Abstract
The involvement of claudins in urothelial carcinogenesis is controversial. In this study, we analyzed Claudin-4 immunoexpression in 50 cases of bladder urothelial carcinomas depending on the main prognostic parameters of the lesions represented by the tumor grade and tumor extension. Claudin-4 immunoexpression scores were significantly higher in high-grade urothelial carcinomas and in tumors with invasion in muscularis propria. The results obtained indicate the involvement of Claudin-4 in the progression of urothelial bladder carcinomas.
Keywords: Bladder carcinoma, Claudin-4, tumor grade
Introduction
Recent studies indicate a 6% incidence of bladder cancer, reported in all cases of cancer, over 90% being urothelial carcinomas [1].
Histopathological evaluation of urothelial carcinomas (UC) is based on the assessment of the tumor grade and stage, which determines the prognosis and has therapeutic implications [2,3].
The heterogeneity of histopathological features is an additional complexity in the context of bladder cancer diversity.
Data on molecular markers involved in UC prognosis are still controversial.
Although the diagnostic and/or prognostic value of claudins is well known in many human tumors, there are few data available on the profile of claudin expression in different varieties of urothelial bladder neoplasms [4,5].
In this study we followed the immunoexpression of Claudin-4 in the bladder UC, depending on the tumor grade and tumor extension pT.
Material and Methods
We studied a number of 50 UCs from the Urology Clinic of the County Emergency Clinical Hospital of Craiova.
The fragments of surgical excision (cystectomy or transurethral tumor resection) were fixed in 10% buffered formalin, processed by routine paraffin inclusion technique and then stained hematoxylin-eosin.
The classification of the lesions according to the WHO (World Health Organization) recommendations indicated in 11 cases low-grade noninvasive urothelial carcinomas (LGNUC), in 14 cases high-grade noninvasive urothelial carcinomas (HGNUC), in 9 cases low-grade invasive urothelial carcinomas (LGIUC) and in 16 cases cases of high-grade invasive urothelial carcinomas (HGIUC) [6].
From the paraffin blocks we performed serial sections that were processed immunohistochemically using the detection system Labeled Streptavidin-Biotin (LSAB) 2 (Dako, Redox, Romania, code K0675).
Visualization of the reactions was done using 3,3’-diaminobenzidine (DAB) tetrahydrochloride (Dako, code 3467), and to validate the reactions we used positive (ovarian carcinoma) and negative external controls (by omitting the primary antibody).
The antibody was used in this study was policlonal rabbit anti-human Claudin-4 (Thermo Fisher Scientific), diluted as 1:400 and with antigen retrieval in citrate buffer pH6.
The analysis of the semi-quantitative expression of Claudin-4 was performed by two specialists using an adapted system [7].
For grading the reactions intensity the score was considered 1 (mild), 2 (moderate) or 3 (strong) while the percentage of positive cells was noted with score 1 (6-25% cells), 2 (26-50% cells), 3 (51-75% cells) and 4 (>75% cells).
The final staining scores (FSS) result by multiplying the intensity and percentage scores and were considered low for values between 1-4 and high for values of 6-12.
The cutoff value for positivity was 5% positive below this value the FSS being considered negative.
The statistical analysis used the chi-square-χ2 test within SPSS10 (Statistical Package for the Social Sciences) software, the p<0.05 values being considered significant.
The study was approved by Ethical Committee of the University of Medicine and Pharmacy of Craiova, and all patients gave a written informed consent.
Results
The study included a number of 50 CUs, which corresponded to the categories pTa in 25 cases, pT1 in 18 cases, pT2 in 5 cases and pT3 in 2 cases.
The immunoreactivity for Claudin-4 was identified in 34 of the investigated cases (68%), with a membranous pattern, respectively in 10 LGNUC cases, 10 HGNUC cases, 6 LGIUC cases and 8 HGIUC cases.
Analysis of Claudin-4 immunoexpression in terms of percentage and labeling intensity for the selected tumors indicated different staining patterns and different FSS values depending on tumor grade and pT category (Table 1).
