Keywords: chronic renal insufficiency, exercise tolerance, exertional fatigue
Abstract
Exertional fatigue, defined as the overwhelming and debilitating sense of sustained exhaustion that impacts the ability to perform activities of daily living, is highly prevalent in chronic kidney disease (CKD) and end-stage renal disease (ESRD). Subjective reports of exertional fatigue are paralleled by objective measurements of exercise intolerance throughout the spectrum of the disease. The prevalence of exercise intolerance is clinically noteworthy, as it leads to increased frailty, worsened quality of life, and an increased risk of mortality. The physiological underpinnings of exercise intolerance are multifaceted and still not fully understood. This review aims to provide a comprehensive outline of the potential physiological contributors, both central and peripheral, to kidney disease-related exercise intolerance and highlight current and prospective interventions to target this symptom. In this review, the CKD-related metabolic derangements, cardiac and pulmonary dysfunction, altered physiological responses to oxygen consumption, vascular derangements, and sarcopenia are discussed in the context of exercise intolerance. Lifestyle interventions to improve exertional fatigue, such as aerobic and resistance exercise training, are discussed, and the lack of dietary interventions to improve exercise tolerance is highlighted. Current and prospective pharmaceutical and nutraceutical strategies to improve exertional fatigue are also broached. An extensive understanding of the pathophysiological mechanisms of exercise intolerance will allow for the development of more targeted therapeutic approached to improve exertional fatigue and health-related quality of life in CKD and ESRD.
INTRODUCTION
Defined as an overwhelming and debilitating sense of sustained exhaustion that impacts the ability to perform activities of daily living, chronic exertional fatigue is one of the most common reported symptoms of chronic kidney disease (CKD) and end-stage renal disease (ESRD) (1, 2). Fatigue becomes apparent in the early stages of CKD, increasing in prevalence and severity as the disease progresses, with up to 97% of patients with ESRD reporting this symptom (1, 2). The prevalence of CKD-related fatigue is noteworthy, as it has significant implications for increased frailty and worsened health-related quality of life (3–5). In addition, fatigue has been reported to be a predictor of CKD-related cardiovascular disease and is associated with a decreased likelihood of renal transplantation and an increased risk of mortality (6, 7).
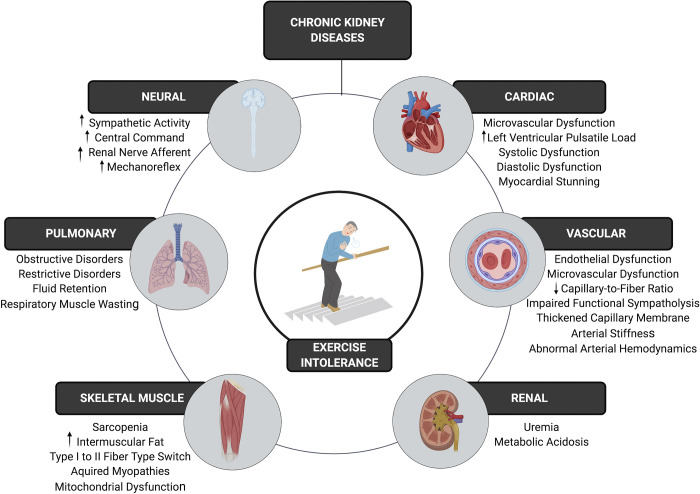
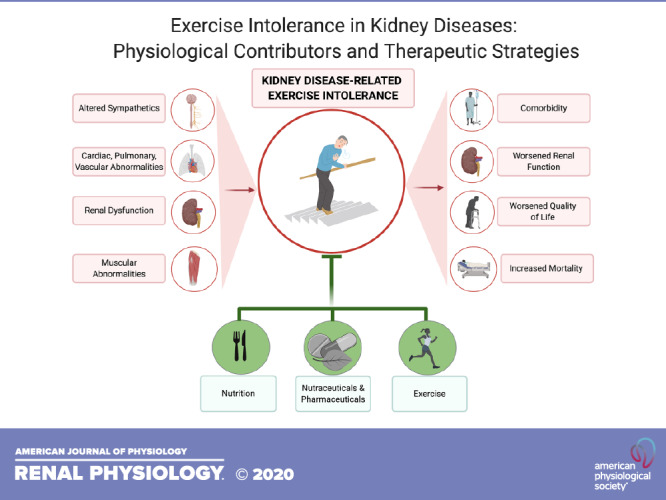
Fatigue is a multidimensional symptom composed of numerous subtypes (8). One subtype of fatigue is exertional fatigue, which represents an increased sense of effort in relation to a task (8). Although fatigue is traditionally considered as a subjective patient-reported symptom, the incidence of exertional fatigue is reflected by substantial decrements in objective measures of exercise capacity and physical function. These objective measures of exertional fatigue are commonly coined as exercise intolerance and are consistently reported in these patients across the spectrum of the disease (5, 9). The reductions in exercise tolerance are evident in mild-moderate CKD, progressively worsen as disease severity increases, and are independently associated with comorbidity, decreased kidney function, decreased quality of life, and mortality (9–13). Despite its substantial prevalence, the underlying multifaceted pathophysiological mechanisms of CKD-related exercise intolerance are not fully understood. With Kidney Disease Outcomes Quality Initiative (KDOQI) clinical guideline recommendations aimed at prioritizing patient-reported quality of life (14), a better understanding of these mechanisms is critical in developing targeted approaches to address exercise intolerance, toward improving quality of life and health-related outcomes in these patients. The purpose of this review is to provide a comprehensive outline of the potential physiological contributors to CKD-related exertional fatigue (Fig. 1) and to highlight current and prospective interventions targeting this symptom.
Figure 1.
The multifaceted pathophysiological mechanisms of kidney disease-related exercise intolerance.
THE OXYGEN TRANSPORT CHAIN: A FRAMEWORK FOR IDENTIFYING DISEASE-RELATED CONTRIBUTIONS TO EXERCISE INTOLERANCE
At rest, the metabolic processes of the human body are met primarily through aerobic metabolism. During activity, an increase in oxygen consumption (V̇o2) is required to meet the metabolic demand of the working muscle. At the beginning of physical activity, an immediate increase in pulmonary blood flow results in a rapid rise in the partial pressure of arterial oxygen. Increasing V̇o2 is then largely dependent on the rate and amount of oxygen delivered to exercising muscle, a function of cardiac output, the oxygen-carrying capacity of the blood, and vascular convective transport (15). Once oxygen is delivered to the working muscle, V̇o2 is reliant on oxygen utilization properties of the skeletal muscle including muscle quantity and quality, fiber type distribution, diffusion distance, and mitochondrial volume (16, 17). At the onset of exercise, there is a single-exponential rise in V̇o2, which is followed by a steady-state condition that is maintained as long as the activity intensity levels remain light to moderate, thereby allowing aerobic metabolism to effectively match energy demand (18). However, high-intensity exercise is severely limited by the capacity of aerobic metabolism and will often result in V̇o2 rising to its most tolerable limit due to symptom-related fatigue, which causes an individual to stop activity.
In a clinical setting, the oxygen transport chain can be comprehensively assessed with a maximal effort, symptom-limited cardiopulmonary exercise test (CPET). This graded exercise test provides breath-by-breath gas exchange measures while progressively increasing exercise intensity in a linear function over time to maximally stress the oxygen delivery and utilization system. The CPET has established itself as a useful clinical tool that reflects organ-specific maladaptive physiological responses that potentially limit exercise tolerance (19). In this respect, CPET has been utilized to establish the presence of exercise intolerance early in CKD, uncovering subclinical central and peripheral abnormalities that may contribute to the progression and development of exertional fatigue over the spectrum of the disease (9, 20).
CENTRAL CONTRIBUTIONS TO EXERCISE INTOLERANCE IN KIDNEY DISEASES
Pulmonary Limitations
Recent data suggest that individuals with low estimated glomerular filtration rate have a higher likelihood of developing lung diseases, especially those with restrictive characteristics (21). Similarly, a recent report described that up to 80% of patients undergoing hemodialysis had undiagnosed chronic obstructive pulmonary disease (22). It is therefore unsurprising that dyspnea is a common symptom reported by patients with CKD, severely impacting quality of life and predicting the risk of hospitalization (23, 24).
Among the different possibilities that may link lungs and kidneys, systemic inflammation in CKD plays an important role in the observed reduced lung function indices (25). Inflammation contributes to pulmonary congestion through a higher permeability of the alveolar-capillary membrane as well as an increase in pulmonary capillary hydrostatic pressure (26, 27). The recurrent inflammatory response also contributes to chronic and progressive damage to the pulmonary alveolar-capillary membrane, helping the deposition of collagen in the airways, reducing the diffusing capacity, and contributing to the development of hypoxia (28, 29). In addition, inflammation contributes to protein energy-wasting syndrome and muscle wasting in CKD, reducing muscle strength not only in the periphery but also in the respiratory muscles, thereby impacting lung function (30–32). The resultant heightened energy demand from the intercostal muscles during exertion could result in a blood flow shunt away from the ambulatory skeletal muscle and toward the lungs (33), thereby reducing oxygen delivery to the working muscle and inducing an earlier onset of fatigue. In the latter stages of the disease, fluid volume overload plays a leading role in both obstructive and restrictive pulmonary abnormalities in these patients (34).
Underlying pulmonary dysfunction also contributes to other lung-related disorders and adverse outcomes in CKD (21, 35). For example, fluid overload and venous congestion associated with right ventricular dysfunction lead to the development of pulmonary hypertension that could ultimately limit gaseous exchange at the alveoli (36). In addition, in renal failure, a reduced expression of pulmonary salt-water transporters such as epithelial Na+ channel (ENaC), Na+-K+-ATPase, and pulmonary aquaporin channels contributes to pulmonary edema that subsequently limits mechanical ventilation and augments small airway dysfunction (37, 38). Local alveoli hypoxia, a well-known contributor to the progression of sleep apnea and chronic obstructive pulmonary disease (COPD), plays a key role in worsening hypoxemia that contributes to the pathogenesis and/or progression of CKD (22, 39, 40). Indeed, underlying hypoxia can exacerbate inflammation and oxidative stress contributing to the development and worsening of proteinuria (41).
