ABSTRACT
Background
Although manganese (Mn) is an essential trace element and a common component of most multivitamins on the market, an adverse effect on blood pressure (BP) has been reported in adults. In addition, the longitudinal relation between prenatal Mn status and childhood BP is still unknown.
Objective
This study investigated the association between prenatal Mn concentrations and risk of elevated BP at ages 3–12 y.
Method
The analyses included 1268 mother-child dyads who were enrolled at birth and followed prospectively at the Boston Medical Center. Maternal RBC Mn concentrations were measured by inductively coupled plasma mass spectrometry, using RBCs collected within 1–3 d after delivery (reflecting late-pregnancy Mn exposure). Child elevated BP was defined as systolic or diastolic BP ≥90th percentile for a given age, sex and height. Multivariate logistic regression models were conducted. Path analysis was applied to mediation estimation.
Results
The median (IQR) maternal RBC Mn concentration was 37.5 (29.2–48.5) μg/L. The rate of child elevated BP at ages 3–12 y was 25%. Both the lowest and highest quartiles of maternal RBC Mn concentrations were associated with higher risk of elevated BP among children aged 6–12 y (OR: 1.52; 95% CI: 1.04, 2.21 and OR: 1.65; 95% CI: 1.13, 2.40, respectively) compared with those in the second and third quartiles. Gestational age and fetal growth mediated the association between low maternal RBC Mn (first quartile) and child elevated BP, explaining 25% of the association, but not for high (fourth quartile) maternal RBC Mn concentrations. No association was found between maternal RBC Mn concentrations and BP among children aged 3–5 y.
Conclusion
We found a nonlinear association between maternal RBC Mn concentrations and elevated BP among children aged 6–12 y from a high-risk, predominantly minority population. Our findings warrant further investigation.
Keywords: manganese, blood pressure, prenatal, birth outcome, childhood, nonlinear
Introduction
In the past decade, in parallel with the obesity epidemic in children, the prevalence of high blood pressure (BP) in children has risen profoundly, especially among minority children (1). Childhood obesity and elevated BP likely persist into adulthood (2, 3), thus representing precursors for cardiovascular diseases (CVDs) in later life (4, 5). Fortunately, if obesity and elevated BP in childhood are addressed by adulthood, this can reduce the risk of future CVD (4, 6). As such, prevention of obesity and high BP in childhood has been increasingly recognized as a public health priority to improve population health across the life span (7).
Growing evidence indicates that oxidative stress is involved in the pathogenesis of hypertension (8, 9). Consistently, trace elements with an antioxidative function play a vital role in preventing CVD (10) and hypertension (11). Manganese (Mn) is an essential trace element for human health, serving as a cofactor for many enzymes such as superoxide dismutase, which is required for scavenging reactive oxygen species (ROS) in mitochondrial oxidative stress (12). The use of Mn-enriched foods and supplements has increased markedly in recent years in the United States due to its presumed beneficial antioxidant effects (12). However, concern has recently been raised about possible adverse cardiovascular effects of excessive Mn exposure (13). Research in adults has yielded inconsistent associations between Mn concentrations and BP, including positive, inverse, and U-shaped (14–16). There is also uncertainty regarding risks and/or benefits of Mn related to gestational hypertension and preeclampsia (13, 17). Importantly, maternal circulating Mn concentration rises throughout pregnancy (13, 18) and easily passes to the placenta into fetal blood, which leads to fetal bioaccumulation (19). These pieces of evidence led us to question whether prenatal Mn concentrations may be linked to BP in later life.
To fill in this research gap, this study prospectively examined the associations of maternal Mn concentrations in RBCs collected in 1–3 d after delivery with the child's birth outcomes and risk of elevated BP and overweight or obesity (OWO) at ages 3–12 y in the Boston Birth Cohort (BBC). Because Mn in RBCs accounts for ∼66% of the Mn in whole blood (20), and the average life span of RBCs is 120 d, maternal RBC Mn concentration may reflect maternal Mn exposure in the third trimester. Study questions of particular interest also included to what degree birth outcomes mediated the maternal Mn-child BP association and whether the maternal Mn-child BP association varied by maternal and child characteristics, including maternal cardiometabolic conditions and child's sex, race/ethnicity, and birth outcomes.
Methods
Study population
The study sample was part of the BBC, with participants enrolled from 2002 to 2013. All of the BBC mother-child dyads were enrolled at birth at the Boston Medical Center. A detailed description of the BBC has been reported previously (21). Supplemental Figure 1 shows the sampling frame of the study sample. Of the total 3163 mother-child dyads who continued pediatric care at Boston Medical Center, this study restricted analyses to children who had BP measurements taken from ages 3–12 y and whose mothers had measured RBC Mn concentrations 1–3 d after delivery. The final analyses included 1268 mother-child dyads with required data. A comparison of the characteristics of the included and excluded samples is presented in Supplemental Table 1. In general, maternal and child perinatal characteristics were comparable. The BBC has been approved by the Institutional Review Boards of Boston Medical Center and Johns Hopkins Bloomberg School of Public Health. Written informed consent was obtained from the mother of each child at study entry and at follow-up.
Ascertainment of Mn in RBCs
Maternal blood samples were collected at 1–3 d after delivery, separated into plasma, RBCs, and WBCs and stored in −80°C freezers. Ascertainment of Mn was carried out on maternal archived RBC samples using inductively coupled plasma mass spectrometry (8900 ICP-QQQ; Agilent Technologies, Inc.) by the Metals Laboratory, Environmental and Chemical Laboratory Services, State of New Jersey Department of Health, Trenton (a certified laboratory of the National Biomonitoring Program of the Centers for Disease Control and Prevention). The interassay coefficient of variation was <5.0%. The limit of detection (LOD) was 4.85 μg/L, and detectable rate was 99.8%. We assigned the value as the LOD divided by the square root of 2 if the concentration was below the LOD (22).
