Abstract
The thiol redox proteome refers to all proteins whose cysteine thiols are subjected to various redox-dependent posttranslational modifications (PTMs) including S-glutathionylation (SSG), S-nitrosylation (SNO), S-sulfenylation (SOH), and S-sulfhydration (SSH). These modifications can impact various aspects of protein function such as activity, binding, conformation, localization, and interactions with other molecules. To identify novel redox proteins in signaling and regulation, it is highly desirable to have robust redox proteomics methods that can provide global, site-specific, and stoichiometric quantification of redox PTMs. Mass spectrometry (MS)-based redox proteomics has emerged as the primary platform for broad characterization of thiol PTMs in cells and tissues. Herein, we review recent advances in MS-based redox proteomics approaches for quantitative profiling of redox PTMs at physiological or oxidative stress conditions and highlight some recent applications. Considering the relative maturity of available methods, emphasis will be on two types of modifications: 1) total oxidation (i.e., all reversible thiol modifications), the level of which represents the overall redox state, and 2) S-glutathionylation, a major form of reversible thiol oxidation. We also discuss the significance of stoichiometric measurements of thiol PTMs as well as future perspectives toward a better understanding of cellular redox regulatory networks in cells and tissues.
Keywords: posttranslational modifications, protein thiols, redox proteomics, site occupancy, stoichiometric quantification, thiol proteome
INTRODUCTION
Cellular redox homeostasis is an essential feature of the physiological steady state that is tightly controlled by continuous signaling for the production and elimination of electrophiles and nucleophiles. A shift from redox homeostasis to either oxidative or reductive stress is often associated with pathological states (1). It has been well recognized that reactive oxygen species (ROS) and the antioxidant defense systems (e.g., antioxidant enzymes, glutathione, protein thiols, and other antioxidants) play an integral role in maintaining redox homeostasis (2). There are multiple endogenous sources of ROS in mammalian cells, including the mitochondrial respiratory chain, membrane-bound NADPH oxidases, endoplasmic reticulum (ER) oxidoreductin-1 (ERO-1), cyclooxygenases (COX), cytochrome P-450, and xanthione oxidase (3). ROS can be further converted to reactive nitrogen species (RNS) in the presence of nitric oxide produced by nitric oxide synthases (4). It has become increasingly clear that ROS and RNS act as important second messengers in modulating cell signaling and various biological processes (3, 5–7). A major mode of ROS/RNS-mediated signaling and regulation is through posttranslational modifications (PTMs) of proteins, in which a number of amino acids including methionine, tyrosine, and, most importantly, cysteine are sensitive to oxidation and other modifications (8, 9).
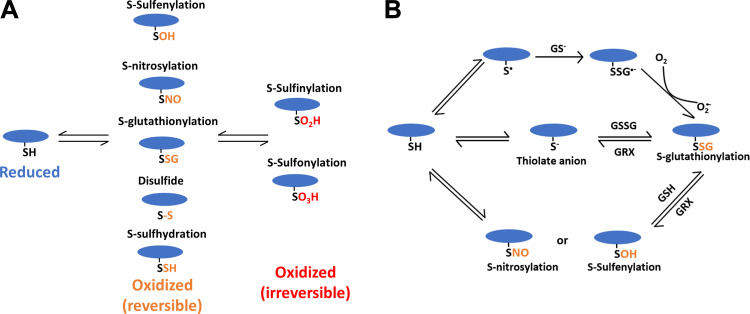
Protein cysteine thiols (also known as the thiol proteome) play a central role on how cells utilize ROS/RNS to regulate cellular activities through dynamic redox-dependent PTMs (10–13). The nucleophilic cysteine thiols can be oxidized to both reversible forms such as S-sulfenylation (SOH), S-nitrosylation (SNO), S-glutathionylation (SSG), disulfide, and S-sulfhydration (SSH) and irreversible forms such as S-sulfinylation (SO2H) and S-sulfonylation (SO3H) (11, 14) (Fig. 1A). For a specific redox PTM, multiple possible pathways exist in vivo, which may involve the interconversion among different forms of PTMs. For instance, both SNO and SOH can serve as an intermediate in the formation of SSG modification (Fig. 1B).
Figure 1.
The thiol redox proteome. A: multiple types of redox modifications on cysteine thiols. While most oxidized posttranslational modifications (PTMs) are reversible, irreversible forms include the formation of sulfinic and sulfonic acids. B: multiple pathways in the formation of protein-S-glutathionylation (SSG).
The reversibility of redox PTMs can serve as “redox switches” (15) on enzyme activity (16, 17), conformation (18, 19), subcellular localization (20), and interactions with other molecules (21), thus providing a mechanism for redox-dependent regulation in cellular physiology (16). For instance, SNO was shown to regulate protein kinases by inhibiting kinase activity or by altering the interaction between a kinase and its substrates (16). Similarly, disulfide formation may affect protein conformation, as highlighted in a recent study where a cell surface integrin (αIIbβ3) involved in blood clotting was shown to be dependent on the state of a disulfide (Cys177-Cys184). The reduction of this disulfide by the oxidoreductase ERp5 induces a conformation change that in turn causes the release of fibrinogen and a possible decrease in blood clot formation (22). Moreover, altered redox modifications may contribute to aging and the pathogenesis of many human diseases (23), such as Parkinson’s disease (24), lung disease (25), and cardiac physiopathology (26). To better understand the landscape of redox regulation of the thiol proteome, it is crucial to have robust proteomics and biochemical methods for accurate identification and quantification of the often low abundant and labile redox PTMs.
A number of biochemical methods had been developed to assess protein thiol redox state, identify redox-sensitive proteins, and reveal the nature of modifications on protein thiols (27–29). For example, Click PEGylation, an immunoblotting-based assay to assess protein redox status, has been demonstrated for distinguishing reduced and oxidized protein thiols (30). These biochemical methods have served as important tools in redox biology. For instance, the SSG modification of endothelial nitric oxide synthase (eNOS), a key mediator of cell signaling that can produce both nitric oxide and superoxide, was discovered by immunoblotting with an anti-eNOS antibody and an anti-GSH antibody (31). Importantly, oxidative stress-induced SSG modification on eNOS decreases its activity in nitric oxide production and increase its ability in generating superoxide. The biotin switch technique (BST) is another broadly applied biochemical method for assaying SNO (32) and other redox PTMs (33, 34). One main limitation of these biochemical approaches is that they are low throughput by nature, typically analyzing a single protein without providing information regarding the exact cysteine site(s) susceptible to modification (35, 36).
Mass spectrometry (MS)-based proteomics has recently become the primary technology for broad identification and quantification of site-specific redox PTMs. Termed “thiol-based redox proteomics”, it has become an emerging area focusing on the thiol redox proteome, all protein thiols subjectable to a variety of PTMs, in order to understand the redox regulation in signaling and metabolism under physiological or pathological conditions. With recent advances in redox proteomics, it is now possible to quantify up to 10,000 unique Cys sites of specific redox PTMs in cells or tissues in a single experiment (37–44). Indeed, a complex landscape of the thiol redox proteome, subcellular redox compartmentalization, and tissue specificity as well as potential novel redox regulators have been revealed. In this review, we provide a brief overview of the general approaches for profiling thiol redox PTMs, with a focus on recent advances in analyzing total reversible cysteine modifications and protein-SSG due to the availability of relative robust approaches for both types of modifications. We also discuss the rationale and significance in performing stoichiometric analysis of redox PTMs along with other future perspectives on thiol redox proteomics.
REDOX PROTEOMICS APPROACHES FOR THIOL PTMS
One of the most critical aspects of thiol-based redox proteomics is to preserve the endogenous redox state of protein thiols during sample storage and processing. In general, tissue samples can be preserved through being flash-frozen and stored at –80°C (41). Several methods have been employed for preserving the thiol redox state of cells and tissues, including trichloroacetic acid (TCA) quenching during cell harvesting to limit the thiolate reactivity and immediate blocking all free thiols with highly reactive alkylating reagents such as N-ethylmaleimide (NEM) or iodoacetamide (IAM) (14, 41, 44, 45). Following the blocking of free thiols, two general strategies have been explored for identifying reversible thiol PTMs. The first is to directly detect the redox PTMs of interest as exemplified by the clickable glutathione approach for protein-SSG, which consists of introducing an azido-glutathione in situ to facilitate a downstream “click chemistry” reaction, avidin pull-down, and MS-based detection of SSG-modified peptides (46, 47). The second strategy, based on an indirect detection concept, has become more popular due to the labile nature of many redox PTMs. In this, all free thiols are initially effectively blocked by alkylation (e.g., NEM), and reversible thiol PTMs are then selectively reduced back to free thiols. The resulting nascent free thiols are either further tagged or captured using different approaches before LC-MS/MS.