Despite these manifestations, very little is known about the exact involvement of lung dysfunction in CKD-related exercise intolerance, and thus, the pulmonary involvement in their dyspnea may be underestimated. Recent data in ESRD patients show that absolute ventilation during exercise is significantly reduced, demonstrating an inability to increase ventilation in response to activity (37). Future studies are warranted to investigate the involvement of lung function in exercise intolerance in kidney diseases.
Cardiac Limitations
Due to the highly nuanced relationship between the heart and kidney, chronic renal dysfunction may induce abnormalities in cardiac structure and function (42). This cardiorenal syndrome is predominantly driven by aberrant neurohumoral, profibrotic, and inflammatory pathways (42, 43). In this respect, IL-1 has recently been implicated as an important mediator of inflammation in nondialysis CKD patients with concurrent heart failure (43). Later in the disease, the repeated hemodynamic challenges of hemodialysis induce global and regional cardiac ischemia, resulting in myocardial stunning (44). Hemodialysis-induced myocardial stunning is highly prevalent, affecting two-thirds of dialysis patients. Myocardial stunning is transient, but when consistently repeated during dialysis, it leads to myocardial hibernation that contributes substantially to myocardial injury and the subsequent development of heart failure (45). As a result, patients with ESRD have elevated risk for developing heart failure (46). Although heart failure with reduced ejection fraction (HFrEF) is highly prevalent in these patients, there is an equally large proportion of patients with heart failure with preserved ejection faction (HFpEF) (46). In HFrEF, the reduction in cardiac output poses a substantial limitation to exercise capacity by hampering oxygen delivery (19). However, in the presence of a normal or preserved ejection fraction, aberrant systolic and diastolic function still play a role in exercise intolerance (19). In this respect, left ventricular global longitudinal strain (GLS) is often utilized to assess variation in systolic function and can detect deviations in contractility and abnormalities due to fibrosis and ischemia while ejection fraction may be preserved. In CKD patients, those with an impaired GLS have reported a lower exercise capacity (47). Similarly, diastolic dysfunction, a signature characteristic of HFpEF, is associated with a lower 6-min walk test distance, reduced CPET exercise time, and a lower peak V̇o2 (V̇o2peak) (47, 48).
Anemia and Iron Deficiencies
Anemia is a common complication of CKD. The causes of CKD-related anemia are multifaceted but are predominantly driven by a relative deficiency of erythropoietin (EPO) production from the kidneys (49). Despite normal serum levels of EPO, the levels are inappropriately low relative to the degree of anemia (49). Anemia is consistently associated with reduced physical function and exercise capacity across the spectrum of CKD and contributes to exercise intolerance by lowering the oxygen-carrying capacity (20, 50). During exercise, typical physiological compensations for anemia include increased cardiac output and/or peripheral oxygen extraction. However, patients with CKD fail to use these compensatory mechanisms, even during low-intensity activities (20). This confirms that anemia is not the sole limiting factor of exercise capacity in these patients and that other mechanisms need to be addressed.
Iron deficiency is also prevalent across the spectrum of kidney disease (51, 52). Chronic inflammation and reduced renal clearance both contribute to increased levels of hepcidin, a key regulator of iron metabolism that inhibits iron absorption form the gut (53). The resultant iron deficiencies impair iron-dependent erythropoiesis, which when severe, leads to iron-deficient anemia (53). Iron deficiencies are detrimental to exercise capacity, as not only do they contribute to reduced iron levels in hemoglobin, thus reducing the oxygen-carrying capacity, but iron also accounts for a large structural and functional component of mitochondrial electron transport chain enzymes (54). Therefore, iron deficiencies may also play a key role in reducing the oxidative capacity of both cardiac and skeletal muscle. Although the link between iron deficiencies and exercise intolerance is more established in the population with heart failure (55, 56), further evidence in patients with CKD and ESRD is warranted.
Fibroblast growth factor 23 (FGF-23), a bone-derived hormone central to vitamin D, calcium, and phosphate balance, is substantially elevated in CKD and has recently been implicated in the development of anemia and iron deficiencies by inhibiting EPO production, enhancing erythrocyte apoptosis, and increasing inflammation-induced hepcidin production (57). Although an abnormal vitamin D status has been linked with worsened physical function and frailty in CKD (5), the potential of FGF-23 as a contributor to intolerance and a possible treatment target to improve exercise capacity is yet to be established.
Elevated Sympathetic Nerve Activity and Abnormal Neurocirculatory Control
The onset and continuance of exercise elicits both a robust activation of the sympathetic nervous system (SNS) to augment cardiac output and vasoconstrict nonessential tissues, and a simultaneous blunting of this SNS response, via local factors at the active tissue site, to effectively conduct oxygen and nutrient-rich blood toward where demand is greatest. In individuals with CKD, a major contribution to a mismatch of oxygen supply and demand is the exacerbation of the exercise pressor reflex (EPR), a sympathoexcitation pathway that combines the input of muscle mechanoreceptors, metaboreceptors, and central command. Elegant work by Park et al. (58) used pharmacological normalization of blood pressure during exercise, revealing significant increases in muscle sympathetic nerve activity (MSNA), a measure of SNS activation. It was further revealed that the mechanoreceptors, and not the metaboreceptors, act as the primary contributor to the increased EPR response (58). This augmented EPR response is perhaps due to CKD-induced increases in circulating oxidative stress (59) and/or angiotensin II (60). A sensitization of vascular α1-adrenergic receptors that contributes to an overall increased blood pressure reactivity may also contribute to this exaggerated EPR (61). One major consequence of an augmented EPR, as it relates to exercise tolerance in CKD, is a reduction in functional sympatholysis, or the ability of the local molecules and metabolites to override or blunt SNS activation of the resistance vessels at the working muscle. Indeed, both patients with CKD and those with ESRD reveal impaired functional sympatholysis during exercise (62, 63).
Renal Dysfunction
An additional contributor to the sympathoexcitatory state of CKD is an increase in renal sympathetic activity that is mediated by an increased afferent firing from the failing kidney (64). It has been suggested that an intrarenal accumulation of uremic toxins stimulate renal afferent nerves (65). The potential role of an increased renal afferent on exercise tolerance in CKD is well demonstrated in a study by Converse et al. (65) that compared healthy controls and hemodialysis patients with and without a bilateral nephrectomy. This study reported that those with native kidneys had significantly increased sympathetic discharge, as measured by MSNA, that consequently increased peripheral vascular resistance and reduced skeletal muscle blood flow. In comparison, patients who had undergone a bilateral nephrectomy had markedly reduced rates of sympathetic discharge directed to the skeletal muscle vasculature that were similar to the healthy controls. In support of this concept, renal denervation in resistant hypertensives has been shown to reduce the blood pressure response to exercise, improve autonomic function, and increase exercise capacity as determined by the amount of work performed in a CPET (66).
A further consequence of impaired renal function that impacts exercise tolerance is metabolic acidosis. Due to renal deficits in acid excretion, metabolic acidosis is present in CKD. Lower levels of serum bicarbonate have been associated with physical function limitations in CKD (67). In addition, patients with CKD with low serum HCO3 levels report an earlier onset of fatigue during submaximal exercise in comparison with patients that have normal serum HCO3 (63). The mechanisms of metabolic acidosis-related exercise intolerance in CKD are still not fully understood. As metabolic acidosis plays a central role in skeletal muscle wasting, it is hypothesized that the resultant reductions in muscle mass and strength limit functional capacity (68). Alternatively, patients with metabolic acidosis have an exacerbated blood pressure response and an increased heart rate response to submaximal exercise (63). It is possible that metabolic acidosis sensitizes the exercise pressor response in these patients, thereby posing a central limitation to exercise capacity (63). Bicarbonate supplementation trials in CKD have shown equivocal findings with respect to improvements in functional capacity (69, 70), thus highlighting the need for further investigations in this area.
Vascular Dysfunction
Vascular endothelial dysfunction, characterized by a reduction in nitric oxide (NO) production and bioavailability, is consistently reported across all stages of CKD (71). The mechanisms of CKD-related vascular endothelial dysfunction appear to evolve throughout the progression of the disease. In the earlier stages of the disease, increased levels of asymmetric dimethyl arginase (that competes with the NO precursor l-arginine) and oxidative stress (72, 73) are significant contributors to endothelial dysfunction. In this respect, local infusion of l-arginine, ascorbic acid, and mitochondria-targeted antioxidants improve microvascular function in moderate CKD (73, 74). However, as uremic toxicity increases with the progression of the disease, transport of the NO precursor l-arginine into the endothelial cell is suggested to be the rate-limiting step in NO production (71, 75). As the endothelium is integral in regulating vascular tone, current work in the field is aimed at investigating whether CKD-related endothelial dysfunction limits skeletal muscle blood flow during exercise, thereby contributing to decreased exercise tolerance. In this respect, endothelial dysfunction assessed by brachial artery flow-mediated dilation has been shown to be associated with reduced V̇o2peak values as well as the exaggerated blood pressure response to exercise observed in patients with mild-to-moderate CKD (76).