Definition of outcomes
All the maternal and child clinical data were retrieved from medical records. Low birthweight was defined as birthweight <2.5 kg. Gestational age was determined by the first day of the last menstrual period or early prenatal ultrasonographic results (21) and further grouped into term birth (≥37 wk) and preterm birth (<37 wk). Standardized birthweight (SBWT), used as a continuous measure of fetal growth, was calculated as sex-, race-, and gestational age–specific birthweight z score according to an internal reference population that comprised >15,000 newborns who were born at the labor and delivery service of the Department of Obstetrics and Gynecology at the Boston Medical Center (23). Fetal growth restriction (FGR) was defined as birthweight for gestational age <10th percentile. Adverse birth outcomes included low birthweight and/or preterm birth.
Child weight, height, and BP were measured by medical staff during pediatric clinic visits and retrieved from the electronic medical records. BMI was calculated as weight (in kg) divided by height (in meters) squared. BMI z scores and percentiles were calculated using US national reference data (24). OWO was defined as BMI ≥85th percentile of age and sex. BP percentiles were computed according to the national reference for age, sex, and height (25). Elevated BP was defined as systolic BP or diastolic BP ≥90th percentile according to guidelines (26).
Maternal covariables
Maternal sociodemographic variables were collected by questionnaire interview. Educational attainment was classified into 2 categories: high school and below compared with college and above. Smoking during pregnancy was categorized as nonsmoker and smoker (including intermittent or continuous smoking). Maternal race/ethnicity was grouped into black and nonblack. Parity was grouped into nulliparous and multiparous. Prepregnancy obesity was defined as prepregnancy BMI ≥30 kg/m2. Maternal diabetes status was classified as nondiabetic compared with diabetic (including gestational diabetes and preexisting diabetes). Hypertensive disorder during pregnancy was defined by the presence of chronic/gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome, a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and low platelet count (21). Maternal cardiometabolic condition included prepregnancy obesity, diabetes, and/or hypertensive disorder during pregnancy.
Statistical analysis
Demographic and clinical data are presented as either mean ± SD or number (percentage) according to maternal RBC Mn quartiles. Comparisons across maternal RBC Mn quartiles were performed by ANOVA for continuous variables and χ2 test for categorical variables.
The shapes of the associations between maternal RBC Mn concentrations and the outcomes of interest (gestational age, birthweight, elevated BP, OWO) are displayed using restricted cubic splines plots with 4 knots located at the 5th, 25th, 75th, and 95th percentiles (27). Linearity assumption tests were conducted using restricted cubic splines function. A Wald χ2 test showed that the relations between maternal RBC Mn concentrations and child's outcomes were nonlinear except for childhood OWO (Supplemental Table 2). Thus, maternal RBC Mn quartiles were included in the regression models.
Multivariate linear regression models were used to examine the relations between maternal RBC Mn quartiles and child's birth outcomes (gestational age, birthweight, and SBWT), with adjustment for maternal age categories, race/ethnicity, education attainment, smoking status, parity, maternal obesity, diabetes, hypertensive disorder, and child's sex. Multivariate logistic regression models were applied to determine if an association was present between maternal RBC Mn quartiles and child's binary birth outcomes (preterm birth, FGR, low birthweight) and childhood outcomes (elevated BP and OWO). To examine the impact of child age on the associations, we performed similar analyses stratified by child age at BP assessment (preschool age: 3–5 y and school age: 6–12 y). As a significant association between maternal RBC Mn concentrations and child elevated BP was observed only at school age (6–12 y), we performed subsequent analyses focused on school-aged children.
We explored whether gestational age and fetal growth mediated the association between maternal RBC Mn concentrations and elevated BP using path analysis, a structural equation model. A path analysis using robust maximum likelihood estimation was implemented using the “PROC CALIS” procedure in SAS (SAS Institute). Standardized β coefficients for total, direct, and indirect were generated simultaneously.
To examine the effect modification of maternal cardiometabolic conditions (prepregnancy obesity, preexisting/gestational diabetes, hypertensive disorder during pregnancy), race/ethnicity, child's sex, birth outcomes (preterm birth and FGR), and OWO, we plotted the relation between maternal RBC Mn concentrations and child elevated BP stratified by those variables using locally weighted regression smoothing plots. In addition, we examined the combined association of maternal cardiometabolic conditions and RBC Mn concentrations with child elevated BP.
Results
A total of 1268 mother-child pairs were included in this study. The median (IQR) ages for mothers and children were 28.4 (23.5–33.6) y and 8.3 (6.1–10.6) y, respectively. Among them, 60.7% of the mothers were black and 49.7% of the children were male. Of the children, 307 (24.2%) and 124 (9.8%) were born prematurely and had FGR, respectively. The range of maternal RBC Mn concentrations was wide in our samples (Supplemental Figure 2). The median (IQR) maternal RBC Mn concentration was 37.5 (29.2–48.5) μg/L. The rate of child OWO and elevated BP at ages 3–12 y was 43.6% and 25.0%, respectively. The maternal and child characteristics by the quartiles of maternal RBC Mn concentrations are shown in Table 1. Mothers with elevated RBC Mn concentrations were more likely to be nonblack, whereas mothers with insufficient RBC Mn concentrations were more likely to be smokers and to have hypertensive disorders during pregnancy. Children whose mothers had insufficient RBC Mn concentrations tended to have lower birthweight and gestational age as well as higher rates of preterm birth (Table 1).
TABLE 1.
