Abstract
Arterial hypertension represents one of the most common pathologies in the adult population. Hypertensive patients have structurally altered arteries, with a higher rigidity that leads to a significant decrease in vascular compliance. At the base of the pathophysiological process stands the inflammation, as a reaction of the organism to injury. Objectives: This study aims to highlight clinical-paraclinical correlations in people diagnosed with arterial hypertension and inflammatory status. Thus, we would like to evaluate possible correlations between the usual inflammatory markers and blood pressure values. Materials and methods: The sample comprises 64 adults who were subsequently divided based on the diagnosis of arterial hypertension, by using Automatic Blood Pressure Monitoring, as following: Study group=26 patients (with arterial hypertension) and Control group: 38 patients (without arterial hypertension). Results: The study has revealed negative correlations between the neutrophil/lymphocyte ratio (NLR) and the general diastolic average (r=-0.248 and p=0.047), the diurnal diastolic average (r=-0.258 and p=0.038) and the diurnal mean arterial pressure (r=-0.249 and p=0.046) and a negative correlation between the red cell distribution width (RDW) and the dipping index (r=-0.402 and p=0.013), each of them accomplishing the level of statistical significance. Conclusions: NLR can be used as a predictor of diastolic blood pressure values and as a factor of prognosis for the evolution of arterial hypertension. RDW is higher in non-dipping patients.
Keywords: Arterial Hypertension, NLR, RDW
Introduction
Arterial hypertension represents one of the most common pathologies in the adult population, causing approximately 10 million deaths and over 200 million years lost due to ill-health (DALY) in 2015 [1].
Although, in recent decades, numerous studies have been conducted, the etiology of adult arterial hypertension has remained incompletely elucidated, this pathology being a challenge for global health systems.
Understanding the pathophysiological mechanisms underlying the natural evolution of essential arterial hypertension remains a goal to which this study subscribes.
The following parameters are involved in modulating blood pressure: the circulating blood volume, the inflammatory cytokines, the properties of the vascular bed (vascular caliber, vascular reactivity), the neuro-hormonal factors etc.
The vascular changes observed in hypertensive patients (increased stiffness and decreased vascular compliance) are not only an important factor in the pathogenesis of arterial hypertension but also a consequence of a constantly high blood pressure.
The correlation between the inflammatory process and the arterial hypertension, observed in different population groups, is based on the effect of persistent inflammation on the vascular endothelium, which is said to be the forerunner of increased blood stiffness and modifications in subsequent blood pressure values [2,3].
Objectives of the study
This study aims to highlight clinical-paraclinical correlations in people diagnosed with arterial hypertension and inflammatory status.
High blood pressure values can cause specific organ damage, as a result of vascular remodeling, highlighted by subclinical inflammation (pre-existing process or, conversely, installed by the persistence of high blood pressure); vascular remodeling can also be responsible for increases in blood pressure.
As the bidirectional relationship between arterial hypertension and subclinical inflammation includes limited data, our study aims to evaluate correlations between common inflammatory markers (C-reactive protein, fibrinogen), several non-specific hematologic markers of inflammation (total leukocyte count, neutrophils/lymphocytes ratio (NLR), red cell distribution width (RDW), total platelet count) and blood pressure parameters obtained using ambulatory blood pressure monitoring (ABPM) (systolic/diastolic blood pressure, mean blood pressure, pulse pressure, dipping index, morning surge).
Material and Methods
For this study we used a sample of 64 adults, both women and men, considered over a period of approximately 6 months (June-December 2019), in the Internal Medicine Department of an adult outpatient clinic in Bucharest (Sanacare Vital Lutheran Clinic).
Patient enrollment was performed after obtaining individual written informed consent.
We included patients with suspected arterial hypertension following the anamnesis, clinical examination or determination of blood pressure values at the medical office, with Systolic Blood Pressure (SBP)≥140mmHg and/or Diastolic Blood Pressure (DBP)≥90mmHg, according to the ESC/ESH Guidelines 2018 for the Management of Arterial Hypertension [1].
Patients who had history of inflammatory diseases or other causes that could determine changes in inflammatory parameters were excluded from the study.
The assessment methods used for these cases were complete clinical examination, together with blood tests (complete blood count, fibrinogen and C-reactive protein) and parameters obtained from Automatic Blood Pressure Monitoring (ABPM/24 hours), using a Meditech (Hungary) ABPM-05 device.
