Abstract
Background
Rotator cuff tears can be asymptomatic in some cases; however, even when the tear size is small, clinical symptoms can be very severe. This suggests that symptoms of rotator cuff tears are related to factors other than the size. Although synovitis has been cited as one of the factors, there is no grading system for synovitis in rotator cuff tears. Moreover, there are few studies that evaluated the relationship between synovitis and clinical features in patients with rotator cuff tears.
Methods
Patients with medium-sized rotator cuff tears, who were scheduled for arthroscopic repair, were recruited for this study. The glenohumeral joint was divided into 4 quarters. Then, vascularity and hypertrophy of the joint were graded in each quarter using a modified scoring system. Clinical assessment was performed preoperatively and at 3 months and 6 months after surgery. Finally, correlation between the severity of synovitis and clinical features was analyzed.
Results
The intraobserver correlation coefficient was 0.815 to 0.918 and the interobserver correlation coefficient was 0.779 to 0.992 for the single measurement. Vascularity was significantly correlated with the range of motion, strength, and constant score within 6 months after surgery. Hypertrophy was correlated with the range of motion within 6 months after surgery.
Conclusions
Synovitis in the shoulder with rotator cuff tears can be graded by using our modified scoring system. The severity of synovitis was closely related to the clinical features after surgery. Therefore, when treating patients with rotator cuff tears, treatment of synovitis should also be considered.
Keywords: Shoulder, Synovitis, Rotator cuff tear, Grading
Many factors are correlated with clinical results of rotator cuff repair, such as patient age, tear size, patient activity, stiffness, and retear.1,2) However, any single factor cannot explain the diverse clinical features after rotator cuff repair. Inflammation, such as synovitis, has been suggested as a contributing factor, but this finding may be nonspecific in variable conditions.3,4,5) Synovitis has been believed to be closely related with the pathogenesis of adhesive capsulitis.6,7) In patients with rotator cuff tears (RCTs), synovitis is commonly observed during surgery. However, the specific pathogenic mechanism of synovitis has not been fully elucidated. Furthermore, based on our literature review, few studies have introduced classification systems for shoulder synovitis or evaluated the relationship between this inflammation and clinical features in patients with RCT.
The purpose of this study was to propose a novel classification system for shoulder synovitis, to assess the reliability of the classification, and to evaluate the clinical relevance of shoulder synovitis with parameters, such as range of motion (ROM), pain, muscle strength, and clinical scores. We hypothesized that synovitis in the shoulder joint could be correlated with pain, limitation of ROM, and clinical scores in preoperative or postoperative periods.
METHODS
Patient Selection
We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of this study was reviewed and approved by Institutional Review Board of Ajou University (IRB No. AJIRB-MED-MDB-15-324). Written informed consents were obtained.
From November 2015 to December 2016, patients scheduled for arthroscopic rotator cuff repair for medium-sized tears (1–3 cm) measured by magnetic resonance imaging (MRI) were included in the study. Patients and their data were collected prospectively. To ensure homogeneity of the study population, only medium-sized tears, which were repaired without high tension by an arthroscopic single-row repair technique, were enrolled in this study.
The exclusion criteria were as follows: (1) stiff shoulder in which passive motion was restricted by more than 20° compared to the contralateral side in any direction or passive forward flexion and passive abduction were less than 120°,8) (2) history of previous operation in the affected side, (3) inflammatory arthritis including rheumatoid arthritis (RA), psoriatic arthritis, and ankylosing spondylitis in any joint or osteoarthritis in the shoulder joint, (4) partial or small RCT, (5) large to massive RCT, (6) confirmed retear in follow-up imaging (MRI or ultrasonography), (7) biceps tendon lesions such as biceps subluxation, dislocation, or a degeneration tear needing tenotomy or tenodesis, (8) a subscapularis tear needing repair, (9) major trauma history, such as falling down or high-speed traffic accidents and prior operations, (10) steroid injections before postoperative 6 months.
Preoperative and Postoperative Evaluations
All operations were performed by 2 senior surgeons, who are arthroscopic specialists (DL and BGL). All enrolled patients were evaluated 1 day before the operation and 3 and 6 months postoperatively. Preoperative and postoperative subjective pain scores were measured with a visual analog scale (VAS). For quantitative assessment of the muscle strength of the rotator cuff, a portable hand-held Myometer (Mecmesin, Nottingham, England) was used. Elevation strength was tested with the patient in the seated position with the arm flexed to 90° in the scapular plane. External and internal rotations were tested with the shoulder in a neutral position and the elbow in 90° of flexion. Shoulder ROM including forward flexion, abduction, external rotation at the side and at 90° of abduction, and internal rotation at 90° of abduction was assessed before and after the operation. All patients were regularly examined at postoperative 3 weeks, 6 weeks, 3 months, and 6 months. We used the preoperative and 3- and 6-month follow-up data in this study.