Characteristics of the study participants (mother-child dyads)1
| Quartile of maternal RBC Mn concentration, μg/L | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 (6.9–29.0) | Q2 (29.2–37.4) | Q3 (37.6–48.4) | Q4 (48.6–109.8) | P value |
| n | 315 | 319 | 317 | 317 | |
| Maternal characteristics | |||||
| Maternal age, y | 0.36 | ||||
| <25 | 94 (29.8) | 94 (29.5) | 100 (31.5) | 118 (37.2) | |
| 25–34 | 157 (49.8) | 162 (50.8) | 152 (48.0) | 148 (46.7) | |
| ≥35 | 64 (20.3) | 63 (19.7) | 65 (20.5) | 51 (16.1) | |
| Race/ethnicity | <0.001 | ||||
| Black | 222 (70.5) | 212 (66.5) | 173 (54.6) | 163 (51.4) | |
| Nonblack | 93 (29.5) | 107 (33.5) | 144 (45.4) | 154 (48.6) | |
| Education | 0.15 | ||||
| High school and less | 192 (61.0) | 205 (64.3) | 197 (62.2) | 219 (69.1) | |
| College and beyond | 123 (39.0) | 114 (35.7) | 120 (37.8) | 98 (30.9) | |
| Smoking | <0.001 | ||||
| Never smoker | 229 (72.7) | 273 (85.6) | 277 (87.4) | 273 (86.1) | |
| Ever smoker | 86 (27.3) | 46 (14.4) | 40 (12.6) | 44 (13.9) | |
| Parity | 0.96 | ||||
| Nulliparous | 132 (41.9) | 130 (40.8) | 127 (40.1) | 127 (40.1) | |
| Multiparous | 183 (58.1) | 189 (59.2) | 190 (59.9) | 190 (59.9) | |
| Prepregnancy obesity2 | 82 (26.0) | 87 (27.3) | 80 (25.2) | 68 (21.5) | 0.36 |
| Hypertensive disorder3 | 67 (21.3) | 50 (15.7) | 43 (13.6) | 36 (11.4) | 0.004 |
| Diabetes4 | 54 (17.1) | 38 (11.9) | 36 (11.4) | 37 (11.7) | 0.10 |
| Child's characteristics | |||||
| Age (y) at BP measure | 7.8 ± 2.3 | 8.0 ± 2.6 | 8.6 ± 2.7 | 8.7 ± 3.0 | <0.001 |
| Sex | 0.92 | ||||
| Male | 156 (49.5) | 157 (49.2) | 161 (50.8) | 164 (51.7) | |
| Female | 159 (50.5) | 162 (50.8) | 156 (49.2) | 153 (48.3) | |
| Birthweight, kg | 2.77 ± 0.85 | 2.99 ± 0.84 | 3.11 ± 0.71 | 3.08 ± 0.70 | <0.001 |
| Gestational age, wk | 37.1 ± 3.9 | 37.9 ± 3.4 | 38.4 ± 2.8 | 38.3 ± 2.7 | <0.001 |
| Low birthweight5 | 100 (31.7) | 70 (21.9) | 49 (15.5) | 64 (20.2) | <0.001 |
| Preterm birth6 | 102 (32.4) | 76 (23.8) | 61 (19.2) | 68 (21.5) | <0.001 |
| Fetal growth restriction7 | 38 (12.1) | 35 (11.0) | 25 (7.9) | 26 (8.2) | 0.21 |
| Overweight/obese8 | 141 (44.8) | 139 (43.6) | 135 (42.6) | 138 (43.5) | 0.96 |
| BMI z score | 0.83 ± 1.18 | 0.72 ± 1.24 | 0.78 ± 1.21 | 0.78 ± 1.14 | 0.69 |
| Elevated BP9 | 89 (28.3) | 70 (21.9) | 69 (21.8) | 89 (28.1) | 0.08 |
1Values are presented as frequency (%) or mean ± SD, n = 1268 mother-child dyads. BP, blood pressure; Q, quartile.
2Prepregnancy obesity was defined as prepregnancy BMI ≥30 kg/m2.
3Hypertensive disorder includes chronic/gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome, a complication of pregnancy characterized by hemolysis, elevated liver enzymes, and low platelet count.
4Diabetes includes preexisting diabetes and gestational diabetes.
5Low birthweight was defined as birthweight <2.5 kg.
6Preterm birth was defined as gestational age <37 wk.
7Fetal growth restriction was defined as birthweight for gestational age <10th percentile.
8Child overweight or obese was defined as BMI ≥85th percentile for age and sex.
9Elevated BP was defined as systolic or diastolic BP ≥90th percentile for age, sex, and height.
Maternal RBC Mn concentrations and birth outcomes
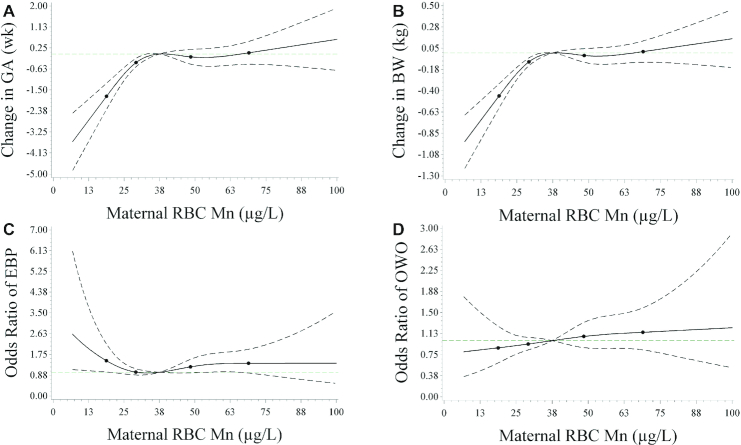
Maternal RBC Mn concentrations were associated with gestational age and birthweight in a nonlinear fashion (Figure 1A, B). Compared with the lowest quartile (Q1) of RBC Mn concentration, all other quartiles (Q2, Q3, Q4) were associated with increased gestational age by 0.64, 1.02, and 0.89 wk, respectively (Table 2), and with reduced risk of preterm birth by 27%, 43%, and 33%, respectively (Supplemental Table 3). Similarly, the higher RBC Mn concentration quartiles (Q2, Q3, Q4) were associated with 0.17, 0.27, and 0.24 kg higher birthweight, respectively (Table 2), and 32%, 55%, and 36% decreased risk of low birthweight, respectively (Supplemental Table 3), compared with Q1 of RBC Mn concentrations. In addition, maternal RBC Mn concentrations had a weak relation with fetal growth. Compared with Q1 RBC Mn concentrations, Q3 RBC Mn concentrations were associated with 0.18 higher SBWT, but this was not true for Q4 RBC Mn concentrations (P = 0.06, Table 2).
FIGURE 1.
