ABSTRACT
Background
Differences in health effects of dietary α-linolenic acid (ALA) and DHA are mediated at least in part by differences in their effects on oxylipins.
Objectives
Time course and sex differences of plasma oxylipins in response to ALA- compared with DHA-rich supplements were examined.
Methods
Healthy men and women, aged 19–34 y and BMI 18–28 kg/m2, were provided with capsules containing ∼4 g/d of ALA or DHA in a randomized double-blind crossover study with >6-wk wash-in and wash-out phases. Plasma PUFA and oxylipin (primary outcome) concentrations at days 0, 1, 3, 7, 14, and 28 of supplementation were analyzed by GC and HPLC-MS/MS, respectively. Sex differences, supplementation and time effects, and days to plateau were analyzed.
Results
ALA supplementation doubled ALA concentrations, but had no effects on ALA oxylipins after 28 d, whereas DHA supplementation tripled both DHA and its oxylipins. Increases in DHA oxylipins were detected as early as day 1, and a plateau was reached by days 5–7 for 11 of 12 individual DHA oxylipins and for total DHA oxylipins. Nine individual DHA oxylipins reached a plateau in females with DHA supplementation, compared with only 4 in males. A similar time course and sex difference pattern occurred with EPA and its oxylipins with DHA supplementation. DHA compared with ALA supplementation also resulted in higher concentrations of 4 individual arachidonic acids, 1 linoleic acid, and 1 dihomo-γ-linolenic acid oxylipin, despite not increasing the concentrations of these fatty acids, further demonstrating that oxylipins do not always reflect their precursor PUFA.
Conclusions
DHA compared with a similar dose of ALA has greater effects on both n–3 and n–6 oxylipins in young, healthy adults, with differences in response to DHA supplementation occurring earlier and being greater in females. These findings can help explain differences in dietary effects of ALA and DHA.
This study was registered at clinicaltrials.gov as NCT02317588.
Keywords: oxylipin, α-linolenic acid, DHA, time course, sex, plasma, human
See corresponding commentary on page 462.
Introduction
Consumption of n–3 PUFAs has been associated with numerous health benefits, including reduced risk of many disorders such as cardiovascular diseases, cancers, inflammatory disorders, prematurity, and depression, although not all findings are consistent (reviewed in references 1–8). This evidence comes primarily from studies with EPA and DHA, but studies with the main dietary 18-carbon n–3 PUFA, namely α-linolenic acid (ALA, 18:3n–3), indicate that it also has beneficial effects that can be different and that are often less potent (1, 2, 8). These effects are generally ascribed at least in part to the oxylipins formed from these PUFAs and likely help explain the different effects of these PUFAs. Oxylipins are biologically active lipids formed from PUFAs released from membrane phospholipids by phospholipase A2 and oxygenated by cyclo-oxygenases, lipoxygenases, and cytochrome P450 enzymes (9).
Conclusions regarding the effects of oxylipins have long been based on data of PUFA composition and a few select oxylipins, but both human and rodent supplementation studies indicate that although effects on oxylipins often reflect PUFA composition, this is not always the case (10–14). Recently more comprehensive direct analysis of the oxylipin profile based on HPLC-MS has become feasible, allowing the direct comparison of supplementation effects on PUFAs and oxylipins. Such studies with combined EPA + DHA supplementation in healthy humans have shown marked increases in plasma EPA and DHA and many oxylipins derived from these PUFAs [reviewed by Ostermann and Schebb (10)]. The concentration of EPA in the supplements was generally higher than DHA in these studies, but in a recent study, DHA supplementation alone primarily increased DHA oxylipins and a small number of EPA oxylipins (15). However, the few studies in humans that have examined the effects of oxylipins produced in response to consumption of ALA suggest that ALA supplementation results in increased plasma ALA and EPA, but relatively small increases in ALA oxylipins (11, 12).
Changes in oxylipins during the time course of supplementation have been reported in several studies with combined EPA + DHA supplementation. In these studies, EPA and DHA oxylipins both increase as early as 3 d or 1 wk after such supplementation (16, 17), whereas it is primarily EPA plasma oxylipins that increase after 6 h of a single low dose of EPA plus DHA (18). In contrast, supplementation with DHA alone increases DHA oxylipins and a small number of EPA oxylipins after as early as 1 wk (15). With respect to ALA supplementation, there has been only 1 study in men that documented oxylipin changes in human plasma over time (12).
Differences in response to supplementation also can be affected by sex. There are known sex differences in plasma PUFAs (19, 20), but little is known about sex differences in oxylipins in response to n–3 PUFA supplementation. The small number of EPA + DHA supplementation studies that have included both males and females were not designed to examine this, and few sex differences were analyzed or reported (15–17, 21–24). Similarly, in a study designed to examine age differences, we reported that supplementation with ALA-rich flaxseed reduced 3 n–6 PUFA–derived oxylipins in females compared with males (11). In rats we have found that serum DHA oxylipins, but not DHA itself, displayed sex differences that were influenced by the diet (13, 14), providing another example of the discrepancy between PUFAs and their oxylipins.
Therefore, the objective of the current study was to systematically examine the time course and sex effects of plasma oxylipins in response to a direct comparison of a similar dose of ALA- and DHA-rich supplements in healthy young men and women. Herein we provide several examples of oxylipin concentrations that do not reflect precursor PUFAs, and demonstrate differing effects of ALA- compared with DHA-rich supplements on the time course of plasma oxylipin changes and on sex differences in their concentrations.
Methods
Participants
Participants for this randomized double-blind crossover trial were recruited from the Winnipeg, Manitoba, area using print, radio, and electronic media. Participants gave written informed consent before any study-related procedures were conducted. This study was approved by the University of Manitoba Research Ethics Board and St Boniface Hospital Research Review Committee. The study was registered at clinicaltrials.gov as NCT02317588. The flow of participants through the study is shown in a consort flow diagram in Supplemental Figure 1.
Volunteers were excluded from participation if they had a clinically diagnosed disease currently affecting the circulatory, respiratory, immune, skeletal, urinary, muscular, endocrine, digestive, nervous, or reproductive system, or a disease condition that had required medical treatment. Additional exclusion criteria included regular use of nonsteroidal anti-inflammatory drugs or n–3 supplements within the past 3 mo, allergy or sensitivity to fish or flax products, current or past cigar/cigarette smoking within the past 12 mo, unstable body weight in the past 6 mo (±3 kg), consumption of >15 alcoholic beverages/wk within the past 3 mo or while participating in the study, current viral, bacterial, or fungal infection, or donation of blood in the past 2 mo.
