Keywords: feedback, sway, threshold
Abstract
Controlling posture requires continuous sensory feedback about body motion and orientation, including from the vestibular organs. Little is known about the role of tilt vs. translation vs. rotation vestibular cues. We examined whether intersubject differences in vestibular function were correlated with intersubject differences in postural control. Vestibular function was assayed using vestibular direction-recognition perceptual thresholds, which determine the smallest motion that can be reliably perceived by a subject seated on a motorized platform in the dark. In study A, we measured thresholds for lateral translation, vertical translation, yaw rotation, and head-centered roll tilts. In study B, we measured thresholds for roll, pitch, and left anterior-right posterior and right anterior-left posterior tilts. Center-of-pressure (CoP) sway was measured in sensory organization tests (study A) and Romberg tests (study B). We found a strong positive relationship between CoP sway and lateral translation thresholds but not CoP sway and other thresholds. This finding suggests that the vestibular encoding of lateral translation may contribute substantially to balance control. Since thresholds assay sensory noise, our results support the hypothesis that vestibular noise contributes to spontaneous postural sway. Specifically, we found that lateral translation thresholds explained more of the variation in postural sway in postural test conditions with altered proprioceptive cues (vs. a solid surface), consistent with postural sway being more dependent on vestibular noise when the vestibular contribution to balance is higher. These results have potential implications for vestibular implants, balance prostheses, and physical therapy exercises.
NEW & NOTEWORTHY Vestibular feedback is important for postural control, but little is known about the role of tilt cues vs. translation cues vs. rotation cues. We studied healthy human subjects with no known vestibular pathology or symptoms. Our findings showed that vestibular encoding of lateral translation correlated with medial-lateral postural sway, consistent with lateral translation cues contributing to balance control. This adds support to the hypothesis that vestibular noise contributes to spontaneous postural sway.
INTRODUCTION
Precision in motion control is critical to survival, and deficits in postural control result in many debilitating and deadly falls. Sensory feedback about body motion is used to determine postural motor behaviors (1–5), including feedback from vestibular, visual, and proprioceptive cues. The vestibular sensory organs, located in the inner ear, include the semicircular canals, which sense three-dimensional angular rotation, and the otolith organs, which sense linear motion and the direction of gravity. Vestibular integrity is important for postural control. In fact, the odds of falling are 12 times higher when both 1) vestibular symptoms are present and 2) subjects are unable to complete a Romberg balance test (6). Suboptimal vestibular function has been associated with an estimated 48,000–152,000 deaths a year due to the vestibular contributions to balance and falls (7).
Numerous studies have examined the role of vestibular cues in postural control. Many do so by measuring differences in postural control between patients with confirmed vestibular deficits (8–11) and normal controls. Some studies have measured changes in postural control due to disruption of vestibular cues using electrical stimulation of the vestibular system using surface electrodes (galvanic vestibular stimulation, GVS) (12–14). Dynamic models have been used to estimate vestibular, proprioceptive, and visual contributions to experimental postural responses to platform motion (3, 5, 15). These models usually define vestibular feedback as the combination of all cues that provide feedback about body tilt relative to gravity but typically take advantage of small-angle linearization to exclude contributions of the known nonlinear sensory integration between signals from the canals and otoliths (16–19). For this paper, a key feature of some models is that postural sway and trial-to-trial postural variability arise from sensory and motor noise (5, 20, 21).
During standing, tilt of the body about the ankles causes rotation and translation of the head, and also tilt relative to gravity. This is almost always true in real life and always true unless motions at the hip and neck counteract the ankle motion to keep the head perfectly still. Despite distinct neural estimates of rotation, tilt and translation provided by the vestibular system, only a few studies have attempted to distinguish the contributions of these specific vestibular cues to postural control (22–24). For example, Serrador et al. (24) implicated the otolith organs by showing a correlation between a decline in otolith-mediated eye movements and increased postural sway.
We recently examined the specific vestibular contributors to balance performance in subjects aged 18–80 yr, with no known vestibular deficits or major health conditions, using a pass/fail assessment of balance on the condition 4 Romberg balance test (eyes closed, stance on foam) (7, 25). Sensory function was assessed using vestibular thresholds, which assay the smallest motion that can be reliably perceived when the seated subject is immobilized on a motorized platform in the dark. Thresholds were measured with different motion directions and stimulus durations, including lateral (Y) translations, vertical (Z) translations, Earth-vertical yaw rotations, and roll tilts. Roll tilt thresholds were assayed with motions lasting 1 s (1 Hz), which primarily elicit semicircular canal responses, and 5 s (“0.2 Hz”), which elicits both otolith organs and semicircular canal responses (details in methods) (26). Using a multiple variable regression, we found that age and roll tilt 0.2-Hz thresholds together were correlated with being unable to complete Romberg condition 4, which had previously been shown to predict those likely to have falling difficulties in the past year (6) and that none of the other threshold measures had a statistically significant contribution (25). This suggests that the ability to sense tilt of the body is important for passing Romberg condition 4, with rotation and translation cues playing a smaller role. Importantly, tilt and rotation thresholds are measured using motions about a head-centered axis, so they do not conflate rotation and translation cues as would occur if tilts occurred about an axis at the feet. Overall results showed that measurable, subclinical intersubject differences in vestibular sensory function contribute to intersubject differences in postural control in conditions that require high reliance on vestibular information for balance, even in healthy subjects who did not report any vestibular symptoms. Additionally, these results validate thresholds as a robust tool to study sensory function at the subclinical level.
In this paper, we build on our recent study by comparing vestibular thresholds to quantitative measures of postural sway. This extension is important because postural sway provides a richer continuous metric of posture compared with pass/fail Romberg results, e.g., a subject taking a step, opening their eyes, or falling into the harness. In particular, pass/fail results are insensitive to interindividual and interconditional differences within those who complete (“pass”) or those who fail to complete a test. To our knowledge, sway has not previously been related to thresholds. Since thresholds serve as a direct assay of neural noise (27–29), likely arising in the sensory periphery or early in central processing (30, 31), and balance control models attribute spontaneous sway primarily to internal noise sources, including sensory noise, there is a theoretical relationship between these thresholds and the sensory noises used in models of postural sway (5, 20, 21, 32, 33), and a relationship between threshold and sway measures is expected.
Our broad hypothesis was that there would be a positive correlation between vestibular perceptual thresholds and postural sway, consistent with individual differences in postural sway arising from individual differences in sensory noise that is quantified by thresholds. On the basis of the geometric assumption that sensory cues are most relevant to postural control in the same plane, we specifically hypothesized 1) a positive correlation between roll tilt 0.2-Hz thresholds and mediolateral (ML) sway, since they both assay function in the frontal plane; 2) a positive correlation between lateral translation thresholds and ML sway, since they both assay function in the frontal plane; 3) a positive correlation between pitch tilt 0.2-Hz thresholds and anterioposterior (AP) sway, since they both assay function in the sagittal plane; and 4) no evidence of a correlation of postural sway with either yaw rotation or vertical translation thresholds. We also expect a positive correlation between fore-aft translation thresholds and AP sway, but fore-aft translation thresholds were not assayed in this study; thus, this could not be tested.
We primarily focused on postural tests that induced the highest reliance on vestibular cues (i.e., eyes closed, unreliable support). In secondary analyses, we examined other tests to determine whether a vestibular influence on postural sway was evident even in conditions with higher visual and proprioceptive reliance. We also studied sensory reweighting to see whether the correlation between thresholds and sway would be larger in conditions that had more vestibular reliance versus less vestibular reliance, consistent with increased vestibular weight causing postural sway to be more dependent on vestibular noise. We found evidence that postural sway was correlated with lateral translation thresholds but not significantly correlated with other thresholds measured.
METHODS
Overview
Two studies were conducted with different subject groups (Table 1). In each study, postural and threshold measures were acquired. Postural center of pressure was measured during standard clinical protocols: the NeuroCom EquiTest (Natus Balance & Mobility, Seattle, WA) for study A and the modified Romberg test of standing balance on firm and compliant support surfaces (34–37) for study B. In both studies, postural sway was calculated for each trial in orthogonal directions. Vestibular perceptual thresholds were assayed to determine the smallest motion that subjects could reliably perceive (38). Subjects sat in the dark on a chair mounted on a motion platform. Subjects completed a number of trials, which consisted of a small motion followed by a response in which subjects had to report their perceived motion. Five threshold measures were assayed in study A and four in study B (Table 1). Multivariate analyses determined the relationship between postural sway and the threshold measures.
Table 1.
Summary of subject group, postural test conditions, and threshold measures
| Study | Subjects | Postural Measures | Threshold Measures |
|---|---|---|---|
| Study A | 12 8 females 4 males Mean age 34 yr (SD = 9, range 21–50) |
SOT 1: Normal vision, fixed support SOT 2: eyes closed, fixed support SOT 3: sway-referenced vision, fixed support SOT 4: normal vision, sway referenced support SOT 5: eyes closed, sway-referenced support SOT 6: sway-referenced vision, sway-referenced support |
Lateral (Y) translation 1 Hz Vertical (Z) translation 1 Hz Yaw rotation 1 Hz Roll tilt 1 Hz Roll tilt 0.2 Hz |
| Study B | 14 8 females 6 males Mean age 39 yr (SD = 12, range 23–61) |
Condition 1: eyes open, fixed support Condition 2: eyes closed, fixed support Condition 3: eyes open, foam support Condition 4: eyes closed, foam support |
Roll tilt 0.2 Hz Pitch tilt 0.2 Hz LARP tilt 0.2 Hz RALP tilt 0.2 Hz |
LARP tilts result from an earth-horizontal head rotation that primarily activates left anterior and right posterior (LARP) canals (i.e., is between pitch and roll). Right anterior left posterior (RALP) tilts result from an earth-horizontal head rotation that primarily activates right anterior and left posterior (RALP) canals. SOT, sensory organization test.
