Abstract
Heat therapy (HT) has emerged as a potential adjunctive therapy to alleviate the symptoms of peripheral artery disease (PAD), but the mechanisms underlying the positive effects of this treatment modality remain undefined. Using a model of diet-induced obesity (DIO) and ischemia-induced muscle damage, we tested the hypothesis that HT would alter body composition, promote vascular growth and mitochondrial biogenesis, and improve skeletal muscle function. Male DIO C57Bl/6J mice underwent bilateral ligation of the femoral artery and were randomly allocated to receive HT or a control intervention for 30 min daily over 3 wk. When compared with a group of lean, sham-operated animals, ligated DIO mice exhibited increases in body and fat masses, exercise intolerance, and contractile dysfunction of the isolated soleus (SOL) and extensor digitorum longus (EDL) muscles. Repeated HT averted an increase in body mass induced by high-fat feeding due to reduced fat accrual. Fat mass was ∼25% and 29% lower in the HT group relative to controls after 2 and 3 wk of treatment, respectively. Muscle mass relative to body mass and maximal absolute force of the EDL, but not SOL, were higher in animals exposed to HT. There were no group differences in skeletal muscle capillarization, the expression of angiogenic factors, mitochondrial content, and the diameter of the gracilis arteries. These findings indicate that HT reduces diet-induced fat accumulation and rescues skeletal muscle contractile dysfunction. This practical treatment may prove useful for diabetic and obese PAD patients who are unable to undergo conventional exercise regimens.
NEW & NOTEWORTHY The epidemic of obesity-related dyslipidemia and diabetes is a central cause of the increasing burden of peripheral artery disease (PAD), but few accessible therapies exist to mitigate the metabolic and functional abnormalities in these patients. We report that daily exposure to heat therapy (HT) in the form of lower-body immersion in water heated to 39 °C for 3 weeks attenuates fat accumulation and weight gain, and improves muscle strength in obese mice with femoral artery occlusion.
Keywords: heat therapy, muscle contraction, obesity, peripheral artery disease
INTRODUCTION
Lower-extremity peripheral artery disease (PAD) is a highly prevalent manifestation of systemic atherosclerosis that is associated with disability and an elevated risk of myocardial infarction, stroke, and cardiovascular death (1, 2). In addition to age, the primary risk factors for PAD include smoking, diabetes, dyslipidemia, and hypertension (3). The proportion of diabetes-related cases is projected to increase substantially in the coming years due to its association with increasing levels of obesity (3). This is important because PAD patients with diabetes have more severe limb symptoms and are at higher risk for cardiac and limb ischemic events compared with those without diabetes (4, 5). In addition, diabetes may reduce treatment effectiveness for PAD, including exercise training (6, 7) and lower-limb revascularization (8, 9).
The quality of life of patients with PAD is markedly worse than in healthy individuals, in part due to the impairment in physical functioning (10). The severe exercise intolerance in these patients limits their capacity for daily physical activity and consequently accelerates functional decline and mobility loss (11, 12). The functional impairment is hemodynamic in origin, but mounting evidence indicates that skeletal muscle alterations also serve an important role (13). Repeated exposure to ischemia-reperfusion during walking activity is thought to elicit a number of detrimental changes in skeletal muscle, including capillary rarefaction, fiber denervation, and atrophy (14, 15). In particular, calf muscle atrophy and the associated decline in muscle strength may help explain increased rates of mobility loss in PAD patients (16, 17). Furthermore, poor leg strength in these patients has been shown to be a predictor of mortality (18, 19).
One therapeutic strategy that may ameliorate vascular and skeletal dysfunction and restore exercise tolerance in PAD is heat therapy (HT). The foundational work of Akasaki et al. (20) in a mouse model of ischemia-induced muscle damage revealed that daily exposure to a far-infrared dry sauna system for 5 wk accelerated blood flow recovery, augmented capillary growth, and enhanced the skeletal muscle expression of endothelial nitric oxide synthase (eNOS). Furthermore, we recently reported that C57BL/6J mice subjected to ligation of the femoral artery and then exposed to HT for 3 wk displayed increased relative muscle mass and maximal absolute force of the soleus muscle when compared with control animals (21). In preclinical models of obesity induced through high-fat diets, HT has been shown to improve whole body glucose tolerance and skeletal muscle glucose uptake while reducing plasma triglyceride, free fatty acid levels, adipocyte size, and skeletal muscle fat accumulation (22–26).
The goal of the present study was to examine the effects of episodic exposure to HT for 3 wk on vascular and skeletal muscle remodeling and exercise tolerance in a mouse model of combined diet-induced obesity (DIO) and ischemia-induced muscle damage. This model incorporates important risk factors for PAD, including hyperlipidemia, endothelial dysfunction, and hypertension and displays impaired compensatory adjustments to the ischemic insult as compared with healthy animals (27, 28). Indeed, mice chronically fed a high-fat diet exhibit 1) contractile dysfunction, particularly in muscles composed predominantly of fast-twitch fibers (29, 30), 2) blunted regeneration following muscle damage induced by cardiotoxin (31, 32) or bupivacaine injection (33); 3) attenuated enlargement of collateral arteries following femoral artery ligation (27), 4) exacerbated ischemia-induced mitochondrial dysfunction and impaired recovery of muscle function (28).
We hypothesized that obese mice subjected to femoral artery ligation and exposed to HT would show improved exercise tolerance when compared with sham-treated animals due to improved body composition (24, 25), augmented collateral growth (20), increased capillary density (20, 34, 35) and mitochondrial content (36–38), and improved skeletal muscle contractile function (21, 35).
METHODS
Animals
All experimental protocols were approved by Purdue University’s Animal Care and Use Committee (1706001586). Male C57BL/6J mice (32 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME). Figure 1 displays a schematic overview of the experimental design.
Figure 1.
Schematic overview of the experimental design. Male C57BL/6J mice were fed a high-fat diet for 10 wk and were then subjected to bilateral ligation of the femoral artery. After recovering from the surgery for 2 wk, the animals were randomly assigned to receive heat therapy (HT) [diet-induced obese mice subjected to HT (DIO-HT)] or a sham treatment [diet-induced obese mice subjected to the control treatment (DIO-CON)]. The HT treatment consisted of placing restrained animals in a water bath heated to 39 °C, whereas animals in the control group were also restrained but were placed in an empty container. Both interventions were applied for 30 min, 6 times/wk, for 3 consecutive wk. Different subsets of animals were used for specific outcome measures. A single group of lean sham-operated mice (LSO) was fed standard chow throughout the duration of the study and underwent a sham operation. These animals were also restrained and subjected to a sham treatment similar to DIO-CON animals. The LSO group did not undergo the assessment of rectal temperature, body composition, indirect calorimetry, or growth of collateral arteries. CAF, capillaries around a fiber; FAL, femoral artery ligation; IMF, isolated muscle function; SO, sham operated.
Upon arrival, the animals were randomly divided into either a diet-induced obese (DIO) or a lean group. Animals allocated to the DIO group were maintained on a high-fat diet (60% of kcal from fat, D12492; Research Diets, New Brunswick, NJ) for the entire duration of the experiment (15–16 wk), whereas lean controls were fed a standard chow. After 10 wk, DIO mice underwent bilateral ligation of the femoral artery and were allowed to recover for 2 wk. Using simple randomization, ligated mice were then allocated to receive HT (DIO-HT) or a control intervention (DIO-CON) for 3 wk (6 days/wk). Lean animals were subjected to a sham operation (LSO) and underwent the control treatment regimen for 3 wk. One group of DIO animals (DIO-CON: n = 8; DIO-HT: n = 7) was used to assess the effect of HT on body composition, skeletal muscle morphology and function, and the expression of a few selected proteins. The impact of the intervention on rectal temperature was determined in a separate subset of animals (DIO-CON: n = 3; DIO-HT: n = 3). A third group (DIO-CON: n = 6; DIO-HT: n = 7) underwent the assessment of metabolic rate by indirect calorimetry. A fourth subset of DIO animals (DIO-CON: n = 13; DIO-HT: n = 12) was employed for the assessment of treadmill exercise performance and collateral artery size as determined using vascular casting and microcomputed tomography (μCT) imaging. All of the experimental outcomes, except for rectal temperature, body composition, indirect calorimetry, and growth of collateral arteries, were also assessed in a single group of lean, sham-operated animals (LSO: n = 8).
Femoral Artery Ligation
Mice were initially anesthetized via a 2.5% isoflurane and oxygen mixture. Ophthalmic lubricant was applied to both eyes to avoid drying during surgery. The hair in the inguinal area was removed with a depilatory agent, followed by cleansing with iodine and an alcohol prep pad. Bilateral ligation of the femoral artery was performed as described by Distasi et al. (39). Briefly, the femoral artery was carefully dissected away from the vein and nerve and ligated with sterile 6-0 silk suture at a point distal to the superficial epigastric artery and proximal to the femoral artery’s trifurcation into the saphenous, popliteal, and geniculate arteries (39). In the LSO group, the femoral artery was dissected free but not ligated. The incision was then closed with a 5-0 sterile absorbable suture, and the animal was given carprofen (5 mg/kg) subcutaneously for pain relief. Prior studies revealed that pedal perfusion and hemoglobin oxygen saturation decline immediately after femoral artery ligation in this model, followed by a progressive recovery that is completed within 7–14 days (40, 41). Leg blood flow, as assessed using fluorescent microspheres, is restored to ∼60% of normal 1 wk after the ligation (40).
