Abstract
Aim:
Surgery with or without chemotherapy represent the only curative option for patients with colon cancer. However, some patients refuse treatment despite the recommendation. This study aims to identify the incidence, risk factors and impact on survival associated with refusal.
Methods:
A National Cancer Data Base (NCDB) analysis between 1998 and 2012 was performed. We identified 924,290 patients with potentially treatable colon cancer. Patients who underwent treatment were compared with patients that refused.
Results:
7152 patients refused surgery. On multivariable analysis, patients were more likely to refuse if they were older (OR = 1.14; 95% CI 1.14–1.15), female (OR = 1.20; 95% CI 1.12–1.28), African American (vs White, OR = 2.30; 95% CI 2.10–2.51) or on Medicaid (vs private, OR = 3.06; 95% CI 2.49–43.77). Overall survival was worse in patients that refused surgery [median survival 6.8 vs 24 months, Cox hazard ratio (HR) 3.41; 95%CI 3.12–3.60]. Furthermore, 11,334 patients with path. stage III disease refused adjuvant chemotherapy.
Conclusions:
Refusal of treatment affects survival and is independently associated with several variables (gender, race, insurance status), therefore raising the concern that socioeconomic factors may drive decisions.
Introduction
Over 1.8 million new colorectal cancer (CRC) cases and 881,000 deaths were estimated to occur worldwide in 2018, accounting for about 1 in 10 cancer cases and deaths.1 In the United States, colorectal cancer is the fourth most common cancer and the second leading cause of cancer-related deaths.1 The primary mode of curative treatment for patients with Stage I, II and III is surgical resection and chemotherapy has been shown to be most beneficial for Stage III disease.2 Most, but not all, patients diagnosed with colon cancer receive treatment. Surgery and chemotherapy are both associated with short and possibly long-term effects of patients’ quality of life and for some patients, despite given the clear benefits, some patients refuse treatment. Despite dramatic reductions in CRC mortality overall, striking disparities in outcomes based on age, race, insurance status and other socioeconomic variables remain.3-5 There is not enough evidence in the literature to suggest which patients are more likely to refuse treatment.
Respecting patients’ wishes is the highest priority; however, physicians may provide more substantial reasoning to convince patients towards undergoing the indicated curative treatment. Modifiable and possibly targetable socioeconomic and demographic variables that affect refusal of treatment, surgery or chemotherapy, in different cancers, including pancreatic, hepatocellular, breast, lung and colorectal has been studied in several retrospective studies.6-10 This has led to a broad number of quality improvement measures and initiatives to increase cancer care utilization and eventually long-term cancer-related outcomes. Data from the population of the United States with colon cancer is lacking in this regard, and the contribution of various socioeconomic and demographic variables to refusal of colon cancer-directed surgery has not been explored by previous studies.
The purpose of this study was to identify the time-related trends and risk factors, both modifiable or targetable, associated with refusal of colon cancer-directed treatment using a large national cancer database. Additionally, we investigated the impact of cancer-related treatment refusal on eventual survival.
Methods
Design and data sources
We conducted a retrospective cohort study using data from the National Cancer Database (NCDB). NCDB was established by the American College of Surgeons and Commission on Cancer in 1989 and includes data from all Commission on Cancer-accredited hospitals in the United States and Puerto Rico. It is estimated to include approximately 70% of new cancer diagnoses and is comprised of more than 30 million records from 1,500 hospitals. The database also includes census tract-level data from the US Census Bureau’s American Community Survey, which provides estimates of patient income, educational attainment, and urban/rural status.
Participants and variables
We included all patients aged 18 or older who were diagnosed with colon cancer with clinically Stage I to III disease (for the analysis regarding refusal of surgery) or pathological proven Stage III (for the analysis of refusal of chemotherapy after surgery) between 1998 and 2012. Demographic data including age, sex, race/ethnicity, and insurance type were collected at patient level, while proxy measures of socioeconomic status were derived from the 2012 American Community Survey for each patient’s home ZIP code. These included ZIP-code level measures of median household income and educational attainment measured as the proportion of patients in the ZIP code with less than a high school diploma. We used the year of diagnosis to assess temporal trends in refusal. Patient urban/rural location was determined at the ZIP code-level from the 2012 American Community Survey and travel distance was measured as the Haversine distance in miles between the center of the patient’s ZIP code and the address of the hospital where they underwent surgery.
Statistical analysis
To identify factors associated with refusal, the Wilcoxon rank-sum test and Chi square or Fisher’s exact test were used to compare baseline characteristics for each outcome of interest. We used univariable logistic regression to calculate unadjusted odds ratios and 95% confidence intervals, and included variables reaching significance level of P < 0.20 in a multivariable logistic regression model. We assessed collinearity using variance inflation factors and evaluated goodness of fit using the Hosmer-Lemeshow test. To identify factors associated with mortality, we looked at patients who received their treatments between 1998 and 2011, as mortality data are not available through the NCDB for patients enlisted during 2012. Overall survival rates were calculated as the time from date of diagnosis to death or last follow-up. Overall survival was estimated by the Kaplan–Meier method and compared using the log-rank test. Cox proportional hazards modeling was used to evaluate the impact of refusal of recommended surgery on survival while adjusting for potential confounders. All analyses were conducted using Stata 13 (StataCorp, College Station, TX).