Aberrant ventricular-arterial coupling due to abnormal arterial hemodynamics is consistently reported across the spectrum of CKD (77–79). At every heartbeat, the left ventricle (LV) generates a forward traveling pulse wave. At sites of impedance mismatch such as a vascular bifurcation or a change in wall diameter (e.g., the narrowing of the conduit artery down to the microvasculature), the forward traveling pulse wave is partially reflected, thus contributing to a reflected waveform (80). The reflected waveform typically arrives back at the heart during diastole and assists coronary perfusion (80). However, the timing and the magnitude of the reflected waveform are adversely affected by arterial stiffness and impedance mismatch due to impaired exercise-induced vasodilation (81) that are both characteristic of CKD (82, 83). Thus, in patients with CKD, a larger and faster traveling reflected waveform will arrive at the heart during late systole, increasing the LV pulsatile load (82). During exercise the arrival of a larger reflected waveform to the heart during late systole increases the cardiac afterload and hampers myocardial perfusion, ultimately increasing the myocardial workload (81). The role of abnormal ventricular-arterial coupling in exercise intolerance is more established in heart failure (19), with studies in CKD still warranted. However, as heart failure is the leading CKD-related cardiovascular disease, it is reasonable to infer that this is applicable to a large proportion of these patients. In addition, several studies in the mild-moderate CKD cohort have shown negative associations between arterial stiffness and V̇o2peak (11, 84).
PERIPHERAL CONTRIBUTIONS TO EXERTIONAL FATIGUE IN KIDNEY DISEASES
Skeletal Muscle Abnormalities
Early work in the field of CKD-related exercise tolerance demonstrated that, in comparison with central contributors, reductions in skeletal muscle strength are stronger predictors of exercise capacity in this patient population (85). This work supported a paradigm shift toward impaired skeletal muscle function being the predominant determinant of CKD-related exercise intolerance. In this respect, skeletal muscle biopsies from patients with CKD show that oxidative type I fibers are significantly atrophied (86). Therefore, the reliance on glycolytic type II fibers is increased during activity, explaining why these patients have a greater lactate accumulation and a dependence on anaerobic metabolism to complete activities of daily living compared with matched controls (20). In addition, myopathies in the form of muscle fiber splitting, nuclear knots, and fat infiltration (87), as well as a reduced capillary-to-fiber ratio (86) have been reported in this cohort and could significantly contribute to declines in strength. Aside from skeletal muscle structural abnormalities, peripheral neuropathies that are characteristic of CKD impair muscle motor neuron recruitment, resulting in reduced force production. Furthermore, in patients with ESRD, an increased accumulation of metabolic byproducts during activity results in a central activation failure of locomotor muscles (88).
An intriguing study by Macdonald et al. (20) compared a group of patients with CKD and age-matched healthy controls who performed submaximal exercise of intensities comparable with activities of daily living. Despite having significantly lower hemoglobin levels, the patients with CKD failed to compensate for anemia by increasing peripheral oxygen extraction (20). Interestingly, the patients with CKD also presented with significantly higher oxygen saturation levels. Therefore, these patients demonstrated a leftward shift in the oxyhemoglobin dissociation curve that is indicative of impairments in peripheral oxygen extraction and utilization (20). With regard to oxygen extraction, the ability to diffuse oxygen from the capillary to the mitochondria is reduced in ESRD (89). Electron micrographs from ESRD quadricep biopsies show a thickened capillary endothelium and basement membrane adjacent to the skeletal muscle, likely due to inflammation or a build up of waste products, which could explain this reduced tissue diffusion capacity (89). Impairments in oxygen utilization could be due to skeletal muscle mitochondrial dysfunction. Recently, abnormalities in mitochondrial structure and function have been reported across the spectrum of CKD (90–93). Dysfunction of skeletal muscle mitochondrial energetics is associated with poor functional capacity throughout the disease (91, 92). However, in patients with mild CKD, abnormal mitochondrial bioenergetics are apparent even before declines in physical function are evident (94). This suggests that mitochondrial dysfunction may be a precursor to a decline in functional capacity, thus highlighting the mitochondria as a therapeutic target to mitigate exercise intolerance early in the disease process. The pleotropic causes of CKD-related mitochondrial dysfunction have not yet been fully elucidated; however, oxidative stress, inflammation, and uremia have all been implicated as key players (90, 91, 95, 96).
Sarcopenia
Patients with CKD often present body composition abnormalities (97), which may further contribute to the already limited exercise capacity, worsening overall clinical status and related outcomes. In addition to the muscle abnormalities previously described in Skeletal Muscle Abnormalities, muscular strength and the absolute amount of lean mass is markedly impaired, particularly in patients with ESRD (98). Such a condition is known as sarcopenia, and as opposed to cachexia, unintentional weight loss is not a requirement for diagnosis (99). Sarcopenia is highly prevalent in patients with CKD, with a variable prevalence between 11% and 28% in those not on dialysis, and in those with more advanced stages of CKD, the prevalence of sarcopenia can reach >65% (100, 101). The causes of sarcopenia in these patients are multifaceted and include anemia, metabolic acidosis, abnormal growth hormone axis and android deficiencies, insulin resistance, inflammation, the hemodialysis process, and disuse (98). In addition to sarcopenia, patients can also present excess adiposity that impairs health (i.e., obesity). This condition is defined as sarcopenic obesity and has been associated with further impairments in exercise capacity than sarcopenia and obesity individually (102). In this respect, recent magnetic resonance images from Gamboa et al. (26) showed significantly increased intermuscular fat (IMF) content in patients with CKD and ESRD compared with individuals without CKD. Interestingly, IMF was inversely correlated with skeletal muscle mitochondrial oxidative capacity, suggesting a role for IMF in mitochondrial myopathy and reduced exercise capacity in these patients (91). CKD-related sarcopenia is consistently associated with reduced exercise capacity and is therefore an integral treatment target to improve functional status in this patient population (103, 104).
IMPROVING EXERCISE TOLERANCE ACROSS THE SPECTRUM OF KIDNEY DISEASES: PROVEN AND PROSPECTIVE INTERVENTIONS
Lifestyle Interventions
Exercise training.
Several decades of research in the chronic renal insufficiency cohort have proven a myriad of health-related benefits following exercise training. With regard to exercise tolerance, aerobic exercise training, for the most part, is efficacious at increasing V̇o2peak (105). For patients with ESRD participating in intradialytic exercise, combined resistance and aerobic training appears to be slightly more efficacious at improving V̇o2peak (106). However, despite significant exercise-related increases in V̇o2peak, these increases are often minimal, falling below the threshold for a clinically meaningful change of 2.8 mL/kg/min (105, 107, 108), and values still remain below age-matched sedentary individuals (9, 109). Furthermore, the improvements in exercise capacity are attenuated in comparison with the training responses observed in matched individuals without CKD (89, 109). This trend is also consistently observed following resistance training interventions in ESRD, where significant increases in muscle mass following training fail to translate into functional improvements in strength and endurance (98, 110–113). In addition, exercise training does not appear to have any effect on habitual physical activity levels in CKD (109, 114). These attenuated training responses are perplexing, but they confirm that we still do not yet have a fully comprehensive understanding on the limitations to exercise capacity and training adaptations in these patients. As a result of these blunted training adaptations alternative exercise interventions are currently being explored and novel, personalized exercise prescriptions as part of a comprehensive lifestyle have been proposed (115). Intradialytic blood flow restricted exercise is one such intervention that has been proposed due to its beneficial effects on exercise capacity and physical function in deconditioned populations (116). Although concerns regarding an augmented pressor reflex during this mode of exercise have been raised (117), limited research has shown that this type of exercise during dialysis has been shown to be hemodynamically safe and tolerable. Future studies are warranted to establish efficacy with regard to improving exercise capacity (116). In addition, numerous dietary, nutra, and pharmaceutical strategies exist to improve various aspects of the oxygen transport chain in disease populations. It is possible that combining exercise training with dietary, nutraceutical, and/or pharmacological strategies that target the disease-related derangements in the oxygen transport chain may result in more pronounced physiological adaptations to exercise in patients with CKD and ESRD. These are areas that warrants future investigation.
Given the role that kidney dysfunction itself may play in exercise intolerance, there has been some debate as to whether exercise training may be detrimental to kidney function in patients without dialysis. This notion is based on reports of acute renal damage associated with large blood flow shunting away from the kidney during exercise in combination with muscle damage associated inflammation (118–120). However, acute kidney injury directly associated with exercise is only evident following extreme bouts of exercise such as marathon running (121, 122) or when muscle damage-inducing exercise is performed in hot environments, accompanied by dehydration and/or consumption of high fructose, caffeinated beverages (118, 121, 123, 124). In patients with CKD, regular exercise training does not appear to worsen estimated glomerular filtration rate (eGFR), and a recent meta-analyses concluded that exercise training did not aggravate proteinuria. The effects of exercise training on kidney function in CKD are still largely unknown. If future trials demonstrate that exercise is efficacious at improving kidney function, this may be an attractive strategy to improve fluid balance, reduce metabolic acidosis, and reduce renal sympathetic activity (64), all of which contribute to exercise intolerance.
Diet.
The role of diet in CKD has received intense scrutiny over the past 3 decades. In the earlier stages of CKD, patients are prescribed a low-protein diet to reduce the buildup of urea and subsequent uremic toxicity (125). Dietary proteins are sources of nitrogen (16%), making them the major target of dietary interventions to slow the progression of CKD and potentially clinical outcomes (126–128). In healthy individuals, the recommended dietary allowance (RDA) is 0.8 g/kg/day to minimize the risk for body nitrogen losses, with most Americans consuming a significantly larger amount than recommended (129); therefore, dietary interventions providing a protein intake below the RDA would be considered a low-protein diet and are prescribed for individuals with CKD.
In a systematic review of 17 studies including 2,996 individuals with CKD, low-protein diets were associated with a lower risk of worsening renal function in those with CKD (130). Importantly, the study did not find a benefit on survival in the low-protein intake group (130). Recent observational studies also suggest that the quality, and particularly the source of dietary protein favoring plant-based protein, could also provide protective effects on survival in patients with CKD (131).