Restricted cubic spline plots of the relation between maternal RBC manganese (Mn) concentrations and birth outcomes and childhood elevated BP and OWO.1 (A) A restricted cubic spline plot of the association between maternal RBC Mn concentrations and gestational age with 4 knots located at the 5th, 25th, 75th, and 95th percentiles. The y-axis represents estimates of the change in gestational age (wk) across maternal RBC Mn concentrations relative to a reference concentration of 37.5 μg/L, median RBC Mn concentration. (B) A restricted cubic spline plot of the association between maternal RBC Mn concentrations and birthweight with 4 knots located at the 5th, 25th, 75th, and 95th percentiles. The y-axis represents estimates of the change in birthweight (kg) across maternal RBC Mn concentrations relative to a reference concentration of 37.5 μg/L, median RBC Mn concentration. (C) The odds ratio of elevated BP according to maternal RBC Mn concentrations, which were estimated using restricted cubic splines with 4 knots located at the 5th, 25th, 75th, and 95th percentiles and the median RBC Mn concentration, 37.5 μg/L, as the reference value. (D) Odds ratios of OWO according to maternal RBC Mn concentrations, which were estimated using restricted cubic splines with 4 knots located at the 5th, 25th, 75th, and 95th percentiles and the median RBC Mn concentration, 37.5 μg/L, as the reference value. Due to a small sample size (n = 4), the curve is truncated at 100 μg/L. The total number of mother-child dyads was 1264 for all panels. 1BP, blood pressure; BWT, birthweight; EBP, elevated blood pressure; GEAA, gestational age; OWO, overweight or obesity. Elevated BP was defined as child systolic or diastolic BP ≥90th percentile for age, sex, and height. OWO was defined as BMI ≥85th percentile for age and sex. Dashed lines indicate 95% CIs. Knots are represented by dots. Covariates included maternal age categories, education, race/ethnicity, smoking status during pregnancy, parity, prepregnancy obesity, diabetes, and hypertensive disorders during pregnancy.
TABLE 2.
Associations between maternal RBC manganese concentrations and birth outcomes1
| Gestational age, wk | Birthweight, kg | Standardized birthweight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile | n | Mean ± SD | β (95% CI) | P value | Mean ± SD | β (95% CI) | P value | Mean ± SD | β (95% CI) | P value |
| Q1 | 315 | 37.1 ± 3.9 | Reference | 2.77 ± 0.85 | Reference | −0.14 ± 1.00 | Reference | |||
| Q2 | 319 | 37.9 ± 3.4 | 0.64 (0.14, 1.13) | 0.01 | 2.99 ± 0.84 | 0.17 (0.05, 0.29) | 0.004 | 0.02 ± 1.06 | 0.12 (−0.03, 0.27) | 0.12 |
| Q3 | 317 | 38.4 ± 2.8 | 1.02 (0.52, 1.51) | <0.001 | 3.11 ± 0.71 | 0.27 (0.15, 0.39) | <0.001 | 0.08 ± 0.97 | 0.18 (0.02, 0.33) | 0.02 |
| Q4 | 317 | 38.3 ± 2.7 | 0.89 (0.39, 1.39) | <0.001 | 3.08 ± 0.70 | 0.24 (0.12, 0.35) | <0.001 | 0.03 ± 1.02 | 0.15 (−0.01, 0.30) | 0.06 |
1The total number of mother-child dyads was 1268 for all models. The range of RBC Mn quartiles: Q1: 6.69–29.0 μg/L; Q2: 29.2–37.4 μg/L; Q3: 37.6–48.4 μg/L; Q4: 48.6–109.8 μg/L. Standardized birthweight was calculated as birthweight z score for gestational age, sex, and race/ethnicity. Adjusted for maternal age categories, education, parity, race/ethnicity, smoking, maternal diabetes, hypertensive disorder, prepregnancy obesity, and child's sex. Q, quartile.
Maternal RBC Mn concentrations and child elevated BP and OWO
We observed a nonlinear association between maternal RBC Mn concentrations and the risk of childhood elevated BP at ages 3–12 y (Figure 1C). Since both Q2 and Q3 of RBC Mn concentrations were similarly associated with the lowest risk of elevated BP (Supplemental Table 4), Q2 and Q3 were combined and used as the reference group. There was a higher risk of elevated BP for children whose mothers had RBC Mn concentrations in the extreme quartiles (Q1 and Q4) relative to those with RBC Mn concentrations in the middle quartiles (Q2–Q3), with an OR of 1.39 (95% CI: 1.01, 1.90) and 1.46 (95% CI: 1.07, 2.00), respectively (Table 3, model 1) after adjusting for maternal age categories, race/ethnicity, education attainment, smoking status, parity, maternal prepregnancy obesity, diabetes, and hypertensive disorder. The associations between low (Q1) RBC Mn concentrations and elevated BP were attenuated after further adjustment for child's fetal growth and gestational age (model 2) and child's BMI z score (model 3) but were strengthened for higher (Q4) RBC Mn concentrations. When stratified by age group, the associations remained in children aged 6–12 y for Q1 (OR: 1.52; 95% CI: 1.04, 2.40) and Q4 (OR: 1.65; 95% CI: 1.13, 2.40) RBC Mn concentrations, respectively, but no associations were found in children aged 3–5 y (Table 3). As such, for the subsequent analyses, we focused on BP at ages 6–12 y. Maternal RBC Mn concentrations were not associated with child OWO risk (Figure 1D and Supplemental Table 5).
TABLE 3.