Results
A total of 924,290 patients with diagnosed clinical stage I-III colon cancer between 1998 and 2012 were identified (Table 1). The median age of patients who underwent surgery was 71 years, 48.2% were male, 33% had private insurance, 85.1% were White and 25.1% were treated at an academic/research program. Of the patients with stage I-III colon cancer, 0.6% were not recommended surgery due to comorbidities, 0.1% died prior to planned surgery, whereas 7,152 (0.77%) patients refused the recommended surgical intervention. A total of 214,943 (24.5%) patients were above the age of 80 and 2.4% refused surgery vs. 0.29% of patients below the age cutoff. In the group >80 years vs < 80 years the racial breakdown for refusal was: (White 83% vs 67.8%, African American 13.3% vs 27.8, Asian/PI 1.8% vs 2.9%). The characteristics of the patients who received or refused surgery or chemotherapy are listed in Table 1. Of note, 814 patients (0.1%) died prior to planned surgery, for 5,612 (0.6%) surgery was not recommended due to patient risk factors. In addition, chemotherapy was not recommended in 11,213 (6.3%) patients because of contraindications due to patient risk factors. Risk factors can’t further be evaluated due to the nature of the NCDB database.
Table 1.
Overview of treatment delivery for patients with colon cancer.
| Frequency | Percent | |
|---|---|---|
| Surgery of the primary was performed | 870,880 | 94.2 |
| Surgery not performed because it was not part of the planned course of treatment | 28,202 | 3.1 |
| Surgery was not recommended/performed, contraindicated due to patient risk factors | 5,612 | 0.6 |
| Surgery not performed because the patient died prior to planned surgery | 814 | 0.1 |
| Surgery was recommended but was refused by the patient | 7,152 | 0.8 |
| Surgery was recommended by physician but not performed, no reason noted | 2,773 | 0.3 |
| Surgery was recommended, but unknown if performed/Unknown if surgery was recommended or performed | 8,857 | 0.9 |
| Total | 924,290 | |
| Chemotherapy administered | 46,116 | 35.0 |
| Single-agent chemotherapy | 81,548 | 45.4 |
| Multi-agent chemotherapy | 16,748 | 9.3 |
| Unknown agent | 46,116 | 35.0 |
| Chemotherapy not recommended/administered because it was contraindicated due to patient risk factors | 11,213 | 6.3 |
| Chemotherapy not administered, patient died prior to planned or recommended therapy | 1,809 | 1.0 |
| Chemotherapy not administered, was recommended by physician but not administered as part of the first course of therapy. No reason stated | 1,440 | 0.8 |
| Chemotherapy not administered, recommended, but patient reused | 11,334 | 6.3 |
| Chemotherapy recommended, unknown if administered | 6,419 | 3.6 |
| Unknown if recommended, or administered | 2,923 | 1.6 |
| Total | 179,550 |
Of the patients who underwent surgery, 179,550 (20.6%) had pathologic stage III disease. Within this group 90.3% received postoperative chemotherapy. However, a total of 11,334 (7.8%) of patients refused chemotherapy (Table 1).
Factors predicting refusal of surgery
Patients refusing surgery were more likely to be female and had a median age of 85 years, which was significantly higher than those patients who underwent surgery (median age 71 years). Overall, 6749 of 7152 (94.4%) patients above 65 years refused surgery. Charlson-Deyo score was reflective of more severe comorbidities in the group of patients that refused surgery. Patients who refused surgery were also more likely to be covered by Medicare and less likely by private insurance. Overall, patients refusing surgery also had less overall income and educational attainment. Patients who initially presented to community programs were also more likely to refuse surgery. On racial comparison, 1.3% of African American refused surgery vs. 0.75% in the White or Asian population. Patients who refused surgery also were more likely to refuse chemotherapy (Table 1). Logistic regression was used to identify factors predicting refusal of recommended surgery. On multivariate analysis, patients were significantly more likely to refuse if they were older, female, African American, on Medicare/Medicaid, had T2 or higher disease or had higher comorbidities. Annual income and level of education did not independently predict if patients refused surgery based on multivariable analysis (Table 2).
Table 2.
Characteristics of patients refusing surgery/chemotherapy.