On the other hand, low-protein diets have been associated with a greater risk for negative nitrogen balance (132), suggesting the presence of a catabolic state, ultimately resulting in reduced lean mass, increasing the risk for sarcopenia. In the Modification of Diet in Renal Disease Study, low protein (0.58 g/kg/day) and very low-protein [0.28 g/kg/day supplemented with ketoacids (dietary supplements containing amino acids without nitrogen)] were feasible and safe for a mean duration of 2.2 years (133). However, the groups receiving low and very-low protein intake dietary recommendations also reduced their caloric intake and tended to present a greater decline in nutritional status, which could, in turn, increase the risk for sarcopenia and overall malnutrition (133). For such reasons, exercise training, particularly resistance training, is recommended in patients with CKD following a low-protein diet due its beneficial effects on preserving lean mass (97). A landmark trial published 20 years ago found that in patients with CKD on a low-protein diet that added resistance training for 12 wk improved the amount of skeletal muscle mass and its related strength (134).
Of note, none of these discussed studies investigated the effects of protein intake on exercise capacity, highlighting the urgent need for dedicated clinical trials. Furthermore, most larger studies investigating the effects of protein intake on slowing the progression of CKD were published decades ago, in which optimal medical therapy was clearly less effective to the current available therapeutic options (135).
With regard to combined diet and exercise training, the timing of macronutrient intake may be important to foster optimal exercise training adaptations. Limited research in this area has been performed in the population with CKD and ESRD. In contrast to findings reported in the aging population, long-term combined oral nutritional supplementation with resistance exercise does not appear to augment protein turnover over and above the improvements observed with oral nutritional supplementation alone in ESRD (136). However, augmented improvements in functional aerobic capacity have been reported in young hemodialysis patients (29 ± 9 years) following exercise training combined with oral nutritional supplementation (137). Despite dietary protein intake restrictions in population with CKD, the anabolic effects of timing the daily protein intake to follow resistance exercise have not yet be elucidated. Whether increased calorie and/or carbohydrate intake before exercise enhances exercise training adaptations is yet to be explored throughout the spectrum of CKD.
Pharmaceutical and Nutraceutical Approaches
In comparison with other patient populations that report exercise intolerance, pharmaceutical and nutraceutical approaches to improving exercise capacity in kidney diseases are relatively unexplored.
Erythropoietin.
With regard to pharmaceutical approaches, EPO has been administered to normalize hematocrit and increase the oxygen-carrying capacity. The findings of these trials are somewhat discouraging. Although small increases in V̇o2peak are observed following EPO chronic administration, values still remain below sedentary age-matched individuals without CKD (89, 138, 139). Even when hematocrit normalization is combined with exercise training, V̇o2peak is still not restored to normative values (89, 139). Nonetheless, complete hematocrit normalization is also inconsistent with current clinical guidelines for anemia in CKD, as it increases the risk of adverse cardiovascular events (140).
Nandrolone decanoate.
Earlier trials explored the anabolic efficacy of nandrolone deaconate to combat muscle wasting and improve exercise capacity (141, 142). This therapy successfully increased muscle mass; however, unexpectedly no significant improvements in V̇o2peak (141) and functional capacity (142) were observed.
Bicarbonate.
Small trials of HCO3 supplementation aimed at reducing metabolic acidosis have shown equivocal findings with respect to improvements in functional capacity, highlighting the need for further investigation in this area (69, 143).
Tetrahydrobiopterin analogs.
In reference to nutraceuticals, tetrahydrobiopterin (BH4) analogs have been explored to improve exercise tolerance in patients with CKD. BH4 is the endothelial NO synthase cofactor required for the production of NO. By increasing NO production, BH4 is hypothesized to improve exercise tolerance by improving vascular tone, neurocirculatory control, and functional sympatholysis. To date, studies in patients with CKD have proved efficacy for BH4 administration in lowering MSNA, systolic blood pressure, and mean arterial pressure during exercise as well as reducing arterial stiffness (144, 145).
Inorganic nitrates and nitrites.
Inorganic nitrate and nitrite supplementation are emerging as a promising strategy to improve exercise intolerance in aging and disease populations. Acute administration of inorganic dietary nitrate in the form of concentrated beetroot juice has been shown to improve the ventilatory threshold, total CPET exercise time, and the total amount of work performed during a CPET in patients with stage 3–4 CKD (146). The proposed mechanisms of inorganic nitrate induced improvements in exercise capacity are through improving vascular function and therefore increasing oxygen delivery to the skeletal muscle (146). In addition, inorganic dietary nitrate supplementation has been shown to improve arterial hemodynamics, thereby reducing the left ventricular pulsatile load during exercise (147). The findings are promising for current trials investigating chronic supplementation (NCT03826147).
With advancements in our knowledge of the physiological contributors to CKD-related exercise intolerance, there is currently a large body of clinical trials underway that are investigating pharma and nutraceutical approaches to address this important problem (Table 1). Future treatments that target the causes of the pathophysiology such as uremia, metabolic acidosis, inflammation, and oxidative stress may be effective strategies for a “whole-systems” approach to improving exercise capacity.
Table 1.
Current pharmaceutical and nutraceutical investigations into improving exercise tolerance in kidney diseases
| Trial | Mechanism of Action to Improve Exercise Capacity | Outcome Measure | Status |
|---|---|---|---|
| AST-120(NCT03788252) | Removes circulating uremic toxin indoxyl sulfate that contributes to sarcopenia | • Body composition • Handgrip strength |
Recruiting |
| CoQ10(NCT03579693) | Ubiquinol antioxidant; improved mitochondrial function | • V̇o2peak • Work efficiency • Mitochondrial energetics • Physical function |
Recruiting |
| Curcumin(NCT04132648) | Anti-inflammatory; improved endothelial function and vascular tone and decreased sympathoexcitation | • Skeletal muscle blood flow during exercise • Sympathetic activity |
Active, not yet recruiting |
| Histadine and β-alanine(NCT02947750) | Increase levels of skeletal muscle carnosine, which acts as an acid buffer and antioxidant | • Muscle pH during exercise • Muscle oxygenation during exercise • Blood pressure response to exercise |
Recruiting |
| Intravenous iron(EudraCT 2018-000144-25) | Improved oxygen-carrying capacity; improved mitochondrial function | • 6-min walk distance • V̇o2peak • Thigh muscle strength • Skeletal muscle metabolism • Mitochondrial function |
Ongoing |
| Mito-Q(NCT02364648) | Mitochondria-targeted ubiquinol; improved mitochondrial function; antioxidant | • V̇o2peak • Vascular function |
Results pending |
| Nicotinamide riboside(NCT03579693) | NAD+ precursor; improved mitochondrial function | • V̇o2peak • Work efficiency • Mitochondrial energetics • Physical function |
Recruiting |
| Sodium bicarbonate(NCT02411773) | Reduced metabolic acidosis leading to improved neurocirculatory control and sympatholysis | • Muscle oxygenation during exercise • Muscle pH • Functional sympatholysis |
Recruiting |
| 6R-BH4(NCT02947750) | Improved endothelial function via increased nitric oxide production; improved neurocirculatory control | • Muscle oxygenation during exercise • Blood pressure response to exercise |
Recruiting |
BH4, tetrahydrobiopterin; NAD, nicotinamide adenine dinucleotide; V̇o2peak, peak oxygen consumption.
CLINICAL INSIGHTS AND CONCLUSIONS
Patients with CKD and ESRD are medically complex and have a high burden of symptoms associated with their chronic illness. Fatigue is a universal concern for patients with CKD and ESRD. In many patients, the feeling of exertional fatigue is constant and incapacitating (148). As a result of exercise intolerance, for many of these patients, any activity including and beyond their activities of daily living may be overwhelming and difficult to perform (149). Although a subjective symptom, objective measures of reduced exercise capacity and exercise intolerance confirm the presence of exertional fatigue that correlates with an increased mortality in this group.
Metabolic derangements due to CKD, underdiagnosed pulmonary disease, cardiac dysfunction, altered physiological response to oxygen consumption, vascular disease, and sarcopenia all contribute to fatigue. A systematic approach is required to investigate reversible causes and should be focused on early in the course of disease. CPET is a useful clinical tool to evaluate exercise intolerance and establish physiological contributors. Using CPET, patients can be phenotyped based on their physiological limitations to exercise capacity, thus fostering the implementation of precision, individually tailored interventions to improve exercise capacity, physical function, and quality of life.
It is assumed that increased physical activity and exercise would provide a mortality benefit to patients with CKD through improvement in the systems highlighted within this review. Although some of the data suggest that some of the physiological adaptations to exercise training are somewhat attenuated, it is important to highlight that exercise is a powerful tool to improve self-reported quality of life and vitality in these patients, with studies also reporting modest reductions in hospitalizations (105, 150). Therefore, nephrology care providers are urged to continue prescribing exercise as part of routine care. Dietary approaches to improve exercise tolerance are lacking in this patient population and critically warranted. Pharmacological and nonpharmacological therapeutic interventions are necessary and urgently needed to increase the exercise tolerance of patients with CKD, and further study is needed to investigate their potential in improving both objective and subjective measures of exertional fatigue.
In conclusion, there are many remaining knowledge gaps in establishing a complete and all-encompassing understanding of the physiological contributors to exercise intolerance across the spectrum of kidney diseases. Building on the current knowledge, a more comprehensive understanding of the pathophysiological mechanisms of kidney disease-related exercise intolerance will help us develop targeted strategies to address this prevalent clinical symptom. Currently, nutra and pharmaceutical interventions that target vascular dysfunction, mitochondrial dysfunction, and metabolic acidosis appear promising. In addition, novel and personalized exercise interventions as part of a comprehensive lifestyle intervention are called for.
GRANTS
D.L.K. is supported by American Heart Association Career Development Award 19CDA34740002. S.C. is supported by American Heart Association Career Development Award 19CDA34660318. P.R-M. is supported by American Heart Association Career Development Award 18CDA341110323. D.L.K., S.C., and P.R-M. are supported by National Institutes of Health Clinical and Translational Science Awards Program UL1TR002649 to Virginia Commonwealth University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.L.K. conceived and designed research; D.L.K. prepared figures; D.L.K., N.B., S.C., R.S.G., P.R-M., R.L.F., J.M.K., and A.A. drafted manuscript; D.L.K., N.B., S.C., R.S.G., P.R-M., R.L.F., J.M.K., and A.A. edited and revised manuscript; D.L.K., N.B., S.C., R.S.G., P.R-M., R.L.F., J.M.K., and A.A. approved final version of manuscript.
REFERENCES
- 1.Jhamb M, Liang K, Yabes J, Steel JL, Dew MA, Shah N, Unruh M. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol 38: 489–495, 2013. doi: 10.1159/000356939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Curtin RB, Bultman DC, Thomas-Hawkins C, Walters BA, Schatell D. Hemodialysis patients' symptom experiences: effects on physical and mental functioning. Nephrol Nurs J 29: 562, 567–574, 2002. discussion 575, 598. [PubMed] [Google Scholar]
- 4.Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. A prospective study of fatigue trajectories among in-centre haemodialysis patients. Br J Health Psychol 25: 61–88, 2020. doi: 10.1111/bjhp.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reese PP, Cappola AR, Shults J, Townsend RR, Gadegbeku CA, Anderson C, Baker JF, Carlow D, Sulik MJ, Lo JC, Go AS, Ky B, Mariani L, Feldman HI, Leonard MB; CRIC Study Investigators. Physical performance and frailty in chronic kidney disease. Am J Nephrol 38: 307–315, 2013. doi: 10.1159/000355568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koyama H, Fukuda S, Shoji T, Inaba M, Tsujimoto Y, Tabata T, Okuno S, Yamakawa T, Okada S, Okamura M, Kuratsune H, Fujii H, Hirayama Y, Watanabe Y, Nishizawa Y. Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clin J Am Soc Nephrol 5: 659–666, 2010. doi: 10.2215/CJN.08151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picariello F, Norton S, Moss-Morris R, Macdougall IC, Chilcot J. Fatigue in prevalent haemodialysis patients predicts all-cause mortality and kidney transplantation. Ann Behav Med 53: 501–514, 2019. doi: 10.1093/abm/kay061. [DOI] [PubMed] [Google Scholar]
- 8.Tiesinga LJ, Dassen TW, Halfens RJ. Fatigue: a summary of the definitions, dimensions, and indicators. Nurs Diagn 7: 51–62, 1996. doi: 10.1111/j.1744-618X.1996.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 9.Kirkman DL, Muth BJ, Stock JM, Townsend RR, Edwards DG. Cardiopulmonary exercise testing reveals subclinical abnormalities in chronic kidney disease. Eur J Prev Cardiolog 25: 1717–1724, 2018. doi: 10.1177/2047487318777777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo C, Tam T, Bo Y, Chang L-Y, Lao XQ, Thomas GN. Habitual physical activity, renal function and chronic kidney disease: a cohort study of nearly 200 000 adults. Br J Sports Med 54: 1225–1230, 2020. doi: 10.1136/bjsports-2019-100989. [DOI] [PubMed] [Google Scholar]
- 11.Howden EJ, Weston K, Leano R, Sharman JE, Marwick TH, Isbel NM, Coombes JS. Cardiorespiratory fitness and cardiovascular burden in chronic kidney disease. J Sci Med Sport 18: 492–497, 2015. doi: 10.1016/j.jsams.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Lopes AA, Lantz B, Morgenstern H, Wang M, Bieber BA, Gillespie BW, Li Y, Painter P, Jacobson SH, Rayner HC, Mapes DL, Vanholder RC, Hasegawa T, Robinson BM, Pisoni RL. Associations of self-reported physical activity types and levels with quality of life, depression symptoms, and mortality in hemodialysis patients: the DOPPS. Clin J Am Soc Nephrol 9: 1702–1712, 2014. doi: 10.2215/CJN.12371213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise capacity as a predictor of survival among ambulatory patients with end-stage renal disease. Kidney Int 65: 719–724, 2004. doi: 10.1111/j.1523-1755.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 14.KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis 66: 884–930, 2015. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Hammer SM, Alexander AM, Didier KD, Huckaby LM, Barstow TJ. Limb blood flow and muscle oxygenation responses during handgrip exercise above vs. below critical force. Microvasc Res 131: 104002, 2020. doi: 10.1016/j.mvr.2020.104002. [DOI] [PubMed] [Google Scholar]
- 16.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 119: 739–744, 2015. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández A, McDonald JR, Lai N, Gladden LB. A prior bout of contractions speeds VO2 and blood flow on-kinetics and reduces the VO2 slow-component amplitude in canine skeletal muscle contracting in situ. J Appl Physiol (1985) 108: 1169–1176, 2010. doi: 10.1152/japplphysiol.01318.2009. [DOI] [PubMed] [Google Scholar]
- 18.Puente-Maestu L. Physiological rationale of commonly used clinical exercise tests. Pulmonology 26: 159–165, 2020. doi: 10.1016/j.pulmoe.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, Garten R, Rodriguez-Miguelez P, Guazzi M, Lavie CJ, Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol 73: 2209–2225, 2019. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald JH, Fearn L, Jibani M, Marcora SM. Exertional fatigue in patients with CKD. Am J Kidney Dis 60: 930–939, 2012. doi: 10.1053/j.ajkd.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Navaneethan SD, Schold JD, Huang H, Nakhoul G, Jolly SE, Arrigain S, Dweik RA, Nally JV, Jr.. Mortality outcomes of patients with chronic kidney disease and chronic obstructive pulmonary disease. Am J Nephrol 43: 39–46, 2016. doi: 10.1159/000444422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plesner LL, Warming PE, Nielsen TL, Dalsgaard M, Schou M, Host U, Rydahl C, Brandi L, Kober L, Vestbo J, Iversen K. Chronic obstructive pulmonary disease in patients with end-stage kidney disease on hemodialysis. Hemodial Int 20: 68–77, 2016. doi: 10.1111/hdi.12342. [DOI] [PubMed] [Google Scholar]
- 23.Incalzi RA, Corsonello A, Pedone C, Battaglia S, Paglino G, Bellia V. Extrapulmonary Consequences of COPD in the Elderly Study Investigators. Chronic renal failure: a neglected comorbidity of COPD. Chest 137: 831–837, 2010. doi: 10.1378/chest.09-1710. [DOI] [PubMed] [Google Scholar]
- 24.Yoshizawa T, Okada K, Furuichi S, Ishiguro T, Yoshizawa A, Akahoshi T, Gon Y, Akashiba T, Hosokawa Y, Hashimoto S. Prevalence of chronic kidney diseases in patients with chronic obstructive pulmonary disease: assessment based on glomerular filtration rate estimated from creatinine and cystatin C levels. Int J Chron Obstruct Pulmon Dis 10: 1283–1289, 2015. doi: 10.2147/COPD.S80673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fedeli U, De Giorgi A, Gennaro N, Ferroni E, Gallerani M, Mikhailidis DP, Manfredini R, Fabbian F. Lung and kidney: a dangerous liaison? A population-based cohort study in COPD patients in Italy. Int J Chron Obstruct Pulmon Dis 12: 443–450, 2017. doi: 10.2147/COPD.S119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairshter RD, Vaziri ND, Mk M. Lung pathology in chronic hemodialysis patients. Int J Artif Organs 5: 97–100, 1982. [PubMed] [Google Scholar]
- 27.Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, Gargani L, Sicari R, Picano E, Mallamaci F. Lung US in CKD Working Group. Pulmonary congestion predicts cardiac events and mortality in ESRD. J Am Soc Nephrol 24: 639–646, 2013. doi: 10.1681/ASN.2012100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herrero JA, Alvarez-Sala JL, Coronel F, Moratilla C, Gamez C, Sanchez-Alarcos JM, Barrientos A. Pulmonary diffusing capacity in chronic dialysis patients. Respir Med 96: 487–492, 2002. doi: 10.1053/rmed.2002.1346. [DOI] [PubMed] [Google Scholar]
- 29.Salerno FR, Parraga G, McIntyre CW. Why is your patient still short of breath? understanding the complex pathophysiology of dyspnea in chronic kidney disease. Semin Dial 30: 50–57, 2017. doi: 10.1111/sdi.12548. [DOI] [PubMed] [Google Scholar]
- 30.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59: 574–580, 2004. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nascimento MM, Qureshi AR, Stenvinkel P, Pecoits-Filho R, Heimburger O, Cederholm T, Lindholm B, Barany P. Malnutrition and inflammation are associated with impaired pulmonary function in patients with chronic kidney disease. Nephrol Dial Transplant 19: 1823–1828, 2004. doi: 10.1093/ndt/gfh190. [DOI] [PubMed] [Google Scholar]
- 32.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care 18: 254–262, 2015. doi: 10.1097/MCO.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 34.Yılmaz S, Yildirim Y, Yilmaz Z, Kara AV, Taylan M, Demir M, Coskunsel M, Kadiroglu AK, Yilmaz ME. Pulmonary function in patients with end-stage renal disease: effects of hemodialysis and fluid overload. Med Sci Monit 22: 2779–2784, 2016. doi: 10.12659/MSM.897480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conger JD, Hammond WS, Alfrey AC, Contiguglia SR, Stanford RE, Huffer WE. Pulmonary calcification in chronic dialysis patients. Clinical and pathologic studies. Ann Intern Med 83: 330–336, 1975. doi: 10.7326/0003-4819-83-3-330. [DOI] [PubMed] [Google Scholar]
