Abstract
Placental ischemia in preeclampsia (PE) results in hypertension and intrauterine growth restriction (IUGR). Stimulation of soluble guanylate cyclase (sGC) reduces blood pressure in the clinically relevant reduced uterine perfusion pressure (RUPP) rat model of PE, implicating involvement in RUPP-induced hypertension. However, the contribution of sGC in the development of IUGR in PE is not known. Thus, this study demonstrated the efficacy of Riociguat, an sGC stimulator, in IUGR reversion in the RUPP rat model of PE, and tested the hypothesis that improvement in fetal weight occurs in association with improvement in placental perfusion, placental morphology, and placental nutrient transport protein expression. Sham or RUPP surgery was performed at gestational day 14 (G14) with administration of vehicle (Sham or RUPP) or the sGC stimulator (Riociguat, 10 mg/kg/day sc; sGC-treated) until G20. Fetal weight was reduced (P = 0.004) at G20 in RUPP but not in sGC-treated RUPP compared with Sham, the control group. At G20, uterine artery resistance index (UARI) was increased (P = 0.010) in RUPP, indicating poor placental perfusion; proportional junctional zone surface area was elevated (P = 0.035), indicating impaired placental development. These effects were ameliorated in sGC-treated RUPP. Placental protein expression of nutrient transporter heart fatty acid-binding protein (hFABP) was increased (P = 0.008) in RUPP but not in sGC-treated RUPP, suggesting a compensatory mechanism to maintain normal neurodevelopment. Yet, UARI (P < 0.001), proportional junctional zone surface area (P = 0.013), and placental hFABP protein expression (P = 0.008) were increased in sGC-treated Sham, suggesting a potential adverse effect of Riociguat. Collectively, these results suggest sGC contributes to IUGR in PE.
Keywords: intrauterine growth restriction, placental nutrient transport, placenta perfusion, preeclampsia, soluble guanylate cyclase
INTRODUCTION
Preeclampsia (PE) affects 5%–7% of pregnancies in the United States and is a leading cause of maternal and fetal morbidity and mortality (1). Although the etiology of PE is not entirely understood, the initiating event may involve improper placentation leading to placental ischemia (2), maternal hypertension (3), and increased risk of intrauterine growth restriction (IUGR), and asymmetric fetal growth (4). Offspring exposed to a pregnancy complicated by PE are at risk for increased blood pressure (BP) and cardiovascular disease after birth; a risk that extends throughout the lifespan of surviving IUGR offspring (5). Thus, PE is not only detrimental to the mother and her developing fetus during pregnancy but is also associated with adverse effects that continue across the lifetime of an individual exposed to PE.
The etiology of PE is multifactorial but is associated with a reduction in nitric oxide (NO) bioavailability (6), impaired signaling of the NO-soluble guanylate cyclase (sGC)- guanosine 3',5'-cyclic monophosphate (cGMP) pathway (7), and decreased urinary cGMP (8). sGC is an enzyme that mediates the synthesis of cGMP (9). cGMP activates cGMP-dependent protein kinase, which leads to vascular smooth muscle cell relaxation and vasodilation (9). The reduction in the NO-sGC-cGMP signaling pathway in PE contributes to vasoconstriction and impaired uterine remodeling (10). Yet, plasma cGMP and urinary concentrations of cGMP are also reduced in pregnancies complicated by IUGR (8, 11), suggesting that deficiencies in NO-sGC-cGMP signaling may also play an important role in the pathogenesis of IUGR.
Rat models that mimic the many characteristics of PE allow for investigation into potential pathways that contribute to the etiology of PE. The rat model of reduced uterine perfusion pressure (RUPP) (12) mimics many facets of PE, including increased maternal BP (13, 14), decreased NO bioavailability (13, 15), decreased renal cGMP (16), and IUGR (17). Additionally, placental perfusion is reduced by ∼40% in late gestation in the RUPP rat (12) mimicking the restriction in uteroplacental blood flow and increased uterine artery resistance that are associated with restricted fetal growth in women with PE (18). Recently Bakrania et al. (19) reported that uterine cGMP concentration is reduced in the RUPP. Moreover, this study also demonstrated that cGMP production is increased and maternal BP is reduced in the RUPP at gestation day 19 (G19) by maternal administration of a sGC activator, a novel class of drug that acts directly on the sGC enzyme to increase sensitivity to NO and ultimately restore cGMP production (19, 20). However, the importance and/or the mechanisms by which the sGG-cGMP signaling pathway contributes to proper fetal growth are unknown.
Nutrient availability and the ability of nutrients to be transported to the fetus are critical for fetal development (21). Maternal-fetal nutrient transfer is influenced by uteroplacental blood flow with the placenta serving as the nutritional interface between maternal and fetal circulation (22). Placental surface area and morphology and expression and activity of placental nutrient transporter proteins are important determinants of fetal growth capacity (21). Clinical (23, 24) and experimental (25–27) studies report that PE and IUGR are associated with impaired placental remodeling and changes in placental morphology. Altered nutrient transporter expression is also reported in human placentas from pregnancies complicated by PE and/or IUGR (28–33) and in experimental models of PE and/or IUGR (48, 49, 50, 56, 61). Yet, the underlying physiological pathways that contribute to impaired fetal growth in PE are not fully understood. Although sGC stimulators are contraindicated during pregnancy (34), the use of an sGC stimulator provides a pharmaceutical tool to investigate the contribution of the sGC signaling pathway in the pathogenesis of impaired fetal growth. Thus, this study tested the hypothesis that Riociguat, an sGC stimulator, prevents the development of IUGR in the RUPP rat model of PE. Furthermore, this study tested the hypothesis that improvement in fetal weight occurs in association with improved placental perfusion, placental morphology, and placental nutrient transporter protein expression.
METHODS
Ethical Approval
All experimental procedures were conducted in accordance with the National Institutes of Health guidelines. An animal use protocol was submitted and approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center for all studies performed in this manuscript. Reporting of animal experiments conforms to the ARRIVE Guideline (35) and complies with the ethical principles of this journal.
Animals
Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle. Four cohorts (four different delivery dates) of first-time, timed pregnant Sprague–Dawley (SD) rats (225–250 g) were purchased from Envigo Inc. (Indianapolis, IN). Upon arrival, each cohort of pregnant rats was placed on a 2020X diet, a soy-free breeding and lactation-specific diet (Envigo, Inc.), and housed singly, with each cage containing a plastic, red hut-like enrichment device and extra bedding. Food and water were available ad libitum. For each cohort, timed pregnant rats were randomly selected to undergo either the RUPP or Sham procedure. All animals undergoing surgical procedures were anesthetized using 2%–5% isoflurane by inhalation with analgesics (Rodent MD’s, Rimadyln, 2 mg/tablet, Bio-Serve, Flemington, NJ) provided for 3 days postsurgery. Rats were monitored twice daily postsurgery until gestation day 20 (G20). The RUPP procedure was performed at day 14 of gestation (G14) to induce PE and IUGR. In brief, in the RUPP procedure, a silver clip (0.203 mm) was placed around the abdominal aorta directly rostral the iliac bifurcation. Because compensation of blood flow to the placenta occurs in pregnant rats through an adaptive increase in ovarian blood flow, we also clipped both branches of the uterine artery with a silver clip (0.100 mm) (17). The Sham procedure involved complete visualization of the uterine horns and implanted embryos to ensure exposure to anesthesia and a comparable surgical procedure as the RUPP. After surgery, Sham and RUPP were randomly assigned to receive either the sGC stimulator, Riociguat (10 mg/kg/day) (methyl(4,6-diamino-2-(1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)(methyl)carbamate, CAS No. 625115-55-1, Cat. No. G6-6188, AChemblock, Burlingame, CA) solubilized in 90% corn oil and 10% dimethyl sulfoxide (DMSO) or vehicle (90% corn oil and 10% DMSO) via subcutaneous injection from G14 to G20, a dose calculated to mimic the increase in cGMP observed in the study by Bakrania et al. (19). The total number of dams included the following experimental groups: 10 vehicle-treated Sham (Sham), 12 vehicle-treated RUPP (RUPP), 12 sGC-treated Sham, and 10 sGC-treated RUPP. Total reabsorption of pups was observed in one RUPP and one sGC-treated RUPP. Thus, the number of litters utilized for fetal morphometric and placental nutrient transporter molecular assessments included: 10 Sham, 11 RUPP, 12 sGC-treated Sham, and 9 sGC-treated RUPP. Final numbers included two to three pregnant rats from each cohort in each experimental group. Placental, fetal body, brain, and liver weights were collected at G20 under anesthesia, placental and fetal location on the uterine horn were recorded, and placental tissues were harvested for morphological and molecular/analysis. Uterine artery resistance index (UARI) was performed at G20 in the last three cohorts of timed pregnant rats. cGMP concentration was assessed in placenta and uterine arteries from dams in the last cohort (n = 3 rats per group). A 12-h urinary cGMP was measured in a separate cohort of timed pregnant rats from urine collected overnight at G19 through G20.
Experimental Details
Dams and offspring were anesthetized with isoflurane at G20 for collection of blood, placental, and fetal tissues and euthanized according to The Guide for the Care and Use of Laboratory Animals (8th edition) and the AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Placental, fetal body, liver, and brain weight were obtained from each viable offspring. Viability of each fetus was determined based on active breathing and movement at G20 by a trained investigator blinded to litter identity. Placentas were sliced in half at their central axis with one half used for morphological assessment and the other half frozen at −80°C for molecular assessment. Placentas for assessment were selected based on representative average placental weight and median location on the uterine horn, and only placentas from dams with viable pups were utilized for morphological or molecular assessment.
Fetal Survival Ratio and Litter Size
At G14, the number of implanted embryos was recorded during the Sham or RUPP procedures, with the number of implanted embryos counted per uterine horn. At G20, the total number of viable fetal pups was recorded. To determine fetal survival ratio, the number of viable offspring at G20 was compared with the number of implanted embryos at G14. Fetal survival ratio = (number of viable offspring at G20/number of implanted embryos at G14) × 100. Placental and viable fetal body weight and location within the uterine horn, in addition to brain weight and liver weight of offspring, were recorded at G20 per dam.
Uterine Artery Resistance Index
UARI was measured by Doppler sonography at G20 on the morning before euthanasia. Only the last three cohorts of timed pregnant rats were used for UARI measurement. Investigator was blinded to animal identity during measurement and analysis of UARI. Dams were anesthetized by isofluorane anesthesia and fixed on the platform of a Vevo 3100 unit (FUJIFILM, Visualsonics, Inc., Toronto, Canada). Doppler velocimetry measurements were taken on uterine arteries from each uterine horn. Waveform images representing the peak systolic velocity (PSV) and end-diastolic velocity (EDV) were captured. Three waveforms were measured per frame. UARI was calculated using the following formula: UARI = (PSV − EDV)/PSV. Average of the three UARI measurements represented the final UARI of each animal. The number of dams per experimental group included the following: eight vehicle-treated Sham (Sham), nine vehicle-treated RUPP (RUPP), nine sGC-treated Sham, and eight sGC-treated RUPP.
Placental Morphology
Placentas of viable offspring at G20 were weighed with a representative placenta per litter selected for morphological assessment. The placenta selected was closest to the average placental weight of the litter and location on the uterine horn, not located directly adjacent to the ovary or cervix. Placentas were fixed in 10% formalin for 24 h at 4°C. Fixed placentas were then washed in 1× phosphate-buffered saline (PBS) three times for 15 min and stored in HistoPrep 70% ethanol (Fisher Scientific, Pittsburgh, PA) at room temperature. Then, eight placentas from each experimental group were selected at random for morphological assessment. Tissues were sectioned in 4-μm thicknesses and stained with hematoxylin and eosin (H&E) by the Histology Core (University of Mississippi Medical Center, Jackson, MS). Slides were digitalized by the Pathology Core (University of Mississippi Medical Center) using a Philips Ultra-Fast Scanner. Slides were visualized and quantified with Philips Image Management System (Philips Electronics, Amsterdam, The Netherlands). Morphological measurements were performed by two different individuals blinded to the identity. To further control for morphological differences based on variation in placental surface area, whole placental surface area was measured in each experimental group (n = 8 rats per group). Five placentas from each experimental group (vehicle or sGC-treated RUPP or Sham) that had a surface area closest to the average for all placentas from all experimental groups were selected for final morphological assessment. Placental tissues were then assessed for surface area of each zone by taking three measurements of width from the junctional and labyrinth zones of each placenta, one at the center, and two at the outer one-thirds and calculating the average surface areas. The average surface area values were determined by the two individuals that were blinded to sample identity and then averaged, and this value represented the final surface area for each zone. Normalization to placental surface area for junctional and labyrinth zones was expressed as the ratio of the relative surface area of the junctional zone or labyrinth zone to junctional zone plus labyrinth zone [J:(J + L) or L:(J + L)] for each placenta.
For junctional zone and labyrinth zone quantification, measurements were taken by demarcating the boundaries of the junctional zone and labyrinth zone at ×10 magnification. The junctional zone separates the labyrinth zone from maternal decidua, and the junctional zone is pale in appearance due to the absence of fetal blood. The inner edge of the junctional zone was identified by the presence of spongiotrophoblasts and glycogen trophoblasts. The outer edge of the junctional zone was identified by morphological detection of trophoblast giant cells. Width of the junctional zone was determined by drawing a line from the inner edge of the junctional zone to the outer edge of the junctional zone. The labyrinth zone is vascular and closest to the fetus. The inner edge of the labyrinth zone was identified by fetal and maternal blood spaces separated by vascular types; the outer edge of the labyrinth zone was identified by the chorionic plate. Measurements of the labyrinth zone were obtained by drawing a line from the outer edge of the labyrinth zone and extending the line to the inner edge of the labyrinth zone. Whole placental surface area was measured by drawing a circle that encompassed the outer edge of the junctional zone to the inner edge of the labyrinth zone and obtaining the total area of the space within.
Placental Nutrient Transporter Protein Expression
Placental tissue was assessed for protein expression of glucose transporter 1 (Glut-1), heart fatty acid-binding protein (hFABP), and ATP-binding cassette (ABCA1) by Western blot in placental tissue harvested at G20. Placentas of viable offspring at G20 were weighed with a representative placenta per litter selected for molecular assessment which was closest to the average placental weight of the litter and location on the uterine horn, not located directly adjacent to the ovary or cervix. Placentas were homogenized in RIPA buffer (Thermo Scientific, Rockford, IL) and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MA). Samples were centrifuged for 12 min at 3,200 rpm. The supernatant was collected, and the protein concentration (1:10 dilution) was measured by Pierce BCA Protein Assay (Thermo Scientific). Protein samples for analysis of Glut-1 protein expression were combined with 2× Laemmli sample buffer (65.8 mM Tris·HCl, 4.2% SDS, 26.3% (wt/vol) glycerol, pH 6.8; Bio-Rad, Hercules, CA) and 5% 2-mercaptoethanol (BME; Sigma Aldrich, St. Louis, MO). The SDS concentration was increased in the 2× Laemmli sample buffer from 2.1 to 4.2% to aid in protein migration. Samples were allowed to sit at room temperature for 1 h to denature protein and reduce background and irreversible aggregation of glucose transporters per manufacturer recommendation (Abcam, Cambridge, UK). Furthermore, 10–20 μg of protein was added to each well in a 10–20% SDS-PAGE gel (Bio-Rad, Hercules, CA). Protein samples for quantification of hFABP and ABCA1 were combined with 2× Laemmli sample buffer (65.8 mM Tris·HCl, 2.1% SDS, 26.3% (wt/vol) glycerol, pH 6.8) and 5% BME and boiled at 100°C for 5 min. A total of 10–20 μg of protein was loaded into each well in a 4%–20% SDS-PAGE gel (Bio-Rad, Hercules, CA) for ABCA1 and hFABP protein assessment. Following SDS-PAGE electrophoresis, resolved proteins were transferred onto a nitrocellulose membrane (Bio-Rad) and incubated in a blocking solution [PBS containing 0.2% Tween-20 (PBST) and 5% nonfat milk] for 1 h at 4°C. Membranes were individually probed using the following primary antibodies: anti-Glut-1 (Abcam) at a dilution of 1:100,000, anti-hFABP (Abcam) at a dilution of 1:500, or anti-ABCA1 (Thermo Scientific, Rockford, IL) at a dilution of 1:500 at 4°C overnight. The blots were thoroughly washed and then exposed to goat anti-rabbit IgG secondary antibody (Li-Cor, Lincoln, NE) at a dilution of 1:15,000 for 1 h at 4°C. Signal was assessed by the Li-Cor Odyssey imager (Li-Cor, Lincoln, NE). Proteins were normalized to total protein (Revert Total Protein Stain, Li-Cor, Lincoln, NE).
cGMP Concentration
To determine if Riociguat dosage was appropriate to stimulate the sGC-cGMP pathway, a marker of sGC stimulation and metabolite of the NO-sGC pathway was measured by overnight urinary collection (12 h) of cGMP in a separate cohort of timed pregnant rats (n = 3 per experimental group). Dams were placed in a metabolic cage from 7 PM to 7 AM on G19-G20. Urinary cGMP concentration was measured by ELISA (R&D Systems, Minneapolis, MN) by the Analytical and Assay Core (University of Mississippi Medical Center, Jackson, MS) and normalized to 12-h urine volume. This cohort of dams was used to quantitate urinary cGMP to avoid the influence of stress induced by the metabolic collection procedure as a biological variable on overall outcomes. Thus, no other maternal or fetal data from these dams were included. To determine if the effect on cGMP production by administration of the sGC stimulator during pregnancy was tissue-specific, the concentration of cGMP was measured in placental tissue and uterine arteries collected from the same animals within the same cohort; cGMP concentration was measured by ELISA (Cayman Chemical, Ann Arber, MI) by the Analytical and Assay Core (University of Mississippi Medical Center). Concentration was normalized to total protein concentration in placental tissue and uterine arteries (Pierce BCA Protein Assay, Thermo Scientific). Placentas for this measurement were selected from placentas not used in morphological or placental nutrient transporter assessment and based on average weight and location on the uterine horn.
Statistics
Graphpad PRISM version 8 (Graph Pad Software, San Diego, CA) was used for all statistical analysis. Comparisons among groups were performed by two-way ANOVA with Sham or RUPP and treatment group (Vehicle/sGC Stimulator) as factors. Where there was a significant interaction, post hoc analysis with uncorrected Fisher’s least-significant difference for pairwise comparisons among groups was used to determine significance between groups. The number of litters used for fetal morphometric and placental analyses only involved litters with viable offspring. Outliers were not excluded to fully capture the effect of drug on maternal and fetal parameters. The level of significance was set at P < 0.05. All results are presented as mean ± standard deviation.
RESULTS
Fetal Morphometrics and Average Number of Offspring per Dam at G20
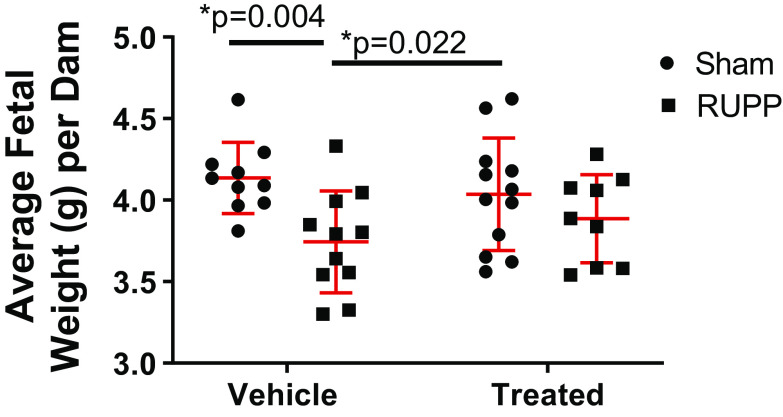
Average fetal weight per dam was significantly reduced at G20 in viable offspring from RUPP compared with Sham (P = 0.004) and sGC-treated Sham (P = 0.022) (Fig. 1). Average fetal weight per dam was not significantly reduced in viable offspring in sGC-treated RUPP compared with Sham or sGC-treated Sham (Fig. 1). Brain, liver, and placental weight did not significantly differ among groups, regardless of treatment (Table 1). When brain and liver weight were normalized to percent body weight, only liver weight differed between RUPP and sGC-treated Sham (0.074% ± 0.01% vs. 0.063% ± 0.01%, respectively; P = 0.025), but sGC-treated did not differ compared with Sham and RUPP counterparts (0.063% ± 0.01% vs. 0.068% ± 0.01%, respectively; P = 0.226).
Figure 1.
Average fetal weight of viable offspring per dam at gestation day 20 (G20) in Sham-operated and reduced uterine perfusion pressure (RUPP) dams treated with vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each data point represents the average fetal weight of viable offspring per dam. n = 10 Sham rats, n = 11 RUPP rats, n = 12 sGC-treated Sham rats, n = 9 sGC-treated RUPP rats. Scatter plots represent means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
Table 1.
Differences in viable fetal body, brain, liver and placenta weight in RUPP and Sham-operated dams in response to vehicle or a sGC stimulator (Riociguat 10 mg/kg/day)
| Gestational Day 20 Morphometrics |
||||
|---|---|---|---|---|
| Vehicle-treated Shamn = 10 | Vehicle-treated RUPPn = 11 | sGC-treated Shamn = 12 | sGC-treated RUPPn = 9 | |
| Body weight, g | 4.14 ± 0.22 | 3.72 ± 0.26*# | 4.04 ± 0.35 | 3.89 ± 0.27 |
| Brain weight, g | 0.19 ± 0.05 | 0.16 ± 0.04 | 0.17 ± 0.04 | 0.16 ± 0.02 |
| Liver weight, g | 0.28 ± 0.05 | 0.27 ± 0.05 | 0.26 ± 0.03 | 0.26 ± 0.04 |
| Placental weight, g | 0.70 ± 0.12 | 0.71 ± 0.13 | 0.68 ± 0.10 | 0.76 ± 0.18 |
All data points are expressed as means ± SD, n = number of rats. *P < 0.05 denotes significant difference vs. Sham; #P < 0.05 denotes significant difference vs. soluble guanylate cyclase (sGC)-treated Sham. Each point represents the average weight per dam. n = 10 Sham, n = 11 reduced uterine perfusion pressure (RUPP), n = 12 sGC-treated Sham, n = 9 sGC-treated RUPP. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison.
Fetal Survival Ratio of Offspring from Vehicle-Treated and sGC-Treated Dams
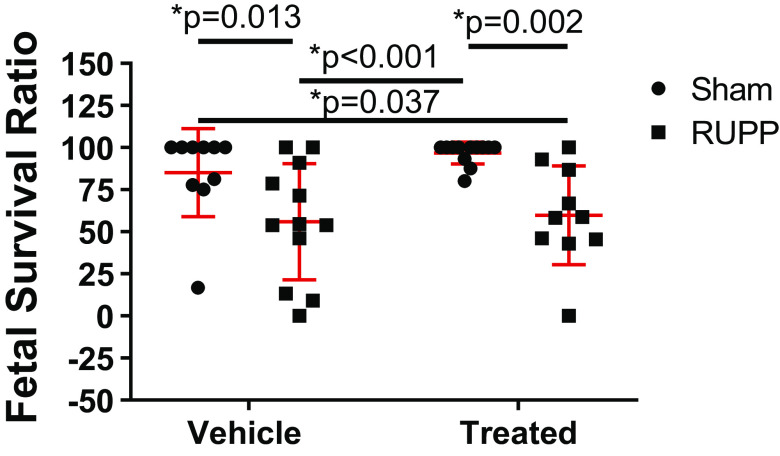
Fetal survival ratio at G20 was significantly reduced in RUPP (P = 0.013) and sGC-treated RUPP (P = 0.037) compared with Sham. Fetal survival ratio was also reduced in RUPP (P < 0.001) and sGC-treated RUPP (P = 0.002) compared with sGC-treated Sham (Fig. 2).
Figure 2.
Fetal survival ratio at gestation day 20 (G20) in reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Each point represents the average fetal survival ratio per dam. Fetal survival ratio = (number of viable offspring at G20/number of implanted embryos at G14) × 100. n = 10 Sham rats, n = 12 RUPP rats, n = 12 sGC-treated Sham rats, n = 10 sGC-treated RUPP rats. Scatter plots are expressed as means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
Uterine Artery Resistance Index
Representative UARI images for each group are shown in Fig. 3A. At G20, UARI was significantly elevated in RUPP compared with Sham (P = 0.010; Fig. 3B). UARI was not significantly increased in sGC-treated RUPP versus Sham, but UARI was significantly increased in sGC-treated Sham compared with Sham (P < 0.001). UARI was significantly reduced in sGC-treated RUPP compared with sGC-treated Sham (P = 0.010; Fig. 3B).
Figure 3.
