Abstract

Drilling fluid and filtrates invasion often alter the near-wellbore flow properties during overbalanced drilling. The utilization of polymeric gels to prevent drilling fluid loss reduces the risk of formation damage caused by this alteration. In this study, the internal and external filter cake evolution by polyacrylamide (PAM) cross-linked with polyethylenimine (PEI) was investigated. The analysis conducted in this study showed that the cross-linked polymer activates and forms a mature gel inside the formation’s pores. Gel also formed a dense uniform structure on the rock’s surface, preventing further fluid loss. A high sealing pressure of up to 1000 psi was achieved, allowing drilling to continue without the need for additional casing string to prevent lost circulation. Moreover, the PAM/PEI formula showed less invasion of filtrate and evolution of a thin shallow internal filter cake that penetrated less than half of the filter disk thickness. In comparison to the full invasion and particle depositions that occurred with the water-based mud (WBM), the PAM/PEI formula is expected to reduce the impact of lost circulation materials (LCMs) on formation damage.
Introduction
Filtration and flow characteristics of drilling fluids are of great importance to address lost circulation problems or designing preventive methods to strengthen the wellbore. The process occurs when the differential pressure forces the fluid particles to penetrate and deposit in and around the wellbore wall and form a low-permeability filter cake.1−3 This process is considered as a time-dependent process that occurs in different stages. The spurt loss, which is the first invasion of fluid into the formation, occurs within seconds at the first exposure of the drilling fluid to the wellbore wall.4 The second stage of filtration shows more invasion of the solid particles due to the overbalanced drilling’s differential pressure. When a high concentration of drilling fluid particles deposit in and around the wellbore, this will lead to the evolution of an internal filter cake, as illustrated in Figure 1.
Figure 1.

Schematic of Mud filtration through mud cake and near-wellbore area.
Another significance of the internal filter cake is referred to as “mud cake wellbore strengthening”; low-permeability internal filter cake imposes a positive change in the effective stress, which can enhance the wellbore strength.5,6 After forming a stable internal filter cake of a low permeability within the pore network, only drilling fluid filtrates will be able to flow, which will lead to the evolution of external filter cake.7,8 Reducing the near-wellbore permeability prevents excessive drilling fluid filtrates invasion, prevents pore pressure increasing behind the filter cake, and reduces formation damage.1
Basically, drilling fluid filtration occurs in a static condition when there is no mud circulation or a dynamic state with mud circulation. The process is the same as explained in the preceding section; however, during the adynamic filtration, the shearing forces suppress the particles in the drilling fluid and cause accumulation of larger particles at the external filter cake, which may increase the thickness of the filter cake.9 During dynamic and static filtration, many factors affect the external and internal mud cake evolution, which are difficult to quantify. For instance, the process could be affected by the physicochemical properties of the suspension, clogging caused by migration of small particles, and the history of the process imposed by the fluid flow on the solid matrix.
In other words, the characteristics of filtration and mud cake evolution depend on many factors such as drilling fluid’s design, fluid rheological properties, rock properties, and operation conditions.10−12 The migration of fine particles into the formation forms the internal filter cake, controlled by the flow characteristics of fluid into the solid matrix. Fine particles might penetrate deeper into the formation if the external filter cake permeability was high, which can negatively impact the formation’s permeability.13,14 A semipermeable external filter cake significantly reduces the filtrates and invasion of the solids. However, the invasion of larger particles is usually localized to the near-wellbore area. Internal filter cake permeability also affects the deeper invasion of the fine particles; therefore, minimizing internal filter cake and quickly forming external cake is very important for fluid loss and controlling of formation damage.13
Regular-drilling fluid systems have large quantities of fine solids that invade the formation, causing damages in productive zones. Bridging materials are often used to improve filtration characteristics and prevent lost circulation, with formation damage resulting from the invasion of fine solid within drilling fluids that pass through the filter cake. The selection of proper lost circulation materials (LCM) should consider many factors such as particle size distribution, targeted fracture size, depth, temperature, and type of mud. However, many attempts for designing suitable drilling fluids for lost circulation treatment have often failed due to many operational and complications such as pumping requirements in deep drilling and increased the risk of bit nozzle clogging.15 For instance, in high-temperature formations, fluid viscosity, particle size, friction coefficient, and Young’s modulus are thermally degraded properties, resulting in ineffective sealing of the fractures.16 Moreover, particle size degradation occurs during the long-term process of drilling fluid circulation, also resulting in severe formation damage.17
