Abstract
Background
Osteoarthritis (OA) and osteoporosis (OP) are the 2 most common bone disorders associated with aging. We can simply assume that older patients have a higher incidence of OA and OP with more severity. Although several papers have conducted studies on the relationship between OA and OP, none of them has demonstrated a conclusive link. In this study, we used radiological knee OA and bone mineral density (BMD; T-score of the total hip and lumbar spine) to analyze the incidence of OA and OP in a large population. We aimed to determine the relationship between OA and OP and investigate the associated risk factors
Methods
This cross-sectional study used data extracted from the 2010–2012 Korea National Health and Nutrition Examination Survey. We evaluated a total of 4,250 participants aged ≥ 50 years who underwent knee radiography and dual-energy X-ray absorptiometry and their laboratory results. The relationship between radiological knee OA and BMD was assessed. The generalized linear model was used to evaluate the relationship between BMD and Kellgren-Lawrence (KL) grade.
Results
The higher KL grade was associated with older age, higher body mass index (BMI), female sex, and lower hemoglobin level (p < 0.001). No significant association was found between OA and the following variables: white blood cell, platelet, total cholesterol, vitamin D, alkaline phosphatase, parathyroid hormone, hypertension, diabetes, asthma, dyslipidemia, smoking status, alcohol consumption, and regular exercise (p > 0.05). After adjusting for confounding factors (age, BMI, diabetes, hypertension, smoking, and alcohol consumption), the average T-scores of total hip and lumbar spine were the highest in the mild OA group with KL grade 2 (−0.22 ± 1.08 and −0.89 ± 1.46, respectively, p < 0.001). The average T-scores of the total hip and lumbar spine significantly decreased as OA progressed from moderate (KL grade 3; −0.49 ± 1.05 and −1.33 ± 1.38, respectively, p < 0.001) to severe (KL grade 4; −0.73 ± 1.13 and −1.74 ± 1.75, respectively, p < 0.001). T-scores of the moderate-to-severe OA group were significantly lower than those of the non-OA group (KL grades 0 and 1, p < 0.001).
Conclusions
Compared with the non-OA group, BMD (T-scores of the total hip and lumbar spine) was higher in the mild OA group and lower in the moderate-to-severe OA group.
Keywords: Osteoarthritis, Osteoporosis, Korea National Health and Nutrition Examination Survey, Kellgren-Lawrence grade
Osteoarthritis (OA) and osteoporosis (OP) are the 2 most common disorders of the bone related to aging. There are several papers on the relationship between OA and OP, none of which have demonstrated a conclusive link between them. It is considered in general that as the age increases, the severity of OA increases and the level of bone mineral density (BMD) decreases. In some studies, the association of OP with Kellgren-Lawrence (KL) grade 0–3 was found, but not with KL grade 4. In another study, a clear association could not be determined because of the small sample size.
According to research by Geusens and van den Bergh,1) the classical phenotype of an OP patient is an older woman with a low body mass index (BMI) and low BMD, and a typical obese OA patient has a normal or high BMD. In particular, there are many cross-sectional and longitudinal studies, which suggest that the risk of OP based on the assessment of BMD is low in OA patients.2,3) A study of 482 women reported that the KL grade 2 or higher group showed higher BMD (z-score) than the OA-free group.4)
However, Dequeker et al.5) supported the classical interpretation that OA and OP are negatively correlated, which has been challenged by recent findings on the multiple convergent relations between OA and OP. Some of the risk factors for OA and OP are divergent, while others are convergent (such as age, sex, genetics, and inflammation).6) A recent review has noted that OA is a heterogeneous disease in terms of stage (early vs. late), and the relationship with BMD also varies with stage.1) There is a report that BMD may decrease in the OA-affected joint.7) Several experimental studies found the role of subchondral bone in OA and OP. Microstructure impairment in the subchondral bone, which is caused by OP, may aggravate OA of the knee in rabbit models.8,9) Some studies focused on the bone turnover of both diseases and showed that the high rate of bone turnover in OP was related to the progression of OA. A prospective study of 450 patients prior to knee arthroplasty reported that BMD was lower with severe knee OA and biomarkers for bone turnover were higher with worse OA grading.10)
As such, OA and OP seem to have many connections with each other, but their relationship has not yet been clearly elucidated. We hypothesized that the degree of relevance between the severity of OP and OA grade would be different due to the heterogenicity of OA and OP. Also, we assumed that it would be possible to infer the bone quality of the knee from hip and spine BMD, which are the current standard BMD measurement sites, because the hip and spine also get stimulated by weight-bearing loading and the subchondral bone of the knee is expected to be affected similarly.