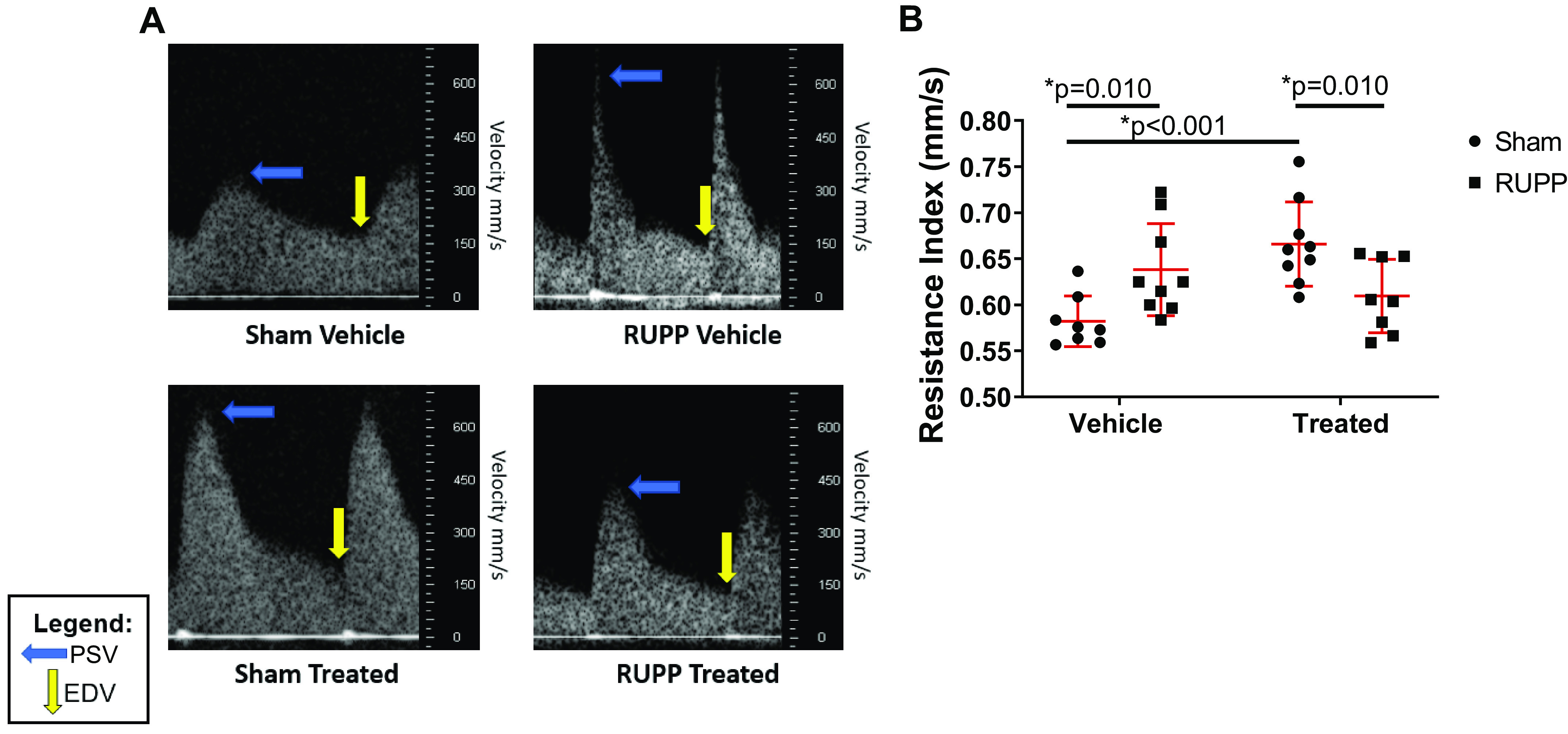
Uterine artery resistance index (UARI) at gestation day 20 (G20) in reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). A: representative UARI images. The blue arrow indicates the point of peak systolic velocity (ESV), and the yellow arrow indicates the point of end-diastolic velocity (EDV). B: UARI was calculated using the following formula: UARI = (PSV − EDV)/PSV. n = 8 Sham rats, n = 9 RUPP rats, n = 9 sGC-treated Sham rats, n = 8 sGC-treated RUPP rats. Each scatter plot represents means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
Placental Morphology
Representative slides of placental morphology for each group are shown in Fig. 4. Whole placental surface area for labyrinth and junctional zones did not differ among experimental groups for placentas selected for study (Table 2). Junctional zone surface area was significantly increased in RUPP (P = 0.036) and sGC-treated Sham (P = 0.035) compared with Sham (Fig. 5A). Junctional zone surface area was no longer elevated in sGC-treated RUPP placenta (Fig. 5A). When normalized to labyrinth zone and junctional zone surface areas, the ratio of junctional zone to labyrinth zone plus junctional zone (J:(J + L)) was significantly increased in RUPP (P = 0.035) and sGC-treated Sham (P = 0.013) compared with Sham (Fig. 5C). Importantly, elevated placental J:(J + L) was no longer significantly increased in sGC-treated RUPP (Fig. 5C). Whereas, sGC-treated Sham was significantly elevated compared with sGC-treated RUPP (P = 0.022; Fig. 5C). Labyrinth zone surface area did not differ among groups, regardless of treatment (Fig. 5B). Normalized labyrinth zone surface area [L:(J + L)] was significantly reduced is RUPP compared with Sham (P = 0.031) but was no longer significantly reduced in sGC-treated RUPP (Fig. 5D). Interesting, L:(J + L) was significantly reduced in sGC-treated Sham compared with Sham (P = 0.012) and sGC-treated RUPP (P = 0.022) (Fig. 5D).
Figure 4.
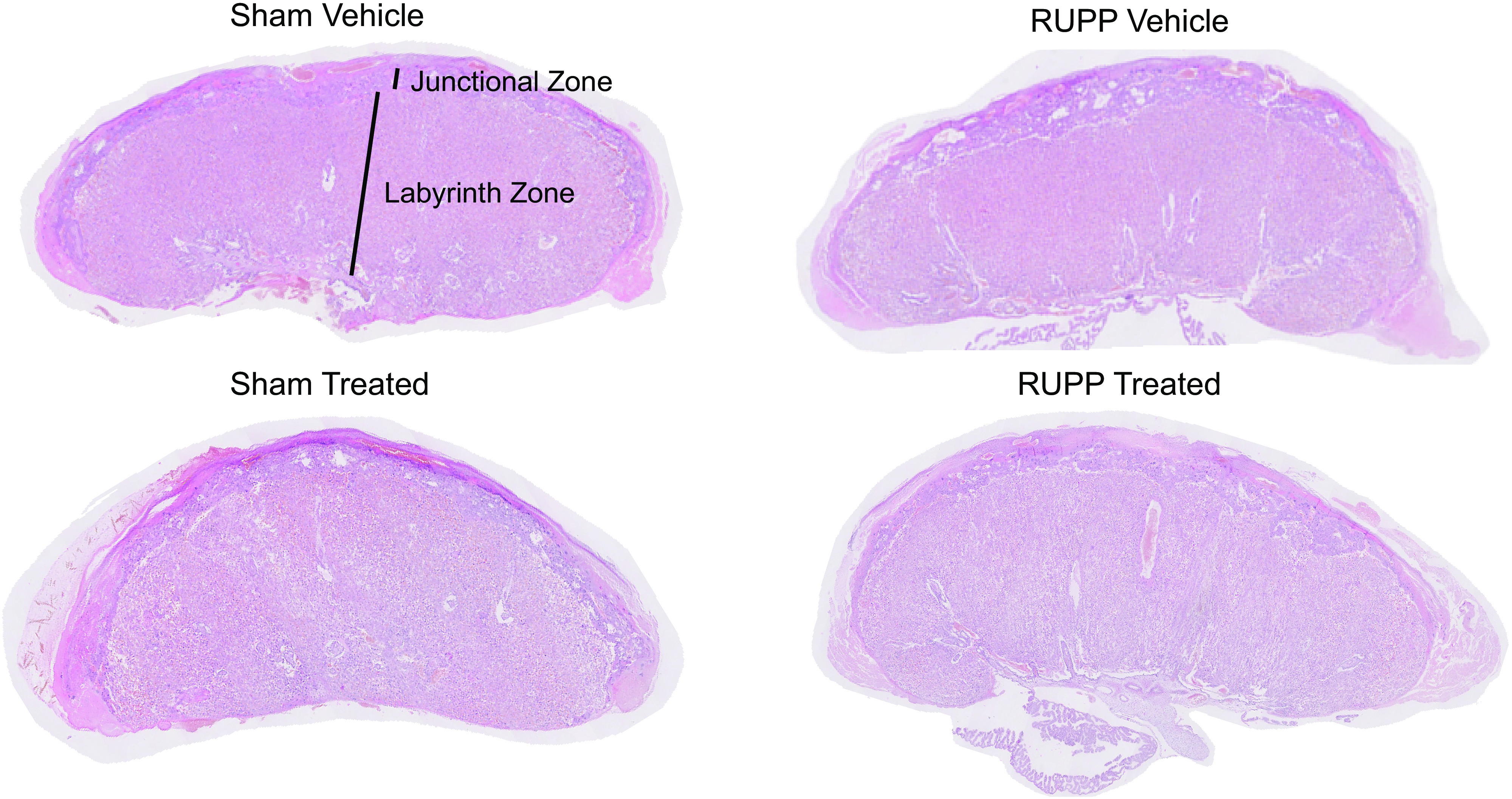
Representative scanned image of one placenta per group for quantification and identification of junctional and labyrinth zones. Placental tissue at gestation day 20 (G20) from reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Placentas for morphological assessment were selected based on average whole placental surface area from randomly selected placentas that were near the average placental weight and median location on the uterine horn at harvest.
Table 2.
Whole placental surface area in RUPP and Sham-operated dams in response to vehicle or a sGC stimulator (Riociguat 10 mg/kg/day)
| Whole Placental Surface Area |
||||
|---|---|---|---|---|
| Vehicle-treated Shamn = 5 | Vehicle-treated RUPPn = 5 | sGC-treated Shamn= 5 | sGC-treated RUPPn = 5 | |
| Surface area, mm2 | 29.42 ± 2.88 | 29.85 ± 4.11 | 29.88 ± 3.81 | 29.54 ± 3.78 |
All data points are expressed as means ± SD, n = number of rats. *P < 0.05 denotes significant difference between groups. Each point represents the average whole placental surface area per dam. Placentas selected based on average whole placental surface area from randomly selected placentas that were near the average placental weight and median location on the uterine horn at harvest. n = 5 Sham, n = 5 reduced uterine perfusion pressure (RUPP), n = 5 soluble guanylate cyclase (sGC)-treated Sham, n = 5 sGC-treated RUPP. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison.
Figure 5.
