Keywords: cutaneous, dorsal root ganglia, mechanoreceptor, nonhuman primate, sensory
Abstract
Cutaneous mechanoreceptors in our hands gather information about the objects we handle. Tactile fibers encode mixed information about contact events and object properties. Neural coding in tactile afferents is typically studied by varying a single aspect of tactile stimuli, avoiding the confounds of real-world haptic interactions. We instead record responses of small populations of dorsal root ganglia (DRG) neurons to variable tactile stimuli and find that neurons primarily respond to force, though some texture information can be detected. Tactile nerve fibers convey information about many features of haptic interactions, including the force and speed of contact, as well as the texture and shape of the objects being handled. How we perceive these object features is relatively unaffected by the forces and movements we use when interacting with the object. Because signals related to contact events and object properties are mixed in the responses of tactile fibers, our ability to disentangle these different components of our tactile experience implies that they are demultiplexed as they propagate along the neuraxis. To understand how texture and contact mechanics are encoded together by tactile fibers, we studied the activity of multiple neurons recorded simultaneously in the cervical DRG of two anesthetized rhesus monkeys while textured surfaces were applied to the glabrous skin of the fingers and palm using a handheld probe. A transducer at the tip of the textured probe measured contact forces as tactile stimuli were applied at different locations on the finger-pads and palm. We examined how a sample population of DRG neurons encode force and texture and found that firing rates of individual neurons are modulated by both force and texture. In particular, slowly adapting (SA) neurons were more responsive to force than texture, and rapidly adapting (RA) neurons were more responsive to texture than force. Although force could be decoded accurately throughout the entire contact interval, texture signals were most salient during onset and offset phases of the contact interval.
NEW & NOTEWORTHY Cutaneous mechanoreceptors in our hands gather information about the objects we handle. Tactile fibers encode mixed information about contact events and object properties. Neural coding in tactile afferents is typically studied by varying a single aspect of tactile stimuli, avoiding the confounds of real-world haptic interactions. We instead record responses of small populations of DRG neurons to variable tactile stimuli and find that neurons primarily respond to force, though some texture information can be detected.
INTRODUCTION
Tactile nerve fibers that innervate the glabrous skin on the palmar surface of the hand encode information about object interactions, such as the location of contacts with the object and the force exerted at each point of contact. The afferent fibers from these mechanoreceptors (“tactile afferents”) also carry information about the objects themselves—their size, shape, and texture. These signals are necessary for not only identifying objects but also dexterous manipulation (1, 2) as evidenced by the impairments that result from the loss of tactile sensation (3–7). Information about contact events and objects is mixed in the responses of nerve fibers and is extracted by downstream structures to give rise to interpretable tactile percepts.
Primary tactile afferents comprise two distinct classes of neurons—rapidly adapting (RA) and slowly adapting (SA)—that respond differently to aspects of a tactile stimulus (8). Studies of neural coding in the nerve have typically varied only a single aspect of the sensory stimulus, whether it be force, speed, or texture (9–16). The overarching theme of these studies is that primary afferents (PAs) encode different stimulus features in distinct yet overlapping ways: scanning speed (17, 18) and contact force are encoded primarily in firing rates (9, 10, 13, 15, 16), whereas texture is encoded in the spatial distribution of the activated fibers (11, 12, 14, 19, 20), and in precisely timed spiking sequences (21). When multiple aspects of tactile stimuli vary at the same time, these different neural codes allow for information to be multiplexed in the responses of single neuron and populations of neurons. For example, the strength and frequency composition of vibratory stimuli are encoded in firing rates and temporal patterning, respectively (22). Sometimes, the same neural code can carry information about multiple aspects of a tactile stimulus. For example, shear force direction and object curvatures are encoded in the latency of the first action potential in a sensory response (23, 24).
The objective of the present study was to further investigate the multiplexing of tactile information in the spiking activity of simultaneously recorded RA and SA afferent neurons. To this end, we varied the scanning speed, contact force, and texture of the stimuli applied to the skin and assessed whether we could decode the force and the texture from the spiking activity of PA neurons recorded simultaneously with penetrating microelectrode arrays in the cervical dorsal root ganglia (DRG) of anesthetized Rhesus monkeys (25, 26). Sensory nerves from the arm converge at the DRG, which provides a focal point for accessing these signals. The DRG offers the additional advantage that, while the peripheral nerve includes both motor and sensory nerve fibers, the DRG contains only sensory neurons. In the present study, we examine how different aspects of a tactile event are encoded in the responses of a population of DRG neurons. Specifically, we record the activity evoked in tactile afferents when we manually apply textured surfaces to the skin at different speeds and contact forces. We then assess the degree to which we can read out information about time-varying contact force and about texture from these neural signals.
METHODS
Two adult male rhesus monkeys (Macaca mulatta) (monkey M, 14-yr-old; monkey B, 10-yr-old) were used in this study. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and are in keeping with the guidelines of the National Institutes of Health for the care and use of laboratory animals.
Surgery
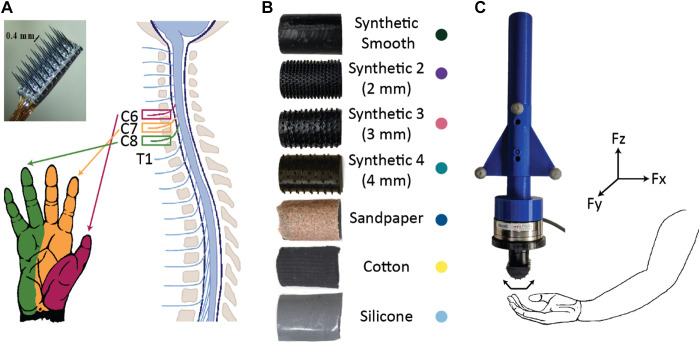
Experiments were conducted under general anesthesia. Anesthesia was induced using ketamine and maintained with isoflurane for the duration of the surgery. With the monkey lying in a prone position and head elevated, laminectomies on C6, C7, and C8 were done to expose the spinal cord, spinal nerve roots, and DRG in the caudal cervical spine on the right side. Three 4 × 8 penetrating microelectrode Blackrock “Utah” arrays (Blackrock Microsystems, Salt Lake City, UT) were implanted into the C6, C7, and C8 DRG in monkey M, and two Modular Bionics probes (Modular Bionics Inc., Berkeley, CA) were implanted into the C7 and C8 DRGs of monkey B (Fig. 1A). Each Modular Bionics probe consisted of four separate shanks with eight electrodes on each shank. The animals used in this study had reached the end of their participation in other research projects, so this was a terminal procedure. We studied anesthetized monkeys because to our knowledge, no system currently exists to perform chronic multielectrode recordings of individual-neuron action potentials from the DRG of awake and behaving Rhesus monkeys.
Figure 1.
Experimental setup. A: three 4 × 8-electrode Blackrock arrays (photograph) were implanted into the C6, C7, and C8 dorsal root ganglia (DRG) of monkey M, and four 8-contact Modular Bionics probes (not pictured) were implanted in the C7 and C8 DRG of monkey B. B: seven textures were used as tactile stimuli: three natural textures (sandpaper, cotton, and silicone) and four synthetic textures (evenly spaced raised dots). The colored dots next to the name of each texture are the color that will be used to represent that texture in all of the figures. C: each texture was attached to a force probe containing motion tracking markers and a force transducer. The force probe was brushed over different regions of the finger-pads and palm while neural responses were recorded. Fx, shear force in direction of stimulus movement; Fy, shear force orthogonal to stimulus movement; Fz, normal force.
Neural Recordings
Neural recordings were conducted with a Ripple Grapevine Neural Interface Processor (Ripple Neuron, Salt Lake City, UT) and recorded continuously at 30 kHz. The raw neural signals were bandpass filtered at 250–7,500 Hz and candidate action potentials were saved for offline sorting by setting a threshold at four times the root-mean-square (RMS) value of the baseline activity on each channel. Individual neural waveforms from well-isolated units were detected using Offline Sorter software (Plexon, Inc., Dallas, TX). Raw spike times were aligned with force traces for analysis.
Force and Velocity Measurements
Force signals were recorded as analog inputs (30 kHz) on the Grapevine system so that they were synchronized to neural events and low-pass-filtered with a cutoff frequency of 150 Hz. The magnitude of the forces parallel with the skin was combined into a scalar shear force measurement. The motion of the force probe was measured with an optical motion capture system at 120 Hz (V120:Trio, NaturalPoint, Inc., Corvallis, OR), synchronized digitally with the Grapevine system.
Unit Identification
Receptive fields (RFs) of units across the arrays were mapped via manual palpation of various regions of the animal’s arm and hand. Each tactile afferent was labeled as rapidly adapting (RA) or slowly adapting (SA) based on its response to brushes and indentations with a cotton swab during the experiment (27). These labels were additionally verified using the firing rate profiles of the trial-averaged neural activity. Units that responded to joint motion movement of the joints but not tactile stimulation were labeled as proprioceptive units and not considered further.
Stimuli
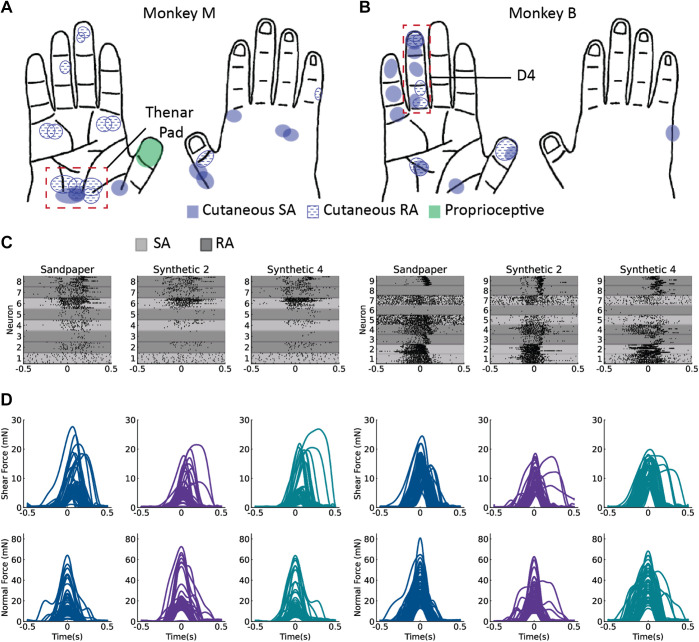
After units had been mapped, a cutaneous region with the highest density of mapped RFs was selected as the target for tactile stimulation (Fig. 2, A and B). A force transducer was attached to a handheld probe with a magnetic attachment for different textured surfaces (Fig. 1B). Natural textures (silicone, cotton, sandpaper) were attached to a 3D-printed half cylinder. Synthetic surfaces were also used. They were 3D-printed half cylinders with a series of evenly spaced raised dots (diameter = 0.75 mm, height = 1 mm, spacing = 2, 3, and 4 mm). Five textures were used in monkey M (silicone, cotton, sandpaper, 2- and 4-mm spaced dots), and seven textures were used in monkey B (silicone, cotton, sandpaper, no dots, 2-, 3-, and 4-mm spaced dots). To perform the experiment, a region of the glabrous skin containing the RFs of many of the units across the electrode arrays was identified—the thenar pad for monkey M and the fourth digit for monkey B (region indicated by the dashed red box in Fig. 2, A and B). The force transducer with attached texture was brushed by the experimenter back and forth over this region of the skin at 1-s intervals with varying force. The application of each texture was recorded continuously in two blocks, with 25–35 trials of contact with a texture per block, each ∼1 s in duration. Force traces were segmented into individual trials for each contact and temporally aligned on the peak normal force (Fig. 2, C and D). Sixty trials for each texture were segmented in the thenar pad of monkey M, and fifty trials per texture were segmented in monkey B for data analysis.
Figure 2.
Receptive fields of individual units were identified through manual palpation of the monkey’s arm and hand. Unit identification for the hand regions in monkey M (A) and in monkey B (B). The dashed red boxes indicate the location of where the tactile stimuli were applied. C: rasters and force traces for 60 applications of each of three textures in monkey M. D: 50 applications for monkey B. Time = 0 is the peak of the normal force. RA, rapidly adapting neuron; SA, slowly adapting neuron.
Rate-Based Encoding of Force
Each trial consisted of a sweep of a texture across the hand (Fig. 4, B and C). Instantaneous firing rates were estimated by binning spikes into 10-ms bins and smoothing with a Gaussian kernel with a width of 20 ms (Fig. 4A). Linear regression models were used to estimate the relationship between contact force and firing rate for both individual neurons and the population of neurons in the recordings. We created these regression models by fitting a linear relationship between neural activity and force using the equation F = βN + ϵ, where F is a matrix of the normal and shear force at each time point, with one column per time point, and N is a matrix of neural firing rates at each time point (one column per time point). The model was fitted by estimating a matrix of coefficients β (two coefficients per neuron: one for shear force and one for normal force), and constants ϵ (one for shear force and one for normal force). To test the generalizability of a linear model for force across textures, a leave-one-texture-out approach was used. In this approach, the linear model was fitted to the firing rates and forces of all trials for all textures except one, then tested by using the firing rates from the held-out texture to predict the corresponding force. Comparisons were made to evaluate how well this predicted force matched the actual force.
Figure 4.
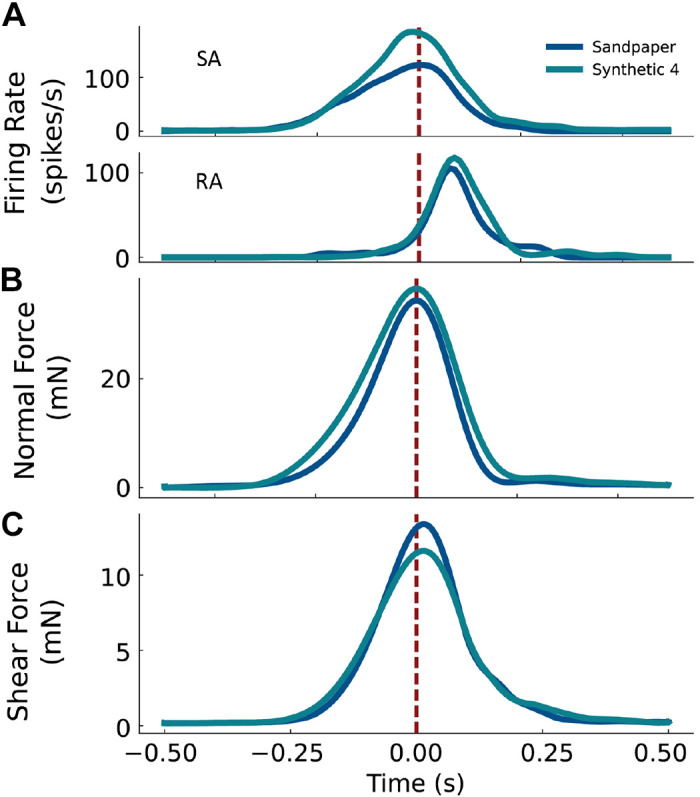
Firing rate during stimulus contact. A: the responses of a representative slowly adapting (SA) neuron (top) and rapidly adapting (RA) neuron (bottom) from monkey B. Trial-averaged normal and (B) shear force for the two textures, sandpaper, and synthetic 4 (C). The vertical dashed line at Time = 0 indicates the peak of the normal force.
