Abstract
Bone tissue engineering (BTE) aims to develop strategies to regenerate damaged or diseased bone using a combination of cells, growth factors, and biomaterials. This article highlights recent advances in BTE, with particular emphasis on the role of the biomaterials as scaffolding material to heal bone defects. Studies encompass the utilization of bioceramics, composites, and myriad hydrogels that have been fashioned by injection molding, electrospinning, and 3D bioprinting over recent years, with the aim to provide an insight into the progress of BTE along with a commentary on their scope and possibilities to aid future research. The biocompatibility and structural efficacy of some of these biomaterials are also discussed.
Keywords: bone defect, orthopedics, tissue engineering, injection molding, electrospinning, 3D printing
Graphical Abstract
1. Introduction:
The occurrence of bone related disorders and diseases has increased drastically over recent years worldwide [1]. This trend is expected to double in the near future, with the aged and obese being at a greater risk [1]. Bones have healing and regenerative potential, but bone-healing cannot be accomplished by itself for large segmental bone defects caused by factors like old age, traffic accidents, non-union fracture, bone tumor resection, and others, constituting serious problems in orthopaedics that can adversely affect the health and quality of life [2].
Bone lesions are termed “critical bone defects” when loss of length exceeds twice the diaphyseal diameter of a long bone [3–5], and remain a considerable challenge in orthopedic surgery and may be a result of trauma, infection, tumor, and developmental abnormalities which contributes to their complexity. There is limited evidence to guide the treatment of critical sized bone defects, with an absence of controlled trials comparing techniques. These defects typically carry a poor prognosis with amputations being a common outcome. Current treatment options include both allograft and autograft bone to replace the defect, the Masquelet technique which takes advantage of the body’s foreign body response to induce a membrane of fibrous tissue around the defect site, and distraction osteogenesis which uses the bone’s natural healing properties to fill in defective bone. All of these techniques have demonstrated successful union in limited case series, but also have specific challenges and complications.
Autologous bone grafting remains the gold standard for bone tissue repair due to their histocompatibility and non-immunogenicity while also providing the imperative properties essential for a bone graft [6]. It has also been shown to promote osteoinduction, osteogenesis and osteoconduction [6]. Despite the many advantages of bone grafting, there exists issues such as secondary damage, significant donor site injury and morbidity, deformity, scarring, in addition to surgical risks such as bleeding, inflammation, and high cost [7]. Autografts are also not applicable in cases involving bone defects which require larger volumes of bone graft than what is available or feasible [8].
Tissue engineering has become an alternate strategy targeting bone repair through the use of a synergistic combination of biomaterial scaffolds, cells, and signaling molecules/growth factors to induce the formation of new bone tissues by eliminating the risks associated with autografts [9]. This review attempts to offer insights into the role of scaffolds, their fabrication methods, efficacy compared to the human bone in terms of biocompatibility and mechanical properties, challenges, and prospective directions for scaffold-based BTE.
2. Scaffolds for BTE
Scaffold-based BTE has garnered a lot of interest among researchers. Bone tissue engineering offers a more sustainable, long term treatment strategy for the reconstitution of bones by enabling the fabrication of implants with a combination of scaffolds, cells, and mechanical/soluble factors. The primary role of scaffolds is to maintain a balance between temporary mechanical functions and mass transport to assist in biological delivery and tissue regeneration [10]. Thus, scaffolds act as temporary extracellular matrixes and assist proliferation, differentiation and biosynthesis of cells on the surface of their own. In addition, scaffolds positioned at the regeneration sites also hinder disturbing cells from invasion into the sites of action [10]. The scaffolds are required to meet several specific criteria to achieve the objective of bone reconstruction. Firstly, the scaffolds must be composed of highly biocompatible materials that do not elicit any adverse permanent immune responses in the host tissue following implantation. Cell seeding and fixation can be facilitated only if the scaffold possesses a certain degree of surface roughness. Further, a stable biological interface can be created only through the bonding of the artificial scaffolds with the host tissue without the formation of any type of scars. Cell migration, vascularization and the diffusion of oxygen and other nutrients are largely dependent on the degree of porosity and pore dimensions. In order to enable proper tissue ingrowth, vascularization and the delivery of nutrients, it is recommended that the scaffolds possess a highly networked and porous geometry, comprising of micro- and macro-pores with more than 60% of the pores having pore diameters ranging between 150 – 400 μm and at least 20% of the pores are smaller than 20 μm [10]. It is also imperative that the artificial scaffolds also possess similar mechanical properties as the bone tissue which is being replaced so as to hamper the effects of stress shielding, which comes into play due to the removal of typical stress from the bone by an implant due to a difference in the stiffnesses between the two. This leads to a drastic reduction in the bone density, also known as bone resorption, following the hypothesis that bone remodeling occurs because of osteocytes mediating bone adaptation in response to mechanical strain. Additionally, the scaffolds must be composed of materials with controlled biodegradability so that the resorption rate of the scaffold is coincidental as much as possible with the rate of bone formation, implying that the scaffold will lend structural support within the body, while the osteoblasts develop their own natural matrix structure around themselves and eventually deteriorate enabling the newly formed bone tissue to take over the mechanical load. The biomaterial to be employed for the fabrication of bone scaffolds is decided based on factors such as ease of fabrication and processability, malleability, and scalability, in addition to the extent of conformation and injectability [10].
Although metallic scaffolds (stainless steel 316 L, Co based alloys, Titanium alloys etc.) have been successfully used to develop implants mimicking native bone tissue [11], they raise the possibility of toxic metallic ion release through corrosion leading to inflammation and allergic responses that decrease biocompatibility and trigger tissue loss [12]. Additionally, metallic scaffolds require surface modification prior to their usage as implants as their metallic surfaces need to be controlled so as stimulate the adhesion and proliferation of cells and the adsorption of essential biomolecules. There is also an imperative need integrate cell-recognizable ligands and signaling growth factors on the scaffold surface to promote cellular communication which facilitates their organization within the porous scaffold [13]. Researchers have consequentially explored bioceramics [14] and/or biopolymers [15] as BTE scaffolds.
Scaffolds comprised of photo-crosslinkable bioglass reinforced akermanite mimicking the Haversian bone were developed using Digital Light Processing-based 3D printing in a one-step process [16], eliminating cytotoxicity arising from the use of UV- or chemical- crosslinking. This study exhibited how scaffold parameters could be altered resulting in varying mechanical and porosity properties (Figure 1) and hence, can be adopted to fabricate bone tissues with varying structures and strength to cater to patients with different ages and diseases [16]. This research can be expanded by investigating other bioceramics that could be fabricated the same way and performing more bone-resident cell studies to understand their individual effects on the formation of new bone tissue, blood vessels and nerves in the scaffolds.