Over the last few years, several researchers have examined the use of other options such as nanoparticles to design drilling fluids with enhanced properties that can endure extreme downhole environments, particularly under HP/HT conditions. Vryzas and Kelessidis summarized some of these nanoparticle additives used in drilling fluid and reported an enhancement in fluid properties and wellbore strengthening; however, there are still many challenges that should be addressed.18 Others looked into the effect of operational conditions and drilling parameters, such as friction coefficients, that increase with declination of particle’s sphericity and increase of surface roughness and particle size in solid materials, which affect lost circulation control.19 A clear understanding of the fluid loss mechanism and particle–particle interaction is important for successful drilling operations. Therefore, much attention has been paid to the development of lost circulation materials to overcome the limitations of the physical properties of both fluid and solid particles.20
Many studies have investigated cross-linkable polymers as LCM because of their high potential in sealing complex loss zones with large fractures at various downhole conditions.21−23 Li et al. showed that the polymer and hydrated bentonite react under the action of an organic cross-linking agent to form a composite gel.24 The cross-linkable polymers are classified based on their origin, natural and synthesized, or on their structure, diversity of monomer structures, and molecular forces. The most commonly used cross-linked polymer is the acrylamide polymer cross-linked with polyethyleneimine (PEI).25
Our research group demonstrated that the utilization of polymeric gels, particularly the polyacrylamide (PAM) cross-linked with polyethylenimine (PEI), is effective in sealing near-wellbore fractures.15,25−27 The PAM/PEI formula is widely used in the oil and gas industry for various applications such as polymer flooding, enhancing sweep efficiency in water flooding, and water shut-off in high water productive zones.15,21−23,28 PAM is a water-soluble polymer, while PEI is a widely used organic cross-linker and environmentally friendly additive.29−31
In this study, for the first time, the PAM/PEI formula is investigated in terms of reducing the risk of formation damage since it does not require adding a large-particle-size material to the seal fractures. These types of gels have been widely used in water shut-off and permeability alteration application in produced wells. Many authors used oxidization treatment employing different oxidizers such as sodium peroxide, calcium peroxide, sodium persulfate, and ammonium persulfate.32−34 The removal of filter cake in some reported cases was up to 93%.35 The evolution of internal and external filter cake is investigated using an optical method and elemental analysis. Carbonate rocks, as an example of naturally fractured formation with high permeability, were used to create disks for the static filtration experiments.
Experimental Section
Fluid Preparation
Nonionic polyacrylamide (PAM) having an average molecular weight (Mw) of 200 000 Da was obtained from a commercial supplier. The as-received sample concentration was 20 wt % with a viscosity of 70 mPa·s for a pure PAM having a concentration of 10 wt % in distilled water. The organic cross-linker used was the highly branched polyethylenimine (PEI) with a concentration of 33.3 wt % and an average molecular weight of 750 000 Da. For drilling fluid formulation, typical drilling fluid additives were used, including barite as a weighting agent, sodium hydroxide to raise the mud’s alkalinity, commercial mud deflocculant (tannin-based dispersant) called Desco, and lignite as a mud dispersant.
The cross-linked polymer of PAM/PEI formula was prepared by adding PAM and PEI with specific concentrations (7.5:1) to form 7.5 wt % PAM solution in distilled water. This concentration was optimized in another study. The formula, as shown in Table 1, with 3.5 lb/bbl bentonite was found to give the best rheology and final gel strength required for sealing application.36 The second fluid used in this study is a WBM with 9.6 ppg; the composition of the water-based mud (WBM) is shown in Table 2. The mud was prepared following API recommendation practices by adding additives in the same order shown in the table and allowing 5 min of high-speed mixing. For the viscosity–temperature profile measurements, a high-pressure/high-temperature (HP/HT) rheometer was used. Measurements were conducted at a constant shear rate of 170 s–1, equivalent to 100 rpm, for better representation of the rheological properties during mud circulation. The samples were heated at 4 °F/min in a temperature range of 70–266 °F (21–130 °C). The maximum temperature was selected based on the gel onset temperature of the cross-linked PAM/PEI established in previous studies.25,37 The onset temperature of gelatinization refers to the inflection point of the viscosity–temperature profile, while the mature gel is reached at the plateau of the curve.37,38
Table 1. Composition of the PBM.
| product | lb/bbl | wt % | vol % |
|---|---|---|---|
| water | 318.6 | 90.5 | 93.5 |
| bentonite | 3.5 | 0.97 | 0.41 |
| PAM | 26.6 | 7.5 | 5.1 |
| PEI | 3.52 | 1 | 1 |
Table 2. Composition of the WBM.