Quantification of junctional and labyrinth zone in placental tissue at gestation day 20 (G20) from reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). A: average surface area (mm2) of the junctional zone. B: average surface area (mm2) of the labyrinth zone. C: surface area of the junctional zone (mm2) normalized to total junctional zone and labyrinth zone surface areas (J:(J + L)). D: surface area of the labyrinth zone (mm2) normalized to total junctional zone and labyrinth zone surface areas (L:(J + L)). Each point represents one placenta that was selected based on average whole placental surface area from randomly selected placentas that were near the average placental weight and median location on the uterine horn at harvest. n = 5 Sham rats, n = 5 RUPP rats, n = 5 sGC-treated Sham rats, n = 5 sGC-treated RUPP rats. Scatter plots represent means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
Nutrient Transporter Protein Expression
Representative images for each protein of interest for each group are shown in Fig. 6, A–C. Placental Glut-1 and ABCA1 protein expression did not significantly differ, regardless of treatment (Fig. 6, A and B). However, placental protein expression of hFABP was significantly increased in RUPP compared with Sham (P = 0.008; Fig. 6C). Placental protein expression of hFABP was not significantly increased in sGC-treated RUPP compared with RUPP and Sham (Fig. 6C). Yet, sGC-treated Sham showed a significant increase in placental hFABP protein expression compared with Sham (P = 0.008; Fig. 6C).
Figure 6.

Placental protein expression of glucose transporter-1 (Glut-1) (A), ATP-binding cassette-1 (ABCA1) (B), and heart fatty acid-binding protein (hFABP) in placental tissue at gestation day 20 (G20) (C) in reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). Representative Western blot image illustrates scatter plot summary, where each point represents one placenta per litter selected based on average weight and location on the uterine horn. n = 10 Sham rats, n = 11 RUPP rats, n = 12 sGC-treated Sham rats, n = 9 sGC-treated RUPP rats. Scatter plots represent means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
cGMP Concentration
Overnight urinary excretion of cGMP was significantly reduced in RUPP compared with Sham (P = 0.003), but it was increased in sGC-treated Sham (P < 0.001) and sGC-treated RUPP (P = 0.018) compared with RUPP (Fig. 7A). Overnight urinary excretion of cGMP was also significant reduced in sGC-treated RUPP compared with sGC-treated Sham (P = 0.011; Fig. 7A). Placental (P = 0.017) and uterine artery (P = 0.003) cGMP concentrations were reduced in RUPP compared with Sham (Fig. 7, B and C, respectively). Placental (P = 0.004) and uterine artery (P < 0.001) cGMP concentration levels were also reduced in RUPP compared with sGC-treated Sham (Fig. 7, B and C, respectively). Yet, placental (P = 0.006) and uterine artery (P = 0.004) cGMP concentration levels were increased in sGC-treated RUPP versus RUPP (Fig. 7, B and C, respectively). cGMP concentration in placenta or uterine artery were not significantly altered in Sham compared with sGC-treated Sham.
Figure 7.
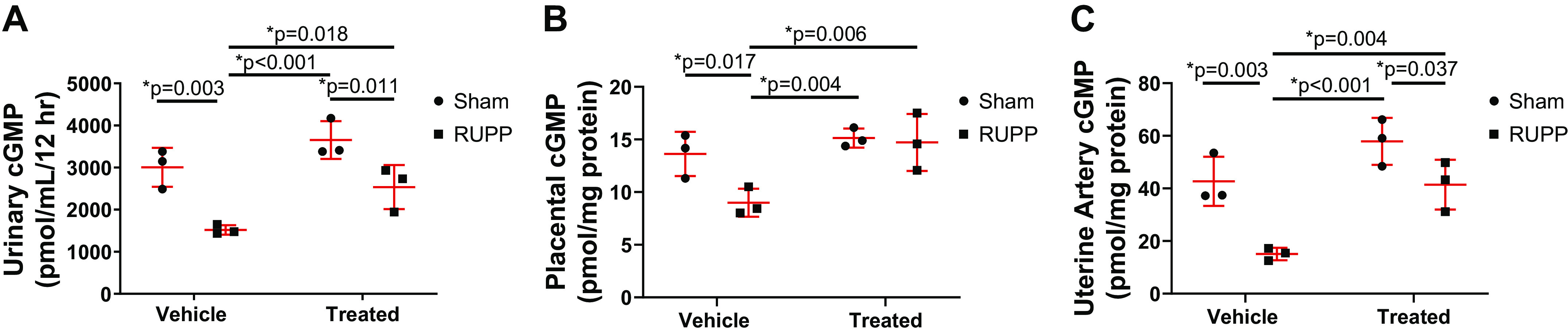
Guanosine 3',5'-cyclic monophosphate (cGMP) concentration in reduced uterine perfusion pressure (RUPP) and Sham-operated dams in response to vehicle or a soluble guanylate cyclase (sGC) stimulator (Riociguat 10 mg/kg/day). A: 12-h overnight urinary cGMP from gestation day (G19) to gestation day 20 (G20). cGMP concentration normalized to 12-h urine volume. Each point represents normalized urine concentration from one dam per experimental group from a separate cohort that was not utilized in additional assessments to avoid the influence of stress as a biological variable. Placental cGMP concentration (B) and uterine artery cGMP concentration from dams in the last cohort (C). n = 3 Sham rats, n = 3 RUPP rats, n = 3 sGC-treated Sham rats, n = 3 sGC-treated RUPP rats. Scatter plots are expressed as means ± SD. Two-way ANOVA with uncorrected Fisher’s least significant difference post hoc analysis was used for pairwise comparison. *P < 0.05 denotes significant difference between groups.
DISCUSSION
This study tested the hypothesis that administration of a sGC stimulator during late pregnancy in a rat model of PE and IUGR induced via placental insufficiency will improve fetal weight in association with improved placental perfusion, placental nutrient transport protein expression, and placental morphology. The major findings from this study include the following: our study demonstrated that UARI was not significantly elevated in sGC-treated RUPP compared with Sham, the vehicle-treated control group, suggesting that targeting the sGC pathway improved uteroplacental blood flow. Yet, UARI was elevated in sGC-treated Sham compared with Sham, implying that administration of the sGC stimulator was associated with a reduction in uteroplacental blood flow. Importantly, fetal body weight was not significantly reduced in sGC-treated RUPP compared with Sham. Proportional placental junctional zone surface area was significantly elevated and proportional labyrinth zone surface area was significantly reduced in RUPP compared with Sham; placental abnormalities not observed in sGC-treated RUPP, suggesting that treatment with an sGC stimulator was associated with placental remodeling. Unlike RUPP, placental hFABP protein expression was not significantly elevated in sGC-treated RUPP compared with Sham. However, placental hFABP protein expression was elevated in sGC-treated Sham—a pattern of change that paralleled the increase in UARI, suggesting an adapted placental functional response to increased UARI in sGC-treated Sham. Lastly, urinary, placental, and uterine artery cGMP concentrations were reduced in RUPP compared with Sham; treatment with an sGC stimulator was associated with a significant increase in urinary, placental, and uterine artery cGMP concentrations in sGC-treated RUPP compared with RUPP. Collectively, these findings indicate that administration of a sGC stimulator during late pregnancy in a rat model of PE and IUGR improved fetal growth in association with improved uteroplacental blood flow and placental remodeling compared with Sham. These data suggest that the sGC-cGMP pathway is associated with the pathogenesis of IUGR in this clinically relevant, and well-characterized model of PE.
Numerous clinical studies demonstrate that UARI is elevated in women with PE (36–38). Experimental studies using animal models that mimic the characteristics of PE also report an increase in UARI (39–41). Santiago-Font et al. (40) and Tam et al. (41) reported that UARI is increased in RUPP compared with Sham. The study by Tam et al. was the first to suggest that a reduction in uteroplacental blood flow in the RUPP model of PE is associated with a reduction in fetal growth in late gestation. Moreover, Tam et al. (41) reported that administration of an endothelin (ET) Type A receptor antagonist in the RUPP is associated with a reduction in UARI in association with improved maternal BP and fetal weight, which indicates a role for ET in the etiology of PE and IUGR. However, this study did not explore the mechanisms by which ET receptor blockade improved impaired fetal growth.
In our study, the increase in UARI at G20 in RUPP was not observed in sGC-treated RUPP compared with Sham. However, UARI was elevated in sGC-treated Sham relative to Sham, suggesting administration of an sGC stimulator had a divergent effect on UARI in Sham compared with RUPP. This finding also suggests that sGC stimulators may elicit harm when utilized in nonpathological conditions, confirming that the use of a sGC stimulator is contraindicated during pregnancy (34). Yet, unlike RUPP, increased UARI in sGC-treated Sham did not correlate with a reduction in fetal weight. IUGR in the RUPP is associated with the many characteristics of PE including systemic inflammation, vascular dysfunction, and enhanced responsiveness to vasoconstrictors such as angiotensin II and ET (42, 43). Therefore, increased UARI in the absence of other confounding factors in PE may not be sufficient to impair fetal growth at G20 in the Sham. Whether increased UARI as an indicator of placental insufficiency will impair fetal growth by birth in sGC-treated Sham is not yet known.