Figure 1:
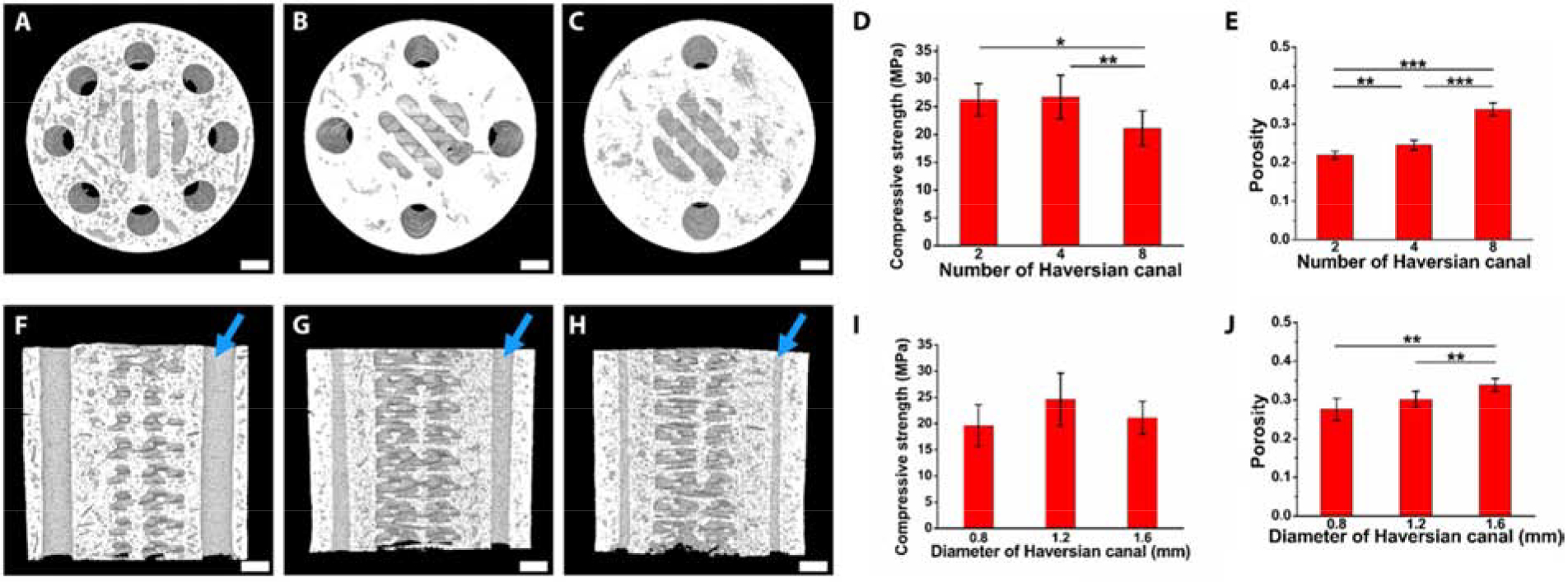
Characterization of Haversian bone-mimicking bioceramic scaffolds by varying the number of Haversian canals (A-C) and their diameters (F-H) (indicated by blue arrows) as seen through micro-CT scans. Samples exhibited varying compressive strength (D, I) and porosity (E,J) for the two cases. Reproduced with permission from [16].
Cojocaru et al. developed bone tissue scaffolds by using a composite made out of a combination of biopolymers dispersed with MNPs [17]. It was observed that the MNPs promoted osteogenesis and increased the osteogenic differentiation while also enhancing cell growth osteogenesis and increased the osteogenic differentiation while enhancing cell growth [17], a concept that has never been explored before, lending novelty to this study. MNPs can also be incorporated into other commonly used bone tissue engineered scaffolding materials such as PCL, PLLA, HA, and alumina to understand how varying scaffolds influences the ability of the MNPs to direct osteoblast growth and differentiation. In the next section, we describe the role of polymer based injection molded scaffolds and their efficacy in BTE.
3. Injection Molding
The ease with which polymeric materials plasticize or toughen through incorporation of additional constituents, makes them desirable as working materials in BTE. Among the most common polymeric product fabrication methods utilized in both industry and research, we find injection molding. This extrusion method is easily tailored by modifying the glass transition temperature through the amalgamation of different polymeric species that may, in turn, adjust the mechanical properties to desired parameters [18]. Due to their biocompatibility and accessible working parameters, the most widely used polymers are PLA and PCL as promising scaffold materials [19–21].
However in the case of injection molding, a major drawback is the difficulty to produce porous surfaces within the monolithic final product that comes from relatively homogeneous solidification. As porosity affects cell proliferation, migration, and tissue formation, this is a key consideration in regenerative medicine [22]. Usage of porogens has solved this problem to an extent. Porogens, or particles with a specific geometry and different melting temperatures in the polymeric matrix, have shown promise in inducing enhanced pore formation [23]. Alternatively, molds can be created with the porogen material, similar to metal-casting, in which the sacrificial material is then removed through dissolution (Figure 2) [19, 24, 25]. This allows for a better tailored material that mimics osseous structures where osteoblasts can be introduced with efficacy and decreased cytotoxicity.