| Characteristics | Surgery |
Refused Surgery |
P-value | Chemotherapy |
Refused Chemotherapy |
P-value |
|---|---|---|---|---|---|---|
| N = 870,880 | N = 7,152 | N = 144,412 | N = 11,334 | |||
| Age at Diagnosis, median (IQR) | 71 (60, 79) | 85 (79, 90) | <0.001 | 65 (55, 74) | 78 (70, 84) | |
| Sex, N(%) | <0.001 | <0.001 | ||||
| Male | 419726 (48.2) | 2602 (36.4) | 70939 (49.1%) | 4630 (40.9%) | ||
| Female | 451154 (51.8) | 4550 (63.6) | 73473 (50.9%) | 6704 (59.1%) | ||
| Race, N(%) | <0.001 | <0.001 | ||||
| White | 740884 (85.1) | 5682 (79.4) | 120572 (83.5%) | 9679 (85.4%) | ||
| African American | 97451 (11.2) | 1236 (17.3) | 17467 (12.1%) | 1244 (11.0%) | ||
| Asian/PI | 20197 (2.3) | 152 (2.1) | 4398 (3.0%) | 286 (2.5%) | ||
| Other/Unknown | 12348 (1.4) | 82 (1.1) | 1975 (1.4%) | 125 (1.1%) | ||
| Insurance Type, N(%) | <0.001 | <0.001 | ||||
| Private | 288693 (33.1) | 624 (8.7) | 61601 (42.7%) | 1866 (16.5%) | ||
| Medicaid | 26222 (3.0) | 195 (2.7) | 5818 (4.0%) | 368 (3.2%) | ||
| Medicare | 509884 (58.5) | 6033 (84.4) | 67872 (47.0%) | 8558 (75.5%) | ||
| Other/Unknown | 4783 (0.5) | 33 (0.5) | 944 (0.7%) | 43 (0.4%) | ||
| Not insured | 41298 (4.7) | 267 (3.7) | 8177 (5.7%) | 499 (4.4%) | ||
| Income ($USD), N(%) | <0.001 | <0.001 | ||||
| <$38,000 | 157208 (18.6) | 1537 (22.1) | 25823 (18.4%) | 2193 (19.9%) | ||
| $38,000-$47,999 | 203520 (24.0) | 1674 (24.1) | 34074 (24.2%) | 2818 (25.5%) | ||
| $48,000-$62,999 | 226073 (26.7) | 1702 (24.5) | 37584 (26.7%) | 3027 (27.4%) | ||
| >$63k | 260521 (30.7) | 2039 (29.3) | 43203 (30.7%) | 2996 (27.2%) | ||
| No HS Diploma, N(%) | <0.001 | <0.001 | ||||
| 21%+ | 142966 (16.9) | 1264 (18.2) | 24004 (17.1%) | 1820 (16.5%) | ||
| 13–20.9% | 223530 (26.4) | 1929 (27.7) | 37452 (26.6%) | 3024 (27.4%) | ||
| 7–12.9% | 282964 (33.4) | 2255 (32.4) | 46637 (33.1%) | 3827 (34.7%) | ||
| <7% | 198297 (23.4) | 1505 (21.6) | 32659 (23.2%) | 2368 (21.5%) | ||
| Patient Urban/Rural Location, N(%) | <0.001 | |||||
| Metro areas | 712138 (85.3) | 6104 (89.2) | ||||
| Urban Metro-Adjacent | 75351 (9.0) | 461 (6.7) | ||||
| Urban Not Metro-Adjacent | 31864 (3.8) | 197 (2.9) | ||||
| Rural | 15282 (1.8) | 82 (1.2) | ||||
| Miles to hospital, median (IQR) | 6.9 (3.3, 15.3) | 4.7 (2.3, 9.4) | <0.001 | 7.5 (3.5, 16.4) | 6.5 (3, 15) | <0.001 |
| Charlson-Deyo Score, N(%) | <0.001 | <0.001 | ||||
| 0 | 390112 (69.7) | 2637 (56.8) | 73105 (74.3%) | 5470 (63.3%) | ||
| 1 | 124510 (22.2) | 1198 (25.8) | 19867 (20.2%) | 2227 (25.8%) | ||
| 2 | 45367 (8.1) | 808 (17.4) | 5459 (5.5%) | 941 (10.9%) | ||
| Hospital Type, N(%) | <0.001 | <0.001 | ||||
| Community Cancer Program** | 218808 (25.1) | 1678 (23.5) | 37485 (26.0%) | 2387 (21.1%) | ||
| Academic/Research Program | 652072 (74.9) | 5474 (76.5) | 106927 (74.0%) | 8947 (78.9%) | ||
| Facility Location, N(%) | <0.001 | <0.001 | ||||
| New England | 57948 (6.7) | 503 (7.0) | 8473 (5.9%) | 881 (7.8%) | ||
| Middle Atlantic | 136493 (15.7) | 1441 (20.1) | 21141 (14.6%) | 1591 (14.0%) | ||
| South Atlantic | 189886 (21.8) | 1305 (18.2) | 32424 (22.5%) | 1989 (17.5%) | ||
| East North Central | 168034 (19.3) | 1782 (24.9) | 28678 (19.9%) | 2665 (23.5%) | ||
| East South Central | 59534 (6.8) | 373 (5.2) | 10090 (7.0%) | 727 (6.4%) | ||
| West North Central | 67972 (7.8) | 470 (6.6) | 11696 (8.1%) | 1085 (9.6%) | ||
| West South Central | 67608 (7.8) | 395 (5.5) | 11429 (7.9%) | 681 (6.0%) | ||
| Mountain | 88952 (10.2) | 643 (9.0) | 5543 (3.8%) | 435 (3.8%) | ||
| Pacific | 57948 (6.7) | 503 (7.0) | 14938 (10.3%) | 1280 (11.3%) | ||
| Received Chemotherapy, N(%) | 249545 (28.7) | 115 (1.6%) | <0.001 | <0.001 |
Factors predicting refusal of chemotherapy
Patients refusing chemotherapy were more likely to be female with a median age of 78 years compared to those patients that received chemotherapy (median age of 65 years). Most patients that refused chemotherapy were white and had lower high school education. Furthermore, those patients also had a higher Charlson-Deyo Score. The majority of patients who refused chemotherapy were initially treated at an academic hospital (Table 2). Logistic regression was used to identify factors that predicted refusal of recommended chemotherapy. Multivariate analysis showed that patients refusing chemotherapy were significantly older, female, African American, on Medicaid, treated at a community hospital and higher Charlson-Deyo Score. Income, high school education and patient location did not independently predict the refusal of chemotherapy (Table 3). Additional analysis was performed following stratification by age. Here, we found that similar factors were associated with refusal of surgical treatment. African American race, Medicaid and Charlson Deyo Score >2 were found to predict refusal of surgery in all patient cohorts (Table 4).
