Abstract

Magnetic nanoparticles (MNPs) have been extensively used as tiny heating sources in magnetic hyperthermia therapy, contrast agents in magnetic resonance imaging, tracers in magnetic particle imaging, carriers for drug/gene delivery, etc. There have emerged many MNP/microbead suppliers since the past decade, such as Ocean NanoTech, Nanoprobes, US Research Nanomaterials, Miltenyi Biotec, micromod Partikeltechnologie GmbH, nanoComposix, and so forth. In this paper, we report the physical and magnetic characterizations on iron oxide nanoparticle products from Ocean NanoTech. Standard characterization tools such as vibrating-sample magnetometry, X-ray diffraction, dynamic light scattering, transmission electron microscopy, and zeta potential analysis are used to provide MNP customers and researchers with an overview of these iron oxide nanoparticle products. In addition, the dynamic magnetic responses of these iron oxide nanoparticles in aqueous solutions are investigated under low- and high-frequency alternating magnetic fields, giving a standardized operating procedure for characterizing the MNPs from Ocean NanoTech, thereby yielding the best of MNPs for different applications.
1. Introduction
Magnetic nanoparticles (MNPs) are nanomaterials with sizes between 1 and 100 nm. Due to their large surface-to-volume ratio and tunable magnetic properties, MNPs have emerged as one of the most important nanomaterials in magnetic, chemical, and biomedical applications. The surface of the MNPs can be functionalized with various coatings from inorganic coatings such as silica1 and carbon2 to organic coatings such as polyethylene glycol3 and dopamine.4 Compared to non-magnetic particles, MNPs can be manipulated by an external magnetic field without any physical contact, which leads to various applications such as drug delivery5 as well as the separation and concentration of certain molecules.6 Under an alternating magnetic field, MNPs can induce a localized temperature increase at the target spot, which makes them promising candidates for hyperthermia applications.7 Under an external magnetic field, MNPs can generate stray fields. By integrating with various magnetic sensors such as magnetoresistance sensors,8,9 hall sensors,10,11 nuclear magnetic resonance (NMR) sensors,11 magnetic resonance imaging (MRI),12 and magnetic particle spectroscopy (MPS),13 MNPs can also serve as magnetic markers in diagnostic applications.
To date, MNPs with various sizes and surface coatings have been successfully commercialized and are available in many companies such as Ocean NanoTech (San Diego, USA), Nanoprobes (New York City, USA), US Research Nanomaterials (Houston, USA), Miltenyi Biotec (Bergisch Gladbach, Germany), micromod Partikeltechnologie GmbH (Rostock, Germany), nanoComposix (San Diego, USA), and so forth. For these aforementioned applications, the quest for a high magnetic moment, uniform size distribution, and colloidal stability of MNPs has pushed the development of various nanoparticle manufacturers. In this paper, we first characterized the magnetic and physical properties of single-core, differently sized iron oxide nanoparticle products from Ocean NanoTech using vibrating-sample magnetometry (VSM), X-ray diffraction (XRD), dynamic light scattering (DLS), transmission electron microscopy (TEM), and zeta potential analysis (summarized in Table 1). In addition, we give application-oriented assessments on these MNP products using a home-built MPS system. Practical suggestions on the applications of these iron oxide nanoparticles with varying core sizes are given at the end of this paper to maximize the use of them.
Table 1. Physical and Magnetic Properties of SHA Series MNPs.
| sample | average sizea (nm) ± SD | zeta potential (mV) ± SD | specific magnetization (emu/g Fe)b,c | specific magnetization (emu/g)c,d | magnetic moment per particle (emu/particle)c | material |
|---|---|---|---|---|---|---|
| SHA-5 | 10.46 ± 3.88 | –0.03 ± 0.005 | 63.84 | ∼44.69 | 1.54 × 10–17 | γ-Fe2O3, Fe3O4 |
| SHA-10 | 18.07 ± 4.72 | 5.03 ± 0.07 | 42.64 | ∼29.85 | 8.23 × 10–17 | γ-Fe2O3, Fe3O4 |
| SHA-15 | 20.69 ± 6.31 | 7.66 ± 0.05 | 83.44 | ∼58.41 | 5.13 × 10–16 | γ-Fe2O3, Fe3O4 |
| SHA-20 | 27.56 ± 11.29 | –0.41 ± 0.33 | 78.08 | ∼54.66 | 1.18 × 10–15 | γ-Fe2O3, Fe3O4 |
| SHA-25 | 28.28 ± 10.38 | 1.15 ± 0.49 | 55.28 | ∼38.70 | 1.58 × 10–15 | γ-Fe2O3, Fe3O4 |
| SHA-30 | 32.60 ± 12.17 | –0.69 ± 0.04 | 51.12 | ∼35.78 | 2.50 × 10–15 | γ-Fe2O3, Fe3O4 |
The average hydrodynamic sizes of SHA series MNPs are based on number-weighted DLS distribution.
The specific magnetization (emu/g Fe) is calculated under a 5000 Oe field.
The specific magnetization (unit: emu/g Fe, emu/g) and magnetic moment per particle (unit: emu/particle) are calculated under a 5000 Oe field based on the nanoparticle concentrations provided by Ocean NanoTech. The concertation of Fe is 5 mg/mL for all SHA series MNPs, while the concentrations of nanoparticles are 34.5, 4.3, 1.35, 0.55, 0.29, and 0.17 nmol/mL for SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30, respectively.
The specific magnetization (emu/g), that is, the magnetic moment per gram of iron oxide nanoparticle is calculated by assuming that iron holds 70% of the nanoparticle weight.
2. Results and Discussion
2.1. Magnetic Properties of SHA Series MNPs
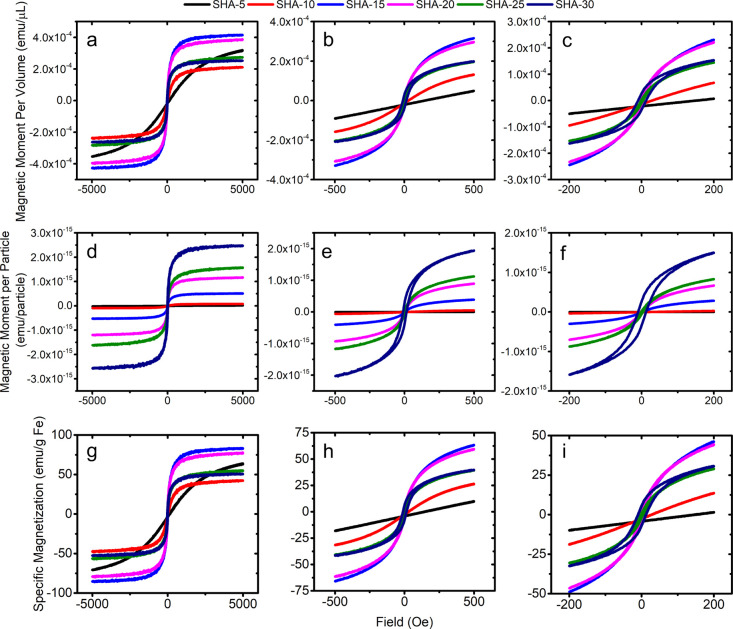
The hysteresis curves of SHA series MNPs are recorded by VSM under field ranges of −5000 to 5000, −500 to 500, and −200 to 200 Oe. The magnetic moment per volume of MNP suspension is averaged over 25 μL of the MNP sample and plotted in Figure 1a–c, with SHA-5, SHA-10, SHA-15, and SHA-20 being superparamagnetic. We also observed coercivities from SHA-25 and SHA-30. Due to the varying particle concentrations in SHA series MNP products (listed in Table 1), the magnetic moment per volume is not comprehensive to represent the magnetic property of each MNP. In addition, the magnetic moment per particle is also summarized in Figure 1d–f, with SHA-30 showing the highest magnetic moment per particle, followed by SHA-25, SHA-20, SHA-15, SHA-10, and SHA-5 showing the lowest magnetic moment per particle. Under a field strength of 5000 Oe, the specific magnetizations (calculated from the magnetic moment per gram of Fe, unit: emu/g Fe) from the highest to the lowest are SHA-15 > SHA-20 > SHA-5 > SHA-25 > SHA-30 > SHA-10, as shown in Figure 1h,i. Note that the magnetization of SHA-5 is not saturated at 5000 Oe, while the remaining SHA series MNPs are saturated. Thus, we use specific magnetization instead of saturation magnetization here. Due to the surface spin canting effect (also called the magnetically anomalous shell or magnetically dead layer) of nanoparticles, the specific magnetizations of MNPs are always lower than that of the bulk material. Herein, the specific magnetizations of SHA series MNPs are lower than the ideal values of bulk γ-Fe2O3 (60–80 emu/g) and Fe3O4 (92–100 emu/g) materials.15−19
Figure 1.
Magnetization curves of SHA series MNPs obtained by VSM at 20 °C. External field sweeps from (a,d,g) −5000 to +5000 Oe, (b,e,h) −500 to +500 Oe, and (c,f,i) −200 to +200 Oe. Magnetization units are represented by emu/μL, emu/particle, and emu/g Fe for (a–c), (d–f), and (g–i), respectively.
Since the magnetic moment of each particle increases with the cube of its magnetic core diameter (or radius), in the ideal case where these SHA series MNPs show similar specific magnetization, the magnetic moment per particle should show a similar trend to the core diameter. However, we do not see the trend of increasing specific magnetizations (under a 5000 Oe field) in SHA series MNPs as the magnetic core size increases, which might be caused by the insufficient oxygenation for larger magnetic core sizes. Thus, as the insufficient oxygenation effect dominates, the SHA-15 MNPs show the highest specific magnetization, and it decreases with increasing core size as seen in Table 1.
The crystal structure of SHA series MNPs is characterized via XRD (Bruker D8 Discover 2D), as shown in Figure 2. It is observed that Fe3O4 and γ-Fe2O3 are the main phases in SHA series MNPs. There are also several diffraction peaks from the solution denoted by the blue dashed lines in Figure 2. The sharp diffraction peaks (labeled by black diamonds) come from the chemicals in the MNP buffer (NaCl, KCl, Na2HPO4, KH2PO4, etc), and the peaks labeled by black rounds come from the Si/SiO2 substrate. The full width at half-maximum of the diffraction peaks is wider for the MNPs compared to their bulk counterparts. The broadening effects are due to the decreased grain size for nanoparticles.
Figure 2.

XRD patterns of SHA series MNPs. The powder diffraction files of FeO, Fe3O4, α-Fe2O3, and γ-Fe2O3 are added for references.
2.2. Hydrodynamic Size and Morphological Characterizations on SHA Series MNPs
Figure 3a–f shows the hydrodynamic size distributions of samples SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30 with mean values of 10.46, 18.07, 20.69, 27.56, 28.28, and 32.6 nm, respectively. The average hydrodynamic sizes of SHA series MNPs are summarized in Table 1. The SHA series MNPs have two organic coating layers: one monolayer of oleic acid and another monolayer of the amphiphilic polymer. The total thickness of the organic layer coating is about 4 nm. This causes the hydrodynamic size of the nanoparticles to be about 8–10 nm larger than their inorganic core size measured by TEM (see Figure 4). In this respect, the mean values for DLS in the SHA series show quite a satisfactory trend and good agreement with the TEM results; the results are arranged in ascending order of their sizes in Figure 3.
Figure 3.
DLS number-weighted distributions of the hydrodynamic size of MNPs from samples (a) SHA-5, (b) SHA-10, (c) SHA-15, (d) SHA-20, (e) SHA-25, and (f) SHA-30 as characterized by DLS. In each figure, the solid green lines are the fitted log-normal distribution curves and the solid red lines are the cumulative distribution curves. The μ values represent the statistical mean of the hydrodynamic sizes of the samples. The standard deviation and R2 values are represented by σ and R2, respectively, for each case.
Figure 4.
TEM images of SHA series MNPs. (a–f) SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30, respectively. Scale bars represent 20 nm. TEM images of SHA series MNPs under different magnifications are given in S5 from the Supporting Information.
The magnetic core morphologies of SHA series MNPs are shown in Figure 4. Some MNPs are agglomerated during the evaporation process of the MNP suspensions. For the MNPs with smaller sizes such as samples SHA-5 and SHA-10, the magnetic core shapes are not strictly spherical, which might cause higher shape anisotropies as well as higher effective magnetic anisotropies in these MNPs. However, larger MNPs show spherical magnetic cores. The contrast of different MNPs from one TEM image is due to the different crystal orientations. When the crystal zone axis is close to the incident electron beam, the MNPs show a darker color.
2.3. Zeta Potential of SHA Series MNPs
The SHA series MNPs have a neutral to slightly alkaline pH between 7.2 and 7.6. The measured zeta potential values for SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30 are −0.03, +5.03, +7.66, −0.41, +1.15, and −0.69 mV, respectively.
2.4. Dynamic Magnetic Responses of SHA Series MNPs under a Low-Frequency Driving Field
The dynamic magnetic responses of SHA series MNPs under a mono-frequency driving field are investigated. The driving field frequency is varied from 50 to 2850 Hz, and the field amplitude is set at 170 Oe (Gauss).20,20−27 Each plastic vial containing 200 μL of SHA series MNPs in 10 nM PBS and 0.03% NaN3 is placed under the alternating magnetic field for MPS measurements. For MNPs suspended in liquid solution under an external magnetic field, they undergo two distinct relaxation mechanisms by which the magnetic moments rotate in response to the field: the Néel relaxation is the flipping of the magnetic moment between easy axes inside a stationary MNP, and on the other hand, the Brownian relaxation is the physical rotation of the entire MNP along with its magnetic moment. In principle, both relaxation mechanisms play important roles in determining the dynamic magnetic responses of MNPs in suspension when subjected to the alternating magnetic field. Depending on the magnetic properties (such as effective anisotropy constant and saturation magnetization),28,29 the physical properties (magnetic core size and the hydrodynamic size including the polymer coatings and anchored biological compounds such as proteins, peptides, cells, and so forth) of MNPs,15,21,30−35 the nanoparticle volume fraction of the suspension (i.e., dipolar interactions),15,36−38 and the physical properties of the suspension (temperature and viscosity),22,23,39−46 MNPs could undergo either Néel or Brownian process-dominated relaxation. It has been reported that for a system of non-interacting iron oxide nanoparticles with negligible polymer coatings, the magnetic dynamics will be dominated by the Brownian process when the core size is above 15 nm and the Néel process dominates when the core size is below 15 nm39,47−49 (see S7 from the Supporting Information).
Under a low-frequency driving field (f < 500 Hz), magnetic moments of SHA series MNPs with diameters from 5 to 30 nm are able to follow the time-varying magnetic field. As shown in S8 from the Supporting Information, all the six SHA series MNPs show similar phase angles to the driving field (f < 500 Hz), and as the field frequency increases, the differences in the phase angles between six samples increase. Larger MNPs with a larger effective relaxation time show a larger phase lag to the driving field.
As summarized in Figure 5, under a low-frequency driving field, the dynamic magnetic responses of six SHA series MNPs from the strongest to the weakest are SHA-30 > SHA-20 > SHA-15 > SHA-25 > SHA-10 > SHA-5. Figure 5a–c summarizes the amplitudes measured at the third, fifth, and seventh harmonics, respectively. Figure 5d–f highlights the corresponding harmonic amplitudes under driving field frequencies of 350, 650, 1250, and 1850 Hz.
Figure 5.
Harmonics generated by SHA series MNPs under a low-frequency driving field. (a–c) Third, fifth, and seventh harmonic amplitudes of SHA series MNPs under different driving field frequencies. (d–f) Harmonic amplitudes at driving field frequencies of 350, 650, 1250, and 1850 Hz.
Figure 6 summarizes the real-time voltage signal obtained from pickup coils at driving field frequencies of 350, 950, and 1850 Hz. The extracted harmonics are plotted along with the total signal in real time. MNPs with stronger dynamic magnetic responses to the driving field generate larger harmonic signals and are thus able to cause the distortions in the total signal (the highlighted dark areas in Figure 6). It is observed that SHA-30 and SHA-20 show the strongest dynamic magnetic responses to the low-frequency driving field, followed by SHA-15 and SHA-25. SHA-5 and SHA-10 show negligible dynamic magnetic responses compared to the former SHA series MNPs, which are mainly due to the low magnetic moments and linear magnetization curves, as shown in Figure 1.
Figure 6.
Recorded real-time dynamic magnetic responses of SHA series MNPs under a low-frequency driving field. The higher harmonics are extracted and plotted in parallel with the total signal obtained from the pickup coils. [(a,d,g,j,m,p)], [(b,e,h,k,n,q)], and [(c,f,i,l,o,r)] are the real-time total signal and higher harmonics under driving field frequencies of 350, 950, and 1850 Hz for samples SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30, respectively. A zoom-in view of the higher harmonics is plotted in S9 from the Supporting Information.
2.5. Dynamic Magnetic Responses of SHA Series MNPs under a High-Frequency Driving Field
In this section, we report the dynamic magnetic responses of SHA series MNPs under high-frequency driving fields. A dual-frequency method is used herein; one excitation field is set at 10 Hz and a magnitude of 170 Oe, and the other high-frequency driving field is set at varying frequencies (from 1 to 20 kHz) and a magnitude of 17 Oe.13,30,39,47,50−52
Under a high-frequency driving field, larger MNPs (i.e., SHA-30) are unable to rotate their magnetic moments to the fast-switching magnetic field; thus, their dynamic magnetic responses are weakened. As shown in S10 from the Supporting Information, there is a constant harmonic phase difference of 50° between SHA-10 and SHA-30 MNPs. As a result, under a high-frequency driving field, the dynamic magnetic responses of six SHA series MNPs from the strongest to the weakest are SHA-15 > SHA-20 > SHA-30> SHA-25 > SHA-10 > SHA-5 (as shown in Figure 7).
Figure 7.
Harmonics generated by SHA series MNPs under a high-frequency driving field. (a–c) Third, fifth, and seventh harmonic amplitudes of SHA series MNPs under different driving field frequencies. (d–f) Harmonic amplitudes at driving field frequencies of 3, 5, 10, and 20 kHz.
Although recent in origin, MNPs of different core sizes have found their applications in various fields of science. This section of the paper is dedicated to identifying the utility of the different-sized and surface-functionalized MNPs in realistic applications. The SHA series particles are amine-functionalized MNPs. As the amine groups are less selective and less specific for antibodies and proteins, they capture a varied range of bacterial pathogens and allow purification of water, food, and urine samples.53 The VSM characterization of the SHA series in Figure 1a–f shows that SHA-5, SHA-10, SHA-15, and SHA-20 are superparamagnetic. Although SHA-25 and SHA-30 show higher magnetic moments, they show remanent magnetizations. For magnetic biosensing, higher-moment particles are preferred in order to generate a higher magnetic signal per particle. However, practical limitations such as colloidal stability (no clustering) should also be considered. For the SHA series, SHA-25 exhibits the second highest magnetic moment/particle with a remanent magnetization of 1.28% M, where M is the specific magnetization under 5000 Oe. Although SHA-30 has a higher magnetic moment/particle compared to SHA-25, a much larger remanence magnetization of 10.93% M is observed from SHA-30. Taking both magnetic moments and remanent magnetizations into consideration, SHA-25 is the optimum candidate from SHA series for biosensing applications. On a different note, for cell separation and sorting and drug/gene delivery, as the property of superparamagnetism is not essential and a higher magnetic moment ensures a larger magnetic torque (force), the highest-magnetic-moment MNP, SHA-30, is probably a better candidate.
For homogeneous bioassays that are based on a conjugation-mediated change in Brownian relaxation time, MNPs should be thermally blocked.13,54−59 Thus, an in-depth study on the Brownian and Néel relaxation times of these MNPs under different driving fields should be carried out.60,61 For magnetic hyperthermia therapy, the dissipated energy or specific absorption rate (SAR) is directly proportional to the imaginary component of AC susceptibility and saturation magnetization (Ms) of MNPs, the applied field frequency, and the amplitude squared.62−66 Thus, a high Ms of MNPs does not guarantee a high SAR, and practical measurements on the hyperthermia performance are required to find out which SHA series MNPs are better suited for hyperthermia applications.
Furthermore, MRI techniques require the MNPs to be injected into the body fluids which then accumulate in the target tissues. Hence, for MRI applications, it is extremely essential for the MNPs to be small as larger MNPs have greater tendency to block the arteries. In this case, SHA-5 and SHA-10 MNPs will be quite useful.67 The dynamic magnetic responses of SHA series MNPs are compared in this paper using a homebuilt MPS system. The harmonics are induced under different driving magnetic fields, which are a result of the joint effects of relaxation mechanisms and the magnetic moment of each MNP. For magnetic particle imaging (MPI) and MPS-based bioassays, larger dynamic magnetic responses (higher harmonic amplitudes) ensure a higher signal-to-noise ratio and sensitivity. Thus, SHA-30 MNPs are suggested for MPI and MPS-based bioassays where the driving field frequencies are below 2 kHz, while SHA-15 MNPs are suggested for these applications where the driving field frequencies are above 2 kHz.
3. Conclusions
In this paper, we characterized the magnetic and physical properties of SHA series MNPs from Ocean NanoTech using standard characterization tools. The VSM results show that SHA-5, SHA-10, SHA-15, and SHA-20 MNPs are superparamagnetic and on the other hand, SHA-25 and SHA-30 are not superparamagnetic, with SHA-30 showing the highest magnetic moment per particle, followed by SHA-25, SHA-20, SHA-15, SHA-10, and SHA-5. Thus, SHA series iron oxide nanoparticles with larger core sizes are preferred for magnetic biosensing and drug delivery where high-moment MNPs are desired for higher magnetic signals and higher magnetic torques. However, SHA-25 and SHA-30 show remnant magnetizations upon the removal of the magnetic field (non-superparamagnetic), and thus, they are not applicable for applications where superparamagnetism is required. The XRD results show that all SHA series MNPs are composed of γ-Fe2O3 and Fe3O4. The dynamic magnetic responses of these iron oxide nanoparticles are investigated using a homebuilt MPS system, where both the responses under low and high driving field frequencies are summarized. It is observed that under low driving field frequencies, the dynamic magnetic responses of SHA series MNPs from the strongest to the weakest are SHA-30 > SHA-20 > SHA-15 > SHA-25 > SHA-10 > SHA-5. However, under high driving field frequencies, due to the larger phase lags of larger MNPs, the dynamic magnetic responses from the strongest to the weakest are modified: SHA-15 > SHA-20 > SHA-30> SHA-25 > SHA-10 > SHA-5. These results give hints on designing MPI and MPS-based bioassays to maximize the use of different MNPs of different core sizes. At the end of this paper, based on the requirements and goals of MNP-based applications, we suggested different SHA MNPs for each application.
4. Materials and Methods
4.1. Materials
The SHA series MNPs are provided by Ocean NanoTech. Six SHA series MNPs with average magnetic core sizes of 5, 10, 15, 20, 25, and 30 nm are characterized in this paper (denoted as SHA-5, SHA-10, SHA-15, SHA-20, SHA-25, and SHA-30, respectively; photographs of SHA series MNPs used in this work can be found in the Supporting Information, S1). The SHA series MNPs are a group of water-soluble iron oxide nanoparticles coated with the amphiphilic polymer and functionalized amine reactive groups. They are very stable in most buffers in the pH range of 4–10 and can be readily conjugated to proteins, peptides, and other carboxylic acid-containing molecules.
4.2. VSM Measurement
25 μL of the SHA series MNP suspension is pipetted onto a filter paper and air-dried before the VSM measurements. Three independent magnetization curves of each sample are obtained at 20 °C, with the external magnetic field swept from −5000 to +5000 Oe (a field step of 10 Oe and an averaging time of 200 ms), −500 to +500 Oe (a field step of 2 Oe and an averaging time of 200 ms), and −200 to +200 Oe (a field step of 1 Oe and an averaging time of 200 ms).
4.3. XRD Measurement
50 μL of the SHA series MNP suspension is pipetted onto a Si/SiO2 slide and air-dried before the XRD characterization. A cobalt radiation source (wavelength ∼1.79 Å) is used for the XRD characterization since it has lower fluorescence, especially for magnetite and maghemite.14 For a convenient comparison, the characterized XRD patterns are converted to copper radiation. The crystal structure of SHA series MNPs is characterized via XRD (Bruker D8 Discover 2D).
4.4. DLS Measurement
The hydrodynamic size distribution of the SHA series MNPs is characterized using a DLS Particle Tracking Analyzer (model: Microtac Nanoflex). 100 μL of the SHA series MNP suspension is diluted in 1.4 mL of deionized (DI) water, reaching a total sample volume of 1.5 mL of the mixture, followed by ultrasonication for 30 min before the DLS characterization.
4.5. TEM Analysis
The morphologies of these SHA series MNPs are characterized using a TEM system (FEI T12 120 kV). Each TEM sample is prepared by putting a droplet (∼10 μL) of the MNP suspension onto a TEM grid (copper mesh coated with an amorphous carbon film). These samples are ready for TEM characterization when the solutions are fully evaporated at room temperature in air.
4.6. Zeta Potential Measurement
A zeta potential analyzer (model: Stabino) is used to characterize the particle charge distribution or the zeta potential of the SHA series MNPs in DI water. 100 μL of SHA series MNPs is diluted in 4.9 mL of DI water, reaching a total sample volume of 5 mL, followed by ultrasonication for 30 min, and then used for zeta potential characterization. This particle charge characterization helps to analyze the surface binding capabilities of these SHA series MNPs.
4.7. MPS Measurement
The dynamic magnetic responses of SHA series MNPs are characterized using a homebuilt MPS system (see the schematic view and photographs of the MPS system in S2 and S3 from the Supporting Information). 200 μL of the SHA series MNP sample is sealed in a plastic vial (a maximum capacity of 300 μL). Two sets of copper coils are used to generate sinusoidal magnetic fields with tunable frequencies and magnitudes. One pair of differentially wound pickup coils (600 windings clockwise and 600 windings counter-clockwise) collects the induced voltage signals due to the dynamic magnetic responses of MNPs under driving magnetic fields. A laptop with LabVIEW controls the frequency and magnitude of the driving magnetic field through a data acquisition card (DAQ, NI USB-6289). The analog voltage signals are sent back from pickup coils to DAQ, sampled at 500 kHz, and converted to the frequency domain after discrete Fourier transform. For each MPS measurement, the MPS system runs for 10 s to collect the baseline signal (noise), followed by inserting the vial containing the MNP sample for another 10 s of signal (total) collection. The induced voltage due to dynamic magnetic responses of MNPs is recovered from the total signal by the phasor theory (see S4 from the Supporting Information). The higher harmonics specific to dynamic magnetic responses of MNPs are extracted for analysis (see S6 from the Supporting Information).
Acknowledgments
This study was financially supported by the Institute of Engineering in Medicine of the University of Minnesota through the FY18 IEM Seed Grant Funding Program. This study was also financially supported by the U.S. Department of Agriculture—National Institute of Food and Agriculture (NIFA) under Award Number 2020-67021-31956. Portions of this work were conducted in the Minnesota Nano Center, which is supported by the National Science Foundation through the National Nano Coordinated Infrastructure Network (NNCI) under Award Number ECCS-1542202. Portions of this work were carried out in the Characterization Facility, University of Minnesota, a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org) via the MRSEC program.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c05845.
Photographs of SHA series iron oxide nanoparticles; MPS system; MPS system setups; phasor theory; magnetic moment per gram of Fe; TEM images of SHA series MNPs captured at various magnifications; magnetic dynamic responses and higher harmonic models; Brownian and Néel relaxations; phase angles of higher harmonics monitored under a low-frequency driving field for SHA series samples; recorded real-time dynamic magnetic responses of SHA series MNPs under a low-frequency driving field; and phase angles of higher harmonics monitored under a high-frequency driving field for SHA series samples (PDF)
Author Contributions
⊥ These authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Crespo P.; de la Presa P.; Marin P.; Multigner M.; Alonso J. M.; Rivero G.; Yndurain F.; Gonzalez-Calbet J. M.; Hernando A. Magnetism in Nanoparticles: Tuning Properties with Coatings. J. Phys.: Condens. Matter 2013, 25, 484006. 10.1088/0953-8984/25/48/484006. [DOI] [PubMed] [Google Scholar]
- Albert E. L.; Che Abdullah C. A.; Shiroshaki Y. Synthesis and Characterization of Graphene Oxide Functionalized with Magnetic Nanoparticle via Simple Emulsion Method. Results Phys. 2018, 11, 944–950. 10.1016/j.rinp.2018.10.054. [DOI] [Google Scholar]
- Torchilin V. P.; Trubetskoy V. S. WHICH POLYMERS CAN MAKE NANOPARTICULATE DRUG CARRIERS LONG-CIRCULATING. Adv. Drug Delivery Rev. 1995, 16, 141–155. 10.1016/0169-409x(95)00022-y. [DOI] [Google Scholar]
- Xie J.; Xu C.; Kohler N.; Hou Y.; Sun S. Controlled PEGylation of Monodisperse Fe3O4 Nanoparticles for Reduced Non-Specific Uptake by Macrophage Cells. Adv. Mater. 2007, 19, 3163. 10.1002/adma.200701975. [DOI] [Google Scholar]
- Chen Y.; Ai K.; Liu J.; Sun G.; Yin Q.; Lu L. Multifunctional Envelope-Type Mesoporous Silica Nanoparticles for PH-Responsive Drug Delivery and Magnetic Resonance Imaging. Biomaterials 2015, 60, 111–120. 10.1016/j.biomaterials.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Xianyu Y.; Wang Y.; Zhang X.; Cha R.; Sun J.; Jiang X. One-Step Detection of Pathogens and Viruses: Combining Magnetic Relaxation Switching and Magnetic Separation. ACS Nano 2015, 9, 3184–3191. 10.1021/acsnano.5b00240. [DOI] [PubMed] [Google Scholar]
- Deatsch A. E.; Evans B. A. Heating Efficiency in Magnetic Nanoparticle Hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. 10.1016/j.jmmm.2013.11.006. [DOI] [Google Scholar]
- Su D.; Wu K.; Krishna V.; Klein T.; Liu J.; Feng Y.; Perez A. M.; Cheeran M. C.; Wang J.-P. Detection of Influenza a Virus in Swine Nasal Swab Samples With a Wash-Free Magnetic Bioassay and a Handheld Giant Magnetoresistance Sensing System. Front. Microbiol. 2019, 10, 1077. 10.3389/fmicb.2019.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K.; Klein T.; Krishna V. D.; Su D.; Perez A. M.; Wang J.-P. Portable GMR Handheld Platform for the Detection of Influenza A Virus. ACS Sens. 2017, 2, 1594–1601. 10.1021/acssensors.7b00432. [DOI] [PubMed] [Google Scholar]
- Aledealat K.; Mihajlović G.; Chen K.; Field M.; Sullivan G. J.; Xiong P.; Chase P. B.; von Molnár S. Dynamic Micro-Hall Detection of Superparamagnetic Beads in a Microfluidic Channel. J. Magn. Magn. Mater. 2010, 322, L69–L72. 10.1016/j.jmmm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M. Z.; Ma X.; Chen T.; Zhang L. e.; Ren W.; Xiang L.; Wu A. Silica-Coated Super-Paramagnetic Iron Oxide Nanoparticles (SPIONPs): A New Type Contrast Agent of T 1 Magnetic Resonance Imaging (MRI). J. Mater. Chem. B 2015, 3, 5172–5181. 10.1039/c5tb00300h. [DOI] [PubMed] [Google Scholar]
- Ma X.-H.; Gong A.; Xiang L.-C.; Chen T.-X.; Gao Y.-X.; Liang X.-J.; Shen Z.-Y.; Wu A.-G. Biocompatible Composite Nanoparticles with Large Longitudinal Relaxivity for Targeted Imaging and Early Diagnosis of Cancer. J. Mater. Chem. B 2013, 1, 3419–3428. 10.1039/c3tb20648c. [DOI] [PubMed] [Google Scholar]
- Wu K.; Su D.; Saha R.; Wong D.; Wang J.-P. Magnetic Particle Spectroscopy-Based Bioassays: Methods, Applications, Advances, and Future Opportunities. J. Phys. D: Appl. Phys. 2019, 52, 173001. 10.1088/1361-6463/ab03c0. [DOI] [Google Scholar]
- Mos Y. M.; Vermeulen A. C.; Buisman C. J. N.; Weijma J. X-Ray Diffraction of Iron Containing Samples: The Importance of a Suitable Configuration. Geomicrobiol. J. 2018, 35, 511–517. 10.1080/01490451.2017.1401183. [DOI] [Google Scholar]
- Wu K.; Tu L.; Su D.; Wang J.-P. Magnetic Dynamics of Ferrofluids: Mathematical Models and Experimental Investigations. J. Phys. D: Appl. Phys. 2017, 50, 085005. 10.1088/1361-6463/aa590b. [DOI] [Google Scholar]
- Shao D.; Zhang S.-L.; Zhao X.-H.; Wang X.-Y. Spin Canting, Metamagnetism, and Single-Chain Magnetic Behaviour in a Cyano-Bridged Homospin Iron (II) Compound. Chem. Commun. 2015, 51, 4360–4363. 10.1039/c4cc10003d. [DOI] [PubMed] [Google Scholar]
- Cao D.; Li H.; Pan L.; Li J.; Wang X.; Jing P.; Cheng X.; Wang W.; Wang J.; Liu Q. High Saturation Magnetization of γ-Fe 2 O 3 Nano-Particles by a Facile One-Step Synthesis Approach. Sci. Rep. 2016, 6, 32360. 10.1038/srep32360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P.; Kang L.; Chang T.; Yang F.; Wang H.; Zhang Y.; Yang J.; Wang K.-s.; Du J.; Yang Z. High Saturation Magnetization Fe3O4 Nanoparticles Prepared by One-Step Reduction Method in Autoclave. J. Alloys Compd. 2017, 728, 88–92. 10.1016/j.jallcom.2017.08.290. [DOI] [Google Scholar]
- Burnham P.; Dollahon N.; Li C. H.; Viescas A. J.; Papaefthymiou G. C. Magnetization and Specific Absorption Rate Studies of Ball-Milled Iron Oxide Nanoparticles for Biomedicine. J. Nanoparticles 2013, 2013, 1–3. 10.1155/2013/181820. [DOI] [Google Scholar]
- Khurshid H.; Shi Y.; Berwin B. L.; Weaver J. B. Evaluating Blood Clot Progression Using Magnetic Particle Spectroscopy. Med. Phys. 2018, 45, 3258–3263. 10.1002/mp.12983. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Reeves D. B.; Perreard I. M.; Kett W. C.; Griswold K. E.; Gimi B.; Weaver J. B. Molecular Sensing with Magnetic Nanoparticles Using Magnetic Spectroscopy of Nanoparticle Brownian Motion. Biosens. Bioelectron. 2013, 50, 441–446. 10.1016/j.bios.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreard I. M.; Reeves D. B.; Zhang X.; Kuehlert E.; Forauer E. R.; Weaver J. B. Temperature of the Magnetic Nanoparticle Microenvironment: Estimation from Relaxation Times. Phys. Med. Biol. 2014, 59, 1109. 10.1088/0031-9155/59/5/1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draack S.; Viereck T.; Kuhlmann C.; Schilling M.; Ludwig F. Temperature-Dependent MPS Measurements. Int. J. Magn. Part. Imag. 2017, 3, 1703018. [Google Scholar]
- Dieckhoff J.; Eberbeck D.; Schilling M.; Ludwig F. Magnetic-Field Dependence of Brownian and Néel Relaxation Times. J. Appl. Phys. 2016, 119, 043903. 10.1063/1.4940724. [DOI] [Google Scholar]
- Draack S.; Lucht N.; Remmer H.; Martens M.; Fischer B.; Schilling M.; Ludwig F.; Viereck T. Multiparametric Magnetic Particle Spectroscopy of CoFe2O4 Nanoparticles in Viscous Media. J. Phys. Chem. C 2019, 123, 6787–6801. 10.1021/acs.jpcc.8b10763. [DOI] [Google Scholar]
- Viereck T.; Draack S.; Schilling M.; Ludwig F. Multi-Spectral Magnetic Particle Spectroscopy for the Investigation of Particle Mixtures. J. Magn. Magn. Mater. 2019, 475, 647–651. 10.1016/j.jmmm.2018.11.021. [DOI] [Google Scholar]
- Wawrzik T.; Yoshida T.; Schilling M.; Ludwig F. Debye-Based Frequency-Domain Magnetization Model for Magnetic Nanoparticles in Magnetic Particle Spectroscopy. IEEE Trans. Magn. 2015, 51, 1–4. 10.1109/tmag.2014.2332371.26203196 [DOI] [Google Scholar]
- Ludwig F.; Heim E.; Schilling M. Characterization of Superparamagnetic Nanoparticles by Analyzing the Magnetization and Relaxation Dynamics Using Fluxgate Magnetometers. J. Appl. Phys. 2007, 101, 113909. 10.1063/1.2738416. [DOI] [Google Scholar]
- Ota S.; Matsugi Y.; Nakamura T.; Takeda R.; