Abstract
Energy has become the key material basis of social development. In this work, liquid capric acid-paraffin was evenly adsorbed in the pore structure of expanded graphite (EG) by a physical adsorption method, and the new composite phase change material of capric acid-paraffin/expanded graphite (CA-P/EG) was prepared. The Fourier transform infrared (FT-IR) curves of CA-P/EG composites did not change after 1000 cycles, and there was no new characteristic absorption peak, indicating that CA-P/EG composites have good chemical stability. The results showed that the optimum content of CA-P/EG in a phase change energy storage gypsum board was 20%, and the wet bending strength and compressive strength were 2.42 and 6.45 MPa, respectively. The water absorption was 16.37%, and the apparent density was 1.410 g/cm3. In addition, the melting and freezing temperatures were 26.40 and 23.10 °C, and the latent heats of melting and freezing were 27.20 and 25.69 J/g, respectively. It was found that the gypsum board has excellent thermal stability after 400 times of melting–freezing cycling and that the heat storage capacity increases with the increase of the CA-P/EG content and the thickness of the gypsum board.
1. Introduction
With the continuous improvement of human requirements for indoor comfort, the corresponding building energy consumption has also been gradually increasing, and environmental pollution has become more serious.1 It is undoubtedly an effective and feasible method to apply phase change material (PCM) to traditional building materials to produce phase change energy storage building materials with load-bearing, energy storage, and temperature control characteristics.2,3 The method makes full use of clean and renewable energy such as solar energy and exerts its own characteristics of energy storage and temperature control, which can effectively increase the thermal inertia of the enclosure structure, reduce the indoor temperature fluctuation, and improve the indoor environment comfort. Gypsum has the advantages of thermal insulation, humidity control, fire prevention, lightweight, easy processing, and low price and has been widely used in building various types of light internal and external walls.4 PCM and gypsum are combined to make a phase change gypsum board, which can not only retain the advantages of gypsum itself but also have the characteristics of energy storage and temperature control of phase change materials.5−7
However, there are few studies on the preparation of the phase change gypsum board by the combination of PCM and gypsum.8−15 Ahmet et al.16 studied the thermal properties of myristic acid, palmitic acid, lauric acid, stearic acid, palmitate stearic acid, and other mixtures to characterize their thermal stabilities. Ahmad et al.17 investigated the thermal properties of energy-saving walls made of different phase change materials and found that the board filled with polyethylene glycol is more suitable for a local environment. Fatty acid phase change energy storage materials and expanded graphite-based composite phase change materials have good temperature control and energy storage characteristics.18−20 Zhao et al.21 studied a kind of polyethylene glycol shape-stabilized phase change material encapsulated by biological porous carbon with a honeycomb structure by a vacuum impregnation method, which enhanced thermal conductivity when used for thermal energy storage. Min et al.22 also prepared a novel poly(ethylene glycol) (PEG)-shaped composite phase change material with radial mesoporous silica spheres for heat storage, which has large latent heat, appropriate phase change temperature, good thermal reliability, good chemical compatibility, and thermal stability, and was a promising candidate material for building heat storage applications. Other studies have also prepared materials with good properties by appropriate methods.23,24 A new type of green building energy storage material can be obtained by combining composite phase change materials and building materials. When applied in the building envelope, the indoor temperature fluctuation and the building energy consumption can be reduced and human comfort can be improved.25,26 Sari et al.27 prepared capric acid–myristic acid/cement-form stable phase change material by the vacuum embedding method. The results showed that the average temperature difference between a phase change laboratory and an ordinary laboratory before the end of heating was 0.78 °C, indicating that it has good temperature regulation performance. Wang et al.28 found that the addition of diatomite-based and ceramsite-based thermal storage aggregates significantly improved the temperature fluctuation and thermal comfort of the laboratory under hot and cold environments, and the indoor temperature fluctuation decreased by 5.94 and 5.74 °C, respectively. Therefore, fatty acid phase change energy storage materials and expanded graphite-based composite phase change materials have good compatibility with cement, concrete, and gypsum.29−33 The composite phase change energy storage building materials can increase the thermal inertia of lightweight envelopes, improve indoor comfort, and reduce building energy consumption.
In this work, phase change gypsum boards were prepared by microencapsulation using capric acid-paraffin/expanded graphite (CA-P/EG) form stable phase change material with a high energy storage density and a low cost. The properties were characterized by a mechanical property test, a water absorption test, scanning electron microscopy (SEM), and differential scanning calorimetry (DSC).
2. Results and Discussion
2.1. Thermal Stability Analysis of CA-P/EG Composite Phase Change Materials
The accelerated melting–freezing cycling test for 1000 times was carried out on CA-P/EG composites, and the thermal conductivity of CA-P/EG composites was 0.455 W/(m·K). The stabilities of thermal and chemical properties of CA-P/EG composites after cycling were measured. The experimental results are shown in Figure 1. It can be seen from Figure 1 that after 1000 cycles, the melting and freezing temperatures of CA-P/EG are 27.14 and 23.80 °C, respectively, and the latent heats of melting and freezing are 130.6 and 126.4 J/g, respectively. By comparing the phase transformation properties of CA-P/EG before and after the melting–freezing cycles, it can be found that the freezing and melting temperatures of CA-P/EG increased by 6.9 and 0.3%, respectively, and the latent heats of freezing and melting decreased by 5.4 and 9.5%, respectively, which may be due to the degradation of the chemical structure and the measurement error of DSC. The latent heat and the temperature of phase transition change slightly, which indicates that CA-P/EG has good thermal stability.
