Keywords: acetylcholine, dorsal root potential, presynaptic inhibition, primary afferent depolarization, tubocurarine
Abstract
Somatosensory input strength can be modulated by primary afferent depolarization (PAD) generated predominantly via presynaptic GABAA receptors on afferent terminals. We investigated whether ionotropic nicotinic acetylcholine receptors (nAChRs) also provide modulatory actions, focusing on myelinated afferent excitability in in vitro murine spinal cord nerve-attached models. Primary afferent stimulation-evoked synaptic transmission was recorded in the deep dorsal horn as extracellular field potentials (EFPs), whereas concurrently recorded dorsal root potentials (DRPs) were used as an indirect measure of PAD. Changes in afferent membrane excitability were simultaneously measured as direct current (DC)-shifts in membrane polarization recorded in dorsal roots or peripheral nerves. The broad nAChR antagonist d-tubocurarine (d-TC) selectively and strongly depressed Aδ-evoked synaptic EFPs (36% of control) coincident with similarly depressed A-fiber DRP (43% of control), whereas afferent electrical excitability remained unchanged. In comparison, acetylcholine (ACh) and the nAChR agonists, epibatidine and nicotine, reduced afferent excitability by generating coincident depolarizing DC-shifts in peripheral axons and intraspinally. Progressive depolarization corresponded temporally with the emergence of spontaneous axonal spiking and reductions in the DRP and all afferent-evoked synaptic actions (31%–37% of control). Loss of evoked response was long-lasting, independent of DC repolarization, and likely due to mechanisms initiated by spontaneous C-fiber activity. DC-shifts were blocked with d-TC but not GABAA receptor blockers and retained after tetrodotoxin block of voltage-gated Na+ channels. Notably, actions tested were comparable between three mouse strains, in rat, and when performed in different labs. Thus, nAChRs can regulate afferent excitability via two distinct mechanisms: by central Aδ-afferent actions, and by transient extrasynaptic axonal activation of high-threshold primary afferents.
NEW & NOTEWORTHY Primary afferents express many nicotinic ACh receptor (nAChR) subtypes but whether activation is linked to presynaptic inhibition, facilitation, or more complex and selective activity modulation is unknown. Recordings of afferent-evoked responses in the lumbar spinal cord identified two nAChR-mediated modulatory actions: 1) selective control of Aδ afferent transmission and 2) robust changes in axonal excitability initiated via extrasynaptic shifts in DC polarization. This work broadens the diversity of presynaptic modulation of primary afferents by nAChRs.
INTRODUCTION
The strength of sensory input must be modifiable over a range to provide appropriate feedback consistent with behavioral needs. One powerful mechanism involves presynaptic control inflow of sensory information via primary afferent depolarization (PAD) that has been associated with presynaptic inhibition. PAD occurs by activation of ionotropic receptors located on primary afferent terminals, producing a local depolarization. PAD electrotonically back-propagates along afferents toward dorsal roots where it can be measured experimentally as a dorsal root potential (DRP). For GABAA receptor (GABAAR) activation, PAD is mediated via Cl− efflux, and it is associated with reduced probability of transmitter release, presumably via sodium channel inactivation and shunting (1, 2; however, see 3).
GABAAR-mediated PAD is predominant in low-threshold afferents (2, 4, 5), whereas cation-conducting N-methyl-d-aspartate (NMDA)/non-NMDA glutamate receptor-evoked PAD is more prominent in high-threshold afferents (5–7). Other ionotropic receptors found on primary afferents include P2X purinergic, 5HT3 serotonergic, TRPV1, and nicotinic acetylcholine receptors (nAChRs). The activation of cation-conducting 5HT3 receptors also produces PAD (8). However, their association with hyperalgesia (9) may instead link PAD to presynaptic facilitation of spike propagation across branch points (3).
Primary afferents contain numerous nAChR subtypes (10–16), supporting a role for complex regulation of afferent excitability. The location of nAChRs along peripheral and central projections may provide important insights into mechanisms of physiological/pharmacological action. Receptor-mediated PAD has a complex pharmacology that may indicate an involvement of nAChRs (17, 18). For example, in adult rat, a large component of the low-threshold afferent evoked presumably GABAAR-generated DRP is resistant to bicuculline (19) and picrotoxin (20). Although there is currently no evidence that nAChRs lead to PAD in thoracic segments (5), nAChR-mediated excitability increases in unmyelinated C-fibers are consistent with axon depolarization (21).
Anatomical evidence provides strong support for central cholinergic control of cutaneous afferent transmission in lamina III: 1) dorsal horn cholinergic interneurons are predominantly located in lamina III (22–24), 2) a descending cholinergic pathway originating in the rostral ventrolateral medulla exclusively projects to this site (25), and 3) laminae IIi/III contain a dense plexus of cholinergic fibers. Cholinergic/GABAergic interneurons with cell bodies in lamina III have repetitive firing patterns following current injection (23), supporting a possible cholinergic involvement in a long-lasting phenomenon such as the DRP (1, 26). Direct cholinergic actions on primary afferents are consistent with the observation of ACh being colocalized in ∼25% of presynaptic GABAergic axo-axonic connections onto primary afferents (27–29). Lamina III is a projection site of Aβ and Aδ cutaneous afferents (30) and a location of interneurons that controls their excitability via PAD (5, 31–34). Lamina III is also the selective projection site of larger-diameter afferents that contain α1,7,9,10 nAChR-sensitive intraspinal α-bungarotoxin-binding sites (35). Thus, both bulbospinal projections and cholinergic spinal interneurons acting on afferent nAChRs could participate in modulating synaptic efficacy by nAChR-mediated PAD.
Primary afferents themselves could presynaptically modulate afferent excitability via autocrine acetylcholine (ACh) release. Both large- and small-diameter afferents express the choline transporters (16, 36), and there is evidence of expression of the ACh synthesis enzyme choline acetyltransferase (ChAT) and a peripheral form (pChAT) in murine models (37–39). To date, no studies have unambiguously demonstrated synaptic ACh release from primary afferents (but see 40, 41). An intriguing possibility is nonvesicular extrasynaptic ACh release (42) along the length of the primary afferent axons to control afferent excitability in an autocrine and paracrine fashion. Extrasynaptic release could tonically modulate membrane excitability and spike propagation at axonal branch points. Presynaptic modulation via actions at branch points is well-documented in invertebrates (43–45), and a GABAAR-dependent mechanism has also been demonstrated in mammalian afferents (3, 19, 46–48).
This study used pharmacology in an in vitro approach to examine nAChR-mediated modulation of afferent excitability, PAD, and synaptic transmission in broad categories based on electrical stimulation-evoked recruitment of Aβ (muscle groups I and II), Aδ (muscle group III) and C (muscle group IV) fiber afferents. By demonstrating two major distinguishable contributions of nAChRs on primary afferents, this work further broadens the diversity of ionotropic receptors involved in presynaptic modulation of primary afferents.
METHODS
All procedures were approved by the Emory University Institutional Animal Care and Use Committee and by the Institutional Committee for Animal Use in the “Centro de Investigación y de Estudios Avanzados” (Mexico). The primary studies were undertaken in mouse FVB or BALB/c strains, and all presented figures are from experiments in these strains. Corroborative experiments assessing generality of primary results were also undertaken in C57BL/6 mice and Sprague–Dawley rats. Midsagittally hemisected spinal cords were isolated from postnatal day (P) 5–12 mice from either the C57BL/6, FVB, or BALB/c background, or 7- to10-day-old Sprague–Dawley rats prepared for in vitro experiments as described previously (49, 50). Effects across strains and between species were broadly similar as described in the Results section. We did not explore sex differences as a variable. Recordings were made in carbogenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 128; KCl 1.9; d-glucose 10; MgSO4 1.3; CaCl2 2.4; KH2PO4 1.2; and NaHCO3 26 (300–310 mOsm at pH 7.4). Bath superfusion was achieved at a rate of ∼20 mL/min.
Stimulation and Recording of Dorsal Roots and Peripheral Nerves
The general experimental setup is shown in Fig. 1A, with example traces of recorded potentials. In some experiments, dorsal lumbar roots (L3, L4, and/or L5) were used for stimulation and recording. In other experiments, hindlimb peripheral nerves (predominantly tibial, sural, and superficial peroneal) were left intact for stimulation and recording. Sural is a cutaneous nerve innervating more distal hindlimb skin, whereas the tibial nerve innervates the plantar surface along with some intrinsic muscles of the foot (51). These nerves are well suited for the use of suction electrodes, and their length facilitates separation of afferent classes (Aβ, Aδ, and C) owing to difference in conduction velocity (49, 50, 52). Systematic assessment of relative afferent recruitment was undertaken in only the mouse. Recruitment of the Aβ, Aδ, and C components of afferent volleys (AVs) by graded stimulation in multiples of threshold was explored by 1) stimulating a peripheral nerve (typically tibial) while recording in the sciatic nerve (n = 10), and 2) stimulating the proximal dorsal root (DR) L4 or L5 and recording the distal stump (n = 6; not shown in Fig. 1A schematic). We observed a similar threshold-dependent patterns of recruitment. The age range was P5–7.
Figure 1.
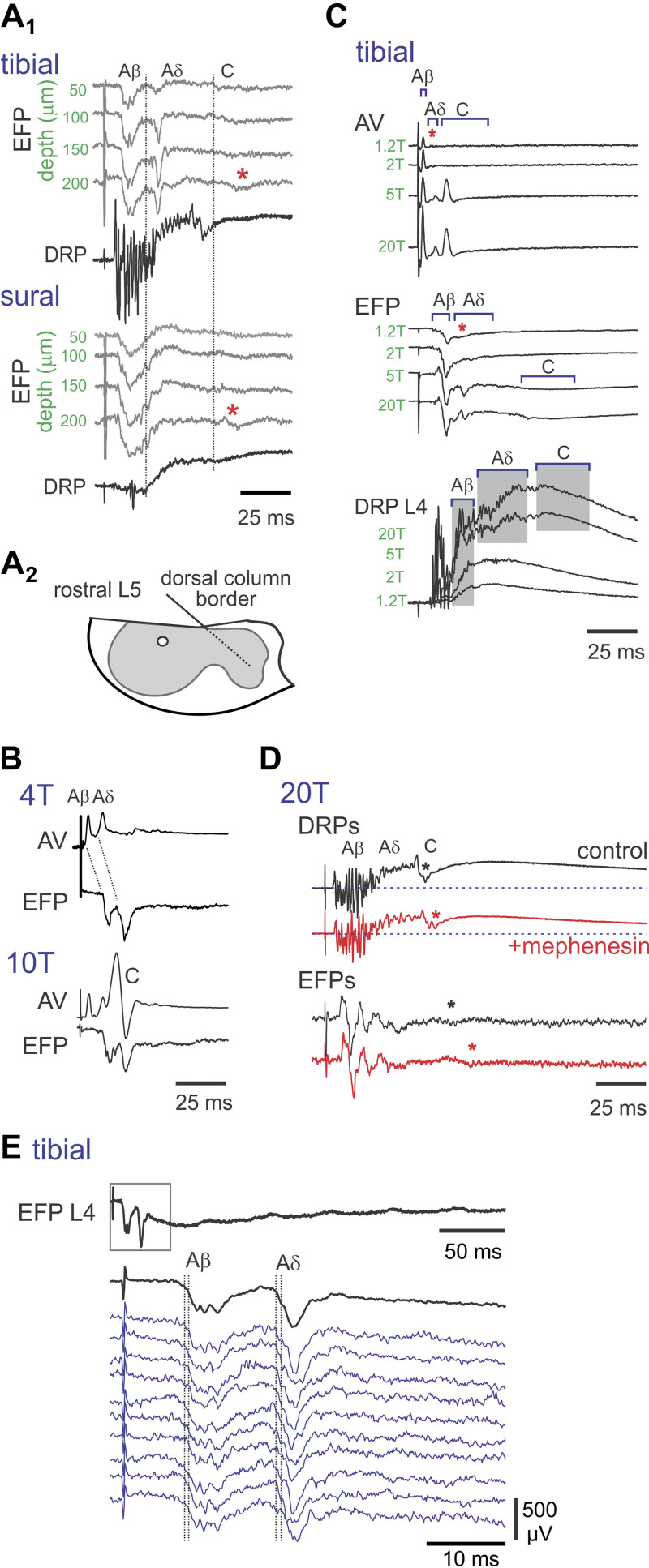
Experimental design and identification of components in evoked responses. A: typical stimulation and recording setup with example traces. Peripheral nerves are isolated for stimulation as well as recording of afferent volley (AV) compound action potentials in sciatic nerve. Dorsal root potentials (DRPs) are monitored at the dorsal root entry zone by means of en passant suction electrodes. Cut dorsal roots record actions of applied drugs independent of spinal circuits. Microelectrodes in the deep dorsal horn are used for recording population synaptic responses as extracellular field potentials (EFPs) generated by afferent stimulation. B: effects of stimulus intensity on afferent fiber recruitment. Electrical stimuli were applied to the tibial nerve, a mixed cutaneous and muscle nerve. Afferent volleys were recorded in the sciatic nerve. B1: stimuli are delivered in multiples of threshold with typical stimulus pulse duration of 200 µs. Two early A- (arrows) and a clear C-fiber volleys are seen (arrows) at 6 T. B2–3: stimuli are delivered at increasing constant current intensities and pulse durations using either cathodic (B1) or anodic current (B2,3). Note that the C-afferent volley component is observed at constant current stimulation as low as 50 µA/50 µs. Longer time period in B3 is shown to display lack of additional afferent volleys, confirming the late component as C-fiber in origin. C: measuring the A- and C-fiber components of the DRP. Shown on the left are sural cutaneous nerve stimulation-evoked DRP responses in the L5 dorsal root when stimulated at current intensities that recruited A-fiber (black traces) or A- and C-fibers (green traces) as monitored by the afferent volley. On the right, the DRP evoked by stimulation at 100 µA/100 µs is shown low-pass filtered to display method for separate amplitude measurement of the A- and C-fiber components of the DRP. Data are from P8–9 mice.
