ABSTRACT
Background
The effect of macronutrient composition on total energy expenditure (TEE) remains controversial, with divergent findings among studies. One source of heterogeneity may be study duration, as physiological adaptation to lower carbohydrate intake may require 2 to 3 wk.
Objective
We tested the hypothesis that the effects of carbohydrate [expressed as % of energy intake (EI)] on TEE vary with time.
Methods
The sample included trials from a previous meta-analysis and new trials identified in a PubMed search through 9 March 2020 comparing lower- and higher-carbohydrate diets, controlled for EI or body weight. Three reviewers independently extracted data and reconciled discrepancies. Effects on TEE were pooled using inverse-variance-weighted meta-analysis, with between-study heterogeneity assessed using the I2 statistic. Meta-regression was used to quantify the influence of study duration, dichotomized at 2.5 wk.
Results
The 29 trials ranged in duration from 1 to 140 d (median: 4 d) and included 617 participants. Difference in carbohydrate between intervention arms ranged from 8% to 77% EI (median: 30%). Compared with reported findings in the prior analysis (I2 = 32.2%), we found greater heterogeneity (I2 = 90.9% in the reanalysis, 81.6% in the updated analysis). Study duration modified the diet effect on TEE (P < 0.001). Among 23 shorter trials, TEE was reduced on lower-carbohydrate diets (−50.0 kcal/d; 95% CI: −77.4, −22.6 kcal/d) with substantial heterogeneity (I2 = 69.8). Among 6 longer trials, TEE was increased on low-carbohydrate diets (135.4 kcal/d; 95% CI: 72.0, 198.7 kcal/d) with low heterogeneity (I2 = 26.4). Expressed per 10% decrease in carbohydrate as %EI, the TEE effects in shorter and longer trials were −14.5 kcal/d and 50.4 kcal/d, respectively. Findings were materially unchanged in sensitivity analyses.
Conclusions
Lower-carbohydrate diets transiently reduce TEE, with a larger increase after ∼2.5 wk. These findings highlight the importance of longer trials to understand chronic macronutrient effects and suggest a mechanism whereby lower-carbohydrate diets may facilitate weight loss.
Keywords: obesity, dietary carbohydrate, low-carbohydrate diet, dietary fat, carbohydrate-insulin model, energy expenditure, feeding study, metabolism
See corresponding commentary on page 468.
Introduction
According to some thinking, on a calorie-per-calorie basis, all sources of metabolizable energy are alike in their effects on body energy stores and weight for practical purposes (1). In this view, any major effects on body weight resulting from macronutrient-focused diets, ranging from very-low-carbohydrate to very-low-fat, result from changes in energy intake, as influenced by hunger, satiety, or other factors, not total energy expenditure (TEE). In support of this view, a recent meta-analysis of 28 feeding trials (2) found that TEE was slightly reduced on lower- versus higher-carbohydrate diets (−25.5 kcal/d, 95% CI: −32.2, −18.8 kcal/d; I2 = 32.2%), a difference that was considered clinically insignificant. However, the median duration of included studies was 4 d, and the potential effect of intervention duration was not reported. Experimental and mechanistic studies suggest that the process of physiological adaptation to lower carbohydrate intake may require at least 2 to 3 wk (3–13), raising the possibility that transient and longer-term metabolic effects may have been conflated.
On a conventional diet, the brain relies upon glucose for energy requirements. With restriction of carbohydrate to <50 to 100 g/d (<10% to 20% of dietary energy), ketones and ketoacids such as B-hydroxybutyrate (BOHB)—derivates of fatty acids that cross the blood–brain barrier—replace glucose as the major energy source for the brain, reducing demand for gluconeogenic substrates from protein, thus preserving lean mass. But even with total elimination of dietary carbohydrate (e.g., fasting), the concentration of BOHB rises slowly, reaching steady state only after 2 to 3 wk (14). Further adaptations that may occur over weeks to months relate to the efficiency of BOHB transport into the brain (15), changes in muscle and liver metabolism (16–18), mitochondrial number and function (19, 20) oxidative stress and inflammation (19–21), and hormonal responses (22, 23). In time-course studies, negative nitrogen balance (indicative of lean mass loss) (24–26), fatigue (27), increased hunger (28), and decreased exercise tolerance (18) are characteristically observed with initiation of a ketogenic diet, but these adverse responses typically resolve after a few weeks. Even with moderate changes in macronutrient proportion (i.e., not sufficient to elicit ketosis), metabolic pathways facilitating a shift from carbohydrate to fat oxidation may adapt over several weeks (29). Thus, a reduction in dietary carbohydrate may transiently suppress TEE through multiple mechanisms, including, but not limited to, reduced voluntary physical activity level, perhaps due to fatigue. If this hypothesis is true, it might help explain heterogeneity among clinical trials and inform the design of weight-loss treatments.
To test the hypothesis, we reanalyzed and updated the prior meta-analysis, with a specific focus on heterogeneity and effect modification by trial duration.
Methods
We followed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for article selection, data extraction, data analysis, and reporting.
Search strategy and study selection
We included all 28 trials in the original meta-analysis, except as described below, and conducted a PubMed search on 9 March 2020 to identify new trials since 2016 that satisfied the search criteria as originally specified (2): (energy expenditure [tiab] or metabolic rate [tiab] or energy balance [tiab] or nutrient balance* [tiab] or fat balance* [tiab] or carbohydrate balance* [tiab] or fat oxidation [tiab] or fat mass [tiab] or body fat [tiab] or body composition [tiab]) AND (dietary carbohydrate [mh] or low-carb* [tiab] or high-carb* [tiab] or dietary fat [mh] or high-fat [tiab] or low-fat [tiab] or dietary protein [mh] or highprotein [tiab] or low-protein [tiab] or macronutrient* [tiab] or diet composition [tiab] or dietary composition [tiab]) AND (indirect calorimetry [mh] or indirect calorimetry [tiab] or calorimeter [tiab] or calorimetry [tiab] or metabolic chamber* [tiab] or respiration chamber* [tiab] or respiratory chamber* [tiab] or doubly labeled water [tiab] or doubly labelled water [tiab]) AND (men [tiab] or women [tiab] or human* [tiab] or subject* [tiab] or volunteer* [tiab] or adults [tiab] or children [tiab] or adolescent* [tiab]).