Associations between maternal RBC manganese concentrations and child elevated blood pressure1
| OR (95% CI) | |||||
|---|---|---|---|---|---|
| Quartile | n | Case, n (%) | Model 1 | Model 2 | Model 3 |
| Age 3–12 y | |||||
| Q1 | 315 | 89 (28.3) | 1.39 (1.01, 1.90)2 | 1.30 (0.94, 1.79) | 1.28 (0.93, 1.78) |
| Q2 + Q3 | 636 | 139 (21.9) | 1.00 | 1.00 | 1.00 |
| Q4 | 317 | 89 (28.1) | 1.46 (1.07, 2.00)2 | 1.49 (1.08, 2.04)2 | 1.47 (1.07, 2.03)2 |
| Age 3–5 y | |||||
| Q1 | 299 | 94 (31.4) | 0.98 (0.72, 1.34) | 0.98 (0.72, 1.34) | 0.98 (0.72, 1.34) |
| Q2 + Q3 | 599 | 186 (31.1) | 1.00 | 1.00 | 1.00 |
| Q4 | 289 | 289 (30.1) | 1.01 (0.74, 1.38) | 1.01 (0.74, 1.38) | 1.01 (0.74, 1.38) |
| Age 6–12 y | |||||
| Q1 | 249 | 63 (25.3) | 1.52 (1.04, 2.21)2 | 1.40 (0.96, 2.06) | 1.44 (0.97, 2.13) |
| Q2 + Q3 | 497 | 92 (18.5) | 1.00 | 1.00 | 1.00 |
| Q4 | 233 | 61 (26.2) | 1.65 (1.13, 2.40)2 | 1.69 (1.16, 2.48)3 | 1.71 (1.16, 2.52)3 |
1The model for ages 3–12 y included 1268 mother-child pairs. The model for ages 3–5 y included 1187 mother-child pairs. The model for ages 6–12 y included 979 mother-child pairs. The range of RBC Mn quartiles: Q1: 6.69–29.0 μg/L; Q2: 29.2–37.4 μg/L; Q3: 37.6–48.4 μg/L; Q4: 48.6–109.8 μg/L. Elevated BP was defined as child systolic or diastolic BP ≥90th percentile for age, sex, and height. Model 1: adjusted for maternal age categories, education, parity, race/ethnicity, smoking, maternal diabetes, hypertensive disorder, and prepregnancy obesity. Model 2: model 1 + gestational age and standardized birthweight. Model 3: model 2 + child's BMI z score. Q, quartile.
2 P < 0.05.
3 P < 0.01.
Birth outcomes mediated the association between RBC Mn and elevated BP at age 6–12 y
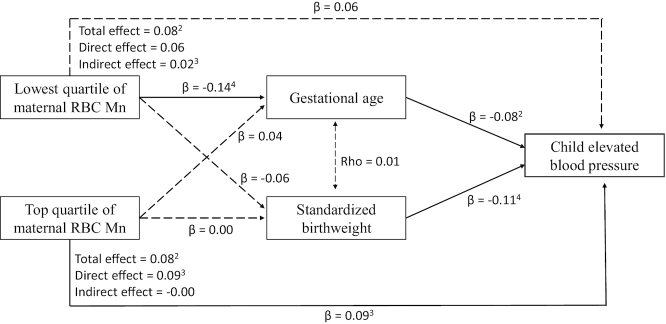
When gestational age and SBWT were controlled for in the multivariate regression models of maternal RBC Mn concentrations and elevated BP at ages 6–12 y, the association between low (Q1) RBC Mn and elevated BP was attenuated (Table 3, model 2). Path analysis further estimated a 25% association between low (Q1) RBC Mn concentrations and elevated BP through gestational age and SBWT (Figure 2). However, there was no mediation effect of birth outcomes on the association between higher (Q4) RBC Mn concentrations and child elevated BP.
FIGURE 2.
Path diagram depicting tested mediation pathways between maternal RBC Mn concentration, gestational age, standardized birthweight, and elevated BP at ages 6–12 y (n = 979).1 1The diagram depicts mediated pathways from maternal RBC Mn concentration, to gestational age and standardized birthweight, and then to childhood elevated blood pressure. Solid arrrows represent a significant direct or indect effect. Dashed single-headed arrows indicate insignificant effect. The dashed double-headed arrow indicates that the variable disturbance term was not significantly correlated in the model. Covariates included maternal age categories, education, race/ethnicity, smoking status during pregnancy, parity, maternal metabolic cardiometabolic conditions, and child's overweight or obesity status. Mn, manganese. The range of RBC Mn quartiles: Q1: 6.69–29.0 μg/L; Q2: 29.2–37.4 μg/L; Q3: 37.6–48.4 μg/L; Q4: 48.6–109.8 μg/L. 2P < 0.05. 3P < 0.01. 4P < 0.001.
Combined association of maternal cardiometabolic condition and RBC Mn concentration with child elevated BP
In general, children whose mothers had higher (Q4) RBC Mn concentrations and prepregnancy obesity (Supplemental Figure 3A), diabetes (Supplemental Figure 3B), hypertensive disorder (Supplemental Figure 3C), or any maternal cardiometabolic condition (Supplemental Figure 3D) had a higher risk of elevated BP. Multivariate regression models confirmed the effect of combined associations between Q1 and Q4 of maternal RBC Mn concentrations and maternal cardiometabolic conditions on child elevated BP. Children whose mothers had any cardiometabolic condition and Q4 of RBC Mn concentrations had the highest risk of elevated BP (OR: 2.89; 95% CI: 1.62, 5.18; P < 0.001), followed by those in the lowest quartile (Q1) (OR: 2.27; 95% CI: 1.32, 3.89; P = 0.003) compared with those in the control group (Supplemental Table 6, model 2).
Effect modification of sex, race, birth outcomes, and child OWO
We plotted the predicted probability of elevated BP according to maternal RBC Mn concentrations stratified by sex (Supplemental Figure 4A), race/ethnicity (Supplemental Figure 4B), preterm birth (Supplemental Figure 4C), FGR (Supplemental Figure 4D), any adverse birth outcome (Supplemental Figure 4E), and child's OWO status (Supplemental Figure 4F). The plotted curves of elevated BP according to maternal RBC Mn concentrations for children who had adverse birth outcomes or OWO were persistently above the curves for children who had normal birth outcomes or were nonobese. However, interaction analysis did not find significant interaction.
Sensitivity analysis
We found 3 samples with RBC Mn values below the LOD. To test the robustness of our findings, we repeated the analysis after removing these 3 samples, although the result did not change substantially (Supplemental Table 7).