Postoperative Rehabilitation
All patients were educated about postoperative rehabilitation. Only shoulder stretching exercises limited to 90° were performed 3 times a day until 3 weeks after the operation and these exercises were gradually increased to the maximum range. Other exercises including external rotation were not allowed. A shoulder abduction brace was applied until 6 weeks after the operation. From 3 weeks after the operation, patients were instructed to increase the range of passive forward flexion to a tolerable level and gradually start other passive exercises such as external rotation. Active exercises were not allowed until 6 weeks after the operation or until full passive ROM was regained. All patients were educated repeatedly on how to perform exercises exactly during hospitalization, and their performance was checked at discharge and at 3 weeks and 6 weeks after the operation on an outpatient basis. Nonsteroidal anti-inflammatory drugs (NSAIDs) were prescribed at the time of discharge, and patients were required to decide whether to take routine pain medication based on the level of pain. Steroid injection was not performed until 6 months postoperatively.
Novel Classification System for Shoulder Synovitis
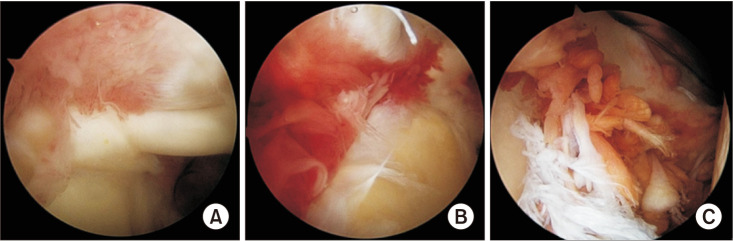
The glenohumeral joint was divided into 4 quarters as follows: anterosuperior (AS), posterosuperior (PS), anteroinferior (AI), and posteroinferior (PI) quarters (Table 1). The severity of joint synovitis was evaluated for scoring in the arthroscopic inspection. The grading system was modified from the method of af Klint et al.9) The synovitis classification system is composed of 2 parameters, hypertrophy and vascularity. Each parameter is divided into 3 classes (0–2) (Table 2, Fig. 1) according to the severity and assessed at 4 quarters respectively in each patient.
Table 1. Four Quarters in Shoulder Joint.
| Quarter | Description |
|---|---|
| Anterosuperior | Area above the leading edge of subscapularis, anterior to biceps long head |
| Posterosuperior | Area above 9 o'clock in glenoid (posterior portal), posterior to biceps long head |
| Anteroinferior | Area under the leading edge of subscapularis, anterior to 6 o'clock in glenoid |
| Posterosuperior | Area under 9 o'clock in glenoid (posterior portal), posterior to 6 o'clock in glenoid |
Table 2. Grading System to Evaluate the Severity of Synovitis.
| Grading system | Visible synovial mass |
|---|---|
| Hypertrophy* | |
| Score 0 | A thin and transparent synovial membrane; Vessels are clearly visible, as is subsynovial tissue (e.g., fibrous capsule, fat, or muscle). |
| Score 1 | Minor thickening, villi are not observed, or long villi are scattered. Its distribution is sparse. |
| Score 2 | Long villi are exuberant. |
| Vascularity | This parameter grades density of visible vessels. |
| Score 0 | Thin and scattered vessels; subsequently fibrotic tissue, containing only few vessels |
| Score 1 | Diffuse increased vascularity, slightly reddish |
| Score 2 | Densely packed vessels, definitely red |
*The hypertrophy score does not take present or past inflammation activity into account; white fibrotic villi score same as active hypervascularized villi. When the distribution of villi varies in a single quarter, the grade of the most severe portion should be scored.
Fig. 1. Grading of the severity of synovitis (hypertrophy: H, vascularity: V). (A) H1 V1. (B) H1 V2. (C) H2 V1.
Repeated Measurements for Assessment of Interobserver and Intraobserver Reliability
Images obtained during surgery were printed in high resolution. Each image was marked by each quarter and interpreted by 2 shoulder specialists (BGL and DL) in a blinded fashion by deleting patient information. Measurement of the parameters (hypertrophy and vascularity) in a randomized set was repeated twice by 1 observer (DL) to assess the repeatability of grading. The other observer (BGL), who was unaware of the prior observer's results, performed a single series of measurements. Intraobserver reproducibility and interobserver reliability were assessed by using the acquired measurement data.