Recorded data were digitized at 5–15 kHz (Digidata 1322 A 16-Bit DAQ, Molecular Devices) with pClamp acquisition software (v. 10.0, Molecular Devices) and stored for offline analysis with pClamp software. Constant current stimulators delivered electrical stimuli at defined intensity and pulse duration every 15–30 s to the dorsal roots or peripheral nerves with monopolar glass suction electrodes having immediately adjacent reference electrodes. Interstimulus intervals >10 s are sufficient to prevent frequency-dependent depression (53). Primary afferent depolarization (PAD) was inferred from antidromically propagating dorsal root potentials (DRPs; gain 1,000×) recorded at L3–L5 dorsal roots using monopolar glass suction electrodes (∼120 µm of internal tip diameter) placed en passant on the dorsal root as close as possible to the entry zone. Recruitment of afferents was monitored via recordings of the afferent volley (AV) in contiguous regions along peripheral nerves or dorsal roots.
DRPs and AVs were recorded using differential amplifiers at direct current (DC; AM Systems Model 3000) or near DC (custom-built amplifiers bandpass-filtered between 0.1 Hz and 3 kHz). In most of the experiments, DC recordings were performed while distal dorsal roots or peripheral nerves were concurrently stimulated, so that changes in the polarization state of the afferents could be monitored simultaneously with stimulus-evoked effects.
Electrical stimulation was provided using two commonly reported methods: 1) constant current intensities and durations or 2) defined by multiples of the threshold (T) of the most excitable afferents measured by the evoked AV (e.g., 4 T, 10 T) using 200- or 500-µs pulse widths. Both provide helpful insight into afferent recruitment in comparison to methods mentioned in past literature. Importantly, owing to immature myelination in neonatal rodents, the classical use of constant current and threshold values to separate various classes of myelinated afferents does not apply (49, 52, 54). For example, measured conduction velocity values for a P6 mouse were Aβ = 3.2, Aδ = 0.5, and C = 0.3 m/s. Thus, relative C-fiber recruitment begins at low relative threshold and constant current values (Fig. 1B), emphasizing the need to record afferent volleys to discriminate between A- and C-fiber recruitment. Although the present study focuses on the actions of A fiber afferents, it is worth noting that, when stimulating hindlimb nerves, the components of the AV representing A- and C-fiber recruitment can coincide with separable components of the DRP (Fig. 1C).
Extracellular Field Potentials
Extracellular field potential (EFP) recordings were obtained to provide a measure of population monosynaptic transmission from primary afferents onto first-order spinal interneurons (49, 50).
EFP electrodes (tip diameter, 2–3 µm) were filled with 2 M NaCl solution with resistances ranging from 4 to 6 MΩ. EFPs were amplified with an Axoclamp-1D (Axon Instruments, Molecular Devices) and low-pass filtered at 2 kHz.
Electrodes were positioned to enter the cord at a 45° angle to the midline at the interface between the dorsal column and the gray matter. The deep dorsal horn was targeted at depths of 80–120 µm below the cut surface of the cord in mice, where the largest short-latency responses were observed by incremental tracking during stimulation of roots or nerves at A-fiber strength (Fig. 2A).
Figure 2.
Relating components of afferent volley (AV), extracellular field potentials (EFP), and dorsal root potentials (DRP). A1: characterization of evoked population synaptic responses (recorded as EFPs). Evoked synaptic responses of tibial and sural nerve stimulated at 10 T. Two large, short-latency EFPs are seen following tibial nerve stimulation, whereas sural evoked responses are not overtly separable. These A-fiber-mediated EFPs are maximal at 150 µm. Longer-latency C-fiber-evoked EFPs are seen at 200 µm (asterisks) and correspond with longer-latency DRPs (aligned with vertical bars). A2: trajectory estimate of the intraspinal electrode placed at dorsal column/gray matter border at rostral L5. B: tibial nerve stimulation at 4 T recruits Aβ and Aδ afferent volleys and are associated with recruitment of two short-latency EFPs and are not associated with C-fiber afferents recruited at 10 T. C: tibial nerve stimulation versus recruitment of afferent volleys and evoked responses. In the sciatic nerve recording, the Aδ component appears present already at 1.2 T (asterisk) and is associated with a later event in the EFP recording (asterisk). Both Aβ and Aδ afferent volleys increase in amplitude with increased T. Note that the large increase in Aδ fiber recruitment at 5 T is associated with a large increase in emergence of the later-arriving EFP and large increase in DRP amplitude. The C-fiber afferent volley is also recruited at 5 T and is associated with a later longer-lasting EFP and DRP. Note also that further increases in the Aβ volley at 20 T is associated with a large increase in the early component of the DRP. D: retained EFPs in the presence of mephenesin support their monosynaptic origin [see Vijayaraghavan et al. (89)]. Tibial nerve stimulation at 20 T recruits A- and C-fiber afferent volleys when recorded at dorsal root entry (superimposed on DRP). Note that EFPs are mephenesin-insensitive (1 mM), although the C volley and corresponding recorded EFPs appear to conduct slower (asterisks). EFPs were recorded 150 µm into the spinal gray caudal L4 200 µm ventral to the dorsal column white matter-gray matter interface. E: average (black) and individual (blue) EFPs evoked by the stimulation of the tibial nerve (4 T). The boxed region of top trace is expanded in an average and individual traces below to demonstrate similar variability in latency of arrival of monosynaptic Aβ and Aδ EFPs. Latencies were measured from the fastest afferent volley recorded in the L4 dorsal root. The pairs of vertical dashed lines correspond to the minimal and maximal latency for each component. Data are from P6–10 mice.
Assessing Recruitment of Aβ, Aδ, and C-Fiber Afferent Volleys in the Sciatic Nerve to Corresponding Synaptic (EFPs) and DRP Responses
Following peripheral nerve stimulation, the recruitment of A-fibers was separable into Aβ and Aδ afferent volleys when recorded in the sciatic nerve. These had corresponding EFPs that were not altered when C-fibers were also recruited (Fig. 2B). As C-fibers generally terminate in more superficial regions of the dorsal horn, observation of longer-latency C-fiber-mediated EFPs were not commonly seen. Examples of C-fiber-evoked EFPs at high stimulation strengths are shown in Fig. 2, A, C, and D. Comparison of afferent volley recruitment to observed EFPs and DRPs was made with graded electrical stimulation (Fig. 2C). A large increase in Aδ fiber recruitment (at 5 T in this example) was associated with the clear emergence of the later-arriving Aδ fiber-mediated EFP and DRP. Concomitant recruitment of C-fiber afferents was also associated with the emergence of later and longer-lasting EFP and DRP components. We used mephenesin to demonstrate that longer-latency EFPs predominantly reflect monosynaptic responses. Mephenesin has been used to isolate monosynaptic from polysynaptic components in the central nervous system (50, 55). The mechanism has not been well described, but mephenesin decreases the firing threshold of interneurons when applied at 1–2 mM, leaving the monosynaptic component unaffected, or less affected (56). As shown in Fig. 2D, restriction to monosynaptic transmission with mephenesin did not abolish these longer-latency EFPs (50). Aβ EFP and Aδ latencies were 2.2 ± 0.3 and 13.6 ± 0.2 ms, respectively. The similar variability in latency of arrival of Aβ and Aδ EFPs further supports the monosynaptic origin of longer-latency responses.
Although this study focuses on ionotropic nicotinic receptor actions, physiologic cholinergic transmission would be expected to also act on presynaptic muscarinic receptors (57, 58). Block of muscarinic receptors with atropine (10 μM) did not significantly alter the amplitude of sural nerve stimulation-evoked EFPs (101% ± 20% of control values; n = 3) or DRPs (80% ± 14% of control; n = 4).
Drug Applications and Data Analysis
Drugs were applied by bath superfusion for between 5 and 30 min. Drug reversibility was usually assessed 60 min after drug washout. Washout effects were in general partially reversible. In many experiments, afferent volleys were continuously monitored to discriminate between A- and C-fiber-mediated actions, and to assess whether observed actions include modulation of afferent recruitment.
The effects of ACh on spontaneous firing in individual axons were assessed with spike sorting using Spike 2 (v 7.05, Cambridge Electronic Devices, UK). Individual spike waveforms were initially separated using the waveform templating feature, but subsequently reassigned using clustering algorithms. Spikes that could not be unambiguously defined as unique were not included. Spike counts were exported to SigmaPlot (v12-14, Systat Software) for subsequent analyses.
Table 1 provides a list of the drugs used, their putative site of action, dose, solvent, and supplier. Drug dosage was based either on published studies showing full block in vitro (intact or slice preparation) or at doses at 10× the reported IC50 values determined in cell culture. There were two exceptions. The first was d-TC, with dosage chosen based on lack of observable nonspecific action at GABAARs in our system. The second was with the application of the neurotransmitter ACh whose concentration at nAChRs would be expected to be greatly affected by endogenous cholinesterase degradation. Consequently, the cholinesterase inhibitor neostigmine was commonly coapplied with ACh to ensure endogenous action.
Table 1.
Drugs used
| Drug | Actions/Receptors Targeted | Dose, µM | Source |
|---|---|---|---|
| nAChR agonists | |||
| ACh | Endogenous transmitter | 20–100 | Sigma-Aldrich |
| Nicotine* | Agonist at all nAChRs except α9-containing channels where it is an antagonist (59) | 5–40 | Sigma-Aldrich |
| Epibatidine* | Agonist at all nAChRs except α9α10 heteromeric channels where it is an antagonist (59) | 0.1 | Sigma-Aldrich |
| NAChR antagonists | |||
| d-Tubocurarine (d-TC) | Broad nAChR competitive antagonist α1–4,7,9,10 | 10–30 | Sigma-Aldrich |
| Mecamylamine | Noncompetitive open-channel block of α3, α6, and α9 nAChRs | 50 | Sigma-Aldrich |
| Methyllycaconitine (MLA) | Antagonist at α7 and α9 nAChRs | 0.2–1.0 | Tocris |
| Other | |||
| Neostigmine | Cholinesterase inhibitor (60) | 20–30 | Sigma-Aldrich |
| Atropine | Muscarinic receptor antagonist | 10 | Sigma-Aldrich |
| Picrotoxin | Antagonist at GABAARs by complex mechanisms | 25 | Tocris |
| Muscimol | Non-selective agonist at GABAARs | 10 | Tocris |
| GABA | Endogenous transmitter | 100 | Sigma-Aldrich |
| Nipecotic acid | GABA uptake inhibitor | 100 | Sigma-Aldrich |
| Tetrodotoxin (TTX) | Voltage-gated Na+ channel blocker | 0.1 | Sigma-Aldrich |
Measures of DRP area (from 0 to 200 ms, after low-pass filtering at 300 Hz) and EFP peak amplitude (low-pass-filtered at 500 Hz) were obtained from multi-episode averages (between 4 and 12, but typically five episodes). Examples of evoked actions in figures are similarly from multi-episode averages of between 4 and 10 unless otherwise stated. Primary studies were undertaken in mouse FVB or BALB/c strains and were pooled as effects were similar (Tables 2 and 3). Unless otherwise described, Student’s t test was used to determine statistical significance of drug effects on the DRP and EFP. Power was set at 0.8 with α level of P < 0.05 considered as significant. Values are reported as means ± SD unless otherwise stated.
Table 2.