Discussion
To our knowledge, this is the first study to prospectively investigate the relation between perinatal maternal Mn concentration, birth outcomes, and childhood BP under a life-course framework. Our study documented a nonlinear relation between maternal RBC Mn concentrations and both gestational age and birthweight; that is, the benefit of the maternal RBC Mn concentration plateaued once it reached around the fourth quartile. We also showed a nonlinear relation between maternal RBC Mn concentrations and child elevated BP at ages 6–12 y, in which both insufficient Mn (in the first quartile) and higher Mn (in the fourth quartile) concentrations were associated with a higher risk of elevated BP. The highest risk of elevated BP was found in children whose mothers had excess RBC Mn concentrations coupled with cardiometabolic disorders, whereas the lowest risk was found in those whose mothers had midrange RBC Mn concentrations and a favorable health profile. Moreover, gestational age and fetal growth partially mediated the association between insufficient maternal RBC Mn (first quartile) concentrations and child's elevated BP. The results of this study underscore the potential role of prenatal Mn concentrations on offspring's BP status later in life.
Previous studies reported a nonlinear relation between prenatal Mn concentrations and birthweight (28) and gestational age (29). We found that maternal RBC Mn concentrations in the lowest (Q1) quartile were associated with shorter gestation and lower birthweight. Although our data did not support a U-shaped relation as shown in a previous study (28), we did find that higher Mn concentrations in the top (Q4) quartile did not confer additional benefit, suggesting a ceiling effect of Mn exposure on birth outcomes. One potential explanation for our finding is that Mn is an essential micronutrient for fetal growth (30), whereas excess Mn has no benefit and may even impose toxicity. Due to the observational nature of our study, our findings remain to be confirmed by future studies.
In adults, inconsistent relations between Mn concentrations and BP have been reported. In a cross-sectional study, urine Mn concentrations were positively associated with an increased risk of high BP (14). In contrast, a Korean study found that high BP was associated with a low intake of Mn (15). Another study also reported that Mn concentrations assessed in toenails were inversely associated with BP in elderly men (31). In addition, a longitudinal study showed a U-shaped relation between Mn and BP (16). The inconsistency of the findings from these previous studies may be due to differences in study designs, population characteristics, types of biospecimens, and Mn measurement methods across studies. Our study corroborates the detrimental effect of insufficient or excess prenatal Mn on elevated BP in offspring at ages 6–12 y. However, no association was found between RBC Mn concentrations and offspring BP at ages 3–5 y, which may be due to the high variability in BP among younger children (32).
One of the potential mechanisms underlying these associations is oxidative stress, which has been implicated in the pathogenesis of hypertension (9). It is well known that high-dose and/or inadequate removal of ROS, especially superoxide anion, results in oxidative stress (33). Mn is an essential element of superoxide dismutase 2, which is localized in the mitochondrial matrix to break down the disproportionate amount of superoxide anion (33). Studies have reported that nonoptimal (both low and high) Mn exposure may result in increased ROS generation and oxidative stress (34). Furthermore, chronic exposure to Mn can affect DNA methylation and histone modification (35), which is involved in epigenomic regulation of hypertension (36).
Another potential mechanism underlying the association between insufficient Mn and elevated BP is via its effect on gestational length and fetal growth. Exposure to an adverse environment during fetal life is known to have a substantial effect on BP levels (37). Our analysis showed that insufficient Mn was associated with short gestation and low birthweight. Furthermore, gestational age and fetal growth mediated 25% of the association between insufficient maternal RBC Mn concentrations (first quartile) and child elevated BP, suggesting that a higher risk of elevated BP can be attributed to insufficient prenatal Mn concentrations, perhaps partly due to short gestation and slow fetal growth.
Consistent with a previous study that found that blood Mn concentration was negatively associated with preeclampsia and high-risk pregnancy (38), our data showed that mothers with hypertensive disorders during pregnancy had lower RBC Mn concentrations. We did not observe a “U-shaped” association between maternal RBC Mn concentrations and maternal hypertensive disorders during pregnancy in the study population. One reason may be that pregnant mothers have to provide enough Mn to their fetus due to its requirement for fetal growth and development. A study has confirmed that Mn can readily pass to the fetus via the placenta and that maternal blood Mn concentrations rise throughout pregnancy, which parallels fetal growth (18).
The range of maternal RBC Mn concentrations was very wide in our sample. Some mothers had extremely low concentrations, whereas others had very high concentrations. This may be due to the fact that some mothers were users of some over-the-counter multivitamin tablets, which may have contained Mn. Reference ranges of Mn in whole blood have been reported for healthy adults (4–15 μg/L) (39), but no reference range has been established for pregnant women. In this study, Mn was measured in RBCs. The median (IQR) RBC Mn concentration was 39.5 (29.2–48.5) μg/L. When taking into account the average hematocrit of 36% (range: 28–40%) in the third trimester (40), the median (IQR) of RBC Mn concentration in our population was equivalent to 13.5 (10.5–17.5) μg/L in whole blood, which is similar to the concentrations seen in the third trimester in the Maternal-Infant Research on Environmental Chemicals (MIREC) study (41) and in Japanese pregnant women (13).
Notably, the rate of elevated BP (systolic or diastolic BP ≥90th percentile) was 25% in our study sample, which is around 1.8-fold higher compared with the data for US children aged 5–18 y from the National Health and Nutrition Examination Survey (42). We speculate that adverse birth outcomes and OWO may have contributed to this difference. It is well established that suboptimal fetal growth, short gestation, and OWO contribute to hypertension (37, 43, 44). Our study sample is a predominantly minority, low-income, inner-city population with higher rates of preterm birth (24.2%), low birthweight (22.3%), and OWO (43.6%), which might account for the increased rate of elevated BP. This is supported by the fact that the rate of elevated BP in our study (25%) is similar to the rate (25.9%) seen among children aged <13 y from a school-based study performed by the University of Texas McGovern Medical School, in which the rate of OWO was 34% (45). Likewise, the rate that we observed is lower than the rate of 29.2% found in a prospective study of US children aged 6–18 y (46). Notwithstanding, it is worthwhile to notice that BP in children may vary markedly between visits. Multiple measurements over time should be obtained before diagnosing hypertension (26). However, the definition of elevated BP in this study was based on BP measurements in a single visit, which may have led to misclassification. In addition, BP in our study was measured using automatic devices during clinic visits, whereas normative BP standards are based on auscultatory measurements. The alternative ways of measuring BP may also affect BP classification.