| products | lb/bbl | wt % | vol % |
|---|---|---|---|
| water | 314.764 | 78.1632 | 89.93 |
| caustic | 0.5 | 0.12416 | 0.093 |
| bentonite | 20 | 4.96 | 2.38 |
| lignite | 4 | 0.99 | 0.76 |
| mud deflocculant | 4 | 0.99 | 0.71 |
| calcium carbonate | 55 | 13.65 | 5.82 |
| barite | 4.43 | 1.10 | 0.29 |
Experimental Setup and Filter Disk Preparation
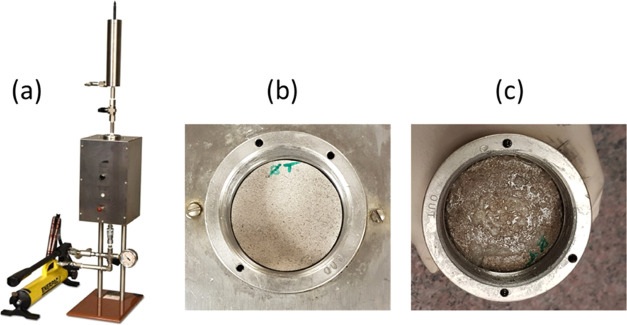
For the static filtration experiments or the permeability plugging test (PPT), a permeability plugging apparatus (PPA) was used with filter disks, as shown in Figure 2. Quarter-inch-thick disks with 2.5 in. diameter were cut from a heterogeneous carbonate core. The process of coring and disk preparation is shown in Figure 3. Static filtration experiments were conducted under a differential pressure of 500 psi across the disks and at a temperature of 266 °F. Another two experiments were conducted using homogeneous aloxite ceramic filter disks with a mean pore throat of 50 μm (No. 170-53) and 10 μm (No. 170-55).
Figure 2.
Experimental setup: (a) PPA, (b) carbonate disk before experiment, (c) carbonate disk after experiment.
Figure 3.
Process of carbonate coring and filter disks preparation: (a) rock after coring, (b) core, 2.5 × 3 in., and (c) disks, 2.5 × 0.25 in.
Results and Discussion
Fluid Properties
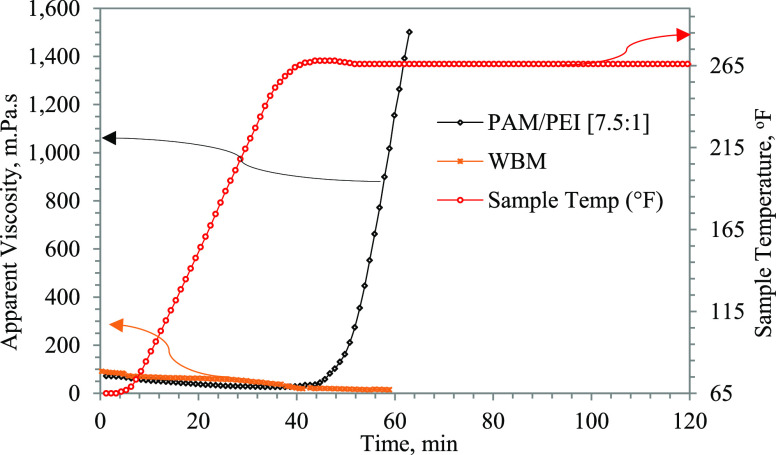
The filtration process depends mainly on the rheological properties of the drilling fluids and the size and content of solids in it, as explained in the preceding discussion. The two fluids used in this study, the PAM/PEI formula and the WBM, were designed to have the same rheological behavior. The difference is that the WBM contains different solid particles, as shown in Tables 1 and 2, and sized calcium carbonate (CaCO3) as primary LCM. On the other hand, the PAM/PEI main sealing mechanism is its gelling property attained at the preset onset temperature (266 °F). Both fluids have apparent viscosities from 15 to 20 mPa·s at 266 °F. Figure 4 shows the measurements of viscosity conducted at a constant shear rate of 170 s–1 (100 rpm) over a temperature range of 70–266 °F (21–130 °C).
Figure 4.
Viscosity–temperature profile of WBM and PAM/PEI fluid.
