ABSTRACT
Background
The current nutritional composition of the “American diet” (AD; also known as Western diet) has been linked to the increasing incidence of chronic diseases, including inflammatory bowel disease (IBD), namely Crohn disease (CD).
Objectives
This study investigated which of the 3 major macronutrients (protein, fat, carbohydrates) in the AD has the greatest impact on preventing chronic inflammation in experimental IBD mouse models.
Methods
We compared 5 rodent diets designed to mirror the 2011–2012 “What We Eat in America” NHANES. Each diet had 1 macronutrient dietary source replaced. The formulated diets were AD, AD-soy-pea (animal protein replaced by soy + pea protein), AD-CHO (“refined carbohydrate” by polysaccharides), AD-fat [redistribution of the ω-6:ω-3 (n–6:n–3) PUFA ratio; ∼10:1 to 1:1], and AD-mix (all 3 “healthier” macronutrients combined). In 3 separate experiments, 8-wk-old germ-free SAMP1/YitFC mice (SAMP) colonized with human gut microbiota (“hGF-SAMP”) from CD or healthy donors were fed an AD, an AD-“modified,” or laboratory rodent diet for 24 wk. Two subsequent dextran sodium sulfate–colitis experiments in hGF-SAMP (12-wk-old) and specific-pathogen-free (SPF) C57BL/6 (20-wk-old) mice, and a 6-wk feeding trial in 24-wk-old SPF SAMP were performed. Intestinal inflammation, gut metagenomics, and MS profiles were assessed.
Results
The AD-soy-pea diet resulted in lower histology scores [mean ± SD (56.1% ± 20.7% reduction)] in all feeding trials and IBD mouse models than did other diets (P < 0.05). Compared with the AD, the AD-soy-pea correlated with increased abundance in Lactobacillaceae and Leuconostraceae (1.5–4.7 log2 and 3.0–5.1 log2 difference, respectively), glutamine (6.5 ± 0.8 compared with 3.9 ± 0.3 ng/μg stool, P = 0.0005) and butyric acid (4:0; 3.3 ± 0.5 compared with 2.54 ± 0.4 ng/μg stool, P = 0.006) concentrations, and decreased linoleic acid (18:2n–6; 5.4 ± 0.4 compared with 8.6 ± 0.3 ng/μL plasma, P = 0.01).
Conclusions
Replacement of animal protein in an AD by plant-based sources reduced the severity of experimental IBD in all mouse models studied, suggesting that similar, feasible adjustments to the daily human diet could help control/prevent IBD in humans.
Keywords: inflammatory bowel disease, soy protein, pea protein, American diet, Firmicutes:Bacteroidetes ratio
Introduction
Chronic inflammatory and metabolic conditions are on the rise and their incidence has been, in part, attributed to environmental factors/modern diets (1–3). However, addressing the role of diet in chronic intestinal diseases directly in humans is challenging. An “American diet” (AD), also known as the Western diet, is characterized as rich in animal protein and refined carbohydrate with an increased omega (ω)-6:ω-3 PUFA ratio (from 1:1 to 10–25:1) (4), and has been postulated to be one of the most important factors contributing to the increased incidence of inflammatory bowel diseases (IBDs), namely Crohn disease (CD) and ulcerative colitis (UC) (5–7).
CD and UC are chronic digestive inflammatory disorders that predominantly affect the ileum (ileitis, ileocolitis in 75% of CD patients) and the colon (colitis in 100% of UC patients) (8). Within the factors that affect both IBDs, diet is considered one of the most important, associated with influencing disease severity, progression, and flare-ups, via modification of gut microbiota composition (6, 9, 10). Despite significant progress in our understanding of the role of various macronutrients of the human diet in IBD-intestinal inflammation, most studies have focused on mouse models of dextran sodium sulfate (DSS) colitis to contribute to our current understanding of UC. However, less is known about the importance of diet on chronic small intestinal inflammation, namely ileitis. The objective of this study was to identify which of the 3 major macronutrients (protein, fat, carbohydrate) in the AD has the greatest impact on preventing chronic inflammation in experimental mouse models of IBD, including a mouse line prone to CD-like ileitis, colonized with gut microbiota from CD patients.
Complementing chemical-colitis models, the use of genetic models with highly penetrant, chronic phenotypes has become increasingly important to study the effect of diet on the prevention of the human disease, or its treatment (therapeutic effect) once developed. Therefore, to further contribute to our understanding of dietary prevention/treatment of chronic ileitis, we used the genetic line SAMP1/YitFC (SAMP), a mouse model of CD-like ileitis which closely resembles the human condition and in which spontaneous ileitis occurs even in the absence of gut microbiota [i.e., mice raised in germ-free (GF) conditions] (11, 12). In addition, our group has previously demonstrated that even though gut bacteria are not essential for the induction of chronic ileitis, they have strong potential to modulate the severity of intestinal inflammation in SAMP mice (10–12).
Since the 1960s, the National Center for Health Statistics has conducted a series of annual surveys released in 2-y cycles to interrogate a variety of health/nutrition measurements of the American population. Now known as the NHANES (13), the dietary interview component “What We Eat in America” has been deemed the standard to understand obesity and related diseases in the United States, and has been used to formulate research diets to examine the role of concurrent trends in dietary habits on human diseases (13).
With respect to dietary macronutrients, numerous studies have investigated the effect of different nutrient sources/ingredients on the severity of intestinal inflammation and independently have found that plant-based proteins—for instance, soy (14–20), various soy bioactive compounds (21, 22), and pea protein (23, 24)—as well as dietary fibers as a source of “healthier” carbohydrates (25, 26), and dietary fats comprised of a balanced 1:1 ratio of ω-6:ω-3 PUFA (27–30), have a beneficial anti-inflammatory impact on chemically induced colitis.
Herein, we tested the effect of mouse diets formulated to mirror the 2011–2012 cycle NHANES report (13), 3 of which replaced 1 macronutrient source with a “healthier” alternative based on their hypothesized health benefits as reported in experimental IBD, with emphasis on the treatment and prevention of chronic ileitis in SAMP mice.
Methods
Mouse lines and animal husbandry
Age/sex-matched (littermate controls) inbred GF and specific-pathogen-free (SPF) SAMP and C57BL/J6 (B6; SAMP reference genome) mice (Cleveland Digestive Diseases Research Center, Mouse Models Core) were individually housed and maintained on nonedible Aspen bedding in our GF-grade NesTiso caging system (31). Mice were kept on a 12-h:12-h light:dark cycle in a species-appropriate temperature/humidity-controlled room and maintained in AAALAC-accredited Animal Research Center rooms at Case Western Reserve University (CWRU). SAMP mice are a unique mouse model that spontaneously develop CD-like “cobblestone” ileitis (chronic inflammation of the distal small intestine) with 100% penetrance and within a well-defined time course (preileitis, disease induction, and chronic ileitis) (11).
All experiments were conducted according to guidelines to minimize artificial microbiome heterogeneity and promote reproducibility/study power (31, 32). To ensure a homogenous microbiota composition in all cages and avoid housing artifacts, a fecal homogenization [as described (33)] was performed 1 wk before all experiments involving SPF mice. Animals were humanely killed by carbon dioxide narcosis. All described procedures were approved by the Institutional Animal Care and Use Committee and the Institutional Review Board (IRB) at CWRU, in accordance with the Guide for Care and Use of Laboratory Animals. See details below and in the Supplemental Methods.
Mouse diets
Supplemental Tables 1 and 2 describe the semipurified murine diets prepared by Research Diets Inc. Mice were randomly assigned to either an AD containing animal protein, saturated fat, and refined carbohydrates, or to 1 of 4 isocaloric ADs, 3 of which were modified only by the dietary source of a single macronutrient—AD-soy-pea (animal proteins replaced by soy and pea protein), AD-CHO (“refined carbohydrate” by polysaccharides), and AD-fat (redistribution of the ω-6:ω-3 PUFA ratio; from ∼10:1 to 1:1)—and 1 called AD-mix (in which all 3 macronutrients were replaced by the “healthier” alternatives). The AD was designed to mimic the composition of the 2011–2012 cycle of NHANES (13) “What We Eat in America.” AD-soy-pea and AD-mix provided an isoflavone content of 0.49–1.4 mg/d (∼5 g food intake/d), comparable to US estimates of 1–3 mg/d for individuals consuming a “Western” diet (34), and that of soy-based US laboratory rodent diets (0.3–0.55 mg/g feed; 1.5–2.75 mg isoflavones/d, assuming ∼5 g intake/d).
Laboratory rodent pellets, standard to our facility (Labdiet® Rat/Mouse 18%VacPac-5LQ6, Charles River), and which have been used in our facility to characterize the SAMP mouse CD-like ileitis phenotype in various studies (35–37), served as the control diet (“con”) (Supplemental Table 3). All diets were vacuum packed and double irradiated to reach GF standards.
Mouse experiments
Figure 1 shows an overview of mouse experiments.
FIGURE 1.