Rate-Based Encoding of Texture
We used a classification procedure to quantify how much information about texture was present in neural activity. We were specifically interested in signals carried by individual neurons, by all neurons, and by neurons of each submodality (SA vs. RA). To measure the texture information in neural activity, we built multinomial logistic regression models (28) to model the relationship between neural activity and texture, then used these models to predict texture from neural activity for each type of neural signal. The higher the model’s accuracy, the more texture information is present in the neural activity. To build these models, neuronal responses were binned into 10-ms bins. Each model performed a classification at each time bin to assess the evolution of the texture signal over time. To train the model, the vector of neural activity across the population (or, for individual neurons, the scalar firing rate) was labeled with the corresponding texture and the model parameters were fit by finding the parameter values that would best reconstruct the texture labels. A leave-one-out cross-validation scheme was used, so that the classifiers were trained on all but one trial for each texture, then tested on the set of held-out trials. To test the decoding accuracy of the model, we classified texture from the neural activity on the held-out trials. The logistic regression models approximated the probability distribution across all textures from neural activity at each 10-ms time bin. The accuracy of the decoder, and thus the strength of the texture information present in the neural signal, was reported as the model’s probability assigned to the true texture given the observed firing rates. These decoder accuracies are shown in Fig. 6.
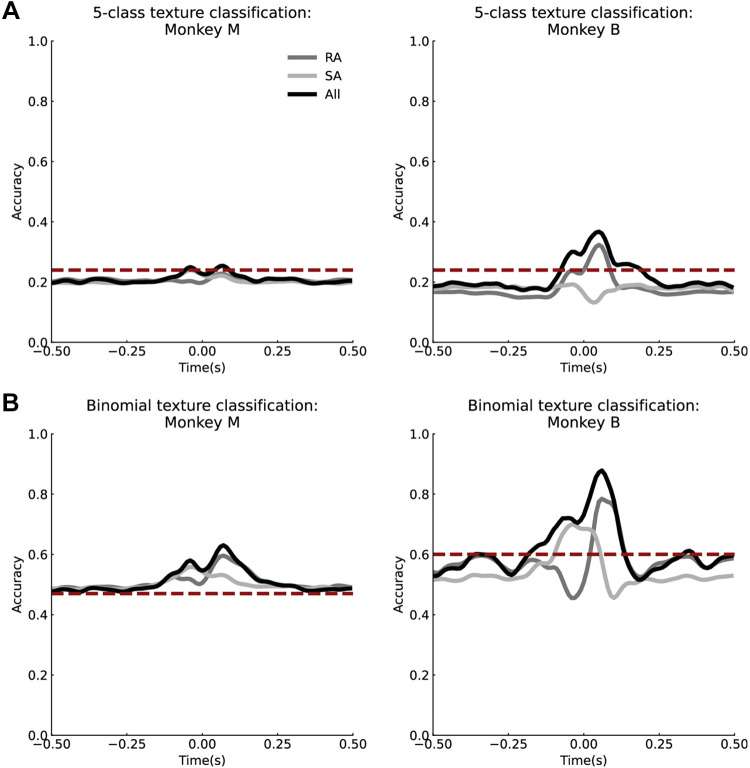
Figure 6.
Texture decoding from neural activity. A: average texture classification accuracy over time for rapidly adapting (RA), slowly adapting (SA), and all neurons across five textures for monkey M (left) and monkey B (right). All trials were used, and thus a wide range of contact forces. B: texture decoding for two textures that have similar normal and shear force profiles for monkey M (left) and monkey B (right). Time = 0 is the peak of the normal force. The dashed red lines represent the baseline texture classification accuracy using only the relationship between the shear and normal force.
Contact-Phase Variations in Texture Encoding
The normal and shear force profiles were segmented into three 50-ms epochs, representing sequential phases of the contact event corresponding to the onset, peak, and offset of the normal or shear force. The middle of the onset epoch was found by finding the time of the maximum rate of increase in force (i.e., maximum of the first derivative of the force with respect to time). Similarly, the middle of the offset epoch was identified as the time of maximum rate of decrease in force, and the peak was identified as the time at which the derivative of force with respect to time was zero. Average firing rates were measured in each 50-ms epoch. A logistic regression classifier was trained on each epoch separately to measure texture information encoded in the average firing rates in each epoch. To demultiplex force and texture, a logistic regression was then trained on the onset and offset epochs combined. These classifiers were trained with the same leave-one-set-out approach as described in the Rate-Based Encoding of Texture section.
Temporal Encoding of Texture
To assess the degree to which PAs covaried in their responses, we computed the cross-correlation functions for pairwise combinations of the instantaneous firing rates for every pair or neurons across two textures (synthetic 2 and synthetic 4). To mitigate the effects of variations in contact force on the texture-specific response of neurons and thus highlight the temporal pattern, we computed cross-correlations on a set of force-matched trials. To establish the role of temporal patterning across neurons in texture encoding, we repeated the classification analysis, but this time based not only on firing rates but also on joint firing rates for every pairwise combination of neurons. The joint firing rate is the product of the instantaneous firing rates of a pair of neurons in each 10-ms bin, which emphasizes responses that covary. As a control for the contribution of timing, we repeated the classification analysis after shuffling the time bins on each trial. We reasoned that if information about texture was present in the joint activity of neurons, then by including pairwise correlations as an additional neural signal, classification accuracy should improve.
RESULTS
We recorded simultaneously from small groups of primary afferent neurons in the DRG of two anesthetized Rhesus monkeys. In monkey M, 55 neurons were identified across the three arrays. In monkey B, 18 cutaneous neurons with RFs on the palmar surface of the hand were identified. Although we did not thoroughly explore the units with RFs in other parts of the limb in monkey B, we found approximately 12 additional units that were proprioceptive or were cutaneous with RFs on the arm.
We limited delivery of the tactile stimuli to the RF locations of well-isolated units (Supplemental Fig. 1; see https://figshare.com/s/0c26c98965d8ab61063a, 0% of interspike intervals < 1 ms) on the glabrous skin of the hand. Our analysis focused on eight neurons in monkey M that innervated the palm, and nine neurons in monkey B that innervated the fourth finger. Five of the eight neurons in monkey M were classified as RA, and the other three were classified as SA. Five out of nine neurons in monkey B were determined to be RA, and the remaining four were SA. Textured surfaces were brushed across the thenar pad of monkey M and the fourth finger (D4) of monkey B (Fig. 2, A and B). The normal and shear forces measured during application of the tactile stimulus as well as the responses of each neuron are shown for a subset of the textures in Fig. 2, C and D.