Twelve individuals (6 males, 6 nonpregnant, nonlactating females) aged 19–34 y with a BMI between 18 and 28 kg/m2 and blood pressure <140/90 mmHg were enrolled by MG into the study. Sample size was based on pilot data of 5 individuals supplemented with an ALA- or EPA/DHA-rich supplement for 6 wk. Using pre- and post-supplementaion means and (SD) of oxylipins derived from ALA [9-hydroxy-octadecatrienoic acid: 278, 522 (165)], and DHA [17-hydroxy-docosahexaenoic acid (HDoHE): 222, 789 (262)], an n = 7 and 3, respectively, provided a power of >0.80 with an α = 0.05. An n = 12 was used to provide sufficient power for oxylipins present at low concentrations and to account for potential dropouts. These individuals also displayed normal blood lipid profiles, plasma creatinine, liver enzymes (aspartate transaminase, alanine transaminase), and normal menses and could be on birth control if female; a stable level of activity and stable regimen if taking vitamin/mineral/dietary supplements for the past month and while participating in the study; and willingness to follow the study protocol. Study population parameters are listed in Table 1.
TABLE 1.
Study population parameters at enrollment1
| Parameter | Total population (range) (n = 12) | Males (n = 6) | Females (n = 6) |
|---|---|---|---|
| Age, y | 25 ± 1 (19–34) | 25 ± 1 | 25 ± 1 |
| Body height, cm | 172 ± 2 (155–188) | 179 ± 3 | 165 ± 2 |
| Body weight, kg | 73 ± 2.4 (57–98) | 76 ± 4.4 | 70 ± 1.8 |
| BMI, kg/m2 | 24.6 ± 0.5 (20.6–27.8) | 23.5 ± 0.8 | 25.7 ± 0.5 |
| Systolic BP, mmHg | 114 ± 2 (104–126) | 115 ± 3 | 114 ± 3 |
| Diastolic BP, mmHg | 76 ± 1 (68–88) | 75 ± 2 | 77 ± 2 |
Values reported as mean ± SEM. BP, blood pressure.
Study design
All participants had a minimum 6-wk wash-in phase where they were instructed to avoid consumption of foods high in ALA, EPA, and DHA (defined as >0.3 g ALA/serving, or >0.1 g EPA + DHA/serving), and which was followed for the duration of the study. The wash-in phase was followed by a 28-d supplementation phase, another minimum 6-wk wash-out phase, and a second 28-d supplementation phase. Due to the crossover design the participants were randomly assigned to one of the supplementations during their first phase and then assigned to the other supplementation during their second phase according to the statistician-generated randomization code. The wash-in and wash-out phases varied in length to ensure females began the supplementation phase 9 ± 2 d after the beginning of menses.
At day 0 of each phase, participants attended the clinic after an overnight fast for baseline (day 0) testing. Height, weight, and blood pressure were measured and blood was collected. On days 1, 3, 7, 14, and 28 of each phase, participants also came into the clinic fasted and provided a blood sample. Weight and blood pressure were also measured at day 28 of each supplementation phase. Completion of activity questionnaires and 3-d food records was used to monitor participant compliance and determine background diet and activity levels. Plasma fatty acid composition was also used to verify participant compliance.
Oil supplementation
Similar daily doses (4.2 g ALA or 4.3 g DHA) were achieved by consumption of 7 flax oil capsules (NOW Foods; Natural Product Number 80002313) (ALA group) or 8 DHA oil capsules (Super DHA gems; Carlson Laboratories; Natural Product Number 80012587) (DHA group) per day. Flax oil capsules (1000 mg) contained 600 mg ALA, and DHA oil capsules (1000 mg) contained 540 mg DHA and 110 mg EPA as per manufacturer's specifications, and confirmed by our GC analyses (Supplemental Table 1). Capsules were prepackaged by a pharmacy (Taché Pharmacy) into 3 or 4 capsules per pack with 2 packs designated per day, provided in opaque packaging to achieve blinding. At the day 0 clinic visit participants were provided with a 2-wk supply of study capsules and instructed to consume the capsules with water, 1 pack with a morning meal and the second pack with an afternoon or evening meal. Participants were provided with a second 2-wk supply of study capsules at their day 14 visit and instructed to report any missed doses at each study visit and to bring back any missed capsules to their day 14 and day 28 visits. An individual not involved in the study visits or analyses oversaw the randomization of dispensed capsules and counted the returned capsules.
Sample collection and plasma biochemistry analysis
Blood samples were collected from participants after completing an overnight fast via venepuncture of an arm vein by a registered nurse or trained phlebotomist, and were immediately processed. For plasma preparation for fatty acid and oxylipin analysis, blood samples collected in tubes (BD Vacutainer Blood Collection Tubes; Becton Dickinson) containing EDTA were centrifuged for 10 min at 1100 × g at 4°C; supernatants were then aliquoted and frozen at −80°C until analyzed. All plasma samples for fatty acid and oxylipin analysis had antioxidant cocktail (0.2 mg/mL BHT, 0.2 mg/mL EDTA, 2 mg/mL triphenylphosphine, 2 mg/mL indomethacin in a solution of 2:1:1 methanol:ethanol:water) added at 3.3% of sample volume. Blood for plasma samples for biochemistry analysis was drawn into lithium heparin–containing tubes and analyzed by Shared Health Services at St Boniface Hospital using a Roche Cobas analyzer and kits from Roche Diagnostics.
Analysis of oxylipins and fatty acids
Plasma samples (400 μL) were analyzed in duplicate for oxylipins by HPLC/MS/MS as we have previously described (25, 26). Briefly, deuterated internal standards (Cayman Chemicals) were added to plasma samples and adjusted to pH <3.0 prior to solid-phase extraction using Strata-X-SPE (Phenomenex) columns preconditioned with methanol, followed by pH 3.0 water. Samples were loaded on the columns, washed, and free oxylipins were eluted with 100% methanol. The eluate was then dried and resuspended in solvent A (water/acetonitrile/formic acid, 70/30/0.02 v/v/v) for oxylipin analysis by HPLC/MS/MS, using a Luna 5-μm C18 column (100 Å, 250 × 2.0 mm, Phenomenex) on a Shimadzu Nexera XR HPLC, coupled to an ABSciex QTRAP 6500 MS operating in negative mode with electrospray ionization. Oxylipins were eluted with the following gradient: 100% solvent A between 0 and 1 min, and then solvent B (acetonitrile/isopropyl alcohol, 50/50, v/v) was increased linearly to 25% at 3 min, to 45% at 11 min, to 60% at 13 min, to 75% at 18 min, and to 90% at 18.5 min. Solvent B was then held at 90% until minute 20, dropped to 0% by 21 min, and held until 25 min. Quantification of oxylipins was performed using the stable isotope dilution method (27). The level of quantification was set at >5 times above baseline. Further details of all oxylipins scanned, mass transitions, internal standards, standard curve slopes, and retention times are provided in references 25 and 26.