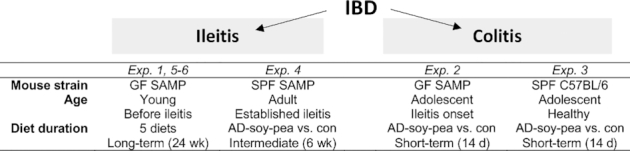
Overview of the experimental study design. AD-soy-pea, American diet modified by protein source (soy and pea isolates); con, control; Exp, experiment; GF, germ-free; IBD, inflammatory bowel disease; SAMP, SAMP1/YitFC mouse line; SPF, specific-pathogen-free.
Prevention of spontaneous chronic ileitis (Experiment 1)
Groups of male and female 7-wk-old GF-SAMP mice (i.e., before ileitis onset) were colonized with human gut microbiota (“hGF-SAMP”) from a CD donor (“CDdonor1”) and randomly assigned to the AD or 1 of the 4 aforementioned AD-modified diets for 24 wk (n = 5–6 mice/group). Body weight (BW), food intake, blood glucose, myeloperoxidase (MPO) activity, ileitis severity, and fecal bacterial profiles were evaluated. We have previously shown that GF-SAMP can harbor 95% ± 0.03% (mean ± SD) genus-level engraftment of human gut taxa (3). Herein, we used the same colonization strategy (3). In brief, establishment of human gut microbiota in GF mice was performed 2 wk before starting diet treatments, via oral gavage (200 μL/10 g BW; 108–9CFU/mouse), using anaerobically prepared, cryopreserved human fecal microbiota, as described (3, 38). De-identified fresh human stools were procured under an IRB-approved protocol (IRB#NHR-11-22). All IBD donors were in complete remission and not taking biologics/corticosteroids. Herein, donor stool selection was based on the “proinflammatory” effect on ileitis severity in GF-SAMP after transplantation, as described (3).
DSS-colitis (Experiments 2 and 3)
Groups of male and female 12-wk-old GF-SAMP colonized with feces (as aforementioned) from “CDdonor1” (Experiment 4), and 20-wk-old SPF-B6 mice (Experiment 5), were fed AD-soy-pea or rodent diet for 16 d total (n = 7–9 mice/group). On day 7 of the diet, colitis was induced with 3% DSS (TdB Consultancy AB) offered ad libitum and mice resumed with water for 2 d, then were killed. Colon length, BW, and colitis severity were evaluated. Following the BW considerations for euthanasia (which includes loss of 10%–20% of initial weight as a physiological indicator of stress or immune demise), animals were killed and samples collected as previously recommended (39–41) and published (42).
Treatment of established chronic ileitis (Experiment 4)
Groups of male and female 24-wk-old SPF-SAMP mice (i.e., established ileitis) were fed AD-soy-pea or rodent diet for 6 wk (n = 7–8 mice/group). Food intake, BW, MPO activity, and ileitis severity were evaluated.
Prevention of spontaneous chronic ileitis in the context of the gut microbiome (Experiments 5 and 6)
Previously, we determined that the human gut microbiota can be classified as proinflammatory, anti-inflammatory, or neutral, based on the effects on hGF-SAMP ileitis (3). To test the impact of different donor microbiota on the anti-inflammatory activity, groups of male and female 7-wk-old GF-SAMP mice (i.e., before ileitis onset) were colonized with human gut microbiota (as described in Experiment 1) from a CD (“CDdonor2,” Experiment 5) or healthy control (“HCdonor,” Experiment 6) donor and fed either the AD, AD-soy-pea, or con diet for 24 wk (n = 6–7 mice/group).
Indicators of metabolic function
Basal and fasting glucose concentrations (after 6-h daytime deprivation of food) were measured in blood collected via tail vein using a OneTouch Ultra2 Blood Glucose meter (LifeScan) at 12 and 24 wk in hGF-SAMP.
Quantification of intestinal inflammation in vivo
MPO activity was assessed in mouse feces (measured in triplicate) as previously described (33, 36). A fluorescently labeled small molecule [fluorescein isothiocyanate (FITC)-dextran] was used to assess intestinal permeability, as described (43).
Quantification of intestinal inflammation postmortem
Intestinal tissue samples (terminal ilea, colon) were collected after killing for blinded stereomicroscopy 3-D pattern profiling (3D-SM) (37) and histological assessment of formalin-fixed tissues using validated methodology (37, 44). Inflammatory indexes for histological villous distortion, active/chronic inflammation, and total inflammation were assessed in a semiquantitative fashion.
Microbiome analysis
Genomic fecal DNA extraction (Illumina TruSeq DNA) and DNA quality control were performed by the Genomics Core, CWRU. Only qualified DNA (quality, quantity) was used to construct 16S libraries, verified by Invitrogen Qubit Fluorometer (Life Technologies), OD260/280 & OD260/230 NanoDrop (Thermo Scientific), and agarose gel electrophoresis (agarose gel: 1%, voltage: 150 V, time: 40 min). Differences in fecal bacterial composition for hGF-SAMP (“CDdonor1”) at week 24 and respective donor inoculum (in technical replicates) were assessed by V3–V4 16S rRNA gene sequences using the Illumina MiSeq platform to generate 25,000 ± 175 (mean ± SD). reads per sample having a mean length of 252 bp passed the quality control filter . Library preparation, sequencing, quality control, and primary bioinformatics analysis were performed by Beijing Genomics Institute (45) as described (3).
Metagenome analysis
Fecal DNA (Experiments 1, 5, and 6; mice and donor inocula in technical replicates) was extracted with a ZymoBIOMICS Mini Prep Kit. DNA libraries were prepared using the Illumina Nextera XT library preparation kit, with a modified protocol. Library quantity was assessed with Qubit (ThermoFisher). Libraries were sequenced on an Illumina HiSeq platform, read length 2 × 150 bp. Unassembled sequencing reads were analyzed by the CosmosID bioinformatics platform (CosmosID Inc.), as described elsewhere (46–49), for multikingdom microbiome analysis/profiling of antibiotic resistance and virulence genes and quantification of organisms’ relative abundance. Briefly, the system utilizes curated genome databases and a high-performance data-mining algorithm that disambiguates hundreds of millions of metagenomic sequence reads into the discrete microorganisms engendering the particular sequences. Similarly, the community resistome and virulome, the collection of antibiotic resistance– and virulence-associated genes in the microbiome, were identified by querying the unassembled sequence reads against the CosmosID curated antibiotic resistance–and virulence-associated gene databases. The diversity of each species (α-diversity) was compared with the Shannon diversity index.
MS to validate microbiome predictions
SCFAs, biogenic amines, and untargeted profiling of primary metabolism were quantified in feces/plasma for hGF-SAMP mice by the NIH West Coast Metabolomics Center via GC time-of-flight MS using published methods (50–52).
Statistical analysis
Between-group differences in mean stereomicroscopy, inflammatory scores, and morphometric analyses were assessed globally using a mixed-effects ANOVA model, which considers between-group/within-group effects. Binary analysis (presence/absence of taxa) of metagenome data excluding low abundant taxa (threshold <0.1) was used to calculate percentage taxa engraftment in mice as described (3). Power analysis was computed for all results based on the difference between 2 independent means using G*Power (version 3.1.9.4) software (53). Using MPO and histology data (primary/secondary outcomes) and power analysis for Experiment 1, sample size was estimated for subsequent experiments to achieve a power of 80%. To promote transparency we report study power for the final observed parameters for all other secondary outcomes in the study for the IBD mouse models, following recently reported guidelines for SAMP mice in our laboratory (54). Statistical analysis of operational taxonomic unit–normalized log2-transformed and rescaled data was conducted using Stata version 15.0 (StataCorp LLC.) and R software version 3.4.2 (R Core Team). Data are primarily presented as violin plots (which enables visualization of data distribution) (54). P values < 0.05 were considered significant. Student's t tests and/or 1-factor ANOVAs, or their nonparametric alternatives like Fisher's exact test, were used for continuous data. Nonparametric tests were conducted to identify correlations between linear primary outcomes of inflammation (MPO, histology, MPO log2, FITC) and the variable measured in feces or plasma (microbiome, metagenome, MS) while controlling for diet and donor group. 1-Factor Kruskal–Wallis ANOVA with Dunn's multiple comparison test and Tukey's multiple comparison test identified the significance of differences between means. All results are expressed as means ± SD. Multivariable regression and biplot analysis identified the most important contributors of data heterogeneity that could explain mouse segregation by diet. Logistic regression analysis assessed the impact of diet on gene ontology (GO) and pathways in mice models across the 3 microbiota. Significant GO and GO molecular function (GO MOLFU; a subcategory of GO) pathways were identified by linear regression with respect to MPO (log2). 2-Factor ANOVA and Mann–Whitney U tests (M-Ws) compared diet effect and donor microbiome between murine diets. Integrative analysis was conducted with mixomics and R software.