Patients were subsequently divided based on the final diagnosis of arterial hypertension, obtained from Automatic Blood Pressure Monitoring (SBP≥130mmHg and/or DBP≥80mmHg, according to the ESC/ESH 2018 Guide for Blood Pressure Management) and 2 groups were formed: Study group, with 26 patients (with arterial hypertension) and Control group, with 38 patients (without arterial hypertension) [1].
We used the 2017 Microsoft Excel program and the Pearson correlation coefficient was calculated to test the relationship between continuous variable, ie inflammatory markers and the parameters resulting from the ambulatory blood pressure monitoring.
In addition, linear regression models were applied and the level of statistical significance of each correlation was calculated.
All numerical data were expressed as mean±standard deviation of the mean (SD) and category variables were expressed in frequencies and percentages.
Results
The division of the sample according to gender and age revealed an approximately equal distribution between men and women, with a slight predominance of females (Figure 1).
Figure 1.

The distribution of sample patients by age and sex
The mean age of the study participants was of 59.04±13.71 years (Table 1), while there were no patients in the category 18-24 years and with a predominance of those over 60 years.
Table 1.
Descriptive statistics of the sample
|
Variable |
Mean±standard deviation |
|
Age (years) |
59,04±13,71 |
|
Men (%)/Women (%) |
30 (47%)/ 34 (53%) |
|
C reactive protein (mg/L) |
1,27±2,61 |
|
Fibrinogen (mg/dL) |
331,06±85,93 |
|
Leukocytes (103/dL) |
7194,68±1811,07 |
|
Neutrophils (103/dL) |
3804,93±1236,89 |
|
Lymphocytes (103/dL) |
2332,31±706,09 |
|
Neutrophil/lymphocyte ratio (NLR) |
1,76±0,84 |
|
RDW (%)* |
13,2±1,08 |
|
Platelets (103/dL)* |
258243±70621 |
|
Office SBP (mmHg) |
141,54±15,55 |
|
Office DPB (mmHg) |
88,59±10,64 |
|
General 24h SBP (mmHg) |
128,56±11,43 |
|
General 24h DBP (mmHg) |
72,78±7,40 |
|
Diurnal SBP (mmHg) |
133,39±12,32 |
|
Diurnal DBP (mmHg) |
76,82±8,45 |
|
Nocturnal SBP (mmHg) |
119,03±12,66 |
|
Nocturnal DBP (mmHg) |
64,96±7,73 |
|
General MAP (mmHg) |
91,35±7,39 |
|
Diurnal MAP (mmHg) |
94,96±9,65 |
|
Nocturnal MAP (mmHg) |
83,43±8,07 |
|
General PP (mmHg) |
55,62±10,75 |
|
Diurnal PP (mmHg) |
56,60±10,93 |
|
Nocturnal PP (mmHg) |
54,25±11,34 |
|
Dipping index |
10,07±8,06 |
|
General maximum SBP (mmHg) |
168,76±23,10 |
|
Diurnal maximum SBP (mmHg) |
168,81±22,56 |
|
Nocturnal maximum SBP (mmHg) |
141,34±23,29 |
|
General maximum DBP (mmHg) |
99,45±19,70 |
|
Diurnal maximum DBP (mmHg) |
100,64±18,98 |
|
Nocturnal maximum DBP (mmHg) |
83,37±12,27 |
|
Morning surge |
18,71±12,36 |
Note: *RDW and platelets were only analyzed for 37 patients (16 from the study lot, 21 from the control lot)
In the ambulatory blood pressure monitoring, an important parameter is the 24 hours average BP, which was used to support the diagnosis of arterial hypertension (SBP≥130mmHg and/or DBP≥80mmHg [1], thus highlighting a percentage of 41% of patients with arterial hypertension (26 patients) (Figure 2).
Figure 2.
The distribution of sample patients by sex and lot
Of these, 9 had both parameters elevated (SBP and DPB), 14 had increased SBP (confirming the diagnostic of Isolated systolic arterial hypertension) and 3 had increased DBP (confirming the diagnostic of Isolated diastolic arterial hypertension) [1].
Regarding the gender of the patients diagnosed with arterial hypertension (Figure 2), the proportion was equal.