Statistical Analysis
PASS 12 (PASS 12. NCSS, Kaysville, UT, USA) was used for determining the sample size.10) As the primary goal of this study was to propose a synovitis classification system and to evaluate the clinical utility of the synovitis classification, a Kappa test (two-sided Z test) was performed to assess agreement between the 2 observers' measurements based on the incidence of the respective synovitis scores in our data. In a test for agreement between the 2 raters using Kappa statistics, a sample size of 36 subjects was found to achieve 81% power to detect the true Kappa value of 0.60 in a test of H0: Kappa = 0.85 vs. H1: Kappa ≠ 0.85 when there are 3 categories with frequencies equal to 0.50, 0.30, and 0.20. This power calculation was based on a significance level of 0.05.
Statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). The Mann-Whitney U-test was used to compare preoperative and postoperative scores for continuous variables. General linear regression model analysis was performed to determine the correlation between synovitis and clinical scores. Statistical significance was set at p < 0.05.
RESULTS
Preoperative Clinical Data and Postoperative Clinical Outcome
From November 2015 to December 2016, 54 patients were initially enrolled in this study. In 1 patient who complained of severe pain at 2 months postoperatively, a retear was confirmed by ultrasonography. Three patients did not visit at the 6-month follow-up. Two patients had subscapularis tears, which had not been found on preoperative MRI. Excluding these 6 patients, a total of 48 patients were included. The final study population comprised 25 men and 23 women, with a mean age of 57.6 years (standard deviation [SD], 7.6; range, 38–80). The average body mass index was 24.1 kg/m2 (SD, 2.1; range, 18.4–28.9) and the dominant side was affected in 35 of the 48 patients. Clinical outcomes of the arthroscopic rotator cuff repair are provided in Table 3. Only VAS score, ROM in forward flexion and abduction, muscle strength, and clinical scores were significantly improved at 3 months after the operation. However, at 6 months after surgery, all clinical data showed significant improvement compared to the preoperative status.
Table 3. Clinical Outcomes of Rotator Cuff Repair.
| Variable | Preoperative | POD 3 mo | POD 6 mo |
|---|---|---|---|
| VAS | 5.5 ± 1.67 | 1.9 ± 1.52* | 1.0 ± 1.14* |
| ROM | |||
| Flexion (°) | 143.8 ± 41.68 | 148.1 ± 30.31* | 159.1 ± 21.33* |
| ERs (°) | 66.8 ± 24.27 | 66.9 ± 23.39 | 73.6 ± 16.75* |
| ERa (°) | 73.6 ± 20.73 | 72.0 ± 18.56 | 77.0 ± 14.17* |
| IRa (°) | 59.8 ± 25.60 | 57.8 ± 23.8 | 64.7 ± 20.29* |
| Abduction (°) | 135.1 ± 47.26 | 135.4 ± 39.29* | 147.5 ± 33.93* |
| Strength of abduction (kg) | 4.7 ± 3.68 | 5.2 ± 3.85* | 6.5 ± 5.33* |
| Constant score | 59.7 ± 16.82 | 64.2 ± 15.55* | 69.0 ± 14.61* |
| ASES score | 61.5 ± 19.33 | 74.8 ± 13.16* | 80.4 ± 11.03* |
Values are presented as mean ± standard deviation.
POD: postoperative, VAS: visual analog scale, ROM: range of motion, ERs: external rotation at side, Era: external rotation at 90 abduction, Ira: Internal rotation at 90 abduction, ASES: American Shoulder and Elbow Surgeons.
*Statistically significant (p < 0.05) compared to preoperative data.
Repeatability of Measurement
The intraobserver correlation coefficient ranged from 0.815 to 0.918 for the single measurement and from 0.803 to 0.910 for the average measurement. The interobserver correlation coefficient ranged from 0.779 to 0.992 for the single measurement and from 0.854 to 0.996 for the average measurement. The results demonstrated good intraobserver reproducibility and interobserver reliability, indicating that all measurements can be performed with good repeatability.
Correlation of Severity of Synovitis with Clinical Features
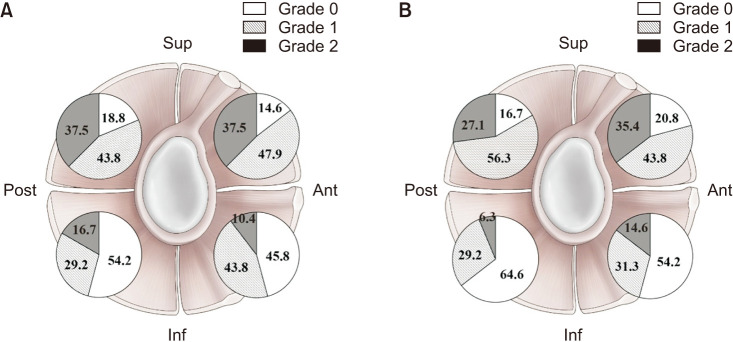
The prevalence of synovitis according to grade is provided in Fig. 2. The incidence of severe synovitis (grade 2) in terms of both vascularity and hypertrophy was higher in the superior area than the inferior area.