Fatty acids, glucose, cholesterol, and amino acids are needed for adequate fetal growth (44). The mechanisms that regulate placental function to maintain fetal demand are not well-known. However, changes in nutrient transporter expression and activity are associated with maternal-fetal nutrient exchange (45), nutrient delivery, and fetal growth (44), suggesting that nutrient transporter expression can influence fetal growth. hFABP is the primary placental nutrient transporter involved in brain development (44, 46). Placental hFABP mRNA expression is elevated in placental tissue from pregnancies complicated by PE (33). Scifres et al. (47) reported that an elevation in serum hFABP predicts the onset of PE. Experimental studies also report that placental hFABP expression is elevated in models of IUGR in rodent (48) and ewes (49) and in vitro models of PE induced by hypoxia (29, 50). Hypoxia is potential mediator of fatty acid transporter expression (29), indicating that an increase in placental hFABP under conditions of maternal under-perfusion and low oxygen may be a compensatory response to maintain normal brain development (51). Thus, an increase in protein expression of placental hFABP may not only be a compensatory response in RUPP to reduced uterine blood flow, but also in sGC-treated Sham.
In all study groups, brain growth was maintained at G20, indicating brain sparing regardless of maternal status, RUPP/Sham, or treatment group. Although the Alexander laboratory observes asymmetrical growth at birth in IUGR offspring, asymmetrical growth was not yet apparent at G20 in this study. Liver and brain weight are crude measurements of brain and liver development and are indicators of asymmetrical organ growth (52). The fetal growth trajectory is determined in early pregnancy but can be altered in response to nutritional insults in later gestation (53). The RUPP model is conducted at the start of the third trimester and is associated with both reduced weight and asymmetrical growth at birth, indicating that the RUPP procedure impairs growth by altering nutrient delivery. The present study suggests that although fetal weight was reduced in offspring from RUPP dams, asymmetrical growth occurs in the final days of gestation, after G20.
Although we hypothesized that placental protein expression of Glut-1 and ABCA1 nutrient transporters would differ between RUPP compared with Sham counterparts, placental protein expression did not vary among groups at G20 regardless of treatment. Glut-l is the main glucose transporter in the placenta (30). Zamudio et al. (54) showed that reduction in placental Glut-1 protein expression is associated with reduction in fetal glucose consumption and reduced fetal growth. Previous studies using different experimental models of IUGR reported conflicting results about placental Glut-1 protein expression. An in vitro study by Baumann et al. (55) showed that hypoxia in BeWo choriocarcinoma cells is associated with a dose-dependent increase in Glut-1 protein expression. In the bilateral uterine ligation model of IUGR, another model of placental insufficiency in the rat, placental Glut-1 protein expression is reduced at G19 (48) and G21 (56). In a clinical study of isolated placental microvillous membrane, maternal-facing cellular membrane protein expression of Glut-1 is significantly reduced in women with pregnancies complicated by PE compared with women with normal pregnancies (57); however, Glut-1 mRNA expression does not differ between PE versus normal pregnancy (57). Jansson et al. (30) and Janzen et al. (31) reported that placental protein expression of Glut-1 does not differ in placental tissue from women with early- and/or late-onset IUGR. However, Janzen et al. (31) also reported that mRNA expression of Glut-1 from the maternal portion of the placenta is significantly reduced in late-onset IUGR compared with controls. Protein expression may not equal mRNA expression or function, but collectively, these studies suggest that placental Glut-1 protein expression is dependent on the severity or the etiology of the maternal insult in both humans and rodents, and Glut-1 expression is often associated with compensatory regulation to meet fetal demand in instances of hypoxia, undernutrition, and IUGR (45).
ABCA1 is a cholesterol transporter also necessary for normal fetal development (58). Like Glut-1, expression of placental ABCA1 also varies in PE (28, 59, 60). A study by Baumann et al. (28) showed that mRNA expression of placental ABCA1 is significantly increased in preterm compared with term, but mRNA expression of placental ABCA1 is downregulated in isolated PE and in PE with IUGR compared with age-matched preterm controls. However, Albrecht et al. (59) showed that placental mRNA and protein expression of ABCA1 do not differ in women with PE compared with third trimester controls. Yet, findings in these studies did not consider the timing of onset or the severity of PE. Plösch et al. (60) reported that mRNA and protein expression of ABCA1 are significantly elevated in placental explants from early-onset but not late-onset PE compared with gestational age-matched controls, suggesting that expression of placental ABCA1 may be dependent upon the timing of disease progression. Unlike our study whereby placental ABCA1 protein expression did not differ at G20, in a mouse model of PE that utilizes knockout of catechol-O-methyltransferase (COMT), COMT(−/−) is associated with IUGR and a significant reduction in placental protein expression of ABCA1 at G18.5 (61). Collectively, these findings suggest that placental expression of ABCA1 may differ based on the experimental model and gestational day at time of study.
The transport of amino acids also varies in PE. Transport of l-arginine is increased in PE (62), whereas placental system A transporter (SNAT) mRNA is not altered in PE (63) but is reduced in pregnancies with small for gestational age babies without PE (64). Regnault et al. (32) reported that amino acid uptake is reduced in severe IUGR in an ovine model of placental insufficiency. Placental SNAT protein expression is also significantly reduced in a rat model of placental insufficiency (48). The transfer of amino acids across the placenta is a complex process due to multiple amino acid transporters that are regulated by several factors (65). Clearly, further studies are needed to determine if placental protein expression adequately denotes nutrient transporter function in the RUPP model. Investigation into the importance of other placental nutrient transporters including amino acid transporters may also indicate benefit related to improved fetal growth.
Numerous clinical (23, 24, 66) and experimental (25–27, 67) studies indicate that PE, placental hypoxia, placental under-perfusion, and IUGR are associated with impaired placental morphology. The placenta serves as the nutritional interface between maternal and fetal circulation (68). The placenta of both rodents and humans allows for direct passage of nutrients and waste between mother and fetus (69). Placental remodeling can impact nutrient and oxygen delivery by altering uteroplacental blood flow and surface area (21), influencing fetal development. The junctional and labyrinth zones compose the fetal component of the rat placenta (69), making these zones of specific interest in this study. The labyrinth zone is responsible for nutrient exchange, and the junctional zone is associated with trophoblast remodeling of the spiral arteries (69). Impairment in junctional zone morphology is associated with improper spiral artery remodeling and placentation (70). However, few studies examine placental morphology of the junctional and labyrinth zones in rat models of PE or IUGR. Wat et al. (27), using a model of reduced uterine perfusion in SD rats from Charles River, reported that a reduction in the ratio of junctional zone to labyrinth zone is associated with IUGR at G19. This study also showed impaired development of the labyrinth zone (27). In a model of IUGR that is associated with reduced plasma volume expansion and placental perfusion induced by a low-sodium diet starting at G14 in the SD rat, Bibeau et al. (25) reported that UARI is elevated at G21 and glycogen cell surface area in the junctional zone is increased but unchanged in the labyrinth zone in association with IUGR at G22 indicative of abnormal junctional zone remodeling. Yet, total and normalized junctional zone surface area were significantly increased, and proportional labyrinth zone surface area was reduced in RUPP at G20 in our study. Collectively, these studies suggest that placental morphology is impaired in association with IUGR (25, 27). Moreover, these findings indicate that differences in outcomes may be gestation day-specific, could vary due to unknown biological variables such as vendor differences which can alter phenotypical outcomes (71, 72), or could be due to subtle differences in the RUPP procedure or perinatal insult. Importantly, in our study UARI and total and proportional junctional zone surface area were no longer significantly elevated and proportional labyrinth zone surface area was no longer reduced in association with attenuation of reduced fetal weight in sGC-treated RUPP compared with Sham. These findings suggest that sGC-cGMP pathway is associated with placental remodeling, which could affect nutrient and oxygen delivery to a developing fetus and influence fetal growth in response to placental ischemia. However, administration of Riociguat in the Sham was associated with adaptive adverse changes in placental morphology correlating with an increase in UARI.
Fetal weight was not reduced in sGC-treated Sham at G20 but whether continued exposure to Riociguat during pregnancy in the Sham would be associated with a reduction in fetal weight is not known. The pathogenesis of reduced uterine blood flow in sGC-treated Sham is also not known. Although use of a sGC stimulator is contraindicated during normal pregnancy (34), specifics related to fetal harm are not reported. Findings from our study suggest that reductions in uteroplacental blood flow alter placental morphology and nutrient transport expression in Sham-operated dams; future goals will determine if longer exposure to a sGC stimulator is associated with a reduction in fetal growth and later increased risk for chronic disease in the offspring.
Finally, urinary cGMP, indicative of renal cGMP, was increased in sGC-treated RUPP compared with RUPP, an effect not observed in sGC-treated Sham compared with their vehicle-treated counterparts. Additionally, cGMP was reduced in placenta and uterine arteries in RUPP compared with Sham; administration of the sGC stimulator was associated with increased production of cGMP in these tissues in the RUPP. George et al. (16) were the first to report that renal cGMP levels are decreased in the RUPP. In this study, George et al. (16) also reported that phosphodiesterase-5 (PDE-5) is significantly increased in the kidney of the RUPP and that administration of the PDE-5 inhibitor sildenafil is associated with a decrease in BP in conjunction with an increase in renal cGMP in the RUPP. These data suggest that sildenafil may be a potential therapeutic target for the treatment of PE. However, this study was published before findings in clinical trials that were discontinued due to increased risk of fetal demise (73). Yet, in the context of our study, these studies indicate the importance of the sGC-cGMP pathway in the etiology of increased BP in PE and the adaptive placental-related changes associated with reduced fetal growth that occurs in response to placental ischemia.