The mean age of patients diagnosed with arterial hypertension was slightly higher (60.38±13.81) as opposed to those without arterial hypertension (57.87±13.62), in the latter group being highlighted a predominance of female patients (N=21), the difference not being statistically significant (F=0,799, p>0.05) (Table 2).
Table 2.
Descriptive statistics of the lots
|
STUDY LOT (n=26) |
CONTROL LOT (n=38) |
P |
|
|
Age (years) |
60,38±13,81 |
57,87±13,62 |
NS |
|
Office SBP (mmHg) |
144,23±15,31 |
139,71±15,65 |
NS |
|
Office DBP (mmHg) |
89,35±12,58 |
88,08±9,23 |
NS |
|
Leukocytes (103/dL) |
7258±2064 |
7151±1644 |
NS |
|
Neutrophils (103/dL) |
3822±1184 |
3794±1287 |
NS |
|
Lymphocytes (103/dL) |
2377±841 |
2302±607,2 |
NS |
|
Neutrophil/lymphocyte ratio (NLR) |
1,75±0,68 |
1,76±0,95 |
NS |
|
RDW (%)* |
13,5±1,43 |
13±0,68 |
NS |
|
Platelets (103/dL)* |
261187,5±89177,89 |
256000±54775,91 |
NS |
|
C reactive protein (mg/L) |
1,03±1,13 |
1,44±3,26 |
NS |
|
Fibrinogen (mg/dL) |
324,2±75,02 |
335,76±93,36 |
NS |
|
General 24h SBP (mmHg) |
139,2±8,34 |
121,26±6,39 |
NS |
|
General 24h DBP (mmHg) |
76,69±7,23 |
70,11±6,31 |
0,0004 |
|
Diurnal SBP (mmHg) |
144,5±9,43 |
125,82±7,30 |
NS |
|
Diurnal DBP (mmHg) |
81,12±8,27 |
73,11±8,107 |
0,0003 |
|
Nocturnal SBP (mmHg) |
128,69±11,67 |
112,42±8,38 |
NS |
|
Nocturnal DBP (mmHg) |
67,81±8,57 |
63,03±6,53 |
0,0203 |
|
General MAP (mmHg) |
97,62±5,45 |
87,08±5,15 |
NS |
|
Diurnal MAP (mmHg) |
102,3±6,83 |
89,86±8,05 |
NS |
|
Nocturnal MAP (mmHg) |
88,23±7,39 |
79,81±6,59 |
NS |
|
General PP (mmHg) |
62,62±11,05 |
50,84±7,53 |
NS |
|
Diurnal PP (mmHg) |
63,31±11,32 |
52,14±8,07 |
NS |
|
Nocturnal PP (mmHg) |
61,15±12,11 |
49,57±8,08 |
0,0001 |
|
Dipping index |
9,80±9,62 |
10±6,99 |
NS |
|
General maximum SBP (mmHg) |
167,68±27,52 |
169,47±20,47 |
NS |
|
Diurnal maximum SBP (mmHg) |
167,33±26,77 |
169,47±20,47 |
NS |
|
Nocturnal maximum SBP (mmHg) |
142,21±22,41 |
139,79±23,87 |
NS |
|
General maximum DBP (mmHg) |
97,83±20,71 |
100,16±19,94 |
NS |
|
Diurnal maximum DBP (mmHg) |
101±18,89 |
100,16±19,94 |
NS |
|
Nocturnal maximum DBP (mmHg) |
80,88±12,02 |
84,35±12,29 |
NS |
|
Morning surge |
19,52±13,57 |
19,19±11,16 |
NS |
Note: *P<0,05 NS=Not significant *RDW and platelets were only analyzed for 37 patients (16 from the study lot, 21 from the control lot).
In the blood tests collected from patients (complete blood count, C-reactive protein, fibrinogen, RDW, total platelet count), no statistically significant difference was found between the two lots (p<0.05).
Furthermore, no statistically significant differences were found regarding the blood pressure values evaluated in the physician's office by the attending medical doctor.
In this study, we carefully analyzed changes in any of the inflammatory parameters (increase of leukocytes, neutrophil/lymphocyte ratio, C-reactive protein, fibrinogen, RDW or platelets).
Next, we tried to find correlations between inflammatory markers and parameters obtained from ambulatory blood pressure monitoring (Table 3).