Heat Therapy
Heat therapy was administered by placing the animal in a customized flat-bottom restrainer and immersing the lower half of the body in a glass container filled with water heated to 39°C for 30 min. The temperature of the water bath was continuously monitored throughout the treatment sessions using a digital thermometer (50-197-8021; Fisher Scientific). We previously showed that this regimen elicited an increase in relative muscle mass and maximal absolute force of the soleus muscle in C57BL/6J mice subjected to bilateral femoral artery ligation (21). Animals assigned to the DIO-CON and LSO groups were also restrained but placed in an empty container. Rectal temperature was measured during the first treatment session in a subset of animals (n = 3/group), using a thermistor probe (RET-3) connected to computer-controlled multichannel thermometer (Iso-Thermex; Columbus Instruments, Columbus, OH).
Body Composition
Magnetic resonance imaging (Echo-MRI 130 analyzer; Echo Medical System, Houston, TX) was used to measure body composition, including fat, lean, free-water, and total-water masses, both pretreatment and weekly throughout the treatment protocols. Data were collected by placing mice in a cylindrical holder, inserting the holder into the sample chamber, and then waiting ∼3 min while body composition was measured twice. The average of two measurements are reported.
Isolated Muscle Function Testing
Isometric contractile properties of the slow-twitch soleus and the fast-twitch EDL muscles were evaluated as described previously (21). Approximately 48 h after the last treatment session, the animals were anesthetized via an isoflurane and oxygen mixture, and both hindlimbs were excised. Left limbs were pinned to a dissecting dish containing a bicarbonate-buffered solution (in mmol/L: 137 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 24 NaHCO3, and 2 CaCl2) equilibrated with 95% O2-5% CO2 (pH ∼7.4). The right limb muscles were snap-frozen in liquid nitrogen for biochemical assays.
The assessment of contractile function of the EDL muscle was performed first, followed by the soleus muscle. The muscles were isolated under a stereo-zoom microscope and a silk suture (18020-40; Fine Science Tools, Foster City, CA) was tied around each end of the tendons. The muscles were then transferred to a glass bath containing a bicarbonate-buffered solution at room temperature continuously bubbled with carbogen (95% O2-5% CO2) and mounted between two platinum electrodes (1200 A Intact Muscle Test System; Aurora Scientific). The optimal muscle length (Lo) was established by adjusting the muscle length in isometric twitch conditions until the maximum twitch force was recorded. Next, the temperature of the water bath was raised to 32°C, and the muscles were allowed to equilibrate for 10 min. The force-frequency relationship was evaluated by recording the isometric forces at stimulation frequencies of 1, 15, 30, 50, 80, 120, 150, 250, and 300 Hz for the EDL muscle and 1, 15, 30, 50, 80, 120, 150, and 200 Hz for the soleus muscle. Each stimulus was separated by a 1-min interval. After the assessment was completed, the muscles were transferred to the dissecting dish, trimmed of connective tissue, blotted dry, and weighed. Muscle cross-sectional area (CSA) was determined by dividing the wet muscle mass by the product of Lo and muscle-specific density (1.056 g/cm3). Specific force (N/cm2) was calculated by dividing the muscle force (N) by the CSA (cm2).
Immunofluorescence Microscopy
After the measurements of contractile function were concluded, the isolated EDL and soleus muscles were placed in a cryomold containing OCT (optimum cutting temperature) compound (Tissue-Tek; Sakura Finetek USA), immersed in a container filled with dry-ice cooled isopentane, and then stored at −80°C. Frozen muscles were cross-sectioned (10 μm) using a Leica CM1850 cryostat (Leica, Wetzlar, Germany) at −23°C, mounted on frosted microscope slides (Thermo Scientific), air-dried for ∼1 h at room temperature, and stored at −80°C for subsequent analyses. Frozen sections were briefly exposed to room air and fixed with 4% paraformaldehyde for 5 min. After 2 × 3 min washes with 1× PBS, the slides were incubated with blocking buffer (5% goat serum, 2% bovine serum albumin, 0.1% Triton X-100, and 0.1% sodium azide in PBS) for 1 h at room temperature. Identification of capillarization in the EDL and soleus muscles was performed in neighboring sections with antibodies against mouse anti-CD31 IgG1 (550300, 1:100; BD Biosciences) and rabbit anti-dystrophin IgG (ab15277, 1:100; Abcam). After 2 × 5 min washes with 1× PBS, sections were stained with appropriate secondary antibodies [Alexa 350 goat anti-rabbit IgG (A11609, 1:500) and Alexa 488 goat anti-mouse IgG1 (A21121, 1:10,000); Thermo Fisher Scientific] diluted in 1× PBS for 1 h at room temperature. Negative controls for the primary antibody against CD31 were used to ensure specificity of staining.
Slides were viewed at ×20 magnification with an Olympus BX53 fluorescence microscope equipped with an Olympus DP72 digital camera and cellSens Dimension software. The entire specimen cross-section was initially selected with the stage navigator. The multi-channel image was then acquired, and two images from each channel were merged using ImageJ (National Institutes of Health).
Analysis of Immunofluorescence Images
Analyses of capillarization in the EDL and soleus muscles were carried out using Adobe Photoshop CC 2015. An average of 599 ± 227 fibers for the EDL and 490 ± 154 fibers for the soleus muscles were initially counted. Fifty EDL and fifty soleus fibers from each animal were then randomly selected for the quantification of capillary numbers around a fiber (CAF). All immunofluorescent images were blinded for both diet and treatment before analysis.
Protein Extraction and Western Blot Analysis
Approximately 5 mg of frozen muscle samples from the EDL and soleus were homogenized in ice-cold homogenization buffer containing 50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, and 1 mM EDTA (RIPA Lysis Buffer; EMD Millipore), with protease inhibitor cocktail (P8340; Sigma-Aldrich) and phosphatase inhibitors (50 mM NaF and 0.2 mM Na3VO4) at a 1:15 dilution of wet muscle weight using a bead mill homogenizer (Bead Ruptor 12; Omni International). The resulting homogenates were centrifuged at 13,500 g for 20 min at 4°C. After supernatants were collected, protein concentration of each sample was determined using a BCA protein assay kit (Thermo Scientific). Samples were subsequently diluted with homogenization buffer and mixed with either reducing sample buffer (4× Laemmli sample buffer with 10% 2-mercaptoethanol) or nonreducing sample buffer (4× Laemmli sample buffer). Except for the mitochondrial oxidative phosphorylation protein blots, all samples were heated to 95°C for 5 min, divided into small aliquots, and stored at −80°C.
Proteins were separated by SDS-PAGE on precast Stain Free 4–15% gels (Bio-Rad) and transferred to PVDF membranes with the Trans-Blot Turbo transfer system (Bio-Rad). Membranes were subsequently blocked with 5% nonfat milk in 1× TBST (1% Tween-20) for 1 h and incubated for ∼4 h with primary antibodies (VEGF 164, AF-493-SP, 1:200, R & D Systems; eNOS, ab199956, 1:1,000, Abcam; OXPHOS, ab110413, 1:1,000, Abcam) at room temperature. The membranes were washed with 1× TBST at room temperature for 3 × 10 min, incubated with horseradish peroxidase-conjugated secondary antibodies diluted in 1× TBST for 1 h at room temperature, and then washed with 1× TBST at least 3 × 10 min, followed by exposure to an enhanced chemiluminescent solution (Clarity Western ECL; Bio-Rad) for 5 min. Membranes were visualized with a densitometer (ChemiDoc Touch Imaging System; Bio-Rad), and band densities were determined using an image analysis software (Image Laboratory version 6.0.1; Bio-Rad). PageRuler Prestained Protein Ladder (Thermo Fisher) was used as a molecular weight marker, and recombinant proteins were used to confirm antibody specificity. Control for equal loading was performed with stain-free technology, and the target protein expression was calculated using band intensity divided by total lane protein after background signal adjustment.
Citrate Synthase Activity
The maximal enzyme activity of citrate synthase (CS) was determined using a colorimetric enzyme assay kit (119692; Abcam), following the manufacturer’s instructions. In brief, ∼5 mg of frozen muscle specimens was homogenized in ice-cold 1× PBS using a bead homogenizer and diluted with extraction buffer. After 20 min of incubation, lysates were centrifuged at 16,000 g for 20 min at 4°C, and the supernatants were transferred into clean tubes and stored at −80°C until further analysis. One hundred microliters of each sample was added into the antibody-coated microplate, sealed with film, placed in a plate shaker at 300 rpm and incubated for 3 h at room temperature. Thereafter, the plate was thoroughly washed three times using the wash buffer. Following the last step, 100 µL of activity solution was added, briefly mixed in a plate shaker, and placed in the microplate reader at 412 nm. The absorbance was measured for 5 min, and maximal CS enzyme activity was expressed as mOD (milli-optical density) per min per mg protein.