Figure 1.
DSC curves of CA-P and CA-P/EG before and after thermal cycling.
It can be seen from Figure 2 that after 1000 melting–freezing cycles, the Fourier transform infrared (FT-IR) curve of the CA-P/EG composite does not change, nor does it produce new characteristic absorption peaks; therefore, its chemical stability is good. According to the test results before and after CA-P/EG melting–freezing cycles, CA-P/EG has good chemical and thermal stabilities after 1000 cycles and has application prospects in building energy-saving, low-temperature solar thermal storage, and other fields.
Figure 2.
FT-IR of CA-P/EG before and after thermal cycling.
2.2. Mechanical Properties of the Phase Change Energy Storage Gypsum Board
According to the standard test for mechanical properties of gypsum, the mechanical properties of the phase change gypsum board with different contents are shown in Tables 1 and 2 and Figure 3. It can be seen that the mechanical properties of the phase change gypsum board decrease gradually with the increase of the CA-P/EG content. When the content of CA-P/EG is 10%, the decreasing speed is faster. The flexural strength decreases from 4.20 to 2.63 MPa, which is a decrease by 37.4%. The compressive strength decreases from 11.6 to 7.5 MPa, which is a decrease by 35.3%. When the content of CA-P/EG increased further, the decreasing speed was reduced because the strength of CA-P/EG is lower than that of gypsum, and CA-P/EG fills the pores of gypsum, prevents the hydration of gypsum, and reduces the mechanical properties of gypsum. However, the flexural strength and compressive strength of the phase change gypsum board with different contents are more than 2 and 4 MPa, which meet the application requirements of the construction industry.
Table 1. Flexural Strength Test Data of the Phase Change Gypsum Board.
| flexural strength (MPa) |
||||||
|---|---|---|---|---|---|---|
| CA-P/EG (wt %) | 1 | 2 | 3 | average value | maximum relative deviation | modified flexural strength (MPa) |
| 0 | 4.10 | 4.45 | 4.05 | 4.20 | 5.9% < 10% | 4.20 |
| 5 | 3.45 | 3.25 | 3.20 | 3.30 | 4.5% < 10% | 3.30 |
| 10 | 2.55 | 2.65 | 2.70 | 2.63 | 3.0% < 10% | 2.63 |
| 15 | 2.70 | 2.55 | 2.45 | 2.57 | 5.1% < 10% | 2.57 |
| 20 | 2.50 | 2.35 | 2.40 | 2.42 | 3.3% < 10% | 2.42 |
Table 2. Compressive Strength Test Data of the Phase Change Gypsum Board.
| compressive strength (MPa) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CA-P/EG (wt %) | 1 | 2 | 3 | 4 | 5 | 6 | average value | maximum relative deviation | corrected compressive strength (MPa) |
| 0 | 11 | 10.7 | 10.5 | 12.3 | 12.7 | 12.3 | 11.6 | 9.5% < 10% | 11.6 |
| 5 | 8.9 | 9.2 | 8.4 | 8.7 | 7.3 | 8.7 | 8.5 | 14% > 10%, 8.5% < 10% | 8.8 |
| 10 | 7.4 | 7.7 | 7.5 | 7.5 | 7.5 | 7.2 | 7.5 | 4% < 10% | 7.5 |
| 15 | 6.7 | 6.7 | 6.9 | 6.8 | 7.0 | 6.7 | 6.8 | 3% < 10% | 6.8 |
| 20 | 6.5 | 6.6 | 6.2 | 6.6 | 6.5 | 6.3 | 6.45 | 4% < 10% | 6.45 |
Figure 3.
Mechanical property curves of the standard phase change gypsum board.
2.3. Water Absorption Performance of the Phase Change Energy Storage Gypsum Board
The water absorption properties of the phase change gypsum board with different contents are shown in Table 3 and Figure 4. It can be seen from Figure 4 that with the increase of the CA-P/EG content, the water absorption property of the phase change gypsum board first increases briefly and then decreases continuously. When the content of CA-P/EG is 5%, the water absorption performance is the best, which is due to the dry density of the phase change gypsum board and the increase of overall porosity. However, good water absorption performance significantly reduces the strength and structure of gypsum. At the same time, the water absorption rate decreases from 18.07 to 16.37% when the content of CA-P/EG is 20%, which is a decrease by 9.4%. The water absorption performance is the worst. The reason for this may be that with the increase of the CA-P/EG content, the pores of gypsum are gradually filled by CA-P/EG to prevent water immersion.