Perspectives and Significance
In conclusion, this article used a well-established and clinically relevant model of PE and IUGR to understand the role of the sGC-cGMP pathway in pathogenesis of IUGR. Findings from this study suggest an important role for a reduction in activity of the sGC-cGMP pathway in the etiology of IUGR in PE. Yet, maternal administration of a sGC stimulator was associated with a reduction in uteroplacental blood flow and adaptive changes in placental morphology at G20 in the sGC-treated Sham indicative of potential harm. Whether prolonged exposure to a sGC stimulator results in reduced weight at birth in sGC-treated Sham or the developmental programming of chronic health in offspring is not yet known. The mechanism(s) by which uterine blood flow was reduced in response to administration of the sGC stimulator, Riociguat, in Sham is also not known highlighting the importance of preclinical studies using models of PE and IUGR with comparison to normotensive controls. To date, the only treatment for PE is early delivery. Thus, understanding the mechanisms involved in the etiology of IUGR in PE may lead to possible therapeutic approaches to improve fetal growth and ultimately health across lifespan in the IUGR offspring and ensure benefit without harm in the mother and her offspring.
GRANTS
This work was supported by the National Institutes of Health (NIH) Grants R56HL143459 and HL143459, with additional funding provided by HL51971, P20GM104357, and P20GM121334. L. E. Coats received funding from NIH T32HL105324.
DISCLAIMERS
The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.E.C., N.B.O., and B.T.A. conceived and designed research; L.E.C., D.R.B.-F., A.M.A., B.A.B., and A.Z.R. performed experiments; L.E.C., B.A.B., N.B.O., and B.T.A. analyzed data; L.E.C. and B.T.A. interpreted results of experiments; L.E.C. prepared figures; L.E.C. drafted manuscript; L.E.C., A.M.A., and B.T.A. edited and revised manuscript; L.E.C., D.R.B.-F., A.M.A., B.A.B., A.Z.R., N.B.O., and B.T.A. approved final version of manuscript.
REFERENCES
- 1.Rana S, Lemoine E, Granger J, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res 127: 1094–1112, 2019. doi: 10.1161/CIRCRESAHA.118.313276. [DOI] [PubMed] [Google Scholar]
- 2.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstin FH, Sibai BM, Suklhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 3.Hermida RC, Ayala DE, Mojón A, Fernández JR, Alonso I, Silva I, Uceida R, Iglesias M. Blood pressure patterns in normal pregnancy, gestational hypertension, and preeclampsia. Hypertension 36: 149–158, 2000. doi: 10.1161/01.HYP.36.2.149. [DOI] [PubMed] [Google Scholar]
- 4.Ødegård RA, Vatten LJ, Nilsen ST, Salvesen KÅ, Austgulen R. Preeclampsia and fetal growth. Obstet Gynecol 96: 950–955, 2000. doi: 10.1016/S0029-7844(00)01040-1. [DOI] [PubMed] [Google Scholar]
- 5.Øglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens 27: 2051–2054, 2009. doi: 10.1097/HJH.0b013e328330052a. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-hour NO (x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO (x) diet. Am J Physiol 277: F48–F57, 1999. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Tang J, Li N, Zhou X, Zhu X, Li W, Liu B, Feng X, Tao J, Han B, Zhang H, Sun M, Zhang H. New conception for the development of hypertension in preeclampsia. Oncotarget 7: 78387–78387, 2016. doi: 10.18632/oncotarget.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baksu B, Davas I, Baksu A, Akyol A, Gulbaba G. Plasma nitric oxide, endothelin‐1 and urinary nitric oxide and cyclic guanosine monophosphate levels in hypertensive pregnant women. Int J Gynaecol Obstet 90: 112–117, 2005. doi: 10.1016/j.ijgo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123: 2263–2273, 2011. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutton EF, Gemmel M, Powers RW. Nitric oxide signaling in pregnancy and preeclampsia. Nitric Oxide 95: 55–62, 2020. doi: 10.1016/j.niox.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Schiessl B, Strasburger C, Bidlingmaier M, Mylonas I, Jeschke U, Kainer F, Friese K. Plasma-and urine concentrations of nitrite/nitrate and cyclic guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch Gynecol Obstet 274: 150–154, 2006. doi: 10.1007/s00404-006-0149-8. [DOI] [PubMed] [Google Scholar]
- 12.Sholook MM, Gilbert JS, Sedeek MH, Huang M, Hester RL, Granger JP. Systemic hemodynamic and regional blood flow changes in response to chronic reductions in uterine perfusion pressure in pregnant rats. Am J Physiol Heart Circ Physiol 293: H2080–H2084, 2007. doi: 10.1152/ajpheart.00667.2007. [DOI] [PubMed] [Google Scholar]
- 13.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennet WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2001. doi: 10.1161/01.HYP.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 14.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin-A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension 37: 485–489, 2001. doi: 10.1161/01.HYP.37.2.485. [DOI] [PubMed] [Google Scholar]
- 15.Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension 35: 367–372, 2000. doi: 10.1161/01.HYP.35.1.367. [DOI] [PubMed] [Google Scholar]
- 16.George EM, Palei AC, Dent EA, Granger JP. Sildenafil attenuates placental ischemia-induced hypertension. Am J Physiol Regul Integr Comp Physiol 305: R397–R403, 2013. doi: 10.1152/ajpregu.00216.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 18.Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E, Roach R, Moore LG. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol 300: R1221–R1229, 2011. doi: 10.1152/ajpregu.91046.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakrania BA, Spradley FT, Patel BR, Travis AB, Sandner P, Granger JP. Soluble guanylate cyclase activators increase cGMP expression and improve vascular function and placental ischemia-induced hypertension. FASEB J 33, 865.13. doi: 10.1096/fasebj.2019.33.1_supplement.865.13. [DOI] [Google Scholar]
- 20.Sandner P, Zimmer DP, Milne GT, Follmann M, Hobbs A, Stasch JP. Soluble guanylate cyclase stimulators and activators. In: Handbook of Experimental Pharmacology. Berlin: Spring Nature, 2018, p. 1–40. doi: 10.1007/164_2018_197. [DOI] [PubMed] [Google Scholar]
- 21.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy 2012: 1–14, 2012. doi: 10.1155/2012/179827.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths SK, Campbell JP. Placental structure, function and drug transfer. BJA Educ 15: 84–89, 2015. doi: 10.1093/bjaceaccp/mku013. [DOI] [Google Scholar]
- 23.Akhlaq M, Nagi AH, Yousaf AW. Placental morphology in pre-eclampsia and eclampsia and the likely role of NK cells. Indian J Pathol Microbiol 55: 17, 2012. doi: 10.4103/0377-4929.94848. [DOI] [PubMed] [Google Scholar]
- 24.Almasry SM, Elfayomy AK. Morphometric analysis of terminal villi and gross morphological changes in the placentae of term idiopathic intrauterine growth restriction. Tissue Cell 44: 214–219, 2012. doi: 10.1016/j.tice.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Bibeau K, Sicotte B, Beland M, Bhat M, Gaboury L, Couture R, St-Louis J, Brochu M. Placental underperfusion in a rat model of intrauterine growth restriction induced by a reduced plasma volume expansion. PloS One 11: e0145982, 2016. doi: 10.1371/journal.pone.0145982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Devl Biol 314: 362–375, 2008. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wat JM, Baczyk D, Kingdom JC. The antithrombin binding regions of heparin mediate fetal growth and reduced placental damage in RUPP model of preeclampsia. Biol Reprod 102: 1102–1110, 2020. doi: 10.1093/biolre/ioaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann M, Korner M, Huang X, Wenger F, Surbek D, Albrecht C. Placental ABCA1 and ABCG1 expression in gestational disease: pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta 34: 1079–1086, 2013. doi: 10.1016/j.placenta.2013.06.309. [DOI] [PubMed] [Google Scholar]
- 29.Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty acid–binding proteins in primary term human trophoblasts. Am J Obstet Gynecol 197: e1–e6, 2007. doi: 10.1016/j.ajog.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 23: 392–399, 2002. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 31.Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta 34: 1072–1078, 2013. doi: 10.1016/j.placenta.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regnault TRH, Friedman J, Wilkening RB, Anthony RV, Hay WW, Jr.. Fetoplacental transport and utilization of amino acids in IUGR—a review. Placenta 26: S52–S62, 2005. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Yan Y, Peng H, Wang P, Wang H, Dong M. Increased expression of fatty acid binding protein 4 in preeclamptic placenta and its relevance to preeclampsia. Placenta 39: 94–100, 2016. doi: 10.1016/j.placenta.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Riociguat Pregnancy and Breastfeeding Warnings. 2010. https://www.drugs.com/pregnancy/riociguat.html.
