Abstract

A new sustainable green protocol for obtaining polyethylene glycol (PEG) conjugates, with a prototype molecule, which in this work was coumarin, by means of click chemistry is presented. The organic solvents commonly used for this type of reaction were replaced by supercritical carbon dioxide (scCO2). The synthesis and characterization of PEG-coumarin were successfully reported using FTIR, 1H NMR, and MALDI TOF. Subsequently, a preliminary study was carried out using the response surface methodology to examine the variables that most affect the use of scCO2 as a reaction medium. The main effects caused by these variables, individually and their binary interaction, have been estimated. The response surface methodology has been used in this work to screen variables using a factorial design 23. The p-values of temperature and pressure were 0.006 and 0.0117, being therefore the most significant variables of the response surface methodology study. Subsequently, a more intensive study has been carried out on the variables that have shown the greatest significant effect on reaction performance where an 82.32% synthesis success was achieved, which broadens the scope of the use of scCO2 as a reaction medium. The conjugated coumarin with mPEG-alkyne and coumarin were evaluated for their in vitro antioxidant activities by the DPPH radical scavenging assay and were found to exhibit substantial activities. The click product showed comparable or even better efficacy than the initial coumarin.
1. Introduction
In the last 15 years, various research teams have studied the applicability of polymer-drug conjugates to deliver drug combinations. The polymer-drug conjugate is a technology in which a drug is covalently bound to a polymeric carrier. In the 1960s, Prof. Frank Davis proposed to conjugate polyethylene glycol (PEG) with a protein, i.e., “PEGylate” a protein, in order to create a conjugate with a hydrophilic polymer with a new protein. Therefore, the new recombined proteins would be less immunogenic in the body and therefore improve their circulation and activity during all their life.1,2 In 1975, a rational model for pharmacologically active polymers was first proposed by Ringsdorf.3 His concept of covalently bound polymer-drug conjugates still forms the basis for much of the work in this area that is performed today.4
Huisgen and co-workers in 1960 studied the cycloaddition using azides and alkynes, which is an important method for the synthesis of 1,2,3 triazoles.5 Independently, Tornøe and Meldal and Sharpless et al. in 2002 discovered that copper catalysis could increase its reaction rate by up to 107.6,7 The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction is generally considered as the most remarkable example of click chemistry, which has emerged as a prominent organic transformation. Polymer science has profited from CuAAC by its simplicity, ease, scope, applicability, and efficiency. In addition, click chemistry has found wide application in drug discovery bioconjugation reactions, and polymer chemistry has pharmaceutical and biomedical applications.8−15 Furthermore, it has greatly facilitated the overall drug discovery process by providing easy access for the synthesis of building blocks for new molecular entities. CuAAC reaction is usually carried out with organic solvents, such as dimethylformamide (DMF), toluene, or tetrahydrofuran (THF).16−18 Traditional organic solvents could potentially cause various health and environmental concerns due to their volatility and toxicity. Recently, the use of supercritical fluids as environmentally benign solvents for chemical synthesis is one of the new approaches in green chemistry. Carbon dioxide is the most widely used gas for supercritical fluid studies because it has moderate critical constants (Pc = 73.8 bar; Tc = 31.1 °C), it is nontoxic, nonflammable, inexpensive, and relatively inert, its removal after the chemical transformation is not energy-consuming, and it is recyclable.19,20 There are a variety of contributions that have studied CuAAC reaction with organic solvents and at high temperatures. However, there are only four papers that use scCO2 to carry out the CuAAC reactions. Therefore, the CuAAC reaction is yet far from being fully developed in scCO2.21−25
The functionalized polymer-drug conjugates are, with a steady increase, being utilized to obtain biodegradable systems in an effort to enhance localized drug delivery and easy removal.4 Although different polymer compositions have been synthesized and studied, some of the simplest polymers, such as poly(ethylene glycol) (PEG), maintain widespread use and versatility. Furthermore, the use of PEG has been established clinically and approved by the Food and Drug Administration (FDA) and Evaluation of Medical Product in Europe (EMEA).26 Because PEG only contains two functional groups limiting the scope for further derivation with targeting ligands, methoxy-poly(ethylene glycol) alkyne (mPEG-alkyne) was used in this.27−30 The bioactive agent chosen for PEG conjugation is coumarin. Natural coumarins or synthetic analogues are of great interest due to their pharmacological properties. Their physiological, bacteriostatic, and antitumor activities make these compounds interesting as novel therapeutic agents.31,32
In the present work, the main objective was to obtain a conjugate polymer-drug in which the interaction has been achieved through a covalent bond in scCO2 by means of click chemistry. This research has been initiated by recent interest in click products that have a broad spectrum of applications in the emerging fields such as drug discovery, chemical biology, and materials science.8−11 The functionalized product was characterized by 1H NMR spectroscopy, MALDI-TOF MS, and FTIR spectroscopy. The next step was to optimize the operating conditions using a factorial design 23, where the most significant influence of the process variables was observed. Once this information was obtained, an additional study was carried out on the most influential variables on the reaction yield. Finally, the antioxidant activities of the click conjugate, the raw coumarin, and the modified coumarin with the azide group were evaluated.
2. Results and Discussion
2.1. Synthesis of mPEG-Coumarin Using scCO2
The initial working conditions were above 31.1 °C and pressures above 73.8 bar with the intention of working in the supercritical solvent region. The molar ratio between alkyne and azide groups was maintained at 1. Copper(II) acetate monohydrate was chosen as a catalyst to conjugate coumarin with mPEG-alkyne (Scheme 1). The choice of this catalyst was derived from its high efficiency in scCO2.23
Scheme 1. Synthesis Reaction Scheme for mPEG-Coumarin in scCO2.

A first preliminary study was carried out at 130 bar, 0.5 molar ratio between the catalyst and alkyne group (C/A), 35 °C, and 24 h. The evidence for click reaction between 4-azidomethyl-7-methoxycoumarin and mPEG-alkyne can be proven by FTIR. The FTIR spectra of the click product, mPEG-alkyne, and 4-azidomethyl-7-methoxycoumarin are shown in Figure 1. The peak at 2167 cm–1 corresponds to the alkyne groups of mPEG-alkyne. The peak at 2110 cm–1 corresponds to the azide group of 4-azidomethyl-7-methoxycoumarin. Both are not observed in the spectra of the mPEG-coumarin click product, which means that the azide and alkyne groups disappear completely due to the coupling reaction between them. In addition, the appearance of the characteristic peaks of the triazole ring at 1464 and 1615 cm–1 was observed. Therefore, the click reaction between 4-azidomethyl-7-methoxycoumarin and mPEG-alkyne was carried out successfully.
Figure 1.
FTIR spectra: (a) mPEG-alkyne; (b) click product mPEG-coumarin in scCO2; (c) 4-azidomethyl-7-methoxycoumarin.
With the aim of getting to know the structure of the mPEG-coumarin product and identify more clearly the presence of the triazole ring and coumarin group in the polymer structure, 1H NMR analysis was used. As shown in Figure S6, the protons of the PEG chain were observed in the range of δi 3.5–3.8. The triazole proton appeared as a singlet at δg 7.85. The aromatic protons of coumarin were observed with a doublet δc 7.59, 7.61 and δb 6.83, 6.84 and multiplet around δe 5.76. The presence of the chemical shifts of the protons corresponding to PEG, triazole, and coumarin confirmed that the conjugate has been successfully synthesized.
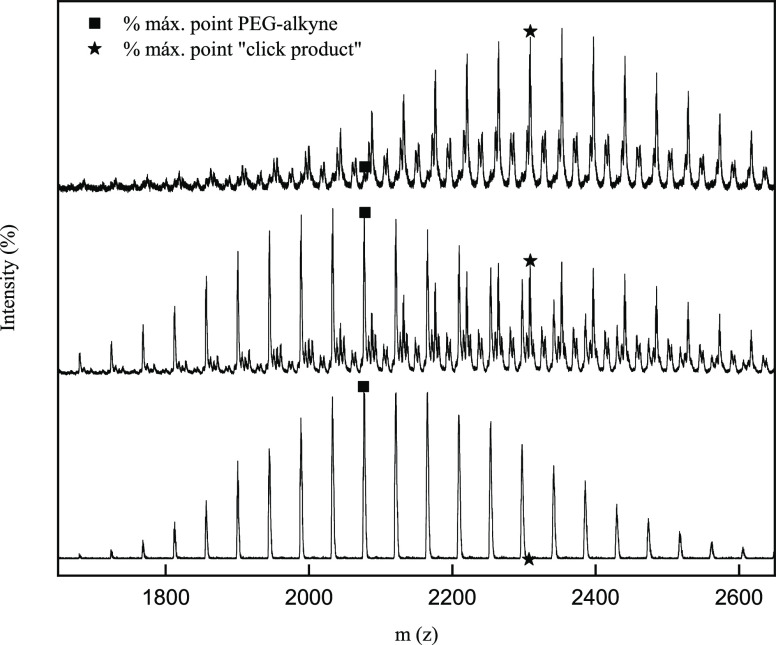
Finally, MALDI-TOF mass spectroscopy was used to complete the characterization of the click product. Through this characterization technique, it will be possible to determine both the structure of the polymer and the synthesized click product as well as the yield achieved in the reaction. Figure 2a shows the mass spectrum for mPEG-alkyne, where it can be verified that the monomer unit of the polymer corresponds to 44 g mol–1 (ethylene oxide) and an average molecular weight of 2122 g/mol–1. In this procedure, the exact distribution of the polymer and its molecular weight are known. Therefore, it will be possible to compare the displacement of the mPEG-alkyne molecular weight signal with the click product (mPEG-coumarin) molecular weight signal and determine the yield of reaction through eq 1, where IPEG-alkyne represents the intensity of the polymer and IClick represents the intensity of mPEG-coumarin. In Figure 2b, a displacement of 232 μm was observed, which corresponds to the molecular weight of azide coumarin. Therefore, the reaction was successfully carried out.
| 1 |
Figure 2.
MALDI TOF MS spectra: (a) mPEG-alkyne; (b) mPEG-coumarin. (square) Peaks of mPEG-alkyne. (star) Peaks of mPEG-coumarin.
The yield obtained was 82.32% for 130 bar, 0.5 molar ratio of catalyst/alkyne, 35 °C, and 24 h. An additional experiment was carried out in order to optimize the reaction time. In a previous study of CuAAC reactions, the absence of the ligand of the catalyst caused a reduction in the observed reaction rate.33 The time was increased from 24 to 48 h, getting a yield of 87.14%. When the time is increased, there is a very low increase in yield. In other words, most of the reaction takes place in the first 24 h.
2.2. Preliminary Study with Factorial Design 23
Once the feasibility of the click product formation process has been demonstrated using supercritical technology with CO2 as solvent, the operation conditions have been optimized through a 23 full factorial design. The yields were determined with MALDI-TOF MS, as mentioned previously, and the spectrum of each run is included in the Supporting Information (Table S1). The studied variables are pressure, molar ratio of catalyst/alkyne, and temperature. The standard experimentation matrix is shown in Table 1.