Effects of nAChR agonists and antagonists on the DRP and EFP
| EFP (Peak amplitude) | FVB and BALB/c (% of control) |
|---|---|
| ACh/neostigmine | [Aβ] 37% ± 21% (19)***[Aδ] 35% ± 26% (14)***[C] 23% ± 20% (8)** |
| Nicotine | [Aβ] 70% ± 13% (14)***[Aδ] 44% ± 17% (14)*** |
| Epibatidine | [Aβ] 69% ± 15% (6)**[Aδ] 45% ± 16% (5)*** |
| d-TC | [Aβ] 106% ± 20% (13)[Aδ] 38% ± 20% (13)*** |
| Mecamylamine | [Aβ] 99% ± 5% (4)[Aδ] 94% ± 9% (3) |
| DRP (Area under curve) | FVB and BALB/c (% of control) |
|---|---|
| Neostigmine | 81% ± 32% (14)* |
| ACh | 74% ± 20% (10)** |
| ACh/neostigmine | 29% ± 23% (22)*** |
| Nicotine | 73% ± 12% (18)*** |
| Epibatidine | 77% ± 16% (9)** |
| d-TC | 56% ± 15% (12)*** |
| Mecamylamine | 88% ± 8% (11)*** |
| Methyllycaconitine (MLA) | 97% ± 12% (9) |
Values are expressed as percent of control and shown as means ± SD with sample size in parentheses. For all panels, *P < 0.05; **P < 0.01; ***P < 0.001.
EFP values are separated into Aβ-, Aδ-, and C-fiber changes, respectively, with sample sizes subsequently listed. C-fiber-evoked EFPs were examined only in the subpopulation of responses seen with ACh/neostigmine. d-TC, d-tubocurarine; DRP, dorsal root potential; EFP, extracellular field potential.
Table 3.
Comparison of changes in evoked responses between BALB/c and FVB strains
| DRP (Area under curve) |
||
|---|---|---|
| BALB/c | FVB | |
| Neostigmine | 80% ± 41% (7) | 82% ± 28% (7) |
| ACh/neostigmine | 20% ± 16% (15)*** | 49% ± 24% (7) *** |
| Nicotine | 74% ± 13% (11)*** | 71% ± 11% (7)*** |
| d-TC | 58% ± 14% (7)*** | 52% ± 18% (5)** |
| EFP (Peak amplitude) |
||
|---|---|---|
| BALB/c | FVB | |
| ACh/neostigmine | [Aβ] 37% ± 26% (12)***[Aδ] 37% ± 27% (11)***[C] 25% ± 23% (6)*** | 35% ± 9% (7)***27% ± 25% (3)*18% ± 4% (2)* |
| Nicotine | [Aβ] 71% ± 12% (9)***[Aδ] 52% ± 14% (9)*** | 67% ± 16% (5)**31% ± 13% (5)*** |
| d-TC | [Aβ] 108% ± 19% (10)[Aδ] 41% ± 21% (10)*** | 100% ± 25% (3)26% ± 13% (3)** |
Values are shown as means ± SD with sample size in parentheses. For all panels, *P < 0.05; **P < 0.01; ***P < 0.001.
ACh and nicotinic receptor agonists and antagonist actions on the DRP. ACh and nicotinic receptor agonist and antagonist actions on EFPs. Values for EFPs are separated into Aβ-, Aδ-, and C-fiber changes, respectively, with sample sizes subsequently listed. C-fiber-evoked EFPs were examined only in the subpopulation of responses seen with ACh/neostigmine. DRP, dorsal root potentials; d-TC, d-tubocurarine; DRP, dorsal root potential; EFP, extracellular field potential.
RESULTS
Table 2 provides the overall results of applied drugs on evoked responses from FVB and BALB/c strains. In all cases, responses were partially reversible after washout of drugs (not shown for clarity). Prominent agonist and antagonist actions were tested between these strains and shown to be comparable (Table 3). This and further exploration of the broader reproducibility of observed nicotinic actions are explored in a section below titled Observed Actions Were Comparable between Mouse Strains, in Rat, and When Performed in Different Labsa.
nAChR Antagonist d-TC Strongly Depresses Aδ Synaptic Transmission and Associated DRPs
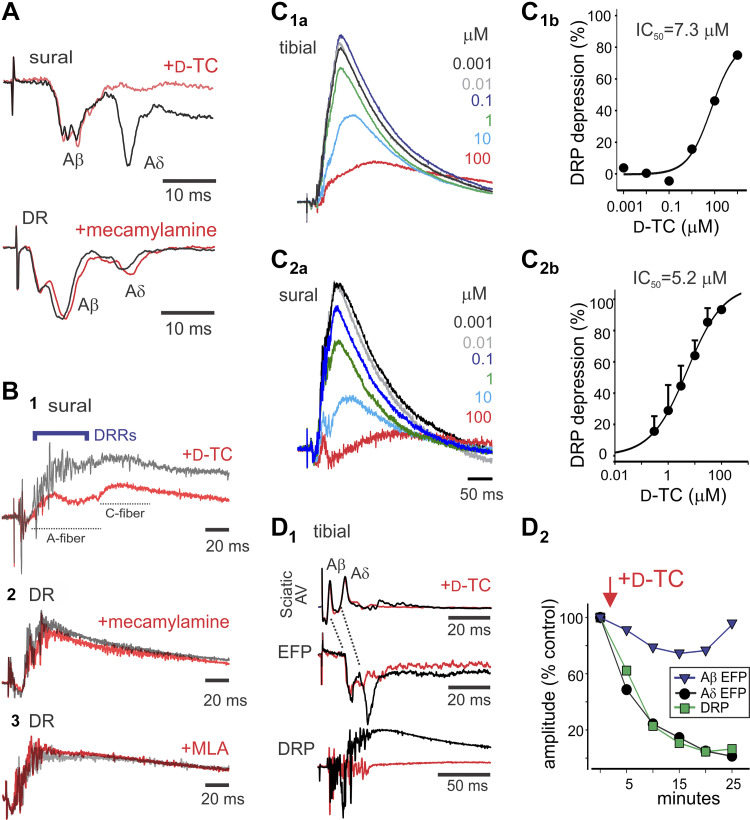
The actions of nAChR antagonists were assessed (Table 2). Deep dorsal horn population monosynaptic responses (EFPs) were commonly temporally distinguishable as Aβ and Aδ components (Fig. 2E) (50). d-TC selectively and strongly depressed the Aδ component of the A-fiber EFP to 38% of control amplitude (n = 13, P < 0.001; Fig. 3A). Mecamylamine was without action (n = 4; Fig. 3B). The effects of nAChR antagonists on DRPs are provided in Table 2 and Fig. 3B. d-Tubocurarine (d-TC) greatly depressed DRPs to 56% of control (n = 13; P < 0.001), with significant but more modest reductions also seen with mecamylamine (88% of control; n = 11; P < 0.001). Methyllycaconitine (MLA) was without effect (n = 9). We examined d-TC actions in greater detail owing to its distinctly strong depression of the DRP. d-TC depressed tibial and sural nerve stimulation-evoked DRPs, with IC50 values of 7.3 and 5.2 μM, respectively (Fig. 3C). d-TC had no effect on afferent recruitment, as there was no evidence of a reduction in the stimulation-evoked afferent volley in sciatic nerve recordings (n = 4; Fig. 3D1). These data are consistent with the hypothesis that Aδ primary afferents release ACh or related agonist as a neurotransmitter. The time course of d-TC selective depression of the Aδ afferent-evoked EFP matched the depression seen in the DRP and was independent of the Aβ-evoked EFP (Fig. 3D2). This suggests that the DRP depressant actions seen with d-TC are causally linked to depression of monosynaptic Aδ afferent-evoked responses.
Figure 3.
The nicotinic acetylcholine receptor (nAChR) antagonist tubocurarine (d-TC) strongly depresses Aδ synaptic transmission and associated dorsal root potentials (DRPs). A: d-TC preferentially depressed the Aδ-fiber component of the extracellular field potentials (EFP), whereas mecamylamine did not alter EFPs. Stimuli are sural (4 T) and dorsal root (DR; 200 µA/500 µA). B: depression of evoked DRPs by nAChR antagonists. d-TC (1) and mecamylamine (2) depressed evoked DRPs, whereas methyllycaconitine (MLA) (3) was without effect (100 μA/100 μs, 10 T, and 100 μA/100 μs, respectively). Note that d-TC depression of DRP was associated with loss of dorsal root reflexes (DRRs). C: DRP was reduced by d-TC in a dose-dependent manner. Tibial (4 T) (C1) and sural (4 T) (C2) stimulation-evoked L3 DRP depression by d-TC. Dose-response curves for d-TC inhibition of tibial (C1b) and sural (C2b) nerve stimulation-evoked DRP. Tibial dose-response curve is from recording in C1a (12 episodes averaged). Sural dose-response curves are from four experiments (means ± SE), with example shown in C2a. D: relating depression of Aδ-mediated EFP to reductions in DRP amplitude. D1: d-TC depression of Aδ afferent EFP and DRP occur with similar magnitude and time course. Shown are the sciatic nerve afferent volley (AV), EFP, and DRP following stimulation of the tibial nerve at 4 T. Averaged responses are control and 20 min after bath application of d-TC. Note that sciatic nerve afferent fiber volleys are unchanged (without evoked DRRs), whereas L4 EFP and DRP depression occur. D2: amplitudes of evoked Aβ and Aδ EFPs and DRP are normalized to compare the relative time course and magnitude of depression produced by d-TC. Note that Aβ synaptic responses are relatively unchanged, whereas depression of Aδ EFP and DRP is closely matched. Data are from P6–8 mice.
ACh and nAChR Agonists Actions Are Widespread
nAChR agonists strongly depress synaptic transmission and associated DRPs.
We next assessed the actions of nAChR agonists on population monosynaptic responses recorded as EFPs (Table 2). When applied with neostigmine, ACh substantially depressed the amplitude of Aβ and Aδ EFPs to 37% (n = 19; P < 0.001) and 35% (n = 14; P < 0.001) of control responses, respectively (Fig. 4A1). Nicotine (n = 14) and epibatidine (n = 6) also significantly depressed Aβ and Aδ-fiber-mediated EFPs, with depression being preferentially greater for Aδ evoked EFPs ([Aβ] 70% versus [Aδ] 44%, and [Aβ] 69% versus [Aδ] 45% of control, respectively; Fig. 4A2,3). These results demonstrate that exogenous pharmacological activation of nAChRs can depress A-fiber afferent synaptic transmission.
Figure 4.
A: nicotinic receptor agonists depress A-fiber-mediated extracellular field potentials (EFPs). A1: although neostigmine had no effect on evoked A-fiber EFPs, subsequent addition of ACh greatly depressed both Aβ and Aδ components (labeled). A2: nicotine led to larger depression of the Aδ EFP. A3: epibatidine preferentially depressed the Aδ-fiber-mediated EFP. Scale bar is 10 ms in all panels. Stimuli for A1–3 are sural (100 µA/100 µA), DR (4 T), and tibial (4 T). B: depression of evoked dorsal root potentials (DRPs) by nicotinic acetylcholine receptor (nAChR) agonists. DRPs were recorded from L4 or L5 dorsal roots and displayed as an average of four evoked responses. ACh following neostigmine greatly depressed the evoked DRP (4 T) (1). Nicotine (2) and epibatidine (3) also depressed the evoked DRP (100 μA/100 μs and 10 T, respectively). C: ACh-induced reduction in dorsal root reflex responses compared in the same animal. C1: DRP depressant action of neostigmine/ACh via stimulation of the tibial nerve (5 T). Note that ACh depresses both A- and C-fiber components. C2: ACh progressive block of dorsal root reflexes (DRRs) (outlined and shaded) as shown in a raster plot of evoked responses occurring every 80 s (events are those in B1 100-Hz high-pass-filtered). DR, dorsal root.
The effects of neostigmine and nAChR agonists on the A-fiber-evoked DRP area are reported in Table 2. Although neostigmine (n = 14) or ACh (n = 10) when applied alone reduced the DRP (81% and 74% of control, respectively), when coapplied (n = 22) the DRP was dramatically reduced to 29% of control (Fig. 4B1). The marked enhancement of ACh action by neostigmine is expected, as neostigmine prevents breakdown of ACh. That neostigmine was able to have actions on its own demonstrates the importance of endogenous cholinesterase activity in limiting ACh actions. As ACh acts on both metabotropic muscarinic and ionotropic nicotinic receptors, we also tested the effects of the specific nAChR agonists nicotine (n = 18) and epibatidine (n = 9). Like ACh, both depressed DRPs (to 73 and 77% of control, respectively; Fig. 4B2,3).
The counterintuitive observation that both ACh agonists and antagonists depress A-fiber-evoked responses is due to actions at distinct differentiable sites as will be demonstrated below.
Asynchronous afferent spiking events could be seen superimposed in dorsal root recordings of the DRP. These events reflect centrally generated dorsal root reflexes (DRRs). When observed, both d-TC (Fig. 3B) and ACh (Fig. 4C) blocked DRRs (n = 6/6 and 5/5, respectively).
ACh and nAChR agonists depolarize primary afferents independent of spinal synaptic actions.
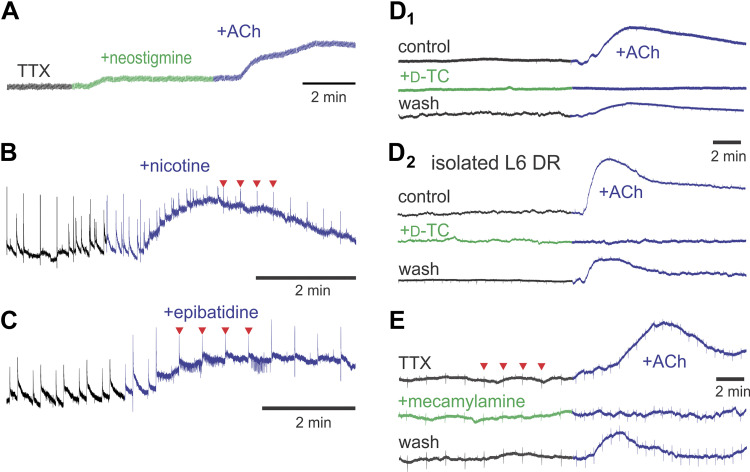
The effect of nAChR activation on overall changes in afferent membrane potential was measured as changes in electrode polarization with DC-coupled electrodes attached to a lumbar dorsal root. Neostigmine led to a depolarizing DC-shift of primary afferents in 10 of 26 experiments (Fig. 5A). That the cholinesterase neostigmine could produce a DC-shift supports the presence of endogenous ACh release. In comparison, ACh always led to a depolarizing DC-shift of dorsal root axons whether applied in the presence of neostigmine (n = 28/28) or alone (n = 17/17; Fig. 5, A and D1, respectively). nAChR agonists also depolarized afferents. Nicotine produced a clear DC depolarization in all cases (n = 3/3; Fig. 5B) and increased spiking activity in two other cases with unstable DC potentials (data not shown). Epibatidine also produced a depolarizing DC-shift of primary afferents (n = 4/5; Fig. 5C) with increased background dorsal root spiking in all five cases.
Figure 5.
ACh and selective nicotinic acetylcholine receptors (nAChR) agonists lead to depolarizing direct current (DC)-shifts in dorsal roots that are blocked by nAChR antagonists. A: in the presence of tetrodotoxin (TTX), both neostigmine and ACh produce depolarizing DC-shift of afferents. B: nicotine produces a desensitizing afferent DC-shift. Note nicotine also induced increase in background spiking activity. C: epibatidine also produces a depolarizing DC-shift with increase in spontaneous background spiking. D: ACh-induced DC-shifts are reversibly blocked by d-TC (D1) including after roots are isolated from the spinal cord (D2) demonstrating that effects are independent of central actions. E: ACh induces depolarizing DC-shift in the presence of TTX and is reversibly blocked by mecamylamine. In all panels, drug applications are denoted by a change in the color of the trace. Recordings in B, C, and E were undertaken coincident with stimulation of the dorsal roots (DRs) (every 20, 30, or 40 s, respectively) with corresponding stimulus artifacts seen as regular vertical events (examples at red arrowheads). Data are from P7–8 mice. d-TC, d-tubocurarine.
To demonstrate that depolarizing actions in primary afferents could occur independent of spinal synaptic actions, we blocked voltage-gated Na+ channels with tetrodotoxin (TTX). Although neostigmine alone could produce a DC depolarization (n = 3/8; Fig. 5A), ACh (applied alone or with neostigmine) consistently depolarized dorsal roots (n = 13/14; z-test on proportions P < 0.02; Fig. 5, A and E). Even when afferents were cut away from the spinal cord and their ganglion cell bodies, ACh depolarized the isolated roots (n = 5/5; 50 µM; Fig. 5D2). These observations demonstrate the presence of extrasynaptic nAChRs in peripheral afferent axons.
To demonstrate that nAChRs are responsible for agonist-induced depolarizing DC-shifts, nAChR agonists and antagonists were coapplied. Owing to known complex features of nAChR receptor desensitization (62), we first undertook repeated applications of agonist to assess the effect of multiple applications on nAChR receptor desensitization. Following second and third applications of ACh, recorded depolarizing shifts were 67% ± 37% (not significant) and 41% ± 27% (P < 0.05) of the original amplitude, respectively (n = 4). These values were taken into consideration in assessing the effects of nAChR antagonists.
d-TC had no effect when applied alone but d-TC prevented the depolarizing DC-shift in primary afferents caused by ACh (to 9% ± 3% of the ACh-induced DC-shift value; n = 4; P < 0.01; Fig. 5D). Two of these instances were from roots isolated from the spinal cord. d-TC also reduced the depolarizing DC-shift of coapplied neostigmine/ACh (to 44% ± 10% of control values; n = 6; P < 0.01).
Like d-TC, mecamylamine did not produce DC-shifts of primary afferents but depressed the depolarizing DC-shift caused by ACh (to 28% ± 15% of ACh-induced DC-shift; n = 3; P < 0.05). A similar effect was seen when undertaken in the presence of TTX (30% ± 16% of control values; n = 5; P < 0.01; Fig. 5E). That nAChR antagonists were without effects on their own suggests an absence of basal extrasynaptic nAChR activity.
ACh-induced depolarizing DC-shifts are not due to overlapping pharmacology with GABAA receptors.
Because d-TC can act as a GABAAR antagonist at higher doses (63) and the GABAAR antagonists picrotoxin and bicuculline can act as antagonists at some nAChRs (64), we tested for overlapping pharmacological actions on both GABAA- and nAChR-mediated depolarizing DC-shifts in afferent fibers. The GABAA receptor agonist muscimol reversibly depolarized primary afferents (n = 15/15; Fig. 6A). Control experiments showed that muscimol-induced DC amplitude changes were not significantly reduced by repeated applications (70% ± 27% and 76% ± 31% the original amplitude for second and third application, respectively; n = 5) even when applied in the presence of TTX (76% ± 24% of muscimol alone; n = 5). Coapplied d-TC did not alter muscimol-induced DC-shifts in TTX (74% ± 36% of muscimol-induced depolarization; n = 5/5). In comparison, muscimol- or GABA-induced DC-shifts were largely abolished with coapplied picrotoxin (13% ± 10% of control; P < 0.01; n = 3; Fig. 6, A and B). Conversely, coapplied picrotoxin did not alter the DC-shift produced by ACh (n = 6; 70% ± 19% of ACh/neostigmine-induced depolarization; Fig. 6B). Last, muscimol still generated depolarizing DC-shifts in the presence of ongoing DC-shifts produced by ACh (with neostigmine; n = 4/4; Fig. 6C). Thus, axons of primary afferents contain pharmacologically distinguishable nAChRs and GABAARs capable of generating depolarizing DC-shifts.
Figure 6.
ACh depolarizing direct current (DC)-shifts are not due to pharmacological actions on GABAARs. A: the GABAA agonist muscimol produces DC-shifts in the absence and presence of tetrodotoxin (TTX), demonstrating direct actions on primary afferents. DC-shifts are insensitive to d-TC but blocked with picrotoxin indicating GABAAR site of origin. B1: conversely, preincubation with picrotoxin does not prevent neostigmine/ACh-induced DC depolarization but does block muscimol-induced DC-shifts. B2: similarly, preincubation of picrotoxin abolishes the nipecotic acid/GABA-induced DC depolarization. C: after a neostigmine/ACh depolarizing DC-shift, muscimol was still able to produce a depolarization supporting an independence of nicotinic acetylcholine receptors (nAChR) and GABAAR sites of action. Data are from P6 mice. d-TC, d-tubocurarine.
nAChR-mediated DC depolarizing shifts modify spontaneous firing rate.
ACh/neostigmine-induced depolarizing DC shifts were often accompanied by changes in axonal spiking (Fig. 7A). A role for direct axonal initiation of spiking was supported by the observation that increased axonal spiking was also seen when afferents were isolated from the spinal cord and their cell bodies (Fig. 7A). Changes in spiking were assessed in individual spike-sorted axons (n = 36 axons from 19 animals). Although spiking activity increased in the majority of axons after ACh was applied, decreases were also seen in some axonal populations (Fig. 7, B and C). There was an overall increase in spike numbers when compared with control (P = 0.01, Wilcoxon signed rank test). That ACh generates depolarizing DC shifts that increase the probability of spontaneous spiking in peripheral axons, even when isolated from the cord, warrants consideration on whether axonal depolarization also occurs intraspinally and the impact these actions may have on sensory circuit excitability.
Figure 7.
Cholinergic modulation of spontaneous firing rate in dorsal roots (DRs) or peripheral nerves. A: example of induced alterations of population axonal firing from an L5 dorsal root in continuity with the spinal cord (top) or from an isolated L6 dorsal root from P8 mouse (400-Hz high-pass). Both cases show increased firing after application of ACh. B: examples of the temporal component of spike modulation in separate axons from an individual mouse with ACh causing either facilitated (axon #1) or depressed activity (axon 2). Spike frequencies are represented as averages in 25-s bins. C: relative change in spiking in 36 axons after addition of ACh/neostigmine. Ratios >1 (to the right of dotted vertical line) represent facilitated spiking (green circles) and those <1 represent overall depressed spiking (red circles). Axonal units chosen for this sample had spike counts of ≥10 spikes per 5- or 6-min epoch (observed either in control, drug application, or washout periods). Although neostigmine alone did not increase overall spiking activity (P = 0.65), there was an overall significant increase in spike numbers in the presence of ACh when compared with neostigmine (P = 0.01). Infinity symbol on the right has denominator values of 0 and represents axons without spiking before ACh application. neo, Neostigmine.
NAChR-mediated DC changes are also seen intraspinally and mirror dorsal root DC depolarizations.
We tested whether the nAChR-mediated DC-shifts of primary afferents were also seen intraspinally by recording DC shifts from extracellular electrodes used to capture Aβ and Aδ stimulation-evoked EFPs in the deep dorsal horn. ACh and nicotine led to intraspinal depolarizations that accurately mirrored DC-shifts seen in dorsal roots (n = 11/11 and 3/3 respectively; Fig. 8, A and B). As with dorsal root recordings, intraspinal DC-shifts were maintained following block of action potential-dependent synaptic transmission with TTX (n = 3; Fig. 8C) and were prevented or greatly attenuated by preapplication of d-TC (n = 5; Fig. 8D). The corresponding time course of DC-shifts in dorsal roots and intraspinally, including in the presence of TTX, suggests that nAChR-mediated intraspinal changes in DC polarization occur on primary afferent axons, although postsynaptic actions on spinal neurons containing nAChRs may also contribute.
Figure 8.
Correspondence between depolarizing direct current (DC)-shifts in roots to intraspinal DC changes and evoked responses. A1: ACh led to a depolarizing DC-shift in the dorsal root (DR; upward deflection) as well as intraspinally as recorded from the extracellular field potential (EFP) electrode within the deep dorsal horn (bottom; downward deflection; DC drift was removed by tilting the trace). Observed DC shifts led to a coincident loss of sural (5 T) stimulation-evoked dorsal root potentials (DRPs) and EFPs, seen as reduced deflections on top of DC recordings. Note that the recovery of evoked responses did not follow DC repolarization after wash. A2: averaged evoked responses before and near the end of ACh application show a near-complete loss of evoked responses. B: nicotine results in similar DC-shifts and depression of evoked responses. C: ACh-induced intraspinal DC-shift remains in the presence of tetrodotoxin (TTX). D: after neostigmine (neo), ACh led to a depolarization in the dorsal root as well as intraspinally (left) that was prevented following preincubation with d-TC (right). Responses are low-pass-filtered (0.2 Hz) to minimize concurrent evoked tibial nerve evoked responses. E: correspondence between ACh-evoked DC depolarizing shifts with depression of evoked responses. E1: ACh-evoked DC-shift in dorsal root recording (top) is redrawn and overlaid, with corresponding peak to trough normalized to changes in DRP amplitude and EFP area to allow time course comparison between these parameters. Time comparison shows that ACh-induced depression of evoked DRP and EFP follows depolarizing DC-shifts. Note also that DRP and EFP recovery begins during the period of DC repolarization (seen in continued presence of agonist) but does not return to pre-ACh application values. E2: ACh-evoked DC-shifts versus depression of EFPs (via peripheral nerve stimulation at 4 T). Population data report on percent depression of EFPs (± SE) at time points reflecting 25%, 50%, 75%, and 100% of the maximal DC shift (n = 11). Data are from P6 mice. d-TC, d-tubocurarine; neo, neostigmine.
nAChR-mediated depression seen in evoked DRPs and EFPs is temporally associated with depolarizing DC-shifts and includes propagation block of slower conducting afferents.