Study Rationale
Studies A and B each had multiple scientific objectives, and the experimental design reflects a compromise between subject testing time and addressing all of these objectives. In study A, a focus was to measure thresholds for multiple axes of motion as a function of frequency (38), which took ∼10 h per subject; thus, it was infeasible to also test thresholds with motions corresponding to the AP plane. In study B, the objectives included testing tilt thresholds with different planes of motion, determining how these thresholds differ in individuals with vestibular migraine, and testing for correlations between these thresholds and sway in both the AP and ML directions. Thus, study B complemented study A by including thresholds pertinent to AP sway. Although testing of vestibular migraine subjects is not yet complete and will be explored in more depth in the future, limited initial results are included here to increase the number of subjects for which correlations are examined, although their inclusion did not alter the conclusions. These compromises and limitations are addressed in detail in discussion and were considered when interpreting results.
Subjects
Twelve healthy volunteers participated in study A. Threshold results for these subjects are published (38), but two subjects from that paper are not included in study A because their posture data files were not available. Study B included nine healthy volunteers and five subjects with vestibular migraine diagnoses. Analyses were performed with vestibular migraine subjects both included and excluded in combined results. Eligibility for healthy subjects was determined using a questionnaire which included questions about back/neck, cardiovascular, neurological, and other physical problems, history of dizziness or vertigo, and pregnancy. Subjects in study A had normal results on clinical vestibular diagnostic tests (caloric, electronystagmography, Hallpike, angular vestibuloocular reflex tests). Both studies were approved by the Massachusetts Eye and Ear Infirmary Human Studies Committee (HSC) and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All subjects gave written informed consent before participation in the study. In study A, there was fewer than 4 yr between postural and threshold testing (7 of 12 tested within 1 yr and 9 of 12 tested within 2 yr). In study B, there was less than 1 mo between postural and threshold testing.
Postural Testing
Postural testing in study A consisted of measuring postural CoP using the NeuroCom EquiTest (Natus Balance & Mobility, Seattle, WA). All subjects performed the standard protocol of the sensory organization test (SOT), including SOTs 1–6 (Table 1). These tests manipulate the relative reliance (i.e., weight) on vestibular, visual, and proprioceptive cues (9, 11, 39). Of special interest was SOT 5, because it causes the brain to increase reliance on vestibular cues (3, 9, 11). Specifically, visual cues are unavailable because the eyes are closed, and proprioceptive cues indicate that the ankle position is near that experienced when upright, with small deviations because sway referencing is determined using low-pass filtered CoP. While cutaneous cues are thought to contribute much less to posture than kinesthetic cues (40), we use “proprioceptive” to encompass both kinesthetic and cutaneous cues. Ideally sway referencing would have been done in the ML plane so that it corresponded to the plane of the threshold measurements. Nonetheless, sensory weighting in the ML direction has been shown to be affected by perturbations in the AP direction (41). Each trial lasted 20 s. Subjects stood with their lateral calcanei separated by ∼0.3 m, following the manufacturer’s clinical protocol. A trial immediately ended if a subject took a step, touched another surface, opened their eyes, fell into the harness, or asked for the trial to end. Two trials were performed for each of SOT 1 and 2 and three trials for each of SOT 3–6. The exception was three subjects who did two trials for all SOTs, since this was at the discretion of the technician in the clinical laboratory. A force plate integrated into the EquiTest system measured ground reaction forces with a sampling rate of 100 Hz, which were stored by a computer for offline analysis. One subject did not complete one of three trials for SOT 5, and another subject did not complete one of three trials for SOT 6.
Postural testing in study B consisted of the modified Romberg test of standing balance on firm and compliant support surfaces (34, 36, 37). In this test, subjects stand with feet together and arms crossed. There are four conditions, each of which must be passed to continue with the test. If a trial was not passed, because a subject took a step, touched another surface, opened their eyes, or asked for the trial to end, a single additional trial was permitted. To pass condition 1, the subject was required to stand on the floor with eyes open for 15 s. To pass condition 2, the subject was required to stand on the floor with eyes closed for 15 s. To pass condition 3, the subject was required to stand on a foam pad (Sunmate medium density, 16 × 18 × 3 in.) with eyes open for 30 s. To pass condition 4, the subject was required to stand on a foam pad with eyes closed for 30 s. Of special interest was condition 4, because it causes the brain to increase reliance on vestibular cues (37). Specifically, visual cues are unavailable because the eyes are closed and the foam alters proprioceptive cues so that they are partly decoupled from deviation of the body from upright and more variable than without foam (42). A force plate (AMTI; AccuSway, Watertown, MA) with 0.005 mm accuracy measured ground reaction forces, which were sampled at 100 Hz. One subject, with vestibular migraine, passed condition 1 on their second attempt, was unable to complete condition 2, and did not perform conditions 3 and 4. Results are presented with this subject included, but they varied only slightly when this subject was excluded. One other subject repeated condition 2 but completed conditions 3 and 4 on their first attempts.
Threshold Testing
The vestibular threshold methods and results for study A have been published (38). Study B used similar methods to study A and other studies in our laboratory (7, 38, 43, 44). Thus, we provide only a brief description of those methods here. Subjects sat upright in the dark on a chair mounted on a Moog 6DOF motion platform. A helmet and five-point harness reduced head and body movement relative to the chair. Subjects wore noise-canceling headphones, which played white noise during motion, which masked device sound and indicated when motion was occurring. Each block of testing consisted of a number of trials, with each trial consisting of a motion followed by a response. An adaptive, one-interval, two-alternative, forced-choice, direction-recognition paradigm was used. After each motion, subjects were required to report the perception of motion direction by pressing buttons in their left or right hand. Single-cycle acceleration motion stimuli were used in which the period (i.e., 1/frequency) of the sinusoid was manipulated, since varying the period changes the contributions of the semicircular canal and otolith organs to roll tilt perception (26). The displacement, peak velocity, and peak acceleration are linearly proportional for these motions for a given period (44, 45). Motion direction was randomly chosen for each trial (e.g., left/right or up/down). Motion amplitude for each trial was selected using an adaptive three-down, one-up (46, 47) parameter estimation by sequential testing (PEST) (48) staircase algorithm, which increased the motion amplitude after a single incorrect response and decreased the motion amplitude after three sequential correct responses.
For each block, binary responses were fitted with a Gaussian cumulative distribution psychometric function (49, 50), which yielded a threshold (σ) and a mean (μ) that is often called the “vestibular bias” (29). Parameters were determined by finding the maximum likelihood estimate for a bias-reduced generalized linear model (brglmfit.m) (49) using the MATLAB Statistic Toolbox (Mathworks, Natick, MA). Compensation for mechanical dynamics is performed by our control software, and we have verified that actual motion is within 4% of desired (51). Subjects with bilateral vestibular nerve sections have much higher vestibular thresholds than those who pass a vestibular clinical exam (38), showing that the vestibular organs are the predominant cue for this threshold task.
In studies A and B, the same frequency and motion axis was used for all trials within a block. In study A, each healthy normal subject participated in 22 blocks of testing over approximately 8 h spread across multiple days (38), consisting of the following: 1) lateral (Y) translations along an earth-horizontal, interaural axis (0.3, 0.5, 1, 2, and 5 Hz); 2) vertical (Z) translations along an earth-vertical axis (0.3, 0.5, 1, 2, and 5 Hz); 3) yaw rotations about an earth-vertical axis (0.2, 0.5, 1, 2, and 5 Hz); and 4) ear-down roll tilts about an earth-horizontal axis passing through the head (0.05, 0.1, 0.2, 0.5, 1, 2, and 5 Hz). For each block, trials began at the same initial amplitudes (38) and were collected until the coefficient of variation for 1/σ was <0.2, which typically required 70–80 trials, where σ is the threshold. The chair always returned to upright before tilt motions.
For study A, we reduced the above data by analyzing the same five threshold measures (Table 1) used in our previous work (7, 25). Four of these thresholds were measured with 1-s motions (1 Hz). These were chosen because vestibular perceptual thresholds exhibit a plateau in peak velocity above roughly 1 Hz (38, 44, 52, 53), which is thought to represent the underlying neural noise (28, 29). In contrast, vestibular perceptual thresholds for most motion axes increase with decreasing frequency below 1 Hz, which is explained by a central high-pass filter associated with perceptual decision making (44, 54), but not with other perceptual tasks like magnitude estimation (55). This filtering is thought to affect sensory noise equally regardless of stimulus frequency but to attenuate low-frequency stimuli more than high-frequency stimuli, resulting in lower signal-to-noise ratios for low-frequency stimuli. This idea is supported by strong correlations between thresholds across frequencies (25). Furthermore, vestibuloocular reflex thresholds for yaw rotation are similar to perceptual thresholds above 1 Hz but are not larger at 0.2 and 0.3 Hz as for perceptual thresholds (30), consistent with perceptual thresholds above 1 Hz corresponding to the underlying neural noise, and with the larger perceptual thresholds at lower frequencies resulting from the central high-pass filter that does not affect motor behavior. Together, this evidence shows that thresholds measured at 1 Hz assay the neural noise that affects motor behavior regardless of the frequency characteristics of the motor behavior. The fifth measure that we focused on was roll tilt 0.2-Hz thresholds, since they reflect a combination of semicircular canal and otolith contributions (26). In contrast, roll tilt thresholds mainly reflect semicircular canal contributions above 1 Hz. This is because the semicircular canals primarily transduce angular velocity and the otolith organs primarily translate tilt position relative to gravity, and as motion frequency deceases, the corresponding peak velocities decrease for a given tilt angle.