Interactive Effects of Force and Texture
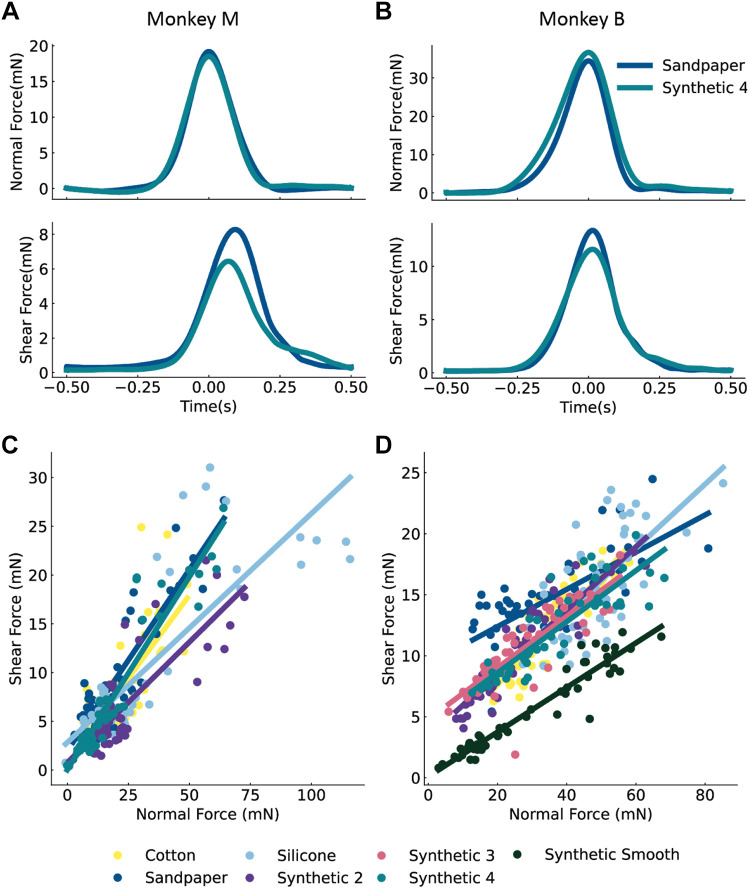
We first examined how the skin responded to our stimuli. Over the course of a tactile stimulus, skin deformations reflect both contact force and texture, and these stimulus features are unlikely to be independent. We determined if differences in texture influence the relationship between shear and normal force. To do this, we examined how the covariation between shear and normal forces varied with the texture. The average force traces for two textures, synthetic 4 and sandpaper, are shown in Fig. 3, A and B. The normal forces were similar for different textures (top) but the shear forces (bottom) were more texture-dependent. This phenomenon was observed across all textures (Fig. 3, C and D). As might be expected, smoother textures such as synthetic smooth and synthetic 2 exhibited smaller shear forces than rougher textures such as sandpaper. Importantly, the fact that shear force depends on both normal force and texture creates a challenge for the nervous system to separately encode these aspects of tactile stimuli.
Figure 3.
Normal and shear forces vary with texture. Normal and shear force of the skin for two textures in monkey M (A) and in monkey B (B). The relationship between the normal force (measured at its peak) and shear force depends on texture. C: shows all such relationships for each texture for monkey M and (D) for monkey B.
Force Encoding
Figure 4 shows the trial-averaged firing rates of a representative RA and SA neuron to two textures (Fig. 4A), as well as trial-averaged normal (Fig. 4B) and shear force (Fig. 4C) profiles. Comparing the forces on the skin elicited by two textures (synthetic 4 and sandpaper), normal force is more similar than shear force. The peak normal force differs by 7% between synthetic 4 higher and sandpaper, but peak shear force for synthetic 4 is 18% lower than the peak shear force for sandpaper. Examining neural responses, peak SA activity was 50% greater for synthetic 4 than for sandpaper, but peak RA activity was more similar between the two textures (12% greater for synthetic 4 than for sandpaper). Furthermore, the SA neuron responds over the entire duration of stimulus application (Fig. 4A, top), reaching its peak within 10 ms of the normal force. In contrast, the RA neuron responds maximally 70–100 ms after the peak normal force, which coincides with the time interval during which the normal and shear forces are decreasing (Fig. 4A, bottom). Taken together, these results show that neurons respond to variations in force and textures in different ways, and that SA and RA neurons respond during different phases of the contact interval.
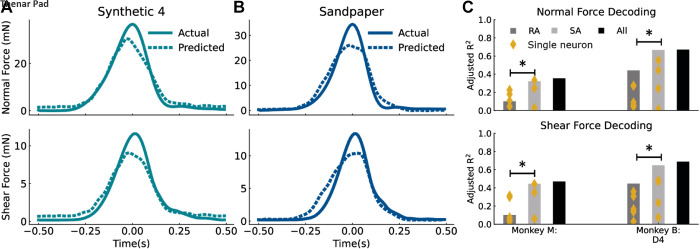
A function achieved by touch receptors is to accurately relay information about object properties such as texture despite variability in contact forces. To understand how this might be achieved, we began by assessing the degree to which these neuronal populations conveyed information about applied force. Because texture influences the relationship between normal and shear force, we determined whether the relationship between contact force and firing rate generalized across textures. To do so, we fitted a linear model to the forces and firing rates of all textures except for one, and then used this model to decode normal and shear force from neural activity on the held-out texture. Force traces decoded from the entire population of neurons for two textures are shown in Fig. 5, A and B. Overall, the neural population provided better force predictions (monkey M adjusted R2 for normal force = 0.35, shear = 0.47; monkey B adjusted R2 for normal force = 0.67, shear = 0.69) than did single neurons (median adjusted R2 for both monkeys for normal and shear force = 0.2), suggesting that the population firing rate of neurons encodes force magnitude, as has been shown previously (9, 10, 15, 16). Interestingly, although the neural population provided for accurate predictions of the normal and shear forces during the onset and offset of contact, the model systematically underestimated peak force. One explanation for this is that the model is biased toward lower forces because the peak force tends to be more variable across trials and account for only a small fraction of the trial duration, whereas force levels during onset and offset of the tactile stimuli are lower in magnitude but are a larger fraction of the trial duration. Because lower force levels are more frequent in the data and force levels are more variable at peak, the model is more likely to underestimate the actual force, particularly when the neural activity is also encoding other aspects of the tactile stimulus. An alternate explanation is that RA afferents exhibit their peak firing rate during the onset and offset of the stimulus, resulting in underestimation of force at the peak and slight overestimation of force during stimulus onset (Fig. 5, A and B). Regardless, a population firing rate codes for both normal and shear force generalized well across textures (Fig. 5C).
Figure 5.