Indirect Calorimetry
Energy expenditure was measured for 24 h at baseline, after the first treatment session, and once weekly throughout the treatment with an Oxymax indirect calorimetry system (Columbus Instruments, Columbus, OH). Mice were singly housed in specially built Plexiglass cages (20 × 10.5 × 12 cm) and had free access to water and food. The animals were acclimatized for 18–24 h before data collection. Metabolic rate (kcal/h) was calculated from the following equation: (3.815 + 1.232 × RER) × VO2, where RER is the respiratory exchange ratio [volume of CO2 produced (mL·kg body wt−1·h−1) per volume of O2 consumed (mL·kg body wt−1·h−1)] and VO2 is the volume of O2 consumed per hour.
Treadmill Test
Exercise testing was conducted at baseline and 36 h after the last treatment session. Prior to both tests, mice were familiarized with the treadmill (Exer3/6 treadmill; Columbus Instruments) daily for 3 consecutive days by running at a constant speed of 10 m/min for 5 min with a fixed 10% slope. The treadmill test consisted of 5 min of running at 10 m/min at a grade of 10%, and the speed was increased by 1 m/min every min until the animal was exhausted. Exhaustion was defined as an inability of the animal to run on the treadmill for 10 s despite mechanical prodding.
Vascular Casting
Approximately 12 h after the last treadmill exercise test, the animals were anesthetized via an isoflurane and oxygen mixture, and the infrarenal abdominal aorta was cannulated with a polyethylene catheter (inner diameter: 0.28 mm; outer diameter: 0.61 mm). Initially, 10 mL of heparinized PBS (10 U/mmol/L) was perfused through the catheter at pressure between 100 and 110 mmHg. The vena cava was severed, allowing exit of the perfusate (27). Next, the catheter was perfused with 10 mL of a vasodilatory solution (0.1 mM adenosine and 0.01 mM sodium nitroprusside in 0.9% saline), followed by 10 mL of 10% neutral buffered formalin (NBF) (27). After fixation, ∼0.8 mL of yellow Microfil vascular casting compound (MV-122; Flow Tech, Carver, MA) was perfused at a 1.25:1:0.5 ratio (MV-diluent/MV-compound/MV-curing agent). The casting agent was allowed to polymerize at 4°C overnight (27). The following day, the lower half of the body was severed, from which the skin and subcutaneous tissues were carefully removed.
Microcomputed Tomography Imaging
The carcasses were transported to Purdue’s Bioscience Imaging Facility (BIF) and were imaged using a Quantum GX2 μCT Imaging System (PerkinElmer, Inc., Waltham, MA). Scans were performed at 9-μm resolution using the following settings: 70 kV, 120 μA, 0.5-mm aluminum filter, 550-ms exposure with three-frame averaging, 360° scan, and a rotation step of 0.7°. Reconstruction of the raw data images was done with NRecon V1.6.1.0 (SkyScan) software and analyzed with Analyze 10.0 (Mayo Clinic), MATLAB R2013a (Image Processing Toolbox; The MathWorks, Natick, MA), and CTan (SkyScan). The primary gracilis artery collateral vessels, running along the adductor magnus and gracilis muscles, were digitally rendered for the assessment of average diameter. We focused on the gracilis collateral pathway because these vessels comprise the primary collaterals after surgical ligation of the distal femoral artery between the origins of the superficial epigastric artery and the geniculate/saphenous/popliteal arteries (39).
Vessel Length Analysis
cThe resulting μCT imaging data sets were loaded into ITK-SNAP for vessel segmentation (42). Using region-growing algorithms, vessels corresponding to the upper and lower gracilis arteries were isolated and rendered as three-dimensional binary masks. To identify the length of each vessel, resultant binary masks were analyzed using an in-house Python script implementing the Vascular Modeling Toolkit (VMTK) library, from which vessel centerlines were extracted. The length of each centerline was used as a measure of overall vessel length, and assuming a cylindrical vessel, average diameters were estimated with the corresponding volume formula and known volumes from each binary mask.
Statistics
Sample sizes were estimated based on our previous report on the effects of HT in a mouse model of PAD (21) as well as other studies that documented the metabolic adaptations to repeated heat stress in preclinical models of obesity (22–26). All data were analyzed using SAS (version 9.4; SAS Institute). Results are presented as means ± SD. Normality was assessed by the Kolmogorov-Smirnov test. For nonnormally distributed variables, log transformations were performed. Weekly measures of body composition, food intake, and energy expenditure, the force-frequency curves, and rectal temperature were analyzed using two-way repeated-measures ANOVA. Muscle masses, maximal tetanic forces, changes in treadmill running time, measures of mitochondrial content, and angiogenesis were analyzed by one-way ANOVA. When indicated by a significant F value, pairwise comparisons were performed using Tukey’s procedure. Multiple comparisons were performed using the Bonferroni adjustment. The average diameter of the gracilis arteries was compared between DIO groups using Student’s t test. Differences were considered statistically significant at P < 0.05.
RESULTS
Rectal Temperature
Changes in rectal temperature during exposure to a single 30-min session of HT or the control regimen are shown in Fig. 2. A significant group-by-time interaction (P < 0.0001) was observed, and post hoc testing revealed that core temperature in the ligated DIO-HT group began to increase relative to the control regimen within 5 min following the onset of treatment. At the end of the session, core body temperature increased by ∼3°C in the HT, whereas no changes were observed in the DIO-CON group.
Figure 2.
Rectal temperature prior to and during a single 30-min session of heat therapy (HT; n = 3) or a control treatment (n = 3). Mice were restrained, and the lower half of the body was immersed in a water bath heated to 39°C for 30 min. Animals in the control group (DIO-CON) were also restrained but placed in an empty container. These measurements were performed on the 1st day of treatment. Data are expressed as means ± SD and were compared using a 2-way repeated-measures ANOVA. Multiple comparisons were performed using a Bonferroni correction. *Significantly different from DIO-CON. DIO-HT, diet-induced obese mice subjected to HT.
Body Composition
A significant group-by-time interaction (P < 0.0001) was observed for the changes in body mass throughout the intervention (Fig. 3A). As expected, ligated DIO mice exhibited greater body mass than the lean sham-operated (LSO) animals fed the standard chow (P < 0.05). Exposure to HT averted a temporal increase in body mass induced by high-fat feeding. Indeed, animals in the DIO-CON group gained ∼5 g during the 3 wk of treatment, whereas a reduction of ∼1 g was noted in the DIO-HT group. This difference was largely the result of distinct rates of fat accrual. A significant group-by-time interaction (P < 0.0001) was observed for fat mass (Fig. 3B), and subsequent post hoc testing revealed that the group exposed to repeated HT had lower values compared with the DIO-CON group at 2 (P = 0.03) and 3 wk (P = 0.01). Whereas a significant group-by-time interaction (P < 0.0001) was also observed for lean mass (Fig. 3C), there were no significant differences between groups throughout the intervention.
Figure 3.
Body mass (A), fat mass (B), and lean mass (C) throughout the 3-wk intervention in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 7) and diet-induced obese mice subjected to the control treatment (DIO-CON; n = 8) groups. Animals in the lean sham-operated (LSO) group (n = 8) were weighted weekly but did not undergo the body composition assessment. Data are expressed as means ± SD and were compared using a 2-way repeated-measures ANOVA. Multiple comparisons were performed using a Bonferroni correction. *Significantly different from DIO-HT; #significantly different from DIO-HT and DIO-CON.
Food Intake and Energy Expenditure
To investigate the possible causes of the marked changes in body composition, animals were housed individually in metabolic chambers of an open-circuit indirect calorimetry system for 24 h at baseline and weekly throughout the intervention. The 24-h chow intake increased similarly (main effect of time, P < 0.001) on both DIO-CON (baseline: 2.8 ± 0.9 g; week 1: 3.7 ± 0.5 g) and DIO-HT (baseline: 2.3 ± 0.9 g; week 1: 3.7 ± 0.8 g) after the first week of treatment but remained stable afterwards (Fig. 4A). The average energy expenditures (EE) for dark and light phases were similar at baseline between the ligated DIO animals exposed to HT (11.4 ± 0.8 kcal·h−1·kg−1) and the DIO-CON group (11.9 ± 0.4 kcal·h−1·kg−1). Figure 4B displays the change from baseline in average EE throughout the 3-wk intervention. The main effect of treatment approached significance (P = 0.054), but there was no group-by-time interaction (P = 0.48). In the DIO-HT group, EE increase by 10% after the first week of treatment and subsequently declined toward baseline levels. Conversely, in the DIO-CON group, EE remained stable for the 2 wk of treatment and then declined by ∼6% in the last week.
Figure 4.