The purpose of this study was to improve the reliability of previous studies that support the relationship between OA and OP by utilizing a large size sample of 4,250 subjects aged ≥ 50 years who underwent BMD testing and knee X-ray. We investigated the relationship between OA and OP, which used to be considered vague, by studying various parameters of the large size data. Based on the results, it would be possible to infer the risk for OA or OP in advance from a patient suffering from either OA or OP and provide appropriate clinical treatment for prevention. Depending on the grade of the disease, more specific patient education and treatment can be helpful.
METHODS
This study was approved by the Korea Centers for Disease Control and Prevention (ethics approval code: IRB 2010-02CON-21-C and 2011-02CON-06-C). All participants provided written informed consent for participation in the survey and for the use of their data for research purposes.
Participants and Study Design
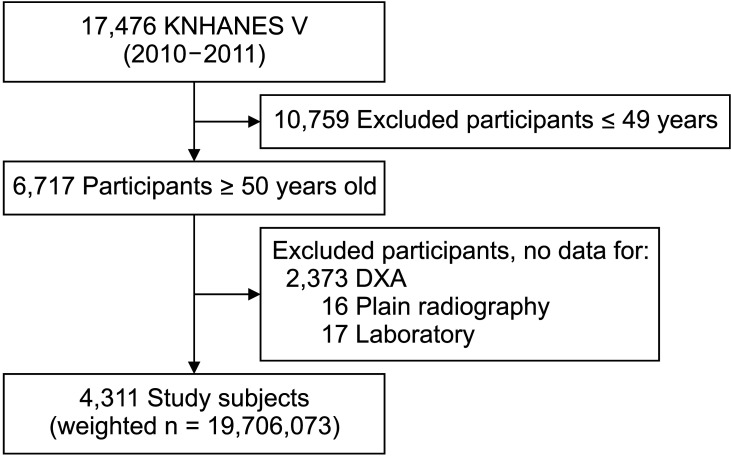
The Korea National Health and Nutrition Examination Survey (KNHANES) is a nationwide cross-sectional health survey, which has been conducted periodically by the Korea Centers for Disease Control and Prevention since 1998. The survey uses a stratified, multistage, clustered probability sampling method to derive a representative Korean population. Regarding the KNHANES V, 8,959 participants from 3,840 households in 2010 (V-1) and 8,517 participants from 3,840 households in 2011 (V-2) responded to the questionnaire-based survey and were categorized by region, sex, age, and average household size. As a cross-sectional study, survey subjects were different each year. Of 8,281 participants who completed a dual-energy X-ray absorptiometry (DXA) and radiographic OA assessment with the KL grading system, 4,311 participants aged ≥ 50 years were included (Fig. 1). The weighted number of participants was 19,706,073.
Fig. 1. Flowchart of study population. KNHANES: Korea National Health and Nutrition Examination Survey, DXA: dual X-ray absorptiometry.