In study B, thresholds were measured for five different tilts, all using 5-s (0.2-Hz) motions. Each block was repeated on a second day, yielding a total of 10 blocks, with 75 trials per block. The chair always returned to upright before motions. Four threshold measures were relevant to the analysis in this paper (Table 1); these tested tilts about a head-centered, earth-horizontal axis: 1) ear-down “roll tilts”; 2) nodding “pitch tilts”; 3) motions that stimulate the left anterior and right posterior semicircular canals (LARP) by rotating forward-left and backward-right with a 45° angle to the sagittal plane; and 4) motions that stimulate the right anterior and left posterior semicircular canals (RALP) by rotating forward-right and backward-left. A fifth condition was also tested, in which subjects experienced roll tilt motion about an axis passing through the base of the platform, just below the subject’s feet. All blocks began at an initial motion amplitude of 4°, which corresponds to a peak velocity of 1.6°/s. As detailed above, 0.2-Hz motions were used because they assay both semicircular canal and otolith contributions (26). Data were pooled across repetitions for each threshold measure, resulting in 150 trials, which corresponds to an expected coefficient of variation of 15% (46). However, one vestibular migraine subject did not return for the second day of testing and thus completed 75 trials for each of the five threshold measures.
Analysis
Postural sway for each trial (56) was calculated separately in the ML and AP directions as the standard deviation of zero-meaned CoP over time. Threshold measures used for analysis were in units of peak velocity. Displacement, peak velocity, and acceleration are linearly related at a given frequency; thus, the choice of units does not affect the analysis, because intersubject relationships remain identical. All postural sway and threshold values were converted to a z score before regression analyses to facilitate comparisons of coefficients.
The primary analysis to investigate the relationship between thresholds and postural sway focused on SOT 5 (study A) and condition 4 (study B) because they induced the highest reliance on vestibular cues (3, 9, 11, 42). We note that SOT 5 and condition 4 differ in the way altered proprioceptive cues are provided, although both result in increased vestibular weight and similar sway characteristics (57). This was done to focus the analysis on our hypothesis of a correlation between vestibular thresholds and postural sway, while reducing other sources of variability. Thus, for study A we conducted a multiple variable linear regression across subjects between ML postural sway and the five threshold measures and an intercept term. Multiple variable analyses were used to ensure false positives did not arise due to correlations between thresholds (25). For study B, we conducted a multiple variable linear regression across subjects between ML postural sway and roll and pitch tilt 0.2-Hz thresholds and an intercept term. The same regression was done for AP sway.
The secondary analysis investigated whether a vestibular influence on postural sway was evident even for conditions with higher visual and proprioceptive reliance. Thus, we conducted the same regressions as for the primary analysis for each of the six SOTs (study A) and four stance conditions (study B), separately for ML and AP. Bonferroni multiple comparison correction was used, with α = 0.05/(6 × 2) = 0.0042 for study A and α = 0.05/(4 × 2) = 0.0063 for study B. Furthermore, to combine data across postural conditions, we used analysis of covariance (ANCOVA) to examine the covariation between each threshold and ML postural sway in SOT 1–6 in study A. The same analysis was done for AP sway. For study B, the same analysis was done across conditions 1–4 for each of roll and pitch tilt threshold and for each of ML and AP. In addition, for study B, the same analysis was done for each of LARP and RALP tilt thresholds and for postural sway in the LARP and RALP planes. These postural sways were determined by applying a 2 × 2 rotation matrix to ML and AP sway with a rotation angle of 45°. Bonferroni multiple comparison correction was used, with α = 0.05/(5 × 2) = 0.0050 for study A and α = 0.05/(4 × 2)=0.0063 for study B. Statistical analyses (e.g., MANCOVA) across all postural conditions and all thresholds could not be used because of the limited degrees of freedom provided by the number of subjects tested. All analyses were repeated using peak-to-peak CoP, which yielded results that were very similar to those found with use of the standard deviation of CoP, and was not pursued further. Similarly, all analyses were repeated using standard deviation of differentiated CoP, but this analysis did not reveal any clear relationship with any threshold and was not pursued further.
Finally, we investigated whether sensory reweighting caused by differences in availability of sensory cues between the SOTs in study A would change the relationship between thresholds and postural sway. Specifically, we hypothesized that an increased vestibular weight would also increase the dependence of sway on vestibular thresholds, since thresholds reflect sensory noise, and it has been hypothesized that spontaneous sway arises from sensory noise. To compare correlation coefficients between SOTs, we used the cocor package (58), which accounts for dependent correlations and overlapping variables. One-sided tests were used because we hypothesized an increase in the correlation when nonvestibular cues were reduced. When two comparisons were relevant to one hypothesis, we used Fisher’s method to determine a combined P value (59).
RESULTS
Study A: Intersubject Variability
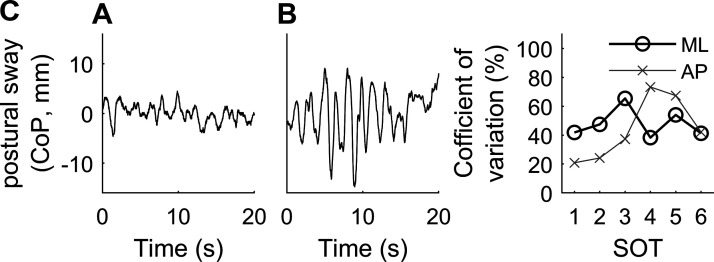
There were substantial intersubject differences in the size of postural sway. Figure 1 shows examples of ML postural center-of-pressure (CoP) from two subjects during SOT 5, in which subjects stood with eyes closed with the platform sway referenced in the AP direction. These intersubject differences were highlighted by large coefficients of variation (CV) for most SOTs (Fig. 1C). Given the healthy and young population tested, this variability is noteworthy. In fact, Fig. 1A shows sway from a subject 10 yr older than shown in Fig. 1B, and both were younger than 35 yr. There was no clear dependence of CV on SOT for ML. For AP, CV was considerably larger for SOTs 4 and 5 versus other SOTs. Substantial intersubject differences were also found in vestibular thresholds, consistent with previously published work (38, 45, 60). CV was 68% for roll tilt 0.2-Hz thresholds, and between 33–49% for the four other thresholds.
Figure 1.
Examples of postural sway from 2 subjects with small (A) and large (B) sway. These examples show mediolateral (ML) sway during sensory organization test (SOT) 5, in which subjects have their eyes closed and stand on a support surface that is sway referenced in the anterioposterior (AP) direction. C: coefficients of variation for postural sway across the subjects in study A for each of the SOTs. CoP, center-of-pressure.
Study A: Relationship between Thresholds and Postural Sway
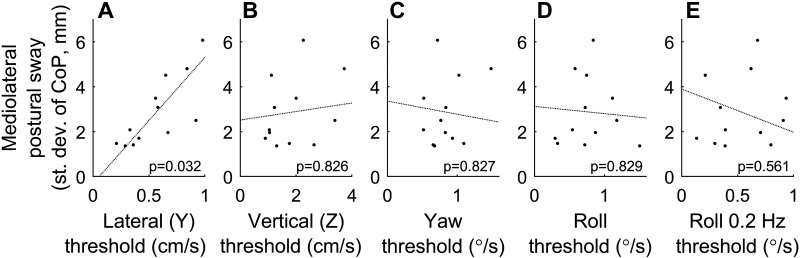
Study A focused on the relationship between postural sway and five thresholds. Some of these thresholds (roll tilt, lateral translation) assay sensory feedback relevant to motion in the ML direction, whereas other (yaw rotation, vertical translation) assay thresholds that provide little feedback relevant to motion in the ML or AP direction. In Fig. 2A, we first show the intersubject relationship between lateral (Y) translation thresholds and ML postural sway for SOT 5. Subjects (dots) with low lateral translation thresholds tend to have less ML postural sway, whereas subjects with high lateral translation thresholds tend to have larger ML postural sway, consistent with lateral translation sensory noise contributing to postural sway. There is no obvious relationship between ML sway and the other four thresholds (Fig. 2, B–E). The partial slope lines and associated P values show the results of a multiple variable linear regression relating the five thresholds to ML postural sway for SOT 5. This analysis indicates a statistically significant correlation between ML postural sway and lateral (Y) translation thresholds (P = 0.032). In discussion, we address the fact that the AP sway referencing used did not correspond to the direction of threshold assays.
Figure 2.
Relationship between the standard deviation of mediolateral (ML) postural center-of-pressure (CoP) sway and vestibular thresholds for sensory organization test (SOT) 5, with each dot representing 1 subject in study A. A: subjects with low lateral translation thresholds tend to have less ML postural sway, whereas subjects with high lateral translation thresholds tend to have larger ML postural sway. Partial slope lines show the results of a multiple variable linear regression between the standard deviation of ML postural CoP and each of the 5 threshold measures simultaneously, with the corresponding P value provided. B–E: there is no obvious intersubject relationship between sway and thresholds for the other 4 thresholds. Some partial slope lines may appear in an unintuitive direction; this occurs because multiple variable regression accounts for interactions between thresholds.