Estimating forces from neural activity. Actual force traces compared with forces estimated using a linear model for synthetic 4 (A) and sandpaper from all neurons [five rapidly adapting (RA), four slowly adapting (SA)] in monkey B (B). C: estimates of normal (top) and shear (bottom) force from neural activity. Four estimations were performed, using different neural signals: individual neurons (yellow dots), all RA neurons, all SA neurons, and all neurons together. *Significantly different decoding performance, P < 0.05, Mann—Whitney U test.
We further compared the relative contributions of SA and RA neurons in predicting contact-force from the ensemble firing rates of neurons grouped by submodality. Within the sample of neurons in each monkey, SA neurons outperformed RA neurons at decoding the time-varying normal and shear forces (P < 0.001, Mann–Whitney U-test with Bonferroni correction). Moreover, force decoding accuracy for SA neurons (monkey M adjusted R2 for normal force = 0.32, shear = 0.44; monkey B normal = 0.67, shear = 0.68), but not RA neurons (monkey M adjusted R2 for normal force = 0.10, shear = 0.10; monkey B normal = 0.44, shear = 0.45), was as good as the entire population of neurons (average difference between adjusted R2 for SA neurons only and all neurons for both forces = 0.01 ± 0.01, between RA and all = 0.27 ± 0.05). Note that although the force decoding accuracy of neurons in monkey M was lower than in monkey B, monkey M had a lower proportion of SA neurons (3 out of 8) than monkey B (4 out of 9). Taken together, these results suggest that SA afferents encode more force information than RA afferents.
Texture Encoding
Next, we examined the degree to which neuronal responses carry information about texture across time-varying levels of contact force. To this end, we trained a multinomial logistic regression classifier to identify the applied texture from the instantaneous firing rate at each time point over the contact interval. For this analysis, we restricted the texture classification to the set of five textures (synthetic 2, synthetic 4, sandpaper, cotton, silicone) that was used in both monkeys.
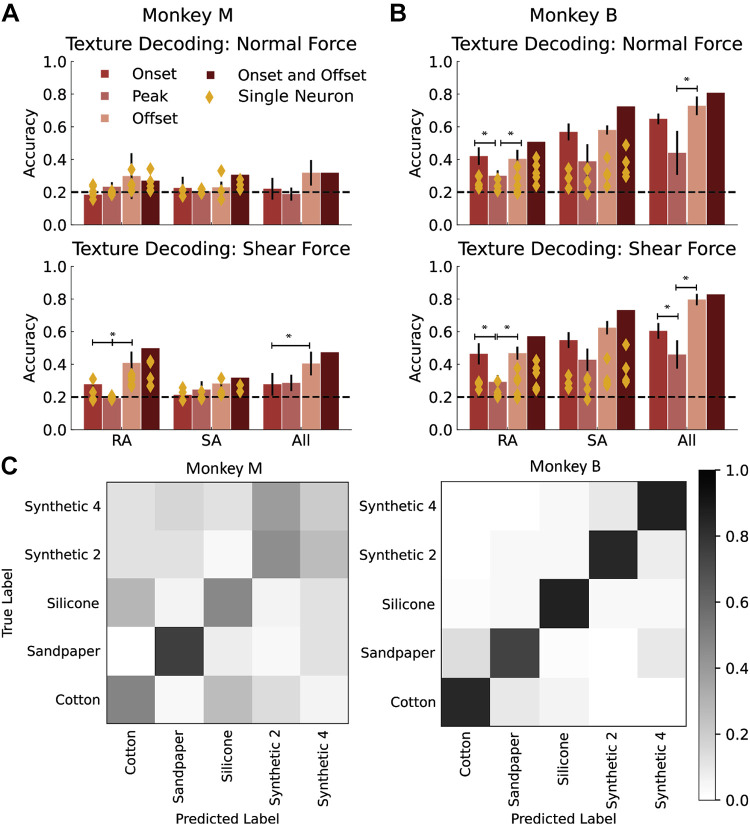
The five-class texture classification accuracy of the population instantaneous firing rate at each time point over the contact interval are shown in Fig. 6, A and B. Instantaneous classification accuracies begin at chance level at the start of contact and increase to peak levels (all neurons, monkey M = 0.25; monkey B = 0.37; chance = 0.2) before and after the midpoint of the stimulus delivery window, which corresponds to the peak in normal force. As the contact interval ends, texture classification accuracies return to chance levels. In addition, although SA afferents appeared to provide the dominant contribution to force encoding (Fig. 5C), RA afferents provided the strongest contribution to texture-classification [peak accuracy for monkey M = 0.23 (RA-only), 0.24 (all); monkey B = 0.32 (RA-only), 0.37 (all)].
Because shear force varies across textures (Fig. 3), and neurons encode shear force through population firing rates (Fig. 5), correlations between contact force and neural activity may be sufficient to explain the time-varying texture-classification profiles shown in Fig. 6, A and B. To determine if this was the case, we attempted to classify the five textures based on the ratio of shear to normal force, which yielded a texture-classification accuracy of 0.24 for both monkeys (vs. neural accuracy of 0.25 for monkey M, 0.37 for monkey B). The similarity in texture classification accuracy between contact force and neural activity suggests that force signals may dominate the neural response across the range of forces and textures represented in these data. This indicates that the texture-specific information conveyed by this small population of neurons was relatively weak and likely confounded by variations in force.
Another possibility as to why neurons in our population encode contact force across textures but not texture across variable forces is that neurons receive texture information entirely through normal and shear forces on the skin. If this is the case, then we would expect that neural activity would fail to discriminate between textures when force conditions are matched. Conversely, if texture discrimination accuracy in the force-matched condition is above chance, then we can conclude that the neural signals convey information for identifying textures.
To test these possibilities, we analyzed the responses evoked by two textures for which the normal and shear force relationship was similar. The following pairs of textures were selected based on the high degree of overlap exhibited in the relationship between normal and shear force shown in Fig. 3, C and D: synthetic 4 and cotton in monkey M, and synthetic 2 and silicone in monkey B. Using these paired textures, we first trained a binomial logistic regression classifier to predict texture using the relationship between shear and normal force (as described above). This established a baseline of how much texture information could be extracted if neurons were purely encoding normal force and shear force. This baseline accuracy is represented by the red dotted line in Fig. 6B (monkey M = 0.47, monkey B = 0.6). If texture classification with neural activity exceeds this baseline accuracy, then neurons encode texture information separately from force information. Thus, we trained a binary logistic regression classifier to classify the texture from the firing rates of all neurons, RA neurons only, and SA neurons only. With force-matching, binomial texture classification accuracy improved (monkey M peak accuracy = 0.63, baseline = 0.47; monkey B = 0.88, baseline = 0.6), suggesting that neural responses carry a texture signal that is independent of normal and shear force signals (Fig. 6B). Interestingly, the peak in texture-classification accuracy for RA neurons (peak accuracy: monkey M = 0.6; monkey B = 0.78) occurred during the offset phase of the contact-interval (Fig. 6B; monkey M = 0.07 s and monkey B = 0.06 s after peak normal force), whereas the peak in classification accuracy for SA neurons (monkey M = 0.56; monkey B = 0.7) occurred during the the onset phase of the normal force (0.04 s before peak normal force for both monkeys). Taken together, these results show that texture-specific signals can be isolated when contact forces are similar, and that RA and SA neurons encode texture information at different timepoints over the duration of the contact interval.