Figure 2 (Top Panel):
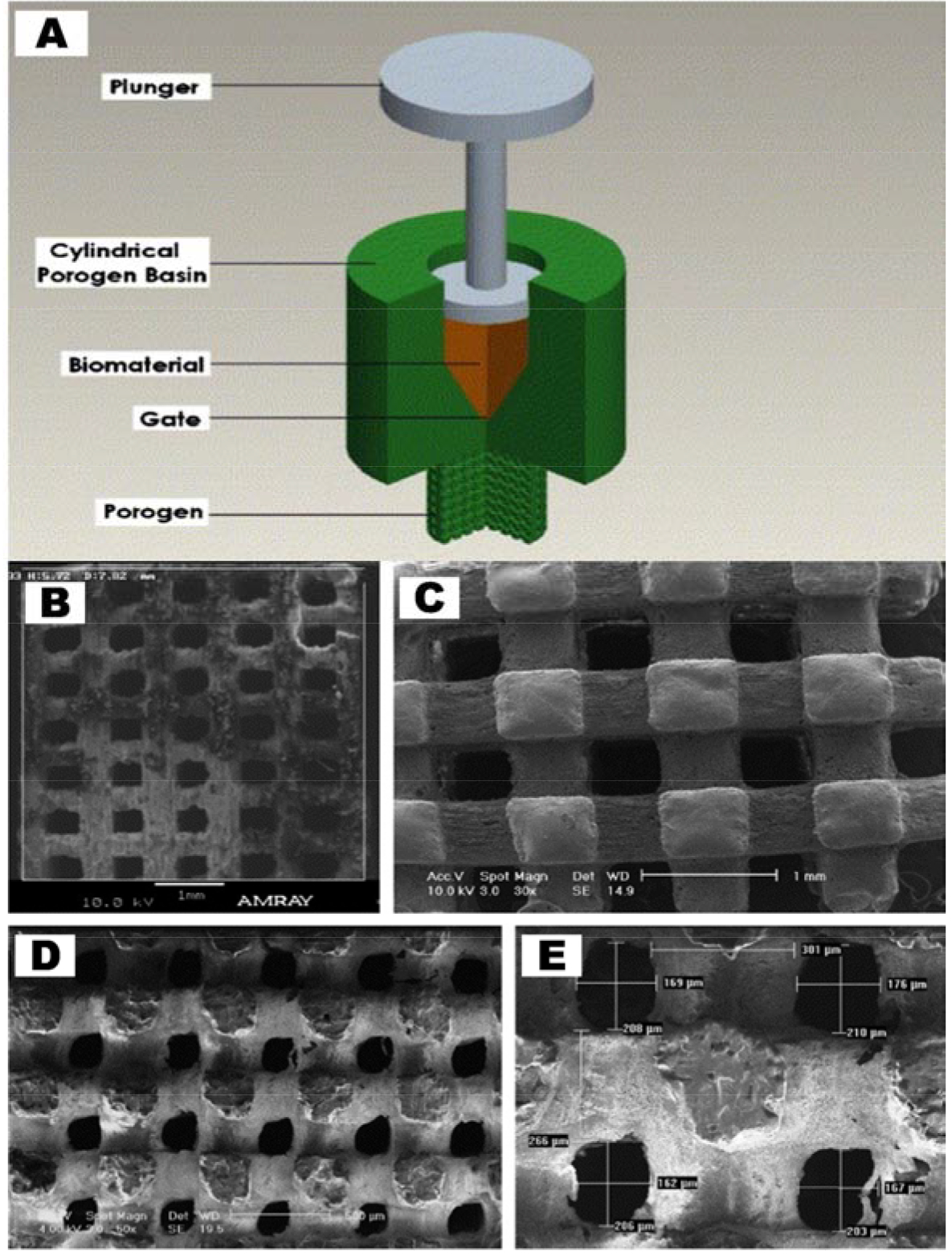
(A) Representative image of injection molding including porogens. (Bottom Panel): (B-E) Corresponding SEM images of porogen-incorporated PCL thermoplastic scaffolds. Both images are reproduced with permission [19].
Another attractive mechanism to induce porosity in injection-molded biomaterials for BTE applications, is microcellular injection molding [26]. Its popularity in regenerative medicine has increased due to its unexpected level of precision and does not cause environmental complications or use organic solvents [27]. This relies on the addition of a supercritical fluid to the polymer melt at an elevated temperature inside the mold, which will begin nucleating bubbles from changing thermodynamic conditions [25, 28].
These methods of introducing pores to a scaffold have been shown to be effective, due to the incorporation of sacrificial materials. Hence, incorporating porosity via injection molding is predominantly managed by secondary constituents that are disposed of without compromising the matrix material. Because of these inherent deficiencies with injection molded scaffolds, we highlight the role of electrospun scaffolds in BTE, in the next section.
4. Electrospun Scaffolds
In BTE there is a need to culture cells on complex surface geometries that mimic the anisotropic nature of ECM of specific tissues, especially in bone [29]. Electrospun scaffolds exhibit an intrinsically extremely porous environment when compared to bulk-polymer, which allows for better cell communication and nutrient transport throughout the scaffold [30]. Current literature reveals that researchers have optimized the electrospinning method for specific applications regarding BTE [31].
Collagen is a natural polymer and main component of ECM; however, it needs a toxic solvent in order to be employed in electrospinning. Türker et al. show how they used a non-toxic solvent to co-electrospin a scaffold with collagen and PLLCL. Figure 3 depicts a schematic of how the desired PLLCL/collagen scaffold was synthesized by the dissolution of PVP from an electrospun scaffold. This group also concluded that these scaffolds could be used in treating bone defects because of its improved cell adhesion and proliferation compared to flat cell-culture surfaces [32].