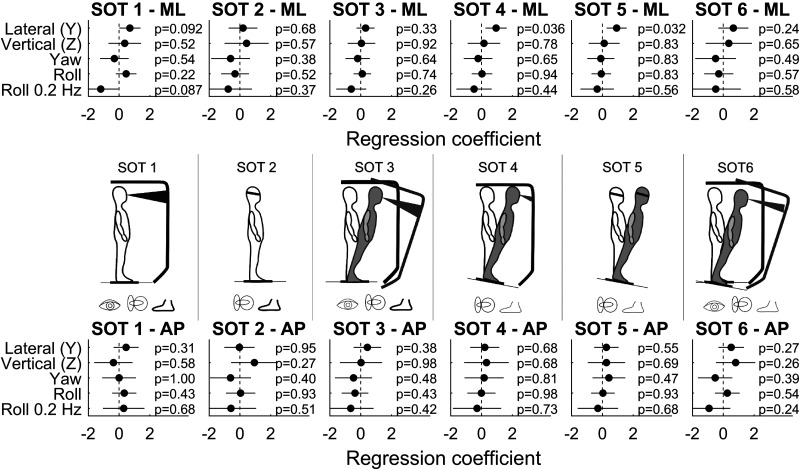
Figure 3 shows the results of the same multiple variable linear regression for each of the six SOTs, for both ML and AP sway. There is a consistently positive regression coefficient between ML sway and lateral translation thresholds in all SOTs, whereas there is no clear relationship between sway and the other thresholds. After Bonferroni multiple comparison correction, none of these individual regressions reached the level of statistical significance (α = 0.05/(6 × 2) = 0.0042). Thus, we conducted additional analyses (ANCOVA) that related each of the threshold measures to postural sway for all six SOTs, separately for ML and AP (Table 2). We found strong evidence for covariation between ML sway across SOTs 1–6 and lateral (Y) translation thresholds (ANCOVA, P = 0.0002). There is also evidence for covariation between AP sway across SOTs 1–6 and lateral (Y) translation thresholds (ANCOVA, P = 0.0012). There is no evidence of a relationship between ML or AP sway and the other thresholds after Bonferroni multiple comparison correction (α = 0.05/(5 × 2) = 0.0050). Together, these results are consistent with postural sway arising from noise in the sense of lateral (Y) translation.
Figure 3.
Results of multiple variable linear regressions between postural sway and the vestibular threshold measures in study A, for each of the 6 sensory organizations (SOTs) and 2 directions [mediolateral (ML) and anterioposterior (AP)]. Each circle shows the estimated coefficient between the corresponding threshold measure and sway, with the 95% confidence intervals shown by black lines.
Table 2.
Results of analyses relating each threshold to sway in the 6 SOTs, performed separately for ML and AP (ANCOVA)
| Lateral (Y) | Vertical (Z) | Yaw | Roll | Roll 0.2 Hz | |
|---|---|---|---|---|---|
| ML | P = 0.0002** | P = 0.24 | P = 0.13 | P = 0.95 | P = 0.53 |
| AP | P = 0.0012** | P = 0.87 | P = 0.25 | P = 0.81 | P = 0.25 |
AP, anterioposterior; ML, mediolateral; SOT, sensory organization test. **P < α after Bonferroni multiple comparison correction.
Study A: Sensory Reweighting
We also examined whether there was evidence of sensory reweighting in our results. We examined whether the correlation coefficient between ML postural sway and lateral (Y) translation thresholds increased with elimination of visual cues and/or alteration of proprioceptive cues, which would be consistent with postural sway becoming more dependent on vestibular noise. Table 3 (row A) shows the comparisons of conditions which altered proprioceptive cues via sway referencing (SOT 4 vs. SOT 1 and SOT 5 vs. SOT 2) but matched visual cues. The correlation coefficients were higher with sway referencing (P = 0.0004, Fisher’s method), consistent with postural sway having a greater dependence on vestibular noise when proprioceptive contributions are altered. Table 3 (row B) shows the comparisons of conditions which eliminated visual cues (SOT 2 vs. SOT 1 and SOT 5 vs. SOT 4) but had matched proprioceptive cues. The correlation coefficients were not significantly different with only visual cues eliminated (P = 0.72, Fisher’s method). Table 3 (row C) shows the comparison of conditions that had eliminated visual cues and altered proprioceptive cues (SOT 5 vs. SOT 1). The correlation coefficients were higher in SOT 5 versus SOT 1 (P = 0.026, Pearson–Filon statistic), consistent with postural sway having a greater dependence on vestibular noise when proprioceptive and visual reliance is reduced.
Table 3.
Evidence that sensory reweighting causes postural sway to have an increased dependence on vestibular noise
| Hypothesized Cause for Lower Correlation | Comparison | Correlation Coefficients between ML Sway and Lateral Translation (r) | P Value for Higher r (one-sided) |
|---|---|---|---|
| A. Altered proprioceptive cues | SOT 4 vs. SOT 1 | +0.74 vs. +0.36 | 0.044 |
| SOT 5 vs. SOT 2 | +0.77 vs. −0.13 | 0.0008 | |
| Combination of the above two comparisons | 0.0004 | ||
| B. Elimination of visual cues | SOT 2 vs. SOT 1 | −0.13 vs. +0.36 | 0.96 |
| SOT 5 vs. SOT 4 | +0.77 vs. +0.74 | 0.36 | |
| Combination of the above two comparisons | 0.72 | ||
| C. Altered proprioceptive cues and elimination of visual cues | SOT 5 vs. SOT 1 | +0.77 vs. +0.36 | 0.026 |
SOT, sensory organization test. P values show evidence that the correlation coefficient is significantly larger (Pearson–Filon statistic (57); combination of two P values is implemented using Fisher’s method). A., B., and C. denote three different hypotheses.
Study B: Thresholds
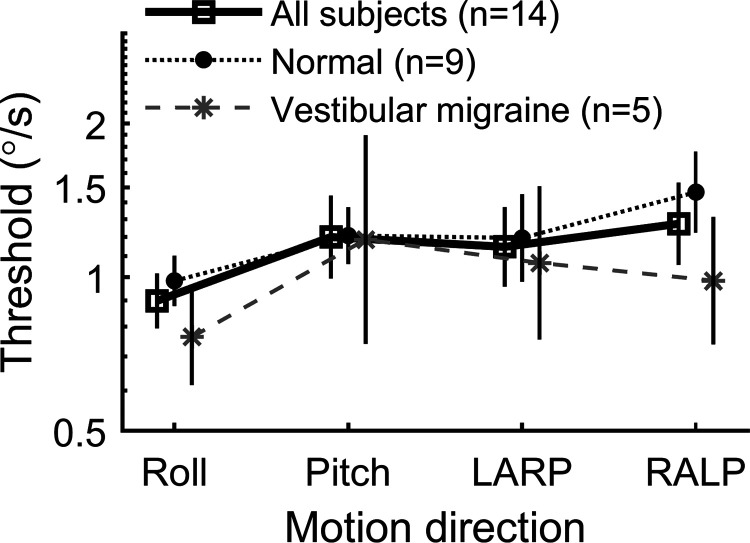
Figure 4 compares roll, pitch, LARP, and RALP tilt thresholds assayed at 0.2 Hz. We found small (relative to the population standard deviation) but statistically significant threshold variations across the four stimulus directions when all subjects, including those with vestibular migraine, were included (repeated measures ANOVA, P = 0.0038 for all subjects, P = 0.0085 for normal subjects, P = 0.15 for VM subjects). Table 4 shows correlation coefficients between thresholds for each of these measures. None of these correlations were statistically significant, likely because of the relatively small number of subjects. However, the moderate correlation coefficients found were similar to those found (0.30–0.51) for a larger group of subjects (25). There was no evidence of a difference between thresholds for roll tilts about a head-centered axis versus an axis passing through the base of the platform, just below the subject’s feet (paired t test, P = 0.089 for all subjects, P = 0.25 for normal subjects, P = 0.16 for VM subjects).
Figure 4.
Tilt thresholds assayed at 0.2 Hz in study B. Left anterior-right posterior (LARP) tilt motions occur forward-left and backward-right. Right anterior-left posterior (RALP) tilt motions occur forward-right and backward-left. Error bars show 95% confidence intervals.
Table 4.
Correlation coefficients across subjects between threshold measures in study B
| Pitch Tilt | LARP Tilt | RALP Tilt | |
|---|---|---|---|
| Roll tilt | 0.52 (P = 0.056) | 0.30 (P = 0.30) | 0.34 (P = 0.24) |
| Pitch tilt | 0.67 (P = 0.0089) | 0.39 (P = 0.17) | |
| LARP tilt | 0.54 (P = 0.046) |
LARP, left anterior and right posterior; RALP, right anterior and left posterior. None were significant after Bonferroni multiple comparison correction (0.05/6 = 0.0083).
Study B: Relationship between Thresholds and Postural Sway
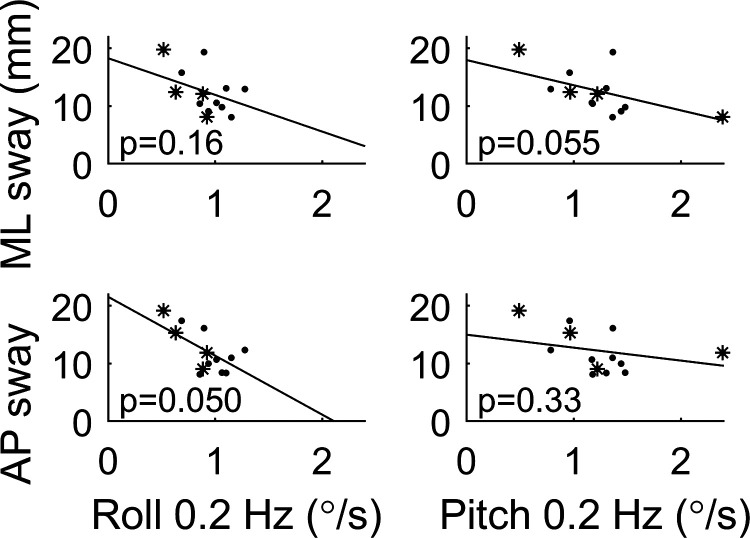
Roll and pitch thresholds assay sensory feedback relevant to motions in the ML and AP directions, respectively. Figure 5 shows the relationship between these thresholds and condition 4 of the modified Romberg foam test (34, 36, 37, 42), for which subjects stand on foam with eyes closed, which increases the reliance on vestibular cues. We highlight this test because it was previously shown that the ability to pass condition 4 was positively correlated with roll tilt 0.2-Hz thresholds (25). Interestingly, there are no statistically significant relationships between ML or AP postural sway and roll and pitch threshold measures (Bonferroni multiple comparison corrected, α = 0.05/(2 × 2) = 0.0125). Potential trends that did not reach the level of statistical significance are addressed in discussion.