The observed depolarizing afferent DC-shifts corresponded with depression of DRPs and EFPs (Fig. 8A2). To determine whether depression of evoked responses was causally linked to DC-shifts in primary afferents, we compared their time course. The time course of the evoked EFP and DRP depression corresponded very well with the depolarizing phase of observed DC-shifts (Fig. 8E1). Overall, the magnitude of EFP depression corresponded particularly well with the early period of DC polarization (Fig. 8E2; n = 11). However, that loss of DC polarization presumably owing to nicotinic receptor desensitization is not mirrored in return of the evoked responses (Fig. 8E1), even for a period following drug wash (Fig. 8A1), suggesting that prolonged synaptic depression is not dependent on continued presence of agonist or DC polarization, and is instead owing to lasting changes in circuit function.
As suggested in Fig. 7B and 8E, nAChR-mediated depolarizing DC-shifts may lead to depolarization block of afferent spiking. A consequence of such a block could be a reduction in afferent recruitment via electrical stimulation. Therefore, we looked for changes in recruited afferent volleys (AVs). When the AV was recorded distal to the dorsal root stimulating electrode, ACh could reversibly depress recruitment of slower conducting afferents (Fig. 9A, n = 5). We then isolated root recordings from spinal cord to directly assess the actions of ACh on afferent recruitment. As shown ACh preferentially depresses slower-conducting AVs (Fig. 9B). To determine whether depressed recruitment was associated with observed DC polarization changes, we compared the time course of their changes (Fig. 9C1). The observed transient ACh-induced depolarizing DC-shifts corresponded closely to changes in AV recruitment (Fig. 9C2), linking afferent depolarization to block of fiber conduction.
Figure 9.
Depolarizing direct current (DC)-shifts in afferent fibers correspond with reduced electrical stimulation-based recruitment of primary afferents. A: ACh also depresses the afferent volley recorded in the tibial nerve distal to the nerve stimulation site (sciatic at 10 T). ACh leads to preferential reduction in C-fiber volley (shaded) and the complete loss of the later-arriving dorsal root reflexes (DRR) (left: horizontal line; 10 events superimposed). Averages of afferent volley responses seen after addition of ACh (with neostigmine) and following wash (right). B: the sciatic nerve was stimulated at 10 T, and recordings were made from the cut L4 dorsal root (DR). Shown are evoked volleys (10 events superimposed) in control and after ACh (with neostigmine). Corresponding rectified integrals are shown divided into earlier A-fiber and later C-fiber afferents. Note that later components of the afferent volley are preferentially reduced. C1: application of ACh subsequent to preincubation in neostigmine leads to a depolarizing DC-shift. The depolarization initially associates with increased spontaneous spiking, and subsequent loss of spiking (arrows) as depolarization increases. Note that ACh-induced polarization changes are transient. C2: normalized ACh-induced DC changes in (same mouse as C1) (blue; y-axis on left) correspond with normalized reduction in afferent volley area (red; y-axis on right; negativity upward). Mice in A–C are P6.
We examined the persistent depression of evoked responses associated with DC shifts in experiments that included at least 10 min of drug wash. Neostigmine/ACh-induced DC shifts led to the emergence or increase in spontaneous spiking during the DC depolarizing phase (n = 13/13). When EFP and DRP amplitude were examined between 10 and 20 min after washout (the duration of recordings), evoked responses began to recover but remained substantially depressed. Interestingly, in one of two experiments with nicotine application, the evoked DC shift was not associated with recruitment of spontaneous firing, and in this experiment, there was little depression in evoked responses during the DC shift and full recovery after wash.
Collectively, these experiments support the presence of extrasynaptic nAChRs on primary afferent axons. We interpret changes in spiking to be preferentially based on membrane depolarization in slower-conducting, predominantly C-fiber axons, with subsequent depolarization block leading to reductions in size of their afferent volley. Although clearly not conclusive, the long-lasting reduction in evoked responses following DC repolarization and drug washout may be linked to lasting depression of central circuits following spontaneous firing in C-fibers observed during axonal depolarization (21; see discussion).
Observed Actions Were Comparable between Mouse Strains, in Rat, and When Performed in Different Labs
As the sample included both FVB and BALB/c mouse strains, we compared whether prominent evoked modulatory actions on DRPs and EFPs differed between them (Table 3). In both strains the DRP was significantly reduced for ACh/neostigmine, nicotine, and d-TC. Responses were comparable and statistically indistinguishable for neostigmine, nicotine, and d-TC. In comparison BALB/c underwent significantly greater ACh/neostigmine DRP depression than FVB mice (P = 0.004; Mann–Whitney rank sum test). Effects on EFP were also comparable between strains including a preferential reduction in Aδ EFP amplitude with nicotine, and an exclusive reduction of this component with d-TC.
We also examined if comparable effects of ACh/neostigmine and d-TC were seen on DRP measures in C57BL/6 mice, as this is the predominant mouse strain for transgenic approaches. Both ACh/neostigmine and d-TC led to significant reductions in DRP area to 22% ± 9% and 44% ± 26%, respectively (for both P < 0.001; n = 7). These values are like those seen in the other combined mouse strains (Table 2; P = 0.7 and P = 0.3, respectively).
To assess whether nAChR-mediated actions were observed across rodent species, we compared observed prominent actions in the Sprague–Dawley rat. As in mouse, strong DRP depression was observed with ACh/neostigmine (7% ± 6% of control; n = 8; P < 0.001) and d-TC (46% ± 10% of control; n = 7, P < 0.001). d-TC also led to a preferential reduction of the Aδ EFPs (19% ± 18% of control; P < 0.001; n = 5). The Aβ EFP was very slightly though significantly reduced to 93% ± 5% of control values (P = 0.04). Together these results demonstrate that nicotinic receptor-mediated depressant actions are conserved across three mouse strains and in separate rodent species.
Last, interlaboratory differences on the effect of ACh/neostigmine on DRPs were compared in BALB/c mice and Sprague–Dawley rats. Substantial reductions were seen regardless of laboratory, being comparable for mouse at 15% ± 6% (n = 8) and 26% ± 23% (n = 7; P = 0.3) but different for rat at 14% ± 3% (n = 3) and 3% ± 2% (n = 5; P < 0.05).
DISCUSSION
Primary afferents express a diversity of ionotropic nAChRs whose activation should lead to depolarization. How nAChR activation is achieved, and whether resultant changes are linked to presynaptic inhibition, facilitation, or more complex signaling events including backpropagating dorsal root reflexes (DRRs) is currently unknown.
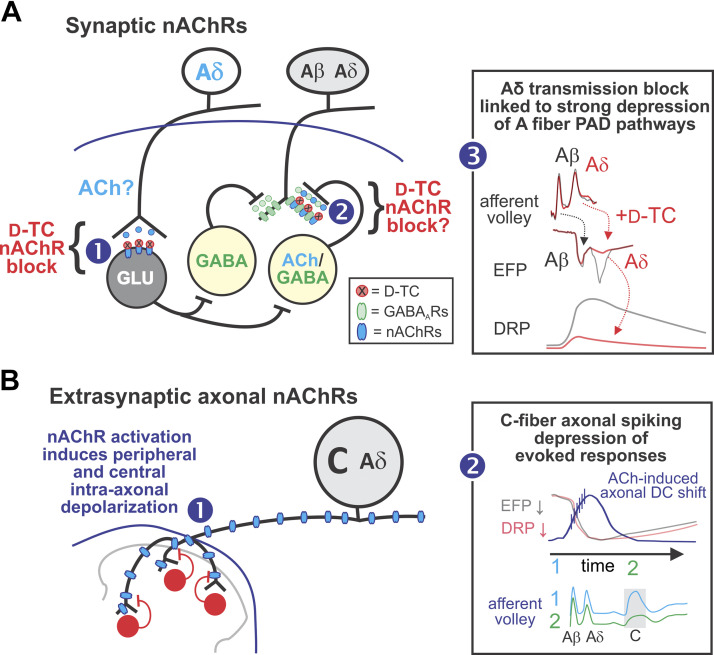
We tested the actions of nAChR ligands and focused on myelinated (Aβ and Aδ) afferent stimulation-evoked population synaptic responses in the deep dorsal horn (recorded as EFPs), primary afferent depolarization (recorded as DRPs), as well as changes in afferent axonal membrane potential (recorded as DC-shifts in population potentials). Overall, results suggest that nAChRs modulate afferent excitability by at least two differentiable mechanisms (summarized in Fig. 10). The first involves depression of Aδ afferents monosynaptic transmission via block of nAChRs, thereby implicating afferent release of ACh or related transmitter to generate synaptic responses (Fig. 10A). The second involves the capacity for axonal extrasynaptic afferent nAChRs to generate depolarizing shifts of sufficient magnitude to initiate spontaneous spiking and long-lasting block of evoked responses. Observed loss of spiking with further depolarization is interpreted to indicate subsequent depolarization block. That conduction block is seen in the predominantly C component of the afferent volley suggests that the observed spontaneous activity occurred preferentially in slower-conducting afferents (Fig. 10B). These two areas are discussed separately below in sections titled Selective nAChR Modulation of Aδ-Evoked Actions and Broad Depression of Afferent Evoked Responses via Activation of Extrasynaptic nAChRs, respectively.
Figure 10.
Summary of primary results and putative mechanisms responsible for synaptic (A) and extrasynaptic (B) nicotinic acetylcholine receptors (nAChR)-mediated actions on afferent signaling. A: nAChR antagonist depression on Aδ afferent synaptic transmission and dorsal root potentials (DRPs). Selective depression of monosynaptic Aδ extracellular field potential (EFPs) with nAChR antagonist d-TC supporting direct actions at this synapse including afferent release of ACh or related agonist acting on postsynaptic nAChRs. As selective depression of Aδ afferents monosynaptic actions is associated with a large reduction in the DRP, these first-order interneurons may act directly on the interneurons mediating primary afferent depolarization (PAD) [1]. Nicotinic AChR antagonists may also reduce PAD via actions on last-order axo-axonic synapses originating from lamina III cholinergic/GABAergic interneurons, but this contribution would be small owing to the well-established prominent contribution of GABAARs in A-fiber-evoked PAD [2]. Summary of observed actions relating selective d-TC block of Aδ afferent transmission to a strong depression of A-fiber-evoked DRP (PAD pathways). B: extrasynaptic axonal nAChR-mediated actions on afferent signaling. Pharmacological extrasynaptic activation of nAChRs generates axonal depolarizing DC shifts both peripherally and intraspinally [1] . Summary of proposed interacting events linking direct current (DC) shifts to loss of evoked responses [2]. The rising phase of the depolarizing DC shift is associated with spontaneous activity in peripheral axons. A prior study demonstrating nAChR-mediated excitability increases in unmyelinated C-fibers (21) is consistent this observation. Subsequent loss of spiking during increasing axonal depolarization is consistent with depolarization block causing conduction block. Conduction block is seen preferentially in slower-conducting axons (Aδ- and C-fibers), particularly in high-threshold C-fibers. nAChR-mediated axonal depolarization coincides with strong depression of Aβ and Aδ EFPs and DRPs. Although the loss of evoked responses corresponds to the rising depolarizing DC shift and observed axonal spiking, depression of evoked responses is long-lasting and does not recover with DC repolarization. d-TC, d-tubocurarine.
Selective nAChR Modulation of Aδ-Evoked Actions
Our major controversial observations are that a population of Aδ afferents use cholinergic transmission and act on a population of first-order interneurons, and that Aδ rather than Aβ afferents are the prominent driver of low-threshold afferent-evoked PAD. Observations of a role for nAChRs in primary afferent depolarization (PAD) is contrary to a recent study that failed to observe such actions (5). Using a similar experimental approach, (5) explored PAD mechanisms in the thoracic cord in P14–18 mice, with focus on activation of genetically identified hairy skin afferents. The present study focused on the actions of hindlimb afferents innervating both hairy and glabrous skin in the lumbar spinal cord of P5–12 mice. Aside from age and location, observed differences may relate to alteration in afferents and circuit excitability associated with age-related injury response differences activated during surgical dissection, as sensory circuits are well known to encode various behavioral pain states that are impacted by nicotinic receptors (65, 66). In the present work, nAChR modulation was found to be broadly similar across three mouse strains, between mice and rats, and between laboratories of the corresponding authors.