- 36.Pabst S, Hammerstingl C, Hundt F, Gerhardt T, Grohe C, Nickenig G, Woitas R, Skowasch D. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS One 7: e35310, 2012. doi: 10.1371/journal.pone.0035310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGuire S, Horton EJ, Renshaw D, Chan K, Krishnan N, McGregor G. Ventilatory and chronotropic incompetence during incremental and constant load exercise in end-stage renal disease: a comparative physiology study. Am J Physiol Renal Physiol 319: F515–F522, 2020. doi: 10.1152/ajprenal.00258.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int 63: 600–606, 2003. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 39.Lyons OD, Bradley TD, Chan CT. Hypervolemia and sleep apnea in kidney disease. Semin Nephrol 35: 373–382, 2015. doi: 10.1016/j.semnephrol.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Mahajan S, Gupta K, Sinha S, Malhotra A, Mahajan S. Effect of kidney transplantation on sleep-disordered breathing in patients with end stage renal disease: a polysomnographic study. Sleep Med 45: 140–145, 2018. doi: 10.1016/j.sleep.2017.11.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon JH, Won JU, Ahn YS, Roh J. Poor lung function has inverse relationship with microalbuminuria, an early surrogate marker of kidney damage and atherosclerosis: the 5th Korea National Health and Nutrition Examination Survey. PLoS One 9: e94125, 2014. doi: 10.1371/journal.pone.0094125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangaswami J, Bhalla V, Blair JE, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WH, McCullough PA. American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the. Circulation 139: e840–e878, 2019. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 43.Buckley LF, Canada JM, Carbone S, Trankle CR, Kadariya D, Billingsley H, Wohlford GF, Kirkman DL, Abbate A, Van Tassell BW. Potential role for interleukin-1 in the cardio-renal syndrome. Eur J Heart Fail 21: 385–386, 2019. doi: 10.1002/ejhf.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saran R, Robinson B, Abbott KC, Agodoa LY, Bragg-Gresham J, Balkrishnan R, , et al. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 73: A7–A8, 2019. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krishnasamy R, Hawley CM, Stanton T, Howden EJ, Beetham KS, Strand H, Leano R, Haluska BA, Coombes JS, Isbel NM. Association between left ventricular global longitudinal strain, health-related quality of life and functional capacity in chronic kidney disease patients with preserved ejection fraction. Nephrology (Carlton) 21: 108–115, 2016. doi: 10.1111/nep.12557. [DOI] [PubMed] [Google Scholar]
- 48.Trankle C, Canada JM, Buckley L, Carbone S, Dixon D, Arena R, Van Tassell B, Abbate A. Impaired myocardial relaxation with exercise determines peak aerobic exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail 4: 351–355, 2017. doi: 10.1002/ehf2.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int 25: 437–444, 1984. doi: 10.1038/ki.1984.36. [DOI] [PubMed] [Google Scholar]
- 50.Odden MC, Whooley MA, Shlipak MG. Association of chronic kidney disease and anemia with physical capacity: the heart and soul study. J Am Soc Nephrol 15: 2908–2915, 2004. doi: 10.1097/01.ASN.0000143743.78092.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macdougall IC. Intravenous iron therapy in patients with chronic kidney disease: recent evidence and future directions. Clin Kidney J 10: i16–i24, 2017. doi: 10.1093/ckj/sfx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, McMurray JJV, Murray H, Tomson CR, Wheeler DC, Winearls CG, Ford I. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380: 447–458, 2019. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 53.Begum S, Latunde-Dada GO. Anemia of inflammation with an emphasis on chronic kidney disease. Nutrients 11: 2424, 2019. doi: 10.3390/nu11102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul BT, Manz DH, Torti FM, Torti SV. Mitochondria and iron: current questions. Expert Rev Hematol 10: 65–79, 2017. [Erratum in Expert Rev Hematol. 2017 Mar;10(3):275]. doi: 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bekfani T, Pellicori P, Morris D, Ebner N, Valentova M, Sandek A, Doehner W, Cleland JG, Lainscak M, Schulze PC, Anker SD, von Haehling S. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol 108: 203–211, 2019. doi: 10.1007/s00392-018-1344-x. [DOI] [PubMed] [Google Scholar]
- 56.Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the studies investigating co-morbidities aggravating heart failure. Int J Cardiol 205: 6–12, 2016. doi: 10.1016/j.ijcard.2015.11.178. [DOI] [PubMed] [Google Scholar]
- 57.Czaya B, Faul C. The role of fibroblast growth factor 23 in inflammation and anemia. Int J Mol Sci 20: 4195, 2019. doi: 10.3390/ijms20174195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park J, Quyyumi AA, Middlekauff HR. Exercise pressor response and arterial baroreflex unloading during exercise in chronic kidney disease. J Appl Physiol (1985) 114: 538–549, 2013. doi: 10.1152/japplphysiol.01037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Girouard H, de Champlain J. Acute and chronic effects of free radicals on alpha1-adrenergic-induced vasoconstriction in mesenteric beds of spontaneously hypertensive rats. J Hypertens 23: 807–814, 2005. doi: 10.1097/01.hjh.0000163150.43201.ac. [DOI] [PubMed] [Google Scholar]
- 60.Barrett-O’Keefe Z, Witman MA, Mcdaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS, Wray DW. Angiotensin II potentiates alpha-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124: 413–422, 2013. doi: 10.1042/CS20120424. [DOI] [PubMed] [Google Scholar]
- 61.Sprick JD, Morison DL, Stein CM, Li Y, Paranjape S, Fonkoue IT, DaCosta DR, Park J. Vascular alpha1-adrenergic sensitivity is enhanced in chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 317: R485–R490, 2019. doi: 10.1152/ajpregu.00090.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sprick JD, Downey RM, Morison DL, Fonkoue IT, Li Y, DaCosta D, Rapista D, Park J. Functional sympatholysis is impaired in end-stage renal disease. Am J Physiol Regul Integr Comp Physiol 316: R504–R511, 2019. doi: 10.1152/ajpregu.00380.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sprick JD, Morison DL, Fonkoue IT, Li Y, DaCosta D, Rapista D, Choi H, Park J. Metabolic acidosis augments exercise pressor responses in chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 317: R312–R318, 2019. doi: 10.1152/ajpregu.00076.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howden EJ, Lawley JS, Esler M, Levine BD. Potential role of endurance training in altering renal sympathetic nerve activity in CKD? Auton Neurosci 204: 74–80, 2017. doi: 10.1016/j.autneu.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 66.Ukena C, Mahfoud F, Kindermann I, Barth C, Lenski M, Kindermann M, Brandt MC, Hoppe UC, Krum H, Esler M, Sobotka PA, Bohm M. Cardiorespiratory response to exercise after renal sympathetic denervation in patients with resistant hypertension. J Am Coll Cardiol 58: 1176–1182, 2011. doi: 10.1016/j.jacc.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 67.Yenchek R, Ix JH, Rifkin DE, Shlipak MG, Sarnak MJ, Garcia M, Patel KV, Satterfield S, Harris TB, Newman AB, Fried LF. Association of serum bicarbonate with incident functional limitation in older adults. Clin J Am Soc Nephrol 9: 2111–2116, 2014. doi: 10.2215/CJN.05480614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kraut JA, Madias NE. Adverse effects of the metabolic acidosis of chronic kidney disease. Adv Chronic Kidney Dis 24: 289–297, 2017. doi: 10.1053/j.ackd.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Kosmadakis GC, John SG, Clapp EL, Viana JL, Smith AC, Bishop NC, Bevington A, Owen PJ, McIntyre CW, Feehally J. Benefits of regular walking exercise in advanced pre-dialysis chronic kidney disease. Nephrol Dial Transplant 27: 997–1004, 2012. doi: 10.1093/ndt/gfr364. [DOI] [PubMed] [Google Scholar]
- 70.Melamed ML, Horwitz EJ, Dobre MA, Abramowitz MK, Zhang L, Lo Y, Mitch WE, Hostetter TH. Effects of sodium bicarbonate in CKD stages 3 and 4: a randomized, placebo-controlled, multicenter clinical trial. Am J Kidney Dis 75: 225–234, 2020. doi: 10.1053/j.ajkd.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martens CR, Kirkman DL, Edwards DG. The vascular endothelium in chronic kidney disease: a novel target for aerobic exercise. Exerc Sport Sci Rev 44: 12–19, 2016. doi: 10.1249/JES.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Renal Physiol 306: F1499–1506, 2014. doi: 10.1152/ajprenal.00058.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kirkman DL, Muth BJ, Ramick MG, Townsend RR, Edwards DG. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am J Physiol Renal Physiol 314: F423–F429, 2018. doi: 10.1152/ajprenal.00321.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or L-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol (1985) 111: 1561–1567, 2011. doi: 10.1152/japplphysiol.00419.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martens CR, Kuczmarski JM, Lennon-Edwards S, Edwards DG. Impaired L-arginine uptake but not arginase contributes to endothelial dysfunction in rats with chronic kidney disease. J Cardiovasc Pharmacol 63: 40–48, 2014. doi: 10.1097/FJC.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 76.Downey RM, Liao P, Millson EC, Quyyumi AA, Sher S, Park J. Endothelial dysfunction correlates with exaggerated exercise pressor response during whole body maximal exercise in chronic kidney disease. Am J Physiol Renal Physiol 312: F917–F924, 2017. doi: 10.1152/ajprenal.00603.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsu JJ, Katz R, Chirinos JA, Jacobs DR, Jr, Duprez DA, Peralta CA. Arterial wave reflections and kidney function decline among persons with preserved estimated glomerular filtration rate: the multi-ethnic study of atherosclerosis. J Am Soc Hypertens 10: 438–446, 2016. doi: 10.1016/j.jash.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 78.London GM, Blacher J, Pannier B, Guérin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension 38: 434–438, 2001. doi: 10.1161/01.HYP.38.3.434. [DOI] [PubMed] [Google Scholar]
