pirro M, Vaudo G, Siepi D, Innocente S, Ciuffetti G, Mannarino E. Renal dysfunction predicts long-term mortality in patients with lower extremity arterial disease. J Intern Med 262: 668–677, 2007. doi: 10.1111/j.1365-2796.2007.01863.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaminski MR, Raspovic A, McMahon LP, Lambert KA, Erbas B, Mount PF, Kerr PG, Landorf KB. Factors associated with foot ulceration and amputation in adults on dialysis: a cross-sectional observational study. BMC Nephrol 18: 293, 2017. doi: 10.1186/s12882-017-0711-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Hare AM, Sidawy AN, Feinglass J, Merine KM, Daley J, Khuri S, Henderson WG, Johansen KL. Influence of renal insufficiency on limb loss and mortality after initial lower extremity surgical revascularization. J Vasc Surg 39: 709–716, 2004. doi: 10.1016/j.jvs.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Prommer HU, Maurer J, von Websky K, Freise C, Sommer K, Nasser H, Samapati R, Reglin B, Guimaraes P, Pries AR, Querfeld U. Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci Rep 8: 5317, 2018. doi: 10.1038/s41598-018-23663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka S, Tanaka T, Nangaku M. Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis (Basel) 1: 80–89, 2015. doi: 10.1159/000381515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung SC, Kuo KL, Huang HL, Lin CC, Tsai TH, Wang CH, Chen JW, Lin SJ, Huang PH, Tarng DC. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int 89: 574–585, 2016. doi: 10.1016/j.kint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Jacobi J, Porst M, Cordasic N, Namer B, Schmieder RE, Eckardt KU, Hilgers KF. Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int 69: 2013–2021, 2006. doi: 10.1038/sj.ki.5000448. [DOI] [PubMed] [Google Scholar]
- 16.Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev 98: 3–58, 2018. doi: 10.1152/physrev.00001.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiss RU, Fahlbusch FB, Jacobi J, Daniel C, Ekici AB, Cordasic N, Amann K, Hartner A, Hilgers KF. Blunted transcriptional response to skeletal muscle ischemia in rats with chronic kidney disease: potential role for impaired ischemia-induced angiogenesis. Physiol Genomics 49: 230–237, 2017. doi: 10.1152/physiolgenomics.00124.2016. [DOI] [PubMed] [Google Scholar]
- 18.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23: 1258–1270, 2012. [Erratum in J Am Soc Nephrol 24: 2127–2129, 2013]. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner L, Klein JD, Sands JM, Baylis C. Urea transporters are distributed in endothelial cells and mediate inhibition of L-arginine transport. Am J Physiol Renal Physiol 283: F578–F582, 2002. doi: 10.1152/ajprenal.00355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao S, Wagner L, Mahaney J, Baylis C. Uremic levels of urea inhibit L-arginine transport in cultured endothelial cells. Am J Physiol Renal Physiol 280: F989–F995, 2001. doi: 10.1152/ajprenal.2001.280.6.F989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 6: 934–949, 2014. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 43: 1522–1535, 2015. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res 67: 38–57, 2017. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49: 393–400, 2010. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, Gonzalez FJ, Murohara T. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol 27: 1297–1304, 2007. doi: 10.1161/ATVBAHA.106.138701. [DOI] [PubMed] [Google Scholar]
- 26.Iwano S, Asanuma F, Nukaya M, Saito T, Kamataki T. CYP1A1-mediated mechanism for atherosclerosis induced by polycyclic aromatic hydrocarbons. Biochem Biophys Res Commun 337: 708–712, 2005. doi: 10.1016/j.bbrc.2005.09.109. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds LM, Wan M, Ding J, Taylor JR, Lohman K, Su D, Bennett BD, Porter DK, Gimple R, Pittman GS, Wang X, Howard TD, Siscovick D, Psaty BM, Shea S, Burke GL, Jacobs DR, Jr, Rich SS, Hixson JE, Stein JH, Stunnenberg H, Barr RG, Kaufman JD, Post WS, Hoeschele I, Herrington DM, Bell DA, Liu Y. DNA methylation of the aryl hydrocarbon receptor repressor associations with cigarette smoking and subclinical atherosclerosis. Circ Cardiovasc Genet 8: 707–716, 2015. doi: 10.1161/CIRCGENETICS.115.001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Nishimura N, Kuo V, Fiehn O, Shahbaz S, Van Winkle L, Matsumura F, Vogel CF. Activation of aryl hydrocarbon receptor induces vascular inflammation and promotes atherosclerosis in apolipoprotein E-/- mice. Arterioscler Thromb Vasc Biol 31: 1260–1267, 2011. doi: 10.1161/ATVBAHA.110.220202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanholder R, De Smet R, Glorieux G, Argiles A, Baurmeister U, Brunet P, Clark W, Cohen G, De Deyn PP, Deppisch R, Descamps-Latscha B, Henle T, Jorres A, Lemke HD, Massy ZA, Passlick-Deetjen J, Rodriguez M, Stegmayr B, Stenvinkel P, Tetta C, Wanner C, Zidek W; the European Uremic Toxin Work Group (EUTox). Review on uremic toxins: classification, concentration, and interindividual variability. Kidney Int 63: 1934–1943, 2003. doi: 10.1046/j.1523-1755.2003.00924.x. [DOI] [PubMed] [Google Scholar]