Population synaptic responses (recorded as EFPs) were monitored in the deep dorsal horn at the depth where the largest signals were elicited by cutaneous or mixed cutaneous/muscle nerve stimulation. When monosynaptic responses were separable into Aβ- and slower-conducting Aδ-fibers, the broad nAChR antagonist d-TC preferentially blocked Aδ afferent-evoked monosynaptic transmission. The selective depression of Aδ afferent-evoked monosynaptic responses suggests that Aδ fibers release an nAChR agonist (e.g., ACh, choline). Several observations indirectly support this possibility: 1) Some primary afferents express the vesicular glutamate transporter, vGluT3 (67), which has been associated with ACh corelease in central nervous system (CNS) neurons (68). 2) Afferent release of ACh has been postulated based on variant immunostaining evidence of ChAT and pChAT in both rat and mouse DRG (37, 39). Although pChAT-dependent ACh synthesis may be compromised owing to its lack of a catalytic region (69), a pChAT-based ACh synthesis has nevertheless been clearly shown (37). 3) The ionotropic glutamate receptor antagonist CNQX did not fully block Aδ-evoked synaptic actions (70). 4) Primary afferents express choline transporters (16, 36) and the released choline may act on postsynaptic choline-sensitive α7 receptors.
Selective nAChR antagonist depression of deep dorsal horn Aδ-evoked EFPs corresponded temporally with strong depression of A- but not C-fiber DRPs. If causally linked, Aδ afferents may contribute significantly to the A-fiber pathways initiating PAD via actions on these first-order deep dorsal horn interneurons (70). Such a viewpoint is contrary to the conventional assumption that A-fiber-evoked DRPs are primarily Aβ-mediated (71, 72), although the relative recruitment of Aβ and Aδ afferents by electrical stimulation has not been well established. In the cat, stimulation-evoked cutaneous DRPs contain two components, with maximal values at 2–4 T and 6–7 T (73). The latter component is unmasked after spinalization and has longer latency and a much broader spinal distribution. In the adult rat, Aδ fibers can be recruited at 1.1 times the recruitment threshold for Aβ afferents, with observed DRPs only partly blocked by GABAA receptor antagonists and unaffected by drugs that potentiate GABAA receptors (barbiturates and benzodiazepines) (70).
Although depressed DRPs were temporally associated with depression of Aδ afferent transmission, block of nAChRs at additional synaptic sites may contribute. Specifically, lamina III cholinergic interneurons project axo-axonic synapses onto primary afferents (22–24) and likely includes Aδ fibers responsible for generating the DRPs. Aδ rapidly adapting (d-hair) mechanoreceptors undergo PAD following cutaneous mechanical stimulation (74) and DRRs have been recorded from Aδ fibers previously (75–77). Lamina III cholinergic interneurons coexpress GABA (23, 29) and ∼25% of presynaptic GABAergic axo-axonic connections onto primary afferents co-express ACh (27–29). Thus, observed nAChR actions might serve to provide modulatory control of presynaptic actions based on the relative strength of coreleased GABA and ACh. Cation-conducting nAChRs have more depolarized reversal potentials (∼0 mV) (78, 79) than anion-conducting GABAA receptors (approximately −30 mV) (80), so activation of nAChRs may better drive suprathreshold depolarizations that produce DRRs. Because DRRs travel both orthodromically and antidromically (81), nAChR-mediated afferent depolarization may play a special role in response modulation (82) including in nAChR-mediated facilitation of antidromic spike propagation (3) for control of peripheral vasodilatation and plasma extravasation in neurogenic inflammation (83, 84).
However, it is unlikely that last-order axoaxonic cholinergic synapses from lamina III cholinergic/GABAergic interneurons can account for the observed DRP depression with d-TC. Other GABAergic interneurons are known to be responsible for the A-fiber DRPs, and these DRPs are predominantly blocked by GABAAR antagonists (2, 4, 5) (however, see 19, 20).
Broad Depression of Afferent Evoked Responses via Activation of Extrasynaptic nAChRs
We also assessed whether nAChRs tonically modulate responses by increasing their activation with agonists and cholinesterase inhibition. d-TC and mecamylamine-sensitive nAChR activation produced depolarizing (negative) DC-shifts of primary afferents. Peripheral nAChR activation-induced DC polarization remained in the dorsal roots after isolation from spinal cord, demonstrating direct extrasynaptic axonal activation of nAChRs. Matching depolarizations were recorded intraspinally in the dorsal horn, and both spinal and peripheral actions were TTX-insensitive. We assume matching central actions are also primarily owing to extrasynaptic nAChR activation on intraspinal afferent axons but additional actions on other central neurons containing nAChRs likely contribute and include the targets of dorsal horn cholinergic interneurons as well as descending cholinergic projections from ventrolateral medulla (23, 25).
Although the time course and magnitude of DC depolarizations coincided with observed depression of A-fiber-evoked responses (EFPs and DRPs), afferent derecruitment via spike conduction block unlikely contributes to the depressant actions seen, as afferent volley conduction block was preferential to slower-conducting afferents (Fig. 9). Observations of applied ACh first increasing then subsequently blocking spontaneous spiking is consistent with depolarizing DC-shifts leading to spike recruitment and then depolarization block that links to conduction block of slower-conducting afferents. That nAChR activation could either decrease or increase afferent spiking in individually spike-sorted axons likely depends on the extent of axonal depolarization produced. Increases in afferent firing predominated (Fig. 7). Time-dependent reductions in DC polarization observed in the continued presence of nAChR agonists are likely due to receptor desensitization (12, 85). That reduction in measured DC polarization did not lead to a similar time course of recovery in evoked responses (Fig. 8) suggests that repolarization or associated recovery from conduction block is not causally linked to the recovery of synaptic transmission. A prior study demonstrated nAChR-mediated excitability increases in unmyelinated C-fibers (21). This is consistent with our observation of emergent spontaneous activity coinciding with an axonal depolarizing DC shift. It thus seems more likely that DC shifts lead to a temporary activation of high threshold fibers that lead to long-lasting depression in A-fiber-evoked responses.
The existence of TTX-insensitive extrasynaptic nAChRs afferent suggesting a role in modulation of sensory signaling. Previous studies on GABAARs support a role in the control of conduction (4, 47, 86, 87). Labrakakis et al. (4) observed depression of Aδ- and C-fiber volleys in dorsal roots following activation of GABAARs or P2X receptors. Regarding P2X receptors, one suggested possibility is that release of neural/glial ATP following injury may act on axonal P2X receptors to modulate spiking (4).
The source(s) of released endogenous nAChR agonists (e.g., ACh and choline) acting along afferent axons is currently unknown. Tonic ACh release from descending projections and spinal interneurons may modulate intraspinal afferents axons. As ChAT/pChAT is expressed in many primary afferents (23, 37, 39) and pChAT does not appear to be found at synaptic terminals (18), synthesized ACh may modulate central and peripheral afferent excitability via nonvesicular release (42). Interestingly, cholinergic signaling in the brain and body constitutes a complexly controlled homeostatic regulatory network, where circulatory ACh levels may serve to globally modulate physiological state, including sensory gain (88, 89). Where access to circulation exists (90), nonneuronal ACh synthesis in vascular endothelial cells may interact with soluble ChAT in plasma to adjust ACh levels that act on primary afferents in a paracrine fashion (89, 91). For example, partial sciatic nerve lesion leads to chronic distal disruption of the blood-nerve barrier (92) and activated T-cells release ACh into the circulation to modulate afferent transmission during inflammatory pain states (93).
Broad Similarity of Effects Observed across Species and Strain
It is increasingly apparent that known species- and strain-related differences in circuit modulation, plasticity, and response to injury have greatly limited the translational potential of preclinical studies, with variability in pain modulation being particularly well-documented. Studies that include examination across species/strains provide greater insight into whether observed mechanisms reflect broadly applicable principles of neural circuit function (94–97). We examined nAChR actions in rat and several strains of mice. Although not all tests were undertaken in all strains, when compared across populations, strong support for the similarity of responses was seen.
Diversity of Primary Afferent nAChRs and Their Receptor Pharmacology
nAChR subunit expression diversity in rat and mouse primary afferents is broad (α2–7,9,10, β2–4) (12, 14, 16, 98) supporting the premise that nAChR modulation of afferents is a widespread feature of afferent excitability control. Nicotinic α7 receptors are predominantly, but not exclusively, expressed in myelinated and unmyelinated CGRP+ fibers and transported both centrally and peripherally (99). nAChR-containing nociceptors are divisible into three populations: α7, α3β4α5, and a population containing both α7 and α3β4α5 receptors. All Aδ nociceptors are thought to contain nAChRs of different composition including α3β4α5 or α3β4 nAChR subtypes, whereas unmyelinated C-fiber nociceptors express α7 nAChRs (16, 100, 101). Taken together, nicotine-evoked α7 and α3β4-like nAChR responses are found in ∼70% of all cutaneous DRG neurons (102), and the majority of visceral afferents also likely contain nAChRs (103). Notably, although ∼70%–80% of rat DRG neurons expressed functional nAChRs, only ∼15%–30% of DRG neurons expressed functional nAChRs in mouse (104).
nAChR receptor pharmacology is outlined in Table 1 (61) and is helpful in identifying α subunits implicated in the modulation of DC-shifts and evoked responses. As primary nAChR antagonist results involve the use of d-TC, a broad nAChR antagonist, it is first worth considering possible actions elsewhere. Specifically, d-TC is also a potent antagonist of 5-HT3 receptors (105, 106) and may also block GABAA receptors, albeit at higher doses than currently used (107–109). d-TC block of GABAA or 5-HT3 receptors would not explain observed depression of monosynaptic transmission (EFPs). Given d-TC's prominent synaptic depressant actions among nAChR antagonists, we cannot exclude the possibility of actions at a currently unidentified binding site that may preferentially depress Aδ transmission (e.g., α1a adrenoceptors; 16, 115). Further, the nAChR antagonist mecamylamine also depressed DRPs (albeit less so) and is without reported actions at GABAA or 5-HT3 receptors (61). Although GABAARs are expressed along primary afferent axons (87, 110), we showed that GABAAR-mediated DC shifts are picrotoxin-sensitive/d-TC-insensitive, whereas nAChR-mediated DC shifts are d-TC-sensitive/picrotoxin-insensitive. Although 5HT3 receptors are also expressed in primary afferents and can generate PAD (111, 112), we previously showed that 5-HT does not alter afferent excitability (49), so d-TC block of 5HT3 receptors would not account for d-TC block of nAChR-induced depolarizing DC-shifts.
As elaborated below in the sections titled Evoked responses and DC shifts, overall results support a role for α3β4, α3β2, α6, and possibly α7 nAChRs in the observed modulation of afferent actions.
Evoked responses.
MLA did not alter evoked DRPs. As MLA is a high-affinity α7 and α9 nAChR antagonist, it would argue against their involvement in modulating evoked responses. Mecamylamine weakly depressed DRPs likely via actions at α3β4, but at the dose used (predominantly 50–100 µM), actions may also or instead be via α3β2 and/or α3α6 chimeric nAChRs (61, 114). In comparison, d-TC strongly depressed DRPs with IC50 values (5.2–7.3 µM), which, given the observations above, is most consistent with actions at α3β2 nAChRs.
DC shifts.
The nAChR agonists ACh, epibatidine, and nicotine produced depolarizing DC-shifts of primary afferents, whereas the nAChR antagonists d-TC and mecamylamine depressed these shifts. α9-Containing nAChRs are not required for DC-shifts, as nicotine and epibatidine act as antagonists at these receptors (59), and direct evidence for functional activation of α9-containing nAChRs in spinal primary afferents remains to be seen (14, 65, 113). Depression of DC-shifts by d-TC and mecamylamine is consistent with actions as α3, α6, and α7-containing nAChRs (59, 61).
GRANTS
This work was supported by CONACyT 59873 (to J.Q.), Mexico (http://www.conacyt.gob.mx), and National Institutes of Health (NIH)NS-065949 (to S.H.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S., J.Q., and S.H. conceived and designed research; J.S., M.H., E.M.-A., D.L.G.-R, J.Q., and S.H. performed experiments; J.S., M.H., E.M.-A., D.L.G.-R, J.Q., and S.H. analyzed data; J.S., M.H., E.M.-A., D.L.G.-R, J.Q., and S.H. interpreted results of experiments; J.S., M.H., D.L.G.-R, J.Q., and S.H. prepared figures; J.S., J.Q., and S.H. drafted manuscript; J.S., M.H., J.Q., and S.H. edited and revised manuscript; J.S., M.H., E.M.-A., D.L.G.-R, J.Q., and S.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brannan O’Neill for help with some of the experiments and Manuel Denton for help with spike sorting and the very helpful reviewer comments.