- 35.NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Physiol 588: 2519, 2015. doi: 10.1113/jphysiol.2010.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao SH, Chigirin N, Hoch V, Ahmed H, Frempong ST, Zhang M, Ruan JL, Kwak-Kim J. Uterine radial artery resistance index predicts reproductive outcome in women with recurrent pregnancy losses and thrombophilia. Biomed Res Int 1: 1–11, 2019. doi: 10.1155/2019/8787010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Mendez MA, Martinez-Gaytan V, Cortes-Flores R, Ramos-Gonzalez RM, Ochoa-Torres MA, Garza-Veloz I, Martinez-Acuña MI, Badillo-Almaraz JI, Martinez-Fierro ML. Doppler ultrasound evaluation in preeclampsia. BMC Res Notes 6: 477, 2013. doi: 10.1186/1756-0500-6-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarasevičienė V, Grybauskienė R, Mačiulevičienė R. sFlt-1, PlGF, sFlt-1/PlGF ratio and uterine artery doppler for preeclampsia diagnostics. Medicina 52: 349–353, 2016. doi: 10.1016/j.medici.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Cunningham MW, Castillo J, Ibrahim T, Cornelius DC, Campbell N, Amaral L, Vaka VR, Usry N, Williams JM, LaMarca B. AT1-AA (angiotensin II type 1 receptor agonistic autoantibody) blockade prevents preeclamptic symptoms in placental ischemic rats. Hypertension 71: 886–893, 2018. doi: 10.1161/HYPERTENSIONAHA.117.10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santiago-Font JA, Amaral LM, Faulkner J, Ibrahim T, Vaka VR, Cunningham MW, Jr, LaMarca BB. Serelaxin improves the pathophysiology of placental ischemia in the reduced uterine perfusion pressure rat model of preeclampsia. Am J Physiol Regul Integr Comp Physiol 311: R1158–R1163, 2016. doi: 10.1152/ajpregu.00192.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tam KBT, George E, Cockrell KL, Arany M, Speed J, Martin JN, Jr, Lamarca BB, Granger JP. Endothelin type A receptor antagonist attenuates placental ischemia–induced hypertension and uterine vascular resistance. Am J Obstet Gynecol 204: e1–e4, 2011. doi: 10.1016/j.ajog.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med 122: 383–392, 2006. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 43.Macdonald-Wallis C, Tilling K, Fraser A, Nelson SM, Lawlor DA. Associations of blood pressure change in pregnancy with fetal growth and gestational age at delivery: findings from a prospective cohort. Hypertension 64: 36–44, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brett K, Ferraro Z, Yockell-Lelievre J, Gruslin A, Adamo K. Maternal–fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 15: 16153–16185, 2014. doi: 10.3390/ijms150916153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaccioli F, Lager S, Powell TL, Jansson T. Placental transport in response to altered maternal nutrition. J Dev Orig Health Dis 4: 101–115, 2013. doi: 10.1017/S2040174412000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knipp GT, Liu B, Audus KL, Fujii H, Ono T, Soares MJ. Fatty acid transport regulatory proteins in the developing rat placenta and in trophoblast cell culture models. Placenta 21: 367–375, 2000. doi: 10.1053/plac.1999.0484. [DOI] [PubMed] [Google Scholar]
- 47.Scifres CM, Catov JM, Simhan H. Maternal serum fatty acid binding protein 4 (FABP4) and the development of preeclampsia. J Clin Endocrinol Metab 97: E349–E356, 2012. doi: 10.1210/jc.2011-2276. [DOI] [PubMed] [Google Scholar]
- 48.Nüsken E, Gellhaus A, Kühnel E, Swoboda I, Wohlfarth M, Vohlen C, Schneider H, Dötsch J, Nüsken KD. Increased rat placental fatty acid, but decreased amino acid and glucose transporters potentially modify intrauterine programming. J Cell Biochem 117: 1594–1603, 2016. doi: 10.1002/jcb.25450. [DOI] [PubMed] [Google Scholar]
- 49.Ma Y, Zhu MJ, Uthlaut AB, Nijland MJ, Nathanielsz PW, Hess BW, Ford SP. Upregulation of growth signaling and nutrient transporters in cotyledons of early to mid-gestational nutrient restricted ewes. Placenta 32: 255–263, 2011. doi: 10.1016/j.placenta.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jadoon A, Cunningham P, McDermott LC. Regulation of fatty acid binding proteins by hypoxia inducible factors 1α and 2α in the placenta: Relevance to pre-eclampsia. Prostaglandins Leukot Essent Fatty Acids 93: 25–29, 2015. doi: 10.1016/j.plefa.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhang W, Chen R, Yang T, Xu N, Chen J, Gao Y, Stetler RA. Fatty acid transporting proteins: roles in brain development, aging, and stroke. Prostaglandins Leukot Essent Fatty Acids 136: 35–45, 2018. doi: 10.1016/j.plefa.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell ML. Fetal brain to liver weight ratio as a measure of intrauterine growth retardation: analysis of 182 stillborn autopsies. Mod Pathol 14: 14–19, 2001. doi: 10.1038/modpathol.3880251. [DOI] [PubMed] [Google Scholar]
- 53.Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed 91: F299–F304, 2006. doi: 10.1136/adc.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, Tutino E, Martin B, Belliappa S, Balana E, Illsley NP. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS ONE 5: e8551, 2010. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumann M, Zamudio S, Illsley NP. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am J Physiol Cell Physiol 293: C477–C485, 2007. doi: 10.1152/ajpcell.00075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das UG, Sadiq HF, Soares MJ, Hay WW, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol 274: R339–R347, 1998. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- 57.Lüscher BP, Marini C, Joerger-Messerli MS, Huang X, Hediger MA, Albrecht C, Baumann MU, Surbek DV. Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia. Placenta 55: 94–99, 2017. doi: 10.1016/j.placenta.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 58.Baardman ME, Kerstjens-Frederikse WS, Berger RM, Bakker MK, Hofstra RM, Plösch T. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod 88: 24–21, 2003. doi: 10.1095/biolreprod.112.102442. [DOI] [PubMed] [Google Scholar]
- 59.Albrecht C, Soumian S, Tetlow N, Patel P, Sullivan M, Lakasing L, Nicolaides K, Williamson C. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta 28: 701–708, 2007.doi: 10.1016/j.placenta.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Plösch T, Gellhaus A, van Straten EM, Wolf N, Huijkman NC, Schmidt M, Dunk CE, Kuipers F, Winterhager E. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia. Placenta 31: 910–918, 2010. doi: 10.1016/j.placenta.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Liao S, Wu H, Chen R. Apolipoprotein A1 mimetic peptide ATI-5261 reverses arterial stiffness at late pregnancy and early postpartum in a COMT−/− mouse model of preeclampsia. Clin Hypertens 24: 11, 2018. doi: 10.1186/s40885-018-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speake PF, Glazier JD, Ayuk PY, Reade M, Sibley CP, D’Souza SW. L-Arginine transport across the basal plasma membrane of the syncytiotrophoblast of the human placenta from normal and preeclamptic pregnancies. J Clin Endo Metab 88: 4287–4292, 2003. doi: 10.1210/jc.2003-030067. [DOI] [PubMed] [Google Scholar]
- 63.Malina A, Daftary A, Crombleholme W, Markovic N, Roberts JM. Placental system A transporter mRNA is not different in preeclampsia, normal pregnancy, or pregnancies with small-for-gestational-age infants. Hypertens Pregnancy 24: 65–74, 2005. doi: 10.1081/PRG-45780. [DOI] [PubMed] [Google Scholar]
- 64.Shibata E, Hubel CA, Powers RW, von Versen-Hoeynck F, Gammill H, Rajakumar A, Roberts JM. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta 29: 879–882, 2008. doi: 10.1016/j.placenta.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cleal JK, Lewis RM. The mechanisms and regulation of placental amino acid transport to the human foetus. J Neuroendocrinol 20:419–426, 2008. doi: 10.1111/j.1365-2826.2008.01662.x. [DOI] [PubMed] [Google Scholar]
- 66.Reijnders IF, Mulders AG, Koster MP. Placental development and function in women with a history of placenta‐related complications: a systematic review. Acta Obstet Gynecol Scand 97: 248–257, 2018. doi: 10.1111/aogs.13259. [DOI] [PubMed] [Google Scholar]
- 67.Sharashenidze A, Kikalishvili L, Turmanidze T, Sanikidze T. Morphological changes of rat placenta in different periods of pregnancy on modeled preeclampsia. Georg Med News 4: 115–120, 2016. doi: 10.1242/dmm.008516. [DOI] [PubMed] [Google Scholar]
- 68.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod 83: 325–331, 2010. doi: 10.1095/biolreprod.110.084517. [DOI] [PubMed] [Google Scholar]
- 69.Soares MJ, Chakraborty D, Rumi MK, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta, 33: 233–243, 2012. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187: 1416–1423, 2002. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 71.Brower M, Grace M, Kotz CM, Koya V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab Anim Res 31: 166–173, 2015. doi: 10.5625/lar.2015.31.4.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heimlich JB, Pollock DM. Variable reactive hyperemia in normotensive strains of rat. Physiol Rep 2: e12052, 2014. doi: 10.14814/phy2.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silva Ferreira RD, Negrini R, Bernardo WM, Simoes R, Piato S. The effects of sildenafil in maternal and fetal outcomes in pregnancy: a systematic review and meta-analysis. PloS One 14: e0219732, 2019. doi: 10.1371/journal.pone.0219732. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]