Table 3.
Correlation table of ABPM variables with inflammatory markers
|
C reactive protein (mg/L) |
Fibrinogen (mg/dL) |
Leukocytes (103/dL) |
NLR |
RDW (%) |
Platelets (103/dL) |
|
|
General 24h SBP (mmHg) |
-0.05613 |
-0.03019 |
0.02710 |
-0.06734 |
0,16585 |
0,06425 |
|
General 24h DBP (mmHg) |
0.01252 |
-0.09995 |
0.20853 |
-0.24865* |
0,24159 |
0,08786 |
|
Diurnal SBP (mmHg) |
-0.09600 |
-0.06564 |
0.10761 |
-0.09766 |
0,31808 |
0,02375 |
|
Diurnal DBP (mmHg) |
-0.02457 |
-0.15700 |
0.23559 |
-0.25882* |
0,30040 |
0,14085 |
|
Nocturnal SBP (mmHg) |
0.04914 |
0.07956 |
-0.12702 |
0.018267 |
-0,10543 |
-0,10759 |
|
Nocturnal DBP (mmHg) |
0.10246 |
0.03274 |
0.05412 |
-0.1454 |
0,02761 |
0,03543 |
|
General MAP (mmHg) |
-0.01668 |
-0.08389 |
0.15470 |
-0.19351 |
0,24720 |
0,03998 |
|
Diurnal MAP (mmHg) |
-0.03609 |
-0.16536 |
0.12634 |
-0.24989* |
0,27620 |
0,11972 |
|
Nocturnal MAP (mmHg) |
0.07694 |
0.05661 |
-0.04509 |
-0.07078 |
-0,04224 |
-0,16899 |
|
General PP (mmHg) |
-0.06556 |
0.02421 |
-0.12323 |
0.09420 |
0,05636 |
-0,10638 |
|
Diurnal PP (mmHg) |
-0.08692 |
0.03492 |
-0.06276 |
0.09027 |
0,13826 |
-0.07472 |
|
Nocturnal PP (mmHg) |
-0.01729 |
-0.06405 |
-0.18315 |
0.11492 |
-0,13669 |
-0,15983 |
|
Maximum SBP (mmHg) |
-0.16632 |
-0.00164 |
0.14897 |
-0.11002 |
0,30956 |
0,14719 |
|
Maximum DBP (mmHg) |
-0.15472 |
-0.03244 |
0.19930 |
-0.09186 |
-0,00841 |
0,06529 |
|
Dipping Index |
-0.15541 |
-0.14435 |
0.22143 |
-0.15228 |
-0,40251* |
0,18079 |
|
Morning surge |
-0,10395 |
-0.20517 |
0.03055 |
-0.16779 |
0,23385 |
0,24056 |
Note: *p<0,05
Even though C-reactive protein, fibrinogen, total leukocyte count and total platelet count did not correlate with any of the parameters obtained from the ambulatory blood pressure monitoring, we found statistically significant correlations between the neutrophil/lymphocyte ratio (NLR) and 3 types of blood pressure parameters: general 24h DBP (r=-0.248, p=0.047), diurnal DBP (r=-0.258, p=0.038) and diurnal MAP (r=−0.249, p=0.046) (Figure 3).
Figure 3.
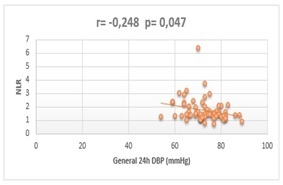
Correlation assessment between NLR and general 24h DBP
All three correlation analysis showed indirect type of dependencies between the considered variables.
The neutrophil/lymphocyte ratio is a suggestive marker for subclinical inflammation, resulting from dividing the total number of neutrophils in the peripheral blood by the total number of lymphocytes in the peripheral blood.
The limits of normal values are still debated but most authors consider the reference range of NLR=[0.79-3.53].
The mean NLR in our study was of 1.76±0.84 (within normal limits).
Regarding the general 24h DBP, the diurnal DBP and the diurnal MAP, their mean values were of 72.78±7.40, 76.82±8.45 and of 95.07±9.67, respectively.
At the same time, there was a statistically significant correlation between the red cell distribution width and the dipping index (r=-0.40251, p=0.013) (Table 3).
This correlation was also negative. The mean RDW of non-dipper patients was of 13.4±1.25 and the mean RDW of dipper patients was of 13±0.83.