Results
Replacing the protein source in an AD prevented experimental chronic ileitis (Experiment 1)
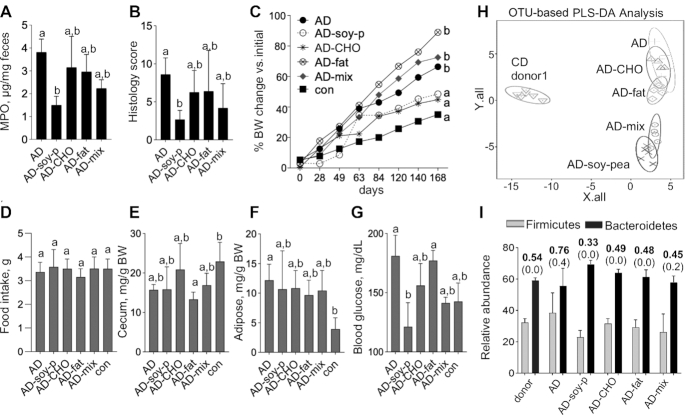
When the sources of protein, carbohydrate, or fat, or a combination of all 3 macronutrients, were replaced by “healthier” alternatives in an AD diet fed to hGF-SAMP for 24 wk, the severity of ileitis (fecal MPO, histology) significantly decreased owing to replacement of animal protein with plant protein (“AD-soy-pea” diet) (MPO: AD compared with AD-soy-pea, P = 0.0005; histology: AD compared with AD-soy-pea, P = 0.01) (Figure 2A, B). Of interest, the AD-mix diet, which also had animal protein replaced by plant protein, had an intermediate, albeit nonsignificant, effect on ileitis (MPO: AD-mix compared with AD-soy-pea, P = 0.08; histology: AD-mix compared with AD-soy-pea, P = 0.15), indicating that adding soy + pea to an AD could reduce the presumed proinflammatory effects of an AD (NHANES) in humans. Compared with mice fed the con diet, there was no difference in the final BW in the AD-soy-pea-fed mice (P = 0.23) (Figure 2C). However, the BW in AD-soy-pea-fed mice was significantly lower than in those fed the AD, AD-fat, and AD-mix diets. Of importance, the addition of soy + pea to the AD diet did not increase food consumption, cecum size, adipose tissue accumulation, or the weight/size of other organs compared with other diets (Figure 2D–F, Supplemental Figure 1), indicating that soy + pea supplementation is safe. Further, use of soy + pea isolate in the AD was accompanied by lower blood glucose concentrations (a well-known beneficial metabolic effect) than in the AD and AD-fat groups (Figure 2G). Supporting the lack of adverse systemic effects, soy + pea diets did not induce histological steatosis, inflammation, or fibrosis in the liver (ANOVA, P = 0.42 across all diets).
FIGURE 2.
A 24-wk (“prevention”) feeding trial shows that soy + pea isolate markedly prevents the development of chronic ileitis in young (7-wk-old) “humanized” germ-free SAMP mice (Experiments 1, 5, and 6). Values are mean ± SD, n = 5–7. (A) Fecal MPO activity measured on days 60 and 120, and (B) ileitis severity measured by histology score for Experiment 1. (C) Percentage change from original BW over time. Day 0: baseline weight at start of diet. (D) Food intake, (E) cecum weight, and (F) adipose tissue (Tukey's test). (G) Blood glucose. (H) 16S microbiome 3-D principal component analysis showing OTU-based PLS-DA analysis, and (I) Firmicutes and Bacteroidetes relative abundance (y axis) for mice and donors with mean (bold) and SD (parentheses) for the Firmicutes:Bacteroidetes ratio shown above histogram bars. a,bMeans without a common letter differed at killing (1-factor Kruskal–Wallis ANOVA with Dunn's multiple comparison test). AD, American diet; AD-CHO, American diet modified by carbohydrate source; AD-fat, American diet modified by fat source; AD-mix, American diet modified by protein, fat, and carbohydrate source; AD-soy-p, American diet modified by protein source (soy and pea isolates); BW, body weight; con, control; MPO, myeloperoxidase; OTU, operational taxonomic unit; PLS-DA, partial least squares-discriminant analysis; SAMP, SAMP1/YitFC mouse line.
16S fecal microbiome profiles revealed that the 2 soy + pea–containing AD diets (AD-soy-pea, AD-mix) were alike and clustered separately compared with the AD and other modified ADs (Figure 2H). Microbiome analysis showed no differences in the ratio of important phyla associated with ileitis in CD patients (55), namely Firmicutes:Bacteroidetes, when comparing mouse diet groups with the gut microbiota of the CD donor, or changes in Proteobacteria, to explain the ileitis improvement (Figure 2I).
To further characterize the anti-inflammatory effect of soy + pea, we then conducted further studies using an acute DSS-colitis model to test the therapeutic and preventative effect on IBD.
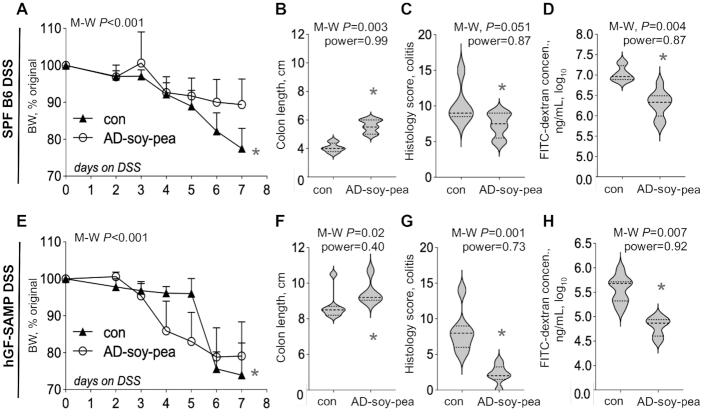
Two-week feeding of AD-soy-pea reduced acute DSS-colitis in 2 mouse lines (Experiments 2 and 3)
At the end of the 2-wk trial, DSS-treated B6 mice fed the AD-soy-pea diet exhibited less colitis (BW, colon length, and histological assessment; Figure 3A–C) having lower post-DSS colonoscopy scores (AD-soy-pea: 2.8 ± 1.6 compared with con: 5.1 ± 1.4; M-W P = 0.03) (for representative photographs, see Supplemental Figure 2), and had improved gut permeability (i.e., less translocation of FITC-dextran from the gut lumen into the plasma), compared with controls (Figure 3D). The AD-soy-pea diet also decreased DSS-mortality (66% of mice fed the rodent diet died compared with 100% of AD-soy-pea-fed mice survived; M-C (Mantel-Cox) P = 0.004). The findings in B6 mice were reproduced in the DSS-colitis experiment performed with hGF-SAMP mice (Figure 3E–H). Despite the prevention of colitis, soy + pea had no major effect on sudden death in the ileitis-prone mice (equal survival rate of 85% in each group), but more mice were killed in the rodent diet group than in the AD-soy-pea group owing to critical BW loss endpoint criteria (mortality; 57% of mice fed the rodent diet compared with 15% of AD-soy-pea-fed mice, M-C P = 0.04).
FIGURE 3.
AD-soy-pea reduces the severity of acute chemical colitis in 12-wk-old hGF-SAMP and 20-wk-old SPF C57BL/6 mice (Experiments 2 and 3). Values are mean ± SD, n = 7–9. (A) Percentage change from original BW (defined as day 0 and as 100%) after induction of DSS-colitis. (B) FITC-dextran, (C) colon length, and (D) colon histology scores. (E) Percentage change from original BW (defined as day 0 and as 100%) after induction of DSS-colitis in hGF-SAMP mice. Note the rapid decline in BW that reached 10%–20% or exceeded 20%, criterion 4 of 5 of the DSS BW loss grade which indicates the need for euthanasia as recommended by Kim et al. (39) and Chassaing et al. (41). (F) FITC-dextran, (G) colon length, and (H) colon histology scores. *Different from con, P < 0.05. Line plots reflect the final BW of all animals for that day. AD-soy-pea, American diet modified by protein source (soy and pea isolates); BW, body weight; B6, C57BL/6J mouse line; con, control; DSS, dextran sodium sulfate; FITC, fluorescein isothiocyanate; hGF, “humanized” germ-free; M-W, Mann–Whitney U test; SAMP, SAMP1/YitFC mouse line; SPF, specific-pathogen-free.
Six-week feeding trial of AD-soy-pea “treated” advanced chronic ileitis in adult mice (Experiment 4)
Older SAMP mice (established cobblestone formation) fed AD-soy-pea had significantly lower fecal MPO and FITC-dextran permeability scores (Figure 4A, B), as well as lower postmortem mesenteric lymph node (MLN). N.3 weight to BW ratios than controls (1.7 ± 0.7 compared with 3.5 ± 0.6 mg/g BW, M-W P = 0.0002). AD-soy-pea-fed mice also had reduced cobblestone lesion severity (percentage abnormal mucosa, 3D-SM) and attenuated severity of CD-ileitis (histology) compared with controls (Figure 4C, D). Compared with the first feeding trial in young mice (Experiment 1), the AD-soy-pea diet promoted a higher BW than that of mice fed a rodent diet (40.8 ± 7.4 compared with 30.4 ± 2.2% BW change, M-W P = 0.001), which was not attributable to differences in food intake (4.95 ± 0.8 compared with 4.45 ± 0.3 g food/d, respectively, M-W P = 0.30). Note that as calculated (see the Statistical analysis section), power estimates for the primary outcome (MPO) in the experiments conducted [to be >0.8, achieved at the end of the study, G*Power software (53)] achieved an even larger effect for MPO (see Figure 4A; power is almost perfect: 0.97). As expected, secondary outcomes could have variable power β values (notice that the power is between 0.65 and 0.77) (Figure 4B–D), which is acceptable for secondary outcome power estimates, as we have extensively described and shown [see Basson et al. (32, 54)].