The biotin switch technique (BST) is the first reported indirect approach for detecting thiol redox PTMs (32). The original BST consists of three steps to capture SNO (Fig. 2A). The first step is to block free protein thiols with S-methyl methanethiosulfonate (MMTS). Next, SNO is reduced to free thiols with ascorbate. Last, nascent free thiols are labeled with thiol-specific biotinylating reagents such as biotin-HPDP, which allows for the purification of these proteins via avidin for either Western blotting or MS analyses. Another common strategy involves the use of Thiopropyl Sepharose 6B resin instead of biotin to facilitate resin-assisted capture (RAC) of thiol-containing peptides or proteins (45, 48). Like BST, the RAC approach also consists of the initial blocking and reduction steps (Fig. 2B). However, RAC directly captures free thiol-containing proteins by forming stable disulfides between protein thiols and the resin, which enables further on-resin digestion and isobaric labeling with reagents such as tandem mass tags (TMT) for multiplexed quantification. The streamlined RAC-TMT workflow has been demonstrated as a robust tool for quantitative profiling of redox PTMs (37, 40, 41, 45, 49–52). It should be noted that both BST and RAC can be applied to different redox PTMs with specific reagents for selective reduction (45). Importantly, RAC displayed significantly improved enrichment specificity (>95% of the obtained peptides containing cysteine residues) compared with BST.
Figure 2.
General strategies in profiling the redox proteome. A: biotin switch technique. B: resin-assisted capture. Note that both approaches are indirect in the sense that a specific redox modification of interest (e.g., S-nitrolsylization, SNO) is selectively reduced back to free thiols, which were then enriched by either biotin-conjugated thiol-reactive reagents (e.g., biotin-HPDP) or thiol-affinity resins (e.g., Thiopropyl-Sepharose 6B).
In general, an effective redox proteomics workflow should meet several requirements for measurements of redox PTMs, including: (18) a high coverage of the thiol redox proteome (e.g., low abundant transcription factors); (53) accurate and site-specific quantification to identify critical cysteine sites; and (54) multiplexing capability for analyzing and quantifying multiple samples simultaneously. Given the unique chemistry of individual thiol PTMs, various techniques have been developed for their detection and quantification. To date, the detection of more transient modifications such as SNO and SOH at physiological levels is still challenging by MS approaches. Since many of the techniques for different PTMs have been previously discussed in detail and reviewed elsewhere (13, 14, 55–57), this review will focus on recent advances in MS-based approaches for analyzing thiol total oxidation as a measure of overall thiol redox state and SSG, the most prevalent thiol PTM, as well as the biological implications of the measurements of these modifications, especially in the aspect of absolute stoichiometric quantification. We also recognize the significance of other types of oxidative protein modifications in physiology and pathology (58). A number of previous reviews have discussed the identification and quantification of different oxidative modifications, including carbonylation and irreversible thiol modifications such as sulfinylation and sulfonylation (58–62).
TOTAL OXIDATION
Herein, we define “total oxidation” as all forms of reversible cysteine thiol modifications. It should be recognized that one limitation of most current approaches in quantifying total oxidation involves a reduction step that converts all reversibly oxidized thiols to free thiol (e.g., by DTT) such that irreversibly oxidized forms will be neglected. Fortunately, the abundances of these irreversible forms such as -SO2H or -SO3H are likely negligible for most Cys sites under physiological conditions (63). However, some enzymes such as peroxiredoxins utilize -SO2H formation on specific Cys sites as a redox switch, as -SO2H can be reversibly reduced by sulfiredoxin (64). Another caveat about the term total oxidation being used as a general measurement of the thiol redox state is that some forms of reversible thiol modifications such as S-acylation are not redox dependent. Similar to irreversible oxidation, the level of S-acylation is generally negligible compared with the other forms of redox modifications (45).
OxICAT is the first reported MS-based quantitative redox proteomics approach (65) for analyzing total oxidation which utilized the isotope-coded affinity tags (ICAT), a duplex reagent containing a cysteine-reactive group, a biotin group, and an isotopic coded link. The light version of the duplex is first used to label the free thiols, while the heavy version is used to label the oxidized thiols after reduction (Fig. 3A). The heavy 13C- versus light 12C-ICAT-labeled peptide intensity ratios measured by MS represent the abundance ratios of oxidized thiols versus reduced thiols on specific Cys sites, thus providing a stoichiometric measurement of total oxidation on individual Cys sites (65, 66). OxICAT has been widely used to study total oxidation in bacteria (65), yeast (67), diatoms (68), and mammalian cells (69). As a modified version of oxICAT, two different samples can be labeled with the duplex ICAT reagents for relative quantification of total oxidation. In this case, protein free thiols were first blocked by iodoacetamide (IAM), and the oxidized thiols were reduced and subsequently labeled with either the light or heavy ICAT reagent; thus, total oxidation levels between two samples can be comparatively analyzed (70–72). Further modification has been introduced to the original OxICAT to enable the quantification of SNO instead of total oxidation (73). Similar to ICAT, an alternative labeling reagent called thiol-reactive iodoacetyl tandem mass tag (iodoTMT) has recently been used to evaluate the relative abundance of reduced and oxidized protein thiols within the same sample (74).
Figure 3.
Quantitative proteomic approaches for profiling total oxidation. A: oxICAT strategy using duplex ICAT. Protein free thiols are first blocked with the light version of ICAT, and reversible PTMs are reduced by DTT and subsequently labeled with heavy ICAT. Following digestion and enrichment of ICAT-containing peptides, the light/heavy ratio indicates the level of reduced/oxidized thiols at a given Cys site. B: stable isotope cysteine labeling with iodoacetamide (SICyLIA). Protein free thiols from two samples are labeled with either heavy or light IAM, and the samples are pooled. Oxidized protein thiols are subsequently reduced by DTT and alkylated with NEM. Following digestion and fractionation, peptide samples are subjected to LC-MS/MS analysis without further enrichment. Note that this approach quantifies the level of reduced protein thiols and calculates the level of total oxidation indirectly. C: RAC-TMT method for total oxidation. The workflow consisted of blocking of protein free thiols with NEM, reduction with DTT, enrichment with Thiol-Sepharose resin on-resin digestion and isobaric labeling, elution, pooling of samples, and finally MS analysis; 10-plex TMT enables the multiplexing capacity of 10 samples currently. D: CPT-TMT-IMAC. In this workflow, free thiols were first blocked by IAM, and oxidized thiols were reduced by TCEP. Then, a CPT is introduced to alkylate nascent free thiols. Following trypsin digestion and TMT labeling, phosphatase was used to remove endogenous phosphorylation, and Cys-containing peptides were enriched by IMAC due to the added phosphate tags. CAM, carbamidomethylation of cysteine thiols by IAM; CPT, cysteine-reactive phosphate tag; IAM, iodoacetamide; ICAT, isotope-code affinity tags; IMAC, immobilized metal-ion affinity chromatography; NEM, N-ethylmaleimide; PTM, posttranslational modification; RAC, resin-assisted capture; -SOX, all forms of reversible modifications; TMT, tandem mass tags.
Instead of targeting the oxidized thiols, isotope-coded tags can also be used to label protein free thiols only. In such cases, a decrease in the level of protein free thiols would indicate an increase in total oxidation. With this concept, Reest et al. (42) recently reported the SICyLIA (stable isotope cysteine labeling with iodoacetamide) approach to assess proteome-wide total oxidation. Protein samples were first extracted in the presence of either light- or heavy-isotope-coded IAM for blocking free thiols (Fig. 3B). Two samples were then pooled, and oxidized thiols were reduced by DTT followed by NEM blocking and tryptic digestion. Due to the complexity of the resulting peptide mixture, an offline high pH reversed-phase LC fractionation was performed before final LC-MS/MS analysis to enhance the overall proteome coverage. The ratio between the light and heavy IAM-labeled peptide pairs indicates the relative levels of reduced thiols, which serves as an indirect readout for changes in total oxidation. Coupled with extensive fractionation, SICyLIA enabled quantification of over 9,000 and 4,000 cysteine-containing peptides in a cell line and mouse kidney, respectively, without specific enrichment of cysteine thiols. A main limitation of this workflow is its limited multiplexing capacity for relative quantification, since only two samples were processed simultaneously. In addition, subtle changes in total oxidation may be easily missed due to the quantification based on total reduced protein thiols.
Along similar lines, Fu et al. (39) reported a quantitative thiol reactivity profiling (QTRP) method by labeling protein thiols directly with an iodoacetamide-alkyne probe (IPM). Oxidized protein thiols were then reduced by DTT and blocked by IAM, followed by tryptic digestion. The IPM-labeled peptides were tagged with either a light or heavy isotope-coded UV-cleavable azido-biotin via click chemistry. The isotopically labeled samples were pooled, enriched by streptavidin, and released by UV exposure for final LC-MS/MS analysis. Similar to SICyLIA, the QTRP method also uses the abundance ratio between the light- and heavy-peptide ratios as readouts to quantify total oxidation. Among ∼ 6,500 peptides identified, more than 900 Cys sites were defined as H2O2 sensitive, with the half-maximal inhibitory concentrations less than 2.5 mM.
