Abstract
This cohort study analyzed inpatient data from multiple US hospitals to characterize patients with and without coronavirus disease (COVID-19) who had diabetic ketoacidosis.
Introduction
Diabetic ketoacidosis (DKA) is a life-threatening, acute complication of diabetes. Despite an increase in DKA hospitalization rates, the age-adjusted DKA in-hospital case-fatality rate has declined over time.1 However, with the advent of coronavirus disease 2019 (COVID-19), a suspected increase in the frequency and severity of DKA has been hypothesized because of the potential diabetogenic effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 To further characterize patients with DKA with and without COVID-19, we analyzed individual-level inpatient data from multiple US hospitals.
Methods
In this cohort study, we extracted individual-level, deidentified data from the Glytec national database (Glytec) to examine severity markers of DKA, insulin requirements, complications, and in-hospital case fatality in patients with and without COVID-19. The study was approved by the Emory University institutional review board. The requirement for informed consent was waived because of the use of deidentified data. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The data reflected a cohort from 175 hospitals located within 17 different states in the United States. Data were collected from February 1 to September 15, 2020, and analysis was performed between October 1, 2020, and January 14, 2021. All patients were treated with the same computerized continuous insulin infusion (CII) algorithm. We included patients with biochemically confirmed DKA (bicarbonate on admission <18 mEq/L [to convert to millimoles per liter, multiply by 1.0], blood glucose >250 mg/dL [to convert to millimoles per liter, multiply by 0.0555], and anion gap >12 mEq/L [to convert to millimoles per liter, multiply by 1.0]). Patients who received CII treatment for less than 4 hours were excluded. We present summarized data stratified by age and COVID-19 diagnosis. Statistical significance for continuous variables was found via t test, analysis of variance, or Kruskal-Wallis test, and statistical significance for categorical variables was found using Fisher exact test or χ2 test. Statistical tests were considered significant with a 2-sided P < .05. Statistical analysis was performed using QI Macros version 2019.10 (KnowWare) and GraphPad version 9.0.0 (Prism).
Results
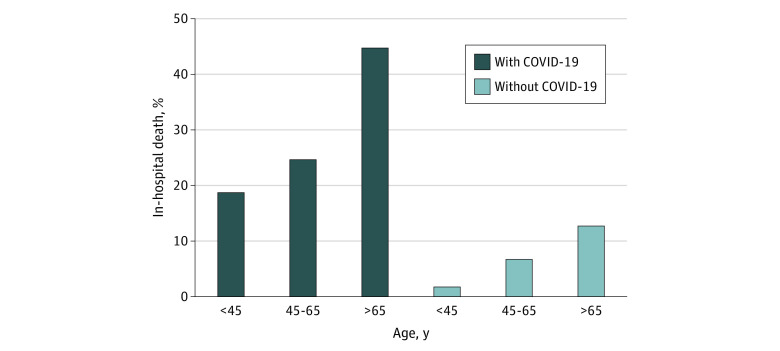
A total of 5029 patients (mean [SD] age, 47 [17.8] years; 2673 [53%] men) with DKA who were admitted between February 1 and September 15, 2020, and treated with the CII protocol were included in this study. Of these patients, 210 (4%) were positive for COVID-19 and 4819 (96%) did not have COVID-19. Compared with patients without COVID-19, those with COVID-19 were older (mean [SD] age, 47 [18] years vs 56 [17] years; P < .001) and had a higher body mass index (calculated as weight in kilograms divided by height in meters squared; mean [SD] body mass index, 28 [8] vs 31 [9]; P < .001) (Table). Among patients with COVID-19, older patients (>65 years) were more likely to have cardiovascular disease and diabetes complications than younger patients (<45 years) (cardiovascular disease: 16 [22%] vs 1 [2%]; P < .001; diabetes complications: 10 [14%] vs 1 [2%]; P = .02). Metabolic parameters (glucose, hemoglobin A1c, potassium, sodium, bicarbonate, and anion gap) were similar on admission for patients with and without COVID-19. Of patients without COVID-19, 262 of 4819 (5%) died in the hospital compared with 64 of 210 (30%) among those with COVID-19. Acute kidney injury occurred more frequently in the patients with COVID-19 (64 of 210 [30%]) vs patients without COVID-19 (498 of 4819 [10%]). Mortality increased with age among patients with and without COVID-19. The overall inpatient mortality was 45% (33 of 73 patients) for patients older than 65 years with COVID-19 and 13% (110 of 860 patients) for those without COVID-19. Increased mortality with COVID-19 was also observed in patients younger than 45 years: 19% (10 of 54 patients) with COVID-19 compared with 2% (41 of 2290 patients) in the non–COVID-19 group (Figure).