REFERENCES
- 1.Fink AJ, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 509: 43–48, 2014. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res 129: 1–37, 1999. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- 3.Lucas-Osma AM, Li Y, Lin S, Black S, Singla R, Fouad K, Fenrich KK, Bennett DJ. Extrasynaptic ∝5GABAA receptors on proprioceptive afferents produce a tonic depolarization that modulates sodium channel function in the rat spinal cord. J Neurophysiol 120: 2953–2974, 2018. doi: 10.1152/jn.00499.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labrakakis C, Tong C‐K, Weissman T, Torsney C, MacDermott AB. AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol. 549: 131–142, 2003. doi: 10.1113/jphysiol.2002.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman AL, Kovatsis EM, Pozsgai RY, Tasnim A, Zhang Q, Ginty DD. Distinct modes of presynaptic inhibition of cutaneous afferents and their functions in behavior. Neuron 102: 420–434, 2019. doi: 10.1016/j.neuron.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci 24: 2774–2781, 2004. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron 35: 135–146, 2002. doi: 10.1016/S0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Garcia JA, King AE. Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. Eur J Neuroscie 8: 2188–2197, 1996. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 9.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019, 2002. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albers KM, Zhang XL, Diges CM, Schwartz ES, Yang CI, Davis BM, Gold MS. Artemin growth factor increases nicotinic cholinergic receptor subunit expression and activity in nociceptive sensory neurons. Mol Pain 10: 31, 2014. doi: 10.1186/1744-8069-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd RT, Jacob MH, McEachern AE, Caron S, Berg DK. Nicotinic acetylcholine receptor mRNA in dorsal root ganglion neurons. J Neurobiol 22: 1–14, 1991. doi: 10.1002/neu.480220102. [DOI] [PubMed] [Google Scholar]
- 12.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol 86: 1773–1782, 2001. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 13.Haberberger RV, Henrich M, Lips KS, Kummer W. Nicotinic receptor alpha 7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem Cell Biol 120: 173–181, 2003. doi: 10.1007/s00418-003-0550-3. [DOI] [PubMed] [Google Scholar]
- 14.Lips KS, Pfeil U, Kummer W. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience 115: 1–5, 2002. doi: 10.1016/S0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh JM, Plazas PV, Watkins M, Gomez-Casati ME, Olivera BM, Elgoyhen AB. A novel alpha-conotoxin, PeIA, cloned from Conus pergrandis, discriminates between rat alpha9alpha10 and alpha7 nicotinic cholinergic receptors. J Biol Chem 280: 30107–30112, 2005. doi: 10.1074/jbc.M504102200. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP, Ginty DD. Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron 103: 598–616.e7, 2019. doi: 10.1016/j.neuron.2019.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demuro A, Palma E, Eusebi F, Miledi R. Inhibition of nicotinic acetylcholine receptors by bicuculline. Neuropharmacology 41: 854–861, 2001. doi: 10.1016/S0028-3908(01)00137-X. [DOI] [PubMed] [Google Scholar]
- 18.Hochman S, Shreckengost J, Kimura H, Quevedo J. Presynaptic inhibition of primary afferents by depolarization: observations supporting nontraditional mechanisms. Ann N Y Acad Sci 1198: 140–152, 2010. doi: 10.1111/j.1749-6632.2010.05436.x. [DOI] [PubMed] [Google Scholar]
- 19.Wall PD. Control of impulse conduction in long range branches of afferents by increases and decreases of primary afferent depolarization in the rat. Eur J Neurosci 6: 1136–1142, 1994. doi: 10.1111/j.1460-9568.1994.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SW, Wall PD. The effect of GABA and 5-HT receptor antagonists on rat dorsal root potentials. Neuroscience Lett 217: 153–156, 1996. doi: 10.1016/0304-3940(96)13097-4. [DOI] [PubMed] [Google Scholar]
- 21.Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. J Neurophysiol 90: 3295–3303, 2003. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- 22.Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol 229: 329–346, 1984. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- 23.Mesnage B, Gaillard S, Godin AG, Rodeau JL, Hammer M, Von Engelhardt J, Wiseman PW, De Koninck Y, Schlichter R, Cordero-Erausquin M. Morphological and functional characterization of cholinergic interneurons in the dorsal horn of the mouse spinal cord. J Comp Neurol 519: 3139–3158, 2011. doi: 10.3110.1002/cne.22668. [DOI] [PubMed] [Google Scholar]
- 24.Olave MJ, Puri N, Kerr R, Maxwell DJ. Myelinated and unmyelinated primary afferent axons form contacts with cholinergic interneurons in the spinal dorsal horn. Exp Brain Res 145: 448–456, 2002. doi: 10.1007/s00221-002-1142-5. [DOI] [PubMed] [Google Scholar]
- 25.Stornetta RL, Macon CJ, Nguyen TM, Coates MB, Guyenet PG. Cholinergic neurons in the mouse rostral ventrolateral medulla target sensory afferent areas. Brain Struct Funct 218: 455–475, 2013. doi: 10.1007/s00429-012-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eccles JC, Kostyuk PG, Schmidt RF. Central pathways responsible for depolarization of primary afferent fibres. J Physiol 161: 237–257, 1962. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phelps PE, Barber RP, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. Postnatal development of neurons containing choline acetyltransferase in rat spinal cord: an immunocytochemical study. J Comp Neurol 229: 347–361, 1984. doi: 10.1002/cne.902290306. [DOI] [PubMed] [Google Scholar]
- 28.Ribeiro-da-Silva A, Cuello AC. Choline acetyltransferase-immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. J Comp Neurol 295: 370–384, 1990. doi: 10.1002/cne.902950303. [DOI] [PubMed] [Google Scholar]
- 29.Todd AJ. Immunohistochemical evidence that acetylcholine and glycine exist in different populations of GABAergic neurons in lamina III of rat spinal dorsal horn. Neuroscience 44: 741–746, 1991. doi: 10.1016/0306-4522(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 30.Willis WD Jr, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press, 1991. [Google Scholar]
- 31.Boyle KA, Gradwell MA, Yasaka T, Dickie AC, Polgár E, Ganley RP, Orr DPH, Watanabe M, Abraira VE, Kuehn ED, Zimmerman AL, Ginty DD, Callister RJ, Graham BA, Hughes DI. Defining a spinal microcircuit that gates myelinated afferent input: implications for tactile allodynia. Cell Rep 28: 526–540.e26, 2019. doi: 10.1016/j.celrep.2019.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escalante A, Klein R. Spinal inhibitory Ptf1a-derived neurons prevent self-generated itch. Cell Rep 33: 108422, 2020. doi: 10.1016/j.celrep.2020.108422. [DOI] [PubMed] [Google Scholar]
- 33.Hughes DI, Sikander S, Kinnon CM, Boyle KA, Watanabe M, Callister RJ, Ba G. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J Physiol 590: 3927–3951, 2012. doi: 10.1113/jphysiol.2012.235655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jankowska E, McCrea D, Rudomin P, Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol 46: 506–516, 1981. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- 35.Ninkovic M, Hunt SP. Alpha-bungarotoxin binding sites on sensory neurones and their axonal transport in sensory afferents. Brain Res 272: 57–69, 1983. doi: 10.1016/0006-8993(83)90364-5. [DOI] [PubMed] [Google Scholar]
- 36.Traiffort E, Ruat M, O'Regan S, Meunier FM. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J Neurochem 92: 1116–1125, 2005. doi: 10.1111/j.1471-4159.2004.02962.x. [DOI] [PubMed] [Google Scholar]
- 37.Bellier JP, Kimura H. Acetylcholine synthesis by choline acetyltransferase of a peripheral type as demonstrated in adult rat dorsal root ganglion. J Neurochem 101: 1607–1618, 2007. doi: 10.1111/j.1471-4159.2007.04458.x. [DOI] [PubMed] [Google Scholar]
- 38.Hanada K, Kishimoto S, Bellier JP, Kimura H. Peripheral choline acetyltransferase in rat skin demonstrated by immunohistochemistry. Cell Tissue Res 351: 497–510, 2013. doi: 10.1007/s00441-012-1536-z. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Mol Pain 3: 41, 2007. doi: 10.1186/1744-8069-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dussor GO, Jones DJ, Hulsebosch CE, Edell TA, Flores CM. The effects of chemical or surgical deafferentation on [3H]-acetylcholine release from rat spinal cord. Neuroscience 135: 1269–1276, 2005. doi: 10.1016/j.neuroscience.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 41.Tata AM, De Stefano ME, Srubek Tomassy G, Vilaro MT, Levey AI, Biagioni S. Subpopulations of rat dorsal root ganglion neurons express active vesicular acetylcholine transporter. J Neurosci Res 75: 194–202, 2004. doi: 10.1002/jnr.10855. [DOI] [PubMed] [Google Scholar]
- 42.Abramochkin DV, Nurullin LF, Borodinova AA, Tarasova NV, Sukhova GS, Nikolsky EE, Rosenshtraukh LV. Non-quantal release of acetylcholine from parasympathetic nerve terminals in the right atrium of rats. Exp Physiol 95: 265–273, 2010. doi: 10.1113/expphysiol.2009.050302. [DOI] [PubMed] [Google Scholar]
- 43.Cattaert D, Manira E. A. Shunting versus inactivation: analysis of presynaptic inhibitory mechanisms in primary afferents of the crayfish. J Neurosci 19: 6079–6089, 1999. doi: 10.1523/JNEUROSCI.19-14-06079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarac F, el Manira A, Cattaert D. Presynaptic control as a mechanism of sensory-motor integration. Curr Opin Neurobiol 2: 764–769, 1992. doi: 10.1016/0959-4388(92)90131-4. [DOI] [PubMed] [Google Scholar]
- 45.Smith DO. Morphological aspects of the safety factor for action potential propagation at axon branch points in the crayfish. J Physiol 301: 261–269, 1980. doi: 10.1113/jphysiol.1980.sp013203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy RA. Presynaptic control of input to the central nervous system. Can J Physiol Pharmacol 58: 751–766, 1980. doi: 10.1139/y80-119. [DOI] [Google Scholar]
- 47.Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci 23: 2002–2007, 2003. doi: 10.1523/JNEUROSCI.23-06-02002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wall PD, McMahon SB. Long range afferents in rat spinal cord. III. Failure of impulse transmission in axons and relief of the failure after rhizotomy of dorsal roots. Philoso Trans R Soc Lond B Biol Sci 343: 211–223, 1994. doi: 10.1098/rstb.1994.0022. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Ramirez DL, Calvo JR, Hochman S, Quevedo JN. Serotonin, dopamine and noradrenaline adjust actions of myelinated afferents via modulation of presynaptic inhibition in the mouse spinal cord. PloS One 9: e89999, 2014. doi: 10.1371/journal.pone.0089999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shreckengost J, Calvo J, Quevedo J, Hochman S. Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord. J Neurosci 30: 5283–5288, 2010. doi: 10.1523/JNEUROSCI.3873-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greene E. Anatomy of The Rat. Philadelphia, PA: The American Philosophical Society, 1935, pp. xi, 370. [Google Scholar]
- 52.Vejsada R, Paleček J, Hník P, Soukup T. Postnatal development of conduction velocity and fibre size in the rat tibial nerve. Int J Deve Neurosci 3: 583–595, 1985. doi: 10.1016/0736-5748(85)90048-6. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Ramirez DL, Calvo JR, Hochman S, Quevedo JN. Serotonin reduces homosynaptic depression by decreasing synaptic efficacy of low threshold afferents in the In vitro mouse spinal cord. In: Society for Neuroscience 2013. San Diego, CA, November 9–13, 2013. [Google Scholar]
- 54.Fitzgerald M. Cutaneous primary afferent properties in the hind limb of the neonatal rat. J Physiol 383: 79–92, 1987. doi: 10.1113/jphysiol.1987.sp016397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lev-Tov A, Pinco M. In vitro studies of prolonged synaptic depression in the neonatal rat spinal cord. J Physiol 447: 149–169, 1992. doi: 10.1113/jphysiol.1992.sp018996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farkas S, Tarnawa I, Berzsenyi P. Effects of some centrally acting muscle relaxants on spinal root potentials: a comparative study. Neuropharmacology 28: 161–173, 1989. doi: 10.1016/0028-3908(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 57.Tata AM, Vilaro MT, Mengod G. Muscarinic receptor subtypes expression in rat and chick dorsal root ganglia. Brain Res Mol Brain Res 82: 1–10, 2000. doi: 10.1016/S0169-328X(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 58.Wanke E, Bianchi L, Mantegazza M, Guatteo E, Mancinelli E, Ferroni A. Muscarinic regulation of Ca2.+ currents in rat sensory neurons: channel and receptor types, dose-response relationships and cross-talk pathways. Eur J Neurosci 6: 381–391, 1994. doi: 10.1111/j.1460-9568.1994.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 59.Verbitsky M, Rothlin CV, Katz E, Elgoyhen AB. Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology 39: 2515–2524, 2000. doi: 10.1016/S0028-3908(00)00124-6. [DOI] [PubMed] [Google Scholar]