Trials were eligible if they met all of the following criteria: 1) compared the effects of lower- versus higher-carbohydrate diets regardless of absolute levels of dietary carbohydrate proportion, 2) controlled energy intake or body weight, 3) controlled dietary protein, 4) provided foods to participants to enhance treatment differentiation, and 5) utilized whole-room calorimetry (WRC) or doubly labeled water (DLW) to measure TEE. Trials were excluded if they had additional interventions (e.g., different levels of prescribed physical activity) the effects of which could not be separated from the dietary intervention.
Our updated analysis excluded 2 studies in the original meta-analysis: Verboeket-van de Venne et al. (30), which was based on the same trial as Verboeket-van de Venne and Westerterp (31) (Klaas Westerterp, Maastricht University, personal communication 2020) and Shepard et al. (32), which had the same participants as Eckel et al. (33) (James Hill, University of Alabama, Birmingham, personal communication 2020). Three new trials published after the original meta-analysis were included, Ebbeling et al. (34), Bush et al. (35) and Begaye et al. (36) for a total of 29 after the above-mentioned exclusions.
Data extraction
Data were extracted independently by 3 co-investigators, with any identified differences resolved through conversation. For trials with ≥3 test diets, the 2 diets with the most extreme differences in carbohydrate content were included. When repeated measurements were reported, the last time point that had data on both diets was used, unless the primary outcome in the trial specified an average of time points and all were either <2.5 or >2.5 wk [as was the case with Ebbeling et al. (34)]. We excluded any time points on which ad libitum (uncontrolled) food intake occurred. When both DLW and WRC were available, we utilized DLW because of the plausibly greater accuracy of the former for adaptive thermogenesis (37). See Supplemental Tables 1 and 2 for additional details, including data extraction and minor methodological differences from the previous meta-analysis.
Effect size calculation for each study
The effect size for each trial was calculated as the mean difference in TEE comparing the lower-carbohydrate versus the higher-carbohydrate diet. Changes from baseline were compared when data were available. Standard errors of the effect size were calculated when data were available from individual participant data or from SDs of each diet along with the estimated correlation between diets in crossover studies (38). When data were unavailable, correlation between diets was imputed using the mean of the correlations for other studies with the same design (r = 0.80 comparing change scores; r = 0.83 comparing end points). Similarly, SEs of changes from baseline were calculated from the SDs of TEE at baseline and end point; correlation was imputed when needed based on the mean correlation between time points (r = 0.88). In the original meta-analysis, a correlation of 0.95 was used to calculate SEs (2), which we utilized in a sensitivity analysis. When data were extracted for subgroups, we calculated a combined effect size for the study, weighted by the inverse variances (39, 40).
Meta-analysis
We performed meta-analysis in Stata version 16 (StataCorp LLC) (41) to calculate the pooled effect sizes. To replicate the original work, we used fixed effects and random effects with DerSimonian-Laird (D-L). For the updated analysis, we used random effects with Restricted Maximum Likelihood (REML) (40, 42–44). Heterogeneity was assessed using the standard Cochran's Q statistic, calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, as follows:
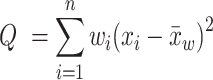 |
(1) |
where n is the number of studies, xi is the effect size for study i, wi is the weight for study i, and 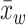 is the pooled weighted effect across studies. The I² statistic (45) describes the percentage of variation across studies due to heterogeneity rather than sampling error, where I² = 100% × (Q − df)/Q.
is the pooled weighted effect across studies. The I² statistic (45) describes the percentage of variation across studies due to heterogeneity rather than sampling error, where I² = 100% × (Q − df)/Q.
We first reanalyzed the effect sizes and CIs presented for the 28 studies included in the original publication meta-analysis (2). We then updated the analysis by re-extracting the data, calculating the effect sizes and CIs, and excluding and adding studies as described above, for the final 29 studies.
Because study duration accounted for a large degree of heterogeneity, we performed the meta-analysis separately for shorter and longer trials. Several authors have proposed 2 to 3 wk as the minimum time required for metabolic adaptation to reduced carbohydrate intake (4, 6–8, 13). We therefore dichotomized the cohort at 2.5 wk (shorter trials, ≤17 d; longer trials, >17 d), which provided an adequate sample to meta-analyze both groups. We also conducted sensitivity analyses dichotomized at 2 and 3 wk (as described below). Because the time course of physiological adaptation has not been precisely determined (and this process may be influenced by carbohydrate difference and baseline participant characteristics), other cutoffs could have been chosen to dichotomize duration. For conservativeness, we also report the P value for the interaction involving study duration using a Bonferroni adjustment for all 11 possible cutoffs among the included trials (i.e., ≤1, 2, 3, 4, 7, 9, 10, 14, 15, 18, and 28 d).
We performed primary analyses on the difference in TEE as presented in the trials. To take into account variability in treatment intensity (i.e., the magnitude of difference in dietary carbohydrate between trials), we also calculated TEE per 10% decrease in carbohydrate as a proportion of energy intake (EI) on the low- versus high-carbohydrate diet, assuming linearity. For example, in Dirlewanger et al. (46), the mean difference in TEE was −65 kcal/d, and the difference in carbohydrate as %EI was 30%, giving (−65/30) × 10 = −21.7 kcal/d per 10% decrease in carbohydrate as %EI. The 95% CIs were also adjusted accordingly. Because the carbohydrate-adjusted mean difference is differently scaled, it cannot be directly compared with the original TEE outcome.
Meta-regression
Using meta-regression with REML random effects, we included potential effect modifiers to determine how they 1) affected the overall estimated means and 2) accounted for variability in effects across studies. To evaluate the heterogeneity, we explored variability due to study duration, differences in carbohydrate amounts between diets, and TEE method (DLW or WRC). We recoded study duration into a binary variable: 0 for study duration <2.5 wk (≤17 d) and 1 for study duration >2.5 wk (>17 d). The difference in carbohydrate as %EI between diets was calculated and re-coded as a mean-centered variable by subtracting the mean difference of 33.2% across studies to aid interpretation of TEE effects at the mean level of the difference in carbohydrate.