A: food intake at baseline and weekly throughout the intervention in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 7) or diet-induced obese mice subjected to the control treatment (DIO-CON; n = 6) groups. B: change from baseline in average energy expenditure (EE) for dark and light phases in the DIO-HT (n = 7) and DIO-CON (n = 6) groups. Data are expressed as means ± SD and were compared using a 2-way repeated-measures ANOVA.
Muscle Masses
Absolute and relative masses of the EDL and soleus muscles are shown in Fig. 5. A significant effect of group (P = 0.0016) was observed for absolute EDL masses, with ligated DIO mice showing greater masses relative to LSO (Fig. 5A). However, when tissue masses were normalized to body weight (Fig. 5B), LSO displayed greater EDL masses relative to the DIO-CON group (P = 0.003) but not the DIO-HT group (P = 0.98). Indeed, relative EDL masses in DIO mice exposed to HT were 16% greater when compared with DIO-CON animals (P = 0.006). A significant effect of group (P = 0.0172) was noted for absolute soleus masses, and subsequent pairwise comparisons revealed that the DIO-CON group had greater masses than LSO (P = 0.01) but not DIO-HT (P = 0.14) (Fig. 5C). There were no group differences for relative soleus masses (P = 0.06; Fig. 5D).
Figure 5.
Absolute and relative masses of the extensor digitorum longus (EDL; A and B) and soleus (C and D) muscles in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 7), diet-induced obese mice subjected to the control treatment (DIO-CON; n = 8), and lean sham-operated (LSO; n = 8) groups. Data are expressed as means ± SD and were compared using a 1-way ANOVA, with Tukey post hoc tests for pairwise comparisons. ×Significantly different from DIO-HT and DIO-CON; φsignificantly different from DIO-CON; *significantly different from LSO and DIO-HT.
Contractile Function
Force-frequency relationships and the maximal tetanic forces of the EDL muscles are displayed in Fig. 6. Long-term exposure to a high-fat diet combined with occlusion of the femoral artery impaired contractile function of the EDL, as revealed by the marked reduction in both absolute and specific tetanic forces in DIO-CON relative to LSO, particularly at frequencies ranging from 80 to 300 Hz (Fig. 6, A and C). In addition, maximal absolute and specific forces were on average 12% and 29% lower in the DIO-CON when compared with LSO. Daily treatment with HT partially restored force development in DIO mice. At frequencies ranging from 120 to 300 Hz, the EDL muscle from DIO-HT mice developed greater absolute force compared with DIO-CON (Fig. 6A). Maximal absolute (P < 0.05) but not specific (P = 0.18) force development was greater in DIO-HT relative to the DIO-CON (Fig. 6, B and D).
Figure 6.
A and C: extensor digitorum longus (EDL) absolute (A) and specific (C) force-frequency relationships in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 7), diet-induced obese mice subjected to the control treatment (DIO-CON; n = 8), and lean sham-operated (LSO; n = 8) groups. Force-frequency curves were compared between groups using a 2-way repeated-measures ANOVA. Multiple comparisons were performed using a Bonferroni correction. B and D: EDL maximal absolute (B) and specific (D) tetanic forces. These data were compared using a 1-way ANOVA, with Tukey post hoc tests for pairwise comparisons. *Significantly different from lean sham-operated (LSO) and DIO-HT; ×significantly different from DIO-HT and DIO-CON.
The soleus muscle was also severely impacted by DIO and the ischemic insult (Fig. 7). A significant group-by-time interaction (P < 0.0001) was observed for the specific but not absolute (P = 0.13) force-frequency relationship, with LSO animals displaying markedly higher forces than the obese, ligated animals (Fig. 7C). Maximal specific soleus force was on average 30% lower (P < 0.05) in ligated DIO animals when compared with LSO (Fig. 7D). Nonetheless, contrary to the observations in the EDL muscle, exposure to HT failed to improve muscle strength. There were no differences in either absolute or specific force between the DIO-CON and DIO-HT groups.
Figure 7.
A and C: soleus absolute (A) and specific (C) force-frequency relationships in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 7), diet-induced obese mice subjected to the control treatment (DIO-CON; n = 8), and lean sham-operated (LSO; n = 8) groups. Force-frequency curves were compared between groups using a 2-way repeated-measures ANOVA. Multiple comparisons were performed using a Bonferroni correction. B and D: soleus maximal (B) absolute and specific (D) tetanic forces. These data were compared using a 1-way ANOVA, with Tukey post hoc tests for pairwise comparisons. ×Significantly different from DIO-HT and DIO-CON.
Capillarization
A significant effect of group (P < 0.01) was observed for the number of capillary contacts per fiber in the EDL muscle (Supplemental Fig. S1; https://doi.org/10.6084/m9.figshare.13067513.v1). Pairwise comparisons revealed that LSO had a significantly lower number of capillary contacts when compared with ligated DIO ligated animals (P = 0.02). Conversely, in the soleus muscle, the number of capillary contacts was comparable between ligated DIO and LSO groups (P = 0.66). Treatment with HT had no impact on capillarization in either the EDL or the soleus muscle. The protein expression of pro-angiogenic factors eNOS and VEGF was also not different among groups in either the EDL or the soleus muscle (Supplemental Fig. S2; https://doi.org/10.6084/m9.figshare.13067528.v1).
Biomarkers of Mitochondrial Content
In the EDL, but not the soleus, a significant effect of group (P < 0.01) was observed for citrate synthase activity, with LSO displaying significantly lower values than ligated DIO animals (Supplemental Fig. S3; https://doi.org/10.6084/m9.figshare.13067540.v1). Protein abundance of mitochondrial oxidative phosphorylation complexes was comparable among the three experimental groups (Supplemental Fig. S4; https://doi.org/10.6084/m9.figshare.13067576.v1).
Treadmill Exercise Performance
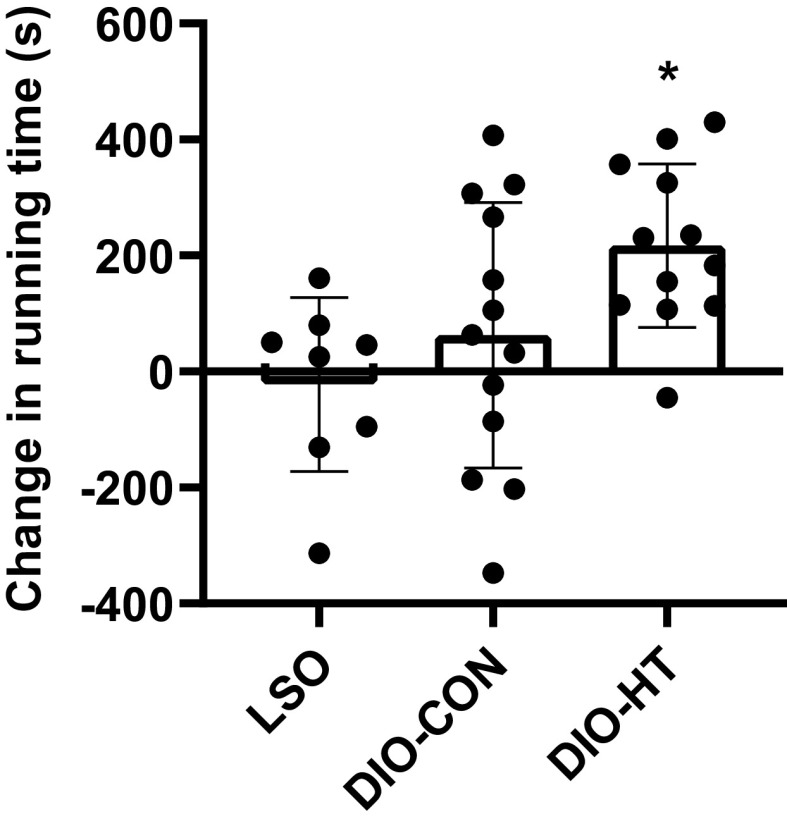
On the baseline assessment, the maximal running time was similar between DIO-CON and DIO-HT (CON: 16.4 ± 1.9 min; HT: 15.7 ± 3.0 min) and markedly higher in the LSO group (29.7 ± 2.6 min). After the intervention, the running time increased by ∼3.5 min in DIO-HT animals and by ∼1 min in the DIO-CON group. A significant effect of group (P = 0.019) was observed for the change from baseline in maximal running time. The change in the DIO-HT group was greater than in LSO (P = 0.02) and tended to be higher than the improvements noted in the DIO-CON group (P = 0.105) (Fig. 8).
Figure 8.
Change from baseline in treadmill running time in the diet-induced obese mice subjected to heat therapy (DIO-HT; n = 12), diet-induced obese mice subjected to the control treatment (DIO-CON; n = 13), and lean sham-operated (LSO; n = 8) groups. An incremental exercise test to exhaustion was conducted prior to the onset of the intervention and 36 h after the last treatment session. Data are expressed as means ± SD and were compared using a 1-way ANOVA with Tukey post hoc tests for pairwise comparisons. *Significantly different from LSO.
Collateral Vessel Diameter
μCT imaging was used to identify and assess the diameter of the gracilis arteries, the major collateral circuit in the mouse hindlimb model with distal femoral artery ligation. There were no group differences (P = 0.11) for the average diameter of the upper or lower gracilis collateral arteries (Supplemental Fig. S5; https://doi.org/10.6084/m9.figshare.13067594.v1).
DISCUSSION
Obesity-associated metabolic dysfunction and diabetes are central players in the increasing burden of PAD worldwide (3). We used a model of prolonged exposure to a high-fat diet, which elicits dyslipidemia, insulin resistance, and other pathological features commonly encountered in obese and diabetic patients, to test the hypothesis that regular exposure to HT reduces body weight gain and fat accrual, promotes vascular growth, and reverses skeletal contractile dysfunction. This hypothesis was derived in part from prior observations that HT raises ATP flux in skeletal muscle (43), effectively combats metabolic abnormalities induced by high-fat feeding (23–25), and augments vascular growth following vascular occlusion (20, 44). We found that daily HT for 30 min over 3 wk reduced body weight gain due to lower fat accrual and partially improved force development in the EDL, but not the soleus muscle. Contrary to our predictions, HT had no impact on skeletal muscle capillarization, angiogenic signaling and biomarkers of mitochondrial content, or the size of the primary collateral network.
The inclusion of a lean, sham-operated group (LSO) in the present study allowed us to document that a combination of diet-induced obesity and femoral artery occlusion induces compensatory responses in skeletal muscle, including increased citrate synthase activity and capillarization in the EDL muscle. Presumably, these coping strategies stem from the excessive supply of fatty acids with a high-fat diet and the subsequent ischemia triggered by the occlusion of the femoral artery. In support of this notion, high-fat feeding has been shown to enhance oxidative capacity (45, 46) and improve oxygen supply due to higher red blood cell supply rates and oxygen saturations (45). Likewise, acute hindlimb ischemia triggers a host of compensatory changes, such as expansion of the collateral network (47) and capillary growth (48). Nonetheless, despite these adaptive responses, contractile function (Figs. 6 and 7) and exercise performance (Fig. 8) in ligated DIO mice were markedly lower than in lean controls. For instance, the maximal running time on the treadmill test was 45% lower in these animals when compared with LSO (Fig. 8). These later findings attest to the notion that the vascular and skeletal muscle compensatory responses to nutrient excess and muscle ischemia are insufficient to restore normalcy (28). Quite contrarily, hindlimb ischemia in C57BL/6J mice may cause a sustained dysfunction of the skeletal muscle microcirculation (49) and permanent impairments in exercise capacity (50).
We previously reported that femoral artery ligation in adult C57BL/6J mice impaired the contractile function of the slow-twitch soleus muscle but not the fast-twitch EDL (21). In contrast to these earlier findings, DIO mice subjected to femoral artery ligation exhibited marked impairments in force development in both the soleus and the EDL (Figs. 6 and 7). On average, maximal specific tetanic forces of the EDL and soleus were reduced by 25% and 30%, respectively, in comparison with LSO. These results suggest that prolonged high-fat feeding impairs the regenerative response to ischemia-induced muscle damage, particularly in the fast-twitch EDL muscle. Interestingly, recent studies revealed that chronic DIO in mice reduces contractile function of the EDL, whereas soleus force production is largely preserved (30, 51). This muscle-specific impairment has been ascribed to the inability of fast-twitch muscles to cope with excessive supply of lipid substrates, thus leading to lipid accumulation and, consequently, contractile dysfunction (24, 29, 30, 51). Along these lines, the EDL muscles of ligated DIO mice generated less absolute force than lean controls (Fig. 6) despite exhibiting greater masses (Figs. 5), which is indicative of a deterioration in muscle quality (30).
One important finding of the present study is that exposure to HT increased relative muscle mass and restored absolute force development of the EDL (Fig. 6) but not the soleus (Fig. 7) muscle. This finding may be explained not only by the preferential detrimental effects of diet on fast-twitch muscles but also the notion that the effects of HT on muscle metabolism are more marked in fast-twitch relative to slow-twitch muscles. The seminal report of Gupte et al. first revealed that the metabolic effects of repeated HT are particularly evident in the fast-twitch EDL when compared with the soleus muscle (24). For example, exposure to a single HT session improved insulin-stimulated glucose uptake in EDL but not the soleus muscle (24). These authors attributed the favorable metabolic effects of HT to the upregulation of heat shock proteins (HSPs), which are more abundantly expressed in slow-twitch muscles (24, 52). Exposure to HT upregulated HSPs in the EDL to levels that are comparable with that of the soleus muscle and, as a result, rescued the metabolic defects caused by overnutrition (24). By elevating energy flux, in particular fatty acid uptake and utilization, HT may abrogate fat accumulation in fast-twitch muscles (22) and consequently minimize the development of contractile dysfunction (52).
Recent reports of reduced lipid accumulation in the liver as well as decreased adipocyte size in epidydimal white adipose tissue (eWAT) (22, 26) following exposure to HT indicate that the positive metabolic adaptations to HT are not confined to skeletal muscle. Archer et al. (22) recently showed that weekly exposure to HT during the final 7 wk of a 15-wk-long high-fat feeding regimen in rats decreased liver triglyceride content by 49% and adipocyte size in the eWAT by 31%. These widespread metabolic effects likely underlie the observed ∼29% reduction in whole body fat content after daily exposure to HT for 3 wk relative to the control intervention (Fig. 3). Our weekly assessments of body composition reveal for the first time that the impact of HT on fat mass is mostly evident in the early phase of the treatment. In fact, at the end of the first week, the fat mass differed by 20% between the ligated DIO groups. This marked fat loss following 1 wk of HT was coupled with a 10% increase in energy expenditure, although this change did not reach statistical significance (main effect of treatment, P = 0.054). Because we have not measured the activity levels of these animals, it is impossible to exclude the possibility that the observed changes in energy expenditure following HT derive from increases in movement. Importantly, Tamura et al. (38) reported that mouse spontaneous physical activity levels do not change during exposure to a hot environmental chamber (40°C) for 60 min.
The functional significance of the improvements in body composition and skeletal muscle contractile function following HT is highlighted by the changes in treadmill exercise performance (Fig. 8). Although not statistically significant (P = 0.104), it is noteworthy that the improvement in treadmill running time after 3 wk of treatment was more than threefold higher in the animals exposed to HT relative to the ligated DIO control group. Considering the weight-bearing nature of treadmill running, it is predictable that the superior improvement in performance in the HT group stems partially from the changes in body weight after the intervention. Whereas CON animals gained more than 5 g during the 3 wk of treatment, the HT group lost ∼1.2 g. Nonetheless, as the genesis of exercise intolerance in DIO and hindlimb ischemia is multifactorial, it is possible that factors other than changes in body mass contributed to the improvements in performance following HT.
Exposure to heat stress has been shown to elicit increased expression of eNOS in a wide range of experimental models, including cultured endothelial cells (53), rat arteries (53), and isolated human arteries (54), and in healthy animals (55). More recently, we (35) and others (34) documented that repeated local and whole body HT elevates eNOS expression in human skeletal muscle. Contrary to these findings, we report herein that 3 wk of HT does affect eNOS expression in either the EDL or the soleus muscle. One possible mechanistic explanation for these discrepant findings is that the combination of DIO and ischemia-induced muscle damage suppressed eNOS responsiveness to HT. Metabolic syndrome chronically reduces nitric oxide bioavailability in the skeletal muscle microcirculation in part via the scavenging actions of oxidative free radicals (56). Similarly, the impairment in vascular remodeling following femoral artery excision has been partially ascribed to elevated oxidant stress within ischemic muscle and in endothelial progenitor cells and the accompanying attenuation of nitric oxide bioavailability (57).
The lack of changes in skeletal muscle capillary contacts following 3 wk of HT in ligated DIO mice is at odds with earlier reports of increased capillary density following repeated dry sauna in a model of femoral artery ligation and excision in apolipoprotein E (apoE)-deficient mice (20, 44). In addition to differences in the experimental model and the HT modality, it is worth noting that we started HT 2 wk after the ligation, whereas these prior studies commenced the treatment immediately after the ischemic insult (20, 44). It has been argued that proper testing of new therapeutics in models of arterial insufficiency should be conducted after the natural compensation to ischemia is resolved because 1) the systemic effects of surgery may impact the treatment outcome, and 2) the mechanisms mediating the early adaptations to acute occlusion may differ from those involved in later stages (47). Capillary growth is a fundamental part of the natural compensatory response to femoral artery ligation, and it is thus possible that a ceiling effect prevented us from capturing the stimulatory actions of HT on skeletal muscle angiogenesis. This notion is supported by the findings of Nwadozi et al. (45), which showed capillary growth and increased numbers of small arterioles at 14 days postligation in C57Bl6/J mice fed a high-fat diet.
Akasaki et al. (20) first reported that apoE-knockout mice exposed to heat stress in a far infrared dry sauna system after being subjected to resection of the femoral artery and vein displayed faster recovery of hindlimb superficial blood flow, as assessed using laser Doppler flowmetry. These findings are noteworthy because the rate of blood flow recovery during serial laser Doppler flowmetry measurements has been interpreted as a surrogate measure of collateralization in murine models of hindlimb ischemia (58). Building upon these findings, we examined the effects of HT on collateral size using μCT imaging after perfusion/fixation. We hypothesized that HT-induced increases in shear stress (59–61), the primary promoter of collateral remodeling (62), would result in growth of the readily identifiable gracilis collaterals. Contrary to these expectations, collateral diameter was comparable between HT and sham-treated groups. Because we focused solely on the gracilis vessels, it is impossible to exclude the possibility that HT promoted the enlargement of other collateral paths (39). In addition, it must be emphasized that the assessment of maximal collateral diameter provides no information about the vasodilatory function of those vessels and the regulation of collateral-dependent blood flow (58). In other words, despite the lack of an observed effect on collateral enlargement, HT may still enhance blood flow to tissues downstream from the occlusion site by improving the vasomotor properties of collateral arteries. To test this hypothesis, future studies must incorporate measures of collateral-dependent blood flow under vasodilation [e.g., calf blood flow during exercise using microspheres (63)] as well as the assessment of vasoresponsiveness of collateral vessels (64).
Motivated by the findings of Tamura et al. (38) that the maximal activity of mitochondrial enzyme citrate synthase and the content of mitochondrial respiratory chain proteins increase following episodic whole body heat stress 5 days/wk for 3 wk in healthy ICR mice, we assessed the impact of HT on mitochondrial content in obese animals with femoral artery ligation. Our findings do not support the notion that HT may augment mitochondrial biogenesis in this model. As delineated above, it is possible that the compensatory stimulation of mitochondrial biogenesis that occurs following the arterial occlusion (65) prevented us from a detecting a treatment effect. Alternatively, it is conceivable that, in contrast to healthy, young animals, obesity and ischemia-induced muscle damage in adult mice may impair the mitochondrial responses to HT. Of importance is that we have not assessed the impact of HT on mitochondrial function, and it is possible that HT may induce favorable changes in mitochondrial respiration in the absence of palpable increases in mitochondrial content.
Limitations
The sham treatment used in the present study consisted of placing restrained animals in an empty container at room temperature. This regimen does not account for factors that may conceivably influence long-term adaptations to repeated water immersion, such as increased hydrostatic pressure (66) and psychological stress (67, 68). It will be valuable for future studies to compare and contrast the adaptations to repeated heated water immersion with those elicited by thermoneutral water immersion. Another important limitation of the current study is that animals in the LSO group were not subjected to HT, and thus it is not possible to determine whether DIO and ischemia-induced muscle damage alter the responsiveness to the treatment. Furthermore, the single group of LSO animals were only assessed for a few outcomes (treadmill testing and muscle-specific measures), thus precluding a thorough comparison between DIO and lean animals for other measures such as body composition, energy expenditure, and collateral size. Lastly, it is also important to acknowledge that despite the marked changes in body composition, it is unclear whether HT had an impact on markers of metabolic health, including glucose and insulin sensitivity. Additional studies are warranted to confirm that the prior observations of improved whole body glucose tolerance and skeletal muscle glucose uptake in models of obesity (22–26) also hold true for combined DIO and ischemia-induced muscle damage.
Conclusions and Clinical Implications
In summary, the findings of the present study add further support to the notion that HT may be a useful adjunctive therapy for PAD. Prior studies revealed that patients with PAD who were exposed to repeated HT in the form of dry sauna and hot water immersion exbibit improved leg pain, hemodynamics, and walking performance (69–73). We report that the salutary effects of HT in a model of DIO and femoral artery ligation are mediated in part by a robust reduction in fat mass and a consequent attenuation of weight gain that occurred in the absence of changes in food intake. These anti-obesity actions of HT may be clinically relevant because a growing number of PAD patients display obesity-associated metabolic abnormalities, including type 2 diabetes (3). In addition, HT partially reversed the skeletal muscle dysfunction induced by prolonged overnutrition and the acute ischemic insult. The increases in relative muscle mass and force development in the EDL muscle described herein align closely with earlier reports that HT improves muscle regeneration following injury (21, 74, 75) and rescues disuse-induced muscle atrophy (76–78). If true for patients with PAD, the observed improvements in contractile function following HT may translate into enhanced functional capacity (79), delayed mobility loss (17), and improved survival (18).
GRANTS
Support for this work was provided by the American Heart Association (16SDH27600003), the National Institutes of Health (1R21AG053687-01A1 and 1F30HL145980-01A1), the Indiana Clinical and Translational Science Institute, the Ralph W. and Grace M. Showalter Research Trust Award, and the Leslie A. Geddes Endowment at Purdue University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.K. and B.T.R. conceived and designed research; K.K., B.R., T.D.L., and B.T.R. performed experiments; K.K., B.R., F.W.D., D.P.G., Q.S., C.J.G., and B.T.R. analyzed data; K.K., B.R., F.W.D., D.P.G., Q.S., C.J.G., and B.T.R. interpreted results of experiments; K.K., F.W.D., C.J.G., and B.T.R. prepared figures; K.K. and B.T.R. drafted manuscript; K.K., B.R., F.W.D., D.P.G., T.D.L., Q.S., C.J.G., and B.T.R. edited and revised manuscript; K.K., B.R., F.W.D., D.P.G., T.D.L., Q.S., C.J.G., and B.T.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Kolapo Ajuwon for his support with the indirect calorimetry measurements.
REFERENCES
- 1.Lee AJ, Price JF, Russell MJ, Smith FB, van Wijk MC, Fowkes FG. Improved prediction of fatal myocardial infarction using the ankle brachial index in addition to conventional risk factors: the Edinburgh Artery Study. Circulation 110: 3075–3080, 2004. doi: 10.1161/01.CIR.0000143102.38256.DE. [DOI] [PubMed] [Google Scholar]
- 2.Sampson UK, Fowkes FG, McDermott MM, Criqui MH, Aboyans V, Norman PE, Forouzanfar MH, Naghavi M, Song Y, Harrell FE Jr., Denenberg JO, Mensah GA, Ezzati M, Murray C. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart 9: 145–158, 2014. e121. doi: 10.1016/j.gheart.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FG, Aboyans V, Fowkes FJ, McDermott MM, Sampson UK, Criqui MH. Peripheral artery disease: epidemiology and global perspectives. Nat Rev Cardiol 14: 156–170, 2017. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 4.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O’Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R, Prevention of Progression of Arterial Disease and Diabetes Study Group; Diabetes Registry Group; Royal College of Physicians Edinburgh. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 337: a1840, 2008. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low Wang CC, Blomster JI, Heizer G, Berger JS, Baumgartner I, Fowkes FGR, Held P, Katona BG, Norgren L, Jones WS, Lopes RD, Olin JW, Rockhold FW, Mahaffey KW, Patel MR, Hiatt WR, EUCLID Trial Executive Committee ETE and Investigators. Cardiovascular and Limb Outcomes in Patients With Diabetes and Peripheral Artery Disease: The EUCLID Trial. J Am Coll Cardiol 72: 3274–3284, 2018. doi: 10.1016/j.jacc.2018.09.078. [DOI] [PubMed] [Google Scholar]
- 6.Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, Hodges JS. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care 34: 2174–2179, 2011. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. J Vasc Surg 59: 1036–1043, 2014. doi: 10.1016/j.jvs.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeRubertis BG, Pierce M, Ryer EJ, Trocciola S, Kent KC, Faries PL. Reduced primary patency rate in diabetic patients after percutaneous intervention results from more frequent presentation with limb-threatening ischemia. J Vasc Surg 47: 101–108, 2008. doi: 10.1016/j.jvs.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Malmstedt J, Leander K, Wahlberg E, Karlström L, Alfredsson L, Swedenborg J. Outcome after leg bypass surgery for critical limb ischemia is poor in patients with diabetes: a population-based cohort study. Diabetes Care 31: 887–892, 2008. doi: 10.2337/dc07-2424. [DOI] [PubMed] [Google Scholar]