For fatty acid analysis, total lipids were extracted from plasma or oils and fatty acids were analyzed as described (28, 29). Briefly, chloroform/methanol (2:1) containing 0.01% BHT and 1,2-dipentadecanoyl-sn-glycero-3-phosphocholine internal standard were added to plasma samples, and then total lipids extracted using chloroform/methanol and 0.73% sodium chloride. Hexane was used to extract the oils from the capsules and triheptanoin internal standard was used for quantification. Extracted total lipids were dried down, and the fatty acids transmethylated using methanolic H2SO4. The resulting FAMES were extracted in toluene, dried down and suspended in hexane, and separated on a 30-m DB-225MS column (Agilent Technologies) using a Bruker 450 gas chromatograph with flame ionization detection (FID). Analyses of both fatty acids and oxylipins were performed in random order.
Statistical analysis
All data were analyzed using SAS 9.3 (SAS Institute, Inc) and are presented as mean ± SEM. One male missed the day 14 and 28 study visits for the DHA supplementation phase and these missing data were not imputed. Anthropometric, blood lipid, fatty acid, and plasma oxylipin (primary outcome) data were analyzed using a series of mixed model approaches on each outcome variable to examine the group differences at each time point. The initial level of each outcome variable (intercept) was assumed to vary across individuals (random effect). Time was specified as a categorical variable to examine the group differences at each assessment, so the effects of time (day or phase) were treated as fixed effects; phase was not significant for any parameter and thus was not included in the reduced models. Supplementation and sex also were fixed effects. If sex or any of the sex interactions were not significant in the full model, the data were analyzed with a reduced model that included time, supplementation, time × supplementation, and the remaining sex/sex interactions as shown in Supplemental Tables 4–11. Adjusted P values (Tukey–Kramer corrections) for differences of least squares means were used to determine significant differences between ALA and DHA supplementation at a particular time point, and to determine significant differences from baseline during ALA supplementation or DHA supplementation. To calculate the plateau for PUFAs and oxylipins during each supplementation phase, quadratic modeling was used to estimate both linear and quadratic slopes. Time was treated as a continuous variable and the outcome as a cubic function of time. P < 0.05 was considered significant.
Results
General findings
All participants completed the study schedule, with the exception of 1 male participant in the DHA supplementation phase who missed days 14 and 28 due to a conflicting schedule. Capsule consumption compliance was 93% as determined by counting of the unused capsules returned by the participants. This was supported by fatty acid analysis that revealed that all individuals were within 2 SDs of the mean for both plasma ALA and DHA at all time points. Similarly, plasma ALA and DHA concentrations were not different between supplementation phases at baseline, indicating that the wash-in and wash-out phases were of sufficient length. Self-reported 3-d food records (Supplemental Table 2) and activity (Supplemental Table 3) indicated similar nutrient intakes and activity levels before the baseline visit and during week 3 of each supplementation phase. There were no effects of supplementation on weight, BMI, blood pressure, or on any serum lipid concentrations (cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, total cholesterol/HDL cholesterol, or LDL cholesterol/HDL cholesterol concentrations) (Table 2).
TABLE 2.
Anthropometrics and fasting plasma lipid concentrations of healthy young male and female adults at days 0 and 28 of ALA and DHA supplementation1
| ALA supplementation | DHA supplementation | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | |||||
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Body weight, kg | 77.3 ± 6.0 | 70.4 ± 2.8 | 76.6 ± 6.0 | 70.4 ± 2.5 | 76.5 ± 6.3 | 69.4 ± 2.3 | 76.9 ± 7.4 | 70.4 ± 2.6 |
| BMI, kg/m2 | 23.6 ± 1.1 | 25.5 ± 0.7 | 24.0 ± 1.2 | 25.9 ± 0.8 | 23.8 ± 1.1 | 25.9 ± 0.7 | 23.6 ± 1.1 | 25.8 ± 0.7 |
| Systolic BP, mmHg | 118 ± 2.8 | 114 ± 4.4 | 118 ± 3.5 | 112 ± 5.3 | 119 ± 2.6 | 119 ± 4.4 | 117 ± 6.6 | 113 ± 4.6 |
| Diastolic BP, mmHg | 77 ± 2.0 | 76 ± 2.8 | 74 ± 3.4 | 74 ± 3.1 | 75 ± 1.2 | 79 ± 2.6 | 74 ± 2.8 | 76 ± 2.7 |
| TC, mmol/L | 4.2 ± 0.1 | 4.7 ± 0.3 | 4.5 ± 0.2 | 4.5 ± 0.4 | 4.3 ± 0.1 | 4.4 ± 0.3 | 4.6 ± 0.3 | 4.4 ± 0.2 |
| TAG, mmol/L | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.3 | 0.7 ± 0.0 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| HDL-C,2 mmol/L | 1.4 ± 0.1 | 2.1 ± 0.3 | 1.4 ± 0.1 | 2.3 ± 0.4 | 1.3 ± 0.1 | 2.1 ± 0.3 | 1.4 ± 0.1 | 2.1 ± 0.4 |
| LDL-C,2 mmol/L | 2.4 ± 0.2 | 2.2 ± 0.2 | 2.7 ± 0.2 | 2.0 ± 0.1 | 2.6 ± 0.2 | 1.9 ± 0.2 | 2.7 ± 0.3 | 1.8 ± 0.2 |
| C/HDL-C2 | 3.3 ± 0.3 | 2.4 ± 0.2 | 3.4 ± 0.3 | 2.2 ± 0.3 | 3.5 ± 0.3 | 2.3 ± 0.2 | 3.3 ± 0.3 | 2.4 ± 0.3 |
| LDL-C/HDL-C2 | 1.9 ± 0.2 | 1.2 ± 0.2 | 2.1 ± 0.3 | 1.1 ± 0.2 | 2.1 ± 0.3 | 1.1 ± 0.2 | 2.0 ± 0.3 | 1.1 ± 0.3 |
Data were analyzed using a mixed model with individuals as a random effect, and time, supplementation, and sex as fixed effects. No supplementation or time effects were significant. Values reported as mean ± SEM; n = 6 for each group, except n = 5 for male day 28 time point in the DHA supplementation phase. ALA, α-linolenic acid; BP, blood pressure; C, cholesterol; TAG, triacylglycerols; TC, total cholesterol, .