FIGURE 4.
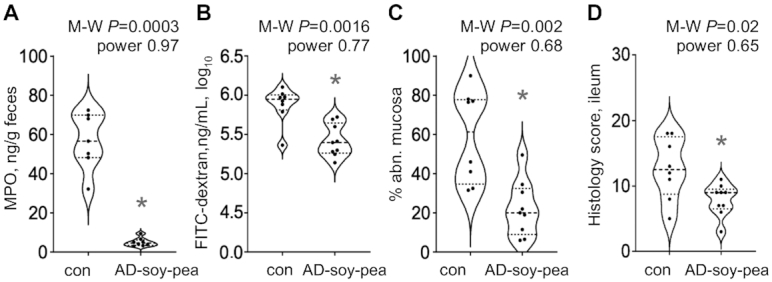
A 6-wk AD-soy-pea feeding (“treatment”) trial reduces the severity of advanced chronic ileitis in adult (24-wk-old) specific-pathogen-free SAMP mice (Experiment 4). Values are mean ± SD, n = 7–8. (A) Fecal MPO and (B) FITC-dextran recovered in plasma at week 6. (C) 3D-SM percentage abnormal mucosa and (D) histology scores for terminal ileum. *Different from con, P < 0.05. AD-soy-pea, American diet modified by protein source (soy and pea isolates); con, control; FITC, fluorescein isothiocyanate; MPO, myeloperoxidase; M-W, Mann–Whitney U test; SAMP, SAMP1/YitFC mouse line; 3D-SM, stereomicroscopy 3-D pattern profiling.
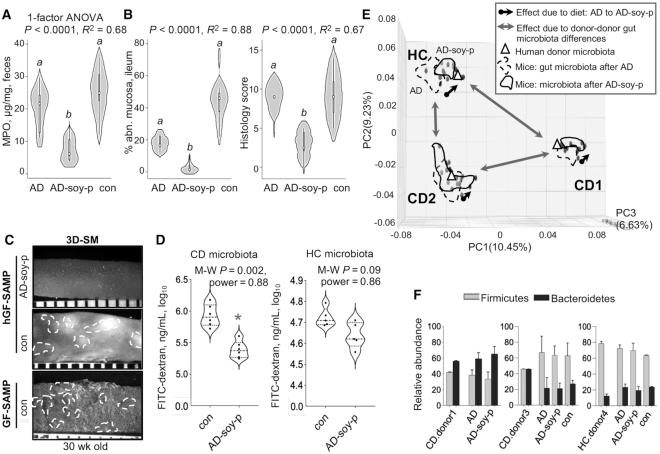
Twenty-four-week feeding of AD-soy-pea prevented chronic ileitis regardless of human microbiota (Experiments 1, 5, and 6)
Remarkably, we observed the same anti-inflammatory effect of soy + pea on fecal MPO in mice across the 3 human gut microbiotas, with almost complete suppression of cobblestone lesions in mice at 31 wk of age (Figure 5A–C). Of interest, mice colonized with CD human gut microbiota exhibited improved gut permeability when fed the AD-soy-pea diet (5.3 ± 0.13 ng/mL log10 compared with rodent diet 5.9 ± 0.17 ng/mL log10, M-W P = 0.002) (Figure 5D).
FIGURE 5.
The effects of AD, AD-soy-pea, and rodent diet after 24 wk on chronic CD-ileitis in young GF SAMP mice colonized with human gut microbiota (hGF-SAMP) (Experiments 1, 5, and 6). Values are mean ± SD, n = 5–7. (A) Fecal MPO measured 3 d after starting diets. The differences in MPO between hGF-SAMP treatment groups continued through to week 24. (B, C) Ileitis severity: (B) 3D-SM percentage abnormal mucosa (left panel) and histology scores (right panel) for terminal ilea, and (C) representative 3D-SM photographs (ileum) for hGF-SAMP and age-matched GF-SAMP. (D) FITC-dextran recovered in plasma of GF-SAMP colonized with CD or HC microbiota. (E) Metagenomic sequencing PC analysis plots for mice groups and respective human donors. Arrows show differences in the magnitude of effect between diet and donor microbiome. (F) Firmicutes:Bacteroidetes ratio across 3 different donor-mouse groups (mean ± SD). a,bLabeled means without a common letter differ (1-factor Kruskal–Wallis ANOVA with Dunn's multiple comparison test). *Different from control, P < 0.05. AD, American diet; AD-soy-p, American diet modified by protein source (soy and pea isolates); CD, Crohn disease; con, control; FITC, fluorescein isothiocyanate; GF, germ-free; HC, healthy control; hGF, “humanized” germ-free; MPO, myeloperoxidase; PC, principal component; SAMP, SAMP1/YitFC mouse line; 3D-SM, stereomicroscopy 3-D pattern profiling.
To fully characterize the effect of soy + pea on the human microbiome, mycobiome, and virome, we conducted fecal metagenome analysis. Across gut microbiota donors, bacterial metagenomics showed that the diet had a modest effect compared with the major donor–donor effect (note the arrow distances in the 3D-principal component analysis) (Figure 5E). Of additional relevance, the Firmicutes:Bacteroidetes ratio, which has been associated with increased risk of IBD in humans (55), revealed again that the beneficial effect of soy + pea (observed across all humanized mouse groups) occurred irrespective of the baseline Firmicutes:Bacteroidetes ratio of the transplanted gut microbiota (Figure 5F). AD-soy-pea also did not alter the abundance of Proteobacteria, Actinobacteria, or Verrucomicrobia (Supplemental Figure 3A). Diet had a very similar effect on Shannon α-diversity across mouse groups; however, the direction of effect varied by donor microbiome (Supplemental Figure 3B).
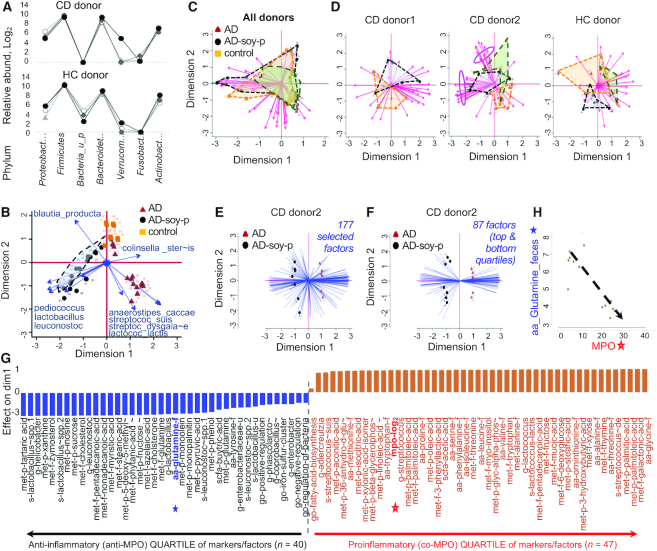
Confirming the reproducibility of human gut microbiota engraftment in mice, phylum- and family-level taxa signature plots showed a similar presence and abundance of most human bacterial taxa in the transplanted mice (Figure 6A, Supplemental Figure 4A), with recovery of human taxa in mice ranging from 89.6% to 100% (96% ± 4.8% family level, 98% ± 2.9% genus level) (Supplemental Table 4). Although microbiome differences could be attributed to diet for each donor, collectively most microbiome analysis at the family level indicated that very few taxa were significantly associated with administration of the AD-soy-pea diet (Supplemental Figure 4B), suggesting that other mechanisms, independent of bacterial abundance, are relevant (e.g., metabolic). Supplemental Table 5 shows the variation of effect of diet and the interaction with donor gut microbiota at the family level.
FIGURE 6.
Integration of metagenomic, MS, and inflammation severity data for all “humanized” germ-free-SAMP groups fed an AD, AD-modified, or rodent diet (Experiments 1, 5, and 6). Values are mean ± SD relative abundance data (log2), n = 5–7. (A) Line plots at phylum level comparing donor inocula with mean abundance in mouse fecal samples. (B) Biplot showing the contribution of the most influential species to the overall variance in dimensions 1 and 2 based on diet effect across all 3 donor microbiomes in mice (blue arrow lines indicate factor direction/magnitude to explain data variance; longer lines denote higher variance). (C) Biplots showing the contribution of all factors across all donors, and (D) at donor level, to the variance in dimensions 1 and 2 for functional GO pathways. Arrow lines indicate factor direction/magnitude to explain data variance; longer lines denote higher variance. Pink ovals show the unique set of vectors separating the AD-soy-p from other diets for CDdonor2. (E) Biplot of 14 observations and 177 factors, including the significant GO and GO Molecular function pathways, and significant taxa (family to species level). Dimension 1 contributes to 0.45, and dimension 2 to 0.14% of the variance (total variance explained: 59.96%). (F) Biplot of 14 observations and 87 factors (Panel E; dimension 1) as the most influential contributors (top 50%) to heterogeneity of variance. Dimension 1 contributes to 80.5 and dimension 2 to 0.05% of the variance (total variance explained: 85.64%). (G) Ranking of top- and bottom-quartile (“most influential”) variables (Panel F) from most positive to most negative for the differentiation of mouse data due to diet effect (AD compared with AD-soy-p, CD donor2). (H) Correlation plot between fecal glutamine (solid blue star) and inflammation (MPO; open red star) depicted in Panel G. AD, American diet; AD-soy-p, American diet modified by protein source (soy and pea isolates); CD, Crohn disease; GO, gene ontology; HC, healthy control; MPO, myeloperoxidase; SAMP, SAMP1/YitFC mouse line.
Linear regression analyses confirmed donor-dependent differences for the effect of diet on human gut taxa, with the exception of a positive correlation of Lactobacillaceae and Leuconostraceae with the AD-soy-pea diet (regression coefficient: 6.9; 95% CI: 4.2, 9.5; P < 0.001), which was also evident across the 3 microbiomes at the species level (Figure 6B). By comparison, the AD diet resulted in changes in the abundance of Anaerostipes caccae, Lactococcus lactis, Streptococcus suis, Streptococcus dysgalactiae, and Adlercreutzia equolifaciens. For example, A. equolifaciens (1 of 2 species of the genus) was decreased by AD-soy-pea by more than half in all mice (1-factor ANOVA, for each of the 3 human microbiotas, P ≤ 0.008).
Metagenomic analysis also revealed AD-soy-pea effects on fecal bacteriophage composition. Specifically, soy + pea resulted in a complete absence of 9 Lactococcus phage spp. (bIL286, bIL309, bIL310, bIL311, bIL312, bILBK5-T, P335 sensu lato, TP901–1, ul36; Fisher exact P < 0.0001) which are known to depend on the presence of L. lactis as their necessary host, indicating that changes in the bacteriophages are secondary to changes in bacterial composition. The effects on fungi were less pronounced, with Candida albicans being the only fungus identified, present in CDdonor1. No differences in C. albicans abundance were observed due to soy + pea (M-W P = 0.43).
Diet and modulatory association between gut microbiota, metabolites, and inflammation
Targeted metabolic validation using MS in fecal/plasma samples of human gut microbiota–colonized mice revealed significant differences in fecal and plasma amino acid concentrations (Table 1), including higher fecal butyric acid (4:0) (SCFA) in AD-soy-pea than in AD mice (3.3 ± 0.5 compared with 2.5 ± 0.4 ng/μg stool, M-W P = 0.006). We then used the metagenome data to infer to what extent the diet modified the overall metabolic activity of the human gut microbiota composition in the SAMP ileitis model. Using MPO data as a reliable continuous indicator of active intestinal inflammation in mice, linear regression revealed that independently from diet and donor, the MPO in the feces was negatively correlated with fecal abundance of Leuconostocaceae (intercept = −0.15, adj. P = 6.5 × 10−12), glutamine (intercept = −0.61, adj. P = 0.002), tyrosine (intercept = −0.59, adj. P = 0.05), and butyric acid (intercept = −1.10, adj. P = 0.03), as well as the plasma concentration of methionine (intercept = −2.8, adj. P = 0.01). Fecal MPO was also positively correlated with Eggerthellaceae (intercept = 0.484, adj. P = 0.0006) and plasma concentrations of linoleic acid (18:2n–6; intercept = −0.061, adj. P = 0.002) and glutamic acid (intercept = 0.71, adj. P = 0.01).
TABLE 1.
Effect of diet on fecal and plasma amino acid and SCFA concentrations for “humanized” germ-free-SAMP mice fed AD or AD-soy-pea1
| % change vs. ''referent'' AD diet2 | Feces, ng/μg | Plasma, ng/μL | ||||||
|---|---|---|---|---|---|---|---|---|
| Feces | Plasma | AD-soy-pea | AD | P 3 | AD-soy-pea | AD | P 3 | |
| Amino acids | ||||||||
| Alanine | ↓ 91.7% (−3.6) | ns | 7.6 ± 0.5 | 11.2 ± 0.1 | 0.005 | 5.2 ± 0.1 | 5.5 ± 0.2 | 0.07 |
| Asparagine | ns | ns | 5.2 ± 0.5 | 5.1 ± 0.2 | 0.59 | 3.0 ± 0.0 | 3.1 ± 0.1 | 0.30 |
| Aspartate | ns | ns | 6.3 ± 0.6 | 6.0 ± 0.8 | 0.53 | 0.5 ± 0.4 | 0.3 ± 0.1 | 0.63 |
| Cysteine | ns | ns | 7.7 ± 0.1 | 7.1 ± 0.8 | 0.25 | 4.0 ± 0.1 | 4.0 ± 0.6 | 0.12 |
| Glutamic acid | ↑ 90% (+0.9) | ↓ 62.1% (−1.4) | 13.0 ± 0.6 | 12.1 ± 0.7 | 0.02 | 6.0 ± 0.6 | 7.4 ± 1.0 | 0.01 |
| Glutamine | ↑ 260% (+2.6) | ↑ 70% (+0.7) | 6.5 ± 0.8 | 3.9 ± 0.3 | 0.0005 | 6.7 ± 0.2 | 6.0 ± 0.6 | 0.005 |
| Glycine | ↓ 89.1 (−3.2) | ns | 5.7 ± 0.4 | 8.9 ± 0.1 | 0.005 | 3.8 ± 0.1 | 3.8 ± 0.2 | 0.73 |
| Histidine | ↓ 18.7% (−0.3) | ns | 6.3 ± 0.1 | 6.6 ± 0.3 | 0.02 | 3.7 ± 0.2 | 3.1 ± 1.6 | 0.69 |
| Isoleucine | ↓ 93.7% (−4.0) | ns | 5.4 ± 1.1 | 9.6 ± 0.3 | 0.005 | 4.0 ± 0.1 | 4.0 ± 0.2 | 0.93 |
| Leucine | ↓ 94.1% (−4.1) | ↓ 12.9% (−0.2) | 6.6 ± 1.1 | 10.7 ± 0.3 | 0.006 | 4.8 ± 0.2 | 5.1 ± 0.2 | 0.01 |
| Linoleic acid | ns | ↓ 89.1% (−3.2) | 12.1 ± 2.0 | 12.0 ± 0.3 | 0.9 | 5.4 ± 0.4 | 8.6 ± 0.3 | 0.01 |
| Lysine | ns | ns | 9.4 ± 0.4 | 9.1 ± 0.8 | 0.45 | 6.6 ± 0.1 | 6.1 ± 1.7 | 0.71 |
| Methionine | ↓ 90.5% (−3.4) | ↑ 30% (+0.3) | 7.6 ± 0.7 | 9.5 ± 0.1 | 0.005 | 6.2 ± 0.2 | 5.9 ± 0.1 | 0.01 |
| Ornithine | ↓ 89.8% (−3.3) | ↓ 24.3% (−0.4) | 5.5 ± 0.6 | 8.8 ± 0.3 | 0.005 | 3.2 ± 0.1 | 3.6 ± 0.1 | 0.005 |
| Phenylalanine | ↓ 89.8% (−3.3) | ↓ 18.7% (−0.3) | 6.1 ± 0.8 | 9.4 ± 0.3 | 0.005 | 3.5 ± 0.1 | 3.8 ± 0.2 | 0.03 |
| Proline | ↓ 89.8% (−3.3) | ↓ 12.9% (−0.2) | 6.1 ± 0.5 | 9.2 ± 0.7 | 0.005 | 3.4 ± 0.1 | 3.6 ± 0.2 | 0.02 |
| Serine | ↓ 88.3% (−3.1) | ns | 5.3 ± 0.6 | 8.4 ± 0.7 | 0.005 | 3.5 ± 0.1 | 3.5 ± 0.1 | 0.82 |
| Threonine | ↓ 91.1% (−3.5) | 24.3% (−0.4) | 6.1 ± 0.5 | 9.6 ± 0.4 | 0.0005 | 4.2 ± 0.1 | 4.6 ± 0.2 | 0.006 |
| Tryptophan | ↓ 78.3% (−2.2) | ns | 5.9 ± 0.4 | 8.1 ± 0.3 | 0.005 | 4.0 ± 0.2 | 4.3 ± 0.3 | 0.09 |
| Tyrosine | ↑ 150% (+1.5) | ns | 4.8 ± 0.9 | 3.3 ± 0.7 | 0.004 | 3.9 ± 0.3 | 3.9 ± 0.3 | 0.68 |
| Valine | ↓ 93.3% (−3.9) | ns | 6.0 ± 0.9 | 9.9 ± 0.3 | 0.005 | 4.9 ± 0.1 | 5.1 ± 0.2 | 0.08 |
| SCFAs | ||||||||
| Acetic acid | ↓ 73.2% (−1.9) | — | 4.9 ± 0.4 | 6.8 ± 0.3 | 0.0005 | — | — | — |
| Butyric acid | ↑ 80% (+0.8) | — | 3.3 ± 0.5 | 2.5 ± 0.4 | 0.006 | — | — | — |
| Formic acid | ↓ 6.7% (−0.1) | — | 2.1 ± 0.1 | 2.2 ± 0.2 | 0.03 | — | — | — |
| Isovaleric acid | ns | — | 2.0 ± 0.2 | 1.9 ± 0.0 | 0.45 | — | — | — |
| Propionic acid | ns | — | 4.2 ± 0.3 | 3.9 ± 0.3 | 0.16 | — | — | — |
AD, American diet; AD-soy-pea, American diet modified by protein source (soy and pea isolates); ns, nonsignificant; SAMP, SAMP1/YitFC mouse line.
Percent change of analyte in mice receiving AD-soy-pea compared to mice receiving ''referent'' AD diet (P < 0.05). Value in parenthesis indicates Log2 difference = ''AD-soy-pea'' minus ''AD''. SCFA concentrations measured in feces only.
Log2-transformed MS data (Experiments 1, 5, and 6). Mann–Whitney U test, n = 5–7.
Logistic regression analyses of GO, GO MOLFU, and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways (against MPO) were not consistently affected by soy + pea in mice across the 3 microbiotas. However, donor-level analysis revealed variable segregation of pathways due to diet, indicating a donor-dependent effect of diet on functional pathways (note the overlap of mice across donors in Figure 6C and segregation of mice for each donor in Figure 6D). Using CDdonor2 as the best example to identify and show the combination of variables that best differentiated the mice with and without the AD-soy-pea diet, biplots indicated that diet modified numerous taxa, amino acids, and metabolic pathways in a linear fashion, having either a positive correlation with MPO activity (concurrent increase, proinflammatory) or a negative correlation (decrease, anti-inflammatory effect on ileitis) (Figure 6E, F). The most influential markers are listed and ranked in Figure 6G, with an example of the strong negative correlation between fecal glutamine and MPO shown in Figure 6H to facilitate interpretation of multiomics results.
Discussion
In this study we investigated the potential effects of replacing major macronutrients (protein, carbohydrate, fat) in ADs to alter the diets’ intestinal proinflammatory effect, and associated metabolic alterations of the gut microbiota in acute and chronic intestinal inflammation. Of the macronutrients replaced, we have shown, in long-/short-term feeding trials using preclinical, treatment, and chemically induced IBD mouse models, including a GF mouse model of CD-ileitis colonized human gut microbiota, that the most influential dietary modification on intestinal inflammation was the replacement of animal protein by plant protein (soy + pea isolates). We have also shown that soy + pea improved gut barrier integrity in both ileitis and chemically induced colitis, with the “intermediate effect” of the AD-mix diet highlighting the importance of dietary background for supplement bioactivity (56, 57).
The addition of soy + pea protein to the AD triggered compositional changes in the human gut microbiota, increasing lactic acid bacteria (e.g., Lactobacillaceae), reducing novel species such as the equol-producing bacterium A. equolifaciens, as well as promoting changes in the fecal and plasma concentrations of metabolites such as glutamine, butyric acid, and linoleic acid, that collectively may have contributed to the improved gut barrier integrity, and prevented and treated chronic ileitis in mice. These findings are especially clinically relevant to human IBD because we also showed that the vast majority of microbial taxa at the genus level, and their abundances in engrafted mice, matched those of their respective human donor feces. The anti-inflammatory effects of AD-soy-pea were seen on ileitis regardless of the Firmicutes:Bacteroidetes ratio of the transplanted human gut microbiota.
The abundance and relative ratios among the phyla Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria have been used to phenotypically describe the alteration of the gut microbiome in IBD (55). For instance, CD and UC have been associated with clear taxonomic shifts, including a depletion of Firmicutes and Bacteroidetes and enrichment in Proteobacteria and Actinobacteria (55). Specifically, an expansion of Proteobacteria, including genus members with adherent or invasive properties (e.g., Escherichia coli), is considered to drive proinflammatory changes in IBD (58). Human and animal studies have shown that dietary soy can alter the Firmicutes:Bacteroidetes ratio, in part by increasing the abundance of Lactobacilli and Bifidobacterium (59–61). Although our study also found increases in Lactobacillaceae, the effect of AD-soy-pea was not due to an overall reduction in Proteobacteria or alteration in the Firmicutes:Bacteroidetes ratio as reported (55, 58), suggesting that the mechanism of action of the diet is not entirely attributable to changes in gut microbiota composition.
At the species level, the reduction of A. equolifaciens is clinically relevant because in vitro studies indicate that the species uses daidzein (soy isoflavone) to produce equol, a metabolite hypothesized to have health benefits in humans (62), albeit shown in mice to perpetuate DSS-induced colitis (63). Although the role of A. equolifaciens remains unclear (63, 64), our results indicate that the species [and potentially the equol production from soy as shown in vitro (65, 66)] is not necessarily the mechanism by which soy + pea promoted the anti-inflammatory effects in our chronic ileitis model. The reduction of this species may have been indirectly caused by the diet favoring other bacterial species that, in turn, could have competed with or displaced Adlercreutzia. Although we did not measure equol, we focused on amino acids, SCFAs, and metabolic enrichment of genes related to amino acid metabolism known to be modified by the human gut microbiota in chronic ileitis (3) and by diet, namely soy (67–69).
Although consumption of soy and pea protein in the United States is not a major part of the market share compared with animal protein, soy flour is very popular owing to the reduced costs and large-scale production in the United States (70). Pea protein has seen significant growth in key sectors such as dietary supplements, baked goods, and beverages, mainly driven by both the rising demand for gluten-free products as well as a rising awareness among vegetarians of nutrition enrichment (70). Soy protein is an easily digestible nonanimal complete protein source (contains all 9 essential amino acids) that has a high protein digestibility–corrected amino acid score (PDCAAS) of 1.0, which is on par with egg and dairy (71). Because of this, soy has been used for decades in the food industry as an alternative protein and meat analog and is one of the most used protein sources in commercial laboratory rodent diets. Soybeans are also a source of biologically active isoflavones (e.g., genistein, daidzein), a class of phytoestrogens which are estrogenic (72, 73) and have been shown to confer benefit in some studies of chemically induced experimental colitis (14, 15, 17, 74, 75), but not others (63, 75, 76), for which we controlled through our diet formulation (Methods). Pea protein, with a PDCAAS of 0.94 comparable with that of soy, is also a complete protein, and although low in methionine, has the highest leucine and branched-chain amino acid arginine content of all plant-based proteins (77). Pea protein is also unique because of its high content of dietary fiber, polyphenolics, and glycoproteins, all of which are known to be beneficial for health (78).
Metagenome metabolic predictions and MS data showing differences in amino and fatty acid concentrations may indicate that the effect of soy + pea on ileitis is attributed to alterations in bacterial function rather than composition. Of importance, our findings are in line with that of our previous work in GF mice groups transplanted with human gut microbiota from different donors, where we showed a strong association between synthesis and degradation of amino acid alterations [predictive functional profiling, via Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (79)], especially leucine and severity of CD-ileitis, as well as linoleic acid which was present a few days after fecal microbiota transplantation (FMT), and not attributed to differences in virulence factors (3). Further, our findings are in agreement with studies in patients with CD reporting negative correlations between clinical disease activity and the concentrations of plasma amino acids (glutamine, tyrosine, valine, methionine, leucine, tryptophan, alanine) (80) and serum linoleic acid (30).
Collectively, our findings hold important implications for human dietetics indicating that patients could add/replace animal protein with soy + pea and expect to reduce the potential proinflammatory effects of certain diets, especially in IBD-susceptible individuals. In context, IBD susceptibility has been shown to increase the likelihood of potential negative effects due to diet (e.g., Splenda worsens SAMP-ileitis) (10). The therapeutic potential of soybean food products and soybean-derived bioactives in IBD patients is still unclear, and, with the exception of soy lecithin, has not been tested in IBD-focused clinical studies. In addition, studies investigating the effect of soy supplementation, specifically isoflavones as an alternative to hormone replacement therapy in postmenopausal women, have yielded inconsistent results (positive or negative) and interpretation of findings is limited given the notable differences in study design (81). Limited evidence also exists at the population level, with 1 Japanese case-control study concluding that increased soy intake was associated with increased UC risk (OR: 4.76; P = 0.02) (82), whereas 1 Chinese study found soy had an anti-inflammatory effect (83). However, differences in soy and isoflavone consumption patterns between Asian and Western populations limit extrapolation of such evidence (64). Thus, the concepts herein described in various genetic mouse/microbiota models require further characterization in humans, which is currently ongoing in CD patients.
This study is unique because we used a chronic ileitis model in the context of its native murine microbiome and that of human gut microbiota, and because we designed the modified ADs so that the original dietary sources of the macronutrients from the referent diet (NHANES) were completely replaced using current dietetic principles. Findings are encouraging to further determine the mechanisms by which both plant grains could potentially alter gene expression of bacteria, which, in turn, could influence local and systemic immunity, and the metabolism and availability of amino acid concentrations, that we demonstrated are positively and negatively correlated with MPO activity.
In conclusion, by emulating what humans eat, the present study demonstrated that the replacement of animal protein with soy + pea protein in an AD controls chronic ileitis regardless of the Firmicutes:Bacteroidetes ratio. Our study supports the concept that replacing animal protein by plant-based soy + pea protein may have an anti-inflammatory effect in humans affected by IBD, or other chronic inflammatory disorders, namely CD.