We and others have recently reported that the RAC (resin-assisted capture)-TMT (tandem mass tags) workflow (45, 75) as a general multiplexed quantification approach for various reversible redox PTMs, including SNO (50, 76), SSG (40, 52, 75), and total oxidation (40). For quantification of total oxidation (Fig. 3C), protein thiols are initially blocked with high concentration (e.g., 100 mM) of NEM at pH 6.0, and all reversible thiol modifications are reduced back to free thiols by DTT. Nascent free thiols are subsequently captured on Thiopropyl-Sepharose 6B resin. Multiplexed quantification is achieved by parallel processing of up to 10 or 16 samples and on-resin isobaric labeling with TMT reagents. The final cysteine-containing peptides from each sample are released from the resin by DTT reduction, and they are subsequently combined for LC-MS/MS analyses. The streamlined workflow has several advantages, including high enrichment specificity, quantification accuracy, multiplexing capacity within a single analysis, and ease of implementation. The RAC-TMT approach was first demonstrated for quantitative profiling of thiol oxidation in cyanobacteria in response to a light-dark cycle where ∼2,200 cysteine-containing peptides were quantified and ∼50% of peptides displayed significant redox changes in response to the light-dark cycle (40). More recently, RAC-TMT was applied to profile thiol oxidation in mouse macrophages (43) and rat lung tissues (43), where ∼4,000 and ∼7,000 Cys sites, respectively, were quantified. In another study, RAC was coupled with LC-MS/MS with a data-independent acquisition (DIA) mode to quantify the temporal changes of total oxidation of ∼4,200 Cys sites in A431 cells, a squamous cell skin cancer model, induced by epidermal growth factor (EGF) stimulation (37). All of these studies demonstrate the utility of RAC-TMT in providing a landscape view of cellular redox signaling networks and uncovering functional protein Cys sites susceptible to oxidative perturbations.
Another interesting approach took advantage of recent advances in phosphoproteomics by introducing a cysteine-specific phosphonate-adaptable tag (CysPAT) to convert the cysteine thiol to a phosphate group (77). The original CysPAT reagent was further improved as cysteine-reactive phosphate tags (CPT) and applied for profiling of total oxidation across 10 different mouse tissues in young and old animals (44), providing a comprehensive quantitative map of the mouse thiol redox proteome in vivo in the context of aging. In this CPT-TMT-IMAC workflow (Fig. 3D), CPT is used as an additional tagging step to convert nascent free thiols to phosphate groups. Following digestion and TMT labeling, the peptide mixtures were further treated with phosphatase, to remove endogenous phosphorylation, and enriched by immobilized metal-ion affinity chromatography (IMAC) before LC-MS/MS.
It should be noted that the RAC-TMT and CPT-TMT-IMAC approaches share a significant degree of conceptual similarity for quantitative profiling of total oxidation. The main difference lies in their enrichment strategies for cysteine-containing peptides, with RAC offering a direct capture on resin, whereas the CPT strategy needs an additional chemical tagging step to convert the Cys thiol to a phosphate group and a phosphatase treatment step to remove endogenous phosphorylation in order to facilitate IMAC enrichment of Cys-containing peptides. Both approaches are amendable to subsequent peptide-level fractionation if needed for achieving deeper proteome coverage.
PROTEIN S-GLUTATHIONYLATION
Besides the measurements of total oxidation, protein S-glutathionylation (SSG), the covalent binding of the tripeptide glutathione (GSH) to protein thiols, has become an increasingly studied thiol PTM by redox proteomics. SSG has emerged as an important type of thiol PTMs that regulates transcription, mitochondrial metabolism, apoptosis, and other processes (54, 78–84). The S-glutathionylation process may occur from reaction with oxidized GSH (GSSG) via thiol-disulfide exchange or through reactions between protein thiyl radical or other oxidized forms such as SNO and SOH and GSH (13, 31, 57, 85) (Fig. 1B). Enzyme- catalyzed formation of SSG is also possible. For example, the endoplasmic reticulum (ER) resident protein glutathione S-transferase Pi (GSTP1) was reported to catalyze SSG modifications of ER proteins (86). Given the high abundance of cellular GSH, it has been estimated that 2–6% of GSH is attached to proteins at steady state (87), suggesting that SSG is a major form of protein thiol oxidation. A well-documented role of SSG is its protective effect in preventing ROS-induced irreversible oxidation of intracellular proteins. Recently, this protective role of SSG has also been observed for extracellular cytokines such as IL-1β (88). ROS promotes the formation of SSG on Cys188 of IL-1β, which could be reduced through extracellular glutaredoxin 1 (Grx1) to reverse its bioactivity.
Several strategies have been used to detect SSG. One relies on anti-GSH antibody to pull down SSG-containing proteins (89, 90). Another approach is built upon the original BST concept with glutaredoxin 1 (GRX1) as a selectively deglutathionylation enzyme for indirect detection (53, 91, 92). Similar to the profiling of total oxidation described above, we have established a robust RAC-TMT workflow that allows for site-specific identification and multiplexed quantification of SSG (41, 52). Instead of using DTT as the reducing reagents for total oxidized thiols, a reduction cocktail containing a mutant of Grx1 (Grx1C14S) (93), GSSG, NADPH, and glutathione reductase was used to selectively reduce SSG back to protein thiols. The RAC-TMT approach has been widely applied in studying SSG from multiple sample types including macrophages (38, 49, 52), liver (75), muscle (41, 94), and lung (95).
In addition, a proteomic approach for direct detection of SSG has also been described (46, 47, 96). In this method (Fig. 4), a mutant of GSH synthetase (GS M4), which catalyzes the production of azido-GSH (N3-GSH) using azido‐alanine as a substrate in vivo, is introduced to a biological system of interest. GSH with the azido functional group does not interfere with its reactivity and thus can be incorporated into protein thiols as SSG-modified proteins, providing a handle to tag SSG modification directly. The azido-SSG-containing proteins are then tagged with a cleavable biotin-alkyne through click chemistry and further enriched by streptavidin-agarose resin. After tryptic digestion, the modified peptides are eluted by acidic cleavage of the linker and analyzed by LC-MS/MS. Unlike indirect approaches, the direct detection strategy alleviates the potential false discovery of SSG modifications due to nonspecific reduction in the indirect approach. Recently, a duplex workflow using a light- or heavy-labeled azido‐alanine was introduced, enabling a pairwise relative quantification based on the MS1 peak area of the isotopically labeled peptides (97). However, several limitations still exist in this approach. First, ectopic expression of a mutant GSH synthetase, a prerequisite for performing such an assay, may not be feasible for in situ SSG profiling (e.g., in tissues). Second, the levels of SSG detected may not reflect the in vivo levels at physiological conditions, given the need of introducing exogenous azido-alanine and the expressing of mutant enzyme GS M4. Last, the observed coverage for these studies was in the range of 1,000–2,000 Cys-containing peptides, much lower compared with the RAC-TMT and other recent approaches.
Figure 4.
Direct detection of S-glutathionylation (SSG). A glutathione synthetase mutant (GS M4) utilizes either light or heavy azido-alanine to produce isotope-coded N3-GSH, which can be incorporated into protein. Proteins with these GSH modifications are extracted, pooled, and tagged with biotin. Following enrichment, digestion, and cleavage of linkers, the GSH-modified proteins can be detected by MS directly.
SITE OCCUPANCY: THE IMPORTANCE OF STOICHIOMETRIC QUANTIFICATION
Since many PTMs are known to dynamically regulate protein function such as activity, structural conformation, and binding or interaction with other molecules, it is highly desirable to perform absolute stoichiometric quantification for the site occupancy of each PTM. PTM site occupancy (i.e, the percentage of the modified form in all molecules with and without modifications) is a critical aspect of quantification that is often overlooked or challenging; however, the significance of such site occupancy data is compelling for assessing the functional consequences of PTMs. In thiol redox proteomics, while most studies were focused on relative fold changes across experimental conditions, a number of studies have demonstrated the ability to perform absolute stoichiometric quantification of site occupancies of thiol PTMs (38, 40, 41, 44, 65).
Figure 5A illustrates the importance of measuring site occupancy. As shown, fold changes often do not provide a full picture of redox regulation, since Cys residues with very different occupancies could show the same fold change between two conditions. For instance, cysteine residues with a low (e.g., 1%), moderate (e.g., 10%), and high (e.g., 50%) basal occupancy, respectively, may display an identical twofold change under oxidative stress. If the thiol PTM leads to the inactivation of an enzyme, a twofold change in the 50% basal occupancy scenario would lead to a complete inhibition, whereas the same fold change on the 1% basal occupancy case would have little functional consequence. On the other hand, fold change measurements may be biased toward low-occupancy sites due to the fact that these sites have much more room to go higher, resulting in higher fold changes as shown in protein phosphorylation (98), and that a high-occupancy site (e.g., 50%) could never have more than a twofold change. Thus, it is critical to have stoichiometric quantification of site occupancies of PTMs to gain a full picture of the functional impact of PTM regulation. Moreover, the interpretation of site occupancy data may be dependent on the functional consequences of redox PTMs (e.g., activation or inhibition). For PTMs that result in an inhibitory effect (loss of function), a certain threshold of site occupancy may be required to have a significant impact. Some examples include the high SNO occupancy on caspase-3 and high SSG occupancy on eNOS that were reported to inhibit the protease (99) and NO production (35), respectively. For gain-of-function modification, a high occupancy may not be necessary, since any significant increase in the level of a PTM regardless of its occupancy would be effective in triggering downstream responses. Moreover, the site occupancy data may provide valuable information for inferring the possible functions of these Cys residues when assisted by examining protein crystal structures. Indeed, it was observed that Cys residues at enzymatic active sites generally had a higher level of occupancy compared to nonactive Cys residues within the same proteins, suggesting site occupancy as a potential indicator of protein functional sites (38). Finally, stoichiometric data on PTMs is important to assess their functional significance. For example, recent data on the stoichiometry of lysine acetylation make it still difficult to assess their significance because most acetylation sites are of low occupancy (median 0.02%) (100). On the other hand, similar to protein phosphorylation (101, 102), a large dynamic range (e.g., several orders of magnitude) of occupancies of redox PTMs have been observed (38, 44).
Figure 5.
Stoichiometric quantification of redox posttranslational modifications (PTMs). A: site occupancy vs. fold change. A Cys site with very different occupancies could experience the same fold change under oxidative stress. SOH), S-sulfenylation; SNO, S-nitrosylation; SSG, S-glutathionylation. B: resin-assisted capture-tandem mass tags (RAC-TMT) approach for stoichiometric quantification of total oxidation. Sample preparation of total oxidation samples includes N-ethylmaleimide (NEM) blocking of free thiols, and selective reduction of total oxidation by DTT. An aliquot of the same sample is used for total thiol profiling, in which no blocking is performed and all reversible modifications, indicated by -SOX (all forms of reversible modifications), are reduced by DTT. Subsequent steps including resin-based enrichment, tryptic digestion, isobaric labeling (tandem mass tags, or TMTs in this case), as well as elution of the peptides from the resin are performed for all samples at the same time. All samples are pooled and then analyzed by LC-MS. The site occupancy of total oxidation is calculated as the ratio between total oxidation intensity and total thiol intensity of the TMT reporter ions. C: subcellular redox compartmentalization in mouse macrophages revealed by stoichiometric quantification of SSG and total oxidation. Left: mean SSG occupancy (%); right: mean occupancy of total oxidation (%). [Adapted from Duan et al. (38) with permission from Elsevier.]
Conceptually, the site occupancy of a given thiol PTM can be determined if both the abundance of the peptide bearing the PTM and the total abundance of the same peptide are quantified. The OxICAT approach (Fig. 3A) was the first to demonstrate the ability to perform stoichiometric quantification of total oxidation for individual Cys sites by quantifying the ratios of oxidized thiols versus reduced thiol through the duplex ICAT (65, 66). More recently, the RAC-TMT workflow has been demonstrated as an effective approach for stoichiometric quantification of different types of redox PTMs (Fig. 5B) (38, 40, 41, 43, 45). This requires one of the TMT channels to serve as the “total thiol” channel where free thiols remain unblocked and all oxidized thiols are subsequently reduced before enrichment (Fig. 5B). The total thiol sample and all oxidized thiol samples are processed in parallel for all the subsequent steps, including resin-assisted capture, on-resin tryptic digestion, isobaric labeling, and elution. MS-based analysis of the peptides produces MS/MS spectra that contain a series of TMT reporter ion intensities, representing the levels of total oxidation samples and the total thiol sample for the corresponding peptide. The site occupancy of total oxidation for each sample can be calculated as the ratio of the reporter ion intensity in the given channel against the intensity from the total thiol channel. To quantify the site occupancy of specific redox PTMs, selective reducing reagents can be used in the reduction step [e.g., Grx1 for SSG (41)]. Using this method, we have quantified total oxidation in cyanobacteria modulated by light-dark conditions (40), where site occupancies of total oxidation were observed to shift from 5 to 20% under light condition to 20 to 40% under dark conditions. This method was also applied to quantify SSG site occupancy in mouse skeletal muscle following fatiguing contractions, revealing a median SSG occupancy ∼4.5% (41). Interestingly, the level of increase in SSG following fatiguing contractions depends on the cellular compartment, with the largest increases occurring in the mitochondria (1.03%) and smallest in the nucleus (0.47%). Another recent study reported simultaneous quantification of SSG and total oxidation occupancies and a mean occupancy of 4.0% for SSG and 11.9% for total oxidation were observed in mouse macrophages with ∼4,000 Cys sites quantified (38). A clear redox compartmentalization was also observed where mean occupancies for different subcellular compartments correlated with their respective redox potentials (Fig. 5C). The lowest average occupancies were observed in the more reducing organelles such as the mitochondria (nonmembrane) and nucleus, whereas the highest average occupancies were found in more oxidizing organelles such as ER and lysosome. Similar to RAC-TMT, the CPT-TMT-IMAC has also been demonstrated for stoichiometric quantification of total oxidation in mouse tissues (44). Other thiol-reactive tags such as iodoTMT (6-plex) were also explored for stoichiometric quantification of redox PTMs. For instance, Shakir et al. (103) described an OxiTMT approach in which both the total oxidation level and the total thiol level could be quantified by using different iodoTMT reagents.
As previously stated, protein cysteine residues can be modified in several different forms such as SOH, SSG, SNO, SSH, or disulfide. The quantification of site occupancy for total oxidation is just the first step to profiling the landscape of the redox state of the thiol proteome. A detailed quantification of site occupancies of each type of PTMs will be required in order to reveal a detailed map of the redox proteome and the biological implications of site-specific PTMs. Furthermore, aberrant site occupancy data on specific proteins may provide potential links between genetic mutation information and pathological and metabolic outcomes. Such information would be valuable for elucidating the role of redox regulation in pathogenesis and identifying novel therapeutic targets.
PERSPECTIVES AND FUTURE DIRECTIONS
Significant advances in MS-based redox proteomics have been achieved with relatively good proteome coverage (1,000 up to 10,000 Cys sites). Along with the more common access to MS instrumentation, broad applications of these redox proteomics approaches are anticipated for investigating the roles of redox PTMs in signaling and regulation. The general goal of such redox proteomics approaches is to achieve quantitative, in-depth thiol redox proteome profiling and to provide advanced tools for the unbiased discovery of redox-dependent pathways and networks as well as novel functional regulators and as well as for integration with omics approaches in systems biology applications. For instance, the recent application of the RAC-TMT approach to macrophages upon exposure of different engineered nanoparticles is an example of such global discovery where redox-dependent pathways impacted by SSG were identified (49). That study supports ROS generation as a potential initiating event in modulating cellular responses and macrophage immunosuppression (104, 105). The recent large-scale profiling of the thiol redox proteome of different mouse tissues is another example of discovery work where the comprehensive Oximouse database serves as a framework for understanding the mechanisms of redox regulation in physiology and aging. Despite the advances of these MS-based redox proteomics approaches, the access to specialized MS instrumentation and expertise in proteomics are required. Alternative biochemical approaches such as click-PEGylation (30) will be great complementary tools for assessing the redox state of target proteins, especially when MS-based capability is not available.
An important aspect of redox proteomics is its ability to identify the exact site(s) of modification, which is essential for functional follow-up studies. Ideally, measurements of redox PTMs will not only quantify the relative fold changes of PTMs to identify redox-sensitive Cys residues in response to perturbations but also report the absolute stoichiometry (or site occupancy) of the PTMs of interest to delineate the details of redox regulation. However, the complexity of the thiol PTMs (e.g., multiple PTM forms existing at a single Cys site) makes such stoichiometric quantification a significant analytical challenge. Moreover, some of the PTMs such as SNO and SOH are extremely transient and labile. Although the significance of SNO and SOH in signaling and regulation has been well recognized (106, 107), there is still a lack of effective proteomics methods to measure endogenous SNO and SOH modifications at physiological conditions due to the challenging nature of these PTMs. More advanced methods are required in order to measure the transient changes as well as occupancies of these important PTMs.