We used the meta regress command in Stata 16 (41) to perform random-effects meta-regression allowing for residual heterogeneity (i.e., variance among studies that cannot be explained by the covariates) (47). The proportion of residual variation due to heterogeneity between studies, given by 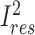 , was calculated as follows:
, was calculated as follows:
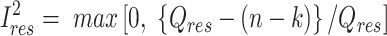 |
(2) |
where 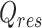 is the weighted sum of squares of the residuals from a fixed-effects meta-regression, n is the number of studies, and k is the number of covariates included in the model. The R2 estimates the proportion of between-study variance (τ2) explained by the covariates included in the model.
is the weighted sum of squares of the residuals from a fixed-effects meta-regression, n is the number of studies, and k is the number of covariates included in the model. The R2 estimates the proportion of between-study variance (τ2) explained by the covariates included in the model.
Sensitivity analyses
We conducted 6 sensitivity analyses to examine potential biases that might have arisen in the trials or from the analytic approach for the meta-analysis. First, we used a higher correlation (0.95) when calculating the SE for differences between diets in the crossover studies, as well as for changes from baseline, consistent with the original meta-analysis (2). Second, we examined how outcomes changed with a shorter adaptation period, dichotomizing studies at ≤14 d versus >14 d, thus moving 1 trial [Eckel et al. (33)] from the shorter to the longer group. Third, we examined how outcomes changed with a longer adaptation period, dichotomizing studies at ≤21 d versus >21 d, thus moving 1 trial [Abbott et al. (48)] from the longer to the shorter group. Fourth, for Rumpler et al. (49), we used final data only, rather than change, because the baseline (Day 0) data were obtained after prior exposure to the test diets. Fifth, we used data for Hall et al. (50) with revised Respiratory Quotient, as proposed by Hall et al. (51). Sixth, we addressed a methodological issue specific to Hall et al. (50). In this non-randomized crossover design, all participants received the higher-carbohydrate diet first. However, due to a miscalculation, they were underfed and progressively lost weight throughout the trial. Consequently, mean weight was about 2.3 kg less during the last 2 weeks on the lower-carbohydrate diet vs last 2 weeks on the higher-carbohydrate diet. To estimate the potential impact, we used the “expert mode” of the NIH Body Weight Planner (52) to calculate how energy requirement would change for a hypothetical individual with the characteristics of the average participant (male, age 33 years, initial weight 87.4 kg) following 2.3 kg weight loss over 28 days (assuming height of 180 cm and physical activity level of 1.6). The anticipated suppression of energy expenditure indicated by this calculation, 50 kcal/d, was added to the lower-carbohydrate diet in that trial.
Verification of analyses and transparency
All calculations of effect sizes and SEs (in Microsoft Excel; Microsoft Corporation) and meta-analyses (in Stata 16) were verified by a second statistician. The full dataset and statistical code used in this study are available at Open Science Framework (53).
Results
Trial characteristics
A total of 29 eligible trials with 617 participants were identified (Figure 1) over a 38-y period, from 1982 to 2020. Study duration varied widely, from 1 to 140 d (median, 4 d; mean, 13.0 d), including 23 shorter trials and 6 longer trials. The difference in dietary carbohydrate between intervention arms ranged from 8% to 77% of total energy intake (median, 30%; mean, 33.2%). Four trials measured TEE by DLW (1 shorter, 3 longer trials) and 25 by WRC (22 shorter, 3 longer trials). In addition to these design characteristics considered in statistical models, trials also varied in other aspects (e.g., randomization, crossover vs parallel, use of a run-in period, dietary energy level) and baseline participant characteristics (e.g., weight status, sex, age, fitness level, and health status).
FIGURE 1.
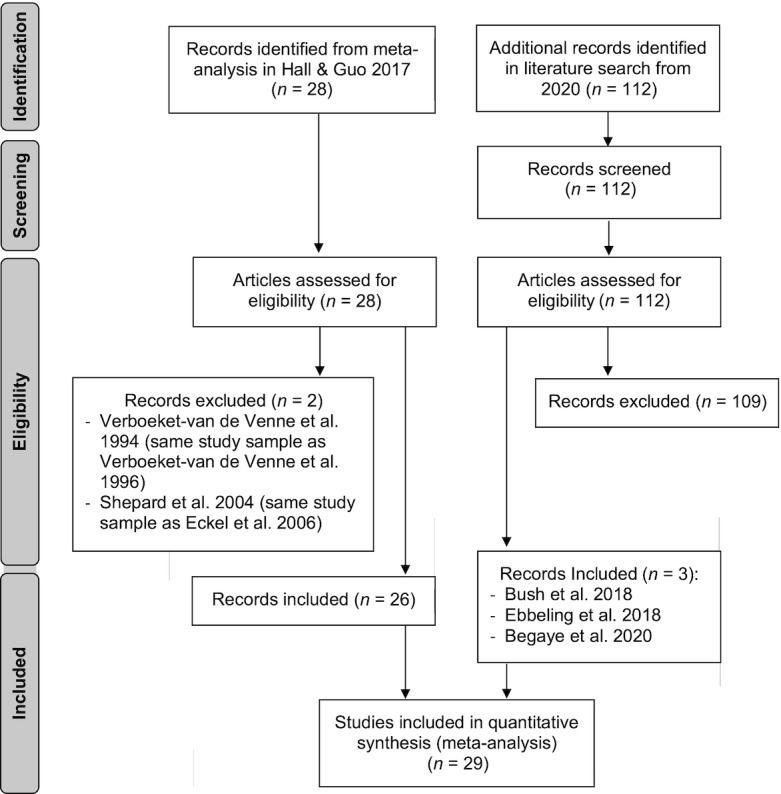
PRISMA flow chart of trials comparing the effects of lower- and higher-carbohydrate diets on total energy expenditure. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Reproduction of original meta-analysis
In the original meta-analysis (2) with 28 trials, the reported mean difference in a D-L random-effects model was −25.5 kcal/d (95% CI: −32.2, −18.8 kcal/d), favoring the higher-carbohydrate diet. Reanalyzing the original data, we obtained a D-L random-effects mean estimate of −38.6 kcal/d (95% CI: −63.5, −13.7 kcal/d), an REML random-effects mean estimate of −45.0 kcal/d (95% CI: −82.6, −7.3 kcal/d), and a fixed-effects mean estimate of −25.5 kcal/d (95% CI: −32.2, −18.8 kcal/d). We observed a discrepancy in estimates of heterogeneity. Whereas the original meta-analysis reported an I2 of 32.2%, we calculated Cochran's Q = 297.1 (df = 27) and I2 = 90.9% from the fixed-effects and D-L random-effects models and I2 = 96.3% from the REML model.