Figure 3:
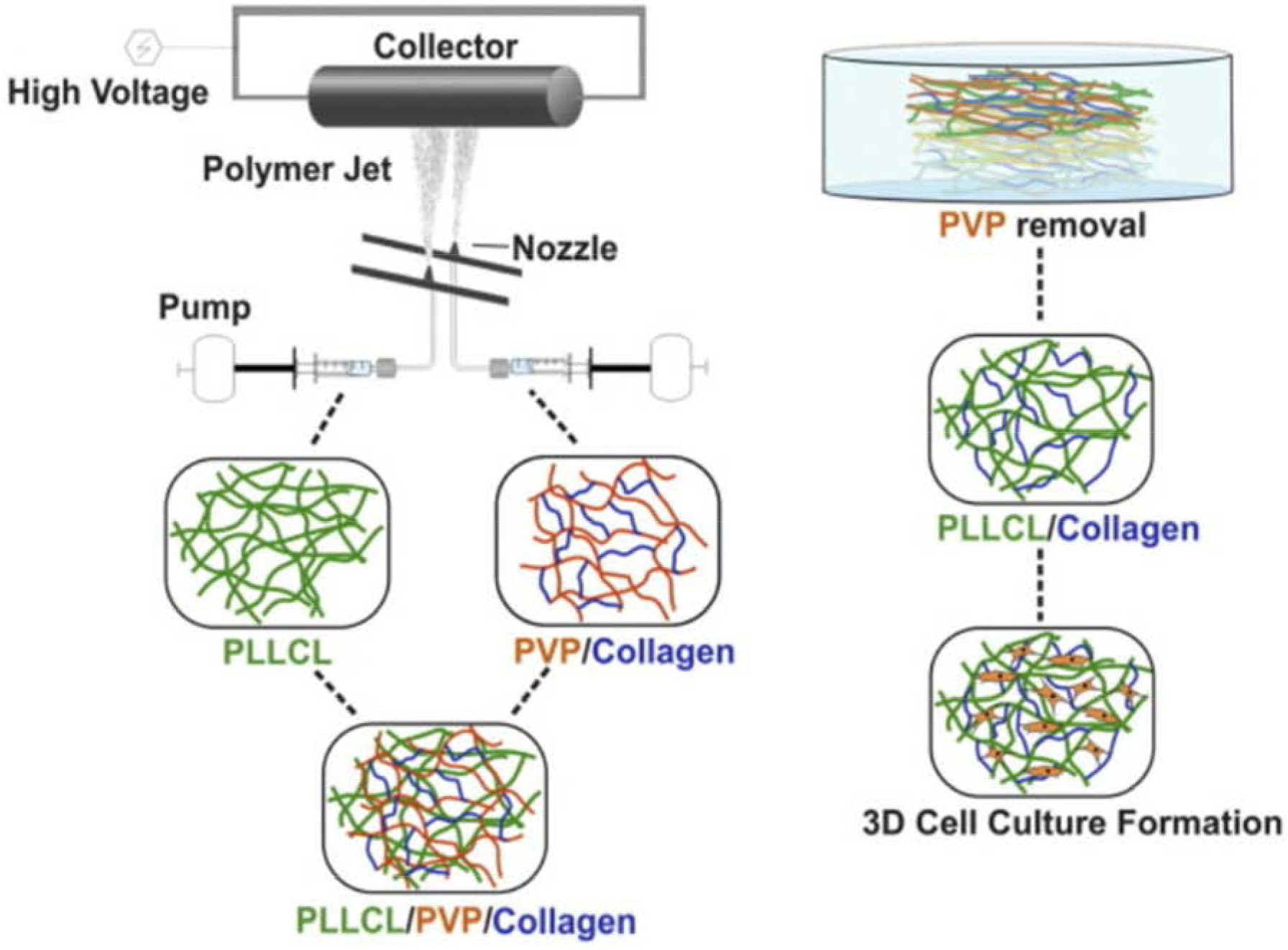
Schematic representing co-electrospinning and dissolution of unwanted PVP on the desired scaffolds. Figure reproduced with permission from [32].
The degradation rate of the scaffold material should be considered when determining which material to utilize, especially in drug delivery systems. Belgheisi et al. used the degradation properties of an electrospun scaffold to control the release of vitamin D3, a vital nutrient that aids in the body’s uptake of calcium. They further concluded that optimized vitamin D3 loaded PCL/clay scaffolds had potential for applications in bone tissue engineering because of the controlled release of vitamin D3 [33].
Though electrospun scaffolds provide little structural support, they greatly influence the bioactivity of a scaffold due to their relatively large surface area. Future research may incorporate alternative compounds such as growth factors and cell-signaling molecules into the scaffold matrix to aid in bone’s natural regenerative process. Electrospun scaffolds may also act as a periosteal layer when designing a bone implant; in this case, the bioactivity of both sides of the scaffold are critical. While one side of the scaffold interfaces with muscle and connective tissues, the other interfaces with engineered tissue and should promote osteogenesis and vascularization. Alternative additives may influence the degradation rate of the scaffold and may be favorable to drug delivery researchers who want to administer specific drug doses over extended periods.
5. 3D-Printing for Mimicking Bone
Three-dimensional bioprinting is the most advanced and strategic technique in the treatment of critical size bone defects as demonstrated by Lipskas et al. in their development of a minimally invasive approach to repair faulty bone and cartilage using robot-assisted extrusion 3D bioprinting [34]. In their study, Lipskas et al. used a viscous alginate-poly(ethylene glycol)diacrylate hydrogel to restore defective bone by 3D printing that material over the contours of the defect as seen in Figure 4 (Top Panel). This novel study introduced a feasible selection for focal defect restoration and as prospective technique for 3D printing in vivo. Future studies may focus on optimizing nozzle path generation algorithms to counteract nozzle inclined effects and reduce dimensional errors. Resources should be allocated to optimizing printing parameters and creating a protocol for 3D scanning/printing at defect sites in-situ.
Figure 4 (Top Panel):
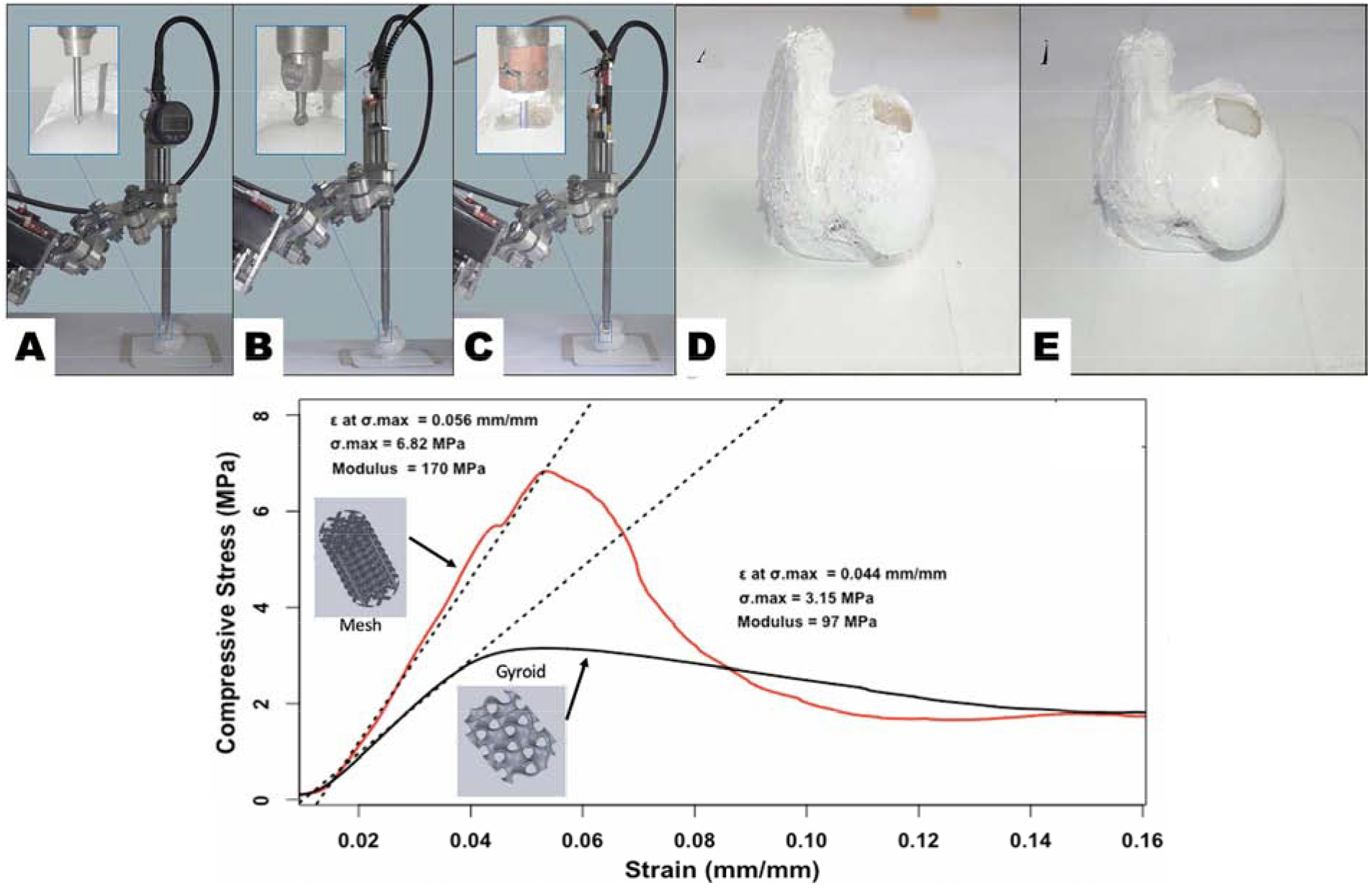
Experimental arrangement (A) Surface registration. (B) bone milling. (C) 3D printing. Bone samples pre and post 3D printing process (D) Milled defect sample. (E) Hydrogel infill sample. Both figures are reproduced with permission from [34]. (Bottom Panel): Compressive strength graph of two 3D printed pattern types based on data obtained from mechanical testing at UTEP conducted for reference.
Increasing trends have been observed in the usage of 3D printable biomaterials to create alternative methods for improvement of defects in the underlying bone structure. However, a persisting challenge is the achievement of mechanical properties that mimic native bone [35]. An experiment to analyze the current state of 3D printed bio-alternatives and compare their efficacy with native human bone was conducted in our laboratory by Alvarez-Primo et al., in order to produce viable evidence of what a frame of reference for what a range of mechanical properties of the tested materials and manufacturing methods normally produce. PLA samples were 3D printed in a gyroid or mesh lattice (Figure 4: Bottom Panel) via FDM in compliance with ASTM D695.1291. and stated specifications for mechanical testing. Both geometries underwent compression testing. Upon statistical analysis, there was no evidence of any functional difference between samples, however the volumetric differences between groups and their respective 3D print raster patterns did play a