Table 1. Experimental Matrix and Results for the Full 23 Factorial Designa.
| run | P (bar) | T (°C) | C/A molar ratio | density of scCO2b (g/L) | yield (%) |
|---|---|---|---|---|---|
| 1 | 1 | –1 | –1 | 769 | 67.67 |
| 2 | –1 | 1 | 1 | 560 | 40.90 |
| 3 | 0 | 0 | 0 | 675 | 60.23 |
| 4 | –1 | –1 | –1 | 680 | 45.52 |
| 5 | 1 | 1 | 1 | 725 | 59.52 |
| 6 | 1 | 1 | –1 | 725 | 45.62 |
| 7 | 1 | –1 | 1 | 769 | 82.32 |
| 8 | –1 | –1 | 1 | 680 | 50.89 |
| 9 | –1 | 1 | –1 | 560 | 37.45 |
| 10 | 0 | 0 | 0 | 675 | 57.96 |
Experimental conditions: azide-to-alkyne molar ratio, 1:1; reaction time, 24 h.
Density of scCO2 determined by the equation of Bender.34
The analysis of the main effects and their interactions for the chosen response is shown in Table 2. Statistically significant effects are underlined according to p-values calculated. According to the results shown in Table 2, the yield of click reaction is mainly affected by pressure and temperature.
Table 2. Estimated Effects, Interactions, and ANOVA Analysis from 23 Factorial Design for Click Reaction in scCO2.
| yield
(%) |
||
|---|---|---|
| factor of interaction | p-effects (±s) | p-value |
| pressure | 20.025 ± 2.86369 | 0.0060 |
| temperature | –15.795 ± 2.86369 | 0.0117 |
| C/A molar ratio | 9.275 ± 2.86369 | 0.0479 |
| pressure–temperature | –6.765 ± 2.86369 | 0.0992 |
| pressure–C/A molar ratio | 4.865 ± 2.86369 | 0.1879 |
| temperature–C/A molar ratio | –0.435 ± 2.86369 | 0.8140 |
The yield of reaction was investigated by means of a Pareto chart (Figure 3). The length of each bar indicates the standardized effect of the selected factor on the different responses, and its color represents if the contribution was positive or negative. The positive effects (gray color) present a favorable effect on the response, while the negative effects (black color) show an antagonistic effect on it. The effects of increasing the C/A molar ratio, increasing the pressure, and both promote the increase of the yield. The temperature has a negative effect on the yield.
Figure 3.
Standardized Pareto chart for the yield.
CO2 density is directly dependent on pressure and temperature. The density of scCO2 was calculated as a function of pressure and temperature with the equation of Bender.34 The density of CO2 increases between 100 and 130 bar and decreases between 35 and 45 °C; therefore, CO2 density increases with the pressure and decreases with the temperature, as shown the Table 1. Slight changes in pressure and temperature in the process with CO2 will produce significant variations in its density and hence in the solubility of CO2 with different kinds of compounds. According to the literature, in the case of polymers with high molecular weights, which are generally insoluble in CO2, a gradual change in the solubility of the CO2 polymer mixture has been reported when there are sudden changes in CO2 density.35−37 On the other hand, CO2 is also able to plasticize many polymers owing to its capability to solubilize into the polymer. This effect appears when the CO2 density increases into polymer causing the plasticization effect. A pressure increase or a temperature decrease favors the density upgrade. This effect improves the yield of reaction due to a better interaction between the polymer and organic compound.38,39
On this basis, temperature has a global negative effect and pressure has a positive effect in the experimental range analyzed according to the Pareto chart. Therefore, an increase in pressure and a decrease in temperature, at a constant C/A molar ratio, acted increasing the yield of reaction.
The effect of the catalyst was evaluated for 0.1 and 0.5 C/A molar ratios. The minimum value of C/A chosen in the factorial design was 0.1 as a large decrease in yield was observed when the ratio decreased. The ratio was not increased up to 0.5 because it will later present the problem of removing it from the click product. Figure 4 shows the MALDI-TOF MS spectra for the click reaction product using a ratio catalyst and in the absence of it. It is observed that when the C/A molar ratio was zero, the reaction was not carried out. Furthermore, the increase in the C/A molar ratio causes an increase in performance, as shown in Figure 4, as the intensity of the peaks corresponding to the click product (mPEG-coumarin) and the signal corresponding to mPEG.
Figure 4.
MALDI-TOF MS spectra: (a) 0.5 C/A molar ratio (run 5); (b) 0.1 C/A molar ratio (run 4); (c) the absence of the catalyst. (square) Peaks of the polymer. (star) Peaks of the click product.
For the purpose of determining whether the association between the response and each term included in the model is statistically significant, the p-value of the term was compared with the significance level for assessing the null hypothesis. Binary interaction between pressure and temperature had a p-value of 0.09, binary interaction between pressure and molar C/A ratio had a value of 0.1879, and the binary interaction between temperature and molar C/A ratio had a value of 0.8140. These binary factors are not statistically significant at the confidence level (0.05) with the terms of the current model. Thus, these values indicate that the binary interaction between pressure, temperature, and C/A molar ratio had no statistically significant association with the yield. In fact, based on evidence, it could be more clearly observed in the Pareto chart (Figure 3).
2.3. Study of the Most Influential Variables of the Cycloaddition in scCO2
Since pressure is the operational variable most easily controllable by the experimental setup, further experiments were carried out to obtain more detailed information within the pressure range chosen for the factorial design. Temperature was not considered in this additional study since its increase causes a negative effect on the yield and it could lead out of the supercritical region. The effect of pressure was re-evaluated because it was the most significant variable in the yield according to the Pareto chart (Figure 3), widening the range between 80 and 170 bar. In addition, the scCO2 density for pressure and temperature conditions was determined with the Bender equation as shown in Table 3.
Table 3. Effect of Pressure in the Cycloaddition Reactiona.
| run | press. (bar) | density of scCO2b (g/L) | yield (%) |
|---|---|---|---|
| 11 | 80 | 332.90 | 14.30 |
| 12 | 90 | 602.95 | 32.55 |
| 13 | 150 | 801.66 | 83.59 |
| 14 | 170 | 826.55 | 84.47 |
Experimental conditions: azide-to-alkyne molar ratio, 1:1; reaction time, 24 h; temperature, 35 °C; C/A molar ratio, 0.5.
Density of scCO2 determined by the equation of Bender.34
With increasing pressure of CO2, the CO2 is diffused between the polymer chains and its sorption into polymer increases the free volume and mobility of the polymer segment, making it more accessible to carry out functionalization with the natural drug.40 However, changes in CO2 density values from 130 bar are gradually smaller, as shown in Figure 5. For this reason, when the pressure is increased above 130 bar, the yield of cycloaddition reaction is relatively constant. Numerous studies of sorption of CO2 into polymer41−43 have shown that by increasing the pressure values close to the critical region, the sorption values increase. However, once the critical region is exceeded, these values remain practically constant from which the performance remains practically constant.
Figure 5.
Effect of pressure and density on the yield of cycloaddition reaction. Experimental conditions: azide-to-alkyne molar ratio, 1:1; reaction time, 24 h; temperature, 35 °C; C/A molar ratio, 0.5. (square) Values of density of scCO2 determined by the equation of Bender34 and (circle) values of the yield of reaction.
In consequence of this study of the response surface, which is carried out for the first time for polyethylene glycol, the optimal conditions were 130 bar, 0.5 C/A molar ratio, 35 °C, and 24 h, obtaining a yield of 82.32%.
Finally, the yield of cycloaddition reaction using supercritical fluid was compared with a work published recently using the same polymer selected in this work and a similar coumarin. Behl et al.32 using THF, as a solvent, and N,N,N′,N″,N″-pentamethyl diethylenetriamine (PMDTA), as a ligand, in order to carry out the click reaction obtained a yield of 73%. Therefore, the use of supercritical fluids is a promising way to carry out the CuAAC reaction without the necessity of the ligand and organic solvents.
2.4. In Vitro Antioxidant Evaluation
In the present study, the antioxidant activities of the synthesized coumarin and conjugated coumarin have been assessed in vitro by the 1,1-dipheyl-2-picrylhydrazyl (DPPH) radical scavenging assay, and ascorbic acid was employed as a reference standard. The test compounds and the standard ascorbic acid were tested at five different test concentrations of 10, 20, 30, 40, and 50 μM. All the analyzed compounds showed significant scavenging activities ranging from 33.96 to 78.18%, in comparison to the standard drug ascorbic acid (95.41% at 50 μM), and the results of antioxidant capacity exhibited significant activity as shown Figure 6.
Figure 6.
Increase in percentage antioxidant activity of different compounds with the increase in test concentrations.
The click product derived from 4-azidomethyl-7-methoxycoumarin shows potent activity (IC50 11.95 μM) as compared to the standard. However, the coumarin azide (AMMC) shows a higher IC50 value than the click product, although the difference is practically negligible. The compound BMMC (4-bromomethyl-7-methoxycoumarin) with a bromo- substituent on coumarin exhibits less activity than AMMC, which shows an activity very close to the IC50 value of the click product.44
The antioxidant activities of 4-bromomethyl-7-methoxycoumarin, 4-azidomethyl-7-methoxycoumarin and click product by the DPPH assay method, expressed as mean ± SD (standard deviation), along with IC50 values obtained by regression analysis, are shown in Table 4.
Table 4. In Vitro Antioxidant Evaluation of 4-Bromomethyl-7-methoxycoumarin, 4-Azidomethyl-7-methoxycoumarin, and Click Product.
| DPPH
method – mean percentage free radical scavenging activity ±
SDa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| compound | 10 μM | 20 μM | 30 μM | 40 μM | 50 μM | IC50 ± SD | ||||||
| BMMCb | 33.96 | ±0.14 | 55.85 | ±0.05 | 55.20 | ±0.02 | 56.66 | ±0.01 | 58.53 | ±0.05 | 22.55 | ±0.05 |
| AMMCc | 47.76 | ±0.14 | 58.83 | ±0.36 | 68.49 | ±0.05 | 64.51 | ±0.05 | 63.39 | ±0.05 | 11.57 | ±0.24 |
| click product | 49.11 | ±0.01 | 56.13 | ±0.10 | 59.85 | ±0.01 | 68.56 | ±0.05 | 72.34 | ±0.05 | 11.95 | ±0.56 |
| ascorbic acid | 45.78 | ±0.37 | 67.73 | ±0.29 | 78.91 | ±0.98 | 87.45 | ±0.41 | 95.42 | ±0.45 | 11.42 | ±0.17 |
The results are expressed as mean values ± SD (standard deviation), n = 3, p < 0.05.
4-Bromomethyl-7-methoxycoumarin.
4-Azidomethyl-7-methoxycoumarin.
3. Conclusions
In conclusion, the synthesis and characterization of PEG-alkyne with a prototype molecule demonstrated by copper-mediated alkyne-azide cycloaddition via scCO2 were successfully reported in which the power of different conditions in scCO2 was studied. The optimization was carried out demonstrating the influence of pressure, temperature, and C/A molar ratio on the yield of click reaction in scCO2. Temperature and pressure played a key role in the process and have a strong significant effect on reaction yield. Finally, a temperature of 35 °C and pressure of 130 bar were selected as the optimal experimental variables in order to maximize the yield of reaction. The synthesized compound showed promising antioxidant activity (IC50 = 11.95 μM) when compared to ascorbic acid.
The advantage of the presented ecofriendly approach of CO2-based supercritical technologies shows enormous potential in the production of different enhanced drug formulations. The pharmaceutical industry must take advantage of the opportunities that these technologies offer.
4. Materials and Methods
4.1. Materials
The following materials were used to carry out the different syntheses: 4-bromomethyl-7-methoxycoumarin (97%, Sigma Aldrich), sodium azide (>99.5%, Sigma Aldrich), methoxy polyethylene glycol (mPEG, Mn = 2000 g/mol, Sigma Aldrich), propargyl bromide (80% in toluene, Sigma Aldrich), sodium hydride (NaH, 60% in mineral oil, Sigma Aldrich), ethyl acetate (anhydrous, 99.8%, Sigma Aldrich), and carbon dioxide (industrial grade ≥99%, Carburos Metálicos). The catalyst used to carry out the cycloaddition reaction was copper(II) acetate monohydrate (Sigma Aldrich).