During the test, the samples are gradually heated at 4 °F/min until the onset temperature is reached and then kept at a constant temperature. There was a small drop in viscosity with heating for both the WBM and the PAM/PEI formula. This is normal for the PAM/PEI fluid since the gelation process has not been initiated; however, when the onset temperature of gelatinization (266 °F) is reached, the PAM/PEI fluid exhibited a sudden sharp increase in viscosity due to the cross-linking process between the PAM and the PEI. The result is a mature high-strength polymeric gel. The gelling process of PAM/PEI fluid is its main sealing mechanism. The increased viscosity helps reduce filtration until the mature gel is formed inside the pores and completely seals the loss zone.
Fluid Filtrates and Evolution of the External Mud Cake
One of the commonly used granular types of LCM is the sized calcium carbonate (CaCO3). The varying wide particle size distribution of the CaCO3 and its good mechanical and chemical characteristics work as an excellent bridging material that enhances mud cake consistency and increases the tolerance to high differential pressures.
The results of the filtration control evaluation of the WBM and PAM/PEI. The WBM contains 55 lb/bbl of calcium carbonate with particle size distribution of d10 = 2 μm, d50 = 15 μm, and d90 = 100 μm. The results of filtration with time are shown in Figure 5. Filtration experiments were conducted under a 500 psi differential pressure for the WBM and PAM/PEI formula using carbonate and ceramic filter disks; two disks with different pore throat sizes were used, the 170-55 disk and 170-53 disk with pore throat sizes of 10 and 50 μm, respectively. The PAM/PEI formula stopped filtration through the carbonate core and both types of disks in a few minutes and peaked at 2–5.5 cm3 for both disks with different permeabilities. As the gelation process takes place once the temperature of the fluid reaches the gel onset temperature (266 °F), the fluid rheology increases rapidly due to the formation of gel structure and filtration stops immediately. The WBM, however, exhibited a progressive filtration over time because of the high-temperature effect and the deterioration of mud properties with heating.
Figure 5.
Filtrate volume from the static filtration experiments.
When a differential pressure of 500 psi was applied across the filter disk during the filtration experiment, the fluids were filtrated through the initial rock permeability and later through the subsequently formed internal and external filter cakes. The structure of the external filter cake formed by PAM/PEI was a dense wavy surface with a uniform structure. Figure 6 shows external filter cake formation on different disks; the thickness of filter cakes was around 2–4 mm for all samples.
Figure 6.
External filter cake formation on different filter disks.
Evolution of the Internal Filter Cake
Figure 6 shows the scanning electron microscopy (SEM) images of the internal filter cake formed inside carbonate cores during the HP/HT static filtration test by the WBM and PAM/PEI formula. From the SEM images, it can be observed that the unaltered carbonate disk has clear open void spaces, as indicated in Figure 7a. The carbonate grains in the spectrum of the SEM showed different sizes, varying from 5 to 30 μm. It is also shown that there are many connected void spaces, which contributed to the effective porosity of the carbonate core.
Figure 7.
SEM images for the (a) carbonate blank core and the internal filter cake after the PPA test with (b) WBM and (c) PAM/PEI.
Figure 7b shows the internal filter cake formulated by the WBM. The calcium carbonate contained in the WBM and the other solids in the mud, invaded carbonate void spaces, and filled it as shown in the SEM images in Figure 7b. The calcium carbonate particulates distributed evenly inside the open voids and formed a permeable internal filter cake, which led to the progressive continuous filtration over time, as shown in Figure 5.
Similarly, the PAM/PEI formula filtrated inside the carbonate disk and formed an internal filter cake. However, in this case, the composition of the internal filter cake was different, which affected its formation process. The PAM/PEI gels exhibited a dense surface and neat structural pattern and resulted in the absence of the porous structures in the micrographs of the PAM/PEI gel, as shown in Figure 6c. Since SEM usually shows the high-atomic-number component with a darker color, the PAM/PEI gel in the black areas, indicated by the arrows in Figure 6c, has filled all of the open voids of the carbonate and remained there to seal it completely. The gel not only forms on the surface of the rock, as shown in the external filter cake, but it also penetrates and plugs the pores from inside as well. This allows drilling to continue without the risk of lost circulation.
For further analysis of the internal filter cake, energy-dispersive X-ray (EDX) analysis was used to identify the elemental composition on three samples, the blank unaltered carbonate and the carbonate after being tested with WBM and PAM/PEI. The elemental composition of the analysis is shown in Figure 8. Generally, the Ca, O, and C contents showed dominating percentages on all samples with up to 90 wt %. Other minerals distinguishing components such as Al, Si, Mg, Na, and K were more prominent in the samples with the WBM, since it contains bentonite and barite.