significant role in the expression of compressive strength, with the mesh pattern reaching a higher ultimate compressive strength and compressive modulus in relation to the gyroid sample. Deformation of the polymeric samples presented an elastic response without brittle fracture. PLA on its own, although a greatly biocompatible material, may require addition of a reinforcing phase or secondary constituent to form a polymer blend which would in turn increase compressive modulus altogether. Healthy femoral compact bone’s ultimate compressive strength lays within a broad range between 100 – 200 MPa [36, 37], which is incomparable to current models developed through additive manufacturing techniques [38–40]. The variation in compressive strength, and mechanical properties altogether are seen to be correlated to the porosity present in samples, as well as the shrinking that occurs as a consequence of the sintering process in 3D printed powder structures [41]. 3D printed PLA samples in this study provided results comparable to the higher range of polymeric bone structure replacements, however there is a need for blend improvement, manufacturing material quality control, and non-destructive analysis of microscopic features attributed to the volumetric differences in directionally preferential designs [38, 40, 41]. In the following section we describe some biocompatibility evaluation for these engineered BTE scaffolds.
6. Biocompatibility Studies
Orthopedic biomaterial development has been focused on two main constituents: metals/alloys and nonmetallic materials [42]. From this conventionality, maximizing the functionality of each group has progressed into inducing increased cellular adherence and proliferation [42]. Due to the corrosive nature of metallic implants, the use of ceramics and polymers to substitute their load bearing function, has been extensively advanced through the addition of natural polymers (chitosan, hyaluronic acid, collagen, keratin, etc) [42]. Enhancement of biocompatibility has been observed in mimicking the bone tissue matrix through incorporation of nanomaterials or nanophase treatment that improves cellular interaction [42]. Polymers, through chemical synthesis and their geometric flexibility, provide an adaptable framework [42]. In one such study, it was shown that a lower amount concentration of HA NP’s mixed within a polymeric alginate-matrix was optimal in bone regeneration, where excessive amounts of HA NP’s resulted in an insufficient amount of area for cell growth that leads to growth abnormalities [43]. Thus, molecular concentrations play a vital role in what produces the most conducive environment for tissue proliferation, a limiting factor that requires extensive testing.
Stem cell-based bone regeneration has proven to be a promising alternative to healing an osteopathic injury. Mesenchymal cells (MSCs) found in bone marrow, have been known to repair cartilage and bone pertaining to the skeletal system [44]. Among all MSCs, human synovial fluid mesenchymal stem cells (SF-MSCs) are the most popular candidate for bone regeneration as they have proven to possess the greatest osteogenic potential of all [45]. PEKK is a thermoplastic commonly used in 3D-printed scaffolds to mimic bone because of its similar properties. In one study, PEKK was seeded with SF-MSCs and utilized in an in-vivo study as a 3D scaffold, then observed by Scanning Electron Microscopy (SEM) to illustrate growth of filopodia and lamellipodia on its surface, indicating the affluence for cell proliferation [45]. The potency in in-vivo was similarly studied with the implantation of PEKK in a rabbit’s bone and continuously observed for growth at 12 weeks post-surgery. The group containing SF-MSCs seeded on PEKK scaffolds (PEKK + SF) had the greatest volume of bone regeneration at 12 weeks [45]. The study demonstrated the advancement of polymer and stem cell integration to create a substantial cohesive environment that allows full tissue regeneration for an orthopedic application.