Heterogeneity related to study duration and macronutrient difference
Table 1
displays between-study heterogeneity and variability in meta-regression models accounting for the 3 covariates of interest, with the 29 trials in the updated analysis. The overall heterogeneity between studies without effect modifiers was I2 = 81.6. Study duration accounted for the most variability in TEE differences across studies (R2 = 57.2%) and reduced the residual heterogeneity furthest among the univariate meta-regressions, to 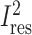 = 65.9%. Effect modification by study duration remained significant (P < 0.001) with Bonferroni correction for all 11 possible ways of dichotomizing duration. Additional inclusion of the difference in carbohydrate between experimental diets (P = 0.002) increased the between-study variability explained in the model to 76.5%, reducing
= 65.9%. Effect modification by study duration remained significant (P < 0.001) with Bonferroni correction for all 11 possible ways of dichotomizing duration. Additional inclusion of the difference in carbohydrate between experimental diets (P = 0.002) increased the between-study variability explained in the model to 76.5%, reducing 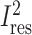 to 51.0%. TEE method (DLW or WRC) did not account for significant additional heterogeneity in models that also included study duration.
to 51.0%. TEE method (DLW or WRC) did not account for significant additional heterogeneity in models that also included study duration.
TABLE 1.
Effect of study design aspects (effect modifiers) on heterogeneity in TEE outcome among 29 trials comparing lower- and higher-carbohydrate diets1
| Modifiers | P | Qres2 | I2res,3 % | τ2 remaining4 | R 2,5 % |
|---|---|---|---|---|---|
| Overall (no modifier) | — | 124.2 | 81.6 | 6036 | — |
| Univariate | |||||
| Study duration | <0.001 | 84.7 | 65.9 | 2586 | 57.2 |
| Difference in carbohydrate | 0.001 | 82.1 | 71.8 | 3534 | 41.5 |
| TEE method6 | 0.004 | 103.6 | 73.4 | 3660 | 39.4 |
| Multivariate | |||||
| Study duration | <0.001 | 54.3 | 51.0 | 1420 | 76.5 |
| Difference in carbohydrate | 0.002 | ||||
| Study duration | <0.001 | 84.1 | 67.1 | 2659 | 56.0 |
| TEE method | 0.919 | ||||
| Difference in carbohydrate | <0.001 | 56.3 | 46.4 | 1173 | 80.6 |
| TEE method | <0.001 | ||||
| Study duration | 0.011 | 49.7 | 46.2 | 1141 | 81.1 |
| Difference in carbohydrate | 0.001 | ||||
| TEE method | 0.130 |
TEE, total energy expenditure.
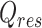 is Cochran's Q statistic for heterogeneity remaining after effect modifiers included.
is Cochran's Q statistic for heterogeneity remaining after effect modifiers included.
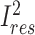 is the percentage of variation across studies due to heterogeneity remaining after modifiers included.
is the percentage of variation across studies due to heterogeneity remaining after modifiers included.
τ2 is the between-study variability remaining after modifiers included.
R 2 is the percentage of variability (τ2) explained by the modifiers in each model.
Whole-room calorimetry vs doubly labeled water.
Updated meta-analysis considering study duration and macronutrient differences
As study duration accounted for the most heterogeneity across studies, we performed meta-analysis separately for the shorter- and longer-duration trials. As shown in Table 2 and Figure 2, results from the REML random-effects models indicated that the lower-carbohydrate diet had modestly lower TEE among the shorter-duration studies of −50.0 kcal/d (95% CI: −77.4, −22.6 kcal/d; P < 0.001, I2 = 69.8) and substantially higher TEE among the longer studies of 135.4 kcal/d, (95% CI: 72.0, 198.7 kcal/d; P < 0.001, I2 = 26.4). Taking into account dietary differences, the results per 10% decrease in carbohydrate as %EI, respectively, are −14.5 kcal/d (95% CI: −21.0, −7.9 kcal/d; P < 0.001) and 50.4 kcal/d (95% CI: 31.4, 69.4 kcal/d; P < 0.001). Results of the 6 sensitivity analyses indicated that the primary findings were unchanged in terms of direction and statistical significance. Consistent with the hypothesized duration of the adaptive process, the mean effect among longer studies was weakened by dichotomizing at 2 wk and strengthened by dichotomizing at 3 wk.
TABLE 2.