Table 3. Water Absorption of the Phase Change Gypsum Board.
| water absorption (%) |
||||||
|---|---|---|---|---|---|---|
| CA-P/EG (wt %) | 1 | 2 | 3 | average value | maximum relative deviation | modified water absorption (%) |
| 0 | 18.1 | 17.7 | 18.4 | 18.07 | 2.0% < 10% | 18.07 |
| 5 | 19.0 | 18.4 | 18.7 | 18.70 | 1.6% < 10% | 18.70 |
| 10 | 18.6 | 16.6 | 18.7 | 17.97 | 7.6% < 10% | 17.97 |
| 15 | 17.5 | 17.5 | 17.4 | 17.47 | 0.4% < 10% | 17.47 |
| 20 | 16.5 | 16.4 | 16.2 | 16.37 | 1.0% < 10% | 16.37 |
Figure 4.
Water absorption curve of the phase change gypsum board.
2.4. Apparent Density Analysis of the Phase Change Energy Storage Gypsum Board
Table 4 shows the apparent density of the phase change energy storage gypsum board with different contents. It can be seen from Table 4 that the apparent density of the phase change gypsum board decreases with the increase of the CA-P/EG content; when the content of CA-P/EG is 5, 10, 15, and 20%, the porosity of the phase change energy storage gypsum board decreases by 5.8, 8.4, 17.2, and 21.8%, respectively. This indicates that CA-P/EG is evenly filled in the pores of the gypsum board, its influence on the total volume of the gypsum board is small, the dry density of CA-P/EG is lower than that of gypsum, and that the quality of the phase change energy storage gypsum board per unit volume decreases.
Table 4. Apparent Density of the Phase Change Gypsum Board.
| apparent density (g/cm) |
||||||
|---|---|---|---|---|---|---|
| CA-P/EG (wt %) | 1 | 2 | 3 | average value | maximum relative deviation | modified apparent density (g/cm3) |
| 0 | 1.812 | 1.802 | 1.794 | 1.803 | 0.5% < 10% | 1.803 |
| 5 | 1.715 | 1.701 | 1.682 | 1.699 | 1.0% < 10% | 1.699 |
| 10 | 1.655 | 1.629 | 1.668 | 1.651 | 1.3% < 10% | 1.651 |
| 15 | 1.418 | 1.576 | 1.486 | 1.493 | 5.6% < 10% | 1.493 |
| 20 | 1.426 | 1.380 | 1.423 | 1.410 | 2.1% < 10% | 1.410 |
2.5. Microstructural Analysis of the Phase Change Energy Storage Gypsum Board
Figure 5 shows the SEM images of the CA-P/EG composite phase change material, the common gypsum board, and the phase change gypsum board with a CA-P/EG content of 20%. It can be observed from Figure 5a that the surface of CA-P/EG becomes relatively smooth and dense. It can be seen from Figure 5b that gypsum boards are interlaced in lamellar and flaky forms with a large number of pores in the interior. As can be seen from Figure 7c, the pores of the phase change gypsum board are uniformly filled with it due to the addition of the CA-P/EG composite phase change material, and the appearance becomes dense and compact, which confirms the above conclusion that the water absorption performance of the phase change gypsum board gradually becomes poor, and the apparent density gradually decreases.
Figure 5.
SEM images of an ordinary gypsum board and the phase change gypsum board ((a), CA-P/EG; (b), common gypsum board; and (c), phase change energy storage gypsum board).
Figure 7.
Mass loss of the CA-P/EG phase change gypsum board before and after thermal cycling.
2.6. Thermal Performance of the Phase Change Energy Storage Gypsum Board
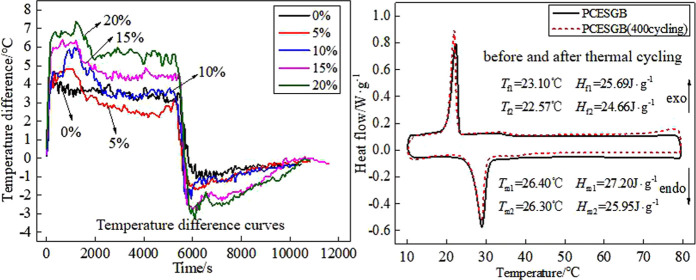
Figure 6 shows the DSC curve of the phase change gypsum board with the CA-P/EG content of 20%. It can be seen from Figure 6 that the melting temperature (Tm) and the melting latent heat (Hm) of the phase change gypsum board are 26.40 °C and 27.20 J/g, respectively, and the freezing temperature (Tf) and the freezing latent heat (Hf) are 23.10 °C and 25.69 J/g, respectively. Compared with the thermal properties of CA-P/EG, it can be found that the melting temperature of CA-P/EG decreases by 2.4%, while the freezing temperature increases by 3.8%. However, it is still in the required temperature range in the field of building energy-saving, which may be due to the force generated by gypsum pores, which needs to be overcome by CA-P/EG in the processes of melting and freezing. In addition, the latent heats of melting and freezing of the phase change energy storage gypsum board are far less than those of CA-P/EG, but it can still meet the requirements of building energy conservation. This is due to the fact that the latent heat of the phase change energy storage gypsum board is proportional to the content of CA-P/EG.
Figure 6.
DSC diagram of the phase change gypsum board.
2.7. Thermal Stability Analysis of the Phase Change Energy Storage Gypsum Board (BCESGB)
Thermal stability is not only an important index to evaluate the service life of phase change energy storage building materials but also an important factor affecting their performance. Figure 7 shows the mass loss rate curve of the phase change gypsum board with different contents of CA-P/EG. It can be seen from Figure 7 that the mass loss of the phase change gypsum board first increases and then gradually tends to be constant. Moreover, the higher the content of CA-P/EG, the greater the leakage rate. When the content of CA-P/EG is 0, 5, 10, 15, and 20%, the mass loss rate is 0.19, 0.53, 0.57, 0.72, and 0.86%, respectively, which are all less than 1%. This is because gypsum has a pore structure that can inhibit the seepage of phase change materials. The leakage of the gypsum sample is not obvious, indicating that the thermal cycle stability of the phase change gypsum board is good. The DSC curve of the phase change gypsum board with a CA-P/EG content of 20% after thermal cycling is shown in Figure 8. It can be seen from Figure 8 that after 400 melting–freezing cycles, the freezing and melting temperatures of the composite are reduced by 0.53 and 0.10 °C, respectively, and the latent heats of freezing and melting are reduced by about 4.0 and 4.6%, respectively; the changes of the latent heat and the temperature are very small, which indicates that the prepared phase change gypsum board has good thermal stability.
Figure 8.
DSC diagram of the phase change gypsum board before and after thermal cycling.
2.8. Heat Storage Performance of the Phase Change Energy Storage Gypsum Board
Heat storage performance is important to evaluate the use effect of phase change energy storage building materials.34−36Figure 9 shows the temperature curve of the phase change gypsum board with different contents of CA-P/EG. It can be seen from Figure 9 that the internal and external surface temperatures of the gypsum board keep increasing until they tend to become constant under the irradiation of an 800 W far-infrared heater. At the beginning of heating, the temperature curves of the phase change gypsum board and the pure gypsum board are basically consistent due to the good heat effect; the latent heat causes the phase change of the gypsum board, and a lot of heat is absorbed and stored in the middle period of the temperature increase, which reduces the heat conduction per unit time and the temperature fluctuation amplitude and produces an obvious inflection point in the temperature-change curve. In the late heating period, the inner and outer surface temperatures of the gypsum boards changed rapidly until they were consistent with the ambient temperature. At the same time, the thermal hysteresis phenomenon occurred, which was obvious with the increase of the CA-P/EG content. When the inner surface temperature of the pure gypsum board and the phase change gypsum board reaches 32 °C, the time required for the phase change energy storage gypsum board with the CA-P/EG contents of 5, 10, 15, and 20% is 170, 260, 430, and 540 s longer than that for the pure gypsum board, respectively. This indicates that the higher the content of CA-P/EG, the slower the rate of temperature rise and the stronger the heat storage capacity. After the far-infrared heater is turned off, the temperature of the inner and the outer surface of the gypsum board decreases gradually until it is close to the ambient temperature. When the inner surface temperature of the pure gypsum board drops to 20 °C, the time required is 2520 s. The time consumed by the phase change energy storage gypsum board with the CA-P/EG contents of 5, 10, 15, and 20% is 660, 910, 1265, and 1375 s longer than that by the pure gypsum board, respectively, as a result of the latent heat effect of phase change, indicating that the higher the content of CA-P/EG, the more obvious the thermal hysteresis, the lower the rate of temperature reduction, and the stronger the capacity of heat storage and temperature regulation.
Figure 9.

Temperature-change curves of the CA-P/EG phase change gypsum board with different contents. ((a) Pure gypsum board, (b) the 5% phase change gypsum board, (c) the 10% phase change gypsum board, (d) the 15% phase change gypsum board, and (e) the 20% phase change gypsum board).
The temperature difference curves of the external and internal surfaces of the CA-P/EG phase change energy storage gypsum board with different contents are shown in Figure 10. The temperature difference curves of the five kinds of gypsum boards are basically consistent, and with the increase of the CA-P/EG content, the maximum temperature difference between the outer and inner surfaces of the gypsum board increases gradually. The maximum temperature difference of the pure gypsum board is 4.1 °C at the heating stage, and the maximum temperature difference of the phase change energy storage gypsum board with the CA-P/EG contents of 5, 10, 15, and 20% is 4.8, 6, 6.4, and 7.4 °C, respectively. Compared with the pure gypsum board, the phase change energy storage gypsum board increased by 17, 46, 56, and 80%, respectively. But the maximum temperature difference of the phase change energy storage gypsum board with the CA-P/EG contents of 5, 10, 15, and 20% increases by 0.6, 1.1, 1.7, and 2.2 °C, respectively, at the cooling stage. This may be because the different temperatures of the heat sources on the inner and outer surfaces lead to inconsistency in the heating and cooling rates.
Figure 10.
Temperature difference curves between the outer surface and the inner surface of the CA-P/EG phase change gypsum board with different contents.