Table 3.
Factors predicting Refusal of Surgery or Chemotherapy.
| Characteristics | Surgery |
|
Chemotherapy |
|||||
|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | P-value | Multivariable Analysis | P-value | Univariable Analysis | P-value | Multivariable Analysis | P-value | |
| Age at Diagnosis, median (IQR) | 1.14 (1.14–1.14) | 0.001 | 1.14 (1.14–1.15) | 0.001 | 1.11 (1.10–1.11) | 0.001 | 1.11 (1.10–1.11) | 0.001 |
| Sex | ||||||||
| Male | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| Female | 1.63 (1.55–1.71) | 0.001 | 1.20 (1.12–1.28) | 0.001 | 1.40 (1.34–1.45) | 0.001 | 1.14 (1.08–1.19) | 0.001 |
| Race | ||||||||
| White | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| African American | 1.65 (1.55–1.76) | 0.001 | 2.30 (2.10–2.51) | 0.001 | 0.89 (0.83–0.94) | 0.001 | 1.24 (1.15–1.38) | 0.001 |
| Asian/PI | .98 (.83–1.15) | 0.819 | 1.37 (1.12–1.68) | 0.002 | 0.81 (0.72–0.91) | 0.001 | 1.03 (0.88–1.19) | 0.727 |
| Other | .87 (.69–1.08) | 0.197 | 1.14 (.86–1.51) | 0.361 | 0.79 (0.66–0.95) | 0.10 | 1.07 (0.85–1.34) | 0.531 |
| Insurance type, N (%) | ||||||||
| Private | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| Medicaid | 3.44 (2.93–4.04) | 0.001 | 3.06 (2.49–3.77) | 0.001 | 2.10 (1.86–2.34) | 0.001 | 2.29 (2.0–2.62) | 0.001 |
| Medicare | 5.47 (5.04–5.95) | 0.001 | 1.24 (1.11–1.39) | 0.001 | 4.16 (3.95–4.38) | 0.001 | 1.021 (0.95–1.10) | 0.546 |
| Other/unknown | 3.19 (2.25–4.54) | 0.001 | 2.17 (1.42–3.31) | 0.001 | 1.50 (1.10–2.05) | 0.01 | 0.97 (0.68–1.38) | 0.860 |
| Not Insured | 2.99 (2.59–3.45) | 0.001 | 2.30 (1.88–2.80) | 0.001 | 2.01 (1.82–2.23) | 0.001 | 2.1 (1.84–2.37) | 0.001 |
| Income | ||||||||
| <38,000 | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| 38,000–47,999 | .84 (.78-.90) | 0.001 | .92 (.84–1.02) | 0.112 | 0.97 (0.92–1.03) | 0.371 | 0.95 (0.87–1.02) | 0.151 |
| 48,000–62,999 | .84 (.72-.83) | 0.001 | .91 (.82–1.01) | 0.066 | 0.95 (0.89–1.00) | 0.069 | 0.91 (0.84–0.97) | 0.022 |
| >63 k | .80 (.75-.86) | 0.001 | .93 (.82–1.04) | 0.215 | 082 (0.77–0.86) | 0.001 | 0.80 (0.73–0.88) | 0.001 |
| Education/no HS diploma | ||||||||
| 21%+ | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| 13–20.9% | .98 (.91–1.05) | 0.505 | 1.02 (.93–1.12) | 0.692 | 1.06 (1.00–1.13) | 0.041 | 1.04 (0.96–1.12) | 0.316 |
| 7–12.9% | .90 (.84-.97) | 0.003 | .95 (.85–1.05) | 0.309 | 1.08 (1.02–1.15) | 0.007 | 1.04 (0.96–1.14) | 0.289 |
| <7% | .86 (.79-.93) | 0.001 | .91 (.80–1.04) | 0.160 | 0.96 (0.90–1.02) | 0.167 | 1.01 (0.91–1.12) | 0.769 |
| Patient urban/rural location, N (%) | ||||||||
| Metro areas | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| Urban metro-adjacent | .71 (.65-.78) | 0.001 | .84 (.74-.94) | 0.004 | 1.06 (0.99–1.13) | 0.087 | 0.96 (0.89–1.05) | 0.391 |
| Urban not metro-adjacent | .72 (.63-.83) | 0.001 | .82 (.69-.99) | 0.034 | 1.14 (1.04–1.26) | 0.004 | 1.07 (0.95–1.20) | 0.249 |
| Rural | .63 (.50-.78) | 0.001 | .63 (.47-.83) | 0.001 | 1.15 (1.01–1.31) | 0.033 | 0.91 (0.77–1.07) | 0.264 |
| Stage | ||||||||
| 0 | 1.00 Reference | 1.00 Reference | ||||||
| 1 | 1.29 (1.14–1.47) | 0.001 | .97 (.83–1.15) | 0.783 | ||||
| 2 | .48 (.41-.58) | 0.001 | .28 (.23-.35) | 0.001 | ||||
| 3 | .49 (.41-.59) | 0.001 | .40 (.32-.51) | 0.001 | ||||
| Hospital Type | ||||||||
| Academic | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| Community | 1.09 (1.04–1.16) | 0.001 | 1.01 (.94–1.09) | 0.770 | 1.34 (1.25–1.38) | 0.001 | 1.15 (1.09–1.22) | 0.001 |
| Charlson score | ||||||||
| 0 | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| 1 | 1.42 (1.33–1.52) | 0.001 | 1.13 (1.05–1.22) | 0.001 | 1.5 (1.42–1.58) | 0.001 | 1.19 (1.13–1.26) | 0.001 |
| 2 | 2.64 (2.43–2.85) | 0.001 | 1.85 (1.70–2.01) | 0.001 | 2.3 (2.14–2.5) | 0.001 | 1.64 (1.52–1.78) | 0.001 |
Table 4.