Meta-analysis on TEE difference among 29 trials comparing lower- and higher-carbohydrate diets1
| Variable | Estimated mean difference | 95% CI | P |
|---|---|---|---|
| Primary analysis | |||
| TEE difference | |||
| Shorter duration | −50.0 | −77.4, −22.6 | <0.001 |
| Longer duration | 135.4 | 72.0, 198.7 | <0.001 |
| TEE difference per 10% difference in carbohydrate | |||
| Shorter duration | −14.5 | −21.0, −7.9 | <0.001 |
| Longer duration | 50.4 | 31.4, 69.4 | <0.001 |
| Sensitivity analyses | |||
| Using correlation of 0.95 for crossover studies | |||
| Shorter duration | −51.0 | −77.2, −24.8 | <0.001 |
| Longer duration | 133.0 | 67.2, 198.8 | <0.001 |
| Using short vs long duration cutoff at 14 d | |||
| Shorter duration | −48.6 | −76.3, −20.8 | 0.001 |
| Longer duration | 111.1 | 27.6, 194.5 | 0.009 |
| Using short vs long duration cutoff at 21 d | |||
| Shorter duration | −45.9 | −73.5, −18.3 | 0.001 |
| Longer duration | 156.5 | 84.5, 228.5 | <0.001 |
| Using final data (instead of change) in Rumpler et al. (49) | |||
| Shorter duration | −50.0 | −77.4, −22.6 | <0.001 |
| Longer duration | 128.5 | 66.4, 190.6 | <0.001 |
| Adjusting for ∆RQ in Hall et al. (50, 51) | |||
| Shorter duration | −50.0 | −77.4, −22.6 | <0.001 |
| Longer duration | 125.1 | 65.6, 184.6 | <0.001 |
| Accounting for progressive weight loss in Hall et al. (50) | |||
| Shorter duration | −50.0 | −77.4, −22.6 | <0.001 |
| Longer duration | 144.6 | 74.7, 214.5 | <0.001 |
Difference is lower-carbohydrate diet minus higher-carbohydrate diet; see Methods for details of models. RQ, Respiratory Quotient; TEE, total energy expenditure. Shorter duration, ≤17 d; longer duration, >17 d
FIGURE 2.
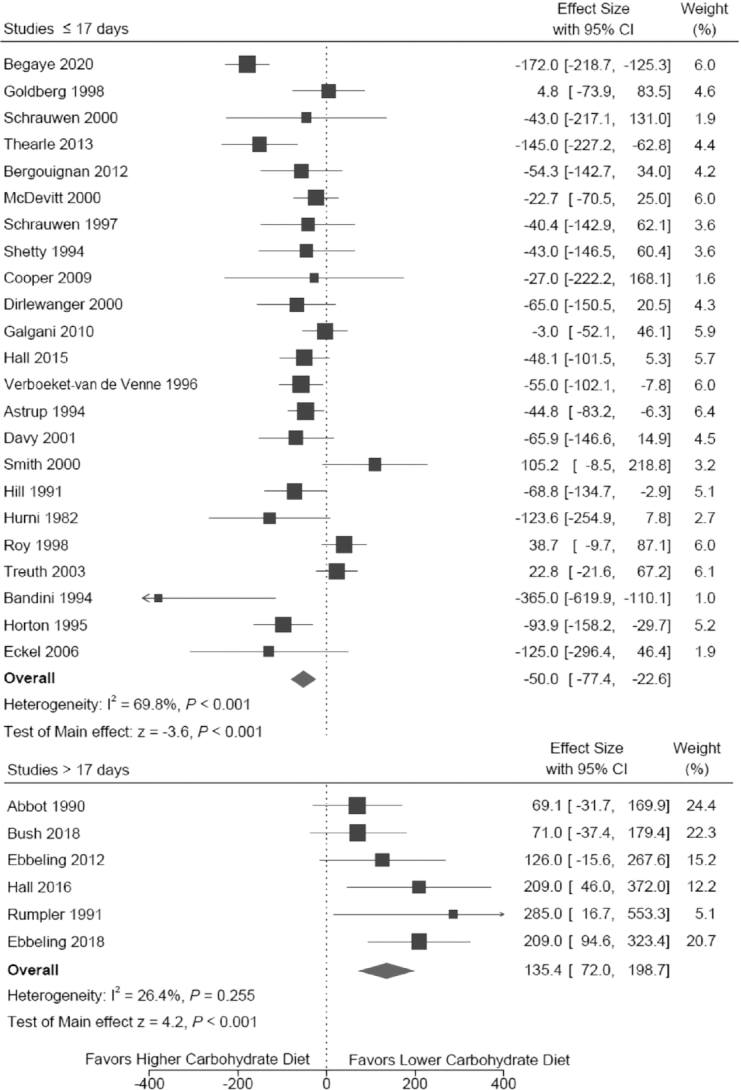
Forest plot of total energy expenditure effect among 29 trials comparing lower- and higher-carbohydrate diets. Trials are listed according to intervention duration in ascending order (i.e., shortest duration at top). Full citations for the individual trials can be found in Supplementary Data.
Discussion
In this updated and reanalyzed meta-analysis, we found that heterogeneity among trials comparing the effect of lower- versus higher-carbohydrate diets on TEE is greater than previously reported (2), consistent with hypothesized effect modification by trial duration and/or additional factors. With control for duration and macronutrient difference, heterogeneity decreased substantially in our study. Among trials <2.5 wk, the lower-carbohydrate diets slightly reduced TEE, with reduced remaining heterogeneity. By contrast, among trials of >2.5 wk, the lower-carbohydrate diet substantially increased TEE—by ∼50 kcal/d for every 10% decrease in carbohydrate as %EI—with minimal residual heterogeneity. These results suggest that the shorter versus longer studies have examined different physiological states. The former consist of trials in which participants experienced varying degrees of metabolic adaptation to carbohydrate reduction; the latter consist of trials of sufficient duration to allow for adequate adaptation and to produce a consistent finding.
This finding supports a prediction of the carbohydrate-insulin model (4, 54–58) and suggests a mechanism whereby dietary carbohydrate reduction could aid in the prevention and treatment of obesity. According to this model, the high insulin-to-glucagon ratio with a diet high in glycemic load (mathematical product of glycemic index and carbohydrate amount) shifts the partitioning of metabolic fuels from oxidation in lean tissue to storage in adipose tissue. If the effects observed here persist over the long term, then reducing dietary carbohydrate intake by half from 60% of energy intake (a typical level for low-fat diets) would increase energy expenditure by ∼150 kcal/d, counterbalancing (if not compensated for by other factors) much of the secular increase in energy intake thought by some to underlie the obesity epidemic (59).
Consistent with our findings, short- versus long-term effects often differ in studies of dietary interventions (60). For instance, the rapid initial weight loss with very-low-calorie diets is not indicative of the effectiveness of these highly restrictive approaches for chronic obesity treatment. Thus, apart from mechanistic examination of the adaptive process per se, short-term studies comparing diets with differing macronutrient composition are likely to yield misleading estimates of long-term effects.