4.2. Synthesis of 4-Azidomethyl-7-methoxycoumarin
The synthesis of this compound was carried out according to the literature.45−48 A mixture of NaN3 (1.2 g) and 4-bromomethyl-7-methoxycoumarin (1 g) in acetone/acetonitrile (1:1, 120 mL) solution was added into a 250 mL flask (Scheme S1). The mixture was stirred at 50 °C for 48 h. Then, solvents were removed under vacuum. The organic extracts were washed with water to precipitate the 4-bromomethyl-7-methoxycoumarin that did not react. The product was filtered, washed with heptane, and dried under vacuum (81% yield). The FTIR and 1H NMR spectra are included in the Supporting Information (Figures S1 and S2). The characteristic peaks of FTIR appearing at 2110, 1694, 1604, 1432, 1135, and 670 cm–1 belong to the C–N3, C=O, C=O, ether groups, and Br–CH, respectively. The FTIR spectra were consistent with the structure of coumarin and confirmed the results of 1H NMR. 1H NMR (CDCl3, ppm, 500 MHz): δa 3.89 (s, 3H), δb 6.87–6.9 (dd, J = 8.5 Hz, 2.5 Hz, 3H), δc 7.44–7.45 (d, J = 8.5 Hz, 1H), δd 6.863–6.868 (d, 2.5 Hz, 1H), δe 6.37 (s, 1H), δf 4.51 (s, 2H).
4.3. Synthesis of Methoxy-PEG Alkyne (mPEG-Alkyne)
The synthesis was performed using a reactor with 250 mL capacity. The first step was to add dropwise mPEG to a mixture of sodium hydride (610.5 mg, 15.4 mmol) in 100 mL of dry THF at room temperature. The first stage was ended when the gas formation stopped, around 30 min. Once the formation of hydrogen has been consumed, propargyl bromide (80% in toluene 1.71 mL, 15.4 mmol) was added (Scheme S2). The operation conditions were 50 °C and 100 rpm for 24 h in order to ensure that hydroxyl groups lost the protons and the alkoxide was formed correctly.32 Ending the synthesis, the solvent was removed easily using a rotary evaporator. Ethyl acetate was used as the wash liquid, and finally, the product was dried under vacuum to get a white solid (62% yield). The characteristic peaks of FTIR appearing at 2167, 1108, 1750, and 2885 cm–1 belong to C≡C, C–O, and CH2, respectively (Figure S3). The FTIR spectra were consistent with the structure of the polymer and confirmed the results of 1H NMR (Figure S4). 1H NMR (CDCl3): δa 2.42 (s, 1H), δb 3.31 (s, 2H), δa 3.5–3.8 (m, 154H), δa 4.17 (s, 1H). The MALDI TOF of mPEG-alkyne is shown in Figure S5.
4.4. Synthesis of the Click Product in Supercritical CO2
The procedure for synthesizing the click product (mPEG-coumarin) in supercritical media consisted of introducing the reagents into the reactor. Equimolar amounts of mPEG-alkyne and 4-azidomethyl-7-methoxycoumarin were added with molar ratios of catalyst/alkyne (C/A) of 0.1 and 0.5. In Figure 7, the schematic diagram of the experimental setup is shown. scCO2 was pumped out after the reactor was hermetically sealed. Afterward, the system was heated up within the temperature range studied. Once the reaction was complete, the heating was switched off and the reactor depressurized with a flow rate of 3 L/min.
Figure 7.
Schematic diagram of the experimental setup for reaction in the supercritical medium. V-1, V-3: check valves; V-2: purge valve. P-1: pump; E-1: cooler; C-1: batch reactor; V-4: valve of depressurization; TIC: temperature digital controller; FI: flow indicator; PI: pressure indicator.
4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
Infrared (IR) spectra were recorded on a Varian 640-IR Fourier transform IR spectrophotometer with 16 scans per experiment at a resolution of 32 cm–1 in the range of 4000–400 cm–1, using the software Varian Resolution.
4.6. Nuclear Magnetic Resonance of Protons (1H NMR)
1H NMR was measured with a Varian Gemini FT-500 spectrometer using CDCl3 as solvent. NMR spectra were acquired at 25 °C. Chemical shifts are given in ppm relative to TMS (1H, 0.0 ppm) or CDCl3 (1H, 7.2 ppm). NMR measurements were performed in the Department of Organic Chemistry at the University of Castilla-La Mancha.
4.7. MALDI-TOF MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was carried out using a Bruker Autoflex II TOF/TOF spectrometer (Bremen, Germany) using CDCl3 as solvent and dithranol (1,8,9-trihydroxyanthracene) as the matrix material. MALDI-TOF MS measurements were performed in the Department of Organic Chemistry at the University of Castilla-La Mancha.
4.8. Experimental Design
A statistical experimental design based on central composite design (CCD) was used. The effects of the operating variables in the cycloaddition reaction in scCO2 (pressure, temperature, and catalyst to alkyne group molar ratio) and their possible interactions on the yield as a response variable were studied using a 23 full factorial design with two central points.49,50 The levels of each factor are indicated in Table 5 and were selected on the basis of preliminary studies of click chemistry in scCO2.21
Table 5. Levels of Factors in the Experimental Design.
| factor | lower level (−1) | higher level (+1) |
|---|---|---|
| temperature (°C) | 35 | 45 |
| pressure (bar) | 100 | 130 |
| C/A molar ratio | 0.1 | 0.5 |
A statistical analysis was performed for these results using the commercial software Statgraphics 5.1 Plus (Manugistics, Inc. Rockville, MD, USA). Analysis of variance (ANOVA) provided a study of the variation present in the results of the experiments carried out. The test of statistical significance, p-value, was determined according to the total error criteria considering a confidence level of 95%. The influence of a factor will be significant if the value of the critical level (p) is lower than 0.05.