Fig. 2. The prevalence of synovitis according to grade in terms of hypertrophy (A) and vascularity (B). Sup: superior, Ant: anterior, Inf: inferior, Post: posterior.
Preoperative Correlation
Table 4 shows the relationship between preoperative clinical features and vascularity in the 4 quarters. The grade of vascularity was significantly correlated with the preoperative flexion and abduction in the AS and AI quarters. Synovitis in the anterior half of the joint (AS and AI quarters) was correlated with 4 of the 6 clinical features. However, in the posterior half of the joint, synovitis was correlated with forward flexion and constant score only in the PI quarter. Synovitis in the PS quarter showed no correlation with any features. Constant score was significantly correlated with the grade of vascularity in AI and PI quarters. The grade of vascularity in the AS quarter was related with preoperative American Shoulder and Elbow Surgeons (ASES) shoulder score.
Table 4. Correlation between Vascularity and Clinical Features in Preoperative Period.
| Vascularity | Anterosuperior | Anteroinferior | Posterosuperior | Posteroinferior | ||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | |
| Flexion | –17.101 (7.980) | 0.038* | –27.207 (7.368) | 0.001* | –8.932 (9.752) | 0.365 | –24.191 (9.936) | 0.019* |
| Abduction | –18.780 (9.092) | 0.045* | –26.553 (8.699) | 0.004* | –7.601 (11.120) | 0.498 | –20.738 (11.605) | 0.081 |
| ERs | –6.197 (4.685) | 0.193 | –8.794 (4.617) | 0.063 | –2.868 (5.591) | 0.611 | –10.671 (5.810) | 0.073 |
| ERa | –6.208 (4.042) | 0.132 | –4.159 (4.124) | 0.319 | –1.809 (4.863) | 0.712 | –4.859 (5.184) | 0.354 |
| IRa | –8.099 (5.124) | 0.121 | –7.622 (5.170) | 0.148 | –6.815 (6.097) | 0.270 | –4.916 (6.606) | 0.461 |
| VAS | 0.370 (0.337) | 0.279 | 0.034 (0.344) | 0.921 | 0.021 (0.401) | 0.958 | 0.005 (0.432) | 0.990 |
| Constant score | –5.652 (3.293) | 0.093 | –9.956 (3.073) | 0.002* | –3.845 (3.951) | 0.336 | –10.580 (3.985) | 0.011* |
| ASES score | –8.904 (3.536) | 0.016* | –4.755 (3.736) | 0.210 | –3.007 (4.417) | 0.500 | 0.820 (4.772) | 0.864 |
| Strength | 0.363 (0.761) | 0.636 | –0.206 (0.766) | 0.790 | 0.773 (0.888) | 0.389 | –0.722 (0.956) | 0.454 |
SE: standard error, Sig: significance, ERs: external rotation at side, Era: external rotation at 90 abduction, Ira: Internal rotation at 90 abduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Surgeons.
*Statistically significant, p < 0.05.
The grade of hypertrophy was significantly associated with flexion and abduction in the AI quarter, but not in the other quarters (Table 5). Constant score was significantly correlated with hypertrophy in AI and PI quarters.
Table 5. Correlation between Hypertrophy and Clinical Features in Preoperative Period.
| Hypertrophy | Anterosuperior | Anteroinferior | Posterosuperior | Posteroinferior | ||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | |
| Flexion | –11.297 (9.161) | 0.224 | –27.587 (8.408) | 0.002* | –2.845 (8.656) | 0.744 | –14.037 (8.337) | 0.099 |
| Abduction | –8.587 (10.502) | 0.418 | –27.737 (9.797) | 0.007* | 1.798 (9.838) | 0.856 | –12.067 (9.597) | 0.215 |
| ERs | –2.669 (5.293) | 0.617 | –6.290 (5.258) | 0.238 | –1.511 (4.931) | 0.761 | –2.465 (4.886) | 0.616 |
| ERa | –2.287 (4.597) | 0.621 | –5.234 (4.573) | 0.259 | 0.568 (4.287) | 0.895 | –4.165 (4.209) | 0.328 |
| IRa | –0.192 (5.852) | 0.974 | –11.331 (5.638) | 0.051 | 1.870 (5.436) | 0.732 | –4.466 (5.360) | 0.409 |
| VAS | 0.023 (0.380) | 0.951 | 0.501 (0.375) | 0.189 | –0.060 (0.353 | 0.866 | –0.089 (0.351) | 0.801 |
| Constant score | –2.311 (3.763) | 0.542 | –9.626 (3.517) | 0.009* | –1.141 (3.511) | 0.747 | –7.098 (3.321) | 0.038* |
| ASES score | –1.202 (4.199) | 0.776 | –7.554 (4.075) | 0.070 | –0.879 (3.907) | 0.823 | 0.592 (3.879) | 0.879 |
| Strength | 0.950 (0.835) | 0.261 | 0.047 (0.853) | 0.956 | 0.937 (0.775) | 0.233 | –0.418 (0.780) | 0.595 |
SE: standard error, Sig: significance, ERs: external rotation at side, Era: external rotation at 90 abduction, Ira: Internal rotation at 90 abduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Surgeons.