Demographic and Anthropometric Characteristics
All participants completed a 4-part questionnaire administered by trained government research teams. A standardized interview and established questionnaire were used to collect information including basic demographics, personal and family medical history, and lifestyle (e.g., hypertension, diabetes, asthma, dyslipidemia, smoking status, alcohol consumption, and regular exercise). Participants were categorized into groups based on the frequency of alcohol consumption over the past year (never, mild-to-moderate, and heavy [> 30 g/day]), cigarette smoking (current smoker), and moderate physical activity (regular exerciser: for at least 30 minutes a session at least 5 times a week). Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as weight (kg)/height (m2). In addition, laboratory results including complete blood count (hemoglobin [Hb] , white blood cell [WBC], and platelet [PLT]) and total cholesterol were also analyzed (Table 1). The subjects were stratified into 5 groups by the KL grade. Groups were compared in terms of categorical variables using the chi-square test and in terms of continuous variables using the analysis of variance. In statistical analysis, the multivariate analysis was considered more effective since it involves more than 1 variable at a time and controls confounding effects or interaction, whereas the univariate analysis involves measurement of 1 variable at a time. Hence, we used the results obtained from multivariate analysis when both univariate and multivariate analysis results were available, and the univariate analysis results were used when multivariate analysis results were not obtained (Table 1).
Table 1. General Characteristics of Participants According to the Kellgren-Lawrence Grade.
| Kellgren-Lawrence grade | 0 (n = 1,531) | 1 (n = 1,054) | 2 (n = 594) | 3 (n = 732) | 4 (n = 401) | Total (n = 4,311) | p-value | |
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||||
| Weighted no, of participants | 7,600,956 | 4,788,482 | 2,407,090 | 3,237,667 | 1,671,878 | 19,706,073 | ||
| Age (yr) | 59.5 ± 7.8 | 64.4 ± 8.8 | 66.5 ± 8.9 | 68.4 ± 8.7 | 71.7 ± 8.1 | 64.1 ± 9.5 | < 0.001 | < 0.001 |
| Male sex (%) | 51.6 | 57.7 | 53.6 | 25.1 | 16.7 | 46.0 | < 0.001 | < 0.001 |
| Body mass index (kg/m2) | 23.4 ± 2.9 | 23.8 ± 3.1 | 24.3 ± 2.9 | 24.8 ± 3.3 | 25.2 ± 3.9 | 24.0 ± 3.2 | < 0.001 | 0.006 |
| Serum | ||||||||
| Hemoglobin (g/dL) | 14.0 ± 1.5 | 14.1 ± 1.4 | 14.1 ± 1.4 | 13.6 ± 1.3 | 13.2 ± 1.4 | 13.9 ± 1.4 | < 0.001 | 0.001 |
| White blood cell (103/mm3) | 6.06 ± 1.72 | 6.16 ± 1.74 | 6.18 ± 1.81 | 6.15 ± 1.66 | 6.22 ± 1.78 | 6.13 ± 1.73 | 0.424 | - |
| Platelet (103/mm3) | 248.2 ± 58.7 | 247.2 ± 59.6 | 243.5 ± 61.8 | 258.6 ± 61.6 | 263.1 ± 65.4 | 250.3 ± 60.7 | < 0.001 | 0.418 |
| Total cholesterol (mg/dL) | 195.9 ± 36.7 | 191.4 ± 35.9 | 194.5 ± 39.8 | 195.4 ± 36.3 | 199.4 ± 41.9 | 194.8 ± 37.4 | 0.005 | 0.431 |
| Vitamin D (ng/mL) | 18.8 ± 6.4 | 19.1 ± 6.7 | 19.6 ± 6.7 | 19.3 ± 7.2 | 18.4 ± 7.3 | 18.7 ± 7.0 | 0.094 | - |
| Alkaline phosphatase (U/L) | 243 ± 74 | 249 ± 77 | 249 ± 72 | 256 ± 75 | 263 ± 81 | 249 ± 75 | 0.100 | - |