Figure 5.
Relationship between postural center-of-pressure (CoP) sway in condition 4 of the Romberg test and vestibular thresholds, in which subjects stand on foam with eyes closed. Each dot represents 1 subject in study B. Vestibular migraine (VM) subjects are shown as stars, with 1 subject missing because they did not complete condition 4. Partial slope lines show the results of a multiple variable linear regression between sway and the threshold for all subjects, with the corresponding P value provided. Normal subject results are in Fig. 6.
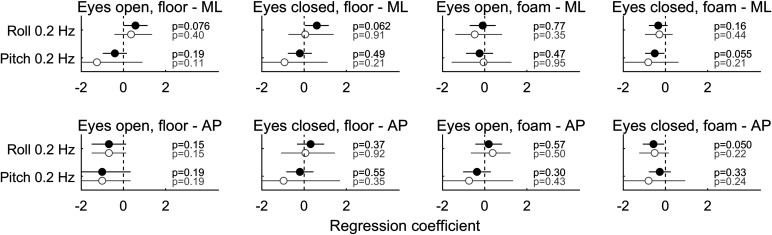
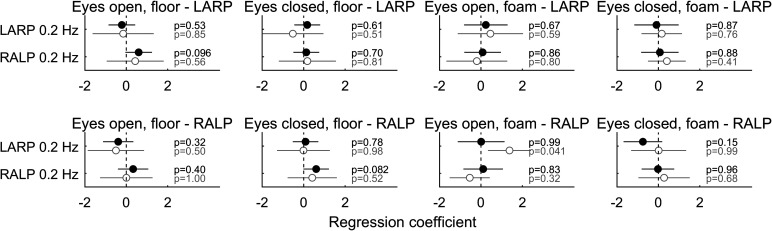
Figure 6 shows the results of the same multiple variable linear regression for each of the posture conditions for both ML and AP. Similarly, Fig. 7 shows the same analysis between LARP and RALP tilt thresholds and postural sway along the same LARP and RALP planes. Neither of these figures shows any clear relationships, and there were no significant correlations (Bonferroni multiple comparison corrected, α = 0.05/(4 × 2) = 0.0063). Results differed only slightly when analyses were performed with vestibular migraine subjects excluded and did not change conclusions (gray symbols in Fig. 6 and Fig. 7). Furthermore, there were no clear relationships found through an ANCOVA analysis (Table 5).
Figure 6.
Results of multiple variable linear regressions between postural sway and roll and pitch thresholds, for each of the 4 Romberg conditions and 2 directions [mediolateral (ML) and anterioposterior (AP)] in study B. Each circle shows the estimated coefficient between the corresponding threshold measure and sway, with the 95% confidence intervals shown by black lines. Results in gray are for normal subjects only, whereas results in black are normal and vestibular migraine (VM) subjects together.
Figure 7.
Results of multiple variable linear regressions between postural sway aligned with the anterior and posterior canal planes [left anterior-right posterior (LARP) and right anterior-left posterior (RALP)] and LARP and RALP tilt thresholds for each of the 4 Romberg conditions and 2 directions [mediolateral (ML) and anterioposterior (AP)] in study B.
Table 5.
Results of analyses relating each threshold with CoP sway in the 4 postural conditions, performed separately for ML, AP, LARP, and RALP (ANCOVA)
| Roll Tilt 0.2 Hz | Pitch Tilt 0.2 Hz | LARP Tilt 0.2 Hz | RALP Tilt 0.2 Hz | |
|---|---|---|---|---|
| ML | P = 0.63 | P = 0.052 | ||
| AP | P = 0.39 | P = 0.056 | ||
| LARP | P = 0.48 | P = 0.086 | ||
| RALP | P = 0.81 | P = 0.12 |
LARP, left anterior and right posterior; RALP, right anterior and left posterior. None were significant after Bonferroni multiple comparison correction (0.05/6 = 0.0083).
AP, anterioposterior; CoP, center-of-pressure; LARP, left anterior and right posterior; ML, mediolateral; RALP, right anterior and left posterior. Note that P = 0.052 and P = 0.056 for pitch 0.2-Hz thresholds arose because of negative covariation, which is the opposite of our hypothesis.
DISCUSSION
Our primary finding was strong evidence of a positive correlation between ML postural sway and lateral (Y) translation vestibular thresholds in study A, consistent with lateral head translation playing an important role in postural control. We did not find a statistically significant correlation between postural sway and any of the other threshold measures in study A or B: yaw rotation, vertical translation, roll tilt, pitch tilt, LARP tilt, or RALP tilt. The results of studies A and B are consistent and point to a role for lateral translation cues in controlling sway, with no evidence of a contribution from other vestibular cues including tilt cues. Of course, a lack of statistical significance does not prove the lack of an effect, and limitations that may have masked effects are discussed below. We also found that the relationship between ML postural sway and lateral (Y) translation thresholds strengthened in SOTs that are known to decrease reliance on proprioceptive cues, consistent with postural sway becoming more dependent on vestibular lateral translation noise in those SOTs.
In study A, there was also a statistically significant correlation between lateral translation thresholds and AP postural sway. Without measuring fore-aft thresholds it is difficult to completely understand this relationship, but it may arise because there are moderate correlations between thresholds in different directions (25) or because of correlations and interactions between ML and AP sway.
Since no study has previously compared thresholds for roll, pitch, and RALP and LARP tilt (to our knowledge), we compared these as an additional result. Although our N was adequate to find statistically significant variations in thresholds between roll, pitch, and RALP and LARP tilts, these were generally small to moderate effects (the effect size divided by the standard deviation ranged between 0.09 and 0.79 across all subjects), as in another recent study comparing pitch and roll tilt thresholds (61).
The Role of Sensory Noise in Postural Control
Our results support the broader hypothesis of a positive correlation between postural sway and sensory noise. Sensory noise is any part of the sensory signal that does not represent the physical stimulus. This noise can arise from sources such as membrane excitability, ion channel opening and closing, sensory transduction and amplification, synaptic transmission, and central processing (27). Early postural models proposed that sensory weighting of vestibular, visual, and proprioceptive cues provided the most precise estimate of motion, given the sensory noise present in each channel (32, 33). More recently, these concepts were extended to predict that spontaneous postural sway arises primarily from sensory noise, with smaller contributions from motor noise, and experimentally verified (5, 20, 21). In parallel, other studies related perceptual sensory thresholds to postural sway (15). A critical link between these studies is that signal detection theory (28, 29) relates perceptual thresholds to the standard deviation of the noise corrupting the underlying perception. A few studies have suggested that a common noise source affects oculomotor behavior and perception (30, 31, 62–67). Together with our studies showing a correlation between perceptual thresholds and performance in a balance test (7, 25) and in a manual control task (68), this supports the hypothesis that perceptual thresholds arise primarily from a common noise source that is either sensory or early in central processing.
We are aware of three potential mechanisms by which sensory noise could contribute to postural sway. One possibility is that sensory noise feeds directly through sensorimotor pathways and results in the generation of motor commands, with noise characteristics directly dependent on the sensory noise. A second possibility is that the brain performs subconscious decision-making associated with the sensory integration process whereby combined noise from multiple sensory signals contributes to the generated motor command. A third possibility is that sensory noise may trigger conscious, perceptual decision-making processes that alter the characteristics of the balance control system (e.g., cocontraction) and thus modify the influence of sensory noise on postural sway. Any, or all, of these may contribute.
The Role of Vestibular Lateral Translation Cues in Postural Control
A few published findings are consistent with our results suggesting that vestibular lateral translation cues play a role in postural control. One study found that gymnasts between 7 and 20 yr have lower lateral translation thresholds than age-matched controls, whereas their rotation thresholds are not significantly different (69), suggesting that their (presumed) enhanced balance function comes from having access to superior (lower variability) encoding or processing of translational motion. Another study measured postural sway and otolith-mediated ocular counterroll (OCR) eye movements and found that the OCR gain decreased with age, and there was a correlation between reduced OCR and increased ML postural sway (24), both with fixed and foam support surfaces. Those authors concluded that a decline in utricular otolith function contributes to impairment in postural control. Our results build on this study in two ways. First, since we measured both otolith-mediated translation and otolith-mediated tilt responses, we were able to show that translation cues specifically correlate with ML sway. Second, thresholds are measured in units that relate to body kinematics, and thus are directly comparable to postural kinematics. We illustrate the importance of this fact in the following paragraph.
A comparison of the magnitude of typical motion experienced during quiet stance and corresponding thresholds provides further evidence for the importance of linear translation cues for postural control. Day et al. (70) reported that the standard deviation of lateral shoulder velocity was 0.4–0.6 cm/s during quiet stance with eyes closed. In comparison, lateral translation thresholds are 0.48 cm/s for 1 Hz (38). Day et al. reported that the standard deviation of lateral shoulder displacement was 0.2 cm, which, assuming an inverted pendulum with a height of 160 cm, corresponds to a tilt standard deviation of 0.07°. This is much less than the roll tilt threshold of 1.91° for very slow tilts that provide a negligible semicircular canal cue and primarily stimulate the otolith organs (26). Similarly, the lateral shoulder velocity of 0.4–0.6 cm/s results in a roll velocity of the head of ∼0.14–0.21°/s. This is less than the roll rotation threshold of 0.42°/s for supine rotations that provide a semicircular canal cue and no otolith organs cue (26). Furthermore, in general, the output of a feedback system can be more variable than the sensor noise, since noise that appears in the output will be fed back to the input and combined with additional noise. Thus, sway caused by sensory and motor noise will be sensed by the vestibular system, which will add additional noise. These comparisons are consistent with our results showing that lateral translation feedback is used for postural control.