Decoding Texture across Variable Forces
Although the texture signals are confounded by variations in contact force, the strongest texture signals appear during the rising and falling phases of the contact force (Fig. 6), which may represent particularly opportune time intervals for extracting texture signals, demultiplexing them from force signals.
Given that the texture signal is strongly confounded by force, we next attempted to identify whether texture information could be demultiplexed from the firing rates of the small sample of neurons at all. Based on the peaks in texture classification accuracy for RA and SA neurons in Fig. 6, C and D, we examined whether we could discriminate texture across variable normal and shear forces by focusing on specific phases of the contact interval. To do so, we computed the average firing rate in 50-ms windows centered on the onset, peak, and offset of the shear and normal forces. Using a logistic regression classifier, we predicted texture from the average firing rate in each of these epochs for the same five textures used in the Texture Encoding section. We found that the firing rates of both individual neurons and the entire population of neurons during the offset times of shear force were significantly more informative about texture than were the responses during peak force application (mixed ANOVA with Tukey multiple comparisons post hoc test, P = 0.001), but this relationship was not observed when response epochs were identified from the onset, peak, and offset of the normal force (Fig. 7, A and B). Based on these findings, we attempted to classify texture using the neural activity during onset and offset of the shear force. This resulted in better texture classification accuracy (monkey M: RA = 0.5, SA = 0.32, all = 0.32; monkey B: RA = 0.57, SA = 0.73, all = 0.83; chance = 0.2), even across all textures and forces (Fig. 7, C and D). Thus, for this small set of neurons, signals at force onset and offset are more informative about texture than are those at peak force.
Figure 7.
Texture classification from neural response epochs. Texture classification accuracy using neural activity in a 50-ms window around the onset only, peak only, offset only, or onset and offset of normal force (A) and shear force (B) by afferent type. C: confusion matrices for texture classification using average firing rates in 50-ms windows centered around the onset and offset of shear force across the entire range of forces and five textures. *Significantly different texture classification accuracy, mixed ANOVA, P < 0.05, RA, rapidly adapting neurons; SA, slowly adapting neurons.
Temporal Encoding of Texture
As temporal spiking patterns in tactile nerve fibers—on the order of milliseconds—have been shown to carry information about texture (20), we assessed whether we could improve texture classification performance by taking spike timing into account. In particular, we assessed spike timing codes that involve the joint firing of pairs of neurons.
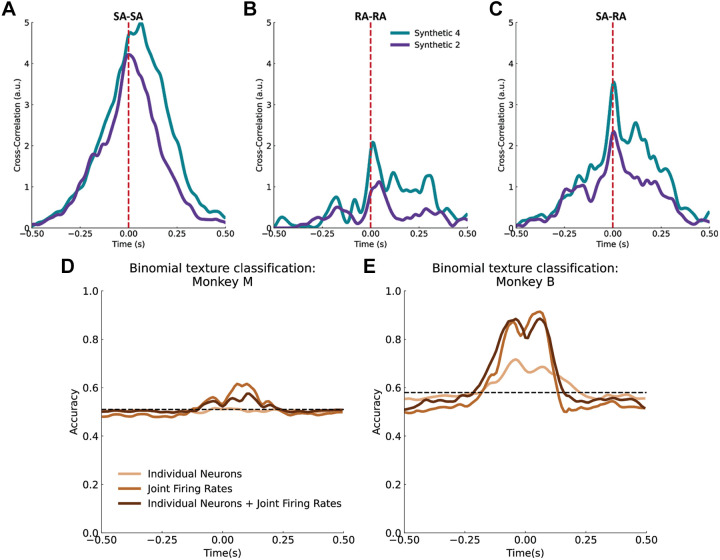
First, we examined whether neuronal responses under our stimulus conditions exhibited temporal patterning, as had been previously shown under tightly controlled stimulus presentations (11, 20, 21, 29). To do so, we computed the cross-correlation between the (time-varying) responses of pairs of neurons to each of two textures. For this analysis, we used two periodic textures, synthetic 2 (2-mm spaced dots) and synthetic 4 (4-mm spaced dots), because these are likely to evoke easily discernible periodic responses. We found that for both SA and RA neurons, the cross-correlation exhibited a peak at zero lags (Fig. 8, A and C). Note that the periodicity of the textures is not observed in the cross-correlations presumably because the scanning speed was not constant over the trial. Nonetheless, the cross-correlation analysis suggests that individual neurons produced responses that covaried in time with neighboring neurons.
Figure 8.
Temporal patterning of neural responses. Example cross-correlation of neural responses to a set of force-matched trials for two textures between two slowly adapting (SA) neurons (A), between two rapidly adapting (RA) neurons (B), and a RA and a SA neuron (C). This shows that pairs of neurons are correlated, and that correlation depends on texture. Texture decoding using all pairwise joint firing rates, all single unit activity in the population, and both single unit and joint firing rate activity for monkey M (D) and for monkey B (E). Dashed black lines indicate chance, computed as average classification accuracy using firing rates with shuffled time bins. a.u., Arbitrary units.
Next, we assessed whether this temporal patterning contained texture information and could thus be used to improve texture classification performance. To do so, we computed a joint firing rate for each pair of neurons by taking the product of their firing rates in 10-ms bins over each trial. Joint firing rates amplify coincident neural activity and thus enhance any temporal patterning in the response that is common across neurons. As expected from the cross-correlation analysis, we found that including joint firing rates in the classification analysis improved performance compared with when only individual-neuron firing rates were used (62% vs. 51% for monkey M and and 93% vs. 72% for monkey B; Fig. 8, D and E). Thus, temporal patterning across neurons contains information that can contribute to texture identification, even under stimulation conditions that vary.
DISCUSSION
During everyday manual interactions with objects, exploratory parameters—contact force, location, and scanning speed—vary constantly. Despite changes in these parameters, we can still extract the stable features of the objects we interact with—their shape, size, texture, and weight. How is it that information about object features can be extracted independently from information about contact mechanics? To help understand this remarkable processing, we examined how small populations of cutaneous afferent neurons encode contact parameters and object features. Specifically, we asked whether contact force and surface textures can be extracted from the activity of a small population of DRG neurons when the contact force and contact speed varied.
Force Encoding
Although shear force was more dependent on variations in texture than was the normal force, both could be decoded accurately from population neuronal firing rates of peripheral afferents (PAs) using a simple linear regression model (Fig. 5). The monotonic relationship between force and firing rate is consistent with previous studies, showing that firing rate increases systematically with indentation depth (15, 30, 31) and, more generally, with stimulus amplitude, regardless of the stimulus type (sinusoidal vibrations, mechanical noise, etc.) (13). Previous studies have shown that RA neurons are more sensitive to transient changes in contact force, whereas SA neurons, at a first approximation, track time-varying force levels (10, 16) as well as the direction of shear forces (32). In agreement with these studies, we found that SA neurons were better predictors of both normal and shear force, but the time-varying force could be reconstructed fairly accurately from the combined firing rates of RA and SA neurons.