Overall sex effect.
ALA compared with DHA supplementation effects on n–3 fatty acids and oxylipins
The data were tested for interactions between sex and supplementation or time, and if there were no such interactions, female and male data were combined to examine supplementation and time effects. All data and statistical details including effect sizes for Figures 1– 4 can be found in Supplemental Tables 4–11.
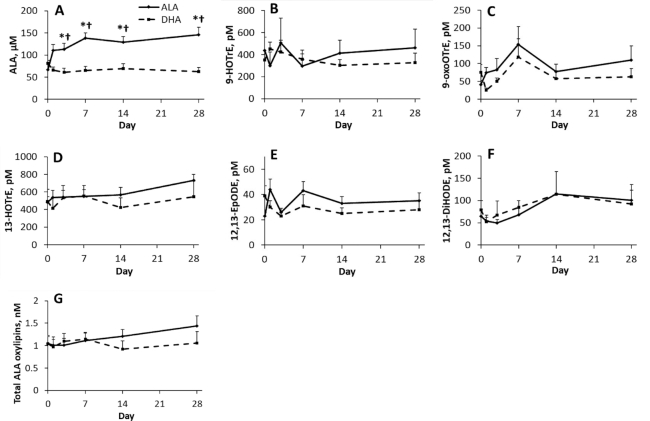
FIGURE 1.
Effect of time and supplementation of ALA or DHA on plasma (A) ALA, (B–F) individual ALA oxylipins, and (G) total ALA oxylipins in healthy young male and female adults. Where there is a supplementation by time interaction, †denotes value is different from DHA supplementation at that time point, and *denotes value is different from baseline for same supplementation based on significant adjusted P values (Tukey–Kramer corrections) for differences of least squares means. Data points are mean ± SEM for sexes combined and analyzed using a mixed model with individuals as a random effect, and time, supplementation, and sex as fixed effects; n = 12 for each time point, except for days 14 and 28 during the DHA supplementation phase, where n = 11. ALA, α-linolenic acid; DiHODE, dihydroxy-octadecadienoic acid; EpODE, epoxy-octadecadienoic acid; HOTrE, hydroxy-octadecatrienoic acid; oxoOTrE, oxo-octadecatrienoic acid.
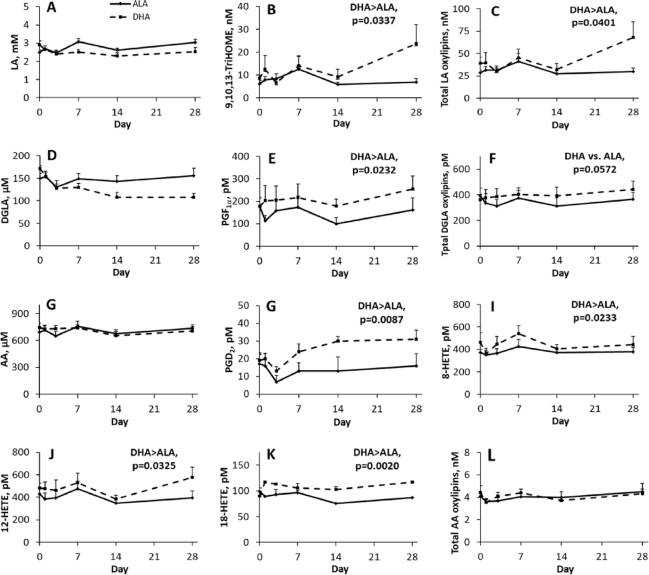
FIGURE 4.
Effect of time and supplementation of ALA and DHA in healthy young male and female adults on plasma (A) LA, (D) DGLA and (G) AA, and on individual or total n–6 oxylipins (B, C, E, F, H–L) with a main effect of supplementation. *Denotes value is different from baseline for same supplementation based on significant adjusted P values (Tukey–Kramer corrections) for differences of least squares means. Data points are mean ± SEM for sexes combined and analyzed using a mixed model with individuals as a random effect, and time, supplementation, and sex as fixed effects; n = 12 for each time point, except for days 14 and 28 during the DHA supplementation phase, where n = 11. AA, arachidonic acid; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; HETE, hydroxy-eicosatetraenoic acid; LA, linoleic acid; TriHOME, trihydroxy-octadecenoic acid.
ALA, but not DHA supplementation, increased plasma ALA concentrations, so from day 3 onward the plasma ALA concentrations were higher in the ALA- compared with the DHA-supplemented individuals (Figure 1). However, oxylipins derived from ALA were unchanged when the ALA supplement was provided. As a result, there were no differences in total ALA oxylipins at any time point during the ALA supplementation. Consistent with the lack of effect of DHA supplementation on plasma ALA concentrations, it also did not increase any individual or total ALA oxylipins (Figure 1).
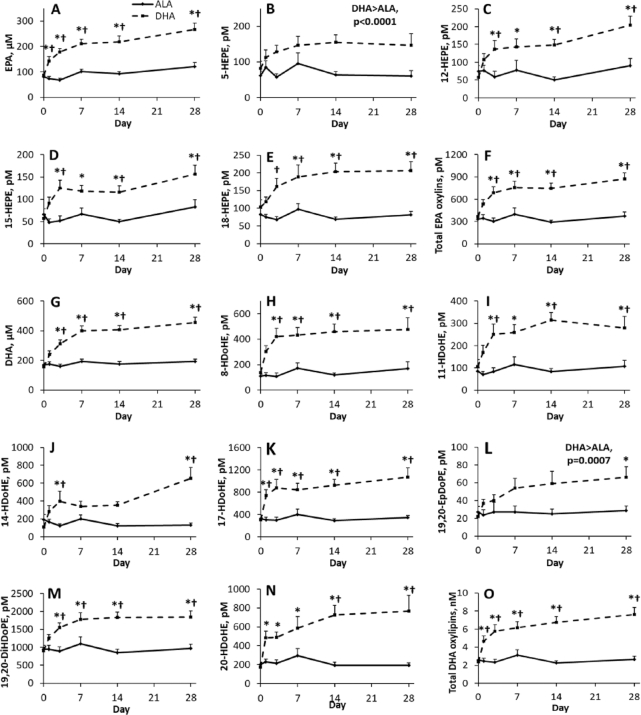
ALA supplementation did not increase EPA or DHA, whereas DHA supplementation increased EPA and DHA from days 1 and 3, respectively. As a result, as shown in Figure 2, EPA and DHA were higher with DHA compared with ALA supplementation from days 1 and 3 onward. DHA supplementation, but not ALA supplementation, also resulted in increases in most EPA and DHA oxylipins, similar to their effects on their precursor PUFA (Figure 2). Some DHA oxylipins (e.g., 13-, 17-, 20-HDoHE) and total DHA oxylipins were significantly increased as early as 1 d of supplementation.