On careful analysis of Table 2, statistically significant differences (p<0.05) can be observed in the values of general 24h DBP, diurnal DBP, nocturnal DBP and nocturnal PP, with higher values in the group of those diagnosed with arterial hypertension (76.69±7.23mmHg compared to 70.11±6.31mmHg in general 24h DBP, 81.12±8.27mmHg compared to 73.11±8.10mmHg in diurnal DBP, 67.81±8.57mmHg versus 63.03±6.53mmHg for nocturnal DBP and 61.15±12.11mmHg compared to 49.57±8.08mmHg for nocturnal PP).
The other blood pressure parameters obtained following the ambulatory blood pressure monitoring did not show any statistically significant differences between the two lots.
Discussions
Investigating the relationship between various inflammatory markers, such as C reactive protein, fibrinogen, total leukocyte count, neutrophil/lymphocyte ratio, RDW, total platelet count and parameters obtained from automatic blood pressure monitoring (general/diurnal/nocturnal systolic blood pressure, general/diurnal/nocturnal diastolic blood pressure, general/diurnal/nocturnal mean arterial pressure, general/diurnal/nocturnal pulse pressure, maximum systolic/diastolic blood pressure, dipping index and morning surge), there was a negative correlation between the NLR and the general 24h DBP, the diurnal DBP and the diurnal MAP and a negative correlation between the RDW and the dipping index.
During the cardiac cycle, only a third of the beating volume moves to the capillaries during systole, the rest of the volume remaining in the arteries, dilating them and thus maintaining blood pressure at an optimum level.
At the end of the ventricular systole, the arterial walls undergo a process of passive recoil so that the blood can reach the capillaries during diastole as well.
This mobility of the arteries during the cardiac cycle is influenced by the tone of the arteriolar smooth muscles, which is modulated by the phenomenon of self-regulation of vascular tone, a mechanism that allows the perfusion to adapt to tissue metabolic needs, despite variations in mean arterial pressure (60-180mmHg) [4,5].
The mean arterial pressure has three components (myogenic, metabolic and endothelial).
The myogenic component refers to the response of the smooth muscle fibers in the vascular wall to stretching, independent of the innervation.
Thus, a decrease in intravascular pressure causes a decrease in flow and stretching of smooth muscle fibers, which determines their relaxation and an increase in arteriolar flow.
Increased intravascular pressure determines the stretching of smooth muscle fibers and causes muscle constriction with decreased arteriolar flow.
Metabolic component comes into play when local tissue flow decreases and local metabolites increase (some with vasodilating effect, such as adenosine, lactate, K+), which can lead to decreased local resistance and tendency to increase perfusion (active hyperemia).
The relationship between systemic inflammation, vascular cell activation and changes in the arterial wall has been demonstrated, with inflammatory mediators having a direct pathogenic effect in altering the mechanisms of vascular tone regulation.
In addition to the increase of the level of cytokines, chemokines and adhesion molecules, the mechanical stress in the arterial wall, along with the pro-inflammatory effects of humoral factors, such as angiotensin II (which in addition to regulating vascular tone also has pro-inflammatory effects on the arterial wall by promoting the release of reactive oxygen species and reduction of nitric oxide generation), activate the resident cells from the media and the adventitia.
Thus, the vascular smooth muscle go through phenomena of differentiation, proliferation, migration to the intima and production of collagen matrix [5].
Mean arterial pressure is a variable greatly influenced by the diastolic blood pressure (MAP=1/3 SBP+2/3 DBP), so that an increasing change in the DBP will cause a similar increase in the MAP.
The same relationship takes place in the opposite direction.
Thus, it can be understood why the NLR index influences not only the diurnal DBP but also the diurnal MAP.
However, in our study we did not find a statistically significant correlation between the NLR index and the general MAP (r=-0.19351, p>0.05), although the NLR had a negative correlation with the general 24h DBP.
Furthermore, no statistically significant correlations were found between the NLR and the nocturnal DBP, respectively nocturnal MAP.
Red cell distribution width is a widely used parameter, especially in the management of various types of anemia; the high level of RDW in atherosclerotic cardiovascular pathologies was argued by its effect of activating neuro-hormonal mediators, oxidative stress and chronic inflammation on erythropoiesis [4].