A main strength of this reanalysis is the ability to test a physiological hypothesis with adequate power, revealing an effect of macronutrients not apparent in the original analysis (2). We used a conservative statistical adjustment to examine how study duration affects outcome, and then conducted sensitivity analyses to examine plausible sources of bias. In addition, we have made the database with the trials publicly available to facilitate transparency and further examination.
Several limitations warrant consideration. First, many of the trials have low quality related to small size, lack of randomization, limited methodological detail (especially for the older studies), and other issues. Second, we cannot rule out the possibility of dietary nonadherence to the test diets. Participants in studies conducted at least partially outside of a metabolic ward could have underconsumed study foods or consumed foods off protocol. Dietary nonadherence would tend to inflate DLW estimation of TEE on a lower- versus higher-carbohydrate diet due to dependency of this method on estimated Food Quotient as a proxy for Respiratory Quotient. (This problem would not apply to WRC measurement of TEE, because Respiratory Quotient is determined directly.) Conversely, WRC may underestimate TEE due to suppression of nonresting energy expenditure (the component of TEE considered to be most involved in adaptive thermogenesis) (37). Reassuringly, we found no significant heterogeneity arising from the TEE measurement method after adjustment for study duration. Third, although ongoing adaptations beyond 3 wk cannot be excluded, we had insufficient power to test this possibility. To the extent that the longer studies included incompletely adapted participants, effect estimates could be underestimated. Fourth, we did not examine quantitative aspects of macronutrients that might affect insulin secretion or metabolism, such as glycemic index. According to preliminary data potentially consistent with the carbohydrate-insulin model, TEE by WRC decreased after 12 wk (by 136 kcal/d) with a diet high in sugar-sweetened beverages, whereas TEE either did not decrease or increased (by 127 kcal/d) with 2 comparison diets controlled for macronutrients that were high in either meat or fish, respectively (61). Dietary fatty acid type, specifically the relative amounts of saturated versus unsaturated fats, may also have metabolic effects of relevance to energy balance and adiposity (62). Fifth, only 1 trial examined effect modification by individual-level baseline biological characteristics (34), too few for meta-analysis. The carbohydrate-insulin model predicts that the largest increase in TEE with carbohydrate restriction will occur among individuals with the highest insulin secretion response to carbohydrate, defined as insulin concentration 30 min into a standard oral-glucose-tolerance test (63–66). Information about such subgroup susceptibility may inform a “personalized” approach to weight control, wherein a low-carbohydrate diet might be targeted to those most likely to benefit.
In view of the complexity of the physiological mechanisms and interindividual variability in response—potentially related to behavioral factors or biological factors such insulin secretion—high-quality studies with different designs will be necessary to further elucidate how macronutrients affect energy metabolism and fat storage, with attention to potentially susceptible subgroups, and translate findings to clinical interventions and public health messages. Important scientific and practical information could be obtained from trials that variously control energy intake (allowing body weight to change), control body weight (adjusting energy intake accordingly), or permit ad libitum food intake (controlling for confounding dietary and environmental factors when feasible). In addition, studies with participants habituated to a low-carbohydrate diet prior to randomization would also be of interest (the converse of most trials to date). An adequate trial duration will also be needed with this design, as consumption of a low-carbohydrate diet may protect for at least 1 mo against adverse effects of dietary carbohydrate on metabolism through persistent reduction in insulin secretion (65).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dariush Mozaffarian for advice on study design and interpretation and for editorial comments on an earlier version of the manuscript. We also thank Kevin Hall for review of, and comments on, an earlier version of this manuscript and Melissa Su Ching Lee for help with coding the meta-analyses in Stata. The authors’ responsibilities were as follows—DSL: designed the study, collected and interpreted data, and participated in manuscript preparation; SLD: designed the study, participated in maintaining quality control of the data, conducted the statistical analyses, interpreted the data, and participated in manuscript preparation; BH: assisted with data collection, conducted statistical analyses, and participated in manuscript preparation; CBE: designed the study, collected and interpreted data, and participated in manuscript preparation; DBA: interpreted data, provided statistical supervision, and participated in manuscript preparation; and all authors: read and approved the final manuscript.
Notes
This work was funded by a grant from the New Balance Foundation.
Author disclosures: DSL and CBE have conducted research studies examining the carbohydrate-insulin model funded by the NIH and philanthropic organizations unaffiliated with the food industry; DSL received royalties for books on obesity and nutrition that recommend a low-glycemic-load diet. In the last 36 mo, DBA has received personal payments or promises for same from the American Society for Nutrition; Alkermes, Inc.; American Statistical Association; Biofortis; California Walnut Commission; Columbia University; Fish & Richardson, PC; Frontiers Publishing; Henry Stewart Talks; IKEA; Indiana University; Laura and John Arnold Foundation; Johns Hopkins University; Law Offices of Ronald Marron; MD Anderson Cancer Center; Medical College of Wisconsin; NIH; Sage Publishing; The Obesity Society; Tomasik, Kotin & Kasserman LLC; University of Alabama at Birmingham; University of Miami; Nestle; and WW (formerly Weight Watchers International, LLC). Donations to a foundation have been made on his behalf by the Northarvest Bean Growers Association. DBA has been an unpaid member of the International Life Sciences Institute North America Board of Trustees. DBA's institution, Indiana University, has received funds to support his research or educational activities from Soleno Therapeutics; NIH; Eli Lilly, Alliance for Potato Research and Education; American Federation for Aging Research; Dairy Management, Inc.; Herbalife; Laura and John Arnold Foundation; National Cattlemen's Beef Association, Oxford University Press, the Sloan Foundation, The Gordan and Betty Moore Foundation, and numerous other for-profit and nonprofit organizations to support the work of the School of Public Health and the university more broadly.
The funder had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation of the manuscript; or decision to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the study sponsors.
Supplemental Tables 1 and 2, with Supplemental References, are available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
The full dataset and statistical code used in this study are available at Open Science Framework (53).
Abbreviations used: BOHB, B-hydroxybutyrate; D-L, DerSimonian-Laird; DLW, doubly-labeled water; EI, energy intake; REML, Restricted Maximum Likelihood; TEE, total energy expenditure; tiab, title/abstract; WRC, whole-room calorimetry.