*Statistically significant, p < 0.05.
Postoperative Correlation
Vascularity in the AS quarter was significantly correlated with flexion, pain, and ASES at 3 months after the operation, but there was no significant correlation at 6 months after the operation. In the AI quarter, there was a significant correlation between vascularity and flexion, abduction, and constant score at 3 months after the operation and flexion and abduction, internal rotation, and constant score at 6 months after the operation (Table 6). At 6 months after the operation, there was no significant correlation between vascularity and clinical features in AS and PS quarters. Vascularity in the anterior half of the joint (especially in the AI quarter) was more correlated with postoperative clinical features than that in the posterior half. PI vascularity was correlated with only forward flexion at 3 months and 6 months after the operation and constant score at 6 months after the operation.
Table 6. Correlation between Vascularity and Clinical Features at 3 and 6 Months after Surgery.
| Vascularity | Anterosuperior | Anteroinferior | Posterosuperior | Posteroinferior | ||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | |
| 3 mo | ||||||||
| Flexion | –13.675 (5.639) | 0.019* | –19.384 (5.284) | 0.001* | –13.439 (6.751) | 0.053 | –15.307 (7.218) | 0.040* |
| Abduction | –14.217 (7.164) | 0.053 | –17.310 (7.052) | 0.018* | –11.818 (8.595) | 0.176 | –6.724 (9.383) | 0.477 |
| ERs | –7.065 (4.108) | 0.093 | –5.510 (4.186) | 0.195 | –6.527 (4.884) | 0.188 | –0.980 (5.354) | 0.856 |
| ERa | –5.531 (3.570) | 0.129 | –5.087 (3.606) | 0.165 | –5.604 (4.220) | 0.191 | –3.233 (4.602) | 0.486 |
| IRa | –7.462 (4.646) | 0.115 | –6.821 (4.696) | 0.153 | –8.077 (5.479) | 0.148 | –0.114 (6.034) | 0.985 |
| VAS | 0.656 (0.282) | 0.025* | 0.020 (0.300) | 0.947 | 0.233 (0.349) | 0.507 | –0.092 (0.376) | 0.809 |
| Constant score | –5.522 (2.973) | 0.070 | –6.561 (2.943) | 0.031* | –5.361 (3.534) | 0.136 | –6.495 (3.772) | 0.092 |
| ASES score | –5.535 (2.311) | 0.021* | –2.526 (2.441) | 0.306 | –2.581 (2.858) | 0.371 | 0.777 (3.099) | 0.803 |
| Strength | –0.587 (0.761) | 0.445 | –0.022 (0.771) | 0.977 | –0.011 (0.900) | 0.990 | –1.315 (0.947) | 0.172 |
| 6 mo | ||||||||
| Flexion | –7.054 (4.583) | 0.132 | –18.917 (3.800) | < 0.001* | –7.584 (5.048) | 0.142 | –24.333 (4.993) | < 0.001* |
| Abduction | –7.895 (7.337) | 0.289 | –22.050 (6.856) | 0.003* | –4.213 (8.168) | 0.609 | –14.352 (9.848) | 0.154 |
| ERs | –1.830 (3.423) | 0.596 | –2.092 (3.570) | 0.562 | –3.311 (3.739) | 0.382 | 1.132 (4.669) | 0.810 |
| ERa | –1.900 (2.884) | 0.514 | –4.359 (2.939) | 0.147 | –3.383 (3.141) | 0.289 | –2.349 (3.925) | 0.553 |
| IRa | –6.112 (4.490) | 0.182 | –10.951 (4.445) | 0.019* | –6.264 (4.955) | 0.214 | –6.798 (6.156) | 0.277 |
| VAS | 0.092 (0.227) | 0.688 | –0.167 (0.235) | 0.483 | 0.217 (0.247) | 0.386 | 0.113 (0.308) | 0.716 |
| Constant score | –4.249 (3.092) | 0.178 | –9.283 (2.926) | 0.003* | –2.537 (3.462) | 0.468 | –8.491 (4.073) | 0.044* |
| ASES score | –1.125 (2.081) | 0.592 | 0.063 (2.180) | 0.977 | –0.519 (2.296) | 0.823 | 2.976 (2.796) | 0.294 |
| Strength | –1.156 (1.185) | 0.336 | –1.109 (1.240) | 0.377 | 1.156 (1.307) | 0.382 | –3.477 (1.526) | 0.029* |
SE: standard error, Sig: significance, ERs: external rotation at side, ERa: external rotation at 90 abduction, IRa: Internal rotation at 90 abduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Surgeons.
*Statistically significant, p < 0.05.
Hypertrophy in the AI quarter was significantly associated with flexion and abduction at 3 months after the operation (Table 7). In the PI quarter, only constant score was correlated with hypertrophy at 3 months after the operation. Hypertrophy in the AI quarter was also correlated with flexion and internal rotation at 6 months after the operation. Based on these results, hypertrophy in the AI quarter was more closely related to the prognosis of the ROM than that in the AS, PS and PI quarters.
Table 7. Correlation between Hypertrophy and Clinical Features at 3 and 6 Months after Surgery.
| Hypertrophy | Anterosuperior | Anteroinferior | Posterosuperior | Posteroinferior | ||||
|---|---|---|---|---|---|---|---|---|
| B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | B (SE) | Sig | |
| 3 mo | ||||||||
| Flexion | –8.580 (6.545) | 0.197 | –18.241 (6.128) | 0.005* | –8.938 (6.057) | 0.147 | –8.356 (6.029) | 0.173 |
| Abduction | –4.583 (8.280) | 0.583 | –17.062 (7.959) | 0.038* | –5.989 (7.675) | 0.439 | –2.893 (7.657) | 0.707 |
| Ers | –2.933 (4.695) | 0.535 | –2.724 (4.729) | 0.568 | –2.719 (4.367) | 0.537 | 0.595 (4.352) | 0.892 |
| ERa | –3.085 (4.047) | 0.450 | –4.823 (4.036) | 0.238 | –2.572 (3.769) | 0.499 | –1.618 (3.753) | 0.668 |
| IRa | –0.909 (5.310) | 0.865 | –10.157 (5.124) | 0.054 | 0.358 (4.940) | 0.943 | –1.193 (4.900) | 0.809 |
| VAS | 0.089 (0.331) | 0.789 | 0.198 (0.332) | 0.554 | 0.047 (0.308) | 0.880 | 0.147 (0.305) | 0.633 |
| Constant score | –1.630 (3.422) | 0.636 | –6.273 (3.322) | 0.066 | –2.591 (3.167) | 0.418 | –6.306 (3.021) | 0.043* |
| ASES score | –1.513 (2.721) | 0.581 | –3.606 (2.694) | 0.188 | –1.141 (2.533) | 0.655 | –0.515 (2.519) | 0.839 |
| Strength | 0.622 (0.847) | 0.467 | –0.263 (0.856) | 0.761 | –0.333 (0.791) | 0.676 | –1.005 (0.771) | 0.200 |
| 6 mo | ||||||||
| Flexion | –7.187 (4.777) | 0.141 | –18.247 (4.310) | < 0.001* | –4.686 (4.452) | 0.300 | –7.992 (4.542) | 0.087 |
| Abduction | –3.855 (7.732) | 0.621 | –15.453 (7.902) | 0.058 | –1.264 (7.118) | 0.860 | 5.859 (7.393) | 0.433 |
| ERs | –1.104 (3.572) | 0.759 | 0.018 (3.832) | 0.996 | 1.418 (3.275) | 0.668 | 3.977 (3.374) | 0.246 |
| ERa | –1.536 (3.009) | 0.613 | –3.856 (3.170) | 0.232 | 0.253 (2.771) | 0.928 | 3.540 (2.842) | 0.221 |
| IRa | –2.050 (4.780) | 0.671 | –12.255 (4.710) | 0.013* | –0.406 (4.398) | 0.927 | 2.167 (4.592) | 0.640 |
| VAS | 0.086 (0.236) | 0.719 | 0.041 (0.253) | 0.871 | –0.062 (0.217) | 0.777 | 0.110 (0.227) | 0.631 |
| Constant score | –1.463 (3.292) | 0.660 | –6.004 (3.392) | 0.085 | –2.801 (2.994) | 0.356 | –0.089 (3.173) | 0.978 |
| ASES score | 1.142 (2.166) | 0.601 | 0.283 (2.329) | 0.904 | –0.405 (1.995) | 0.840 | 1.498 (2.075) | 0.475 |
| Strength | 0.437 (1.248) | 0.728 | –0.697 (1.334) | 0.604 | –0.721 (1.141) | 0.531 | –1.594 (1.172) | 0.182 |
SE: standard error, Sig: significance, ERs: external rotation at side, ERa: external rotation at 90 abduction, IRa: Internal rotation at 90 abduction, VAS: visual analog scale, ASES: American Shoulder and Elbow Surgeons.