4.9. In Vitro Antioxidant Activity
The in vitro radical scavenging of the newly synthesized compounds was carried out by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical. The hydrogen atom or electron-donating ability of the compounds was measured from the bleaching of the purple-colored methanol solution of 1,1-diphenyl-1-picrylhydrazyl (DPPH), where 2 mL of each methanolic solution of two compounds was tested with various concentrations (10, 20, 30, 40, and 50 μM) that was mixed with 2 mL of a methanolic solution of DPPH (0.1 μM) employed as the control, as indicated by Venkata Sairam et al.51 The solution was incubated at 37 °C, since this is approximately the corporal temperature, for 30 min, and the measurements were done at λ = 516 nm because the sorption spectrum gave a peak at this wavelength (Figure S6). The percentage free radical scavenging activity was calculated according to eq 2.
| 2 |
The IC50 values for the drug compounds as well as standard preparation were calculated. IC50 is the concentration of the drug required for 50% inhibition. The IC50 (μM) value was calculated by interpolation from linear regression analysis.52
The measurement of the samples at different concentrations was done in triplicate.
Acknowledgments
We gratefully acknowledge funding from the Ministry of Economy and Competitiveness through funding the projects Ref. CTQ2016-79811-P. The authors also acknowledge the support of the Ministry of Economy and Competitiveness for the fellowship of Ms. López Quijorna (Ref. BES-2017-079770).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05466.
Full experimental details and synthesis and characterizations of the synthesized conjugates (FTIR, 1H NMR, and MALDI-TOF MS spectra) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Davis F. F. The Origin of Pegnology. Adv. Drug Delivery Rev. 2002, 54, 457–458. 10.1016/S0169-409X(02)00021-2. [DOI] [PubMed] [Google Scholar]
- Hoffman A. S. The Early Days of PEG and PEGylation (1970s–1990s). Acta Biomater. 2016, 40, 1–5. 10.1016/j.actbio.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Ringsdorf H. Structure and Properties of Pharmacologically Active Polymers. J. Polym. Sci., Polym. Symp. 1975, 51, 135–153. 10.1002/polc.5070510111. [DOI] [Google Scholar]
- Larson N.; Ghandehari H. Polymeric Conjugates for Drug Delivery. Chem. Mater. 2012, 24, 840–853. 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisgen R.; Mloston G.; Langhals E. The First Two-Step 1,3-Dipolar Cycloadditions: Non-Stereospecificity. J. Am. Chem. Soc. 1986, 108, 6401–6402. 10.1021/ja00280a053. [DOI] [Google Scholar]
- Tornøe C. W.; Meldal M.Peptidotriazoles: Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions on Solid-Phase. In Peptides: The Wave of the Future: Proceedings of the Second International and the Seventeenth American Peptide Symposium, June 9--14, 2001, San Diego, California, U.S.A.; Lebl M., Houghten R. A., Eds.; Springer Netherlands: Dordrecht, 2001; pp. 263–264, 10.1007/978-94-010-0464-0_119. [DOI] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]
- van Dijk M.; Rijkers D. T. S.; Liskamp R. M. J.; van Nostrum C. F.; Hennink W. E. Synthesis and Applications of Biomedical and Pharmaceutical Polymers via Click Chemistry Methodologies. Bioconjugate Chem. 2009, 20, 2001–2016. 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]
- Crescenzi V.; Cornelio L.; Di Meo C.; Nardecchia S.; Lamanna R. Novel Hydrogels via Click Chemistry: Synthesis and Potential Biomedical Applications. Biomacromolecules 2007, 8, 1844–1850. 10.1021/bm0700800. [DOI] [PubMed] [Google Scholar]
- Nwe K.; Brechbiel M. W. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother. Radiopharm. 2009, 24, 289–302. 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. C.; Sharpless K. B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discovery Today 2003, 8, 1128–1137. 10.1016/S1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Zhang L.; Yang L.; Zhu F.; Ding M.; Lin F.; Wang Z.; Li Y. “Click” Chemistry in Polymeric Scaffolds: Bioactive Materials for Tissue Engineering. J. Controlled Release 2018, 273, 160–179. 10.1016/j.jconrel.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Lallana E.; Fernandez-Trillo F.; Sousa-Herves A.; Riguera R.; Fernandez-Megia E. Click Chemistry with Polymers, Dendrimers, and Hydrogels for Drug Delivery. Pharm. Res. 2012, 29, 902–921. 10.1007/s11095-012-0683-y. [DOI] [PubMed] [Google Scholar]