For effective profiling of the thiol redox proteome, the robustness and reproducibility of the measurements is a critical requirement. The inherent stochastic sampling nature of commonly used shotgun proteomics is a significant factor leading to the often inconsistency of protein/peptide identifications between different analyses. To date, isobaric labeling (e.g., TMT)-based multiplexing strategies have served as important tools for achieving reproducible quantification by enabling the analysis of 10 or more samples (e.g., 10-plex or even 16-plex in the new generation of reagents) simultaneously in a single experiment. Such a multiplexing strategy (e.g., RAC-TMT) enables both relative quantification of redox PTMs across conditions and the site occupancies of the PTMs (38, 41, 43, 44). Current methods applied for most redox proteomics enable a moderate coverage of 2,000–10,000 Cys sites depending on cell types or tissues (38, 43, 44), and further advances are still needed to achieve true proteome-wide coverage.
Another important aspect of redox proteomics is its potential for functional discovery of novel redox-sensitive proteins. For instance, Su et al. (108) recently reported the discovery of a redox switch in Akt from global redox proteome profiling. In that work, the two Cys residues (Cys60 and Cys77) in the pleckstrin homology domain of Akt were observed as reversibly oxidized, and the oxidation of these sites was confirmed as critical for Akt activation by stabilizing the PIP3 binding pocket and thus impacting the Akt recruitment to the plasma membrane. However, the elucidation of the functional consequences, as well as the mechanisms by which these modifications lead to specific functional outcomes for each individual protein, is still a daunting task despite the fact that the number of Cys sites of redox PTMs has been kept expanding. For instance, the Cys93 of human hemoglobin β-chain has long been known as a site of SSG modification under multiple disease states including diabetes (109) and chronic renal failure (110), but it was only recently that the structural perturbation induced by SSG modification was revealed (111). Such changes include a weakening in both intersubunit and intrasubunit interactions, possibly leading to functional abnormalities such as tighter oxygen binding of hemoglobin-SSG (111). Similarly, glycolytic glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is another well-known enzyme whose activity is subjected to redox control via SSG (112), disulfide (113), and SNO (114). One mechanism by which redox PTM regulates GAPDH activity is by altering the structural stability, as revealed in a recent study showing that SSG modification on the catalytic cysteine of GAPDH disrupts the native folding and thus promotes the formation of insoluble aggregates (115). Therefore, the structural consequences of redox PTMs are highly context dependent, in agreement with previous findings that enzymatic activities could be decreased or enhanced following modification (116, 117). To date, the number of redox-sensitive Cys sites with experimentally validated function information is still relatively low (118), and the large majority of Cys sites discovered by redox proteomics still do not have any clear functional information. Significant efforts are required to expand the repertoire of redox-dependent regulators in signaling and metabolism (119). Dysregulated redox PTMs on such proteins may not only cause protein dysfunction but may also serve as important targets for therapeutic interventions (120).
In addition to oxidation, other forms of modification on cysteine residues have been reported. For instance, alkylation of multiple Cys residues (Cys151/257/273/288/297) on Kelch-like ECH-associated protein-1 (KEAP1) by itaconate, the most abundant endogenous metabolite in human macrophages after LPS stimulation, had recently been demonstrated (121). Alkylation on KEAP1 was shown to disrupt its association with Nrf2, the accumulation of which promotes the expression of anti-inflammatory genes. Another metabolite that can covalently modify Cys residues is palmitic acid (C16:0), a common component in various lipids that function in membrane organization and signal transduction. S-palmitoylation has been observed in many membrane proteins such as claudin-3 and CD20 at multiple accessible Cys sites (122). To date, these metabolite-derived thiol modifications remain understudied at the proteome level.
One of the long-term goals of thiol redox proteomics will be establishing advanced capabilities for providing a true, detailed landscape of the thiol redox proteome with specificity in identifying and quantifying multiple types of redox PTMs and their site occupancies in cells, tissues, or clinical specimens. The ability to perform single-cell or near-single-cell measurements (123) for redox PTMs as well as subcellular measurements will be highly desirable. Such capabilities for performing stoichiometric measurements of multiple types of redox PTMs in tissues with in-depth proteome coverage will be particularly powerful for uncovering the role of redox PTMs in disease pathogenesis and for facilitating data integration with other omics measurements, including other PTMs such as phosphorylation and acetylation, using systems biology approaches (124). It is also clear that different forms of PTMs could occur at the same cysteine residue for a given protein at any given time (125, 126), but quantitative measurement of these dynamic modifications simultaneously at the proteome level is still rare (127). Such a comprehensive profiling of the thiol proteome for specific PTM types, along with occupancy quantification, would provide critical information on functional regulation, specificity of signals (e.g., SNO vs. SSG), and the sensitivity or dynamics of these PTMs under oxidative stress conditions. It also holds the potential to reveal cross-talks between redox and other PTMs such as phosphorylation, as well as the metabolome. Advances in understanding the redox regulatory networks and their interactions with other signaling mechanisms will provide important mechanistic insights into redox regulation and oxidative stress underlying many diseases, and eventually contribute to the development of better therapeutic strategies.
GRANTS
Portions of this work were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01 DK122160 and U24 DK112349.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.Z. and W.J.Q. prepared figures; T.Z., X.L. and W.J.Q. drafted manuscript; T.Z., M.J.G. and W.J.Q. edited and revised manuscript; T.Z., M.J.G., X.L. and W.J.Q. approved final version of manuscript.
REFERENCES
- 1.Ursini F, Maiorino M, Forman HJ. Redox homeostasis: the golden mean of healthy living. Redox Biol 8: 205–215, 2016. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4: 278–286, 2008. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 3.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–462, 2014. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartesaghi S, Radi R. Fundamentals on the biochemistry of peroxynitrite and protein tyrosine nitration. Redox Biol 14: 618–625, 2018. doi: 10.1016/j.redox.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry 49: 835–842, 2010. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannaa A, Hanisch FG. Redox proteomes in human physiology and disease mechanisms. J Proteome Res 19: 1–17, 2020. doi: 10.1021/acs.jproteome.9b00586. [DOI] [PubMed] [Google Scholar]
- 9.Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol 33: 8–13, 2015. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benhar M, Forrester MT, Stamler JS. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol 10: 721–732, 2009. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 11.Chung HS, Wang S- B, Venkatraman V, Murray CI, Van Eyk JE. Cysteine oxidative posttranslational modifications: emerging regulation in the cardiovascular system. CircRes 112: 382–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mieyal JJ, Chock PB. Posttranslational modification of cysteine in redox signaling and oxidative stress: focus on s-glutathionylation. Antioxid Redox Signal 16: 471–475, 2012. doi: 10.1089/ars.2011.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulsen CE, Carroll KS. Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chemical Reviews 113: 4633–4679, 2013. doi: 10.1021/cr300163e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held JM, Gibson BW. Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes. Mol Cell Proteomics 11: R111.013037, 2012. doi: 10.1074/mcp.R111.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 11: 997–1014, 2009. doi: 10.1089/ars.2008.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess DT, Stamler JS. Regulation by S-nitrosylation of protein post-translational modification. J Biol Chem 287: 4411–4418, 2012. doi: 10.1074/jbc.R111.285742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu M, Zhang T, Ji W, Silva-Sanchez C, Song WY, Assmann SM, Harmon AC, Chen S. Redox regulation of a guard cell SNF1-related protein kinase in Brassica napus, an oilseed crop. Biochem J 474: 2585–2599, 2017. doi: 10.1042/BCJ20170070. [DOI] [PubMed] [Google Scholar]
- 18.Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem 279: 29857–29862, 2004. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Zhu M, Song WY, Harmon AC, Chen S. Oxidation and phosphorylation of MAP kinase 4 cause protein aggregation. Biochim Biophys Acta 1854: 156–165, 2015. doi: 10.1016/j.bbapap.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang SB, Venkatraman V, Crowgey EL, Liu T, Fu Z, Holewinski R, Ranek M, Kass DA, O'Rourke B, Van Eyk JE. Protein S-nitrosylation controls glycogen synthase kinase 3beta function independent of its phosphorylation state. Circ Res 122: 1517–1531, 2018. doi: 10.1161/CIRCRESAHA.118.312789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valek L, Heidler J, Scheving R, Wittig I, Tegeder I. Nitric oxide contributes to protein homeostasis by S-nitrosylations of the chaperone HSPA8 and the ubiquitin ligase UBE2D. Redox Biol 20: 217–235, 2019. doi: 10.1016/j.redox.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passam F, Chiu J, Ju L, Pijning A, Jahan Z, Mor-Cohen R, Yeheskel A, Kolsek K, Tharichen L, Aponte-Santamaria C, Grater F, Hogg PJ. Mechano-redox control of integrin de-adhesion. Elife 7, 2018. doi: 10.7554/eLife.34843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go YM, Jones DP. Redox theory of aging: implications for health and disease. Clin Sci (Lond) 131: 1669–1688, 2017. doi: 10.1042/CS20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Garcia A, Zavala-Flores L, Rodriguez-Rocha H, Franco R. Thiol-redox signaling, dopaminergic cell death, and Parkinson's disease. Antioxid Redox Signal 17: 1764–1784, 2012. doi: 10.1089/ars.2011.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofman G, Tipple TE. Thiol-redox regulation in lung development and vascular remodeling. Antioxid Redox Signal 31: 858–873, 2019. doi: 10.1089/ars.2018.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res 111: 1091–1106, 2012. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 27.Cobley JN, Husi H. Immunological techniques to assess protein thiol redox state: opportunities, challenges and solutions. Antioxidants (Basel) 9 9: 315, 2020. doi: 10.3390/antiox9040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YJ, Chang GD. Quantitative display of the redox status of proteins with maleimide-polyethylene glycol tagging. Electrophoresis 40: 491–498, 2019. doi: 10.1002/elps.201800335. [DOI] [PubMed] [Google Scholar]
- 29.Rudyk O, Eaton P. Biochemical methods for monitoring protein thiol redox states in biological systems. Redox Biol 2: 803–813, 2014. doi: 10.1016/j.redox.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Leeuwen LAG, Hinchy EC, Murphy MP, Robb EL, Cocheme HM. Click-PEGylation—a mobility shift approach to assess the redox state of cysteines in candidate proteins. Free Radic Biol Med 108: 374–382, 2017. doi: 10.1016/j.freeradbiomed.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468: 1115–1118, 2010. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 2001: pl1–pl1, 2001. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 33.Li R, Kast J. Biotin switch assays for quantitation of reversible cysteine oxidation. Methods Enzymol 585: 269–284, 2017. doi: 10.1016/bs.mie.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Murray CI, Van Eyk JE. Chasing cysteine oxidative modifications: proteomic tools for characterizing cysteine redox status. Circ Cardiovasc Genet 5: 591–591, 2012. doi: 10.1161/CIRCGENETICS.111.961425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerby P, Swiader A, Auge N, Parant O, Vayssière C, Uchida K, Salvayre R, Negre-Salvayre A. High glutathionylation of placental endothelial nitric oxide synthase in preeclampsia. Redox Biol 22: 101126, 2019. doi: 10.1016/j.redox.2019.101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J, Weisbrod RM, Shao D, Watanabe Y, Yin X, Bachschmid MM, Seta F, Janssen-Heininger YMW, Matsui R, Zang M, Hamburg NM, Cohen RA. The redox mechanism for vascular barrier dysfunction associated with metabolic disorders: glutathionylation of Rac1 in endothelial cells. Redox Biol 9: 306–319, 2016. doi: 10.1016/j.redox.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behring JB, van der Post S, Mooradian AD, Egan MJ, Zimmerman MI, Clements JL, Bowman GR, Held JM. Spatial and temporal alterations in protein structure by EGF regulate cryptic cysteine oxidation. Sci Signal 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan J, Zhang T, Gaffrey MJ, Weitz KK, Moore RJ, Li X, Xian M, Thrall BD, Qian WJ. Stochiometric quantification of the thiol redox proteome of macrophages reveals subcellular compartmentalization and susceptibility to oxidative perturbations. Redox Biol 36: 101649, 2020. doi: 10.1016/j.redox.2020.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu L, Liu K, Sun M, Tian C, Sun R, Morales Betanzos C, Tallman KA, Porter NA, Yang Y, Guo D, Liebler DC, Yang J. Systematic and quantitative assessment of hydrogen peroxide reactivity with cysteines across human proteomes. Mol Cell Proteomics 16: 1815–1828, 2017. doi: 10.1074/mcp.RA117.000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Nguyen AY, Dai Z, Su D, Gaffrey MJ, Moore RJ, Jacobs JM, Monroe ME, Smith RD, Koppenaal DW, Pakrasi HB, Qian WJ. Proteome-wide light/dark modulation of thiol oxidation in cyanobacteria revealed by quantitative site-specific redox proteomics. Mol Cell Proteomics 13: 3270–3285, 2014. doi: 10.1074/mcp.M114.041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramer PA, Duan J, Gaffrey MJ, Shukla AK, Wang L, Bammler TK, Qian WJ, Marcinek DJ. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol 17: 367–376, 2018. doi: 10.1016/j.redox.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Reest J, Lilla S, Zheng L, Zanivan S, Gottlieb E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun 9: 1581, 2018. doi: 10.1038/s41467-018-04003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Zhang T, Johnston CJ, Kim SY, Gaffrey MJ, Chalupa D, Feng G, Qian WJ, McGraw MD, Ansong C. Protein thiol oxidation in the rat lung following e-cigarette exposure. Redox Biol 37: 101758, 2020. doi: 10.1016/j.redox.2020.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, Li J, Long J, Mills EL, Szpyt J, He Z, Du G, Garrity R, Reddy A, Vaites LP, Paulo JA, Zhang T, Gray NS, Gygi SP, Chouchani ET. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180: 968–983, 2020. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J, Gaffrey MJ, Su D, Liu T, Camp DG 2nd, Smith RD, Qian W-J. Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat Protoc 9: 64–75, 2014. doi: 10.1038/nprot.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samarasinghe KT, Munkanatta Godage DN, VanHecke GC, Ahn YH. Metabolic synthesis of clickable glutathione for chemoselective detection of glutathionylation. J Am Chem Soc 136: 11566–11569, 2014. doi: 10.1021/ja503946q. [DOI] [PubMed] [Google Scholar]
- 47.VanHecke GC, Abeywardana MY, Ahn YH. Proteomic identification of protein glutathionylation in cardiomyocytes. J Proteome Res 18: 1806–1818, 2019. doi: 10.1021/acs.jproteome.8b00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu T, Qian W-J, Strittmatter EF, Camp DG 2nd, Anderson GA, Thrall BD, Smith RD. High-throughput comparative proteome analysis using a quantitative cysteinyl-peptide enrichment technology. Anal Chem 76: 5345–5353, 2004. doi: 10.1021/ac049485q. [DOI] [PubMed] [Google Scholar]
- 49.Duan J, Kodali VK, Gaffrey MJ, Guo J, Chu RK, Camp DG 2nd, Smith RD, Thrall BD, Qian WJ. Quantitative profiling of protein S-glutathionylation reveals redox-dependent regulation of macrophage function during nanoparticle-induced oxidative stress. ACS Nano 10: 524–538, 2016. doi: 10.1021/acsnano.5b05524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol 27: 557–559, 2009. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kramer PA, Duan J, Qian WJ, Marcinek DJ. The measurement of reversible redox dependent post-translational modifications and their regulation of mitochondrial and skeletal muscle function. Front Physiol 6: 347, 2015. doi: 10.3389/fphys.2015.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su D, Gaffrey MJ, Guo J, Hatchell KE, Chu RK, Clauss TR, Aldrich JT, Wu S, Purvine S, Camp DG, Smith RD, Thrall BD, Qian WJ. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radic Biol Med 67: 460–470, 2014. doi: 10.1016/j.freeradbiomed.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aesif SW, Janssen-Heininger YM, Reynaert NL. Protocols for the detection of s-glutathionylated and s-nitrosylated proteins in situ. Methods Enzymol 474: 289–296, 2010. doi: 10.1016/S0076-6879(10)74017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anathy V, Roberson EC, Guala AS, Godburn KE, Budd RC, Janssen-Heininger YM. Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal 16: 496–505, 2012. doi: 10.1089/ars.2011.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bachi A, Dalle-Donne I, Scaloni A. Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem Rev 113: 596–698, 2013. [Erratum in Chem Rev 115: 3677, 2015]. doi: 10.1021/cr300073p. [DOI] [PubMed] [Google Scholar]