- 10.Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, Hirsch AT. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 13: 15–24, 2008. doi: 10.1177/1358863X07084911. [DOI] [PubMed] [Google Scholar]
- 11.Garg PK, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Tan J, McDermott MM. Physical activity during daily life and functional decline in peripheral arterial disease. Circulation 119: 251–260, 2009. doi: 10.1161/CIRCULATIONAHA.108.791491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg PK, Tian L, Criqui MH, Liu K, Ferrucci L, Guralnik JM, Tan J, McDermott MM. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation 114: 242–248, 2006. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res 116: 1527–1539, 2015. doi: 10.1161/CIRCRESAHA.116.303566. [DOI] [PubMed] [Google Scholar]
- 14.Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD, Febbraio MA. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg 41: 802–807, 2005. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Regensteiner JG, Wolfel EE, Brass EP, Carry MR, Ringel SP, Hargarten ME, Stamm ER, Hiatt WR. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 87: 413–421, 1993. doi: 10.1161/01.CIR.87.2.413. [DOI] [PubMed] [Google Scholar]
- 16.Herman SD, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, Liao Y, McDermott MM. Baseline lower extremity strength and subsequent decline in functional performance at 6-year follow-up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc 57: 2246–2252, 2009. doi: 10.1111/j.1532-5415.2009.02562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, Liao Y, Criqui MH. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 120: 1048–1055, 2009. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott MM, Liu K, Tian L, Guralnik JM, Criqui MH, Liao Y, Ferrucci L. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol 59: 1159–1167, 2012. doi: 10.1016/j.jacc.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh N, Liu K, Tian L, Criqui MH, Guralnik JM, Ferrucci L, Liao Y, McDermott MM. Leg strength predicts mortality in men but not in women with peripheral arterial disease. J Vasc Surg 52: 624–631, 2010. doi: 10.1016/j.jvs.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akasaki Y, Miyata M, Eto H, Shirasawa T, Hamada N, Ikeda Y, Biro S, Otsuji Y, Tei C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J 70: 463–470, 2006. doi: 10.1253/circj.70.463. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Reid BA, Ro B, Casey CA, Song Q, Kuang S, Roseguini BT. Heat therapy improves soleus muscle force in a model of ischemia-induced muscle damage. J Appl Physiol 127: 215–228, 2019. doi: 10.1152/japplphysiol.00115.2019. [DOI] [PubMed] [Google Scholar]
- 22.Archer AE, Rogers RS, Von Schulze AT, Wheatley JL, Morris EM, McCoin CS, Thyfault JP, Geiger PC. Heat shock protein 72 regulates hepatic lipid accumulation. Am Physiol Regul Integr Comp Physiol 315: R696–R707, 2018. doi: 10.1152/ajpregu.00073.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung J, Nguyen AK, Henstridge DC, Holmes AG, Chan MH, Mesa JL, Lancaster GI, Southgate RJ, Bruce CR, Duffy SJ, Horvath I, Mestril R, Watt MJ, Hooper PL, Kingwell BA, Vigh L, Hevener A, Febbraio MA. HSP72 protects against obesity-induced insulin resistance. Proc Natil Acad Sci USA 105: 1739–1744, 2008. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes 58: 567–578, 2009. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia 23: 259–265, 2007. doi: 10.1080/02656730601176824. [DOI] [PubMed] [Google Scholar]
- 26.Rogers RS, Morris EM, Wheatley JL, Archer AE, McCoin CS, White KS, Wilson DR, Meers GM, Koch LG, Britton SL, Thyfault JP, Geiger PC. Deficiency in the heat stress response could underlie susceptibility to metabolic disease. Diabetes 65: 3341–3351, 2016. doi: 10.2337/db16-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiStasi MR, Mund JA, Bohlen HG, Miller SJ, Ingram DA, Dalsing MC, Unthank JL. Impaired compensation to femoral artery ligation in diet-induced obese mice is primarily mediated via suppression of collateral growth by Nox2 and p47phox. Am J Physiology Heart Circ Physiol 309: H1207–H1217, 2015. doi: 10.1152/ajpheart.00180.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan TE, Schmidt CA, Green TD, Spangenburg EE, Neufer PD, McClung JM. Targeted expression of catalase to mitochondria protects against ischemic myopathy in high-fat diet-fed mice. Diabetes 65: 2553–2568, 2016. doi: 10.2337/db16-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K, Kakigi R, Okada T, Sakurai T, Kawamori R, Watada H. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep 5: e13250, 2017. doi: 10.14814/phy2.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallis J, Hill C, James RS, Cox VM, Seebacher F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J Appl Physiol 122: 170–181, 2017. doi: 10.1152/japplphysiol.00836.2016. [DOI] [PubMed] [Google Scholar]
- 31.D’Souza DM, Trajcevski KE, Al-Sajee D, Wang DC, Thomas M, Anderson JE, Hawke TJ. Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol Rep 3: e12506, 2015. doi: 10.14814/phy2.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu X, Zhu M, Zhang S, Foretz M, Viollet B, Du M. Obesity impairs skeletal muscle regeneration through inhibition of AMPK. Diabetes 65: 188–200, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown LA, Lee DE, Patton JF, Perry RA Jr, Brown JL, Baum JI, Smith-Blair N, Greene NP, Washington TA. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol 215: 46–57, 2015. doi: 10.1111/apha.12537. [DOI] [PubMed] [Google Scholar]
- 34.Hesketh K, Shepherd SO, Strauss JA, Low DA, Cooper RJ, Wagenmakers AJM, Cocks M. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am J Physiol Heart Circ Physiol 317: H114–H123, 2019. doi: 10.1152/ajpheart.00816.2018. [DOI] [PubMed] [Google Scholar]
- 35.Kim K, Reid BA, Casey CA, Bender BE, Ro B, Song Q, Trewin AJ, Petersen AC, Kuang S, Gavin TP, Roseguini BT. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J Appl Physiol (1985) 128: 483–492, 2020. doi: 10.1152/japplphysiol.00701.2019. [DOI] [PubMed] [Google Scholar]
- 36.Hafen PS, Abbott K, Bowden J, Lopiano R, Hancock CR, Hyldahl RD. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J Appl Physiol 127: 47–57, 2019. doi: 10.1152/japplphysiol.01098.2018. [DOI] [PubMed] [Google Scholar]
- 37.Hafen PS, Preece CN, Sorensen JR, Hancock CR, Hyldahl RD. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J Appl Physiol (1985) 125: 1447–1455, 2018. doi: 10.1152/japplphysiol.00383.2018. [DOI] [PubMed] [Google Scholar]
- 38.Tamura Y, Matsunaga Y, Masuda H, Takahashi Y, Takahashi Y, Terada S, Hoshino D, Hatta H. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 307: R931–R943, 2014. doi: 10.1152/ajpregu.00525.2013. [DOI] [PubMed] [Google Scholar]
- 39.Distasi MR, Case J, Ziegler MA, Dinauer MC, Yoder MC, Haneline LS, Dalsing MC, Miller SJ, Labarrere CA, Murphy MP, Ingram DA, Unthank JL. Suppressed hindlimb perfusion in Rac2-/- and Nox2-/- mice does not result from impaired collateral growth. Am J Physiol Heart Circ Physiology 296: H877–H886, 2009. doi: 10.1152/ajpheart.00772.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoefer IE, van Royen N, Rectenwald JE, Bray EJ, Abouhamze Z, Moldawer LL, Voskuil M, Piek JJ, Buschmann IR, Ozaki CK. Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation 105: 1639–1641, 2002. doi: 10.1161/01.CIR.0000014987.32865.8E. [DOI] [PubMed] [Google Scholar]
- 41.Mees B, Wagner S, Ninci E, Tribulova S, Martin S, van Haperen R, Kostin S, Heil M, de Crom R, Schaper W. Endothelial nitric oxide synthase activity is essential for vasodilation during blood flow recovery but not for arteriogenesis. Arterioscler Thromb Vasc Biol 27: 1926–1933, 2007. [Erratum in Arterioscler Thromb Vasc Biol. 2013 Mar;33(3):e102]. doi: 10.1161/ATVBAHA.107.145375. [DOI] [PubMed] [Google Scholar]
- 42.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31: 1116–1128, 2006. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Goto A, Egawa T, Sakon I, Oshima R, Ito K, Serizawa Y, Sekine K, Tsuda S, Goto K, Hayashi T. Heat stress acutely activates insulin-independent glucose transport and 5'-AMP-activated protein kinase prior to an increase in HSP72 protein in rat skeletal muscle. Physiol Rep 3: e12601, 2015. doi: 10.14814/phy2.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyauchi T, Miyata M, Ikeda Y, Akasaki Y, Hamada N, Shirasawa T, Furusho Y, Tei C. Waon therapy upregulates Hsp90 and leads to angiogenesis through the Akt-endothelial nitric oxide synthase pathway in mouse hindlimb ischemia. Circ J 76: 1712–1721, 2012. doi: 10.1253/circj.CJ-11-0915. doi:. [DOI] [PubMed] [Google Scholar]