Factors Predicting Refusal of Surgery – Stratified by age group.
| 50s |
60s |
70s |
80s |
|||||
|---|---|---|---|---|---|---|---|---|
| Multivariable Analysis | P-value | Multivariable Analysis | P-value | Multivariable Analysis | P-value | Multivariable Analysis | P-value | |
| Sex, N(%) | ||||||||
| Male | 1.00 Reference | 1.00 Reference | 1.00 Reference | |||||
| Characteristics | 0.73 (0.54–0.99) | 0.049 | 1.06 (0.91–1.22) | 0.410 | 1.20 (1.09–1.32) | <0.001 | ||
| Race, N(%) | ||||||||
| White | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| African American | 2.2 (0.16–3.0) | <0.001 | 2.81 (2.12–3.72) | <0.001 | 2.48 (2.06–3.00) | <0.001 | 2.28 (1.99–2.16) | <0.001 |
| Asian/PI | 1.1 (0.39–3.01) | 0.860 | 1.03 (0.45–2.34) | 0.951 | 1.43 (0.92–2.23) | 0.113 | 1.70 (1.27–2.28) | <0.001 |
| Other/Unknown | 1.34 (0.42–4.24) | 0.621 | 1.63 (0.67–3.98) | 0.286 | 1.22 (0.65–2.30) | 0.523 | 1.24 (0.83–1.87) | 0.296 |
| Insurance Type, N(%) | ||||||||
| Private | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| Medicaid | 5.25 (3.38–8.14) | <0.001 | 4.79 (2.97–7.72) | <0.001 | 1.74 (1,05–2.90) | 0.032 | 2.07 (1.37–3.14) | 0.001 |
| Medicare | 5.74 (3.8–8.7) | <0.001 | 2.06 (1.42–2.98) | <0.001 | 1.03 0.82–1.31) | 0.787 | 1.39 (1.15–1.68) | 0.001 |
| Other/Unknown | 1.15 (0.16–8.34) | 0.889 | 5.52 (2.34–13.02) | <0.001 | 2.05 (0.82–5.10) | 0.123 | 2.06 (1.03–4.10) | 0.041 |
| Not insured | 4.14 (2.66–6.44) | <0.001 | 4.57 (2.83–7.36) | <0.001 | 1.80 (1.13–2.88) | 0.014 | 1.71 (1.1702.49) | 0.006 |
| Income($USD), N(%) | ||||||||
| <$38,000 | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| $38,000-$47,999 | 0.93 (0.62–1.4) | 0.727 | 0.97 (0.69–1.41) | 0.942 | 0.87 (0.70–1.09) | 0.229 | 0.91 (0.78–1.06) | 0.221 |
| $48,000-$62,999 | 0.66 (0.40–1.09) | 0.107 | 1.02 (0.69–1.52) | 0.913 | 0.83 (0.66–1.06) | 0.145 | 0.91 (0.78–1.06) | 0.229 |
| >$63k | 0.62 (0.32–1.19) | 0.153 | 1.13 (0.70–1.83) | 0.612 | 0.93 (0.71–1.23) | 0.615 | 0.92 (0.77–1.10_ | 0.365 |
| No HS Diploma, (%) | ||||||||
| 21%+ | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| 13–20.9% | 1.24 (0.85–1.82) | 0.266 | 1.13 (0.81–1.59) | 0.455 | 0.90 (0.73–1.11) | 0.328 | 1.08 (0.93–1.26) | 0.298 |
| 7–12.9% | 0.74 (0.43–1.26) | 0.265 | 0.78 (0.44–1.04) | 0.078 | 0.89 (0.62–1.00) | 0.057 | 1.02 (0.86–1.20) | 0.841 |
| <7% | 0.77 (0.37–1.60) | 0.480 | 0.80 (0.47–1.36) | 0.407 | 0.73 (0.54–0.99) | 0.046 | 0.98 (0.81–1.19) | 0.850 |
| Charlson Deyo Score | 1.00 Reference | |||||||
| 0 | 1.00 Reference | 1.00 Reference | 1.00 Reference | 1.00 Reference | ||||
| 1 | 1.28 (0.96–1.74) | 0.094 | 1.21 (1.02–1.44) | 0.027 | 1.19 (1.07–1.33) | 0.001 | ||
| 2 | 3.20 (2.33–4.40) | <0.001 | 2.41 (2.00–2.90) | <0.001 | 2.02 (.80–2.27) | <0.001 | ||
| Hospital Type, N(%) | ||||||||
| Academic/Research Program | 1.00 Reference | 1.00 Reference | 1.00 Reference | |||||
| Community Cancer Program | 0.91 (0.66–1.26) | 0.570 | 0.82 (0.63–1.07) | 0.144 | 0.86 (0.73–1.02) | 0.076 | ||
Impact on survival
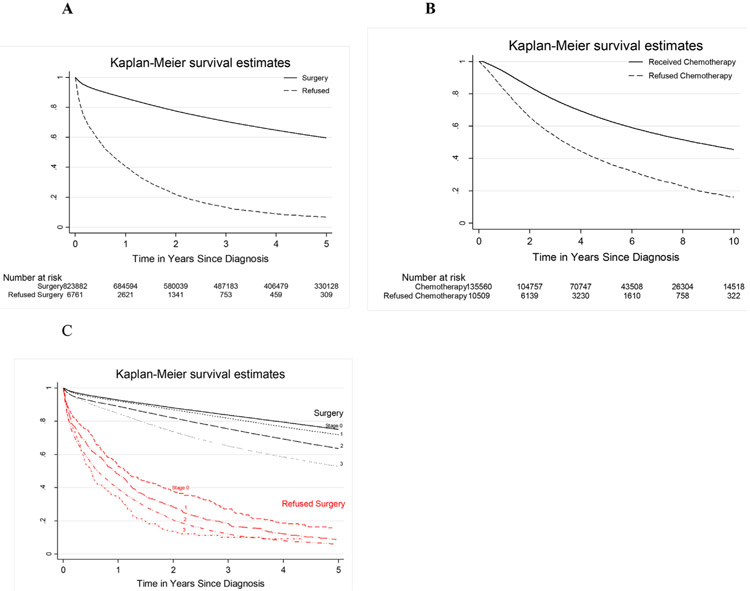
To evaluate the impact of the decision to refuse surgery on survival, we compared patients who underwent surgery and patients who refused the recommended surgery for every stage. One-, 3-, and 5-year overall survival rate for clinical stage I-III who underwent surgery were significantly higher than those patients who refused surgery (Fig. 1A).
Fig. 1. Survival Estimates of Patients Receiving/Refusing Surgery or Chemotherapy.
Kaplan Meier survival curves of patients undergoing/refusing surgery (A) or chemotherapy. Panel C represents survival for every stage.
Patients that refused chemotherapy had a median survival of 3.35 years vs 8.46 years in those receiving chemotherapy for stage III disease. One-, 3-, 5- and 10- year overall survival rates for pathological stage III disease were significantly prolonged in patients receiving chemotherapy versus patients who refused (93, 76, 64 and 45% versus 82, 54, 38 and 16%, respectively) (Fig. 1B). The median survival for patients with Stage 0, I, II or III were 11.4, 9.9, 7.8, 6.6 years respectively. In comparison; patients who refused surgery had a median survival of 1.1, 0.9, 0.7, 0.5 years for Stage 0 – III respectively (Fig. 1C).
Discussion