| Parathyroid hormone (pg/mL) | 67.4 ± 6.4 | 67.9 ± 26.6 | 67.7 ± 27.7 | 68.5 ± 26.2 | 76.0 ± 36.0 | 68.3 ± 30.1 | 0.032 | 0.312 |
| Alcohol consumption (%) | < 0.001 | 0.234 | ||||||
| None | 31.4 | 33.4 | 37.4 | 49.0 | 59.8 | 37.9 | ||
| Mild to moderate | 62.0 | 58.8 | 56.7 | 47.3 | 38.0 | 56.1 | ||
| Heavy (daily) | 6.6 | 7.8 | 5.9 | 3.7 | 2.3 | 6.0 | ||
| Current smoker (%) | 24.2 | 22.8 | 17.9 | 10.2 | 7.9 | 19.4 | < 0.001 | 0.477 |
| Regular exercise (%) | 90.7 | 89.1 | 88.7 | 87.9 | 87.2 | 89.3 | 0.420 | - |
| Hypertension (%) | 31.6 | 35.5 | 41.1 | 46.7 | 66.6 | 39.1 | < 0.001 | 0.578 |
| Asthma (%) | 4.8 | 5.7 | 8.9 | 8.6 | 8.7 | 9.4 | 0.075 | - |
| Dyslipidemia (%) | 20.3 | 18.9 | 20.8 | 24.6 | 26.8 | 21.3 | 0.006 | 0.685 |
| Diabetes (%) | 12.3 | 14.1 | 13.8 | 15.7 | 21.6 | 14.3 | 0.003 | 0.209 |
| T-score | ||||||||
| Total femur | –0.46 ± 0.98 | –0.34 ± 1.04 | –0.22 ± 1.08 | –0.49 ± 1.05 | –0.73 ± 1.13 | –0.51 ± 1.05 | < 0.001 | < 0.001 |
| Lumbar spine | –1.22 ± 1.26 | –1.08 ± 1.43 | –0.89 ± 1.46 | –1.33 ± 1.38 | –1.74 ± 1.75 | –1.35 ± 1.38 | < 0.001 | < 0.001 |
Values are presented as mean ± standard deviation.
Assessment and Definition of Radiographic Knee OA
Plain radiographs of bilateral knees, including weight-bearing anteroposterior and lateral views (30° flexion), were taken using an SD 3000 Synchro Stand (Accele Ray; Shinyoung Co., Seoul, Korea). Two independent radiologists (SWL and YMJ) graded radiographic changes concerning OA using the KL grading system. The definition of KL grading system is as follows: grade 0 (none), definite absence of X-ray changes in OA; grade 1 (doubtful), doubtful joint space narrowing and possible osteophytic lipping; grade 2 (minimal), definite osteophytes and possible joint space narrowing; grade 3 (moderate), moderate multiple osteophytes, definite narrowing of joint space, some sclerosis, and possible deformity of bone ends; and grade 4 (severe), large osteophytes, marked narrowing of joint space, severe sclerosis, and definite deformity of bone ends.
Concordant grades of both radiologists were accepted. The higher grade was accepted when there was a difference of 1 grade between the radiologists. If the discrepancy was greater than 1 grade, a third radiologist (JYJ) was consulted. Interrater agreement within 1 grade between radiologists was 92.8%. Radiographic knee OA was defined as a KL grade ≥ 2 in 1 knee.
BMD Measurement
Areal BMD and body composition were measured using DXA (Discovery QDR 4500W; Hologic Inc., Belford, MA, USA). BMD was defined as weight per area of bone (g/cm2). T-score was calculated using the reference for Asians. Osteopenia and OP were defined as a T-score of less than −1.0 and −2.5, respectively.
Statistical Analysis
All statistical analyses were conducted with PASW statistics ver. 18 (SPSS Inc., Chicago, IL, USA), and two-sided p-value < 0.05 was considered statistically significant. Survey sample weights were applied to reflex representative estimates of the noninstitutionalized Korean population. Sample weights were calculated by considering the sampling rate, response rate, location, age, and sex proportion of the reference population. The number of weighted participants was 17,138,845.