The temperature damping rate γ is used to evaluate the heat storage capacity and energy-saving effect of the phase change gypsum board, which is to set the time required for the internal surface temperature of the pure gypsum board to increase from t0 to TP as τ1, and the time required for the phase change gypsum board to be set as τ2, and the ratio between the difference and τ1 is set as the temperature damping rate γ, and the calculation formula is as follows
| 1 |
Figure 11 shows the temperature damping rate curves of the CA-P/EG phase change gypsum board with different contents from the initial temperature to 32 °C. As can be seen from Figure 11, the temperature damping rate gradually increases with the increase of the CA-P/EG content. The temperature damping rate of the phase change gypsum board with the CA-P/EG contents of 5, 10, 15, and 20% increases by 0.12, 0.19, 0.31, and 0.39, respectively. This shows that the CA-P/EG composite can effectively improve the temperature-change rate of the gypsum board and the heat storage and temperature regulation ability of the phase change gypsum board.
Figure 11.
Temperature damping rate change curve of the CA-P/EG phase change gypsum board with different contents.
The temperature damping rates of the pure gypsum board and the phase change energy storage gypsum board with the CA-P/EG content of 20% are calculated under different conditions. As can be seen from Table 5, when the power is 400, 800, and 1200 W, the temperature damping ratio of the phase change gypsum board and the CA-P/EG content of 20% is 0.25, 0.39, and 0.52 higher than that of the pure gypsum board, respectively. At the same time, it can be seen that with the increase of the far-infrared heater power, the temperature damping rate increases, and the energy storage and heat insulation capacities are also gradually enhanced. Table 6 shows the temperature damping rates of three different thickness phase change energy storage gypsum boards heated by an 800 W far-infrared heater. It can be seen from Table 6 that the temperature damping rate of the phase change gypsum board with thicknesses of 1, 2, and 4 cm increases by 0.3, 0.48, and 0.54, respectively, compared to the pure gypsum board. The temperature damping rate of the phase change gypsum board with 20% CA-P/EG content increases with the increase of thickness, and its heat storage capacity also increases.
Table 5. Temperature Damping Rate under Different Powers.
| 400 W |
800 W |
1200 W |
||||
|---|---|---|---|---|---|---|
| τ(s) | γ | τ(s) | γ | τ(s) | γ | |
| pure gypsum board | 1805 | 0 | 1400 | 0 | 900 | 0 |
| phase change gypsum board | 2250 | 0.25 | 1940 | 0.39 | 1370 | 0.52 |
Table 6. Temperature Damping Rate of Three Different Thickness Phase Change Gypsum Boards.
| 1 cm |
2 cm |
4 cm |
||||
|---|---|---|---|---|---|---|
| τ(s) | γ | τ(s) | γ | τ(s) | γ | |
| pure gypsum board | 1400 | 0 | 2395 | 0 | 4450 | 0 |
| phase change gypsum board | 1940 | 0.3 | 3540 | 0.48 | 6850 | 0.54 |
3. Conclusions
The mechanical properties of the phase change gypsum board decrease with the increase of the CA-P/EG content, but the flexural strength and the compressive strength of the phase change gypsum board exceed 2 and 4 MPa, respectively, which can meet the requirements of the construction industry. Moreover, the water absorption capacity first increased for a short time and then decreased, and the apparent density decreased continuously. After 400 cycles, the mass loss rate of the gypsum samples with different CA-P/EG contents is less than 1%, indicating that gypsum has good thermal stability. The optimum content of CA-P/EG is 20%, the melting and freezing temperatures are 26.40 and 23.10 °C, respectively, and the latent heats of melting and freezing are 27.20 and 25.69 J/g, respectively. The change of the latent heat and the temperature is very small after 400 cycles, indicating that the thermal stability is good. On the other hand, the increase or the decrease rate of the temperature of the phase change gypsum board decreases with the increase of the CA-P/EG content, the maximum temperature difference between the inner and outer surfaces increases, and the heat storage capacity increases with the increase of the CA-P/EG content and the thickness of the phase change gypsum board.
4. Materials and Methods
4.1. Materials
Capric acid (CA, C10H20O2, 172.27, 98.5%, Chemical Pure), hexadecanol (H, C16H34O, 242.44, 99.0%, Analytical Reagent), and paraffin section (PS, melting point range of 50–52 °C) were supplied by Changzhou Haituo Experimental Instrument Co., Ltd. Expandable graphite (mesh 50, expansion ration: 380 mL/g, carbon content: 98%) was purchased from Qingdao Risheng graphite Co., Ltd. The expandable graphite (mesh 50, expansion ration: 380 mL/g, carbon content: 98%) used in this experiment was purchased from Qingdao Risheng graphite Co., Ltd. The α-type superfine high-strength gypsum powder (mesh 180, the initial/final setting time: 5/28 min, flexural strength: 6.0 MPa, standard consistency: 36%, expansion coefficient: 0.03, bulk density: 1.43 g/cm3), naphthalene superplasticizer (brown-yellow powder, water reduction rate: 12–20%), and poly(vinyl alcohol) 2488 (PVA, mesh 100) were used as experimental materials. A temperature inspection instrument (THJ082K, accuracy ±0.5% FS), a constant temperature and humidity incubator (HWS-80B, accuracy ±0.5%, temperature range 0–100 °C, humidity range 40–90% RH), a PT100 temperature sensor (accuracy ±0.1 °C), a far-infrared heater (NSB-120), a cement flexural testing machine (DKZ-5000), a pressure testing machine (DYE-2000S), a differential scanning calorimeter (DSC, TAQ200, TA company), and scanning electron microscopy (SEM, Zeiss evo18, Germany) were used in this experiment.
4.2. Preparation of the Phase Change Energy Storage Gypsum Board
A certain mass of EG was weighed in a beaker, and then the liquid CA-P weighed according to the mass ratio of 7:1 was added to the EG. The thermal conductivity of the CA-P/EG phase change material is 0.455 W/(m·K) and the diffusion coefficient is 1.0 × 10–4 m2/s. After being evenly stirred with a glass rod, the beaker sealed with a film was placed in a drying oven at 65 °C for 24 h and stirred every 8 h to ensure that CA-P was evenly absorbed into the pores of the EG. Then, on being cooled to room temperature, the CA-P/EG composite was obtained. Next, 0.6% naphthalene superplasticizer; 0.47% water binder ratio; 0.6% PVA dispersant; and 0.5, 10, 15, 20, and 25% mass fractions of the CA-P/EG composite were selected in this work. The weighed superplasticizer and dispersant were fully dispersed in ultrapure water, and then the gypsum powder and the CA-P/EG composite were evenly added into the ultrapure water solution within 30 s. After standing for 40 s, the mixture was fully stirred using a mixing rod until the slurry began to thicken. Then, the slurry was slowly injected into the trial mold coated with the release agent, vibrated, and compacted. When the initial setting was completed, the surface was scraped with a scraper, and the gypsum was heated and hardened until demolding after the final setting. After being demolded, the samples were cured to the specified time in the laboratory (20 ± 2 °C, 65 ± 5% RH) to prepare the standard phase change energy storage gypsum board, and the compressive strength, flexural strength, and other properties of the gypsum board were tested. The fluidity of the slurry decreased gradually with the increase of the CA-P/EG content. According to the above method, the phase change energy storage gypsum board was prepared by changing the specific mass ratio and mold specification, and the relevant performance test was carried out.
4.3. Performance of the Phase Change Energy Storage Gypsum Board
According to the physical and mechanical properties’ test method, the 2 h wet flexural strength and compressive strength of the standard phase change energy storage gypsum board and the ordinary gypsum board were measured using a cement bending tester and a pressure testing machine. The phase change energy storage gypsum board and the ordinary gypsum board after the mechanical property test were made into three samples with the size of 4 × 4 × 1 cm3, and their mass was measured. The apparent densities ρ of the three samples were calculated using the m/v, and the arithmetic mean value was taken as the apparent density of the sample. Then, the above three samples of the same size were dried in a drying oven at 40 °C to a constant weight M1 and then placed in a closed container containing ultrapure water to fully soak the samples. After 24 h, the samples were taken out and wiped with a saturated wet towel, and the mass M2 was quickly weighed. The water absorption S of the three samples was calculated according to (M2 – M1)/M1, and the arithmetic average value was taken as the water absorption rate of the gypsum sample.
The microstructure of the phase change gypsum board and the ordinary gypsum board was observed using a scanning electron microscope. The thermal performance and thermal cycle stability of the phase change gypsum board were analyzed using differential scanning calorimetry under a nitrogen atmosphere. The temperature range was 10–80 °C, and the heating/cooling rate was set at 5 °C/min. The samples of the same size were dried to a constant weight in a drying oven at 40 °C and placed in a constant temperature and humidity incubator at 5 °C for 20 min. Then, they were placed in the drying oven at 65 °C for 20 min. In addition, differential scanning calorimetry was used to test the thermal properties of the phase change gypsum board, and the thermal stability of the phase change gypsum board was characterized by microscopic analysis.
The heat storage performance test device of the phase change energy storage gypsum board is shown in Figure 12. To test the heat storage performance of the phase change gypsum board, in a winter indoor environment (the indoor temperature was about 15 °C), the far-infrared heater was used to simulate solar radiation, which was placed 25 cm in front of the sample, and the wall-mounted thermal resistance was evenly arranged at the center of the inner and outer surfaces of the sample. After the whole test device was installed, the far-infrared heater was turned on, and the heating curve of the sample was recorded. When the heater was turned on for 1.5 h, the power was turned off to cool the sample in the natural environment, and the cooling curve of the sample was recorded.
Figure 12.

Schematic diagram of the phase change energy storage gypsum board experimental device (①, the gypsum sample; ②, a fixed table; ③, the far-infrared heater; ④, a temperature inspection instrument; ⑤, thermal resistance; and ⑥, a computer).
Acknowledgments
This work was supported by financial assistance from the Natural Science Foundation of China (51966004 and 51666004) and the Program of Qingjiang Excellent Talents (JXUSTQJYX2017003).
The authors declare no competing financial interest.
References
- Zhang W. Y.; Zhang X. G.; Huang Z. H.; Wen R. L.; Huang Y. T.; Wu X. W.; Min X.; et al. Preparation and characterization of capric-palmitic-stearic acid ternary eutectic mixture/ expanded vermiculite composites as form-stabilized thermal energy storage materials. J. Mater. Sci. Technol. 2018, 34, 379–386. 10.1016/j.jmst.2017.06.003. [DOI] [Google Scholar]
- Liang C. H.; Huang X.; Li Y.; Di Y. H. Application of phase change materials in buildings. Build. Energy Environ. 2004, 4, 23–26. [Google Scholar]
- Zhang X. G.; Qiao J. X.; Zhang W. Y.; Cheng F.; Yin Z. Y.; Huang Z. H.; Min X. Thermal behavior of composite phase change materials based on polyethylene glycol and expanded vermiculite with modified porous carbon layer. J. Mater. Sci 2018, 53, 13067–13080. 10.1007/s10853-018-2531-x. [DOI] [Google Scholar]
- Liu F. L.; Zhu J. Q.; Ma B. G.; Zhou W. B.; Li Y. Y. Research progress on Preparation of phase change gypsum board and its application in building wall. J. Chin. Ceram. Soc. 2016, 44, 1178–1191. [Google Scholar]
- Zhang S. L.; Wu W.; Wang S. F. Experimental investigations of Alum/expanded graphite composite phase change material for thermal energy storage and its compatibility with metals. Energy 2018, 161, 508–516. 10.1016/j.energy.2018.07.075. [DOI] [Google Scholar]
- Shen C.; Xu L. L.; Li W. H. Research progress of phase change energy storage materials in the field of building energy conservation. Mater. Rep. 2015, 29, 100–104. [Google Scholar]
- Shamsi H.; Boroushakia M.; Geraeib H. Performance evaluation and optimization of encapsulated cascade PCM thermal storage. J. Energy Storage 2017, 11, 64–75. 10.1016/j.est.2017.02.003. [DOI] [Google Scholar]
- Peng Z.; Yue C. S.; Qiu G. B.; Wu C. Y.; Li J. J. Latest research progress and application of phase change energy storage materials. Mater. Rep. 2018, 32, 248–252. [Google Scholar]
- Huang X.; Alva G.; Liu L. K.; Fang G. Y. Preparation, characterization and thermal properties of fatty acid eutectics/bentonite/expanded graphite composites as novel form-stable thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 166, 157–166. 10.1016/j.solmat.2017.03.026. [DOI] [Google Scholar]
- Yang L.; Qiao Y. H.; Liu Y.; Hou L. Q.; Wang M. Y.; Liu J. P. Research progress of phase change heat storage and night ventilation technology. Chin. Sci. Bull. 2018, 63, 629–640. 10.1360/N972017-00788. [DOI] [Google Scholar]
- Hawes D. W.; Feldman D.; Banu D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. 10.1016/0378-7788(93)90040-2. [DOI] [Google Scholar]
- Hawes D. W.; Feldman D. Absorption of phase change materials in concrete. Sol. Energy Mater. Sol. Cells 1992, 27, 91–101. 10.1016/0927-0248(92)90112-3. [DOI] [Google Scholar]
- Feldman D.; Banu D.; Hawes D. W.; Ghanbari E. Obtaining an energy storing building material by direct incorporation of an organic phase change material in gypsum wallboard. Sol. Energy Mater. 1991, 22, 231–242. 10.1016/0165-1633(91)90021-C. [DOI] [Google Scholar]
- Hu X. F.; Xiao D. Study on temperature characteristics of phase change energy storage building materials based on ANSYS. Mater. Rep. 2009, 23, 83–86. [Google Scholar]
- Sayyar M.; Weerasiri R. R.; Soroushian P.; Lu J. Experimental and numerical study of shape-stable phase-change nano-composite toward energy-efficient building constructions. Energy Build. 2014, 75, 249–255. 10.1016/j.enbuild.2014.02.018. [DOI] [Google Scholar]
- Ahmet S.; Hayati S.; Adem O. Thermal properties and thermal reliability of eutectic mictures of some fatty acids as latent heat storage materials. Energy Convers. Manage. 2004, 45, 365–376. 10.1016/S0196-8904(03)00154-7. [DOI] [Google Scholar]
- Ahmad M.; Bontemps A.; Sallee H.; Quenard D. Experimental investigation and computer simulation of thermal behaviour of wallboards containing a change material. Energy Build. 2006, 38, 357–366. 10.1016/j.enbuild.2005.07.008. [DOI] [Google Scholar]
- Sari A. Fabrication and thermal characterization of kaolin-based composite phase change materials for latent heat storage in buildings. Energy Build. 2015, 96, 193–200. 10.1016/j.enbuild.2015.03.022. [DOI] [Google Scholar]
- Huang X.; Cui Y. D.; Yin G. Q.; Feng G. Z.; Wu Z. M. Preparation and properties of lauric acid expanded graphite composite phase change materials. CIESC J. 2015, 66, 370–374. [Google Scholar]