*Statistically significant, p < 0.05.
DISCUSSION
In this study, we suggested a synovitis classification system with 2 different parameters (vascularity and hypertrophy). In order to express the severity of synovitis, hypertrophy and vascularity were graded from 0 to 2 in a semi-quantitative manner and the inter- and intraobserver reliability of this method was demonstrated to be satisfactory at 0.7 or higher. With this classification system, we examined the relation of synovitis with clinical features before surgery and at 3 and 6 months after surgery.
Synovitis in patients with RCT was associated with preoperative and postoperative ROM (especially forward flexion and abduction) and constant score. However, synovitis was not related to pain or muscle strength. The higher the vascularity, the lower the preoperative and postoperative forward flexion, abduction, constant score, and ASES score. When analyzed by quarter, vascularity of the AI quarter was most correlated with clinical features in the preoperative and postoperative periods. Vascularity of the PS quarter was independent of the clinical features, whereas vascularity of the inferior half (PI and AI quarters) was associated with clinical features. A similar pattern was also found in the results of the hypertrophy analysis. The higher the hypertrophy, the lower the preoperative and postoperative features, such as forward flexion, abduction, and constant score, and these associations were observed in the inferior half (PI or AI quarters). In addition, anterior half synovitis of the joint showed correlations with more clinical features than the posterior half synovitis did. The prevalence of the quarter of synovitis revealed that severe vasculitis or hypertrophy corresponding to grade 2 occurred in superior areas such as AS and PS quarters. On the other hand, grade 0 occurred relatively more frequently in the inferior area. Moreover, the anterior half synovitis was more correlated with postoperative clinical features than posterior half synovitis was. These findings imply that each quarter of the glenohumeral joint could show different synovitis grade and AI synovitis can be most correlated with clinical results than the other quarters, which will need to be confirmed in further research.
The study of synovitis in the shoulder was limited to RA or pyogenic arthritis, but recently, microscopic and macroscopic pathologic findings of synovitis in the shoulder have been reported. Jo et al.5) claimed that unlike our 4 quarters, the joints were divided into anterior, posterior, and inferior areas and performed histological evaluation in each compartment. Macroscopic synovitis was assessed as hypertrophy, hyperemia, and density, which differs from our study. However, the clinical relevance of the degree of synovitis was not addressed in the study. Our study has advantage in that it has found a correlation between the severity of synovitis and clinical features before surgery and 6 months after surgery.
In this study, we could not conclude how the treatment of synovitis could affect the prognosis of RCT. If synovitis is the result of the pathology of RCT, surgical repair of RCT will result in an improvement in synovitis as well, but if synovitis is the cause of pain and limitation of motion as adhesive capsulitis, treatment of synovitis is expected to improve the symptom of RCT. We often encounter patients with RCT who were treated using steroids and their symptoms, such as pain or painful arc, have been improved for more than 6 months.11) Recent studies showed that the asymptomatic condition lasts for more than 2 years if the cuff tear size does not increase during follow-up.12) Therefore, it can be assumed that synovitis may be related with pain and limitation of motion in RCT. However, the relationship between progression of tear size and synovitis remains unclear, and further studies are needed.
Another question is whether surgical synovectomy is helpful in RCT with synovitis. It has been reported that treatment with synovectomy for patients with RA, who have not responded to medication, has relieved symptoms for more than 5 years.13,14) Therefore, it is necessary to determine whether the long-term anti-inflammatory medication or synovectomy could be helpful for synovitis associated with RCT.
This study has several limitations. First, we did not evaluate subacromial bursitis, which is frequently associated with RCT with synovitis. Unlike synovitis in which hypertrophy or vascularity are well distinguished, bursitis is relatively difficult to distinguish by visual inspection. Second, the severity of synovitis was graded semi-quantitatively only by visual inspection, not histologically. It was reported that visual grading is more useful in identifying synovitis, such as hyperemia or density, which is difficult to distinguish with histological classification.5) It is even more efficient to use visual grading rather than histological evaluation for physicians who practice arthroscopy. In addition, visual grading is a reliable method because it has been used in the knee for a long time.9) Finally, the follow-up period was only 6 months. In general, studies on patients with RCT tend to involve a 2-year follow-up after surgery, but the aim of this study was to determine how synovitis affects clinical features of patients with RCT. Considering that drugs such as NSAIDs administered to control inflammation after surgery do not need to be taken until 6 months after surgery, the inflammatory response in RCT almost disappears in about 6 months. In order to obtain more reliable evidence, it is necessary to perform a re-examination for the shoulder through diagnostic arthroscopy at 6 months after surgery, but it is difficult to persuade asymptomatic patients to participate in such a study. The 4-quarter grading system for synovitis using vascularity and hypertrophy showed reliable interobserver and interobserver agreement. Thu, synovitis in the shoulder with RCT can be graded by using this system.