Texture Encoding
The large neural signal of force suggests that when object-contact forces are varying, extracting texture information from the instantaneous firing rate of neurons is difficult. Other studies have shown that spike timing carries important information about the vibrations in the skin elicited by surface characteristics of a stimulus (21, 22). We found some evidence that temporal patterning in terms of synchronicity between pairs of neurons contained information about texture (Fig. 8). However, the role of temporal codes in texture encoding needs to be further studied because temporal patterning in our small population of neurons can be obscured by fluctuations in speed and temporal variations in the region of the tactile stimuli contacting the skin. In addition, these variations are more easily accounted for in large populations of neurons where many of the neurons exhibit similar texture-specific temporal responses. Therefore, perhaps in part as a result of a small number of neurons and the temporal information in our recordings, our ability to decode texture-specific features from the neuronal signals was relatively poor. Such variations in force, scanning speed, and texture are also present during naturally occurring object manipulations. Nonetheless, we were able to decode texture when normal and shear forces were matched across textures, indicating that PAs do convey information about texture. Previous studies have shown that RAs encode detailed texture information (29, 33, 34), whereas SAs encode coarse stimulus features (34). Our results agreed with these studies, with RAs encoding texture information at the onset and offset of the contact interval, whereas SAs encode texture information best at the peak of the contact force (Fig. 7). However, this information is confounded by force. Texture signals were strongest during shear force transients, consistent with studies showing that people increase the shear forces they apply to objects during tactile exploration (30). Similarly, studies in rodent whisking have found that mechanoreceptors also respond strongly to shear deformations, and that information about object properties is most salient in small temporal windows around object contact (35, 36). Thus, shear force modulation appears to be a crucial aspect of tactile perception. More specifically, previous studies have shown that mechanical deformations resulting from vibratory stimuli propagate throughout the skin in object-specific ways (37). Combined, these studies suggest that the interaction between shear forces and texture deforms the skin by inducing vibrations that depend upon contact mechanics, object surface characteristics, and the mechanical properties of skin (38). Since cutaneous RA afferents have been shown to respond primarily to vibratory stimuli (39–41), and transient, impulse-like stimuli best enable vibrations to propagate through the skin without damping, it is perhaps unsurprising that texture discrimination is best at transients of shear force. Given the small number of neurons and variable nature of the tactile stimuli in the experiments presented here, however, the responses of RA afferents to transients in shear force warrants further study.
In general, although texture classification accuracy in our study was far below human performance levels (42), the results reported here were obtained with only a small sample of neurons. We presume that performance would increase substantially with larger numbers of neurons. Although taking temporal patterns in the spiking response that were common across neurons improved texture classification, we anticipate that the boost in performance would be enhanced when temporal patterns are shared across a larger PA population.
Conclusion
Across all sensory modalities, the nervous system faces the challenge of extracting information from a highly variable, multi-factored stimulus. In the skin, populations of PAs combine information about exploratory parameters and about various features of contacted objects. This mixing occurs at the single-cell level, as the responses of most PAs depend on both texture and force information. Here, we showed that force information can be extracted reliably from the responses of a small population of PAs, and that SA fibers are more informative about force than are RA fibers. In contrast, information about texture occupies a high-dimensional perceptual space (43) and could not be determined unambiguously from the responses of our small population of PAs when force is also varying. Our results suggest that texture signals are most prominent when shear force is changing, perhaps due to the increased RA response during these epochs, and are carried in part in temporal spiking patterns common across neurons. We propose that demultiplexing texture signals from contact mechanics requires the implementation of sophisticated computations (44) on the responses of a larger population of nerve fibers. Whatever the mechanism for extracting texture information in the face of real-world changing forces might be, the present results highlight the challenge the nervous system faces in extracting texture signals in the somatosensory nerves during natural texture exploration.
GRANTS
This work was supported by the National Science Foundation Graduate Research Fellowship under Grant 1747452. We also thank the National Institutes of Health for support (National Institute of Neurological Disorders and Stroke Grants NS072342-01 (to D.J.W.) and NS101325 (to S.J.B.) and National Institute of Child Health and Human Development Grant R01HD090125 (to A.P.B.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.B. and D.J.W. conceived and designed research; M.L. and D.J.W. performed experiments; M.L. analyzed data; M.L., A.B., S.B., and D.J.W. interpreted results of experiments; M.L. prepared figures; M.L. drafted manuscript; M.L., A.B., S.B., and D.W. edited and revised manuscript; M.L., A.B., S.B., and D.W. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Dr. Robert Gaunt for conducting the surgeries, and Jeremy Winberry, Tyler Simpson, and Dr. Devapratim Sarma for assistance with data collection and experiment setup.
REFERENCES
- 1.Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56: 550–564, 1984. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan MA, Whitehouse JM, LaMotte RH. Tactile detection of slip: surface microgeometry and peripheral neural codes. J Neurophysiol 63: 1323–1332, 1990. doi: 10.1152/jn.1990.63.6.1323. [DOI] [PubMed] [Google Scholar]
- 3.Augurelle A-S, Smith AM, Lejeune T, Thonnard J-L. Importance of cutaneous feedback in maintaining a secure grip during manipulation of hand-held objects. J Neurophysiol 89: 665–671, 2003. doi: 10.1152/jn.00249.2002. [DOI] [PubMed] [Google Scholar]
- 4.Carteron A, McPartlan K, Gioeli C, Reid E, Turturro M, Hahn B, Benson C, Zhang W. Temporary nerve block at selected digits revealed hand motor deficits in grasping tasks. Front Hum Neurosci 10: 596, 2016. doi: 10.3389/fnhum.2016.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Nunzio AM, Dosen S, Lemling S, Markovic M, Schweisfurth MA, Ge N, Graimann B, Falla D, Farina D. Tactile feedback is an effective instrument for the training of grasping with a prosthesis at low- and medium-force levels. Exp Brain Res 235: 2547–2559, 2017. doi: 10.1007/s00221-017-4991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillon GS, Horch KW. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans Neural Syst Rehabil Eng 13: 468–472, 2005. doi: 10.1109/TNSRE.2005.856072. [DOI] [PubMed] [Google Scholar]