FIGURE 2.
Effect of time and supplementation of ALA and DHA in healthy young male and female adults on plasma (A) EPA, (B–E) examples of individual EPA oxylipins with differences from baseline and/or between supplementations, (F) total EPA oxylipins, (G) DHA, (H–N) examples of individual DHA oxylipins with differences from baseline and/or between supplementations, and (O) total DHA oxylipins. Where there is a supplementation by time interaction, †denotes value is different from DHA supplementation at that time point, and *denotes value is different from baseline for same supplementation based on significant adjusted P values (Tukey–Kramer corrections) for differences of least squares means. Data points are mean ± SEM for sexes combined and analyzed using a mixed model with individuals as a random effect, and time, supplementation, and sex as fixed effects; n = 12 for each time point, except for days 14 and 28 during the DHA supplementation phase, where n = 11. ALA, α-linolenic acid; DiHDoPE, dihydroxy-docosapentaenoic acid; EpDoPE, epoxy-docosapentaenoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid.
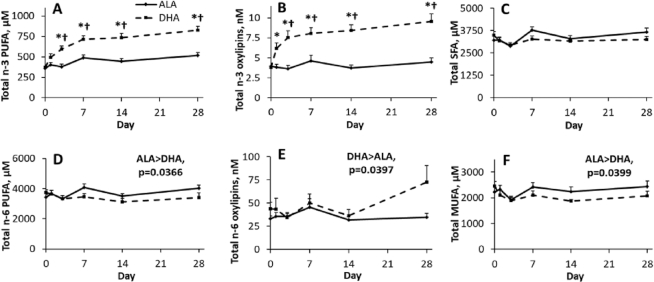
Overall, plasma total n–3 PUFAs were not altered by ALA supplementation, whereas DHA increased total n–3 PUFAs from day 3 onward, resulting in higher total n–3 PUFAs with DHA supplementation from day 3 onward (Figure 3). This contrast was also observed for total n–3 oxylipins, which were unaltered by ALA supplementation, but were increased 2-fold and were higher by day 3 of DHA supplementation (Figure 3).
FIGURE 3.
Effect of time and supplementation of ALA and DHA in healthy young male and female adults on plasma total (A, D) n–3 and n–6 PUFAs, and (B, E) oxylipins and (C, F) total SFAs and MUFAs. Where there is a supplementation by time interaction, †denotes value is different from DHA supplementation at that time point, and *denotes value is different from baseline for same supplementation based on significant adjusted P values (Tukey–Kramer corrections) for differences of least squares means. Data points are mean ± SEM for sexes combined and analyzed using a mixed model with individuals as a random effect, and time, supplementation, and sex as fixed effects; n = 12 for each time point, except for days 14 and 28 during the DHA supplementation phase, where n = 11. ALA, α-linolenic acid.
ALA compared with DHA supplementation effects on n–6 fatty acids and oxylipins
n–6 PUFA–derived oxylipins also were altered by supplementation, but fewer oxylipins were affected and the effects were much smaller. Similar to effects on n–3 oxylipins, supplementation effects also did not always parallel effects on their precursor PUFA. For example, the concentrations of plasma total n–6 PUFAs were higher, but total n–6 oxylipins were lower with ALA compared with DHA supplementation (Figure 3).
These patterns were reflected in some individual n–6 PUFAs and their oxylipins (Figure 4). Plasma linoleic acid (LA) and dihomo-γ-linolenic acid (DGLA) were not altered by supplementation (concentrations were numerically lower with DHA supplementation), but 9,10,13-trihydroxy-octadecenoic acid, PGF1α, and total LA oxylipins were higher in DHA- compared with ALA-supplemented participants. Plasma arachidonic acid (AA) also was not altered by either supplementation. This was reflected in the lack of effect of either supplementation on total AA oxylipins throughout the 28-d supplementation phase, and on most individual oxylipins. However, 4 individual plasma AA oxylipins were higher with DHA compared with ALA supplementation, namely PGD2 and 8-, 12-, and 18-hydroxy-eicosatetraenoic acid.
ALA compared with DHA supplementation effects on other fatty acids
Changes to other fatty acids also occurred with supplementation, but these were typically smaller. Supplementation had few effects on individual SFAs (Supplemental Tables 4–6) and had no effects on plasma total SFAs (Figure 3C). Several individual MUFAs were higher with ALA compared with DHA supplementation (Supplemental Tables 4–6), resulting in higher plasma total MUFAs in ALA- compared with DHA-supplemented participants (Figure 3F).
Trends over time in fatty acid and oxylipin concentrations
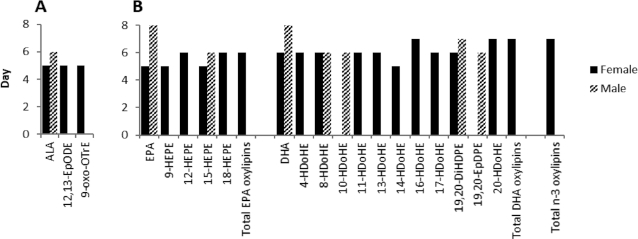
To determine trends in the increases in plasma n–3 PUFAs and their oxylipins over time with ALA or DHA supplementation, n–3 PUFAs and their oxylipins were subjected to plateau analysis. With ALA supplementation, plasma ALA increased rapidly and reached a plateau at days 5 and 6 for females and males, respectively. In comparison, a plateau in ALA oxylipins was detected in females for 2 of the 5 ALA oxylipins present, with 12,13-epoxy-octadecadienoic acid and 9-oxo-octadecatrienoic acid reaching a plateau at 5 d (Figure 5). ALA supplementation also increased docosapentaenoic acid (22:5n–3; DPAn–3), reaching a plateau at 5 d in females (not shown), but 17-keto DPAn–3, the only DPAn–3 oxylipin in the analysis, was not detected in plasma.
FIGURE 5.
Days to reach plateau concentrations in healthy young male and female adults for plasma (A) ALA and ALA oxylipins during ALA supplementation, and for (B) EPA and DHA and their individual and total oxylipins and total n–3 oxylipins during DHA supplementation. n = 12. Only fatty acids and oxylipins where the plateau was observed (significant) are shown. To calculate the plateau for PUFA and oxylipins during each supplementation phase, quadratic modeling was used to estimate both linear and quadratic slopes. ALA, α-linolenic acid; DiHDPE, dihydroxy-docosapentaenoic acid; EpDPE, epoxy-docosapentaenoic acid; EpODE, epoxy-octadecadienoic acid; HDoHE, hydroxy-docosahexaenoic acid; HEPE, hydroxy-eicosapentaenoic acid; oxoOTrE, oxo-octatrienoic acid.
DHA supplementation rapidly increased EPA and DHA, reaching plateaus at days 5 and 6, respectively, in females, and at day 8 for both PUFAs in males (Figure 5). Similarly, plateaus for EPA and DHA oxylipins were reached in 5–7 d for 4 EPA (of 7 present) and 9 (of 12 present) DHA oxylipins in females, and in 6–7 d for 1 EPA and 4 DHA oxylipins in males.
Sex differences in oxylipins and their precursor PUFAs
Oxylipins that displayed an overall main effect of sex were always at higher concentrations in females, with 16 of 62 oxylipins displaying a main sex effect. All others had no main effect of sex, and 3 had interactions with treatment or time (Supplemental Table 11). Oxylipins higher in females included those derived from all major PUFAs, that is, 5 of 10 LA oxylipins, 1 of 3 DGLA oxylipins, 3 of 30 AA oxylipins, 1 of 5 ALA oxylipins, 1 of 6 EPA oxylipins, and 5 of 12 DHA oxylipins. By comparison, none of their precursor PUFAs displayed significant overall sex effects (Supplemental Table 7).
In addition, the plateau analyses for time effects indicate that supplementation usually increased plasma oxylipin and fatty acid concentrations earlier in females (see above and Figure 5). This analysis also revealed that more oxylipins reached a plateau with supplementation in females compared with males: that is, in females compared with males, 2 compared with 0 ALA oxylipins reached a plateau with ALA supplementation, and 13 compared with 5 n–3 oxylipins reached a plateau with DHA supplementation (Figure 5). Further, total EPA, total DHA, and total n–3 oxylipins reached a plateau in plasma by 6–7 d in females, whereas no plateau was detected for these totals in males (Figure 5).
Discussion
In this study with similar amounts of ALA and DHA provided over a 28-d phase, DHA supplementation had much greater effects on the appearance of plasma n–3 oxylipins than ALA supplementation. Although ∼4 g/d of supplemented DHA rapidly increased EPA and DHA oxylipins, a similar dose of ALA had no effects on ALA oxylipins and did not alter EPA or DHA oxylipins in men and women consuming a typical Canadian diet. Supplementation with ALA increased plasma ALA ∼2-fold, but did not increase ALA oxylipins. However, DHA concentrations were already approximately twice as high as ALA at baseline, and supplementation with DHA resulted in a ∼3-fold increase of both plasma DHA and DHA oxylipins. These differences in their effects on oxylipins and PUFAs could be due in part to the fact that relative to the background dietary intake, the amount of supplemented PUFA was proportionally much greater for DHA than for ALA; for ALA the background intake was ∼1.5 g/d whereas for DHA it was less than ∼0.1 g/d. This suggests that the amount of ALA in the typical diet is sufficient to saturate ALA oxylipin levels, whereas dietary amounts of DHA are low enough that both DHA and its oxylipins are increased by DHA supplementation. However, we cannot rule out increased turnover of ALA or its oxylipins as a potential explanation for the lack of change in ALA and ALA oxylipins, as has been suggested for DHA by carbon-13 studies of DHA turnover (30, 31).
The effects of ALA supplementation on blood ALA changes in this study are consistent with previous studies using a similar dose for the same time period (3.7 g/d for 6 wk) (32), a slightly lower dose (3 g/d) for 12 wk (33), or a much higher dose (14–15 g/d) for 4–12 wk (32, 34). These studies also documented increased blood concentrations of EPA, but not DHA, with ALA supplementation. Similarly, Greupner et al. (12) provided 12.9 g/d ALA for 12 wk to males only and observed higher EPA and unchanged DHA in RBC fatty acids. They also measured plasma oxylipins and found that 2 DHA oxylipins were higher and 1 was lower . In a previous 4-wk study we showed that 6 g/d ALA from flaxseed did not alter ALA oxylipins and could have reduced some EPA and DHA oxylipins (11). The current study also showed that supplemental ALA has no effects on oxylipins in both men and women. In contrast, DHA significantly increased EPA and DHA oxylipins, consistent with previously reported findings—reviewed by Ostermann and Schebb (10). Hence this direct comparison study confirms that the effects of ALA supplementation on n–3–derived oxylipins are much less than with DHA supplementation.
An objective of the current study was to examine the time course for changes in plasma oxylipins during ALA compared with DHA supplementation. We found that EPA and DHA oxylipins reached a plateau in <1 wk of DHA supplementation, although a plateau was not detected for all oxylipins. Nevertheless, it shows that the increase in oxylipins is rapid and largely complete by 1 wk with DHA supplementation. In contrast, with ALA supplementation a plateau was detected for only 2 ALA oxylipins, and no plateau was reached for total ALA oxylipins. This is consistent with a previous study that showed that much higher levels of ALA for a longer supplementation period increase ALA, and possibly DHA, oxylipins only to a small extent (12).
The current study also demonstrated that whereas the effects of n–3 PUFA supplementation over time occur rapidly (within days) for n–3 oxylipins, the effects on n–6 oxylipins are subtler. DHA compared with ALA supplementation resulted in higher concentrations of several individual AA-, LA-, and DGLA-derived oxylipins, as well as total LA oxylipins and total n–6 PUFA–derived oxylipins. In contrast, ALA supplementation did not alter AA-derived oxylipins. The current study therefore demonstrates a greater and more rapid human plasma oxylipin response to DHA compared with ALA supplementation.
Another objective of this study was to examine sex differences in plasma oxylipins and in response to dietary n–3 PUFA supplementation. We observed that ∼25% of oxylipins displayed a sex difference, and in all such cases, oxylipin concentrations were higher in females. These findings differ from what we found in rats, where serum oxylipins displaying a sex difference were higher in males (more than two-thirds of serum oxylipins had a sex effect and >90% of these were higher in males) (14). Thus, species differences must be considered when comparing sex effects on oxylipins.
The time course and plateau analysis also showed that females increased more n–3 oxylipins and did so sooner. This is consistent with the report by Fischer et al. (16) suggesting that DHA oxylipins are increased more in females than males with EPA plus DHA supplementation. This indicates that supplementation in females could be more effective when used as an intervention for health benefits. Sex effects on fatty acids previously have been demonstrated, with studies showing higher plasma DHA in females than males (19, 20).