Table. Diabetic Ketoacidosis Metabolic Parameters on Admission, Insulin Requirements, and Complications Among Patients With and Without Coronavirus Disease 2019 (COVID-19) Across 175 US Hospitals.
| Characteristic | Full sample | Stratified by age | |||||||||
| Patients with COVID-19, mean (SD) (n = 210) | Patients without COVID-19, mean (SD) (n = 4819) | P value | Patients with COVID-19, mean (SD) | P value | Patients without COVID-19, mean (SD) | P value | |||||
| <45 y (n = 54) | 45-65 y (n = 83) | >65 y (n = 73) | <45 y (n = 2290) | 45-65 y (n = 1669) | >65 y (n = 860) | ||||||
| Men, No. (%) | 120 (57) | 2553 (53) | .32 | 31 (57) | 52 (63) | 37 (51) | .28 | 1208 (53) | 919 (55) | 426 (50) | <.001 |
| Age, y | 56 (17) | 47 (18) | <.001 | 33 (7) | 55 (5) | 74 (6) | <.001 | 31 (8) | 55 (6) | 73 (6.8) | <.001 |
| Cardiovascular disease, No. (%) | 26 (12) | 247 (6) | <.001 | 1 (2) | 9 (11) | 16 (22) | <.001 | 23 (1) | 126 (8) | 98 (1) | <.001 |
| Heart failure, No. (%) | 18 (9) | 122 (3) | <.001 | 1 (2) | 6 (7) | 11 (15) | .03 | 21 (1) | 62 (4) | 39 (5) | <.001 |
| Diabetes complications, No. (%)a | 23 (11) | 280 (7) | .004 | 1 (2) | 12 (14) | 10 (14) | .045 | 91 (4) | 136 (8) | 53 (6) | <.001 |
| BMI | 31 (9) | 28 (8) | <.001 | 32 (11) | 31 (7) | 30 (8.4) | .43 | 27 (9) | 27 (7) | 27 (8.3) | <.001 |
| Diabetes or HbA1c ≥6.5%, No. (%) | 191 (91) | 4486 (93) | .24 | 52 (96) | 74 (89) | 65 (89) | .28 | 2194 (96) | 1540 (92) | 752 (87) | <.001 |
| HbA1c, No. (%) | 11.3 (2.7) | 11.2 (2.8) | .59 | 12.5 (2.5) | 11.4 (2.9) | 10.5 (2.4) | <.001 | 11.6 (2.6) | 11.1 (2.8) | 10.2 (2.9) | <.001 |
| BG, mg/dL | 523 (228) | 588 (265) | <.001 | 536 (225) | 518 (231) | 521 (232) | .68 | 570 (257) | 603 (274) | 604 (266) | <.001 |
| Sodium, mEq/L | 133 (8) | 131 (7) | .003 | 132 (5.8) | 132 (7.3) | 136 (8.6) | <.001 | 131 (6) | 131 (7) | 133 (7.6) | <.001 |
| K, mEq/L | 4.7 (0.9) | 4.9 (1.0) | <.001 | 4.7 (1.0) | 4.7 (1.0) | 4.7 (0.7) | .30 | 4.9 (1.0) | 5.0 (1.1) | 5.1 (1.1) | <.001 |
| HCO3, mEq/L | 12.2 (4.5) | 11.1(4.5) | .04 | 10.5 (4.3) | 12.5 (5.0) | 13.0 (3.9) | .02 | 10.3 (4.6) | 10.6 (4.6) | 11.7 (4.4) | <.001 |
| Anion gap, mEq/L | 27 (8) | 27 (8) | .11 | 28 (9) | 26 (8) | 26 (7) | .28 | 28 (8) | 27 (8) | 26 (8) | <.001 |