- 79.Verbeke F, Marechal C, Van Laecke S, Van Biesen W, Devuyst O, Van Bortel LM, Jadoul M, Vanholder R. Aortic stiffness and central wave reflections predict outcome in renal transplant recipients. Hypertension 58: 833–838, 2011. doi: 10.1161/HYPERTENSIONAHA.111.176594. [DOI] [PubMed] [Google Scholar]
- 80.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension 56: 555–562, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157321. [DOI] [PubMed] [Google Scholar]
- 81.Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure-flow and pressure-volume relations in humans. Hypertension 56: 563–570, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157339. [DOI] [PubMed] [Google Scholar]
- 82.Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR, Appel LJ, He J, Kusek JW, Rahman M; CRIC Study Investigators. Arterial stiffness, central pressures, and incident hospitalized heart failure in the chronic renal insufficiency cohort study. Circ Heart Fail 7: 709–716, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park J, Middlekauff HR. Abnormal neurocirculatory control during exercise in humans with chronic renal failure. Auton Neurosci 188: 74–81, 2015. doi: 10.1016/j.autneu.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Craenenbroeck AH, Van Craenenbroeck EM, Van Ackeren K, Hoymans VY, Verpooten GA, Vrints CJ, Couttenye MM. Impaired vascular function contributes to exercise intolerance in chronic kidney disease. Nephrol Dial Transplant 31: 2064–2072, 2016. doi: 10.1093/ndt/gfw303. [DOI] [PubMed] [Google Scholar]
- 85.Diesel W, Noakes TD, Swanepoel C, Lambert M. Isokinetic muscle strength predicts maximum exercise tolerance in renal patients on chronic hemodialysis. Am J Kidney Dis 16: 109–114, 1990. doi: 10.1016/S0272-6386(12)80563-4. [DOI] [PubMed] [Google Scholar]
- 86.Sakkas GK, Ball D, Mercer TH, Sargeant AJ, Tolfrey K, Naish PF. Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol Dial Transplant 18: 2074–2081, 2003. doi: 10.1093/ndt/gfg325. [DOI] [PubMed] [Google Scholar]
- 87.Diesel W, Emms M, Knight BK, Noakes TD, Swanepoel CR, van Zyl Smit R, Kaschula RO, Sinclair-Smith CC. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis 22: 677–684, 1993. doi: 10.1016/S0272-6386(12)80430-6. [DOI] [PubMed] [Google Scholar]
- 88.Johansen KL, Doyle J, Sakkas GK, Kent-Braun JA. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol Regul Integr Comp Physiol 289: R805–813, 2005. doi: 10.1152/ajpregu.00187.2005. [DOI] [PubMed] [Google Scholar]
- 89.Stray-Gundersen J, Howden EJ, Parsons DB, Thompson JR. Neither hematocrit normalization nor exercise training restores oxygen consumption to normal levels in hemodialysis patients. J Am Soc Nephrol 27: 3769–3779, 2016. doi: 10.1681/ASN.2015091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gamboa JL, Billings FT, Bojanowski MT, Gilliam LA, Yu C, Roshanravan B, Roberts LJ, 2nd, Himmelfarb J, Ikizler TA, Brown NJ. Mitochondrial dysfunction and oxidative stress in patients with chronic kidney disease. Physiol Rep 4: e12780, 2016. doi: 10.14814/phy2.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gamboa JL, Roshanravan B, Towse T, Keller CA, Falck AM, Yu C, Frontera WR, Brown NJ, Ikizler TA. Skeletal muscle mitochondrial dysfunction is present in patients with CKD before initiation of maintenance hemodialysis. Clin J Am Soc Nephrol 15: 926–936, 2020. doi: 10.2215/CJN.10320819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kestenbaum B, Gamboa J, Liu S, Ali AS, Shankland E, Jue T, Giulivi C, Smith LR, Himmelfarb J, de Boer IH, Conley K, Roshanravan B. Impaired skeletal muscle mitochondrial bioenergetics and physical performance in chronic kidney disease. JCI Insight 5, 2020. doi: 10.1172/jci.insight.133289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Watson EL, Baker LA, Wilkinson TJ, Gould DW, Graham-Brown MP, Major RW, Ashford RU, Philp A, Smith AC. Reductions in skeletal muscle mitochondrial mass are not restored following exercise training in patients with chronic kidney disease. FASEB J 34: 1755–1767, 2020. doi: 10.1096/fj.201901936RR. [DOI] [PubMed] [Google Scholar]
- 94.Roshanravan B, Kestenbaum B, Gamboa J, Jubrias SA, Ayers E, Curtin L, Himmelfarb J, de Boer IH, Conley KC. CKD and muscle mitochondrial energetics. Am J Kidney Dis 68: 658–659, 2016. doi: 10.1053/j.ajkd.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Enoki Y, Watanabe H, Arake R, Fujimura R, Ishiodori K, Imafuku T, Nishida K, Sugimoto R, Nagao S, Miyamura S, Ishima Y, Tanaka M, Matsushita K, Komaba H, Fukagawa M, Otagiri M, Maruyama T. Potential therapeutic interventions for chronic kidney disease-associated sarcopenia via indoxyl sulfate-induced mitochondrial dysfunction. J Cachexia Sarcopenia Muscle 8: 735–747, 2017. doi: 10.1002/jcsm.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishikawa M, Ishimori N, Takada S, Saito A, Kadoguchi T, Furihata T, Fukushima A, Matsushima S, Yokota T, Kinugawa S, Tsutsui H. AST-120 ameliorates lowered exercise capacity and mitochondrial biogenesis in the skeletal muscle from mice with chronic kidney disease via reducing oxidative stress. Nephrol Dial Transplant 30: 934–942, 2015. doi: 10.1093/ndt/gfv103. [DOI] [PubMed] [Google Scholar]
- 97.Gollie JM, Harris-Love MO, Patel SS, Argani S. Chronic kidney disease: considerations for monitoring skeletal muscle health and prescribing resistance exercise. Clin Kidney J 11: 822–831, 2018. doi: 10.1093/ckj/sfy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kirkman DL, Mullins P, Junglee NA, Kumwenda M, Jibani MM, Macdonald JH. Anabolic exercise in haemodialysis patients: a randomised controlled pilot study. J Cachexia Sarcopenia Muscle 5: 199–207, 2014. doi: 10.1007/s13539-014-0140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carbone S, Billingsley HE, Rodriguez-Miguelez P, Kirkman DL, Garten R, Franco RL, Lee DC, Lavie CJ. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol 45: 100417, 2020. doi: 10.1016/j.cpcardiol.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirai K, Ookawara S, Morishita Y. Sarcopenia and physical inactivity in patients with chronic kidney disease. Nephrourol Mon 8: e37443, 2016. doi: 10.5812/numonthly.37443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Souza VA, Oliveira D, Barbosa SR, Correa J, Colugnati FA, Mansur HN, Fernandes N, Bastos MG. Sarcopenia in patients with chronic kidney disease not yet on dialysis: analysis of the prevalence and associated factors. PLoS One 12: e0176230, 2017. doi: 10.1371/journal.pone.0176230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ventura HO, Carbone S, Lavie CJ. Muscling up to improve heart failure prognosis. Eur J Heart Fail 20: 1588–1590, 2018. doi: 10.1002/ejhf.1314. [DOI] [PubMed] [Google Scholar]
- 103.D’Alessandro C, Piccoli G, Barsotti M, Tassi S, Giannese D, Morganti R, Cupisti A. Prevalence and correlates of sarcopenia among elderly CKD outpatients on tertiary care. Nutrients 10: 1951, 2018. doi: 10.3390/nu10121951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rao M, Jaber BL, Balakrishnan VS. Chronic kidney disease and acquired mitochondrial myopathy. Curr Opin Nephrol Hypertens 27: 113–120, 2018. doi: 10.1097/MNH.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 105.Heiwe S, Jacobson SH. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev CD003236, 2011. doi: 10.1002/14651858.CD003236.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andrade FP, Rezende PD, Ferreira TD, Borba GC, Müller AM, Rovedder PM. Effects of intradialytic exercise on cardiopulmonary capacity in chronic kidney disease: systematic review and meta-analysis of randomized clinical trials. Sci Rep 9: 18470, 2019. doi: 10.1038/s41598-019-54953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wilkinson TJ, Watson EL, Xenophontos S, Gould DW, Smith AC. The “minimum clinically important difference” in frequently reported objective physical function tests after a 12-week renal rehabilitation exercise intervention in nondialysis chronic kidney disease. Am J Phys Med Rehabil 98: 431–437, 2019. doi: 10.1097/PHM.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 108.Yang H, Wu X, Wang M. Exercise affects cardiopulmonary function in patients with chronic kidney disease: a meta-analysis. Biomed Res Int 2017: 6405797, 2017. doi: 10.1155/2017/6405797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirkman DL, Ramick MG, Muth BJ, Stock JM, Townsend RR, Edwards DG. A randomized trial of aerobic exercise in chronic kidney disease: evidence for blunted cardiopulmonary adaptations. Annals of Physical and Rehabilitation Medicine. In press. doi: 10.1016/j.rehab.2020.101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheema B, Abas H, Smith B, O’Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, Singh FM. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the progressive exercise for anabolism in kidney disease (PEAK) study. Am J Kidney Dis 50: 574–584, 2007. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Cheema B, Abas H, Smith B, O'Sullivan A, Chan M, Patwardhan A, Kelly J, Gillin A, Pang G, Lloyd B, Singh MF. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol 18: 1594–1601, 2007. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 112.Chen JL, Godfrey S, Ng TT, Moorthi R, Liangos O, Ruthazer R, Jaber BL, Levey AS, Castaneda-Sceppa C. Effect of intra-dialytic, low-intensity strength training on functional capacity in adult haemodialysis patients: a randomized pilot trial. Nephrol Dial Transplant 25: 1936–1943, 2010. doi: 10.1093/ndt/gfp739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johansen KL, Painter PL, Sakkas GK, Gordon P, Doyle J, Shubert T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol 17: 2307–2314, 2006. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 114.Pike MM, Alsouqi A, Headley SA, Tuttle K, Evans EE, Milch CM, Moody KA, Germain M, Stewart TG, Lipworth L, Himmelfarb J, Ikizler TA, Robinson-Cohen C. Supervised exercise intervention and overall activity in CKD. Kidney Int Rep 5: 1261–1270, 2020. doi: 10.1016/j.ekir.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilund KR, Viana JL, Perez LM. A critical review of exercise training in hemodialysis patients: personalized activity prescriptions are needed. Exerc Sport Sci Rev 48: 28–39, 2020. doi: 10.1249/JES.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clarkson MJ, Brumby C, Fraser SF, McMahon LP, Bennett PN, Warmington SA. Hemodynamic and perceptual responses to blood flow-restricted exercise among patients undergoing dialysis. Am J Physiol Renal Physiol 318: F843–F850, 2020. doi: 10.1152/ajprenal.00576.2019. [DOI] [PubMed] [Google Scholar]