Figure 8.

EDX analysis of the internal filter cake formation in carbonate by WBM and PAM/PEI.
As shown in Figure 8, the C content in the carbonate after PAM/PEI filtration test showed about 40 wt %, which is almost double the amount detected in blank carbonate rock or after the WBM test, which was 20 and 19.5 wt.% for blank carbonate and carbonate with WBM, respectively. This notably high content of carbon (C) is due to the PAM being mainly composed of acrylamide monomers, which contain mainly carbon and hydrogen with the chemical formula of (C3 H5 N O)n. The EDX analysis agrees with the SEM images and indicates that the PAM/PEI gels filled the pores of the carbonates and formed a uniform internal filter cake. The bonding between the polymeric gel and the carbonate rock could be verified visually from the SEM that shows a thick structured surface of polymers and from the filtration test, where the polymer plug successfully stops mud filtrations with a high maximum sealing pressure (>1000 psi).
Another analysis was conducted using ceramic filter disks with two different permeabilities and different pore throat size. Figure 9 shows the SEM images post the PPT filtration test with PAM/PEI. As shown in SEM images in the void spaces, indicated by the arrows, the permeability of the ceramic filter disk has affected the injectivity of the PAM/PEI fluid. The larger size of the pore throat allowed more PAM/PEI into the filter disk, and the internal filter cake was more notable in the filter disk with a mean pore throat of 50 μm (No. 170-53) compared to the one with the lower permeability and smaller pore size of 10 μm (No. 170-55). However, PAM/PEI fluid was successful in stopping loss circulation in both cases, and filter disk pores were plugged.
Figure 9.
SEM images for the internal filter on ceramic filter disks Nos. 170-53 and 170-55 before and after PPA test with PAM/PEI.
Formation Damage and Secondary Filtration
Potential formation damage in carbonate cores that may be caused by the PAM/PEI or CaCO3 was also investigated using SEM images. To reflect on the effect of formation damage, the spectrum of SEM was selected in two areas: one close to the entering side of filtration and another one close to the exit side; the arrows in Figure 10 indicate the areas. The post-PPT analysis of SEM images was compared with the images obtained from unaltered carbonate samples to check for the LCM invasion. To investigate the internal filter cake after the filtration tests and the deeper invasion of the WBM containing CaCO3, the carbonate core was cut cross-sectionally, as shown in Figure 9. The top three SEM images show the section of the core close to the direction of the flow, which represents the internal filter cake. The bottom three SEM images show the deeper invasion of the mud after the fluid passes the internal filter cake. The arrow on top of the filter disks indicates the direction of filtration through the core.
Figure 10.
SEM images for the invasion of WBM filtrates inside carbonate disk.
The CaCO3, since it is a granular type of LCMs with different particle sizes, tends to form an internal filter cake from the particles that escape the permeability of the external filter cake, as shown in the three SEM images in Figure 9 (top). The 1500 times magnification shows that the pores of the carbonate of size 10–30 μm are filled with CaCO3 particles and WBM solids. Furthermore, the bottom three SEM images also show some fine particles of CaCO3 covering the carbonate pores, indicating that the differential pressure across the core will cause deeper invasion of the WBM solids and LCM into the formation. These CaCO3 particulates, if not removed later with acidizing or any filter cake removals means will affect formation permeability and impair the productivity of the well.
Overall, looking at filtration mechanisms of the WBM during the PPT static filtration, the fluid filtration continuity depends on the permeability of this external filter cake as well as the internal filter cake. The internal filter cake is supposed to reduce the near-wellbore permeability, representing the carbonate core disk. However, in this case, the WBM containing calcium carbonate did not form a low-permeable filter cake; therefore, the fine particles of LCM still penetrated deeper into the pores, as shown in the three images in Figure 10 (bottom). The CaCO3 particulates, if not removed, will remain in the wellbore or formation and permanently reduce the productivity of the well. This invasion makes formation damage difficult to remove.
On the other hand, The PAM/PEI internal mud cake did not penetrate deep inside the core; only about 0.125 in. was affected, as shown in Figure 11. The three SEM images in Figure 10 (top) show thick polymer gel covering all carbonate pores and sealing them entirely with a thick-layer, high-strength gel with a very low permeability indicated by the filtration results. The bottom set of SEM images show clear open pores with no visible traces of polymer. This suggests that the resulting internal filter cake from the PAM/PEI formula is limited to small areas near the wellbore, which will reduce contamination of the reservoir and cause less formation damage.