Limitations and strengths
A major limitation of this study is that BP measurements used were abstracted from medical records and not specifically assessed for research purposes, which may be subject to measurement error. However, the error theoretically is random and nondifferential and may bias the results toward the null. Next, Mn concentrations were measured in mothers’ RBCs, which were obtained between 1 and 3 d postpartum. Because the average life span of RBCs is 120 d, the RBC Mn concentrations may only reflect maternal Mn exposure for up to the previous 120 d (i.e., the third trimester). It would be ideal to also collect such data during early pregnancy and childhood. In addition, we were only able to study a subset of BBC mothers who had available RBC Mn concentration data. However, the major demographic and clinical characteristics were similar between the study sample and the overall BBC follow-up sample. Finally, this study was conducted in a high-risk, minority US population in Boston. Caution is needed to compare or generalize our findings to other populations with different characteristics or living in a different geographic location.
Our study has several strengths. First, our data were collected prospectively, and our analyses were adjusted for important confounding factors. Second, to avoid the influence of hemodilution on perinatal maternal Mn measurements, we assessed maternal RBC Mn concentrations because 66% of circulating Mn is found in RBCs (20), and RBC Mn concentrations are free from a hemodilution effect.
Conclusion
We demonstrated a nonlinear association between maternal RBC Mn concentrations and birth outcomes and a nonlinear association between maternal RBC Mn concentrations and child's risk of elevated BP at ages 6–12 y, suggesting that both an insufficiency and an excess of prenatal Mn exposure may contribute to a child's future risk of elevated BP. We also found that birth outcomes partially mediated the association between low maternal RBC Mn concentrations and child elevated BP. Our findings warrant further investigation; if confirmed, optimizing maternal Mn nutrition during pregnancy may be an early prevention strategy for childhood elevated BP.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Linda Rosen and the Clinical Data Warehouse (CDW) for their assistance in obtaining relevant clinical information; CDW service is supported by Boston University's Clinical and Translational Institute and the National Institutes of Health Clinical and Translational Science Awards Program grant U54-TR001012. In addition, we thank all the study participants and the Boston Medical Center Labor and Delivery Nursing Staff for their support and help with the study.
The authors’ contributions were as follows—GW, TLC, and XW: designed the research study; YJ and CP: conducted the research; GW, XH, and HJ: analyzed the data; GW, WYT, MWK, and TRB: wrote and revised the paper; GW and XW: had primary responsibility for the final content of the manuscript, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analyses; and all authors: read and approved the final manuscript.
Notes
Sources of support: The Boston Birth Cohort is supported in part by NIH grants (R01HD086013, 2R01HD041702, R01HD098232, and R01ES031272) and by the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) under grant R40MC27443 and cooperative agreement UJ2MC31074. GW is also supported by grant R03ES029594 from the NIH/National Institute of Environmental Health Science. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by, the NIH, HRSA, HHS, or US government.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–4 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: BBC, Boston Birth Cohort; BP, blood pressure; CVD, cardiovascular diseases; FGR, fetal growth restriction; LOD, limit of detection; Mn, manganese; OWO, overweight or obesity; Q, quartile; ROS, reactive oxidative species; SBWT, standardized birthweight.
Contributor Information
Guoying Wang, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Wan-Yee Tang, Department of Environmental Health and Engineering, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; Department of Environmental and Occupational Health, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Marsha Wills-Karp, Department of Environmental Health and Engineering, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Hongkai Ji, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Tami R Bartell, Mary Ann & J. Milburn Smith Child Health Research, Outreach and Advocacy Center, Stanley Manne Children's Research Institute, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, USA.
Yuelong Ji, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Xiumei Hong, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Colleen Pearson, Department of Pediatrics, Boston University School of Medicine and Boston Medical Center, Boston, MA, USA.
Tina L Cheng, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Xiaobin Wang, Center on the Early Life Origins of Disease, Department of Population, Family and Reproductive Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA; Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- 1. Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24(3):423–30. [DOI] [PubMed] [Google Scholar]
- 2. Li Z, Snieder H, Harshfield GA, Treiber FA, Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32(5):404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deshmukh-Taskar P, Nicklas TA, Morales M, Yang SJ, Zakeri I, Berenson GS. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60(1):48–57. [DOI] [PubMed] [Google Scholar]
- 4. Juhola J, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W et al. Combined effects of child and adult elevated blood pressure on subclinical atherosclerosis: the International Childhood Cardiovascular Cohort Consortium. Circulation. 2013;128(3):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lai CC, Sun D, Cen R, Wang J, Li S, Fernandez-Alonso C, Chen W, Srinivasan SR, Berenson GS. Impact of long-term burden of excessive adiposity and elevated blood pressure from childhood on adulthood left ventricular remodeling patterns: the Bogalusa Heart Study. J Am Coll Cardiol. 2014;64(15):1580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, Srinivasan SR, Daniels SR, Davis PH, Chen W et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–85. [DOI] [PubMed] [Google Scholar]
- 7. Zachariah JP. Improving blood pressure in children is protective over the long term. Circulation. 2013;128(3):198–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russo C, Olivieri O, Girelli D, Faccini G, Zenari ML, Lombardi S, Corrocher R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J Hypertens. 1998;16(9):1267–71. [DOI] [PubMed] [Google Scholar]