Supplementary Material
ACKNOWLEDGEMENTS
We thank John D Ward, Heather Wang, Alicia DePlatchett, Jonathan Craven, and Rachael Murphy for their technical and administrative support, Dr. Gurkan Bebek for bioinformatics consultation, and Dr. Wei Xin for the histological scoring of ileitis severity. ARB especially thanks and acknowledges Mr. and Mrs. Raffner for their unwavering support. The authors’ responsibilities were as follows––ARB: designed and conducted the research with intellectual support from AR-P and wrote the final manuscript with input from AR-P and FC; AR-P: performed the statistical analysis and interpretation of data; AG-N, AL, LB, AW, DK, and GP: provided technical/experimental/intellectual support; LDM: performed the colonoscopy procedure and blinded scoring; AO: performed all histological analysis; and all authors: read, edited, and approved the final manuscript.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK055812, DK091222, and DK097948 (to FC), T32DK083251 and F32DK117585 (to ARB), and P01DK091222 Germ-free and Gut Microbiome Core and R21DK118373 (to AR-P). We acknowledge the support of the Mouse Models, the Histology Imaging, and the Tissue Biorepository Cores of the NIH P30 Silvio O Conte Cleveland Digestive Diseases Research Core Center.
Author disclosures: The authors report no conflicts of interest.
Supplemental Methods, Supplemental Tables 1–5, and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: AD, American diet; AD-fat, American diet modified by fat source; AD-mix, American diet modified by protein, fat, and carbohydrate source; AD-soy-pea, American diet modified by protein source (soy and pea isolates); BW, body weight; B6, C57BL/6J mouse line; CD, Crohn disease; con, control (laboratory rodent diet); CWRU, Case Western Reserve University; DSS, dextran sodium sulfate; FITC, fluorescein isothiocyanate; GF, germ-free; GO, gene ontology; hGF, “humanized” germ-free; IBD, inflammatory bowel disease; IRB, Institutional Review Board; MOLFU, molecular function; MPO, myeloperoxidase; M-W, Mann–Whitney U test; PDCAAS, protein digestibility–corrected amino acid score; SAMP, SAMP1/YitFC mouse line; SPF, specific-pathogen-free; UC, ulcerative colitis; 3D-SM, stereomicroscopy 3-D pattern profiling.
Contributor Information
Abigail Raffner Basson, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Adrian Gomez-Nguyen, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Alexandria LaSalla, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA.
Ludovica Buttó, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Danielle Kulpins, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA.
Alexandra Warner, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA.
Luca Di Martino, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Gina Ponzani, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Abdullah Osme, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Department of Pathology, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Alexander Rodriguez-Palacios, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
Fabio Cominelli, Division of Gastroenterology & Liver Diseases, School of Medicine, Case Western Reserve University, Cleveland, OH, USA; Digestive Health Research Institute, University Hospitals Cleveland Medical Center, Cleveland, OH, USA.
References
- 1. Egger G, Dixon J. Should obesity be the main game? Or do we need an environmental makeover to combat the inflammatory and chronic disease epidemics?. Obes Rev. 2009;10:237–49. [DOI] [PubMed] [Google Scholar]
- 2. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basson AR, Gomez-Nguyen A, Menghini P, Buttó LF, Di Martino L, Aladyshkina N, Osme A, LaSalla A, Fischer D, Ezeji JC et al. Human gut microbiome transplantation in ileitis prone mice: a tool for the functional characterization of the microbiota in inflammatory bowel disease patients. Inflamm Bowel Dis. 2020;26:347–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simopoulos AP. Evolutionary aspects of diet: the omega-6/omega-3 ratio and the brain. Mol Neurobiol. 2011;44:203–15. [DOI] [PubMed] [Google Scholar]
- 5. Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17:742–53. [DOI] [PubMed] [Google Scholar]
- 6. Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rizzello F, Spisni E, Giovanardi E, Imbesi V, Salice M, Alvisi P, Valerii MC, Gionchetti P. Implications of the westernized diet in the onset and progression of IBD. Nutrients. 2019;11:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018;64:20–57. [DOI] [PubMed] [Google Scholar]
- 9. Reddavide R, Rotolo O, Caruso MG, Stasi E, Notarnicola M, Miraglia C, Nouvenne A, Meschi T, De’ Angelis GL, Di Mario F et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018;89:60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez-Palacios A, Harding A, Menghini P, Himmelman C, Retuerto M, Nickerson KP, Lam M, Croniger CM, McLean MH, Durum SK et al. The artificial sweetener Splenda promotes gut Proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn's disease–like ileitis. Inflamm Bowel Dis. 2018;24:1005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, Chieppa M, Arseneau KO, Ley K, Cominelli F. SAMP1/YitFc mouse strain: a spontaneous model of Crohn's disease-like ileitis. Inflamm Bowel Dis. 2011;17:2566–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–18. [DOI] [PubMed] [Google Scholar]
- 13. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy. Adv Nutr. 2016;7:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One. 2018;13:e0199631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo Q, Cheng D, Huang C, Li Y, Lao C, Xia Y, Liu W, Gong X, Hu D, Li B et al. Improvement of colonic immune function with soy isoflavones in high-fat diet-induced obese rats. Molecules. 2019;24:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Metzger CE, Narayanan SA, Zawieja DC, Bloomfield SA. A moderately elevated soy protein diet mitigates inflammatory changes in gut and in bone turnover during chronic TNBS-induced inflammatory bowel disease. Appl Physiol Nutr Metab. 2019;44:595–605. [DOI] [PubMed] [Google Scholar]
- 17. Wang B, Wu C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp Ther Med. 2017;14:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Snelson M, Mamo JCL, Lam V, Giles C, Takechi R. Differential effects of high-protein diets derived from soy and casein on blood–brain barrier integrity in wild-type mice. Front Nutr. 2017;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bitzer ZT, Wopperer AL, Chrisfield BJ, Tao L, Cooper TK, Vanamala J, Elias RJ, Hayes JE, Lambert JD. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo. J Nutr Biochem. 2017;40:201–8. [DOI] [PubMed] [Google Scholar]
- 20. Jiang H, Przybyszewski J, Mitra D, Becker C, Brehm-Stecher B, Tentinger A, MacDonald RS. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-α in colon of dextran sodium sulfate-treated C57BL/6 mice. J Nutr. 2011;141:1239–46. [DOI] [PubMed] [Google Scholar]
- 21. Al-Nakkash L, Kubinski A. Soy isoflavones and gastrointestinal health. Curr Nutr Rep. 2020;9:193–201. [DOI] [PubMed] [Google Scholar]
- 22. Lee I-A, Park Y-J, Joh E-H, Kim D-H. Soyasaponin Ab ameliorates colitis by inhibiting the binding of lipopolysaccharide (LPS) to Toll-like receptor (TLR)4 on macrophages. J Agric Food Chem. 2011;59:13165–72. [DOI] [PubMed] [Google Scholar]
- 23. Utrilla MP, Peinado MJ, Ruiz R, Rodriguez-Nogales A, Algieri F, Rodriguez-Cabezas ME, Clemente A, Galvez J, Rubio LA. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol Nutr Food Res. 2015;59:807–19. [DOI] [PubMed] [Google Scholar]
- 24. Bibi S, de Sousa Moraes LF, Lebow N, Zhu M-J. Dietary green pea protects against DSS-induced colitis in mice challenged with high-fat diet. Nutrients. 2017;9:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han M, Wang C, Liu P, Li D, Li Y, Ma X. Dietary fiber gap and host gut microbiota. Protein Pept Lett. 2017;24:388–96. [DOI] [PubMed] [Google Scholar]
- 26. Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, De Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Lochhead P, Richter JM, Chan AT. Genetic polymorphisms in fatty acid metabolism modify the association between dietary n3: n6 intake and risk of ulcerative colitis: a prospective cohort study. Inflamm Bowel Dis. 2017;23:1898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morampudi V, Bhinder G, Wu X, Dai C, Sham HP, Vallance BA, Jacobson K. DNBS/TNBS colitis models: providing insights into inflammatory bowel disease and effects of dietary fat. J Vis Exp. 2014(84):e51297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–84. [DOI] [PubMed] [Google Scholar]
- 30. Scoville EA, Allaman MM, Adams DW, Motley AK, Peyton SC, Ferguson SL, Horst SN, Williams CS, Beaulieu DB, Schwartz DA et al. Serum polyunsaturated fatty acids correlate with serum cytokines and clinical disease activity in Crohn's disease. Sci Rep. 2019;9:2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rodriguez-Palacios A, Aladyshkina N, Ezeji JC, Erkkila HL, Conger M, Ward J, Webster J, Cominelli F. ‘Cyclical bias’ in microbiome research revealed by a portable germ-free housing system using nested isolation. Sci Rep. 2018;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Basson AR, LaSalla A, Lam G, Kulpins D, Moen EL, Sundrud MS, Miyoshi J, Ilic S, Theriault BR, Cominelli F et al. Artificial microbiome heterogeneity spurs six practical action themes and examples to increase study power-driven reproducibility. Sci Rep. 2020;10:5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodriguez-Palacios A, Aladyshkina N, Cominelli F. Stereomicroscopy and 3D-target myeloperoxidase intestinal phenotyping following a fecal flora homogenization protocol. Protocol Exchange. 2015.doi:10.1038/protex.2015.065. [Google Scholar]