- 60.Burgen AS. The mechanism of action of anticholinesterase drugs. Br J Pharmacol Chemother 4: 219–228, 1949. doi: 10.1111/j.1476-5381.1949.tb00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, Collaborators C. The concise guide to pharmacology 2019/20: ion channels. Br J Pharmacol 176 : S142–S228, 2019. doi: 10.1111/bph.14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dani JA. Neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. Int Rev Neurobiol 124: 3–19, 2015. doi: 10.1016/bs.irn.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston GA. GABAA receptor pharmacology. Pharmacol Ther 69: 173–198, 1996. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 64.Erkkila BE, Weiss DS, Ve W. Picrotoxin-mediated antagonism of alpha3beta4 and alpha7 acetylcholine receptors. Neuroreport 15: 1969–1973, 2004. doi: 10.1097/00001756-200408260-00027. [DOI] [PubMed] [Google Scholar]
- 65.Naser PV, Kuner R. Cellular and circuit basis of cholinergic modulation of pain. Neuroscience 387: 135–148, 2018. doi: 10.1016/j.neuroscience.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Res 953: 53–62, 2002. doi: 10.1016/S0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- 67.Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462: 651–655, 2009. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68..Gras C, Amilhon B, Lepicard EM, Poirel O, Vinatier J, Herbin M, Dumas S, Tzavara ET, Wade MR, Nomikos GG, Hanoun N, Saurini F, Kemel ML, Gasnier B, Giros B, El Mestikawy S. The vesicular glutamate transporter VGLUT3 synergizes striatal acetylcholine tone. Nat Neurosci 11: 292–300, 2008. doi: 10.1038/nn2052. [DOI] [PubMed] [Google Scholar]
- 69.Govindasamy L, Pedersen B, Lian W, Kukar T, Gu Y, Jin S, Agbandje-McKenna M, Wu D, McKenna R. Structural insights and functional implications of choline acetyltransferase. J Struct Biol 148: 226–235, 2004. doi: 10.1016/j.jsb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu T, Yoshimura M, Baba H, Shimoji K, Higashi H. Role of A delta afferent fibers in modulation of primary afferent input to the adult rat spinal cord. Brain Res 691: 92–98, 1995. doi: 10.1016/0006-8993(95)00619-2. [DOI] [PubMed] [Google Scholar]
- 71.Janig W, Schmidt RF, Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res 6: 100–115, 1968. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- 72.Janig W, Schmidt RF, Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res 6: 116–129, 1968. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter D, Engberg I, Funkenstein H, Lundberg A. Decerebrate control of reflexes to primary afferents. Acta Physiol Scand 59: 424–437, 1963. doi: 10.1111/j.1748-1716.1963.tb02758.x. [DOI] [PubMed] [Google Scholar]
- 74.Whitehorn D, Burgess PR. Changes in polarization of central branches of myelinated mechanoreceptor and nociceptor fibers during noxious and innocuous stimulation of the skin. J Neurophysiol 36: 226–237, 1973. doi: 10.1152/jn.1973.36.2.226. [DOI] [PubMed] [Google Scholar]
- 75.Brooks CM, Koizumi K. Origin of the dorsal root reflex. J Neurophysiol 19: 61–74, 1956. doi: 10.1152/jn.1956.19.1.61. [DOI] [PubMed] [Google Scholar]
- 76.Casey KL, Oakley B. Intraspinal latency, cutaneous fiber composition, and afferent control of the dorsal root reflex in cat. Brain Res 47: 353–369, 1972. doi: 10.1016/0006-8993(72)90645-2. [DOI] [PubMed] [Google Scholar]
- 77.Toennies JF. Reflex discharge from the spinal cord over the dorsal roots. J Neurophysiol 1: 378–390, 1938. doi: 10.1152/jn.1938.1.4.378. [DOI] [Google Scholar]
- 78.Adams PR. An analysis of the dose-response curve at voltage-clamped frog-endplates. Pflugers Arch 360: 145–153, 1975. doi: 10.1007/BF00580537. [DOI] [PubMed] [Google Scholar]
- 79.Ballivet M, Nef P, Couturier S, Rungger D, Bader CR, Bertrand D, Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron 1: 847–852, 1988. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- 80.Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl− cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J Neurophysiol 100: 169–184, 2008. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bos R, Brocard F, Vinay L. Primary afferent terminals acting as excitatory interneurons contribute to spontaneous motor activities in the immature spinal cord. J Neurosci 31: 10184–10188, 2011. doi: 10.1523/JNEUROSCI.0068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rees H, Sluka KA, Lu Y, Westlund KN, Willis WD. Dorsal root reflexes in articular afferents occur bilaterally in a chronic model of arthritis in rats. J Neurophysiol 76: 4190–4193, 1996. doi: 10.1152/jn.1996.76.6.4190. [DOI] [PubMed] [Google Scholar]
- 83.Lawand NB, Lu Y, Westlund KN. Nicotinic cholinergic receptors: potential targets for inflammatory pain relief. Pain 80: 291–299, 1999. doi: 10.1016/S0304-3959(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 84.Lobanov OV, Peng YB. Differential contribution of electrically evoked dorsal root reflexes to peripheral vasodilatation and plasma extravasation. J Neuroinflammation 8: 20, 2011. doi: 10.1186/1742-2094-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci 17: 5747–5759, 1997. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhisitkul RB, Villa JE, Kocsis JD. Axonal GABA receptors are selectively present on normal and regenerated sensory fibers in rat peripheral nerve. Exp Brain Res 66: 659–663, 1987. doi: 10.1007/BF00270698. [DOI] [PubMed] [Google Scholar]
- 87.Sakatani K, Chesler M, Hassan AZ, Lee M, Young W. Non-synaptic modulation of dorsal column conduction by endogenous GABA in neonatal rat spinal cord. Brain Res 622: 43–50, 1993. doi: 10.1016/0006-8993(93)90799-S. [DOI] [PubMed] [Google Scholar]
- 88.Soreq H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci 38: 448–458, 2015. doi: 10.1016/j.tins.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Vijayaraghavan S, Karami A, Aeinehband S, Behbahani H, Grandien A, Nilsson B, Ekdahl KN, Lindblom RP, Piehl F, Darreh-Shori T. Regulated extracellular choline acetyltransferase activity-the plausible missing link of the distant action of acetylcholine in the cholinergic anti-inflammatory pathway. PloS One 8: e65936, 2013. doi: 10.1371/journal.pone.0065936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hirakawa H, Okajima S, Nagaoka T, Kubo T, Takamatsu T, Oyamada M. Regional differences in blood-nerve barrier function and tight-junction protein expression within the rat dorsal root ganglion. Neuroreport 15: 405–408, 2004. doi: 10.1097/00001756-200403010-00004. [DOI] [PubMed] [Google Scholar]
- 91.Kawashima K, Fujii T. Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci 106: 167–173, 2008. doi: 10.1254/jphs.FM0070073. [DOI] [PubMed] [Google Scholar]
- 92.Lim TK, Shi XQ, Martin HC, Huang H, Luheshi G, Rivest S, Zhang J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 155: 954–967, 2014. doi: 10.1016/j.pain.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 93.Kawashima K, Fujii T, Moriwaki Y, Misawa H, Horiguchi K. Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Ann N Y Acad Sci 1261: 7–17, 2012. doi: 10.1111/j.1749-6632.2012.06516.x. [DOI] [PubMed] [Google Scholar]
- 94.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, , et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490: 187–191, 2012. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 10: 283–294, 2009. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 96.Shay BL, Sawchuk M, Machacek DW, Hochman S. Serotonin 5-HT2 receptors induce a long-lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J Neurophysiol 94: 2867–2877, 2005. doi: 10.1152/jn.00465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol 233: 597–605, 2012. doi: 10.1016/j.expneurol.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Hone AJ, Meyer EL, McIntyre M, McIntosh JM. Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype. FASEB J 26: 917–926, 2012. doi: 10.1096/fj.11-195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shelukhina I, Paddenberg R, Kummer W, Tsetlin V. Functional expression and axonal transport of alpha7 nAChRs by peptidergic nociceptors of rat dorsal root ganglion. Brain Struct Funct 220: 1885–1899, 2014. doi: 10.1007/s00429-014-0762-4. [DOI] [PubMed] [Google Scholar]
- 100.Rau KK, Johnson RD, Cooper BY. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. J Neurophysiol 93: 1358–1371, 2005. doi: 10.1152/jn.00591.2004. [DOI] [PubMed] [Google Scholar]
- 101.Umana IC, Daniele CA, McGehee DS. Neuronal nicotinic receptors as analgesic targets: it's a winding road. Biochem Pharmacol 86: 1208–1214, 2013. doi: 10.1016/j.bcp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang XL, Albers KM, Gold MS. Inflammation-induced increase in nicotinic acetylcholine receptor current in cutaneous nociceptive DRG neurons from the adult rat. Neuroscience 284: 483–499, 2015. doi: 10.1016/j.neuroscience.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rau KK, Petruska JC, Cooper BY, Johnson RD. Distinct subclassification of DRG neurons innervating the distal colon and glans penis/distal urethra based on the electrophysiological current signature. J Neurophysiol 112: 1392–1408, 2014. doi: 10.1152/jn.00560.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith NJ, Hone AJ, Memon T, Bossi S, Smith TE, McIntosh JM, Olivera BM, Teichert RW. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Front Cell Neurosci 7: 225, 2013. doi: 10.3389/fncel.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson AJ. Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol Sci 34: 100–109, 2013. doi: 10.1016/j.tips.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 106.Yan D, Pedersen SE, White MM. Interaction of D-tubocurarine analogs with the 5HT3 receptor. Neuropharmacology 37: 251–257, 1998. doi: 10.1016/S0028-3908(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 107.Lebeda FJ, Hablitz JJ, Johnston D. Antagonism of GABA-mediated responses by d-tubocurarine in hippocampal neurons. J Neurophysiol 48: 622–632, 1982. doi: 10.1152/jn.1982.48.3.622. [DOI] [PubMed] [Google Scholar]
- 108.Schwartz RD, Mindlin MC. Inhibition of the GABA receptor-gated chloride ion channel in brain by noncompetitive inhibitors of the nicotinic receptor-gated cation channel. J Pharmacol Exp Ther 244: 963–970, 1988. [PubMed] [Google Scholar]
- 109.Wotring VE, Yoon KW. The inhibitory effects of nicotinic antagonists on currents elicited by GABA in rat hippocampal neurons. Neuroscience 67: 293–300, 1995. doi: 10.1016/0306-4522(95)00011-7. [DOI] [PubMed] [Google Scholar]
- 110.Kingery WS, Fields RD, Kocsis JD. Diminished dorsal root GABA sensitivity following chronic peripheral nerve injury. Exp Neurol 100: 478–490, 1988. doi: 10.1016/0014-4886(88)90033-7. [DOI] [PubMed] [Google Scholar]
- 111.Khasabov SG, Lopez-Garcia JA, King AE. Serotonin-induced population primary afferent depolarisation in vitro: the effects of neonatal capsaicin treatment. Brain Res 789: 339–342, 1998. doi: 10.1016/S0006-8993(98)00136-X. [DOI] [PubMed] [Google Scholar]
- 112.Maxwell DJ, Kerr R, Rashid S, Anderson E. Characterisation of axon terminals in the rat dorsal horn that are immunoreactive for serotonin 5-HT3A receptor subunits. Exp Brain Res 149: 114–124, 2003. doi: 10.1007/s00221-002-1339-7. [DOI] [PubMed] [Google Scholar]
- 113.Vincler M, McIntosh JM. Targeting the alpha9alpha10 nicotinic acetylcholine receptor to treat severe pain. Expert Opin Ther Targets 11: 891–897, 2007. doi: 10.1517/14728222.11.7.891. [DOI] [PubMed] [Google Scholar]
- 114.Cachelin AB, Rust G. Unusual pharmacology of (+)-tubocurarine with rat neuronal nicotinic acetylcholine receptors containing beta 4 subunits. Mol Pharmacol 46: 1168–1174, 1994. [PubMed] [Google Scholar]
- 115.Mena-Avila E, Milla-Cruz JJ, Calvo JR, Hochman S, Villalón CM, Arias-Montaño J-A, Quevedo JN. Activation of α-adrenoceptors depresses synaptic transmission of myelinated afferents and inhibits pathways mediating primary afferent depolarization (PAD) in the in vitro mouse spinal cord. Exp Brain Res 238: 1293–1303, 2020. doi: 10.1007/s00221-020-05805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buisson B, Vallejo YF, Green WN, Bertrand D. The unusual nature of epibatidine responses at the alpha4beta2 nicotinic acetylcholine receptor. Neuropharmacology 39: 2561–2569, 2000. doi: 10.1016/S0028-3908(00)00158-1. [DOI] [PubMed] [Google Scholar]