- 56.Duan J, Gaffrey MJ, Qian WJ. Quantitative proteomic characterization of redox-dependent post-translational modifications on protein cysteines. Mol Biosyst 13: 816–829, 2017. doi: 10.1039/C6MB00861E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J, Carroll KS, Liebler DC. The Expanding Landscape of the Thiol Redox Proteome. Mol Cell Proteomics 15: 1–11, 2016. doi: 10.1074/mcp.O115.056051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hawkins CL, Davies MJ. Detection, identification, and quantification of oxidative protein modifications. J Biol Chem 294: 19683–19708, 2019. doi: 10.1074/jbc.REV119.006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang YC, Huang CN, Lin CH, Chang HC, Wu CC. Mapping protein cysteine sulfonic acid modifications with specific enrichment and mass spectrometry: an integrated approach to explore the cysteine oxidation. Proteomics 10: 2961–2971, 2010. doi: 10.1002/pmic.200900850. [DOI] [PubMed] [Google Scholar]
- 60.Lo Conte M, Carroll KS. Chemoselective ligation of sulfinic acids with aryl-nitroso compounds. Agnew Chem Int Ed Engl 51: 6502–6505, 2012. doi: 10.1002/anie.201201812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madian AG, Regnier FE. Proteomic identification of carbonylated proteins and their oxidation sites. J Proteome Res 9: 3766–3780, 2010. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinez-Acedo P, Nuññez E, Gómez FJ, Moreno M, Ramos E, Izquierdo-Álvarez A, Miró-Casas E, Mesa R, Rodriguez P, Martínez-Ruiz A, Dorado DG, Lamas S, Vázquez J. A novel strategy for global analysis of the dynamic thiol redox proteome. Mol Cell Proteomics 11: 800–813, 2012. doi: 10.1074/mcp.M111.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paulech J, Liddy KA, Engholm-Keller K, White MY, Cordwell SJ. Global analysis of myocardial peptides containing cysteines with irreversible sulfinic and sulfonic acid post-translational modifications. Mol Cell Proteomics 14: 609–620, 2015. doi: 10.1074/mcp.M114.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacob C, Holme AL, Fry FH. The sulfinic acid switch in proteins. Org Biomol Chem 2: 1953–1956, 2004. doi: 10.1039/B406180B. [DOI] [PubMed] [Google Scholar]
- 65.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105: 8197–8202, 2008. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Go YM, Roede JR, Walker DI, Duong DM, Seyfried NT, Orr M, Liang Y, Pennell KD, Jones DP. Selective targeting of the cysteine proteome by thioredoxin and glutathione redox systems. Mol Cell Proteomics 12: 3285–3296, 2013. doi: 10.1074/mcp.M113.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brandes N, Reichmann D, Tienson H, Leichere LI, Jakob U. Using quantitative redox proteomics to dissect the yeast redoxome. J Biol Chem 286: 41893–41903, 2011. doi: 10.1074/jbc.M111.296236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenwasser S, Graff van Creveld S, Schatz D, Malitsky S, Tzfadia O, Aharoni A, Levin Y, Gabashvili A, Feldmesser E, Vardi A. Mapping the diatom redox-sensitive proteome provides insight into response to nitrogen stress in the marine environment. Proc Natl Acad Sci U S A 111: 2740–2745, 2014. doi: 10.1073/pnas.1319773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brandes N, Tienson H, Lindemann A, Vitvitsky V, Reichmann D, Banerjee R, Jakob U. Time line of redox events in aging postmitotic cells. Elife 2, 2013. doi: 10.7554/eLife.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.García-Santamarina S, Boronat S, Calvo IA, Rodríguez-Gabriel M, Ayté J, Molina H, Hidalgo E. Is oxidized thioredoxin a major trigger for cysteine oxidation? Clues from a redox proteomics approach. Antioxid Redox Signal 18: 1549–1556, 2013. doi: 10.1089/ars.2012.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.García-Santamarina S, Boronat S, Domènech A, Ayté J, Molina H, Hidalgo E. Monitoring in vivo reversible cysteine oxidation in proteins using ICAT and mass spectrometry. Nat Protoc 9: 1131–1145, 2014. doi: 10.1038/nprot.2014.065. [DOI] [PubMed] [Google Scholar]
- 72.García-Santamarina S, Boronat S, Espadas G, Ayté J, Molina H, Hidalgo E. The oxidized thiol proteome in fission yeast–optimization of an ICAT-based method to identify H2O2-oxidized proteins. J Proteomics 74: 2476–2486, 2011. doi: 10.1016/j.jprot.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 73.Chouchani ET, James AM, Methner C, Pell VR, Prime TA, Erickson BK, Forkink M, Lau Gy, Bright TP, Menger KE, Fearnley IM, Krieg T, Murphy MP. Identification and quantification of protein S-nitrosylation by nitrite in the mouse heart during ischemia. J Biol Chem 292: 14486–14495, 2017. doi: 10.1074/jbc.M117.798744. ]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araki K, Kusano H, Sasaki N, Tanaka R, Hatta T, Fukui K, Natsume T. Redox sensitivities of global cellular cysteine residues under reductive and oxidative stress. J Proteome Res 15: 2548–2559, 2016. doi: 10.1021/acs.jproteome.6b00087. [DOI] [PubMed] [Google Scholar]
- 75.McGarry DJ, Chen W, Chakravarty P, Lamont DL, Wolf CR, Henderson CJ. Proteome-wide identification and quantification of S-glutathionylation targets in mouse liver. Biochem J 469: 25–32, 2015. doi: 10.1042/BJ20141256. [DOI] [PubMed] [Google Scholar]
- 76.Su D, Shukla AK, Chen B, Kim JS, Nakayasu E, Qu Y, Aryal U, Weitz K, Clauss TR, Monroe ME, Camp DG 2nd, Bigelow DJ, Smith RD, Kulkarni RN, Qian WJ. Quantitative site-specific reactivity profiling of S-nitrosylation in mouse skeletal muscle using cysteinyl peptide enrichment coupled with mass spectrometry. Free Radic Biol Med 57: 68–78, 2013. doi: 10.1016/j.freeradbiomed.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang H, Haar Petersen M, Ibañez-Vea M, Lassen PS, Larsen MR, Palmisano G. Simultaneous enrichment of cysteine-containing peptides and phosphopeptides using a cysteine-specific phosphonate adaptable tag (CysPAT) in combination with titanium dioxide (TiO2) chromatography. Mol Cell Proteomics 15: 3282–3296, 2016. doi: 10.1074/mcp.M115.054551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aesif SW, Kuipers I, van der Velden J, Tully JE, Guala AS, Anathy V, Sheely JI, Reynaert NL, Wouters EF, van der Vliet A, Janssen-Heininger YM. Activation of the glutaredoxin-1 gene by nuclear factor kappaB enhances signaling. Free Radic Biol Med 51: 1249–1257, 2011. doi: 10.1016/j.freeradbiomed.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci 34: 85–96, 2009. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A 103: 13086–13091, 2006. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: role in reversible protein s-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal 7: 348–366, 2005. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 84.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cell 25: 332–346, 2008. [PMC free article] [PubMed] [Google Scholar]
- 85.Grek CL, Zhang J, Manevich Y, Townsend DM, Tew KD. Causes and consequences of cysteine S-glutathionylation. J Biol Chem 288: 26497–26504, 2013. doi: 10.1074/jbc.R113.461368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ye ZW, Zhang J, Ancrum T, Manevich Y, Townsend DM, Tew KD. Glutathione S-transferase P-mediated protein S-glutathionylation of resident endoplasmic reticulum proteins influences sensitivity to drug-induced unfolded protein response. Antioxid Redox Signal 26: 247–261, 2017. doi: 10.1089/ars.2015.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brigelius R, Muckel C, Akerboom TP, Sies H. Identification and quantitation of glutathione in hepatic protein mixed disulfides and its relationship to glutathione disulfide. Biochem Pharmacol 32: 2529–2534, 1983. doi: 10.1016/0006-2952(83)90014-X. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X, Liu P, Zhang C, Chiewchengchol D, Zhao F, Yu H, Li J, Kambara H, Luo KY, Venkataraman A, Zhou Z, Zhou W, Zhu H, Zhao L, Sakai J, Chen Y, Ho YS, Bajrami B, Xu B, Silberstein LE, Cheng T, Xu Y, Ke Y, Luo HR. Positive regulation of interleukin-1beta bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep 20: 224–235, 2017. doi: 10.1016/j.celrep.2017.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Butturini E, Boriero D, Carcereri de Prati A, Mariotto S. Immunoprecipitation methods to identify S-glutathionylation in target proteins. MethodsX 6: 1992–1998, 2019. doi: 10.1016/j.mex.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Butturini E, Carcereri de Prati A, Chiavegato G, Rigo A, Cavalieri E, Darra E, Mariotto S. Mild oxidative stress induces S-glutathionylation of STAT3 and enhances chemosensitivity of tumoural cells to chemotherapeutic drugs. Free Radic Biol Med 65: 1322–1330, 2013. doi: 10.1016/j.freeradbiomed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 91.Gianazza E, Eberini I, Ghezzi P. Detection of protein glutathionylation. Methods Mol Biol 519: 397–415, 2009. doi: 10.1007/978-1-59745-281-6_26. [DOI] [PubMed] [Google Scholar]