Figure 11.
SEM images for the invasion of PAM/PEI filtrates inside carbonate disk.
The process mainly depends on the rheological properties and injectivity of the polymer fluid. The injectivity of PAM/PEI fluid also depends on the initial permeability of the carbonate formation in the vicinity of the wellbore, which governs the depth of invasion. Later, when the cross-linked polymer activates, a mature gel forms inside the formation, sealing the pores entirely and preventing a further invasion of fluids. After sealing off the loss zone with PAM/PEI gel, the drilling could be resumed safely with no risk of further mud loss.
Conclusions
This study provides insight into the filtration process of the polymer-based fluids and the evolution of the internal filter cake. Understanding the formation process of internal and external filter cake by these polymeric fluids would highlight the factors affecting deep invasion and formation damage caused by alteration of fluid flow characteristics.
Moreover, the cross-linked polymeric LCM could overcome many limitations faced when regular granular LCM, such as calcium carbonate (CaCO3), were used. This eliminates the impact of filtration factors such as drilling fluid properties, the concentration of LCM, type, and size of solids. Using such cross-linkable polymeric fluids as sealing materials increases the efficiency and maximizes sealant integrity with minimum formation damage. The following are the major concluding points:
The filtration process of PAM/PEI fluid depends mainly on the rheological properties after gelation, which impacts its injectivity and deep invasion.
PAM/PEI fluids are limited to the high-temperature formation to provide sufficient heat to trigger the cross-linking process. The PAM/PEI gelation time could be controlled depending on the depth and temperature of the loss zone.
The internal filter cake formed by PAM/PEI gel is limited to a small area near the wellbore. This will reduce deep invasion and isolate reservoir fluids and cause less formation damage.
The EDX analysis showed that the PAM/PEI gels filled the carbonates’ pores and formed a uniform internal filter cake.
A pressure test up to 1000 psi verified the integrity of the seal. The seal integrity is usually tested by applying a certain pressure and check if the seal will withstand the pressure and maintain its integrity. The pressure at which the seal fails is called the opening pressure.
Calcium carbonate particulates contained in the WBM showed deeper invasion and precipitated inside the pores of the carbonate disks. The filtration continued to propagate with time, which indicates that at high temperatures, CaCO3 is not a good choice. Besides, granular LCM needs to be selected and appropriately designed to prevent further invasion by forming a low-permeable internal filter cake.
Acknowledgments
The authors thank the Qatar National Research Fund (a member of the Qatar Foundation) for funding this study. This paper was made possible by an NPRP Grant No. NPRP10-0125-170240. They also thank SNF Floerger Group, France, for providing the materials for the tests. The statements made herein are solely the responsibility of the authors.
Glossary
Nomenclatures
- Da
daltons
- EDX
energy-dispersive X-ray
- LCM
lost circulation materials
- PAM
polyacrylamide
- PEI
polyethylenimine
- PPA
permeability plugging apparatus
- PPT
permeability plugging test
- SEM
scanning electron microscope
- WBM
water-based mud
The authors declare no competing financial interest.
References
- Ezeakacha C.; Salehi S.; Hayatdavoudi A. Experimental study of drilling fluid’s filtration and mud cake evolution in sandstone formations. J. Energy Resour. Technol. 2017, 139, 022912 10.1115/1.4035425. [DOI] [Google Scholar]
- Vasheghani Farahani M.; Soleimani R.; Jamshidi S.; Salehi S. In Development of a Dynamic Model for Drilling Fluid’s Filtration: Implications to Prevent Formation Damage, SPE International Symposium and Exhibition on Formation Damage Control, 2014.
- Salehi S.Numerical Simulations of Fracture Propagation and Sealing: Implications for Wellbore Strengthening. Doctoral dissertation; Missouri S&T, 2012. [Google Scholar]
- Jiao D.; Sharma M. M. Mechanism of cake buildup in crossflow filtration of colloidal suspensions. J. Colloid Interface Sci. 1994, 162, 454–462. 10.1006/jcis.1994.1060. [DOI] [Google Scholar]
- Nygaard R.; Salehi S. In Critical Review of Wellbore Strengthening: Physical Model and Field Deployment, AADE National Technical Conference and Exhibition, Houston, TX, April 2011; pp 12–14.
- Tran M. H.; Abousleiman Y. N.; Nguyen V. X. In The Effects of Low-Permeability Mudcake on Time-Dependent Wellbore Failure Analyses, IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, 2010.