- 117.Spranger MD, Krishnan AC, Levy PD, O'Leary DS, Smith SA. Blood flow restriction training and the exercise pressor reflex: a call for concern. Am J Physiol Heart Circ Physiol 309: H1440–H1452, 2015. doi: 10.1152/ajpheart.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Junglee NA, Di Felice U, Dolci A, Fortes MB, Jibani MM, Lemmey AB, Walsh NP, Macdonald JH. Exercising in a hot environment with muscle damage: effects on acute kidney injury biomarkers and kidney function. Am J Physiol Renal Physiol 305: F813–F820, 2013. doi: 10.1152/ajprenal.00091.2013. [DOI] [PubMed] [Google Scholar]
- 119.Schlader ZJ, Hostler D, Parker MD, Pryor RR, Lohr JW, Johnson BD, Chapman CL. The potential for renal injury elicited by physical work in the heat. Nutrients 11: 2087, 2019. doi: 10.3390/nu11092087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilund KR, Tomayko EJ, Wu PT, Chung H, Vallurupalli S, Lakshminarayanan B.Fernhall B. Intradialytic exercise training reduces oxidative stress and epicardial fat: a pilot study. Nephrol Dial Transplant 25: 2695–2701, 2010. doi: 10.1093/ndt/gfq106. [DOI] [PubMed] [Google Scholar]
- 121.Mansour SG, Martin TG, Obeid W, Pata RW, Myrick KM, Kukova L, Jia Y, Bjornstad P, El-Khoury JM, Parikh CR. The role of volume regulation and thermoregulation in AKI during marathon running. Clin J Am Soc Nephrol 14: 1297–1305, 2019.doi: 10.2215/CJN.01400219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCullough PA, Chinnaiyan KM, Gallagher MJ, Colar JM, Geddes T, Gold JM, Trivax JE. Changes in renal markers and acute kidney injury after marathon running. Nephrology (Carlton) 16: 194–199, 2011. doi: 10.1111/j.1440-1797.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 123.Chapman CL, Johnson BD, Sackett JR, Parker MD, Schlader ZJ. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am J Physiol Regul Integr Comp Physiol 316: R189–R198, 2019. doi: 10.1152/ajpregu.00351.2018. [DOI] [PubMed] [Google Scholar]
- 124.Chapman CL, Johnson BD, Vargas NT, Hostler D, Parker MD, Schlader ZJ. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J Appl Physiol (1985) 128: 715–728, 2020. doi: 10.1152/japplphysiol.00787.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bellizzi V, Calella P, Carrero JJ, Fouque D. Very low-protein diet to postpone renal failure: pathophysiology and clinical applications in chronic kidney disease. Chronic Dis Transl Med 4: 45–50, 2018. doi: 10.1016/j.cdtm.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fouque D, Aparicio M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat Clin Pract Nephrol 3: 383–392, 2007. doi: 10.1038/ncpneph0524. [DOI] [PubMed] [Google Scholar]
- 127.Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care 20: 77–85, 2017. doi: 10.1097/MCO.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yan B, Su X, Xu B, Qiao X, Wang L. Effect of diet protein restriction on progression of chronic kidney disease: a systematic review and meta-analysis. PLoS One 13: e0206134, 2018. doi: 10.1371/journal.pone.0206134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wolfe RR, Cifelli AM, Kostas G, Kim I-Y. Optimizing protein intake in adults: interpretation and application of the recommended dietary allowance compared with the acceptable macronutrient distribution range. Adv Nutr 8: 266–275, 2017. doi: 10.3945/an.116.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev 10: Cd001892, 2020. doi: 10.1002/14651858.CD001892.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen X, Wei G, Jalili T, Metos J, Giri A, Cho ME, Boucher R, Greene T, Beddhu S. The associations of plant protein intake with all-cause mortality in CKD. Am J Kidney Dis 67: 423–430, 2016. doi: 10.1053/j.ajkd.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ford J, Phillips ME, Toye FE, Luck VA, de Wardener HE. Nitrogen balance in patients with chronic renal failure on diets containing varying quantities of protein. Br Med J 1: 735–740, 1969. doi: 10.1136/bmj.1.5646.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kopple JD, Levey AS, Greene T, Chumlea WC, Gassman JJ, Hollinger DL, Maroni BJ, Merrill D, Scherch LK, Schulman G, Wang SR, Zimmer GS; Modification of Diet in Renal Disease Study Group. Effect of dietary protein restriction on nutritional status in the Modification of Diet in Renal Disease Study. Kidney Int 52: 778–791, 1997. doi: 10.1038/ki.1997.395. [DOI] [PubMed] [Google Scholar]
- 134.Castaneda C, Gordon PL, Uhlin KL, Levey AS, Kehayias JJ, Dwyer JT, Fielding RA, Roubenoff R, Singh MF. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. a randomized, controlled trial. Ann Intern Med 135: 965–976, 2001. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 135.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. Jama 322: 1294–1304, 2019. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dong J, Sundell MB, Pupim LB, Wu P, Shintani A, Ikizler TA. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J Ren Nutr 21: 149–159, 2011. doi: 10.1053/j.jrn.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin-Alemañy G, Espinosa-Cuevas M, Pérez-Navarro M, Wilund KR, Miranda-Alatriste P, Cortés-Pérez M, García-Villalobos G, Gómez-Guerrero I, Cantú-Quintanilla G, Ramírez-Mendoza M, Valdez-Ortiz R. Effect of oral nutritional supplementation with and without exercise on nutritional status and physical function of adult hemodialysis patients: a parallel controlled clinical Ttial (AVANTE-HEMO Study). J Ren Nutr 30: 126–136, 2020. doi: 10.1053/j.jrn.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 138.Lewis NP, Macdougall IC, Willis N, Coles GA, Williams JD, Henderson AH. Effects of the correction of renal anaemia by erythropoietin on physiological changes during exercise. Eur J Clin Invest 23: 423–427, 1993. doi: 10.1111/j.1365-2362.1993.tb00785.x. [DOI] [PubMed] [Google Scholar]
- 139.Painter P, Moore G, Carlson L, Paul S, Myll J, Phillips W, Haskell W. Effects of exercise training plus normalization of hematocrit on exercise capacity and health-related quality of life. Am J Kidney Dis 39: 257–265, 2002. doi: 10.1053/ajkd.2002.30544. [DOI] [PubMed] [Google Scholar]
- 140.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 141.Johansen KL, Mulligan K, Schambelan M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. Jama 281: 1275–1281, 1999. doi: 10.1001/jama.281.14.1275. [DOI] [PubMed] [Google Scholar]
- 142.Macdonald JH, Marcora SM, Jibani MM, Kumwenda MJ, Ahmed W, Lemmey AB. Nandrolone decanoate as anabolic therapy in chronic kidney disease: a randomized phase II dose-finding study. Nephron Clin Pract 106: c125–135, 2007. doi: 10.1159/000103000. [DOI] [PubMed] [Google Scholar]
- 143.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol 8: 714–720, 2013. doi: 10.2215/CJN.08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lin AM, Liao P, Millson EC, Quyyumi AA, Park J. Tetrahydrobiopterin ameliorates the exaggerated exercise pressor response in patients with chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol 310: F1016–F1025, 2016. doi: 10.1152/ajprenal.00527.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 308: R208–R218, 2015. doi: 10.1152/ajpregu.00409.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ramick MG, Kirkman DL, Stock JM, Muth BJ, Farquhar WB, Chirinos JA, Doulias PT, Ischiropoulos H, Edwards DG. The effect of dietary nitrate on exercise capacity in chronic kidney disease a randomized controlled pilot study. Nitric Oxide 106: 17–23, 2021. doi: 10.1016/j.niox.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, Doulias PT, Ischiropoulos H, Townsend RR, Margulies KB, Cappola TP, Poole DC, Chirinos JA. Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation 131: 371–380; discussion 380, 2015. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jefferson NM. A patient's view on exercise and ESKD. Clin J Am Soc Nephrol 14: 171, 2019. doi: 10.2215/CJN.00150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moorman D, Suri R, Hiremath S, Jegatheswaran J, Kumar T, Bugeja A, Zimmerman D. Benefits and barriers to and desired outcomes with exercise in patients with ESKD. Clin J Am Soc Nephrol 14: 268–276, 2019. doi: 10.2215/CJN.09700818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manfredini F, Mallamaci F, D’Arrigo G, Baggetta R, Bolignano D, Torino C , et al. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol 28: 1259–1268, 2017. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]