- 92.Reynaert NL, Ckless K, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. In situ detection of S-glutathionylated proteins following glutaredoxin-1 catalyzed cysteine derivatization. Biochim Biophys Acta 1760: 380–387, 2006. doi: 10.1016/j.bbagen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Bushweller JH, Aaslund F, Wuethrich K, Holmgren A. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14→S) and its mixed disulfide with glutathione. Biochemistry 31: 9288–9293, 1992. doi: 10.1021/bi00153a023. [DOI] [PubMed] [Google Scholar]
- 94.Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Merrihew GE, Egertson JD, Wang L, Bammler TK, Moore RJ, White CC, Kavanagh TJ, Voss JG, Szeto HH, Rabinovitch PS, MacCoss MJ, Qian WJ, Marcinek DJ. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med 134: 268–281, 2019. doi: 10.1016/j.freeradbiomed.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chia SB, Elko EA, Aboushousha R, Manuel AM, van de Wetering C, Druso JE, van der Velden J, Seward DJ, Anathy V, Irvin CG, Lam YW, van der Vliet A, Janssen-Heininger Y. Dysregulation of the glutaredoxin/S-glutathionylation redox axis in lung diseases. Am J Physiol Cell Physiol 318: C304–C327, 2019. doi: 10.1152/ajpcell.00410.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kekulandara DN, Samarasinghe KT, Munkanatta Godage DN, Ahn YH. Clickable glutathione using tetrazine-alkene bioorthogonal chemistry for detecting protein glutathionylation. Org Biomol Chem 14: 10886–10893, 2016. [Erratum in Org Biomol Chem 16: 2576, 2018]. doi: 10.1039/C6OB02050J. [DOI] [PubMed] [Google Scholar]
- 97.VanHecke GC, Yapa Abeywardana M, Huang B, Ahn YH. Isotopically labeled clickable glutathione to quantify protein S-glutathionylation. Chem Biochem 21: 853–859, 2019. doi: 10.1002/cbic.201900528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Presler M, Van Itallie E, Klein AM, Kunz R, Coughlin ML, Peshkin L, Gygi SP, Wuhr M, Kirschner MW. Proteomics of phosphorylation and protein dynamics during fertilization and meiotic exit in the Xenopus egg. Proc Natl Acad Sci USA 114: E10838–E10847, 2017. doi: 10.1073/pnas.1709207114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science 284: 651–654, 1999. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 100.Hansen BK, Gupta R, Baldus L, Lyon D, Narita T, Lammers M, Choudhary C, Weinert BT. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat Commun 10: 1055, 2019. doi: 10.1038/s41467-019-09024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3: ra3–ra3, 2010. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 102.Wu R, Haas W, Dephoure N, Huttlin EL, Zhai B, Sowa ME, Gygi SP. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat Methods 8: 677–683, 2011. doi: 10.1038/nmeth.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shakir S, Vinh J, Chiappetta G. Quantitative analysis of the cysteine redoxome by iodoacetyl tandem mass tags. Anal Bioanal Chem 409: 3821–3830, 2017. doi: 10.1007/s00216-017-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang T, Gaffrey MJ, Thomas DG, Weber TJ, Hess BM, Weitz KK, Piehowski PD, Petyuk VA, Moore RJ, Qian W-J, Thrall BD. A proteome-wide assessment of the oxidative stress paradigm for metal and metal-oxide nanomaterials in human macrophages. NanoImpact 17: 100194, 2020. doi: 10.1016/j.impact.2019.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang T, Gaffrey MJ, Thrall BD, Qian WJ. Mass spectrometry-based proteomics for system-level characterization of biological responses to engineered nanomaterials. Anal Bioanal Chem 410: 6067–6077, 2018. doi: 10.1007/s00216-018-1168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 6: 150–166, 2005. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 107.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15: 411–421, 2014. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 108.Su Z, Burchfield JG, Yang P, Humphrey SJ, Yang G, Francis D, Yasmin S, Shin SY, Norris DM, Kearney AL, Astore MA, Scavuzzo J, Fisher-Wellman KH, Wang QP, Parker BL, Neely GG, Vafaee F, Chiu J, Yeo R, Hogg PJ, Fazakerley DJ, Nguyen LK, Kuyucak S, James DE. Global redox proteome and phosphoproteome analysis reveals redox switch in Akt. Nat Commun 10: 5486, 2019. doi: 10.1038/s41467-019-13114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Niwa T, Naito C, Mawjood AH, Imai K. Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin Chem 46: 82–88, 2000. doi: 10.1093/clinchem/46.1.82. [DOI] [PubMed] [Google Scholar]
- 110.Mandal AK, Woodi M, Sood V, Krishnaswamy PR, Rao A, Ballal S, Balaram P. Quantitation and characterization of glutathionyl haemoglobin as an oxidative stress marker in chronic renal failure by mass spectrometry. Clin Biochem 40: 986–994, 2007. doi: 10.1016/j.clinbiochem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 111.Muralidharan M, Mitra A, Maity D, Pal D, Mandal AK. Structural analysis of glutathionyl hemoglobin using native mass spectrometry. J Struct Biol 208: 107386, 2019. doi: 10.1016/j.jsb.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Mohr S, Hallak H, de Boitte A, Lapetina EG, Brüne B. Nitric oxide-induced S-glutathionylation and inactivation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 274: 9427–9430, 1999. doi: 10.1074/jbc.274.14.9427. [DOI] [PubMed] [Google Scholar]
- 113.Barinova KV, Serebryakova MV, Muronetz VI, Schmalhausen EV. S-glutathionylation of glyceraldehyde-3-phosphate dehydrogenase induces formation of C150-C154 intrasubunit disulfide bond in the active site of the enzyme. Biochim Biophys Acta Gen Subj 1861: 3167–3177, 2017. doi: 10.1016/j.bbagen.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 114.Zaffagnini M, Morisse S, Bedhomme M, Marchand CH, Festa M, Rouhier N, Lemaire SD, Trost P. Mechanisms of nitrosylation and denitrosylation of cytoplasmic glyceraldehyde-3-phosphate dehydrogenase from Arabidopsis thaliana. J Biol Chem 288: 22777–22789, 2013. doi: 10.1074/jbc.M113.475467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zaffagnini M, Marchand CH, Malferrari M, Murail S, Bonacchi S, Genovese D, Montalti M, Venturoli G, Falini G, Baaden M, Lemaire SD, Fermani S, Trost P. Glutathionylation primes soluble glyceraldehyde-3-phosphate dehydrogenase for late collapse into insoluble aggregates. Proc Natl Acad Sci U S A 116: 26057–26065, 2019. doi: 10.1073/pnas.1914484116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cho D-H, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science (New York) 324: 102–105, 2009. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qu J, Nakamura T, Cao G, Holland EA, McKercher SR, Lipton SA. S-nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci U S A 108: 14330–14335, 2011. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun MA, Wang YJ, Cheng H, Zhang Q, Ge W, Guo DJ. RedoxDB—a curated database for experimentally verified protein oxidative modification. Bioinformatics 28: 2551–2552, 2012. doi: 10.1093/bioinformatics/bts468. [DOI] [PubMed] [Google Scholar]
- 119.Moldogazieva NT, Mokhosoev IM, Feldman NB, Lutsenko SV. ROS and RNS signalling: adaptive redox switches through oxidative/nitrosative protein modifications. Free Radic Res 52: 507–543, 2018. doi: 10.1080/10715762.2018.1457217. [DOI] [PubMed] [Google Scholar]
- 120.Ovalle F, Grimes T, Xu G, Patel AJ, Grayson TB, Thielen LA, Li P, Shalev A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat Med 24: 1108–1112, 2018. doi: 10.1038/s41591-018-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, , et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556: 113–117, 2018. doi: 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodenburg RNP, Snijder J, van de Waterbeemd M, Schouten A, Granneman J, Heck AJR, Gros P. Stochastic palmitoylation of accessible cysteines in membrane proteins revealed by native mass spectrometry. Nat Commun 8: 1280–1280, 2017. doi: 10.1038/s41467-017-01461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu Y, Piehowski PD, Kelly RT, Qian WJ. Nanoproteomics comes of age. Expert Rev Proteomics 15: 865–871, 2018. doi: 10.1080/14789450.2018.1537787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med 44: 921–937, 2008. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Go YM, Jones DP. The redox proteome. J Biol Chem 288: 26512–26520, 2013. doi: 10.1074/jbc.R113.464131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ma T, Yoo MJ, Zhang T, Liu L, Koh J, Song WY, Harmon AC, Sha W, Chen S. Characterization of thiol-based redox modifications of Brassica napusSNF1-related protein kinase 2.6-2C. FEBS Open Bio 8: 628–645, 2018. doi: 10.1002/2211-5463.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan KT, Chen YY, Pu TH, Chao YS, Yang CY, Bomgarden RD, Rogers JC, Meng TC, Khoo KH. Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia. Antioxid Redox Signal 20: 1365–1381, 2014. doi: 10.1089/ars.2013.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]