RESULTS
General Characteristics of the Subjects
The mean age of the participants was 63.9 ± 9.3 years. The mean BMI was 24.0 ± 3.2 kg/m2. The mean serum vitamin D level was 18.7 ± 7.0 ng/mL. The prevalence of osteopenia and OP was 47.9% and 22.0%, respectively. The mean ages of participants in the normal, osteopenia, and OP groups were 58.2 ± 7.3, 64.4 ± 8.8, and 66.5 ± 8.9 years, respectively. The prevalence of radiographic knee OA was 40.1%. Concomitant knee OA was present in 31.7% of OP patients. The mean age of the participants with concomitant OA and OP was significantly higher than that of the participants with OA or OP only (72.7 ± 7.3, 67.2 ± 9.8, and 67.9 ± 10.2 years, respectively, p < 0.001).
Clinical Characteristics of the Subjects According to KL Grade
The study participants were grouped according to KL grade. Comparisons of baseline characteristics are shown in Table 1. As mentioned in the Method, we used the results obtained from multivariate analysis when both univariate and multivariate analysis results were available, and the univariate analysis results were used when multivariate analysis results were not obtained.
Higher KL grade was associated with older age, higher BMI, female sex, and lower Hb level (p < 0.001). There were no significant differences in WBC, PLT, total cholesterol, hypertension, diabetes, asthma, and dyslipidemia incidence. Serological markers associated with bone turnover (25-OH vitamin D, alkaline phosphatase [ALP], and parathyroid hormone [PTH]) showed no tendency of linear increment. Also, social behaviors (alcohol consumption, smoking, and regular exercise) had no relevance with KL grade (p > 0.05).
BMD in Relation to the Progression of Radiological Knee OA
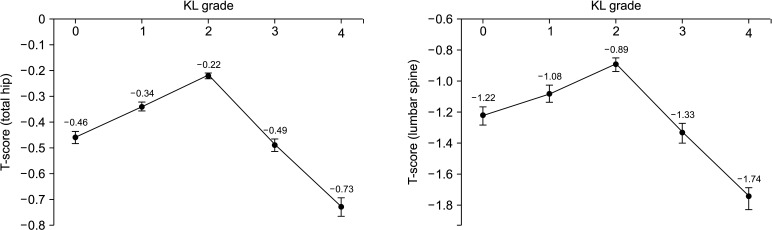
T-scores of total hip and lumbar spine in the mild OA group (KL grade 2) were the highest. Moderate and severe OA groups (KL grades 3 and 4) showed lower T-scores than those of the non-OA group. T- scores of total hip and lumbar spine increased linearly from KL grade 0 to KL grade 2 and rapidly decreased in KL grades 3 and 4 (Fig. 2). It indicates that there was significant difference between T-scores of the normal group (KL grade 0) and mild OA group (KL grades 1 and 2) (p < 0.001) and T-scores significantly decreased in the moderate and severe OA groups (KL grades 3 and 4) (p < 0.001).
Fig. 2. Relationship between bone mineral density (T-scores of hip and lumbar spine) and the Kellgren-Lawrence grade (KL grade). T-scores of mild OA group (KL grade 2) were the highest in all age groups. T-scores of the moderate and severe OA groups (KL grades 3 and 4) were lower than those of the non-OA group. T-scores were adjusted by age, sex, body mass index, alcohol consumption, smoking, exercise, diabetes, dyslipidemia, and hypertension. The p-values for the relationship between KL grade and total hip and lumbar T-scores were < 0.001. Whiskers indicate standard deviation.