- Hein C. D.; Liu X.-M.; Wang D. Click Chemistry, a Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. 10.1007/s11095-008-9616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain Y.; Gupta R.; Yadav P.; Kumari M. Chemical Waltz of Organic Molecules “On Water”: Saline-Assisted Sustainable Regioselective Synthesis of Fluorogenic Heterobioconjugates via Click Reaction. ACS Omega 2019, 4, 3582–3592. 10.1021/acsomega.8b03167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashi-Silab S.; Kiskan B.; Antonietti M.; Yagci Y. Mesoporous Graphitic Carbon Nitride as a Heterogeneous Catalyst for Photoinduced Copper(i)-Catalyzed Azide–Alkyne Cycloaddition. RSC Adv. 2014, 4, 52170–52173. 10.1039/C4RA09954K. [DOI] [Google Scholar]
- Díaz D. D.; Finn M. G.; Sharpless K. B.; Fokin V. V.; Hawker C. J.. Cicloadicción 1,3-Dipolar de Azidas y Alquinos: I: Principales Aspectos Sintéticos. In Anales de la Real Sociedad Española Química; Real Sociedad Española de Química: ISSN 1575–3417, No. 3, 2008, pags. 173–180 2020. [Google Scholar]
- Ellanki A. R.; Islam A.; Rama V. S.; Pulipati R. P.; Rambabu D.; Krishna G. R.; Reddy C. M.; Mukkanti K.; Vanaja G. R.; Kalle A. M.; Kumar K. S.; Pal M. Solvent Effect on Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC): Synthesis of Novel Triazolyl Substituted Quinolines as Potential Anticancer Agents. Bioorg. Med. Chem. Lett. 2012, 22, 3455–3459. 10.1016/j.bmcl.2012.03.091. [DOI] [PubMed] [Google Scholar]
- Kazarian S. G. Polymer Processing with Supercritical Fluids. Polym. Sci. 2000, 42, 78. [Google Scholar]
- Shieh Y.-T.; Su J.-H.; Manivannan G.; Lee P. H. C.; Sawan S. P.; Spall W. D. Interaction of Supercritical Carbon Dioxide with Polymers. I. Crystalline Polymers. J. Appl. Polym. Sci. 1996, 59, 695–705. . [DOI] [Google Scholar]
- Gracia E.; García M. T.; Borreguero A. M.; De Lucas A.; Gracia I.; Rodríguez J. F. Functionalization and Optimization of PLA with Coumarin via Click Chemistry in Supercritical CO2. J. CO2 Util. 2017, 20, 20–26. 10.1016/j.jcou.2017.04.008. [DOI] [Google Scholar]
- Grignard B.; Calberg C.; Jerome C.; Detrembleur C. “One-Pot” Dispersion ATRP and Alkyne-Azide Huisgen’s 1,3-Dipolar Cycloaddition in Supercritical Carbon Dioxide: Towards the Formation of Functional Microspheres. J. Supercrit. Fluids 2010, 53, 151–155. 10.1016/j.supflu.2009.12.014. [DOI] [Google Scholar]
- Zhang W.; He X.; Ren B.; Jiang Y.; Hu Z. Cu(OAc)2·H2O—an Efficient Catalyst for Huisgen-Click Reaction in Supercritical Carbon Dioxide. Tetrahedron Lett. 2015, 56, 2472–2475. 10.1016/j.tetlet.2015.03.102. [DOI] [Google Scholar]
- Grignard B.; Schmeits S.; Riva R.; Detrembleur C.; Lecomte P.; Jérôme C. First Example of “Click” Copper(I) Catalyzed Azide-Alkyne Cycloaddition in Supercritical Carbon Dioxide: Application to the Functionalization of Aliphatic Polyesters. Green Chem. 2009, 11, 1525. 10.1039/b822924d. [DOI] [Google Scholar]
- Meghani N. M.; Amin H. H.; Lee B.-J. Mechanistic Applications of Click Chemistry for Pharmaceutical Drug Discovery and Drug Delivery. Drug Discovery Today 2017, 22, 1604–1619. 10.1016/j.drudis.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Kemptner J.; Marchetti-Deschmann M.; Siekmann J.; Turecek P. L.; Schwarz H. P.; Allmaier G. GEMMA and MALDI-TOF MS of Reactive PEGs for Pharmaceutical Applications. J. Pharm. Biomed. Anal. 2010, 52, 432–437. 10.1016/j.jpba.2010.01.017. [DOI] [PubMed] [Google Scholar]
- D’souza A. A.; Shegokar R. Polyethylene Glycol (PEG): A Versatile Polymer for Pharmaceutical Applications. Expert Opin. Drug Delivery 2016, 13, 1257–1275. 10.1080/17425247.2016.1182485. [DOI] [PubMed] [Google Scholar]
- Sherman M. R.; Williams L. D.; Sobczyk M. A.; Michaels S. J.; Saifer M. G. P. Role of the Methoxy Group in Immune Responses to MPEG-Protein Conjugates. Bioconjugate Chem. 2012, 23, 485–499. 10.1021/bc200551b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.; Müller S. S.; Frey H. Beyond Poly(Ethylene Glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Biomacromolecules 2014, 15, 1935–1954. 10.1021/bm5002608. [DOI] [PubMed] [Google Scholar]
- Zacchigna M.; Cateni F.; Drioli S.; Bonora G. M. Multimeric, Multifunctional Derivatives of Poly(Ethylene Glycol). Polymers 2011, 3, 1076–1090. 10.3390/polym3031076. [DOI] [Google Scholar]
- Hu Y.; Chen W.; Shen Y.; Zhu B.; Wang G.-X. Synthesis and Antiviral Activity of Coumarin Derivatives against Infectious Hematopoietic Necrosis Virus. Bioorg. Med. Chem. Lett. 2019, 29, 1749–1755. 10.1016/j.bmcl.2019.05.019. [DOI] [PubMed] [Google Scholar]
- Behl G.; Sikka M.; Chhikara A.; Chopra M. PEG-Coumarin Based Biocompatible Self-Assembled Fluorescent Nanoaggregates Synthesized via Click Reactions and Studies of Aggregation Behavior. J. Colloid Interface Sci. 2014, 416, 151–160. 10.1016/j.jcis.2013.10.057. [DOI] [PubMed] [Google Scholar]