It is considered that RDW may be a significant marker of subclinical inflammation and an important cardiovascular risk factor.
There are studies that have shown that RDW is elevated in patients with a non-dipping pressure profile [6,7].
In our study, a lower dipping index is associated with an increased RDW and vice versa.
We set out to further study this bilateral relationship, in an attempt to elucidate whether RDW increases as a result of the decrease in the dipping index or whether RDW is altered by the effects of non-dipping pressure status on the body.
We did not find any correlation with blood pressure parameters for C-reactive protein, fibrinogen, total leukocyte count and total platelet count, although there are studies that have shown some associations [5,8].
The lack of statistically significant correlations for C-reactive protein could be explained by not using in the study a highly sensitive C-reactive protein assay (hsCRP), which can be measured up to levels of 0.3mg/L thus being a more appropriate indicator for the characterization of a low-intensity inflammation.
There are clinical studies that have shown a positive correlation between highly sensitive C-reactive protein and intimate-average thickness of the carotid artery, an important marker of atherosclerosis and subsequent of arterial hypertension [9].
We set out to continue the research, emphasizing the respective correlations.
Through the study we also demonstrated a statistically significant difference in terms of general 24h DBP, diurnal DBP, nocturnal DBP and nocturnal PP, with higher values in the study group, as opposed to the control group.
The other parameters in which the diastolic pressure value is involved, such as maximum DBP, MAP, did not meet the level of statistical significance.
No systolic parameters met the level of statistical significance.
Among the limitations of our study we mention the small group of patients, the lack of determination of iron balance, highly sensitive C-reactive protein, adipo-cytokines.
Conclusions
Consistent with data reported in the literature, we can conclude that the neutrophil-lymphocyte ratio can be used as a predictor of diastolic blood pressure values and as a factor of prognosis for the evolution of arterial hypertension.
It is worth mentioning the bidirectional link between the diastolic blood pressure and NLR, two acquirable parameters in hypertensive patients’ evaluation.
Another important correlation was between the red cell distribution width and the dipping index, the pathological mechanisms of higher RDW in the “non-dippers” hypertensive patients have not yet been fully elucidated.
The arterial hypertension represents a complex pathology, one of the leading causes of cardiovascular mortality worldwide, requiring important long-term monitoring.
The subclinical inflammation is not a recent concept in the literature, but its characteristics continues to be a topic of great interest.
To this extent, our study provides evidence that some inexpensive biochemical parameters, part of complete blood cell count, can be correlated with circadian changes of hypertension, in the attempt of proper evaluation of hypertensive patients and prediction of cardiovascular events in this population.
Conflict of interests
None to declare.
References
- 1.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, Group ESCSD ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 2.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62(1):126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 3.Heptinstall RH. Renal biopsies in hypertension. Br Heart J. 1954;16(2):133–141. doi: 10.1136/hrt.16.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altiparmak IH, Erkus ME, Kocarslan A, Sezen H, Gunebakmaz O, Sezen Y, Kaya Z, Yildiz A, Demirbag R. High aortic pulse-wave velocity may be responsible for elevated red blood cell distribution width in overweight and obese people: a community-based, cross-sectional study. Cardiovasc J Afr. 2016;27(4):246–251. doi: 10.5830/CVJA-2016-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanindi A, Topal FE, Topal F, Celik B. Red cell distribution width in patients with prehypertension and hypertension. Blood Press. 2012;21(3):177–181. doi: 10.3109/08037051.2012.645335. [DOI] [PubMed] [Google Scholar]
- 6.Su D, Guo Q, Gao Y, Han J, Yan B, Peng L, Song A, Zhou F, Wang G. The relationship between red blood cell distribution width and blood pressure abnormal dipping in patients with essential hypertension: a cross-sectional study. BMJ Open. 2016;6(2):e010456–e010456. doi: 10.1136/bmjopen-2015-010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349(9050):462–466. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 8.Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- 9.Gomez-Marcos MA, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Gomez-Sanchez L, Rodriguez-Sanchez E, Gomez-Sanchez M, Martinez-Vizcaino V, Garcia-Ortiz L. Relationships between high-sensitive C-reactive protein and markers of arterial stiffness in hypertensive patients. Differences by sex. BMC Cardiovasc Disord. 2012;12:37–37. doi: 10.1186/1471-2261-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]