In contrast to these findings, our previous work found a correlation between roll tilt 0.2-Hz thresholds and the ability to pass Romberg condition 4 (7, 25, 71), but not lateral translation, yaw, vertical, or roll tilt 1-Hz thresholds. One possible explanation for this difference is that different balance metrics (sway vs. pass/fail) depend on different sensory cues or strategies. As one example, perhaps a sensed postural threat can, under certain circumstances, induce a reduction in postural sway displacement. More explicitly, when individuals sense that they have full control of their balance and are not concerned about falling, their balance system may favor reduced energy expenditure and accept the larger sways that occur when control effort is reduced. However, when the same individuals sense that their balance is threatened, their balance system may favor the application of greater control effort to avoid sways approaching stability limits and thus reducing overall sway. Hence, sway could be reduced even as stability is threatened. In fact, an earlier study (72) documented a sway decrease as postural threat (modified by varying the height of a platform between 40 and 160 cm) increased.
Another explanation is differences in both the number and demographics of the subjects used. Whereas Bermudez Rey et al. (7) used 99 subjects ranging 18–80 yr, including ∼20% who could not complete condition 4, subjects were younger in the present studies (21–50 yr for study A, 23–61 yr for study B), and only 1 of 26 could not complete all SOTs/conditions. Methodological explanations may also apply. In the Ref. 7 study and in study B, subjects stood on foam that was unstable in all directions or on a solid surface with feet together. In contrast, in study A presented herein, subjects stood on an AP sway-referenced or solid surface with feet apart. With only AP sway referencing, proprioceptive cues signaling ML sway are accurate, thus making it less likely that vestibular roll tilt thresholds would be correlated with ML sway. However, this explanation would not account for the absence of a correlation in our study B. The relatively small number of subjects in studies A and B may have also prevented finding statistically-significant correlations where relationships existed. Future studies with larger subject populations and matched methods may elucidate the cause of these differences.
A strength of our approach is that it explains intersubject sway differences by sensory function differences. These differences are sometimes a challenge in studying posture, but our study elucidates at least some sources of this variability. From a methodological perspective, larger intersubject differences make it easier to find correlations in the presence of measurement error and thus increase statistical power.
Although related, there are important differences between our study and those that measured thresholds for perception of postural sway (73, 74), which argued that ankle tilt cues are more precise that vestibular tilt cues. In those studies, subjects had to perceive their motion while standing, with their perception being influenced by the combination of normal body sway, a mechanical perturbation applied to the body, and sensory processing. In our studies, thresholds were measured with subjects sitting in a chair with the head restrained such that their perception should reflect only the known stimulus provided by the chair motion. Furthermore, Fitzpatrick and McCloskey (73) measured thresholds for the tilt of the body about an axis passing through the feet, which caused coupled rotation, tilt, and translation of the head. In our studies, we modified the axis and direction of motion and the stimulus frequency to allow rotation, tilt, and translation thresholds to be separately measured. Finally, those studies did not investigate how individual differences in thresholds related to individual differences in postural control.
In study B, there are three trends that, albeit not statistically significant, may motivate future investigation. The first are positive trends (Fig. 6) between roll tilt 0.2-Hz thresholds and ML sway in the first two conditions (solid support, eyes open/closed), which would be consistent with a role for roll tilt cues in controlling ML sway. The second trend is the absence of this positive trend in the last two conditions (foam support, eyes open/closed). The third trend is a consistent negative correlation between pitch 0.2-Hz thresholds and both ML and AP sway (Fig. 6 and Table 5). As noted earlier, there is evidence that postural threat causes a reduction in postural sway displacement (72). Thus, a possible explanation is that compromised sensory function evokes a compensatory strategy that uses increased control effort to reduce sway. This could explain why there was a trend of a relationship between roll tilt 0.2-Hz thresholds and ML sway in the easier conditions but not the more difficult ones, as well as the negative correlation between pitch 0.2-Hz thresholds and sway.
Interpretation of Correlations and Concerns about Covariates
Since our conclusions herein arise from correlations, there is the potential that other variables that are correlated with our measures may actually be the cause of the observed effect. In Ref. 25, we detailed our approach to the interpretation of correlations as expressions of fundamental causes. The same interpretation applies to the results of this study. Briefly, we argued that 1) it is unlikely the correlation arises from posture-causing changes in vestibular thresholds; 2) the only threshold for which there was a statistically significant correlation was one of two thresholds that correspond to motion that provides sensory feedback relevant to ML postural control; 3) if a covariate of vestibular thresholds, e.g., age or muscle strength (75), were the actual cause of changes in postural sway, it is unlikely they would covary with only a single threshold measure out of five.
Sensory Reweighting
Many studies have shown that postural control relies on sensory reweighting of cues, depending on their availability and reliability (3, 9, 11). Our results also support this hypothesis. We found a significantly higher correlation between lateral translation thresholds and ML postural sway when proprioceptive contributions were altered but not when visual cues were eliminated. Consistent with a minimal effect of vision, previous research showed low sensitivity to ML plane visual motion disturbances that evoke upper body sway (76) and whole body center of mass sway (77) when comparable stance widths were used. However, Goodworth and Peterka (77) found that visual-vestibular sensory reweighting was more pronounced for narrower widths, suggesting that a narrower stance width in our current study may have brought out a stronger visual interaction. Nevertheless, the intersubject variability in vestibular thresholds explains more of the intersubject variability in postural sway when subjects cannot rely upon proprioception cues for balance. These results are consistent with an increased vestibular weight also increasing the dependence of sway on vestibular noise. A limitation of the study was that sensory thresholds were measured in the frontal plane whereas sway referencing occurred in the sagittal plane. Nonetheless, an increase in vestibular weighting in the frontal plane has been shown to occur due to perturbations in the sagittal plane (41).
During human subject experiments, some of us have observed subjects whose posture was minimally perturbed from upright even with large platform perturbations. This behavior could be explained by low vestibular thresholds (i.e., low noise/high precision) versus a typical population, which would result in the ability to sense and counteract very small head motions. This empirical observation is supported by published results. Model analysis of postural responses to platform perturbations with eyes closed have been used to determine the weighting of graviceptive and proprioceptive cues. There is substantial intersubject variability in these weights, with the CV ranging between 20 and 44% across different conditions (12, 76, 78).
Relationship to Falls
A correlation between a history of falls and postural sway has been noted by numerous studies (79, 80), and particularly between a history of falls and ML postural sway (81). A recent meta-analysis of studies using wearable sensors found a correlation between mediolateral root mean square acceleration and history of falls (82).
The causes of falls are complex (83). Nonetheless, some studies have found evidence of a sensory role in falls. In the large NHANES (National Health and Nutrition Examination Survey) study, the odds of a history of falls were 12 times higher when vestibular symptoms were present, with vestibular symptoms defined as failing Romberg condition 4 while passing the other Romberg conditions (6). In fact, an estimated 48,000–152,000 deaths a year in the US have been attributed to suboptimal vestibular function (7). Similarly, there is a correlation between history of falls and poorer visual acuity and proprioception (81). Future investigations can determine which measures are most correlated with history of falls and which have potential diagnostic benefit for predicting future fall risk. Similarly, some measures may be important at determining which targeted therapies are applicable in situations where a high fall risk has been identified, such as those specifically targeting proprioceptive (83) and vestibular (85) deficits.
As with the possible mechanisms relating sensory noise to postural sway, it remains an open question about the mechanisms by which sensory noise could relate to falls. For example, humans can experience a distinct awareness of falling with a concomitant startle response (86). Does this reflect a perception that is used to generate a motor response, or does the sense of falling generate parallel conscious perceptual and unconscious motor responses (87)?
Application to Vestibular Prostheses
The development of prosthetic devices to replace or augment deficient vestibular function is a priority. This includes vestibular implants that electrically stimulate the afferent neurons innervating the three semicircular canals (88, 89), and vibrotactile balance prostheses that provide tactile stimulation of the skin in a pattern that indicates body tilt (90). Notably, these devices do not provide translation cues, and our results suggest that the provision of translation cues might contribute to functional performance.
Limitations
A limitation of these studies was that the number of subjects (12 subjects in study A and 14 subjects in study B) was limited. Furthermore, due to the standard testing protocol used, there was a limited amount of sway data (15–30 s) for each subject and condition. These limitations restricted the analysis, since there were insufficient data for multivariate, multiple variable analysis. Nonetheless, the analyses were done rigorously, providing support for the conclusions. The small number of subjects prevented us from using age in our analysis, as we previously did with a larger data set of 105 subjects (25). Ideally, sway referencing would have been done in the frontal plane in study A so that it corresponded to the plane of the threshold measurements. It would have been beneficial to measure actual head motion during postural testing rather than CoP, since this would be a more direct comparison to the kinematic threshold measures. The threshold measures used do not assay signal-dependent noises that increase with stimulus amplitude (31). This is justified because a model of postural responses found that the inclusion of signal-dependent noise sources only slightly improved the explicative power of posture models (5).
Finally, unlike studies in which a single degree-of-freedom lightweight backboard (3, 12) caused head orientation and ankle to be unambiguously linked to center-of-mass motion, the subjects in our study did not have any restriction of the body segments. While head-on-body motion and intersegmental dynamics complicate the relationship between sensory feedback and center-of-mass motion without a backboard, studies have found that sway dynamics for tilts <8° are nearly identical whether a backboard is present or not, both for AP (3) and ML (12) and that sensory integration mechanisms are similar (91).
GRANTS
This research was supported by National Institute on Deafness and Other Communication Disorders Grants R01 DC-04158, R01 DC-014924, and R03 DC-013635.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.K. and D.M.M. conceived and designed research; Y.V. and T.L. performed experiments; F.K., Y.V. and T.L. analyzed data; F.K., A.D.G., R.J.P., and D.M.M. interpreted results of experiments; F.K. and T.L. prepared figures; F.K. and A.D.G. drafted manuscript; F.K., A.D.G., R.J.P., and D.M.M. edited and revised manuscript; F.K., A.D.G., R.J.P., and D.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rakshatha Kabbaligere for performing the pilot data analysis.
REFERENCES
- 1.Goodworth AD, Peterka RJ. Influence of bilateral vestibular loss on spinal stabilization in humans. J Neurophysiol 103: 1978–1987, 2010. doi: 10.1152/jn.01064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35, Suppl 2: ii7–ii11, 2006. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 3.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol 88: 1097–1118, 2002. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 4.Todorov E. Optimality principles in sensorimotor control. Nat Neurosci 7: 907–915, 2004. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Kooij H, Peterka RJ. Non-linear stimulus-response behavior of the human stance control system is predicted by optimization of a system with sensory and motor noise. J Comput Neurosci 30: 759–778, 2011. doi: 10.1007/s10827-010-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001-2004. Arch Intern Med 169: 938–944, 2009. [Erratum in Arch Intern Med 169: 1419, 2009]. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez Rey MC, Clark TK, Wang W, Leeder T, Bian Y, Merfeld DM. Vestibular perceptual thresholds increase above the age of 40. Front Neurol 7: 162, 2016. doi: 10.3389/fneur.2016.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bles W, Vianney de Jong JM, de Wit G. Compensation for labyrinthine defects examined by use of a tilting room. Acta Otolaryngol 95: 576–579, 1983. doi: 10.3109/00016488309139445. [DOI] [PubMed] [Google Scholar]
- 9.Nashner LM, Black FO, Wall C. Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci 2: 536–544, 1982. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterka RJ, Benolken MS. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp Brain Res 105: 101–110, 1995. doi: 10.1007/BF00242186. [DOI] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Woollacott MH. Motor Control Translating Research into Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins, 2012. [Google Scholar]
- 12.Cenciarini M, Peterka RJ. Stimulus-dependent changes in the vestibular contribution to human postural control. J Neurophysiol 95: 2733–2750, 2006. doi: 10.1152/jn.00856.2004. [DOI] [PubMed] [Google Scholar]
- 13.Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol 500: 661–672, 1997. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDougall HG, Moore ST, Curthoys IS, Black FO. Modeling postural instability with Galvanic vestibular stimulation. Exp Brain Res 172: 208–220, 2006. doi: 10.1007/s00221-005-0329-y. [DOI] [PubMed] [Google Scholar]
- 15.Mergner T, Schweigart G, Maurer C, Blumle A. Human postural responses to motion of real and virtual visual environments under different support base conditions. Exp Brain Res 167: 535–556, 2005. doi: 10.1007/s00221-005-0065-3. [DOI] [PubMed] [Google Scholar]
- 16.Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJM. Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19: 316–327, 1999. doi: 10.1523/JNEUROSCI.19-01-00316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during Translation and Tilt. J Neurophysiol 94: 186–198, 2005. doi: 10.1152/jn.00904.2004. [DOI] [PubMed] [Google Scholar]
- 18.Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined Tilt & Translation. J Neurophysiol 94: 199–205, 2005. doi: 10.1152/jn.00905.2004. [DOI] [PubMed] [Google Scholar]
- 19.Merfeld DM, Young LR, Oman CM, Shelhamer MJ. A multidimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestib Res 3: 141–161, 1993. doi: 10.1007/s00221-016-4638-0. [DOI] [PubMed] [Google Scholar]
- 20.Assländer L, Peterka RJ. Sensory reweighting dynamics following removal and addition of visual and proprioceptive cues. J Neurophysiol 116: 272–285, 2016. doi: 10.1152/jn.01145.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodworth AD, Tetreault K, Lanman J, Klidonas T, Kim S, Saavedra S. Sensorimotor control of the trunk in sitting sway referencing. J Neurophysiol 120: 37–52, 2018. doi: 10.1152/jn.00330.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol 563: 229–234, 2005. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mian OS, Dakin CJ, Blouin JS, Fitzpatrick RC, Day BL. Lack of otolith involvement in balance responses evoked by mastoid electrical stimulation. J Physiol 588: 4441–4451, 2010. doi: 10.1113/jphysiol.2010.195222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrador JM, Lipsitz LA, Gopalakrishnan GS, Black FO, Wood SJ. Loss of otolith function with age is associated with increased postural sway measures. Neurosci Lett 465: 10–15, 2009. doi: 10.1016/j.neulet.2009.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karmali F, Bermudez Rey MC, Clark TK, Wang W, Merfeld DM. Multivariate analyses of balance test performance, vestibular thresholds, and age. Front Neurol 8: 578, 2017. [Erratum in Front Neurol 11: 556797, 2020]. doi: 10.3389/fneur.2017.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim K, Karmali F, Nicoucar K, Merfeld DM. Perceptual precision of passive body tilt is consistent with statistically optimal cue integration. J Neurophysiol 117: 2037–2052, 2017. doi: 10.1152/jn.00073.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci 9: 292–303, 2008. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966. [Google Scholar]
- 29.Merfeld DM. Signal detection theory and vestibular thresholds: I. Basic theory and practical considerations. Exp Brain Res 210: 389–405, 2011. [Erratum in Exp Brain Res 218: 159–160, 2012]. doi: 10.1007/s00221-011-2557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haburcakova C, Lewis RF, Merfeld DM. Frequency dependence of vestibuloocular reflex thresholds. J Neurophysiol 107: 973–983, 2012. doi: 10.1152/jn.00451.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouri S, Karmali F. Variability in the vestibulo-ocular reflex and vestibular perception. Neuroscience 393: 350–365, 2018. doi: 10.1016/j.neuroscience.2018.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Kooij H, Jacobs R, Koopman B, Grootenboer H. A multisensory integration model of human stance control. Biol Cybern 80: 299–308, 1999. doi: 10.1007/s004220050527. [DOI] [PubMed] [Google Scholar]
- 33.van der Kooij H, Jacobs R, Koopman B, van der Helm F. An adaptive model of sensory integration in a dynamic environment applied to human stance control. Biol Cybern 84: 103–115, 2001. doi: 10.1007/s004220000196. [DOI] [PubMed] [Google Scholar]
- 34.CDC. Balance Procedures Manual. National Health and Nutrition Examination Survey, 2003, p. 26. https://www.cdc.gov/nchs/data/nhanes/ba.pdf [2017 7 July].
- 35.Goodworth AD, Kunsman M, DePietro V, LaPenta G, Miles K, Murphy J. Characterization of how a walking boot affects balance. J Prosthet Orthot 26: 54–60, 2014. doi: 10.1097/JPO.0000000000000014. [DOI] [Google Scholar]
- 36.Horak FB. Clinical measurement of postural control in adults. Phys Ther 67: 1881–1885, 1987. doi: 10.1093/ptj/67.12.1881. [DOI] [PubMed] [Google Scholar]
- 37.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance: suggestion from the field. Phys Ther 66: 1548–1550, 1986. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 38.Valko Y, Lewis RF, Priesol AJ, Merfeld DM. Vestibular labyrinth contributions to human whole-body motion discrimination. J Neurosci 32: 13537–13542, 2012. doi: 10.1523/JNEUROSCI.2157-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alahmari KA, Marchetti GF, Sparto PJ, Furman JM, Whitney SL. Estimating postural control with the balance rehabilitation unit: measurement consistency, accuracy, validity, and comparison with dynamic posturography. Arch Phys Med Rehabil 95: 65–73, 2014. doi: 10.1016/j.apmr.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simoneau GG, Derr JA, Ulbrecht JS, Becker MB, Cavanagh PR. Diabetic sensory neuropathy effect on ankle joint movement perception. Arch Phys Med Rehab 77: 453–460, 1996. doi: 10.1016/S0003-9993(96)90033-7. [DOI] [PubMed] [Google Scholar]
- 41.Blümle A, Maurer C, Schweigart G, Mergner T. A cognitive intersensory interaction mechanism in human postural control. Exp Brain Res 173: 357–363, 2006. doi: 10.1007/s00221-006-0384-z. [DOI] [PubMed] [Google Scholar]
- 42.Schut IM, Engelhart D, Pasma JH, Aarts R, Schouten AC. Compliant support surfaces affect sensory reweighting during balance control. Gait Posture 53: 241–247, 2017. doi: 10.1016/j.gaitpost.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhuri SE, Karmali F, Merfeld DM. Whole body motion-detection tasks can yield much lower thresholds than direction-recognition tasks: implications for the role of vibration. J Neurophysiol 110: 2764–2772, 2013. doi: 10.1152/jn.00091.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186: 677–681, 2008. doi: 10.1007/s00221-008-1350-8. [DOI] [PubMed] [Google Scholar]
- 45.Benson AJ, Hutt EC, Brown SF. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60: 205–213, 1989. [PubMed] [Google Scholar]
- 46.Karmali F, Chaudhuri SE, Yi Y, Merfeld DM. Determining thresholds using adaptive procedures and psychometric fits: evaluating efficiency using theory, simulations, and human experiments. Exp Brain Res 234: 773–789, 2016. doi: 10.1007/s00221-015-4501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys 63: 1279–1292, 2001. doi: 10.3758/BF03194543. [DOI] [PubMed] [Google Scholar]
- 48.Taylor MM, Creelman CD. PEST: efficient estimates on probability functions. J Acoust Soc Am 41: 782–787, 1967. doi: 10.1121/1.1910407. [DOI] [Google Scholar]
- 49.Chaudhuri SE, Merfeld DM. Signal detection theory and vestibular perception: III. Estimating unbiased fit parameters for psychometric functions. Exp Brain Res 225: 133–146, 2013. doi: 10.1007/s00221-012-3354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCullagh P, Nelder JA. Generalized Linear Models. London, UK: Chapman & Hall/CRC, 1989, p. 532. [Google Scholar]
- 51.Karmali F, Lim K, Merfeld DM. Visual and vestibular perceptual thresholds each demonstrate better precision at specific frequencies and also exhibit optimal integration. J Neurophysiol 111: 2393–2403, 2014. doi: 10.1152/jn.00332.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soyka F, Robuffo Giordano P, Barnett-Cowan M, Bulthoff HH. Modeling direction discrimination thresholds for yaw rotations around an earth-vertical axis for arbitrary motion profiles. Exp Brain Res 220: 89–99, 2012. doi: 10.1007/s00221-012-3120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res 209: 95–107, 2011. doi: 10.1007/s00221-010-2523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merfeld DM, Clark TK, Lu YM, Karmali F. Dynamics of individual perceptual decisions. J Neurophysiol 115: 39–59, 2016. doi: 10.1152/jn.00225.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karmali F, Merfeld DM. Evidence for sensory signal filtering for perceptual decision-making. In: Society for Neuroscience Abstracts 2015.