- Tale R. H.; Gopula V. B.; Toradmal G. K. ‘Click’ Ligand for ‘Click’ Chemistry: (1-(4-Methoxybenzyl)-1-H-1,2,3-Triazol-4-Yl) Methanol (MBHTM) Accelerated Copper-Catalyzed [3+2] Azide-Alkyne Cycloaddition (CuAAC) at Low Catalyst Loading. Tetrahedron Lett. 2015, 56, 5864. 10.1016/j.tetlet.2015.09.010. [DOI] [Google Scholar]
- Bender E. Equations of State for Ethylene and Propylene. Cryogenics 1975, 15, 667–673. 10.1016/0011-2275(75)90100-9. [DOI] [Google Scholar]
- Sato Y.; Fujiwara K.; Takikawa T.; Sumarno; Takishima S.; Masuoka H. Solubilities and Diffusion Coefficients of Carbon Dioxide and Nitrogen in Polypropylene, High-Density Polyethylene, and Polystyrene under High Pressures and Temperatures. Fluid Phase Equilib. 1999, 162, 261–276. 10.1016/S0378-3812(99)00217-4. [DOI] [Google Scholar]
- Choo Y.-S.; Yeo W.-H.; Byun H.-S. Phase Equilibria and Cloud-Point Behavior for the Poly(2-Phenylethyl Methacrylate) in Supercritical CO2 with Monomers as Co-Solvent. J. CO2 Util. 2019, 31, 215–225. 10.1016/j.jcou.2019.03.008. [DOI] [Google Scholar]
- Panayiotou C.; Sanchez I. C. Swelling of Network Structures. Polymer 1992, 33, 5090–5093. 10.1016/0032-3861(92)90064-4. [DOI] [Google Scholar]
- Trupej N.; Hrnčič M. K.; Škerget M.; Knez Ž. Solubility and Binary Diffusion Coefficient of Argon in Polyethylene Glycols of Different Molecular Weights. J. Supercrit. Fluids 2015, 103, 10–17. 10.1016/j.supflu.2015.04.022. [DOI] [Google Scholar]
- Gutiérrez C.; Garcia M. T.; Curia S.; Howdle S. M.; Rodriguez J. F. The Effect of CO2 on the Viscosity of Polystyrene/Limonene Solutions. J. Supercrit. Fluids 2014, 88, 26–37. 10.1016/j.supflu.2014.01.012. [DOI] [Google Scholar]
- Martín A.; Cocero M. J. Micronization Processes with Supercritical Fluids: Fundamentals and Mechanisms. Adv. Drug Delivery Rev. 2008, 60, 339–350. 10.1016/j.addr.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Pasquali I.; Andanson J.-M.; Kazarian S. G.; Bettini R. Measurement of CO2 Sorption and PEG 1500 Swelling by ATR-IR Spectroscopy. J. Supercrit. Fluids 2008, 45, 384–390. 10.1016/j.supflu.2008.01.015. [DOI] [Google Scholar]
- Daneshvar M.; Kim S.; Gulari E. High-Pressure Phase Equilibria of Polyethylene Glycol-Carbon Dioxide Systems. J. Phys. Chem. 1990, 94, 2124–2128. 10.1021/j100368a071. [DOI] [Google Scholar]
- Aionicesei E.; Škerget M.; Knez Ž. Measurement and Modeling of the CO2 Solubility in Poly(Ethylene Glycol) of Different Molecular Weights. J. Chem. Eng. Data 2008, 53, 185. 10.1021/je700467p. [DOI] [Google Scholar]
- Shaikh M. H.; Subhedar D. D.; Shingate B. B.; Kalam Khan F. A.; Sangshetti J. N.; Khedkar V. M.; Nawale L.; Sarkar D.; Navale G. R.; Shinde S. S. Synthesis, Biological Evaluation and Molecular Docking of Novel Coumarin Incorporated Triazoles as Antitubercular, Antioxidant and Antimicrobial Agents. Med. Chem. Res. 2016, 25, 790–804. 10.1007/s00044-016-1519-9. [DOI] [Google Scholar]
- Oh C.; Yi I.; Park K. P. Nucleophilic Vinylic Substitution of Halocoumarins and Halo-l,4-Naphthoquinones with Morpholine. J. Heterocycl. Chem. 1994, 31, 841–844. 10.1002/jhet.5570310426. [DOI] [Google Scholar]
- Chaurasia C. S.; Kauffman J. M. Synthesis and Fluorescent Properties of a New Photostable Thiol Reagent “BACM.”. J. Heterocycl. Chem. 1990, 27, 727–733. 10.1002/jhet.5570270347. [DOI] [Google Scholar]
- Sivakumar K.; Xie F.; Cash B. M.; Long S.; Barnhill H. N.; Wang Q. A Fluorogenic 1,3-Dipolar Cycloaddition Reaction of 3-Azidocoumarins and Acetylenes. Org. Lett. 2004, 6, 4603–4606. 10.1021/ol047955x. [DOI] [PubMed] [Google Scholar]
- Kusanur R. A.; Kulkarni M. V.; Kulkarni G. M.; Nayak S. K.; Guru Row T. N.; Ganesan K.; Sun C.-M. Unusual Anisotropic Effects from 1,3-Dipolar Cycloadducts of 4-Azidomethyl Coumarins. J. Heterocycl. Chem. 2009, 47, 91–97. 10.1002/jhet.273. [DOI] [Google Scholar]
- Cabezas L. I.; Mazarro R.; Gracia I.; De Lucas A.; Rodríguez J. F.. Optimizing the Bulk Copolymerization of D,L-Lactide and Glycolide by Response Surface Methodology. 2013, 7 ( (11), ), 886–894, 10.3144/expresspolymlett.2013.86. [DOI]
- Parameswaran R. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. JMR 1979, 16, 291. [Google Scholar]
- Venkata Sairam K.; Gurupadayya B. M.; Vishwanathan B. I.; Chandan R. S.; Nagesha D. K. Cytotoxicity Studies of Coumarin Analogs: Design, Synthesis and Biological Activity. RSC Adv. 2016, 6, 98816–98828. 10.1039/C6RA22466K. [DOI] [Google Scholar]
- Rajesh P.; Natvar P. In Vitro Antioxidant Activity of Coumarin Compounds by DPPH, Super Oxide and Nitric Oxide Free Radical Scavenging Methods. J. Adv. Pharm. Educ. Res. 2011, 1, 52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.