Table 1.
FSS values of Claudin-4 in urothelial carcinomas
|
stage/ No. cases |
Tumor grade/ No. of positive cases |
Low FSS |
High FSS |
|
pTa/25 |
LGNUC/10 |
3.8 |
- |
|
HGNUC/10 |
- |
8.4 |
|
|
pT1/18 |
LGIUC/6 |
4.6 |
- |
|
HGIUC/5 |
- |
8 |
|
|
pT2/5 |
HGIUC/3 |
- |
8 |
|
pT3/2 |
HGIUC/0 |
- |
- |
**FSS: final staining score; LGNUC: low grade noninvasive urothelial carcinoma; HGNUC: high grade noninvasive urothelial carcinoma; LGIUC: low grade invasive urothelial carcinoma; HGIUC: high grade invasive urothelial carcinoma
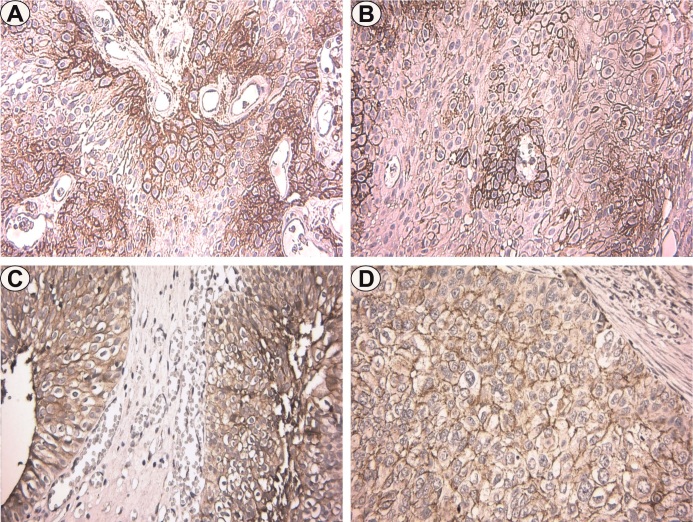
In the LGNUC included in the pTa category, we found positivity with membranous pattern and disposition in the basal layers, adjacent to the connective axes.
The percentage of positive cells varied between 25-50%, the intensity was moderate, with FSS values varying between 2-4 and an average value of 3.8 (Figure 1A).
Figure 1.
Immunoexpression of Claudin-4 in urothelial carcinomas. A. LGNUC, pTa, x400; B. HGNUC, pTa, x400; C. LGIUC, pTa, x400; D. HGIUC, pTa, x400
In HGNUC also included in the pTa category, the staining pattern was also membranous and available in the basal layers, but also randomly in the rest of the tumor layers.
The percentage of positive cells varied between 40-65%, but the staining intensity was strong, with FSS values varying between 6-9 and an average of 8.4 (Figure 1B).
In LGIUC corresponding to the pT1 stage we observed positivity also with membranous pattern, predominantly in the basal layers.
The percentage of positive cells varied between 50-75%, the intensity was moderate, with FSS values varying between 4-6 and an average value of 4.6 (Figure 1C).
In HGIUC from the pT1, and pT2 categories, the immunostaining had a membranous pattern with a random disposition.
The percentage of positive cells ranged from 80-85% for pT1/T2 tumors, with mean FSS values of 8 for both tumor categories (Figure 1D).
In HGIUC from the pT3 stage we found the absence of staining.
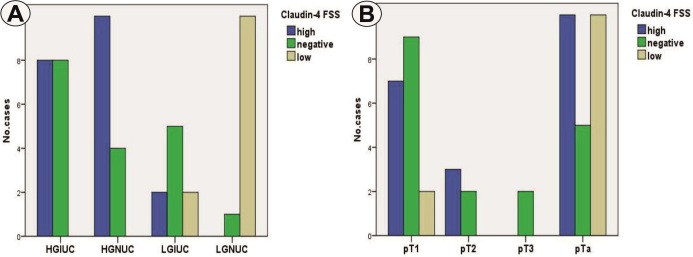
The statistical analysis indicated the association of the high grade of UC with the high values of FSS, aspects that were significant (p=0.000, χ2 test) (Figure 2A).