| Osmolality >300 mOsm/kg, No. (%)b | 66 (31) | 1494 (31) | .88 | 15 (28) | 24 (29) | 27 (37) | .44 | 580 (25) | 533 (32) | 381 (44) | <.001 |
| Lactic acid, mg/dL | 3.8 (3.2) | 3.7 (3.2) | .69 | 3.2 (3.3) | 3.6 (3.3) | 4.3 (3.2) | .15 | 2.9 (2.4) | 3.8 (3.2) | 4.7 (3.9) | <.001 |
| Creatinine, mg/dL | 2.1 (2.1) | 1.8 (1.7) | .046 | 1.4 (1.2) | 2.2 (2.7) | 2.5 (1.9) | .02 | 1.5 (4.5) | 2.0 (1.8) | 2.2 (1.7) | <.001 |
| Acute kidney injury, No. (%) | 64 (30) | 498 (10) | <.001 | 7 (13) | 31 (37) | 26 (36) | .005 | 149 (7) | 220 (13) | 129 (15) | <.001 |
| LOS, median (MAD) | 8.3 (5.9) | 3.4 (1.5) | <.001 | 5.2 (2.2) | 11.9 (7.5) | 10.2 (6.8) | .003 | 2.9 (1.1) | 4.0 (1.9) | 5.1 (2.7) | <.001 |
| In-hospital death, No. (%) | 64 (30) | 262 (5) | <.001 | 10 (19) | 21 (25) | 33 (45) | .002 | 41 (2) | 111 (7) | 110 (13) | <.001 |
| DKA treatment | |||||||||||
| Insulin units/h, median (MAD) | 5.0 (3.3) | 3.6 (2.5) | <.001 | 5.9 (3.0) | 4.8 (3.0) | 4.5 (2.7) | <.001 | 3.4 (2.0) | 3.9 (2.1) | 3.5 (2.0) | <.001 |
| Length of treatment, h | 34 (20) | 23 (8) | <.001 | 30 (15) | 37 (21) | 37 (24) | .54 | 23 (9) | 24 (10) | 23 (10) | .02 |
| BG <10 minutes late, No. (%) | 7006 (65) | 110 024 (72) | <.001 | 1710 (69) | 3070 (66) | 2226 (62) | .99 | 51 218 (73) | 39 373 (72) | 19 228 (70) | <.001 |
| TTR of BG <250 mg/dL, h | 5.8 (2.9) | 4.4 (2.3) | <.001 | 4.5 (2.6) | 6.0 (3.3) | 6.6 (2.1) | <.001 | 3.8 (1.9) | 5.3 (2.9) | 5.2 (2.9) | <.001 |
| TTR of BG <180 mg/dL, h | 9.9 (3.6) | 7.1 (2.4) | <.001 | 9.6 (4.3) | 8.6 (3.9) | 9.9 (3.3) | .09 | 8.0 (3.3) | 8.9 (3.6) | 8.4 (3.3 | <.001 |
| TTR anion gap <12 mEq/L, h | 25 (17) | 15 (8) | <.001 | 19 (17) | 29 (16) | 81 (68) | <.001 | 15 (7) | 15 (8) | 17 (101) | <.001 |
| K <3.5 mEq/L during first 48 h, No. (%) | 104 (43) | 2880 (52) | .004 | 34 (33) | 45 (43) | 28 (27) | .02 | 1550 (52) | 945 (33) | 430 (15) | <.001 |
| BG <70 mg/dL during first 48 h, No. (%) | 21 (10) | 385 (8) | .30 | 2 (3.7) | 11 (13.3) | 8 (11) | .18 | 168 (7.3) | 143 (8.6) | 74 (8.6) | .28 |
Abbreviations: BG, blood glucose; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COVID-19, coronavirus disease 2019; DKA, diabetic ketoacidosis; HbA1c, hemoglobin A1c; HCO3, bicarbonate; K, potassium; LOS, length of stay; MAD, median absolute deviation; TTR, time to resolution.