- 30.Falero-Perez J, Sorenson CM, Sheibani N. Cyp1b1-deficient retinal astrocytes are more proliferative and migratory and are protected from oxidative stress and inflammation. Am J Physiol Cell Physiol 316: C767–C781, 2019. doi: 10.1152/ajpcell.00021.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2: 329–333, 2007. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 32.Diebold LP, Gil HJ, Gao P, Martinez CA, Weinberg SE, Chandel NS. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab 1: 158–171, 2019. doi: 10.1038/s42255-018-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClung JM, Reinardy JL, Mueller SB, McCord TJ, Kontos CD, Brown DA, Hussain SN, Schmidt CA, Ryan TE, Green TD. Muscle cell derived angiopoietin-1 contributes to both myogenesis and angiogenesis in the ischemic environment. Front Physiol 6: 161, 2015. doi: 10.3389/fphys.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D'Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7: 89–104, 2011. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 35.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA 99: 9990–9995, 2002. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narasimhan SS, Zulick E, Novikov O, Parks AJ, Schlezinger JJ, Wang Z, Laroche F, Feng H, Mulas F, Monti S, Sherr DH. Towards resolving the pro- and anti-tumor effects of the aryl hydrocarbon receptor. Int J Mol Sci 19: 1888, 2018. doi: 10.3390/ijms19051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res 58: 5707–5712, 1998. [PubMed] [Google Scholar]
- 38.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (CH-223191) prevents 2,3,7,8-TCDD-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol 69: 1871–1878, 2006. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 39.Mathew RO, Bangalore S, Lavelle MP, Pellikka PA, Sidhu MS, Boden WE, Asif A. Diagnosis and management of atherosclerotic cardiovascular disease in chronic kidney disease: a review. Kidney Int 91: 797–807, 2017. doi: 10.1016/j.kint.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 40.O'Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000. Circulation 109: 320–323, 2004. doi: 10.1161/01.CIR.0000114519.75433.DD. [DOI] [PubMed] [Google Scholar]
- 41.Yuan J, Zou XR, Han SP, Cheng H, Wang L, Wang JW, Zhang LX, Zhao MH, Wang XQ, on behalf of the C-STRIDE study group. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol 18: 23, 2017. doi: 10.1186/s12882-017-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lekawanvijit S, Kompa AR, Krum H. Protein-bound uremic toxins: a long overlooked culprit in cardiorenal syndrome. Am J Physiol Renal Physiol 311: F52–F62, 2016. doi: 10.1152/ajprenal.00348.2015. [DOI] [PubMed] [Google Scholar]
- 43.Vanholder R, Glorieux G, De Smet R, Lameire N.; European Uremic Toxin Work G . New insights in uremic toxins. Kidney Int Suppl 63: S6–S10, 2003. doi: 10.1046/j.1523-1755.63.s84.43.x. [DOI] [PubMed] [Google Scholar]
- 44.Piroddi M, Bartolini D, Ciffolilli S, Galli F. Nondialyzable uremic toxins. Blood Purif 35: 30–41, 2013. doi: 10.1159/000350846. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Takayanagi K, Kojima M, Katome T, Taguchi K, Kobayashi T. Direct impairment of the endothelial function by acute indoxyl sulfate through declined nitric oxide and not endothelium-derived hyperpolarizing factor or vasodilator prostaglandins in the rat superior mesenteric artery. Biol Pharm Bull 42: 1236–1242, 2019. doi: 10.1248/bpb.b19-00177. [DOI] [PubMed] [Google Scholar]
- 46.Adelibieke Y, Shimizu H, Muteliefu G, Bolati D, Niwa T. Indoxyl sulfate induces endothelial cell senescence by increasing reactive oxygen species production and p53 activity. J Ren Nutr 22: 86–89, 2012. doi: 10.1053/j.jrn.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 47.Carmona A, Guerrero F, Buendia P, Obrero T, Aljama P, Carracedo J. Microvesicles derived from indoxyl sulfate treated endothelial cells induce endothelial progenitor cells dysfunction. Front Physiol 8: 666, 2017. doi: 10.3389/fpls.2017.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, Yu HT, Kim MC, Cho JY, Lee CJ, Kim HC, Park S, Lee WW. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci Rep 7: 3057, 2017. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo KL, Zhao JF, Huang PH, Guo BC, Tarng DC, Lee TS. Indoxyl sulfate impairs valsartan-induced neovascularization. Redox Biol 30: 101433, 2020. doi: 10.1016/j.redox.2020.101433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int 65: 442–451, 2004. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu VC, Young GH, Huang PH, Lo SC, Wang KC, Sun CY, Liang CJ, Huang TM, Chen JH, Chang FC, Chen YL, Kuo YS, Chen JB, Chen JW, Chen YM, Ko WJ, Wu KD; The NSARF group. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: modulation by statin. Angiogenesis 16: 609–624, 2013. doi: 10.1007/s10456-013-9339-8. [DOI] [PubMed] [Google Scholar]