DISCUSSION
T-scores of total hip and lumbar spine in the mild OA group (KL grade 2) were the highest, while moderate and severe OA groups (KL grades 3 and 4) showed lower T-scores than those of the non-OA group. Although the results of the present study seem to be in conflict with those of previous studies, similar findings have also been observed in previous population-based studies. In the Rotterdam study, as in our study, BMD was higher in KL grade 2 and lower in KL grade 3 than in KL grade 0 in women.11) The study documented that higher BMD levels were observed with increasing KL scores, except for the highest knee KL score category in women. They inferred that the systemic factor may have influenced the unexpected correlation; however, there was no further study. In the Framingham study using 473 elderly women, BMD was higher in participants with KL grades 1 and 2 than in those without OA; however, it was lower in KL grades 3 and 4.2) The researchers speculated that the unanticipated correlation was caused by the small number of participants in KL grades 3 and 4. In a multicenter OA study, in knees without OA, higher BMDs were associated with increases in the grade of joint space narrowing; on the other hand, in knees with existing OA, progression of OA was not significantly related to BMD.12) The present study analyzed a relatively large number of KL grade 3 or 4 participants (n = 1,133) and showed that the relationship between OA and OP could be reversed according to the severity of OA. It is noteworthy to focus on the shared mechanism of OA and OP in order to identify the cause of BMD decrease in the severe knee OA group. Although OA has been recognized as a disease of the cartilage, the roles of alteration of subchondral bone and low grade inflammation in OA have been recently elucidated.13,14)
OA and OP seem to have many connections, but their relationship has not been clearly elucidated. We assumed that the extent of relationship between the severity of OP and the grade of OA would be different due to the heterogenicity of OA and OP. As the hip and spine are affected by weight-bearing loading as the subchondral bone of the knee is, we also assumed that BMD of the knee could be inferred from the values of hip and spine, which are the standard BMD measurement sites.
The linear relationship between OA and Hb appeared to be affected by age, and the linear relationship between OA and BMI appeared to be due to the increasing knee joint loading with weight increase. We assumed in the beginning, based on the results of several previous studies, that the factors such as vitamin D, ALP, and PTH would have certain association with the KL grade due to the turnover of the subchondral bone, but it turned out otherwise. This may be because these factors are not only specific to bone turnover but also greatly influenced by other endocrine diseases, which were not controlled in this study. Although it was not at a statistically significant level (p = 0.094), vitamin D showed an increasing trend in mild OA (KL grades 1 and 2) and a decreasing trend in moderate to severe OA (KL grades 3 and 4). This implies that vitamin D can be considered as a factor, which is more specific to bone turnover compared to other factors. Other factors such as lab results (WBC, PLT, and total cholesterol), social behavior (alcohol consumption, smoking, and exercise), and underlying diseases (hypertension, asthma, dyslipidemia, and diabetes) did not show any association. Thus, they were not considered as significant risk factors for OA and OP.
The results of this paper are of clinical significance. First, an increase in T-score was often seen in mild stage OA patients, which is likely to leave room for misinterpretation of bone quality. In treating mild OA patients, it is not appropriate to interpret a high T-score as an indication of good bone quality. It is more appropriate to consider that the T-score was temporarily elevated as a result of high turnover of the subchondral bone in OA. If T-score decreases significantly as moderate to severe OA progresses, it is necessary to educate patients that the likelihood of OP exacerbation is higher. This study also showed the need to prevent and treat OP more actively in the early stage OA patients. Second, in the case where total knee arthroplasty (TKA) is necessary, we expect our study findings could be used as evidence supporting provision of more aggressive treatment for OP before and after the surgery to enhance the success rate of TKA. In general practice sites, there is a strong tendency to view and treat each disease separately, rather than to consider OA and OP together in connection. However, as Geusens and van den Bergh1) have shown in their study, the 2 diseases share the pathophysiology by complex mechanism. And based on the relationship between OA and OP shown in this study, it is thought to be beneficial to prevent implant failure by preemptive OP management before and after TKA. Third, BMD assessed using DXA, the internationally standardized measuring instrument, did not accurately show bone quality in mild OA patients. In mild OA, T-score may increase as a result of sclerosis. However, in fact, it is often found that the inside of the bone is quite empty in patients with high T-scores, which implies that DXA does not accurately represent the bone quality. Our results showed that it is necessary to obtain a more accurate measurement of bone quality, and further research in this field is also needed.