- 7.Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler J, Tyler DJ. A neural interface provides long-term stable natural touch perception. Sci Transl Med 6: 257ra138, 2014. doi: 10.1126/scitranslmed.3008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol 11: 455–461, 2001. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 9.Birznieks I, Jenmalm P, Goodwin AW, Johansson RS. Encoding of direction of fingertip forces by human tactile afferents. J Neurosci 21: 8222–8237, 2001. doi: 10.1523/JNEUROSCI.21-20-08222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birznieks I, Macefield VG, Westling G, Johansson RS. Slowly adapting mechanoreceptors in the borders of the human fingernail encode fingertip forces. J Neurosci 29: 9370–9379, 2009. doi: 10.1523/JNEUROSCI.0143-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor CE, Johnson KO. Neural coding of tactile texture: comparison of spatial and temporal mechanisms for roughness perception. J Neurosci 12: 3414–3426, 1992. doi: 10.1523/JNEUROSCI.12-09-03414.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin AW, Wheat HE. Effects of nonuniform fiber sensitivity, innervation geometry, and noise on information relayed by a population of slowly adapting type i primary afferents from the fingerpad. J Neurosci 19: 8057–8070, 1999. doi: 10.1523/JNEUROSCI.19-18-08057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muniak MA, Ray S, Hsiao SS, Dammann JF, Bensmaia SJ. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J Neurosci 27: 11687–11699, 2007. doi: 10.1523/JNEUROSCI.1486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips JR, Johansson RS, Johnson KO. Responses of human mechanoreceptive afferents to embossed dot arrays scanned across fingerpad skin. J Neurosci 12: 827–839, 1992. doi: 10.1523/JNEUROSCI.12-03-00827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poulos DA, Mei J, Horch KW, Tuckett RP, Wei JY, Cornwall MC, Burgess PR. The neural signal for the intensity of a tactile stimulus. J Neurosci 4: 2016–2024, 1984. doi: 10.1523/JNEUROSCI.04-08-02016.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheat HE, Salo LM, Goodwin AW. Cutaneous afferents from the monkeys fingers: responses to tangential and normal forces. J Neurophysiol 103: 950–961, 2010. doi: 10.1152/jn.00502.2009. [DOI] [PubMed] [Google Scholar]
- 17.Delhaye BP, O'Donnell MK, Lieber JD, McLellan KR, Bensmaia SJ. Feeling fooled: texture contaminates the neural code for tactile speed. PLoS Biol 17: e3000431, 2019. doi: 10.1371/journal.pbio.3000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edin BB, Essick GK, Trulsson M, Olsson KA. Receptor encoding of moving tactile stimuli in humans. I. Temporal pattern of discharge of individual low-threshold mechanoreceptors. J Neurosci 15: 830–847, 1995. doi: 10.1523/JNEUROSCI.15-01-00830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. J Physiol 310: 117–144, 1981. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber AI, Saal HP, Lieber JD, Cheng J-W, Manfredi LR, Dammann JF, Bensmaia SJ. Spatial and temporal codes mediate the tactile perception of natural textures. Proc Natl Acad Sci USA 110: 17107–17112, 2013. doi: 10.1073/pnas.1305509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackevicius EL, Best MD, Saal HP, Bensmaia SJ. Millisecond precision spike timing shapes tactile perception. J Neurosci 32: 15309–15317, 2012. doi: 10.1523/JNEUROSCI.2161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey MA, Saal HP, Dammann JF, Bensmaia SJ. Multiplexing stimulus information through rate and temporal codes in primate somatosensory cortex. PLoS Biol 11: e1001558, 2013. doi: 10.1371/journal.pbio.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin AW, Macefield VG, Bisley JW. Encoding of object curvature by tactile afferents from human fingers. J Neurophysiol 78: 2881–2888, 1997. doi: 10.1152/jn.1997.78.6.2881. [DOI] [PubMed] [Google Scholar]
- 24.Johansson RS, Birznieks I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat Neurosci 7: 170–177, 2004. doi: 10.1038/nn1177. [DOI] [PubMed] [Google Scholar]
- 25.Stein RB, Weber DJ, Aoyagi Y, Prochazka A, Wagenaar JB, Shoham S, Normann RA. Coding of position by simultaneously recorded sensory neurones in the cat dorsal root ganglion. J Physiol 560: 883–896, 2004. doi: 10.1113/jphysiol.2004.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umeda T, Seki K, Sato M-A, Nishimura Y, Kawato M, Isa T. Population coding of forelimb joint kinematics by peripheral afferents in monkeys. PloS One 7: e47749, 2012. doi: 10.1371/journal.pone.0047749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan MA, LaMotte RH. Tactile discrimination of shape: responses of slowly and rapidly adapting mechanoreceptive afferents to a step indented into the monkey fingerpad. J Neurosci 7: 1682–1697, 1987. doi: 10.1523/JNEUROSCI.07-06-01682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Böhning D. Multinomial logistic regression algorithm. Ann Inst Stat Math 44: 197–200, 1992. doi: 10.1007/BF00048682. [DOI] [Google Scholar]
- 29.Freeman AW, Johnson KO. Cutaneous mechanoreceptors in macaque monkey: temporal discharge patterns evoked by vibration, and a receptor model. J Physiol 323: 21–41, 1982. doi: 10.1113/jphysiol.1982.sp014059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callier T, Saal HP, Davis-Berg EC, Bensmaia SJ. Kinematics of unconstrained tactile texture exploration. J Neurophysiol 113: 3013–3020, 2015. doi: 10.1152/jn.00703.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janko M, Wiertlewski M, Visell Y. Contact geometry and mechanics predict friction forces during tactile surface exploration. Sci Rep 8: 4868, 2018. doi: 10.1038/s41598-018-23150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birznieks I, Wheat HE, Redmond SJ, Salo LM, Lovell NH, Goodwin AW. Encoding of tangential torque in responses of tactile afferent fibres innervating the fingerpad of the monkey. J Physiol 588: 1057–1072, 2010. doi: 10.1113/jphysiol.2009.185314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bensmaïa SJ, Hollins M. The vibrations of texture. Somatosens Mot Res 20: 33–43, 2003. doi: 10.1080/0899022031000083825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blake DT, Johnson KO, Hsiao SS. Monkey cutaneous SAI and RA responses to raised and depressed scanned patterns: effects of width, height, orientation, and a raised surround. J Neurophysiol 78: 2503–2517, 1997. doi: 10.1152/jn.1997.78.5.2503. [DOI] [PubMed] [Google Scholar]
- 35.Bush NE, Solla SA, Hartmann MJ. Whisking mechanics and active sensing. Curr Opin Neurobiol 40: 178–188, 2016. doi: 10.1016/j.conb.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Severson KS, Xu D, Yang H, O’Connor DH. Coding of whisker motion across the mouse face. eLife 8: e41535, 2019. doi: 10.7554/eLife.41535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao Y, Hayward V, Visell Y. Spatial patterns of cutaneous vibration during whole-hand haptic interactions. Proc Natl Acad Sci USA 113: 4188–4193, 2016. doi: 10.1073/pnas.1520866113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delhaye B, Barrea A, Edin BB, Lefèvre P, Thonnard J-L. Surface strain measurements of fingertip skin under shearing. J R Soc Interface 13: 20150874, 2016. doi: 10.1098/rsif.2015.0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freeman AW, Johnson KO. A model accounting for effects of vibratory amplitude on responses of cutaneous mechanoreceptors in macaque monkey. J Physiol 323: 43–64, 1982. doi: 10.1113/jphysiol.1982.sp014060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson RS, Vallbo AB. Detection of tactile stimuli. Thresholds of afferent units related to psychophysical thresholds in the human hand. J Physiol 297: 405–422, 1979. doi: 10.1113/jphysiol.1979.sp013048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbot WH, Darian-Smith I, Kornhuber HH, Mountcastle VB. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol 31: 301–334, 1968. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- 42.Manfredi LR, Saal HP, Brown KJ, Zielinski MC, Dammann JF, Polashock VS, Bensmaia SJ. Natural scenes in tactile texture. J Neurophysiol 111: 1792–1802, 2014. doi: 10.1152/jn.00680.2013. [DOI] [PubMed] [Google Scholar]
- 43.Lieber JD, Bensmaia SJ. High-dimensional representation of texture in somatosensory cortex of primates. Proc Natl Acad Sci USA 116: 3268–3277, 2019. doi: 10.1073/pnas.1818501116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lieber JD, Bensmaia SJ. Emergence of an invariant representation of texture in primate somatosensory cortex. Cereb Cortex 30: 3228–3239, 2020. doi: 10.1093/cercor/bhz305. [DOI] [PMC free article] [PubMed] [Google Scholar]