- 52.Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 77: 713–722, 2009. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Juan SH, Lee JL, Ho PY, Lee YH, Lee WS. Antiproliferative and antiangiogenic effects of 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, in human umbilical vascular endothelial cells. Eur J Pharmacol 530: 1–8, 2006. doi: 10.1016/j.ejphar.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 54.Monteiro EB, Soares EDR, Trindade PL, de Bem GF, Resende AC, Passos M, Soulage CO, Daleprane JB. Uraemic toxin-induced inflammation and oxidative stress in human endothelial cells: protective effect of polyphenol-rich extract from acai. Exp Physiol 105: 542–551, 2020. doi: 10.1113/EP088080. [DOI] [PubMed] [Google Scholar]
- 55.Pei J, Juni R, Harakalova M, Duncker DJ, Asselbergs FW, Koolwijk P, Hinsbergh VV, Verhaar MC, Mokry M, Cheng C. Indoxyl sulfate stimulates angiogenesis by regulating reactive oxygen species production via CYP1B1. Toxins (Basel ) 11: 454, 2019. doi: 10.3390/toxins11080454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brito JS, Borges NA, Esgalhado M, Magliano DC, Soulage CO, Mafra D. Aryl hydrocarbon receptor activation in chronic kidney disease: role of uremic toxins. Nephron 137: 1–7, 2017. doi: 10.1159/000476074. [DOI] [PubMed] [Google Scholar]
- 57.Lekawanvijit S, Kompa AR, Wang BH, Kelly DJ, Krum H. Cardiorenal syndrome: the emerging role of protein-bound uremic toxins. Circ Res 111: 1470–1483, 2012. [Erratum in Circ Res 114: e23, 2014]. doi: 10.1161/CIRCRESAHA.112.278457. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Wang K, Zou QY, Magness RR, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin differentially suppresses angiogenic responses in human placental vein and artery endothelial cells. Toxicology 336: 70–78, 2015. doi: 10.1016/j.tox.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichihara S, Yamada Y, Gonzalez FJ, Nakajima T, Murohara T, Ichihara G. Inhibition of ischemia-induced angiogenesis by benzo[a]pyrene in a manner dependent on the aryl hydrocarbon receptor. Biochem Biophys Res Commun 381: 44–49, 2009. doi: 10.1016/j.bbrc.2009.01.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LaFoya B, Munroe JA, Albig AR. A comparison of resveratrol and other polyphenolic compounds on Notch activation and endothelial cell activity. PLoS One 14: e0210607, 2019. doi: 10.1371/journal.pone.0210607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szende B, Tyihak E, Kiraly VZ. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med 32: 88–92, 2000. doi: 10.1038/emm.2000.16. [DOI] [PubMed] [Google Scholar]
- 62.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis 12: 267–274, 2009. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 63.Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5: 628–635, 2010. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- 64.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem 49: 32–40, 2003. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 65.Elkin M, Miao HQ, Nagler A, Aingorn E, Reich R, Hemo I, Dou HL, Pines M, Vlodavsky I. Halofuginone: a potent inhibitor of critical steps in angiogenesis progression. Faseb J 14: 2477–2485, 2000. doi: 10.1096/fj.00-0292com. [DOI] [PubMed] [Google Scholar]
- 66.Grant DS, Lelkes PL, Fukuda K, Kleinman HK. Intracellular mechanisms involved in basement-membrane induced blood-vessel differentiation invitro. In Vitro Cell Dev Biol 27: 327–336, 1991. doi: 10.1007/BF02630910. [DOI] [PubMed] [Google Scholar]
- 67.Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement-membrane collagen-synthesis prevent endothelial-cell alignment in Matrigel in-vitro and angiogenesis in-vivo. Lab Invest 71: 575–582, 1994. [PubMed] [Google Scholar]
- 68.Kinsella JL, Grant DS, Weeks BS, Kleinman HK. Protein-kinase-C regulates endothelial-cell tube formation on basement-membrane matrix. Exp Cell Res 199: 56–62, 1992. doi: 10.1016/0014-4827(92)90461-G. [DOI] [PubMed] [Google Scholar]
- 69.Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol 158: 3408–3416, 1997. [PubMed] [Google Scholar]
- 70.Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9: 267–285, 2005. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta - a quantitative assay of angiogenesis invitro. Lab Invest 63: 115–122, 1990. [PubMed] [Google Scholar]