This study has several limitations. First, the present study did not determine the cause-effect relationship between OA and OP due to the cross-sectional nature of the database. A prospective, longitudinal study is needed to validate the results. Second, the authors could not assess the personal medication information, which can be associated with OA, OP, and other comorbidities. Lack of information of potential underlying medical conditions might have influenced the results. Third, the risk of recall bias, resulting from the self-reported comorbidities, should be considered. Fourth, because our study used only radiological OA grades, we could not predict the same results in symptomatic OA with knee pain.
In conclusion, the complexity of the relationship between OA and OP was revealed in this study. BMD was the highest when the radiological knee OA was mild, and it was significantly lower in the moderate and severe OA than in the non-OA group.
ACKNOWLEDGEMENTS
We appreciate contribution of Sheen-Woo Lee, Yu Mi Jeong, and Ji Young Jeon (Department of Radiology, Gil Medical Center, Gachon University College of Medicine) for this study.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Geusens PP, van den Bergh JP. Osteoporosis and osteoarthritis: shared mechanisms and epidemiology. Curr Opin Rheumatol. 2016;28(2):97–103. doi: 10.1097/BOR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27(4):1032–1037. [PubMed] [Google Scholar]
- 3.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46(1):92–99. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Sowers M, Lachance L, Jamadar D, et al. The associations of bone mineral density and bone turnover markers with osteoarthritis of the hand and knee in pre- and perimenopausal women. Arthritis Rheum. 1999;42(3):483–489. doi: 10.1002/1529-0131(199904)42:3<483::AID-ANR13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 5.Dequeker J, Aerssens J, Luyten FP. Osteoarthritis and osteoporosis: clinical and research evidence of inverse relationship. Aging Clin Exp Res. 2003;15(5):426–439. doi: 10.1007/BF03327364. [DOI] [PubMed] [Google Scholar]
- 6.Bultink IE, Lems WF. Osteoarthritis and osteoporosis: what is the overlap? Curr Rheumatol Rep. 2013;15(5):328. doi: 10.1007/s11926-013-0328-0. [DOI] [PubMed] [Google Scholar]
- 7.Im GI, Kim MK. The relationship between osteoarthritis and osteoporosis. J Bone Miner Metab. 2014;32(2):101–109. doi: 10.1007/s00774-013-0531-0. [DOI] [PubMed] [Google Scholar]
- 8.Bellido M, Lugo L, Roman-Blas JA, et al. Subchondral bone microstructural damage by increased remodelling aggravates experimental osteoarthritis preceded by osteoporosis. Arthritis Res Ther. 2010;12(4):R152. doi: 10.1186/ar3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Chen S, Chen W, et al. Ultrastructural change of the subchondral bone increases the severity of cartilage damage in osteoporotic osteoarthritis of the knee in rabbits. Pathol Res Pract. 2018;214(1):38–43. doi: 10.1016/j.prp.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Linde KN, Puhakka KB, Langdahl BL, et al. Bone mineral density is lower in patients with severe knee osteoarthritis and attrition. Calcif Tissue Int. 2017;101(6):593–601. doi: 10.1007/s00223-017-0315-y. [DOI] [PubMed] [Google Scholar]
- 11.Burger H, van Daele PL, Odding E, et al. Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age: the Rotterdam Study. Arthritis Rheum. 1996;39(1):81–86. doi: 10.1002/art.1780390111. [DOI] [PubMed] [Google Scholar]
- 12.Nevitt MC, Zhang Y, Javaid MK, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis. 2010;69(1):163–168. doi: 10.1136/ard.2008.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA. Age-related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant. 2017;26(9):1520–1529. doi: 10.1177/0963689717721201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]