- 56.Maurer C, Peterka RJ. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J Neurophysiol 93: 189–200, 2005. [Erratum in J Neurophysiol 93: 3720, 2005]. doi: 10.1152/jn.00221.2004. [DOI] [PubMed] [Google Scholar]
- 57.Allum JH, Zamani F, Adkin AL, Ernst A. Differences between trunk sway characteristics on a foam support surface and on the Equitest ankle-sway-referenced support surface. Gait Posture 16: 264–270, 2002. doi: 10.1016/S0966-6362(02)00011-5. doi: 10.1016/S0966-6362(02)00011-5. [DOI] [PubMed] [Google Scholar]
- 58.Diedenhofen B, Musch J. cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One 10: e0121945, 2015. [Erratum in PLoS One 10: e0131499, 2015]. doi: 10.1371/journal.pone.0121945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosteller F, Fisher RA. Questions and answers. Am Stat 2: 30, 1948. doi: 10.2307/2681650. [DOI] [Google Scholar]
- 60.Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med 57: 1088–1096, 1986. [PubMed] [Google Scholar]
- 61.Suri K, Clark TK. Human vestibular perceptual thresholds for pitch tilt are slightly worse than for roll tilt across a range of frequencies. Exp Brain Res 238: 1499–1509, 2020. doi: 10.1007/s00221-020-05830-x. [DOI] [PubMed] [Google Scholar]
- 62.Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci 27: 6832–6842, 2007. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mukherjee T, Battifarano M, Simoncini C, Osborne LC. Shared sensory estimates for human motion perception and pursuit eye movements. J Neurosci 35: 8515–8530, 2015. doi: 10.1523/JNEUROSCI.4320-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osborne LC, Hohl SS, Bialek W, Lisberger SG. Time course of precision in smooth-pursuit eye movements of monkeys. J Neurosci 27: 2987–2998, 2007. doi: 10.1523/JNEUROSCI.5072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005. doi: 10.1038/nature03961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoppik D, Nagel KI, Lisberger SG. Cortical mechanisms of smooth eye movements revealed by dynamic covariations of neural and behavioral responses. Neuron 58: 248–260, 2008. doi: 10.1016/j.neuron.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tarnutzer AA, Bockisch CJ, Straumann D. Head roll dependent variability of subjective visual vertical and ocular counterroll. Exp Brain Res 195: 621–626, 2009. doi: 10.1007/s00221-009-1823-4. [DOI] [PubMed] [Google Scholar]
- 68.Rosenberg M, Galvan-Garza R, Clark T, Sherwood D, Young L, Karmali F. Human manual control precision depends on vestibular sensory precision and gravitational magnitude. J Neurophysiol 120: 3187–3197, 2018. doi: 10.1152/jn.00565.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartmann M, Haller K, Moser I, Hossner EJ, Mast FW. Direction detection thresholds of passive self-motion in artistic gymnasts. Exp Brain Res 232: 1249–1258, 2014. doi: 10.1007/s00221-014-3841-0. [DOI] [PubMed] [Google Scholar]
- 70.Day BL, Steiger MJ, Thompson PD, Marsden CD. Effect of vision and stance width on human body motion when standing: implications for afferent control of lateral sway. J Physiol 469: 479–499, 1993. doi: 10.1113/jphysiol.1993.sp019824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beylergil SB, Karmali F, Wang W, Bermudez Rey MC, Merfeld DM. Vestibular roll tilt thresholds partially mediate age-related effects on balance. Prog Brain Res 248: 249–267, 2019. doi: 10.1016/bs.pbr.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 72.Adkin AL, Frank JS, Carpenter MG, Peysar GW. Postural control is scaled to level of postural threat. Gait Posture 12: 87–93, 2000. doi: 10.1016/S0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- 73.Fitzpatrick R, McCloskey D. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol 478: 173–186, 1994. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teasdale N, Nougier V, Barraud P-A, Bourdin C, Debû B, Poquin D, Raphel C. Contribution of ankle, knee, and hip joints to the perception threshold for support surface rotation. Percept Psychophys 61: 615–624, 1999. doi: 10.3758/BF03205534. [DOI] [PubMed] [Google Scholar]
- 75.Fukagawa NK, Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol Series A Biol Sci Med Sci 50A: 64–67, 1995. doi: 10.1093/gerona/50A.Special_Issue.64. [DOI] [PubMed] [Google Scholar]
- 76.Goodworth AD, Peterka RJ. Contribution of sensorimotor integration to spinal stabilization in humans. J Neurophysiol 102: 496–512, 2009. doi: 10.1152/jn.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goodworth AD, Peterka RJ. Influence of stance width on frontal plane postural dynamics and coordination in human balance control. J Neurophysiol 104: 1103–1118, 2010. doi: 10.1152/jn.00916.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goodworth AD, Peterka RJ. Identification of sensory contributions to stance control in transtibial amputees. In: American Orthotic & Prosthetics Association National Assembly. Orlando, FL: 2013.
- 79.Fernie GR, Gryfe CI, Holliday PJ, Llewellyn A. The relationship of postural sway in standing to the incidence of falls in geriatric subjects. Age Ageing 11: 11–16, 1982. doi: 10.1093/ageing/11.1.11. [DOI] [PubMed] [Google Scholar]
- 80.Overstall PW, Exton-Smith AN, Imms FJ, Johnson AL. Falls in the elderly related to postural imbalance. Br Med J 1: 261–264, 1977. doi: 10.1136/bmj.1.6056.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lord SR, Rogers MW, Howland A, Fitzpatrick R. Lateral stability, sensorimotor function and falls in older people. J Am Geriatr Soc 47: 1077–1081, 1999. doi: 10.1111/j.1532-5415.1999.tb05230.x. [DOI] [PubMed] [Google Scholar]
- 82.Montesinos L, Castaldo R, Pecchia L. Wearable inertial sensors for fall risk assessment and prediction in older adults: a systematic review and meta-analysis. IEEE Trans Neural Syst Rehabil Eng 26: 573–582, 2018. doi: 10.1109/TNSRE.2017.2771383. [DOI] [PubMed] [Google Scholar]
- 83.Horak F, Nashner L, Diener H. Postural strategies associated with somatosensory and vestibular loss. Exp Brain Res 82: 167–177, 1990. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- 84.Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL. Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 11: 521–534, 2012. doi: 10.1016/S1474-4422(12)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han BI, Song HS, Kim JS. Vestibular rehabilitation therapy: review of indications, mechanisms, and key exercises. J Clin Neurol 7: 184–196, 2011. doi: 10.3988/jcn.2011.7.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanders OP, Savin DN, Creath RA, Rogers MW. Protective balance and startle responses to sudden freefall in standing humans. Neurosci Lett 586: 8–12, 2015. doi: 10.1016/j.neulet.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lackner JR, DiZio P. Vestibular, proprioceptive, and haptic contributions to spatial orientation. Annu Rev Psychol 56: 115–147, 2005. doi: 10.1146/annurev.psych.55.090902.142023. [DOI] [PubMed] [Google Scholar]
- 88.Chiang B, Fridman GY, Dai C, Rahman MA, Della Santina CC. Design and performance of a multichannel vestibular prosthesis that restores semicircular canal sensation in rhesus monkey. IEEE Trans Neural Syst Rehabil Eng 19: 588–598, 2011. doi: 10.1109/TNSRE.2011.2164937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Merfeld DM, Lewis RF. Replacing semicircular canal function with a vestibular implant. Curr Opin Otolaryngol Head Neck Surg 20: 386–392, 2012. doi: 10.1097/MOO.0b013e328357630f. [DOI] [PubMed] [Google Scholar]
- 90.Sienko KH, Vichare VV, Balkwill MD, Wall IIC. Assessment of vibrotactile feedback on postural stability during pseudorandom multidirectional platform motion. IEEE Trans Biomed Eng 57: 944–952, 2010. doi: 10.1109/TBME.2009.2036833. [DOI] [PubMed] [Google Scholar]
- 91.Goodworth AD, Peterka RJ. Sensorimotor integration for multisegmental frontal plane balance control in humans. J Neurophysiol 107: 12–28, 2012. [Erratum in J Neurophysiol 108: 2338, 2012]. doi: 10.1152/jn.00670.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]