Newly diagnosed cancer can often lead to a high degree of depression, anxiety, fear or distress in a patient’s life.11 Together with their doctors, patients have to decide on appropriate treatment strategies. Patients learn of possible side effects and complications that go along with the conventional current treatment strategies and as a result often become overwhelmed. Refusal of surgery or chemotherapy for potentially treatable disease therefore remains a major problem and plays a significant role in overall survival rate of patients with cancer.6,12-14 The current study examined patient- and hospital-related risk factors associated with refusal of treatment in patients with colorectal cancer. We found that the number of patients refusing steadily declined between the years 1998 and 2012, however this was statistically not significant. Overall 0.77% (7,152) refused surgery for resectable colorectal cancer and 7.85% of patients who underwent surgery (11,334) refused chemotherapy for stage III disease. Not surprisingly, all patients had significantly decreased survival, stage by stage, once they declined treatment. Patient who refused surgery/chemotherapy were more likely to be female, older, African American, on Medicaid and evaluated at a non-academic community hospital.
Racial disparities in regards to cancer treatment have been shown to be present in a variety of solid malignancies.6,15-18 Rapp et al. performed a SEER (Surveillance, Epidemiology and End Results database) analysis on patients with primary stage I and II lung, prostate, breast, and colon cancers who were diagnosed between 2007 and 2014. In regard to colon cancer, they found 235 patients (0.34%) refused surgery. Patients who refused surgery were more likely to be older, a non-White race/ethnicity, to live without a life partner and to have a Stage II diagnosis.19 Efforts have been made to minimize underuse of cancer treatment based on race and other socioeconomic factors.7,9
Studies of black and white medicare patients undergoing the same surgical procedure displayed similar mortality, suggesting that racial disparities are not because of differences in hospital or surgical care. Higher levels of distrust among black are not surprising and have been reported in prior studies of medical research, distrust of the health care system and health care providers.20-23 Unfortunately, after accounting for comorbidities and socioeconomic factors, racial differences often persist as an independent predictor for refusal of treatment.6,24 African Americans suffer from lack of satisfaction with physician communication and overall more skepticism towards their diagnosis, treatment modalities and prognosis.25,26 Black patients were more likely to believe that physicians would ask them to participate in harmful research or expose them to unnecessary risks. African Americans were more likely to believe that their physicians would not explain research fully or would treat them as part of an experiment without their consent.27,28 These differences are generally attributed to current and historical evidence of inequitable treatment of Blacks by the health care system, as well as racial differences in patientߝprovider communication, insurance coverage, and physician characteristics.29,30
For instance, recent studies have shown that the African-American population is more heavily burdened by this disease and has an overall 40–50% excess mortality from colorectal cancer compared to Caucasians.3,31 The reason for this is multifactorial and potential reasons that have been investigated include poorer access to quality health insurance, lower health care utilization, lower socioeconomic status, treatment inequalities and biologic factors.29,32 Even after adjusting for stage, age, sex and socioeconomics the disparity remains. African Americans are more likely to underutilize screening tools and present with advanced stages. They are less frequently referred to a medical oncologist and less likely to receive adequate therapy.33 A multi institutional study of colon cancer patients in Chicago has shown that African Americans were less likely to travel further distances for visits with their physician.26 Similar data was found in our analysis, patients in rural areas were more likely to refuse surgery. Landrum et al. have shown that patients within the Veterans Health System were less likely to be given the option of surgery. Reasons for underuse of surgery in this population was advanced age, comorbid illnesses and performance status.13 It is important to evaluate functional performance status rather than chronological age before making recommendations for or against treatment. Physicians should recognize age as a possible bias once counseling elderly patients against the use of treatment.13 Within our study population there were 5,612 patients who did not undergo surgery and 11,213 patients for whom chemotherapy was not recommended secondary to risk factors from comorbidities. Unfortunately, the database does not provide additional information regarding comorbidities. A reason why elderly patients refuse treatment could be related to frailty, predicted longer hospital stay, overall life expectancy and unclear benefits from treatment. Often older patients choose not to undergo a treatment if it is not in-line with their goals of care or could subsequently lead to a significant functional or cognitive decline requiring placement in a nursing home. The literature has shown that there are wide variety of patient factors that can lead to disparities and the use of surgery including gender, age and insurance status.8