- Tien C.; Bai R.; Ramarao B. Analysis of cake growth in cake filtration: Effect of fine particle retention. AIChE J. 1997, 43, 33–44. 10.1002/aic.690430106. [DOI] [Google Scholar]
- Civan F. In Formation Damage Mechanisms and Their Phenomenological Modeling-an Overview, European Formation Damage Conference, 2007.
- Elkatatny S.; Mahmoud M.; Nasr-El-Din H. A. Filter cake properties of water-based drilling fluids under static and dynamic conditions using computed tomography scan. J. Energy Resour. Technol. 2013, 135, 042201 10.1115/1.4023483. [DOI] [Google Scholar]
- Calçada L.; Scheid C.; de Araujo C. A. O.; Waldmann A.; Martins A. Analysis of dynamic and static filtration and determination of mud cake parameters. Braz. J. Pet. Gas 2011, 5, 159–170. 10.5419/bjpg2011-0016. [DOI] [Google Scholar]
- Riveland F. A.Investigation of Nanoparticles for Enhanced Filtration Properties of Drilling Fluid; Institutt for petroleumsteknologi og anvendt geofysikk, 2013.
- Williams M.; Cannon G. E. In Evaluation of Filtration Properties of Drilling Mud, Drilling and Production Practice 1938, American Petroleum Institute: Amarillo, Texas, 1938; p 9.
- Cargnel R.; Luzardo J. In Particle Size Distribution Selection of CaCO3 in Drill-in Fluids: Theory and Applications, Latin American and Caribbean Petroleum Engineering Conference; SPE, 1999.
- Sacramento R. N.; Yang Y.; You Z.; Waldmann A.; Martins A. L.; Vaz A. S. L.; Zitha P. L. J.; Bedrikovetsky P. Deep bed and cake filtration of two-size particle suspension in porous media. J. Pet. Sci. Eng. 2015, 126, 201–210. 10.1016/j.petrol.2014.12.001. [DOI] [Google Scholar]
- Magzoub M. I.; Salehi S.; Hussein I. A.; Nasser M. S. Loss circulation in drilling and well construction: The significance of applications of crosslinked polymers in wellbore strengthening: A review. J. Pet. Sci. Eng. 2020, 185, 106653 10.1016/j.petrol.2019.106653. [DOI] [Google Scholar]
- Lee L.; Dahi Taleghani A. Simulating Fracture Sealing by Granular LCM Particles in Geothermal Drilling. Energies 2020, 13, 4878. 10.3390/en13184878. [DOI] [Google Scholar]
- Ghosh B.; AlCheikh I. M.; Ghosh D.; Ossisanya S.; Arif M. Development of Hybrid Drilling Fluid and Enzyme–Acid Precursor-Based Clean-Up Fluid for Wells Drilled with Calcium Carbonate-Based Drilling Fluids. ACS Omega 2020, 5, 25984–25992. 10.1021/acsomega.0c03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vryzas Z.; Kelessidis V. C. Nano-based drilling fluids: A review. Energies 2017, 10, 540 10.3390/en10040540. [DOI] [Google Scholar]
- Li X.; Jiang G.; Shen X.; Li G. Application of Tea Polyphenols as a Biodegradable Fluid Loss Additive and Study of the Filtration Mechanism. ACS Omega 2020, 5, 3453–3461. 10.1021/acsomega.9b03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.-B.; Guo Y.-L.; Chen W.-Q. Effect of Solid Particles on the Lost Circulation of Drilling Fluid: A Numerical Simulation. Powder Technol. 2020, 363, 408–418. 10.1016/j.powtec.2019.12.029. [DOI] [Google Scholar]
- Gibson J.; Javora P. H.; Adkins M. In Pre-Cross-Linked Pills Provide Efficient and Consistent Fluid Loss Control, SPE European Formation Damage Conference, SPE: Noordwijk, The Netherlands, 2011; p 10.
- Hashmat M. D.; Sultan A. S.; Rahman S.; Hussain S. M. S.; Ali S. A. In Flowing Gels for Loss Circulation Prevention, SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, SPE: Dammam, Saudi Arabia, 2017; p 9.