This study also provides more evidence that oxylipin concentrations do not necessarily reflect dietary or plasma fatty acid concentrations and cannot be predicted based on their precursor fatty acid levels in the diet or in plasma. Although EPA and DHA levels seem to correspond with the levels of their respective oxylipins, this is not always the case for LA, ALA, or AA and their oxylipins. For example, ALA supplementation increased plasma ALA concentrations, but not the concentrations of ALA oxylipins. Also, DHA compared with ALA supplementation resulted in similar concentrations of n–6 PUFAs, but several n–6 oxylipins were higher with DHA supplementation. Another example of the discrepancy between oxylipins and their precursor PUFAs is that the higher concentrations of oxylipins in females are not reflected in their corresponding PUFAs. Such differences between blood PUFA and oxylipin concentrations have been reported in other human studies (10), as well as in serum and multiple tissues in rats, in which discrepancies between DHA and its oxylipins are also observed (13,14, 35–37). No clear pattern for these discrepancies has emerged, likely due to differences between PUFA, supplementation dose, sex, species, tissues, and/or length of intervention in these studies.
Several limitations in our study exist. Although the supplementation dose for ALA might be achievable with dietary changes, the dose for DHA would be more difficult to achieve with diet alone. Conclusions from this study also are limited to the dose that was used, because plasma oxylipin concentrations depend on the dose of the PUFA provided during dietary intervention (38). Conclusions also are limited to plasma, because oxylipin pools in plasma would turn over at different rates than in tissues. These tissues, in addition to blood cells and the endothelium, could influence the overall plasma oxylipin content, but the relative contribution of each remains to be determined. This study also only applies to healthy young adults, and results could vary with age and health status. The DHA capsules also contained EPA, so 0.9 g/d EPA was also consumed during the DHA supplementation phase, and likely influenced the formation of EPA oxylipins, as observed in rats when provided pure sources of EPA or DHA (13). Also, the oxylipins reported herein are the free oxylipins, which represent only a portion of the total oxylipins present in plasma. Free oxylipins are presumed to be the most active form and are most commonly analyzed, but several reports also describe functions for esterified oxylipins (39, 40). However, dietary EPA and DHA supplementation has been shown to affect free and esterified oxylipins similarly (10, 23).
In summary, this side-by-side comparison demonstrates that ∼4 g/d DHA compared with ALA has greater effects on increasing plasma n–3 PUFA oxylipins and altering n–6 oxylipins. It also demonstrates that n–3 PUFA supplementation increases n–3 PUFA–derived oxylipins sooner in females, reaching a plateau by 1 wk of supplementation with the doses used in this study. However, effects on n–6 PUFA oxylipins are much subtler, and do not necessarily follow PUFA patterns, confirming that PUFA data alone are insufficient to predict oxylipin changes. These findings can help explain the differing effects of dietary ALA and DHA on health, and indicate that the responses to these PUFAs can be greater for females.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tanja Winter and Dennis Labossiere for technical support, and Dr Depeng Jiang, Biostatistical Consulting Unit, and Dr Audrey Schnell, The Analysis Factor, for statistical consultations.
The authors’ responsibilities were as follows—MG, PZ, CGT, HMA: were involved in the design of the study; CGT, PZ: were responsible for human ethics and regulatory approvals; MG: conducted the study and collected the data; MG, HMA: analyzed the data and wrote the manuscript; and all authors edited, read, and approved the final manuscript.
Notes
Supported by the Canadian Institutes of Health Research (CIHR) (RPA 132173 & MOP 133667) and Research Manitoba. MG was supported by scholarships from CIHR, the University of Manitoba Graduate Fellowship, and the Graduate Enhancement of Tri-Council Stipends (GETS) program. Infrastructure support was from the St Boniface Hospital Foundation.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1–11 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AA, arachidonic acid (20:4n–6); ALA, α-linolenic acid (18:3n–3); DGLA, dihomo-γ-linolenic acid; DPAn–3, docosapentaenoic acid (22:5n–3); HDoHE, hydroxy-docosahexaenoic acid; LA, linoleic acid (18:2n–6).
Contributor Information
Melissa Gabbs, Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
Peter Zahradka, Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Department of Physiology and Pathophysiology, University of Manitoba, Winnipeg, Manitoba, Canada.
Carla G Taylor, Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, University of Manitoba, Winnipeg, Manitoba, Canada; Department of Physiology and Pathophysiology, University of Manitoba, Winnipeg, Manitoba, Canada.
Harold M Aukema, Department of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; Canadian Centre for Agri-Food Research in Health and Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
References
- 1. Wei J, Hou R, Xi Y, Kowalski A, Wang T, Yu Z, Hu Y, Chandrasekar EK, Sun H, Ali MK. The association and dose-response relationship between dietary intake of α-linolenic acid and risk of CHD: a systematic review and meta-analysis of cohort studies. Br J Nutr. 2018;119(1):83–9. [DOI] [PubMed] [Google Scholar]
- 2. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KHO, Alabdulghafoor FK, Summerbell CD, Worthington H V, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;2018(7):CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo X-F, Li K-L, Li J-M, Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59(20):3380–93. [DOI] [PubMed] [Google Scholar]
- 4. Li D, Wahlqvist ML, Sinclair AJ. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac J Clin Nutr. 2019;28(1):1–5. [DOI] [PubMed] [Google Scholar]
- 5. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta Mol Cell Biol Lipids. 2015;1851(4):469–84. [DOI] [PubMed] [Google Scholar]
- 6. Manson JAE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Appleton KM, Sallis HM, Perry R, Ness AR, Churchill R. Omega-3 fatty acids for depression in adults. Cochrane Database Syst Rev. 2015;2015(11):CD004692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;2018(11):CD003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr. 2015;6(5):513–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostermann AI, Schebb NH. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct. 2017;8(7):2355–67. [DOI] [PubMed] [Google Scholar]