SI conversion factors: To convert anion gap to millimoles per liter, multiply by 1.0; BG to millimoles per liter, multiply by 0.0555; HbA1c to proportion of total hemoglobin, multiply by 0.01; creatinine to micromoles per liter, multiply by 76.25; HCO3 to millimoles per liter, multiply by 1.0; K to millimoles per liter, multiply by 1.0; lactic acid to millimoles per liter, multiply by 0.111; osmolarity to millimoles per kilogram, multiply by 1.0; sodium to millimoles per liter, multiply by 1.0.
Diabetes nephropathy, neuropathy, or retinopathy.
Estimated effective osmolality equals 2(Na+) plus glucose, divided by 18.
Figure. Diabetic Ketoacidosis–Related Mortality Among Patients With and Without COVID-19 Across 175 US Hospitals.
Patients with COVID-19 had higher insulin requirements and a prolonged duration of CII with a longer time to resolution of DKA. Hypokalemia and hyperosmolality were common in both groups. The proportion of hypoglycemia was similar in both groups.
Discussion
This study consisted of a large cohort of patients with DKA during the COVID-19 pandemic, which provides perspective for the presentation, severity of disease, response to therapy, and outcomes among patients with and without COVID-19 treated with a single protocol. We found that patients with COVID-19 had a higher body mass index in all age strata, higher insulin requirements, prolonged time to resolution of DKA, and a much higher rate of mortality compared with patients without COVID-19.
As previously reported, among patients without COVID-19, most DKA cases (62% [113 709 of 184 55]) occurred among younger adults3; however, among patients with COVID-19, 74% (156 of 210) were older than 45 years. The expected DKA mortality in patients without COVID-19 with confirmed diagnosis is approximately 3% to 8%,4 with a 2020 small case series reporting a mortality rate as high as 50% in patients with COVID-19.5 Similarly, in this analysis, the mortality observed in patients with COVID-19 was high and increased across age strata, with older adults (>65 years) having a mortality of 45%.
This cohort has the advantage of including patients with biochemical confirmation of DKA managed with the same protocol across multiple US hospitals. However, a limitation in this data set is the lack of individual-level data on admission diagnosis, race, duration of diabetes, additional disease severity markers, or COVID-19–specific therapy, such as the use of corticosteroids.
Several transformations in diabetes care are occurring during the COVID-19 pandemic to reduce the number of patient interactions.6 However, it is not known whether fewer interactions may increase mortality by causing a delay in DKA resolution. The cause for the considerably higher mortality in the COVID-19–positive population is unknown. Contributing factors could include obesity and a more severe stress state (as suggested by higher insulin requirements). These findings are worrisome and warrant further investigation.
References
- 1.Benoit SR, Zhang Y, Geiss LS, Gregg EW, Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2018;67(12):362-365. doi: 10.15585/mmwr.mm6712a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383(8):789-790. doi: 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit SR, Hora I, Pasquel FJ, Gregg EW, Albright AL, Imperatore G. Trends in emergency department visits and inpatient admissions for hyperglycemic crises in adults with diabetes in the US, 2006-2015. Diabetes Care. 2020;43(5):1057-1064. doi: 10.2337/dc19-2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasquel FJ, Tsegka K, Wang H, et al. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: a retrospective, hospital-based cohort study. Diabetes Care. 2020;43(2):349-357. doi: 10.2337/dc19-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020;14(6):1563-1569. doi: 10.1016/j.dsx.2020.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 2020;dc202219. doi: 10.2337/dc20-2219 [DOI] [PMC free article] [PubMed] [Google Scholar]