- 34. Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol. 2010;31:400–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corridoni D, Kodani T, Rodriguez-Palacios A, Pizarro TT, Xin W, Nickerson KP, McDonald C, Ley KF, Abbott DW, Cominelli F. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110:16999–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Menghini P, Corridoni D, Buttó LF, Osme A, Shivaswamy S, Lam M, Bamias G, Pizarro TT, Rodriguez-Palacios A, Dinarello CA et al. Neutralization of IL-1α ameliorates Crohn's disease-like ileitis by functional alterations of the gut microbiome. Proc Natl Acad Sci U S A. 2019;116(52):26717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Palacios A, Kodani T, Kaydo L, Pietropaoli D, Corridoni D, Howell S, Katz J, Xin W, Pizarro TT, Cominelli F. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat Commun. 2015;6:7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodriguez-Palacios A, Khoretonenko MV, Ilic S. Institutional protocols for the oral administration (gavage) of chemicals and microscopic microbial communities to mice: analytical consensus. Exp Biol Med (Maywood). 2019;244:459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp. 2012(60):e3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eichele DD, Kharbanda KK. Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol. 2017;23:6016–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15.25.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miles JP, Zou J, Kumar MV, Pellizzon M, Ulman E, Ricci M, Gewirtz AT, Chassaing B. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm Bowel Dis. 2017;23:1133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–66 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn's disease in mice. Gastroenterology. 2001;121:1428–36. [DOI] [PubMed] [Google Scholar]
- 45. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ponnusamy D, Kozlova EV, Sha J, Erova TE, Azar SR, Fitts EC, Kirtley ML, Tiner BL, Andersson JA, Grim CJ et al. Cross-talk among flesh-eating Aeromonas hydrophila strains in mixed infection leading to necrotizing fasciitis. Proc Natl Acad Sci U S A. 2016;113:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ottesen A, Ramachandran P, Reed E, White JR, Hasan N, Subramanian P, Ryan G, Jarvis K, Grim C, Daquiqan N et al. Enrichment dynamics of Listeria monocytogenes and the associated microbiome from naturally contaminated ice cream linked to a listeriosis outbreak. BMC Microbiol. 2016;16:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345:1048–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hasan NA, Young BA, Minard-Smith AT, Saeed K, Li H, Heizer EM, McMillan NJ, Isom R, Abdullah AS, Bornman DM et al. Microbial community profiling of human saliva using shotgun metagenomic sequencing. PLoS One. 2014;9:e97699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S, Nikolau B. Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J. 2008;53:691–704. [DOI] [PubMed] [Google Scholar]
- 51. Moreau NM, Goupry SM, Antignac JP, Monteau FJ, Le Bizec BJ, Champ MM, Martin LJ, Dumon HJ. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:395–403. [DOI] [PubMed] [Google Scholar]
- 52. Darwin, WipaCharles, Cord-Ruwisch R. Concurrent lactic and volatile fatty acid analysis of microbial fermentation samples by gas chromatography with heat pre-treatment. J Chromatogr Sci. 2018;56:1–5. [DOI] [PubMed] [Google Scholar]
- 53. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 54. Basson AR, Cominelli F, Rodriguez-Palacios A. Patterns of ‘analytical irreproducibility’ in multimodal diseases. bioRxiv. 2020. doi:10.1101/2020.03.22.002469. [Google Scholar]
- 55. Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hintze KJ, Benninghoff AD, Cho CE, Ward RE. Modeling the Western diet for preclinical investigations. Adv Nutr. 2018;9:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Määttänen P, Lurz E, Botts SR, Wu RY, Yeung CW, Li B, Abiff S, Johnson-Henry KC, Lepp D, Power KA et al. Ground flaxseed reverses protection of a reduced-fat diet against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2018;315:G788–G98. [DOI] [PubMed] [Google Scholar]
- 58. Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD—what role do Proteobacteria play?. Nat Rev Gastroenterol Hepatol. 2012;9:219–30. [DOI] [PubMed] [Google Scholar]
- 59. Zhu Y, Lin X, Li H, Li Y, Shi X, Zhao F, Xu X, Li C, Zhou G. Intake of meat proteins substantially increased the relative abundance of genus Lactobacillus in rat feces. PLoS One. 2016;11:e0152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep. 2015;5:15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huang H, Krishnan HB, Pham Q, Yu LL, Wang TTY. Soy and gut microbiota: interaction and implication for human health. J Agric Food Chem. 2016;64:8695–709. [DOI] [PubMed] [Google Scholar]
- 62. Magee PJ. Is equol production beneficial to health?. Proc Nutr Soc. 2011;70:10–8. [DOI] [PubMed] [Google Scholar]
- 63. Sakai T, Furoku S, Nakamoto M, Shuto E, Hosaka T, Nishioka Y, Sone S. Soy isoflavone equol perpetuates dextran sulfate sodium-induced acute colitis in mice. Biosci Biotechnol Biochem. 2011;75:593–5. [DOI] [PubMed] [Google Scholar]
- 64. Juritsch AF, Moreau R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr Rev. 2018;76:618–38. [DOI] [PubMed] [Google Scholar]
- 65. Mace TA, Ware MB, King SA, Loftus S, Farren MR, McMichael E, Scoville S, Geraghty C, Young G, Carson WE 3rd et al. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci Rep. 2019;9:5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Florez AB, Vazquez L, Rodriguez J, Redruello B, Mayo B. Transcriptional regulation of the equol biosynthesis gene cluster in Adlercreutzia equolifaciens DSM19450T. Nutrients. 2019;11:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, Zhou G. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song S, Hooiveld GJ, Li M, Zhao F, Zhang W, Xu X, Muller M, Li C, Zhou G. Dietary soy and meat proteins induce distinct physiological and gene expression changes in rats. Sci Rep. 2016;6:20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luiking YC, Deutz NE, Jakel M, Soeters PB. Casein and soy protein meals differentially affect whole-body and splanchnic protein metabolism in healthy humans. J Nutr. 2005;135:1080–7. [DOI] [PubMed] [Google Scholar]
- 70. Grand View Research . Protein ingredients market size, share & trends analysis report by product (plant protein, animal/dairy protein), by application (food & beverages, personal care & cosmetics), and segment forecasts, 2020–2027. Report ID 978-1-68038-451-2. San Francisco (CA): Grand View Research; 2020. [Google Scholar]
- 71. Hughes GJ, Ryan DJ, Mukherjea R, Schasteen CS. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: criteria for evaluation. J Agric Food Chem. 2011;59:12707–12. [DOI] [PubMed] [Google Scholar]
- 72. Mallien AS, Soukup ST, Pfeiffer N, Brandwein C, Kulling SE, Chourbaji S, Gass P. Effects of soy in laboratory rodent diets on the basal, affective, and cognitive behavior of C57BL/6 mice. J Am Assoc Lab Anim Sci. 2019;58:532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kraemer WJ, Solomon-Hill G, Volk BM, Kupchak BR, Looney DP, Dunn-Lewis C, Comstock BA, Szivak TK, Hooper DR, Flanagan SD et al. The effects of soy and whey protein supplementation on acute hormonal responses to resistance exercise in men. J Am Coll Nutr. 2013;32:66–74. [DOI] [PubMed] [Google Scholar]
- 74. Vanden Braber NL, Novotny Nuñez I, Bohl L, Porporatto C, Nazar FN, Montenegro MA, Correa SG. Soy genistein administered in soluble chitosan microcapsules maintains antioxidant activity and limits intestinal inflammation. J Nutr Biochem. 2018;62:50–8. [DOI] [PubMed] [Google Scholar]
- 75. Seibel J, Molzberger AF, Hertrampf T, Laudenbach-Leschowski U, Degen GH, Diel P. In utero and postnatal exposure to a phytoestrogen-enriched diet increases parameters of acute inflammation in a rat model of TNBS-induced colitis. Arch Toxicol. 2008;82:941–50. [DOI] [PubMed] [Google Scholar]
- 76. Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997;26:63–70. [DOI] [PubMed] [Google Scholar]
- 77. Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145:372–9. [DOI] [PubMed] [Google Scholar]
- 78. Ahnen RT, Jonnalagadda SS, Slavin JL. Role of plant protein in nutrition, wellness, and health. Nutr Rev. 2019;77(11):735–47. [DOI] [PubMed] [Google Scholar]
- 79. Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Thurber RLV, Knight R et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chiba T, Suzuki K, Matsumoto T. Plasma-free amino acid profiles in Crohn's disease: relationship with the Crohn disease activity index. Clin Med Insights Gastroenterol. 2018;11:1179552218791173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beavers KM, Jonnalagadda SS, Messina MJ. Soy consumption, adhesion molecules, and pro-inflammatory cytokines: a brief review of the literature. Nutr Rev. 2009;67:213–21. [DOI] [PubMed] [Google Scholar]
- 82. Ohfuji S, Fukushima W, Watanabe K, Sasaki S, Yamagami H, Nagahori M, Watanabe M, Hirota Y, Japanese Case-Control Study Group for Ulcerative Colitis . Pre-illness isoflavone consumption and disease risk of ulcerative colitis: a multicenter case-control study in Japan. PLoS One. 2014;9:e110270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wu SH, Shu XO, Chow W-H, Xiang Y-B, Zhang X, Li H-L, Cai Q, Ji B-T, Cai H, Rothman N et al. Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet. 2012;112:996–1004, e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.