- Yuan Y. G.; Yuan Y. P.; Zhang N.; Li T. Y.; Cao X. L. Preparation and properties of lauric acid palmitic acid stearic acid/expanded graphite composite phase change materials. CIESC J. 2014, 65, 286–292. [Google Scholar]
- Zhao Y. J.; Min X.; Huang Z. H.; Liu Y. G.; Wu X. W.; Fang M. H. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. 10.1016/j.enbuild.2017.10.078. [DOI] [Google Scholar]
- Min X.; Fang M. H.; Huang Z. H.; Liu Y. G.; Huang Y. T.; Wen R. L.; Qian T. T.; Wu X. W. Enhanced thermal properties of novel shape-stabilized PEG composite phase change materials with radial mesoporous silica sphere for thermal energy storage. Sci. Rep. 2015, 5, 12964 10.1038/srep12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y. J.; Min X.; Ding Z. P.; Chen S.; Ai C. Z.; Liu Z. L.; Yang T. Z.; Wu X. W.; Liu Y. G.; Lin S. W.; Huang Z. H.; Gao P.; Wu H.; Fang M. H. Metal-Based Nanocatalysts via a Universal Design on Cellular Structure. Adv. Sci. 2020, 7, 1902051 10.1002/advs.201902051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X.; Sun B.; Chen S.; Fang M. H.; Wu X. W.; Liu Y. G.; Abdelkader A.; Huang Z. H.; Liu T.; Xi K.; Kumar R. V. A textile-based SnO2 ultra-flexible electrode for lithium-ion batteries. Energy Storage Mater. 2019, 16, 597–606. 10.1016/j.ensm.2018.08.002. [DOI] [Google Scholar]
- Cheng F.; Wen R. L.; Zhang X. G.; Huang Z. H.; Huang Y. T.; Fang M. H.; Liu X. G.; Wu X. W.; Min X. Synthesis and characterization of beeswax-tetradecanol-carbon fiber/expanded perlite form-stable composite phase change material for solar energy storage. Composites, Part A 2018, 107, 180–188. 10.1016/j.compositesa.2017.12.022. [DOI] [Google Scholar]
- Zhou Y.; Yu C. W. F.; Zhang G. Study on heat-transfer mechanism of wallboards containing active phase change material and parameter optimization with ventilation. Appl. Therm. Eng. 2018, 144, 1091–1108. 10.1016/j.applthermaleng.2018.04.083. [DOI] [Google Scholar]
- Sari A.; Bicer A.; Karaipekli A.; Al-Sulaiman F. A. Preparation, characterization and thermal regulation performance of cement based-composite phase change material. Sol. Energy Mater. Sol. Cells 2018, 174, 523–529. 10.1016/j.solmat.2017.09.049. [DOI] [Google Scholar]
- Wang R.; Ren M.; Gao X. J.; Qin L. Preparation and properties of fatty acids based thermal energy storage aggregate concrete. Constr. Build. Mater. 2018, 165, 1–10. 10.1016/j.conbuildmat.2018.01.034. [DOI] [Google Scholar]
- Shi T.; Sun W. Application technology progress of phase change energy storage building materials. J. Chin. Ceram. Soc. 2008, 7, 1031–1036. [Google Scholar]
- Bentz D. P.; Randy T. Potential applications of phase change materials in concrete technology. Cem. Concr. Compos. 2007, 29, 527–532. 10.1016/j.cemconcomp.2007.04.007. [DOI] [Google Scholar]
- Zhang N.; Yuan Y. P.; Wang X.; Cao X.; Yang X.; Hu S. Preparation and characterization of lauric-myristic-palmitic acid ternary eutectic mixtures/expanded graphite composite phase change material for thermal energy storage. Chem. Eng. J. 2013, 231, 214–219. 10.1016/j.cej.2013.07.008. [DOI] [Google Scholar]
- Zuo J. G.; Li W. Z.; Weng L. D. Thermal properties of lauric acid/1-tetradecanol binary system for energy storage. Appl. Therm. Eng. 2011, 31, 1352–1355. 10.1016/j.applthermaleng.2011.01.008. [DOI] [Google Scholar]
- Koschenz M.; Lehmann B. Development of a thermally activated ceiling panel with PCM for application in lightweight and retrofitted buildings. Energy Build. 2004, 36, 567–578. 10.1016/j.enbuild.2004.01.029. [DOI] [Google Scholar]
- Sari A. Thermal energy storage characteristics of bentonite-based composite PCMs with enhanced thermal conductivity as novel thermal storage building materials. Energy Convers. Manage. 2016, 117, 132–141. 10.1016/j.enconman.2016.02.078. [DOI] [Google Scholar]
- Yang X.; Yuan Y.; Zhang N.; Cao X. L.; Liu C. Preparation and properties of myristic-palmitic-stearic acid/expanded graphite composites as phase change materials for energy storage. Sol. Energy 2014, 99, 259–266. 10.1016/j.solener.2013.11.021. [DOI] [Google Scholar]
- Zhang H.; Gao X. N.; Chen C. X.; Xu T.; Fang Y. T.; Zhang Z. G. A capric-palmitic-stearic acid ternary eutectic mixture/expanded graphite composite phase change material for thermal energy storage. Composites, Part A 2016, 87, 138–145. 10.1016/j.compositesa.2016.04.024. [DOI] [Google Scholar]