- 11. Caligiuri SPB, Aukema HM, Ravandi A, Pierce GN. Elevated levels of pro-inflammatory oxylipins in older subjects are normalized by flaxseed consumption. Exp Gerontol. 2014;59:51–7. [DOI] [PubMed] [Google Scholar]
- 12. Greupner T, Kutzner L, Nolte F, Strangmann A, Kohrs H, Hahn A, Schebb NH, Schuchardt JP. Effects of a 12-week high-α-linolenic acid intervention on EPA and DHA concentrations in red blood cells and plasma oxylipin pattern in subjects with a low EPA and DHA status. Food Funct. 2018;9(3):1587–600. [DOI] [PubMed] [Google Scholar]
- 13. Leng S, Winter T, Aukema HM. Dietary LA and sex effects on oxylipin profiles in rat kidney, liver, and serum differ from their effects on PUFAs. J Lipid Res. 2017;58(8):1702–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leng S, Winter T, Aukema HM. Dietary ALA, EPA and DHA have distinct effects on oxylipin profiles in female and male rat kidney, liver and serum. J Nutr Biochem. 2018;57(Jul):228–37. [DOI] [PubMed] [Google Scholar]
- 15. Schuchardt JP, Ostermann AI, Stork L, Fritzsch S, Kohrs H, Greupner T Hahn A, Schebb NH. Effect of DHA supplementation on oxylipin levels in plasma and immune cell stimulated blood. Prostaglandins Leukot Essent Fatty Acids. 2017;121:76–87. [DOI] [PubMed] [Google Scholar]
- 16. Fischer R, Konkel A, Mehling H, Blossey K, Gapelyuk A, Wessel N, von Schacky C, Dechend R, Muller DN, Rothe M et al. Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway. J Lipid Res. 2014;55(6):1150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Yang N, Ai D, Zhu Y. Systematic metabolomic analysis of eicosanoids after omega-3 polyunsaturated fatty acid supplementation by a highly specific liquid chromatography-tandem mass spectrometry-based method. J Proteome Res. 2015;14(4):1843–53. [DOI] [PubMed] [Google Scholar]
- 18. Schuchardt JP, Schneider I, Willenberg I, Yang J, Hammock BD, Hahn A, Schebb NH. Increase of EPA-derived hydroxy, epoxy and dihydroxy fatty acid levels in human plasma after a single dose of long-chain omega-3 PUFA. Prostaglandins Other Lipid Mediat. 2014;109–111:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kitson AP, Stroud CK, Stark KD. Elevated production of docosahexaenoic acid in females: potential molecular mechanisms. Lipids. 2010;45(3):209–24. [DOI] [PubMed] [Google Scholar]
- 20. Slater-Jefferies JL, Hoile SP, Lillycrop KA, Townsend PA, Hanson MA, Burdge GC. Effect of sex and dietary fat intake on the fatty acid composition of phospholipids and triacylglycerol in rat heart. Prostaglandins Leukot Essent Fatty Acids. 2010;83(4–6):219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keenan AH, Pedersen TL, Fillaus K, Larson MK, Shearer GC, Newman JW. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J Lipid Res. 2012;53(8):1662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010;51(8):2074–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins Other Lipid Mediat. 2014;113–115:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, Hogg RJ, Trygg J, Hammock BD, Zivkovic AM. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 fatty acids. PLoS One. 2013;8(10):e76575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aukema HM, Winter T, Ravandi A, Dalvi S, Miller DW, Hatch GM. Generation of bioactive oxylipins from exogenously added arachidonic, eicosapentaenoic and docosahexaenoic acid in primary human brain microvessel endothelial cells. Lipids. 2016;51(5):591–9. [DOI] [PubMed] [Google Scholar]
- 26. Monirujjaman M, Devassy JG, Yamaguchi T, Sidhu N, Kugita M, Gabbs M, Nagao S, Zhou J, Ravandi A, Aukema HM. Distinct oxylipin alterations in diverse models of cystic kidney diseases. Biochim Biophys Acta. 2017;1862(12):1562–74. [DOI] [PubMed] [Google Scholar]
- 27. Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom. 1998;9(5):527–32. [DOI] [PubMed] [Google Scholar]
- 28. Fair DE, Ogborn MR, Weiler HA, Bankovic-Calic N, Nitschmann EP, Fitzpatrick-Wong SC, Aukema HM. Dietary soy protein attenuates renal disease progression after 1 and 3 weeks in Han:SPRD-cy weanling rats. J Nutr. 2004;134(6):1504–7. [DOI] [PubMed] [Google Scholar]
- 29. Aukema HM, Holub BJ. Effect of dietary supplementation with a fish oil concentrate on the alkenylacyl class of ethanolamine phospholipid in human platelets. J Lipid Res. 1989;30(1):59–64. [PubMed] [Google Scholar]
- 30. Metherel AH, Bazinet RP. Updates to the n-3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Prog Lipid Res. 2019;76:101008. [DOI] [PubMed] [Google Scholar]
- 31. Metherel AH, Irfan M, Klingel SL, Mutch DM, Bazinet RP. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am J Clin Nutr. 2019;110(4):823–31. [DOI] [PubMed] [Google Scholar]
- 32. Li D, Sinclair A, Wilson A, Nakkote S, Kelly F, Abedin L, Mann N, Turner A. Effect of dietary α-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr. 1999;69(5):872–82. [DOI] [PubMed] [Google Scholar]
- 33. Harper CR, Edwards MJ, DeFilipis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136(1):83–7. [DOI] [PubMed] [Google Scholar]
- 34. Schwab US, Callaway JC, Erkkilä AT, Gynther J, Uusitupa MIJ, Järvinen T. Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. Eur J Nutr. 2006;45(8):470–7. [DOI] [PubMed] [Google Scholar]
- 35. Mendonça AM, Cayer LGJ, Pauls SD, Winter T, Leng S, Taylor CG, Zahradka P, Aukema HM. Distinct effects of dietary ALA, EPA and DHA on rat adipose oxylipins vary by depot location and sex. Prostaglandins Leukot Essent Fatty Acids. 2018;129(141):13–24. [DOI] [PubMed] [Google Scholar]
- 36. Ferdouse A, Leng S, Winter T, Aukema HM. The brain oxylipin profile is resistant to modulation by dietary n-6 and n-3 polyunsaturated fatty acids in male and female rats. Lipids. 2019;54(1):67–80. [DOI] [PubMed] [Google Scholar]
- 37. Ferdouse A, Leng S, Winter T, Aukema HM. Dietary n-6 and n-3 PUFA alter the free oxylipin profile differently in male and female rat hearts. Br J Nutr. 2019;122(3):252–61. [DOI] [PubMed] [Google Scholar]
- 38. Ostermann AI, West AL, Schoenfeld K, Browning LM, Walker CG, Jebb SA, Calder PC, Schebb NH. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: results from a randomized controlled trial in healthy humans. Am J Clin Nutr. 2019;109(5):1251–63. [DOI] [PubMed] [Google Scholar]
- 39. O'Donnell VB, Murphy RC. New families of bioactive oxidized phospholipids generated by immune cells: identification and signaling actions. Blood. 2012;120(10):1985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Slatter DA, Percy CL, Allen-Redpath K, Gajsiewicz JM, Brooks NJ, Clayton A, Tyrrell VJ, Rosas M, Lauder SN, Watson A et al. Enzymatically oxidized phospholipids restore thrombin generation in coagulation factor deficiencies. JCI Insight. 2018;3(6):e98459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.