Figure 2.
A. Distribution of cases depending on tumor type and Claudin-4 FSS;B. Distribution of cases depending on tumor extension and Claudin-4 FSS
In relation to the tumor stage, high values of Claudin-4 FSS predominated only in carcinomas in the pT2 category, and low FSS values were present only in the pTa/T1 categories, aspects that were at the limit of statistical significance (p=0.063, χ2 test) (Figure 2B).
Discussions
The possible role of claudins in human tumorigenesis is mentioned, several studies proving the modification of the molecular structure of tight junctions in different pathological conditions [8], the loss of claudin expression being reported in several malignant diseases [9].
Moreover, the immunohistochemical detection of some claudins has proved useful as a diagnostic tool that can differentiate some types of malignancies, and some claudins can be used as prognostic markers because the loss of their expression is related in some cases to metastatic potential [9].
Claudin-4 is one of the claudins predominantly expressed in the kidneys and urinary tract, including selective segments of the renal nephrons and the entire urothelium from the pelvis to the bladder [10].
Thus, in the normal urothelium, Claudin-4 expression is intense at the membrane level, in the upper layers, progressively decreasing towards the basal layers [1,8,11,12].
However, there are limited studies on Claudin-4 expression in UC, the results of various studies being discordant.
Kökenek Unal TD et al. reported for invasive urothelial carcinomas, loss of expression in 60% of cases analyzed [11].
The authors observed that Claudin-4 expression decreases with increasing histological grade and tumor stage, the positive predictive value of Claudin-4 being statistically significant for high nuclear grade [11].
In our study, Claudin-4 expression was identified in 68% of cases with a membranous pattern. We observed the higher FSS values in high-grade UC and in carcinomas invasive in muscularis propria. We also observed the loss of Claudin-4 expression in pT3 carcinomas.
Boireau S et al. reported decreased Claudin-4 expression in HGIUC, while LGNUC did not present this aspect [4].
On the contrary, Székely E et al. reported an increase in Claudin-4 expression in LGNUC associated with reduced relapse-free survival, supporting the idea of the association between high expression of Claudin-4 and advanced urinary carcinogenesis, suggesting that Claudin-4 expression profile could be used to predict behavior clinic at UC [8].
Another study reports that increased expression of Claudin-3 and Claudin-4 was significantly correlated with advanced disease [12].
Similarly, another study concludes that decreased Claudin-4 expression may indicate increased invasiveness in UC, noting that Claudin-4 expression decreases with increasing histological grade and pathological status [11].
In other studies, Claudin-4 expression was closely related to tumor stage and grade, but did not correlate with tumor recurrence and metastasis [1,4,12].
Moreover, experimental data suggest that tumor cells expressing Claudin-4 could be a therapeutic target for cytotoxic fusion proteins, recognizing Claudin-4 as a docker molecule [13].
Seiler E et al. proposed four molecular subtypes (basal, Claudin-low, luminal and luminal-infiltrated subtypes) that can predict the response to cisplatin-based neoadjuvant chemotherapy and confirmed that the Claudin-low subtype, defined as a subset of tumors with low Claudin-3 and Claudin-4 had the lowest overall survival and did not benefit from this therapy [14].
Conclusions
In this study, Claudin-4 overexpression was associated with high-grade urothelial carcinomas with invasion into the muscularis propria, and negative reactions were associated with deep-invasive throughout the bladder wall carcinomas.
The results support the involvement of Claudin-4 in the progression of urothelial carcinomas, both in the noninvasive and tumor invasion phases.
Conflict of interests
None to declare.
Acknowledgments
Acknowledgements
Marius Matei and Cristiana Eugenia Simionescu contributed equally to the study.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M, van Rhijn BWG, Shariat SF, Soukup V, Sylvester RJ, Zigeuner R. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71(3):447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Stenzl A. Bladder cancer: work in progress. Curr Opin Urol. 2009;19(5):486–487. doi: 10.1097/MOU.0b013e32832f0635. [DOI] [PubMed] [Google Scholar]
- 4.Boireau S, Buchert M, Samuel MS, Pannequin J, Ryan JL, Choquet A, Chapuis H, Rebillard X, Avancès C, Ernst M, Joubert D, Mottet N, Hollande F. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis. 2007;28(2):246–258. doi: 10.1093/carcin/bgl120. [DOI] [PubMed] [Google Scholar]
- 5.Törzsök P, Riesz P, Kenessey I, Székely E, Somorácz A, Nyirády P, Romics I, Schaff Z, Lotz G, Kiss A. Claudins and ki-67: potential markers to differentiate low- and high-grade transitional cell carcinomas of the urinary bladder. J Histochem Cytochem. 2011;59(11):1022–1030. doi: 10.1369/0022155411424606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 7.Wang WS, Yu SL, Yang XS, Chang SD, Hou JQ. Expression and significance of twist and E-cadherin in ovarian cancer tissues. Asian Pac J Cancer Prev. 2013;14(2):669–672. doi: 10.7314/apjcp.2013.14.2.669. [DOI] [PubMed] [Google Scholar]
- 8.Székely E, Törzsök P, Riesz P, Korompay A, Fintha A, Székely T, Lotz G, Nyirády P, Romics I, Tímár J, Schaff Z, Kiss A. Expression of claudins and their prognostic significance in noninvasive urothelial neoplasms of the human urinary bladder. J Histochem Cytochem. 2011;59(10):932–941. doi: 10.1369/0022155411418829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouban A, Ahmed AA. Claudins in human cancer: a review. Histol Histopathol. 2010;25(1):83–90. doi: 10.14670/HH-25.83. [DOI] [PubMed] [Google Scholar]
- 10.Fujita H, Hamazaki Y, Noda Y, Oshima M, Minato N. Claudin-4 deficiency results in urothelial hyperplasia and lethal hydronephrosis. PLoS One. 2012;7(12):e52272–e52272. doi: 10.1371/journal.pone.0052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokenek-Unal TD, Coban I, Oguz-Erdogan AS, Seneldir H, Gurcay N, Alper Mt. Differential Expression of Claudin-1, Claudin-3, and Claudin-4 in Bladder Lesions. J Cancer and Tumor Int. 2015;2(3):117–127. [Google Scholar]
- 12.Nakanishi K, Ogata S, Hiroi S, Tominaga S, Aida S, Kawai T. Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am J Clin Pathol. 2008;130(1):43–49. doi: 10.1309/U77A6BTEXVCA5D0E. [DOI] [PubMed] [Google Scholar]
- 13.Saeki R, Kondoh M, Kakutani H, Tsunoda S, Mochizuki Y, Hamakubo T, Tsutsumi Y, Horiguchi Y, Yagi K. A novel tumor-targeted therapy using a claudin-4-targeting molecule. Mol Pharmacol. 2009;76(4):918–926. doi: 10.1124/mol.109.058412. [DOI] [PubMed] [Google Scholar]
- 14.Seiler R, Ashab HAD, Erho N, van Rhijn BWG, Winters B, Douglas J, Van Kessel KE, Fransen van de Putte EE, Sommerlad M, Wang NQ, Choeurng V, Gibb EA, Palmer-Aronsten B, Lam LL, Buerki C, Davicioni E, Sjödahl G, Kardos J, Hoadley KA, Lerner SP, McConkey DJ, Choi W, Kim WY, Kiss B, Thalmann GN, Todenhöfer T, Crabb SJ, North S, Zwarthoff EC, Boormans JL, Wright J, Dall'Era M, van der Heijden MS, Black PC. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur Urol. 2017;72(4):544–554. doi: 10.1016/j.eururo.2017.03.030. [DOI] [PubMed] [Google Scholar]