The inclusion of nanoparticles in both gelatin scaffolds (hydro-, micro-, nano- gels) and surface treatments of implants (Ti, Mg- alloys, stainless steel, Co-Cr) have significantly improved cellular interactions by promoting osteoblasts and inducing bone tissue regeneration [42]. This extension includes nanosurface modification that has allowed topographical refinement of the grain size, surface energy, and surface functionality through varying coatings that improve cellular adherence. Notably, the use of polydopamine coatings has been able to offer a provisional surface that targets both the improvement to cellular adherence due to interaction with functional groups, whilst also providing the ability to synthesize the compound with antibacterial additives [46]. The use of microwave surface modification is inclusionary in the emerging novel techniques as it offers a homogenous crystalline topography through improved intraparticle interaction, overall increasing surface stability [42]. Refinement of the processing treatment and their long-term efficacy, along with the dynamic molecular interaction and their promotion of growth factors, remain aspects that need to be extensively studied for the overall commercialization of orthopedic implants on the industrial scale.
7. Challenges and Future Direction
The challenges of selecting a biomaterial for BTE come from the paradigm between a material’s mechanical and biological properties. Though researchers prefer to utilize materials with high strengths and stiffnesses to match that of natural bone, materials should also achieve some threshold of bioactivity to minimize the probability of host-rejection as well as promote naturally regenerative processes such as osteogenesis and vascularization. Metals are among the most popular high strength materials and better serve BTE when used on material surfaces to promote wear-resistance such as in hip replacements. However, when designing scaffolds whose main purpose is structural support, i.e. femoral and tibial segmental scaffolds, the aforementioned high strength materials may cause an unwanted phenomenon called stress shielding which can weaken segments of native bone over time. In addition, many high strength metals are prone to corrosion and subsequently mechanical failure. Polymeric and bio-ceramic materials have begun to see use in structural scaffolds because of the ease of processing as well as favorable bioactivity.
The current direction of biomaterials in BTE is creating a polymer composite that matches native bone in mechanical strength and stiffness, yet also contains enough bioactive components to promote formation of new and healthy bone. Popular scaffolds include a high strength polymer such as PCL, PLA, or polyether-ether ketone in conjunction with bioactive minerals loaded into the polymer matrix to promote naturally regenerative processes. Copolymer blends are also favorable for optimizing a biomaterial’s mechanical and biological properties. One of the most popular additives in BTE is HA because of the biomimetic nature of the presence of calcium phosphate. More research should be done on bioactive additives in BTE scaffolds since many copolymers have already been shown to have mechanical properties similar to natural bone.
8. Conclusion
Numerous advances have been made in the field of BTE over recent years. The search for biomaterials that mimic the mechanical and biological properties of native bone have led researchers to study bioceramics and polymer composites that counteract the negative effects of corrosion caused by metallic implants. Furthermore, the designing of these materials to best produce a favorable environment for bone regeneration has been accomplished through the introduction of porogens in injection molding and incorporating bone-benefiting nutrients into electrospun scaffolds that are released as a function of the scaffold’s degradation. As technology in the field of robotics and three-dimensional bioprinting is upgraded, researchers are looking for ways to deposit biopolymers directly onto the contours of the bone to treat defective bone without compromising their mechanical stability and biocompatibility.
Despite the progress in the fabrication of bone tissue scaffolds, there remain several unresolved issues like the ability of a newly formed bone tissue to be support and renew itself, whether a scaffold derived bone could promote hematopoiesis and whether different types of scaffolds should be used and their mechanical properties modulated for different bones. Presently, there is much focus on strategies involving immune-mediated tissue regeneration driven by biomaterial scaffolds or biomaterial scaffolds that could be used to activate a drug release where and when it is needed to circumvent systemic treatment effects. It has also been hypothesized that biomaterial scaffolds can be made to entrap cells, change them and subsequently release them for the fulfilment of specific functions which they would not have achieved otherwise. Significant benefits and a more profound knowledge of native tissues, developmental biology and the natural processes of tissue repair and regeneration could be derived by studying the integration of biomaterial scaffolds with molecules that that can influence cell behavior like inflammatory cytokines, adhesive ligands and ECM molecules for recapacitating the initial phases of tissue repair and remodeling. Bone tissue scaffolds should be designed to incorporate materials or growth factors that can enhance angiogenesis while also providing the necessary porosity to support vascular ingrowth to ensure the regeneration of vascularized bone. Understanding the nature and cellular response mechanisms to microenvironmental cues can potentially direct the design of a number of scaffold features such as the encompassing of bioactive ions and surface topographies by promoting cellular adhesion, proliferation and differentiation, and also the modification of the microstructure of the scaffold or the stiffness to alter the mechanical properties and cellular interactions. Another unmet need in BTE is to fabricate cell laden, vascularized, scaffolds that could be used to rectify large segmental bone defects. To overcome these challenges, it is required that the field of bone tissue engineering to be expanded into newer realms of research involving other areas like nanotechnology, manufacturing technologies, mechanobiology and medical diagnostics.
Acknowledgments
The Joddar Lab (IMSTEL) acknowledges the NSF (CBET 1927628). Matthew Alonzo acknowledges the Eloise E. and Patrick B. Wieland fellowship at UTEP and the Gates Millennium Scholarship Program. Authors also acknowledge support for Materials and Supplies for the study summarized in Figure 4 (bottom panel) obtained from the NSF-MRI (DMR 1826268). Authors also acknowledge the GF acknowledges the National Institute of General Medical Sciences of the National Institutes of Health under RL5GM118969, TL4GM118971 and UL1GM118970.
Abbreviations
- AM
additive manufacturing
- ECM
extracellular matrix
- FDM
fused deposition modeling
- HA
hydroxyapatite
- HA NP
hydroxyapatite nanoparticles
- MNPs
magnetic nanoparticles
- PCL
polycaprolactone
- PEKK
polyetherketoneketone
- PLA
Polylactic acid
- PLLA
poly-L-lactic acid
- PLLCL
L-blocked polycaprolactone
- PVP
polyvinyl pyrrolidone
- SLS
selective laser sintering
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
William Weiss acknowledges he has no financial conflicts of interest, but serves as an editor for Canadian Orthopedic Association Bulletin, is on the editorial board for Arthroscopy: Journal of Arthroscopic and Related Surgery; Bone & Joint 360, and is on the social media board for Arthroscopy Association of North America (AANA). All other authors acknowledge no conflict of interest.
References
- 1.Kargozar Saeid, Milan Peiman Brouki, Baino Francesco, et al. , Nanoengineered biomaterials for bone/dental regeneration, in Nanoengineered Biomaterials for Regenerative Medicine. 2019, Elsevier. p. 13–38. [Google Scholar]
- 2.Qasim Muhammad, Chae Dong Sik, and Lee Nae Yoon, Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. International journal of nanomedicine, 2019. 14: p. 4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SCHMITZ JOHNP and Hollinger Jeffrey O, The critical size defect as an experimental model for craniomandibulofacial nonunions. Clinical Orthopaedics and Related Research®, 1986. 205: p. 299–308. [PubMed] [Google Scholar]
- 4.KEY J ALBERT, The effect of a local calcium depot on osteogenesis and healing of fractures. JBJS, 1934. 16(1): p. 176–184. [Google Scholar]
- 5.McBride J, Clyde M, Banks RE, et al. , Healing of segmental bone defects in goat tibia. J Invest Surg, 1993. 6: p. 369. [Google Scholar]
- 6.Dinçel Yaşar Mahsut, Bone Graft Types, in Bone Grafting-Recent Advances with Special References to Cranio-Maxillofacial Surgery. 2018, IntechOpen. [Google Scholar]
- 7.Sohn Hoon-Sang and Oh Jong-Keon, Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomaterials research, 2019. 23(1): p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares Mariana Quirino Silveira, Dessel Van, Jeroen Jacobs, Reinhilde, et al. , Morphometric evaluation of bone regeneration in segmental mandibular bone defects filled with bovine bone xenografts in a split-mouth rabbit model. International journal of implant dentistry, 2019. 5(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Soares et al demonstrated the comparison in morphometric and bone density changes after using two different bovine bone graft blocks in segmental osseous defects in the mandible of rabbits following different postoperative periods. Both grafts showed an enhanced bone formation and more complex structure compared to untreated defect indicating potential applications towards guided bone regeneration procedures in the maxillofacial region.
- 9.Dzobo Kevin, Thomford Nicholas Ekow, Senthebane Dimakatso Alice, et al. , Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells International, 2018. 2018: p. 2495848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorozhkin Sergey V., Calcium Orthophosphate (CaPO4) Scaffolds for Bone Tissue Engineering Applications. Journal of Biotechnology and Biomedical Science, 2018. 1(3). [Google Scholar]
- 11.Su Yingchao, Cockerill Irsalan, Zheng Yufeng, et al. , Biofunctionalization of metallic implants by calcium phosphate coatings. Bioactive materials, 2019. 4: p. 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Su et al provides a comprehensive review detailing different types of calcium phosphates (CaPs) with their coating methods and in vitro and in vivo performances, and then give insight into the representative biofunctions, including osteointegration, corrosion resistance and biodegradation control, and antibacterial property, provided by CaP coatings for metallic implant materials.
- 12.Eliaz Noam, Corrosion of metallic biomaterials: A review. Materials, 2019. 12(3): p. 407. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review discusses the corrosion performance of the metals most commonly to manufacture implant materials. The article also provides insight into the principles of implant failure, retrieval and failure analysis, followed by description of the most common corrosion processes in vivo.
- 13.Alvarez Kelly, Nakajima Hideo, Metallic Scaffolds for Bone Regeneration. Materials, 2009. 2(3): p. 790–832. [Google Scholar]
- 14.Guo Liying, Du Zhiyun, Wang Yue, et al. , Degradation behaviors of three-dimensional hydroxyapatite fibrous scaffolds stabilized by different biodegradable polymers. Ceramics International, 2020. [Google Scholar]
- 15.George Ann Mary, Peddireddy Sai Preetham Reddy, Thakur Goutam, et al. , Biopolymer-based scaffolds: Development and biomedical applications, in Biopolymer-Based Formulations. 2020, Elsevier. p. 717–749. [Google Scholar]
- 16.Zhang Meng, Lin Rongcai, Wang Xin, et al. , 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Science advances, 2020. 6(12): p. eaaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Zhang et al has developed a new strategy that can be utilized to design structured and functionalized biomaterials through mimicking native complex bone tissue for tissue regeneration.The authors also demonstrated how the compressive strength and porosity of scaffolds can be well controlled by altering the parameters of the Haversian bone-mimicking structure used in the study.
- 17.Cojocaru Florina D, Balan Vera, Popa Marcel I, et al. , Biopolymers-Calcium phosphates composites with inclusions of magnetic nanoparticles for bone tissue engineering. International journal of biological macromolecules, 2019. 125: p. 612–620. [DOI] [PubMed] [Google Scholar]; **Cojucaro et al demonstarted in this novel study how magnetic nanoparticle-diseprsed scaffolds were not only cytocompatible but also promoted osteogenesis and increased the osteogenic differentiation while also enhancing cell growth osteogenesis and increased the osteogenic differentiation while enhancing cell growth.
- 18.Renkó József Bálint, Kemény Dávid Miklós, Nyirő József, et al. , Comparison of cooling simulations of injection moulding tools created with cutting machining and additive manufacturing. Materials Today: Proceedings, 2019. 12: p. 462–469. [Google Scholar]
- 19.Mondrinos Mark J., Dembzynski Robert, Lu Lin, Venkata KC Byrapogu David M. Wootton, Lelkes Peter I., and Zhou Jack. “Porogen-based solid freeform fabrication of polycaprolactone-calcium phosphate scaffolds for tissue engineering.” Biomaterials 27, no. 25 (2006): 4399–4408. [DOI] [PubMed] [Google Scholar]; **Mondrinos et al demonstrated how Drop by Demand Printing can be utilized for the incorporation of injectable porogens into tissue engineering scaffolds.
- 20.Hernandez Ivan, Kumar Alok, and Joddar Binata, A bioactive hydrogel and 3D printed polycaprolactone system for bone tissue engineering. Gels, 2017. 3(3): p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Hernandez et al succesfully developed a a hybrid system consisting of 3D printed polycaprolactone (PCL) filled with hydrogel was developed as an application for reconstructing long bone defects, which are innately difficult to repair due to large missing segments of bone. The hMSCs seeded in the PCL/hydrogel system exhibited cell adhesion and viability indicating the cytocompatibility of the material.The bioactivity of the system was confirmed by the nucleation and growth of apatite crystals, verifying the potential of the PCL/hydrogel system in bone tissue engineering applications.
- 21.Singh Sunpreet, Prakash Chander, Singh Manjeet, et al. , Poly-lactic-Acid: Potential Material for Bio-printing Applications, in Biomanufacturing. 2019, Springer. p. 69–87. [Google Scholar]
- 22.Abbasi Naghmeh, Hamlet Stephen, Love Robert M, et al. , Porous scaffolds for bone regeneration. Journal of Science: Advanced Materials and Devices, 2020. 5(1): p. 1–9. [Google Scholar]
- 23.Annabi Nasim, Nichol Jason W., Zhong Xia, et al. , Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue engineering. Part B, Reviews, 2010. 16(4): p. 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahdokht Akbari Taemeh, Babak Akbari, and Mojtaba Khorramnezhad, Fabrication of polycaprolactone scaffold with gradient porous microstructure for bone tissue engineering. Journal of Tissues and Materials, 2019. 2(3): p. 9–17. [Google Scholar]
- 25.Huang An, Jiang Yongchao, Napiwocki Brett, et al. , Fabrication of poly (ε-caprolactone) tissue engineering scaffolds with fibrillated and interconnected pores utilizing microcellular injection molding and polymer leaching. RSC advances, 2017. 7(69): p. 43432–43444. [Google Scholar]
- 26.Wang Xiaofeng, Salick Max R, Gao Yanhong, et al. , Interconnected porous poly (ɛ-caprolactone) tissue engineering scaffolds fabricated by microcellular injection molding. Journal of Cellular Plastics, 2018. 54(2): p. 379–397. [Google Scholar]
- 27.Llewelyn Gethin, Rees Andrew, Griffiths Christian A, et al. , Advances in microcellular injection moulding. Journal of Cellular Plastics, 2020: p. 0021955X20912207. [Google Scholar]
- 28.Dehghan-Manshadi Ali, Chen Yunhui, Shi Zhiming, et al. , Porous Titanium Scaffolds Fabricated by Metal Injection Moulding for Biomedical Applications. Materials (Basel, Switzerland), 2018. 11(9): p. 1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naderi Parisa, Zarei Moien, Karbasi Saeed, et al. , Evaluation of the Effects of keratin on Physical, Mechanical and Biological Properties of Poly (3-hydroxybutyrate) Electrospun Scaffold: Potential Application in Bone Tissue Engineering. European Polymer Journal, 2020. 124: p. 109502. [Google Scholar]
- 30.Jun I, Han HS, Edwards JR, et al. , Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int J Mol Sci, 2018. 19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]; **This review article summarizes the principles of electrospinning processes for the generation of complex fibrous scaffold geometries mimicking the structural complexity of the ECM of living tissues. The article also elaborates numerous approaches for the formation of three-dimensional fibrous scaffolds arranged in hierarchical structures for tissue engineering applications.
- 31.Lin W, Chen M, Qu T, Li J, & Man Y, Three-dimensional electrospun nanofibrous scaffolds for bone tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2020. 18(4): p. 1311–1321. [DOI] [PubMed] [Google Scholar]
- 32.Türker Esra, Yildiz Ümit Hakan, and Yildiz Ahu Arslan, Biomimetic hybrid scaffold consisting of co-electrospun collagen and PLLCL for 3D cell culture. International journal of biological macromolecules, 2019. 139: p. 1054–1062. [DOI] [PubMed] [Google Scholar]
- 33.Belgheisi Ghazal, Nazarpak Masoumeh Haghbin, and Hashjin Mehran Solati, Bone tissue engineering electrospun scaffolds based on layered double hydroxides with the ability to release vitamin D3: Fabrication, characterization and in vitro study. Applied Clay Science, 2020. 185: p. 105434. [Google Scholar]; ** Belgheisi et al developed Poly (ɛ-caprolactone) (PCL) electrospun scaffolds enriched with vitamin D3 (VD3)-loaded layered double hydroxides (LDHs) nanohybrid, in different concentrations. The results indicated that nanohybrid containing scaffolds could highly support cell adhesion and proliferation.
- 34.Lipskas Julius, Deep Kamal, and Yao Wei, Robotic-assisted 3D bio-printing for repairing bone and cartilage defects through a minimally invasive approach. Scientific Reports, 2019. 9(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Lipskas describes a new technique that applies a combination of 3D bio-printing and robotic-assisted minimally invasive surgery techniques to treat critical size tissue defects, by further reduction of invasiveness in implant, cell and tissue-based surgery. The feasibility of Remote Centre of Motion (RCM) and viscous material extrusion 3D printing was explored with favorable resulting including reasonably high printing accuracy comparable with the values reported for other types of bioprinting.
- 35.Patel Pushpendra P, Buckley Christian, Taylor Brittany L, et al. , Mechanical and biological evaluation of a hydroxyapatite-reinforced scaffold for bone regeneration. Journal of Biomedical Materials Research Part A, 2019. 107(4): p. 732–741. [DOI] [PubMed] [Google Scholar]
- 36.Nibali Luigi, Diagnosis and Treatment of Furcation-Involved Teeth. 2018, John Wiley & Sons Ltd. [Google Scholar]
- 37.Aljohani Ablah and Desai Salil, 3D printing of porous scaffolds for medical applications. American Journal of Engineering and Applied Sciences, 2018. 11(3). [Google Scholar]
- 38.Haleem Abid, Javaid Mohd, Khan Rizwan Hasan, et al. , 3D printing applications in bone tissue engineering. Journal of Clinical Orthopaedics and Trauma, 2020. 11: p. S118–S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldaadaa Alaa, Owji Nazanin, and Knowles Jonathan, Three-dimensional printing in maxillofacial surgery: hype versus reality. Journal of tissue engineering, 2018. 9: p. 2041731418770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ligon Samuel Clark, Liska Robert, Stampfl Jürgen, et al. , Polymers for 3D printing and customized additive manufacturing. Chemical reviews, 2017. 117(15): p. 10212–10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tappa Karthik and Jammalamadaka Udayabhanu, Novel biomaterials used in medical 3D printing techniques. Journal of functional biomaterials, 2018. 9(1): p. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar Sandeep, Nehra Monika, Kedia Deepak, et al. , Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Materials Science and Engineering: C, 2020. 106: p. 110154. [DOI] [PubMed] [Google Scholar]
- 43.Barakat Abeer, 3D printing of hydrogels and nanoparticle containing bio-inks for bone tissue regeneration. 2019.
- 44.Zhang Rui, Ma Jie, Han Jing, et al. , Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. American Journal of Translational Research, 2019. 11(10): p. 6275–6289. [PMC free article] [PubMed] [Google Scholar]
- 45.Lin Yi, Umebayashi Mayumi, Abdallah Mohamed-Nur, et al. , Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Scientific Reports, 2019. 9: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia Luanluan, Han Fengxuan, Wang Huan, et al. , Polydopamine-assisted surfacemodification for orthopaedic implants. Journal of Orthopaedic Translation, 2019. 17: p. 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