- 9. Pedro-Botet J, Covas MI, Martin S, Rubies-Prat J. Decreased endogenous antioxidant enzymatic status in essential hypertension. J Hum Hypertens. 2000;14(6):343–5. [DOI] [PubMed] [Google Scholar]
- 10. Gharipour M, Sadeghi M, Behmanesh M, Salehi M, Nezafati P, Gharpour A. Selenium homeostasis and clustering of cardiovascular risk factors: a systematic review. Acta Biomed. 2017;88(3):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Woo JG, Zhang N. Association between urinary manganese and blood pressure: results from National Health and Nutrition Examination Survey (NHANES), 2011–2014. PLoS One. 2017;12(11):e0188145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguirre JD, Culotta VC. Battles with iron: manganese in oxidative stress protection. J Biol Chem. 2012;287(17):13541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vigeh M, Nishioka E, Yokoyama K, Ohtani K, Matsukawa T. Increased prenatal blood manganese may induce gestational blood pressure. Hypertens Pregnancy. 2016;35(4):583–92. [DOI] [PubMed] [Google Scholar]
- 14. Shiue I. Higher urinary heavy metal, phthalate, and arsenic but not parabens concentrations in people with high blood pressure, U.S. NHANES, 2011–2012. IJERPH. 2014;11(6):5989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi MK, Bae YJ. Relationship between dietary magnesium, manganese, and copper and metabolic syndrome risk in Korean adults: the Korea National Health and Nutrition Examination Survey (2007–2008). Biol Trace Elem Res. 2013;156(1–3):56–66. [DOI] [PubMed] [Google Scholar]
- 16. Bulka CM, Scannell Bryan M, Persky VW, Daviglus ML, Durazo-Arvizu RA, Parvez F, Slavkovich V, Graziano JH, Islam T, Baron JA et al. Changes in blood pressure associated with lead, manganese, and selenium in a Bangladeshi cohort. Environ Pollut. 2019;248:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu T, Zhang M, Guallar E, Wang G, Hong X, Wang X, Mueller NT. Trace minerals, heavy metals, and preeclampsia: findings from the Boston Birth Cohort. JAHA. 2019;8(16):e012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, Legrand M, Davis K, Ettinger AS, Fraser WD et al. Maternal and fetal exposure to cadmium, lead, manganese and mercury: the MIREC study. Chemosphere. 2016;163:270–82. [DOI] [PubMed] [Google Scholar]
- 19. Guan H, Wang M, Li X, Piao F, Li Q, Xu L, Kitamura F, Yokoyama K. Manganese concentrations in maternal and umbilical cord blood: related to birth size and environmental factors. Eur J Public Health. 2014;24(1):150–7. [DOI] [PubMed] [Google Scholar]
- 20. Milne DB, Sims RL, Ralston NV. Manganese content of the cellular components of blood. Clin Chem. 1990;36(3):450–2. [PubMed] [Google Scholar]
- 21. Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang G, DiBari J, Bind E, Steffens AM, Mukherjee J, Bartell TR, Bellinger DC, Hong X, Ji Y, Wang MC et al. In utero exposure to mercury and childhood overweight or obesity: counteracting effect of maternal folate status. BMC Med. 2019;17(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Wang X, Laird N, Zuckerman B, Stubblefield P, Xu X. Polymorphism in maternal LRP8 gene is associated with fetal growth. Am J Hum Genet. 2006;78(5):770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Center for Health Statistics . CDC growth charts. 2000; [Internet]. [Accessed 2018 Nov 26]. Available from: http://www.cdc.gov/growthcharts/. [Google Scholar]
- 25. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 26. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 27. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–57. [DOI] [PubMed] [Google Scholar]
- 28. Eum JH, Cheong HK, Ha EH, Ha M, Kim Y, Hong YC, Park H, Chang N. Maternal blood manganese level and birth weight: a MOCEH birth cohort study. Environ Health. 2014;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Irwinda R, Wibowo N, Putri AS. The concentration of micronutrients and heavy metals in maternal serum, placenta, and cord blood: a cross-sectional study in preterm birth. J Pregnancy. 2019;2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mistry HD, Williams PJ. The importance of antioxidant micronutrients in pregnancy. Oxid Med Cell Longev. 2011;2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, Sparrow D, Vokonas P, Schwartz J. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect. 2012;120(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chiolero A, Bovet P, Paradis G. Screening for elevated blood pressure in children and adolescents: a critical appraisal. JAMA Pediatr. 2013;167(3):266–73. [DOI] [PubMed] [Google Scholar]
- 33. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15(6):1583–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li L, Yang X. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tarale P, Chakrabarti T, Sivanesan S, Naoghare P, Bafana A, Krishnamurthi K. Potential role of epigenetic mechanism in manganese induced neurotoxicity. Biomed Res Int. 2016;2016:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stoll S, Wang C, Qiu H. DNA methylation and histone modification in hypertension. IJMS. 2018;19(4):1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298(6673):564–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Jameil N, Tabassum H, Al-Mayouf H, Aljohar HI, Alenzi ND, Hijazy SM, Khan FA. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. Int J Clin Exp Pathol. 2014;7(5):1900–10. [PMC free article] [PubMed] [Google Scholar]
- 39. US ATSDR . Toxicological profile for manganese. 2012; [Internet]. [Accessed 2020 Feb 1]. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp151.pdf. [Google Scholar]
- 40. Abbassi-Ghanavati M, Greer L, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gyneco. 2009;114(6):1326–31. [DOI] [PubMed] [Google Scholar]
- 41. Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, Monnier P, Morisset AS, Fraser WD, Bouchard MF. Maternal and cord blood manganese (Mn) levels and birth weight: the MIREC birth cohort study. Int J Hyg Environ Health. 2018;221(6):876–82. [DOI] [PubMed] [Google Scholar]
- 42. Sharma AK, Metzger DL, Rodd CJ. Prevalence and severity of high blood pressure among children based on the 2017 American Academy of Pediatrics Guidelines. JAMA Pediatr. 2018;172(6):557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–95S. [DOI] [PubMed] [Google Scholar]
- 44. Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta-analysis. Lancet Public Health. 2017;2(8):e375–e86. [DOI] [PubMed] [Google Scholar]
- 45. Bell CS, Samuel JP, Samuels JA. Prevalence of hypertension in children. Hypertension. 2019;73(1):148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nafiu OO, Zepeda A, Curcio C, Prasad Y. Association of neck circumference and obesity status with elevated blood pressure in children. J Hum Hypertens. 2014;28(4):263–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.