Contributor Information
David S Ludwig, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
Stephanie L Dickinson, Indiana University School of Public Health–Bloomington, Bloomington, IN, USA.
Beate Henschel, Indiana University School of Public Health–Bloomington, Bloomington, IN, USA.
Cara B Ebbeling, New Balance Foundation Obesity Prevention Center, Boston Children's Hospital and Harvard Medical School, Boston, MA, USA.
David B Allison, Indiana University School of Public Health–Bloomington, Bloomington, IN, USA.
References
- 1. Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LA et al. Obesity pathogenesis: an Endocrine Society scientific statement. Endocr Rev. 2017;38:267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall KD, Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang J, Ratamess NA, Faigenbaum AD, Bush JA. Ergogenic properties of ketogenic diets in normal-weig. ht individuals: a systematic review. J Am Coll Nutr. 2020;39:(7):665–75. [DOI] [PubMed] [Google Scholar]
- 4. Ludwig DS, Ebbeling CB. The carbohydrate-insulin model of obesity: beyond “calories in, calories out”. JAMA Intern Med. 2018;178(8):1098–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manninen AH. Very-low-carbohydrate diets and preservation of muscle mass. Nutr Metab (Lond). 2006;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McSwiney FT, Doyle L, Plews DJ, Zinn C. Impact of ketogenic diet on athletes: current insights. Open Access J Sports Med. 2019;10:171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruskin DN, Masino SA. The nervous system and metabolic dysregulation: emerging evidence converges on ketogenic diet therapy. Front Neurosci. 2012;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaw DM, Merien F, Braakhuis A, Maunder E, Dulson DK. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Sports Med. 2020;50(4):641–56. [DOI] [PubMed] [Google Scholar]
- 9. Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am J Clin Nutr. 2019;110(3):562–73. [DOI] [PubMed] [Google Scholar]
- 10. Hawley JA. Fat adaptation science: low-carbohydrate, high- fat diets to alter fuel utilization and promote training adaptation. Nestle Nutr Inst Workshop Ser. 2011;69:59–71. [DOI] [PubMed] [Google Scholar]
- 11. Lundsgaard AM, Holm JB, Sjoberg KA, Bojsen-Moller KN, Myrmel LS, Fjaere E, Jensen BAH, Nicolaisen TS, Hingst JR, Hansen SL et al. Mechanisms preserving insulin action during high dietary fat intake. Cell Metab. 2019;29(1):50. [DOI] [PubMed] [Google Scholar]
- 12. Yeo WK, Carey AL, Burke L, Spriet LL, Hawley JA. Fat adaptation in well-trained athletes: effects on cell metabolism. Appl Physiol Nutr Metab. 2011;36(1):12–22. [DOI] [PubMed] [Google Scholar]
- 13. Phinney SD. Ketogenic diets and physical performance. Nutr Metab. 2004;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Owen OE, Caprio S, Reichard GA Jr, Mozzoli MA, Boden G, Owen RS. Ketosis of starvation: a revisit and new perspectives. Clin Endocrinol Metab. 1983;12(2):359–79. [DOI] [PubMed] [Google Scholar]
- 15. Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int. 2001;38(6):519–27. [DOI] [PubMed] [Google Scholar]
- 16. Fukazawa A, Koike A, Karasawa T, Tsutsui M, Kondo S, Terada S. Effects of a ketogenic diet containing medium-chain triglycerides and endurance training on metabolic enzyme adaptations in rat skeletal muscle. Nutrients. 2020;12(5):1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciapaite J, van den Broek NM, Te Brinke H, Nicolay K, Jeneson JA, Houten SM, Prompers JJ. Differential effects of short- and long-term high-fat diet feeding on hepatic fatty acid metabolism in rats. Biochim Biophys Acta. 2011;1811(7–8):441–51. [DOI] [PubMed] [Google Scholar]
- 18. Phinney SD, Horton ES, Sims EA, Hanson JS, Danforth E Jr, LaGrange BM. Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric, ketogenic diet. J Clin Invest. 1980;66(5):1152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metab. 2018;2018:5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55(11):2211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40(1):238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watanabe M, Singhal G, Fisher FM, Beck TC, Morgan DA, Socciarelli F, Mather ML, Risi R, Bourke J, Rahmouni K et al. Liver-derived FGF21 is essential for full adaptation to ketogenic diet but does not regulate glucose homeostasis. Endocrine. 2020;67(1):95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winzell MS, Magnusson C, Ahren B. Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metabolism. 2007;56(1):122–8. [DOI] [PubMed] [Google Scholar]
- 24. Bortz WM, Wroldson A, Morris P, Issekutz B Jr. Fat, carbohydrate, salt, and weight loss. Am J Clin Nutr. 1967;20(10):1104–12. [DOI] [PubMed] [Google Scholar]
- 25. Phinney SD, Bistrian BR, Wolfe RR, Blackburn GL. The human metabolic response to chronic ketosis without caloric restriction: physical and biochemical adaptation. Metabolism. 1983;32(8):757–68. [DOI] [PubMed] [Google Scholar]
- 26. Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992;41(4):406–14. [DOI] [PubMed] [Google Scholar]
- 27. Bostock ECS, Kirkby KC, Taylor BV, Hawrelak JA. Consumer reports of “keto flu” associated with the ketogenic diet. Front Nutr. 2020;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nymo S, Coutinho SR, Jorgensen J, Rehfeld JF, Truby H, Kulseng B, Martins C. Timeline of changes in appetite during weight loss with a ketogenic diet. Int J Obes. 2017;41(8):1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horton TJ, Drougas H, Brachey A, Reed GW, Peters JC, Hill JO. Fat and carbohydrate overfeeding in humans: different effects on energy storage. Am J Clin Nutr. 1995;62(1):19–29. [DOI] [PubMed] [Google Scholar]
- 30. Verboeket-van de Venne WP, Westerterp KR, ten Hoor F. Substrate utilization in man: effects of dietary fat and carbohydrate. Metabolism. 1994;43(2):152–6. [DOI] [PubMed] [Google Scholar]