- Al-Muntasheri G. A.; Nasr-El-Din H. A.; Zitha P. L. J. Gelation Kinetics and Performance Evaluation of an Organically Crosslinked Gel at High Temperature and Pressure. SPE J. 2008, 13, 337–345. 10.2118/104071-PA. [DOI] [Google Scholar]
- Li J.; Zhou W.; Qi Z.; Luo T.; Yan W.; Xu H.; Cheng K.; Li H. Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel. Energies 2019, 12, 3648. 10.3390/en12193648. [DOI] [Google Scholar]
- Magzoub M. I.; Salehi S.; Hussein I. A.; Nasser M. S. In A Comprehensive Rheological Study for a Flowing Polymer-Based Drilling Fluid Used for Wellbore Strengthening, SPE International Conference and Exhibition on Formation Damage Control, SPE, 2020.
- Shamlooh M.; Hamza A.; Hussein I. A.; Nasser M. S.; Magzoub M.; Salehi S. Investigation of the Rheological Properties of Nanosilica-Reinforced Polyacrylamide/Polyethyleneimine Gels for Wellbore Strengthening at High Reservoir Temperatures. Energy Fuels 2019, 33, 6829–6836. 10.1021/acs.energyfuels.9b00974. [DOI] [Google Scholar]
- Shamlooh M.; Hussein I. A.; Nasser M. S.; Magzoub M.; Salehi S. Development of pH-Controlled Aluminum-Based Polymeric Gel for Conformance Control in Sour Gas Reservoirs. ACS Omega 2020, 5, 24504–24512. 10.1021/acsomega.0c02967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Fang Y.; Chen A.; You Q.; Dai C.; Cheng R.; Liu Y. Gelation behavior study of a resorcinol–Hexamethyleneteramine crosslinked polymer gel for water shut-off treatment in low temperature and high salinity reservoirs. Energies 2017, 10, 913 10.3390/en10070913. [DOI] [Google Scholar]
- Ghriga M. A.; Grassl B.; Gareche M.; Khodja M.; Lebouachera S. E. I.; Andreu N.; Drouiche N. Review of recent advances in polyethylenimine crosslinked polymer gels used for conformance control applications. Polym. Bull. 2019, 6001–6029. 10.1007/s00289-019-02687-1. [DOI] [Google Scholar]
- Reddy B.; Eoff L. S.; Dalrymple E. D.; Brown D. L. Natural polymer-based compositions designed for use in conformance gel systems. SPE J. 2005, 10, 385–393. 10.2118/84510-PA. [DOI] [Google Scholar]
- Hamza A.; Shamlooh M.; Hussein I. A.; Nasser M.; Salehi S. Polymeric formulations used for loss circulation materials and wellbore strengthening applications in oil and gas wells: A review. J. Pet. Sci. Eng. 2019, 180, 197–214. 10.1016/j.petrol.2019.05.022. [DOI] [Google Scholar]
- Reddy B. R. Laboratory characterization of gel filter cake and development of nonoxidizing gel breakers for zirconium-crosslinked fracturing fluids. SPE J. 2014, 19, 662–673. 10.2118/164116-PA. [DOI] [Google Scholar]
- Hanes R. E.; Weaver J. D.; Slabaugh B. F.. Methods and Compositions for Reducing the Viscosity of Treatment Fluids. U.S. Patent US7,082,9952006.
- Gunawan S.; Armstrong C. D.; Qu Q. In Universal Breakers with Broad Polymer Specificity for Use in Alkaline, High-Temperature Fracturing Fluids, SPE Annual Technical Conference and Exhibition; SPE, 2012.
- Sarwar M. U.; Cawiezel K. E.; Nasr-El-Din H. A. In Gel Degradation Studies of Oxidative and Enzyme Breakers to Optimize Breaker Type and Concentration for Effective Break Profiles at Low and Medium Temperature Ranges, SPE Hydraulic Fracturing Technology Conference, SPE, 2011.
- Magzoub M. I.; Salehi S.; Hussein I.; Nasser M. Development of a Polyacrylamide-Based Mud Formulation for Loss Circulation Treatments. J. Energy Resour. Technol. 2020, 143, 073001 10.1115/1.4048682. [DOI] [Google Scholar]
- Mohamed A. I.; Hussein I. A.; Sultan A. S.; El-Karsani K. S.; Al-Muntasheri G. A. DSC investigation of the gelation kinetics of emulsified PAM/PEI system. J. Therm. Anal. Calorim. 2015, 122, 1117–1123. 10.1007/s10973-015-4965-6. [DOI] [Google Scholar]
- El-Karsani K. S.; Al-Muntasheri G. A.; Sultan A. S.; Hussein I. A. Gelation kinetics of PAM/PEI system. J. Therm. Anal. Calorim. 2014, 116, 1409–1415. 10.1007/s10973-014-3754-y. [DOI] [Google Scholar]