- 45.Nwadozi E, Rudnicki M, De Ciantis M, Milkovich S, Pulbere A, Roudier E, Birot O, Gustafsson T, Ellis CG, Haas TL. High-fat diet pre-conditioning improves microvascular remodelling during regeneration of ischaemic mouse skeletal muscle. Acta Physiol 229: e13449, 2020. doi: 10.1111/apha.13449. [DOI] [PubMed] [Google Scholar]
- 46.Thomas MM, Trajcevski KE, Coleman SK, Jiang M, Di Michele J, O’Neill HM, Lally JS, Steinberg GR, Hawke TJ. Early oxidative shifts in mouse skeletal muscle morphology with high-fat diet consumption do not lead to functional improvements. Physiol Rep 2: e12149, 2014. doi: 10.14814/phy2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, Akingba AG, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation 17: 3–20, 2010. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts KC, Nixon C, Unthank JL, Lash JM. Femoral artery ligation stimulates capillary growth and limits training-induced increases in oxidative capacity in rats. Microcirculation 4: 253–260, 1997. doi: 10.3109/10739689709146788. [DOI] [PubMed] [Google Scholar]
- 49.Arpino JM, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, Balint B, O’Neil C, Fraser GM, Goldman D, Ellis CG, Pickering JG. Four-dimensional microvascular analysis reveals that regenerative angiogenesis in ischemic muscle produces a flawed microcirculation. Circ Res 120: 1453–1465, 2017. doi: 10.1161/CIRCRESAHA.116.310535. [DOI] [PubMed] [Google Scholar]
- 50.Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. ATVB 26: 520–526, 2006. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- 51.Eshima H, Tamura Y, Kakehi S, Kakigi R, Hashimoto R, Funai K, Kawamori R, Watada H. A chronic high-fat diet exacerbates contractile dysfunction with impaired intracellular Ca(2+) release capacity in the skeletal muscle of aged mice. J Appl Physiol 128: 1153–1162, 2020. doi: 10.1152/japplphysiol.00530.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geiger PC, Gupte AA. Heat shock proteins are important mediators of skeletal muscle insulin sensitivity. Exerc Sport Sci Rev 39: 34–42, 2011. doi: 10.1097/JES.0b013e318201f236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris MB, Blackstone MA, Ju H, Venema VJ, Venema RC. Heat-induced increases in endothelial NO synthase expression and activity and endothelial NO release. Am J Physiol Heart Circ Physiol 285: H333–H340, 2003. doi: 10.1152/ajpheart.00726.2002. [DOI] [PubMed] [Google Scholar]
- 54.Ives SJ, Andtbacka RH, Kwon SH, Shiu YT, Ruan T, Noyes RD, Zhang QJ, Symons JD, Richardson RS. Heat and alpha1-adrenergic responsiveness in human skeletal muscle feed arteries: the role of nitric oxide. J Appl Physiol 113: 1690–1698, 2012. doi: 10.1152/japplphysiol.00955.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikeda Y, Biro S, Kamogawa Y, Yoshifuku S, Eto H, Orihara K, Kihara T, Tei C. Repeated thermal therapy upregulates arterial endothelial nitric oxide synthase expression in Syrian golden hamsters. Jpn Circ J 65: 434–438, 2001. doi: 10.1253/jcj.65.434. [DOI] [PubMed] [Google Scholar]
- 56.Frisbee JC. Reduced nitric oxide bioavailability contributes to skeletal muscle microvessel rarefaction in the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 289: R307–R316, 2005. doi: 10.1152/ajpregu.00114.2005. [DOI] [PubMed] [Google Scholar]
- 57.Yan J, Tie G, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg 50: 1412–1422, 2009. doi: 10.1016/j.jvs.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoefer IE, van Royen N, Jost MM. Experimental models of arteriogenesis: differences and implications. Lab Anim 35: 36–44, 2006. . doi: 10.1038/laban0206-36. [DOI] [PubMed] [Google Scholar]
- 59.Carter HH, Spence AL, Atkinson CL, Pugh CJ, Naylor LH, Green DJ. Repeated core temperature elevation induces conduit artery adaptation in humans. Eur J Appl Physiol 114: 859–865, 2014. doi: 10.1007/s00421-013-2817-2. [DOI] [PubMed] [Google Scholar]
- 60.Green DJ, Carter HH, Fitzsimons MG, Cable NT, Thijssen DH, Naylor LH. Obligatory role of hyperaemia and shear stress in microvascular adaptation to repeated heating in humans. J Physiol 588: 1571–1577, 2010. doi: 10.1113/jphysiol.2010.186965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naylor LH, Carter H, FitzSimons MG, Cable NT, Thijssen DH, Green DJ. Repeated increases in blood flow, independent of exercise, enhance conduit artery vasodilator function in humans. Am J Physiol Heart Circ Physiol 300: H664–H669, 2011. doi: 10.1152/ajpheart.00985.2010. [DOI] [PubMed] [Google Scholar]
- 62.Schaper W. Collateral circulation: past and present. Basic Res Cardiol 104: 5–21, 2009. doi: 10.1007/s00395-008-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang HT, Laughlin MH, Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol Heart Circ Physiol 279: H1890–1897, 2000. doi: 10.1152/ajpheart.2000.279.4.H1890. [DOI] [PubMed] [Google Scholar]
- 64.Colleran PN, Li Z, Yang HT, Laughlin MH, Terjung RL. Vasoresponsiveness of collateral vessels in the rat hindlimb: influence of training. J Physiol 588: 1293–1307, 2010. . doi: 10.1113/jphysiol.2009.186247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mohiuddin M, Lee NH, Moon JY, Han WM, Anderson SE, Choi JJ, Shin E, Nakhai SA, Tran T, Aliya B, Kim DY, Gerold A, Hansen LM, Taylor WR, Jang YC. Critical Limb Ischemia Induces Remodeling of Skeletal Muscle Motor Unit. Sci Rep 9: 9551, 2019. doi: 10.1038/s41598-019-45923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johansen LB, Jensen TU, Pump B, Norsk P. Contribution of abdomen and legs to central blood volume expansion in humans during immersion. J Appl Physiol (1985) 83: 695–699, 1997. doi: 10.1152/jappl.1997.83.3.695. [DOI] [PubMed] [Google Scholar]
- 67.Miyata S, Koyama Y, Takemoto K, Yoshikawa K, Ishikawa T, Taniguchi M, Inoue K, Aoki M, Hori O, Katayama T, Tohyama M. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS One 6: e19859, 2011. doi: 10.1371/journal.pone.0019859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci 20: 1568–1574, 2000. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. Am J Physiol Heart Circ Physiol 316: H1495–H1506, 2019. doi: 10.1152/ajpheart.00151.2019. [DOI] [PubMed] [Google Scholar]
- 70.Pellinger TK, Neighbors CB, Simmons GH. Acute lower leg heating increases exercise capacity in patients with peripheral artery disease. J Cardiovasc Nurs 34: 130–133, 2019. doi: 10.1097/JCN.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 71.Shinsato T, Miyata M, Kubozono T, Ikeda Y, Fujita S, Kuwahata S, Akasaki Y, Hamasaki S, Fujiwara H, Tei C. Waon therapy mobilizes CD34+ cells and improves peripheral arterial disease. J Cardiol 56: 361–366, 2010. doi: 10.1016/j.jjcc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Tei C, Shinsato T, Kihara T, Miyata M. Successful thermal therapy for end-stage peripheral artery disease. J Cardiology 47: 163–164, 2006. ] [PubMed] [Google Scholar]
- 73.Tei C, Shinsato T, Miyata M, Kihara T, Hamasaki S. Waon therapy improves peripheral arterial disease. J Am Coll Cardiol 50: 2169–2171, 2007. doi: 10.1016/j.jacc.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 74.Oishi Y, Hayashida M, Tsukiashi S, Taniguchi K, Kami K, Roy RR, Ohira Y. Heat stress increases myonuclear number and fiber size via satellite cell activation in rat regenerating soleus fibers. J Appl Physiol (1985) 107: 1612–1621, 2009. doi: 10.1152/japplphysiol.91651.2008. [DOI] [PubMed] [Google Scholar]
- 75.Shibaguchi T, Hoshi M, Yoshihara T, Naito H, Goto K, Yoshioka T, Sugiura T. Impact of different temperature stimuli on the expression of myosin heavy chain isoforms during recovery from bupivacaine-induced muscle injury in rats. J Appl Physiol 127: 178–189, 2019. doi: 10.1152/japplphysiol.00930.2018. [DOI] [PubMed] [Google Scholar]
- 76.Ohira T, Higashibata A, Seki M, Kurata Y, Kimura Y, Hirano H, Kusakari Y, Minamisawa S, Kudo T, Takahashi S, Ohira Y, Furukawa S. The effects of heat stress on morphological properties and intracellular signaling of denervated and intact soleus muscles in rats. Physiol Rep 5: e13350, 2017. doi: 10.14814/phy2.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selsby JT, Dodd SL. Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289: R134–R139, 2005. doi: 10.1152/ajpregu.00497.2004. [DOI] [PubMed] [Google Scholar]
- 78.Tamura Y, Kitaoka Y, Matsunaga Y, Hoshino D, Hatta H. Daily heat stress treatment rescues denervation-activated mitochondrial clearance and atrophy in skeletal muscle. J Physiol 593: 2707–2720, 2015. doi: 10.1113/JP270093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koutakis P, Myers SA, Cluff K, Ha DM, Haynatzki G, McComb RD, Uchida K, Miserlis D, Papoutsi E, Johanning JM, Casale GP, Pipinos II. Abnormal myofiber morphology and limb dysfunction in claudication. J Surg Res 196: 172–179, 2015. doi: 10.1016/j.jss.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]