In patients with RCT, pain and limited ROM, especially abduction and flexion, were found to be associated with the severity of synovitis. These associations persisted for up to 3 months after surgery and disappeared after 6 months. In particular, synovitis in the lower part of the shoulder joint was associated with clinical features. As the severity of synovitis was found to be related to clinical features regardless of the size of the rupture, treatment of synovitis should be considered in patients with RCT.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.McElvany MD, McGoldrick E, Gee AO, Neradilek MB, Matsen FA., 3rd Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43(2):491–500. doi: 10.1177/0363546514529644. [DOI] [PubMed] [Google Scholar]
- 2.Kim CW, Kim JH, Kim DG. The factors affecting pain pattern after arthroscopic rotator cuff repair. Clin Orthop Surg. 2014;6(4):392–400. doi: 10.4055/cios.2014.6.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrams GD, Luria A, Carr RA, Rhodes C, Robinson WH, Sokolove J. Association of synovial inflammation and inflammatory mediators with glenohumeral rotator cuff pathology. J Shoulder Elbow Surg. 2016;25(6):989–997. doi: 10.1016/j.jse.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Gotoh M, Hamada K, Yamakawa H, et al. Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J Orthop Res. 2002;20(6):1365–1371. doi: 10.1016/S0736-0266(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 5.Jo CH, Shin JS, Kim JE, Oh S. Macroscopic and microscopic assessments of the glenohumeral and subacromial synovitis in rotator cuff disease. BMC Musculoskelet Disord. 2015;16:272. doi: 10.1186/s12891-015-0740-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamai K, Akutsu M, Yano Y. Primary frozen shoulder: brief review of pathology and imaging abnormalities. J Orthop Sci. 2014;19(1):1–5. doi: 10.1007/s00776-013-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh CH, Oh JH, Kim SH, Cho JH, Yoon JP, Kim JY. Effectiveness of subacromial anti-adhesive agent injection after arthroscopic rotator cuff repair: prospective randomized comparison study. Clin Orthop Surg. 2011;3(1):55–61. doi: 10.4055/cios.2011.3.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YS, Lee HJ, Park I, Im JH, Park KS, Lee SB. Are delayed operations effective for patients with rotator cuff tears and concomitant stiffness? An analysis of immediate versus delayed surgery on outcomes. Arthroscopy. 2015;31(2):197–204. doi: 10.1016/j.arthro.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.af Klint E, Catrina AI, Matt P, et al. Evaluation of arthroscopy and macroscopic scoring. Arthritis Res Ther. 2009;11(3):R81. doi: 10.1186/ar2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flack VF, Afifi AA, Lachenbruch PA, Schouten HJ. Sample size determinations for the two rater kappa statistic. Psychometrika. 1988;53(3):321–325. [Google Scholar]
- 11.Lee DH, Hong JY, Lee MY, Kwack KS, Yoon SH. Relation between subacromial bursitis on ultrasonography and efficacy of subacromial corticosteroid injection in rotator cuff disease: a prospective comparison study. Arch Phys Med Rehabil. 2017;98(5):881–887. doi: 10.1016/j.apmr.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Khatri C, Ahmed I, Parsons H, et al. The natural history of full-thickness rotator cuff tears in randomized controlled trials: a systematic review and meta-analysis. Am J Sports Med. 2019;47(7):1734–1743. doi: 10.1177/0363546518780694. [DOI] [PubMed] [Google Scholar]
- 13.Kanbe K, Chiba J, Inoue Y, Taguchi M, Iwamatsu A. Analysis of clinical factors related to the efficacy of shoulder arthroscopic synovectomy plus capsular release in patients with rheumatoid arthritis. Eur J Orthop Surg Traumatol. 2015;25(3):451–455. doi: 10.1007/s00590-014-1570-5. [DOI] [PubMed] [Google Scholar]
- 14.Aydin N, Aslan L, Lehtinen J, Hamuryudan V. Current surgical treatment options of rheumatoid shoulder. Curr Rheumatol Rev. 2018;14(3):200–212. doi: 10.2174/1573397112666161201161256. [DOI] [PubMed] [Google Scholar]