The goal of a physician is to provide adequate amount of information on the disease, guidance throughout the care process and identify patients at risk for refusing treatment. One of the most crucial steps is to obtain informed consent, which includes information on material risks that are common to all surgeries and risks specific for the proposed procedure, even if they are rare. The physician has to discuss risks that may cause the patient to refuse surgery and it is also important to identify special circumstances patients are in at time of conversation including work responsibilities, family issues, religious believes or insurance status. It is even more important to understand that surgery can lead to a tremendous amount of anxiety in patients and some display stress in different ways than others. In these cases, the skilled surgeon needs to address the patient concerns and develop a treatment plan that is within the patients’ preferences and values. It is important to refocus the physician-patient interaction from a disease-centric to a patient-centric view to improve patient satisfaction and outcomes. A patient-centered outcome would include studying the mental and functional recovery following major surgery. However most studies do not extend their morbidity/mortality analysis past 30 days which is the current gold standard judging quality of care.34,35 It is of utmost importance to understand why patients refuse surgery in order to target those with a goal to improve survival and outcomes. Communication is crucial in establishing trust with patients, gathering information and assisting patients in decisions about care.25,36 The quality of communication in cancer care has been shown to affect patient experience in terms of satisfaction, decision making and compliance.37 Decision to undergo treatment is an active ongoing process and requires good communication skills and understanding from a physician. Patients who initially refuse treatment may later choose to undergo conventional cancer treatment if given the adequate support, information, and time necessary to make the decision. In addition, we have to re-define high-quality care as optimization of patient-centered outcomes, which have to be incorporated at all local and national levels of patient treatment.
The current study uses the NCDB database to analyze trends regarding refusal of surgery/chemotherapy, however this also causes several limitations. The database does not provide reasoning of refusal of treatment. It is unclear what the reasons were that patients or family members refused surgery. In addition is not clear how the surgeon communicated with the patient before and especially after the patient refused.
The database does not provide insights at the exact level at which refusal occurred (primary care vs surgeon vs medical oncologist). It is important to know that patient demographic variables such as gender, race, income, distance to treatment or insurance status play an important role in the decision for treatment. These findings of patient demographics and refusal of treatment need to be confirmed in prospective institutional studies, where patients who refuse surgery are identified and a detailed interview is conducted to study the reason behind refusal. Once we have a better understanding on why patients refuse treatment, active policies and strategies can be developed which can include patient education, workshops with healthcare providers to improve communication and informed consent. This will hopefully increase the receipt of surgery for patients with colon cancer as a chance of cure and improve their overall survival rate.
Conclusion
Surgery and chemotherapy for colorectal cancer remain the treatments of choice for patients with colorectal cancer. Based on our analysis, there have been several thousand patients that refused treatment despite a significant known survival benefit. Patients who refuse are more likely to be African American, older, female, on Medicare/Medicaid and treated in a non-academic community hospital. Efforts have to be made to target these subgroups of patients to improve survival. All involved care providers need to be aware to this disparity and be able to recognize and address this appropriately. We recommend that all patients should be evaluated by a multidisciplinary team that provides accurate information and treatment strategies in a patient-centered approach.
Acknowledgements
The University of Pittsburgh holds a Physician-Scientist Institutional Award from the Burroughs Wellcome Fund: S.T. C.K.
References
- 1.Kuipers EJ, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upadhyay S, Dahal S, Bhatt VR, Khanal N, Silberstein PT. Chemotherapy use in stage III colon cancer: a National Cancer Database analysis. Ther Adv Med Oncol. 2015;7:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustus GJ, Ellis NA. Colorectal cancer disparity in african americans: risk factors and carcinogenic mechanisms. Am J Pathol. 2018;188:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Shayeb M, Scarfe A, Yasui Y, Winget M. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes. 2012;5:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millan M, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol. 2015;7:204–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tohme S, Kaltenmeier C, Bou-Samra P, Varley PR, Tsung A. Race and health disparities in patient refusal of surgery for early-stage pancreatic cancer: an NCDB cohort study. Ann Surg Oncol. 2018;25:3427–3435. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RS, Lenzner D, Argiris A. Race and health disparities in patient refusal of surgery for early-stage non-small cell lung cancer: a SEER cohort study. Ann Surg Oncol. 2012;19:722–727. [DOI] [PubMed] [Google Scholar]
- 8.Gaitanidis A, et al. Refusal of cancer-directed surgery by breast cancer patients: risk factors and survival outcomes. Clin Breast Canc. 2018;18:e469–e476. [DOI] [PubMed] [Google Scholar]
- 9.Wang N, et al. The effect of socioeconomic status on health-care delay and treatment of esophageal cancer. J Transl Med. 2015;13:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson JO, Rotenstein LS, Berry LL. New diagnosis bundle: improving care delivery for patients with newly diagnosed cancer. JOP. 2016;12:404–406. [DOI] [PubMed] [Google Scholar]
- 12.Gilbar P, Lee A, Pokharel K. Why adjuvant chemotherapy for stage III colon cancer was not given: reasons for non-recommendation by clinicians or patient refusal. J Oncol Pharm Pract. 2017;23:128–134. [DOI] [PubMed] [Google Scholar]
- 13.Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118:3345–3355. [DOI] [PubMed] [Google Scholar]
- 14.Moaven O, et al. Healthcare disparities in outcomes of patients with resectable pancreatic cancer. Am J Surg. 2019;217:725–731. [DOI] [PubMed] [Google Scholar]
- 15.Swords DS, et al. Disparities in utilization of treatment for clinical stage I-II pancreatic adenocarcinoma by area socioeconomic status and race/ethnicity. Surgery. 2019;165:751–759. [DOI] [PubMed] [Google Scholar]
- 16.Suh WN, et al. Risk factors associated with treatment refusal in lung cancer. Thorac Cancer. 2017;8:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson SB, Park HS, Gross CP, Yu JB. Complementary medicine, refusal of conventional cancer therapy, and survival among patients with curable cancers. JAMA Oncol. 2018;4:1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Canc Res Treat. 2019. 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 19.Rapp J, Tuminello S, Alpert N, Flores RM, Taioli E. Disparities in surgery for early-stage cancer: the impact of refusal. Cancer Causes Control. 2019;30: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 20.Corbie-Smith G, Thomas SB, St George DMM. Distrust, race, and research. Arch Intern Med. 2002;162:2458–2463. [DOI] [PubMed] [Google Scholar]
- 21.LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about racism, medical mistrust, and satisfaction with care among African American and white cardiac patients. Med Care Res Rev. 2000;57(Suppl 1):146–161. [DOI] [PubMed] [Google Scholar]
- 22.Akinyemiju T, Meng Q, Vin-Raviv N. Race/ethnicity and socio-economic differences in colorectal cancer surgery outcomes: analysis of the nationwide inpatient sample. BMC Canc. 2016;16:715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund MJ, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Canc Res Treat. 2008;109:545–557. [DOI] [PubMed] [Google Scholar]
- 24.Williams CD, Provenzale DT, Stechuchak KM, Kelley MJ. Impact of race on early-stage lung cancer treatment and survival. J Clin Oncol. 2012;30, 232–232.22067393 [Google Scholar]
- 25.Jacobs EA, Rolle I, Ferrans CE, Whitaker EE, Warnecke RB. Understanding African Americans’ views of the trustworthiness of physicians. J Gen Intern Med. 2006;21:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones LA, et al. Examining racial disparities in colon cancer clinical delay in the Colon Cancer Patterns of Care in Chicago study. Ann Epidemiol. 2017;27: 731–738. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharff DP, et al. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musa D, Schulz R, Harris R, Silverman M, Thomas SB. Trust in the health care system and the use of preventive health services by older black and white adults. Am J Publ Health. 2009;99:1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine (US) Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care, Smedley BD, Stith AY, Nelson AR. RACIAL and ETHNIC DISPARITIES IN DIAGNOSIS and TREATMENT: A REVIEW of the EVIDENCE and A CONSIDERATION of CAUSES - Unequal Treatment - NCBI Bookshelf. 2003. [PubMed] [Google Scholar]
- 30.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Publ Health. 2007;97:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams R, et al. Colorectal cancer in african americans: an update. Clin Transl Gastroenterol. 2016;7:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams LB, Richmond J, Corbie-Smith G, Powell W. Medical mistrust and colorectal cancer screening among african americans. J Community Health. 2017;42:1044–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris AM, et al. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanson M, Pitt D. Informed consent for surgery: risk discussion and documentation. Can J Surg. 2017;60:69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson TN, Berian JR. Incorporating patient-centered outcomes into surgical care. Ann Surg. 2017;265:654–655. [DOI] [PubMed] [Google Scholar]
- 36.Pincus HA, Patel SR. Barriers to the delivery of psychosocial care for cancer patients: bridging mind and body. J Clin Oncol. 2009;27:661–662. [DOI] [PubMed] [Google Scholar]
- 37.Frenkel M. Clinical consultation, a personal perspective: components of a successful integrative medicine clinical consultation. J Soc Integr Oncol. 2008;6: 129–133. [PubMed] [Google Scholar]