- 31. Verboeket-van de Venne WP, Westerterp KR. Effects of dietary fat and carbohydrate exchange on human energy metabolism. Appetite. 1996;26(3):287–300. [DOI] [PubMed] [Google Scholar]
- 32. Shepard TY, Weil KM, Sharp TA, Grunwald GK, Bell ML, Hill JO, Eckel RH. Occasional physical inactivity combined with a high-fat diet may be important in the development and maintenance of obesity in human subjects. Am J Clin Nutr. 2001;73(4):703–8. [DOI] [PubMed] [Google Scholar]
- 33. Eckel RH, Hernandez TL, Bell ML, Weil KM, Shepard TY, Grunwald GK, Sharp TA, Francis CC, Hill JO. Carbohydrate balance predicts weight and fat gain in adults. Am J Clin Nutr. 2006;83(4):803–8. [DOI] [PubMed] [Google Scholar]
- 34. Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bush NC, Resuehr HES, Goree LL, Locher JL, Bray MS, Soleymani T, Gower BA. A high-fat compared with a high-carbohydrate breakfast enhances 24-hour fat oxidation in older adults. J Nutr. 2018;148(2):220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Begaye B, Vinales KL, Hollstein T, Ando T, Walter M, Bogardus C, Krakoff J, Piaggi P. Impaired Metabolic Flexibility to High-Fat Overfeeding Predicts Future Weight Gain in Healthy Adults. Diabetes. 2020;69(2):181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol. 1996;270(3 Pt 2):R496–504. [DOI] [PubMed] [Google Scholar]
- 38. Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins J, Thomas J, . Cochrane handbook for systematic reviews of interventions. London: Cochrane; 2019. [Google Scholar]
- 39. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis. Hoboken (NJ): John Wiley & Sons; 2009. [Google Scholar]
- 40. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. [Google Scholar]
- 41. Stata meta-analysis reference manual, release 16. College Station (TX): StataCorp LLC; 2019; [Internet]. Available from: https://www.stata.com/manuals/meta.pdf. [Google Scholar]
- 42. Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: Fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3–28. [Google Scholar]
- 43. Raudenbush SW. Analyzing effect sizes: random-effects models. Cooper H, Hedges LV, Valentine JC, . New York: Russell Sage Foundation; 2009. [Google Scholar]
- 44. Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, Viechtbauer W, Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Syn Meth. 2019;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- 45. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dirlewanger M, di Vetta V, Guenat E, Battilana P, Seematter G, Schneiter P, Jéquier E, Tappy L. Effects of short-term carbohydrate or fat overfeeding on energy expenditure and plasma leptin concentrations in healthy female subjects. Int J Obes. 2000;24(11):1413–8. [DOI] [PubMed] [Google Scholar]
- 47. Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J. 2008;8(4):493–519. [Google Scholar]
- 48. Abbott WG, Howard BV, Ruotolo G, Ravussin E. Energy expenditure in humans: effects of dietary fat and carbohydrate. Am J Physiol. 1990;258(2 Pt 1):E347–51. [DOI] [PubMed] [Google Scholar]
- 49. Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr. 1991;53(2):430–6. [DOI] [PubMed] [Google Scholar]
- 50. Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hall KD, Guo J, Chen KY, Leibel RL, Reitman ML, Rosenbaum M, Smith SR, Ravussin E. Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am J Clin Nutr. 2019;109(5):1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. National Institute of Diabetes and Digestive and Kidney Diseases . Body weight planner [Internet]. [Accessed 2020 Oct 1]. Available from: https://www.niddk.nih.gov/bwp.
- 53. Dickinson S, Ludwig D, Ebbeling C, Allison D, Henschel B. Do lower-carbohydrate diets increase total energy expenditure? An updated and reanalyzed meta-analysis of 29 controlled feeding studies. Open Science Framework. 2020; [Internet]. [Accessed 2020 Nov 24]. Available from: https://osf.io/2hyqr/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287(18):2414–23. [DOI] [PubMed] [Google Scholar]
- 55. Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating?. JAMA. 2014;311(21):2167–8. [DOI] [PubMed] [Google Scholar]
- 56. Friedman MI, Stricker EM. The physiological psychology of hunger: a physiological perspective. Psychol Rev. 1976;83(6):409–31. [PubMed] [Google Scholar]
- 57. Taubes G. The science of obesity: what do we really know about what makes us fat? An essay by Gary Taubes. BMJ. 2013;346:f1050. [DOI] [PubMed] [Google Scholar]
- 58. Shimy KJ, Feldman HA, Klein GL, Bielak L, Ebbeling CB, Ludwig DS. Effects of dietary carbohydrate content on circulating metabolic fuel availability in the postprandial state. J Endocr Soc. 2020;4(7):bvaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wright JD, Kennedy-Stephenson J, Wang CY, McDowell MA, Johnson CL. Trends in intake of energy and macronutrients—United States, 1971–2000. MMWR Morb Mortal Wkly Rep. 2004;53(4):80–2. [PubMed] [Google Scholar]
- 60. Ludwig DS, Astrup A, Bazzano LA, Ebbeling CB, Heymsfield SB, King JC, Willett WC. Ultra-processed food and obesity: the pitfalls of extrapolation from short studies. Cell Metab. 2019;30(1):3–4. [DOI] [PubMed] [Google Scholar]
- 61. Mitchell CM, Piaggi P, Krakoff J, Votruba SB. Diets high in fish and sugar-sweetened beverages affect energy expenditure and energy balance (3901). American College of Sports Medicine; 2020; [Internet]. [Accessed 2020 Nov 24]. Available from: https://virtualmeeting.ctimeetingtech.com/acsm2020/attendee/eposter/poster/1663. [Google Scholar]
- 62. Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63(7):2356–68. [DOI] [PubMed] [Google Scholar]
- 63. Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87(2):303–9. [DOI] [PubMed] [Google Scholar]
- 64. Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297(19):2092–102. [DOI] [PubMed] [Google Scholar]
- 65. Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity. 2015;23(11):2216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364(9436):778–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


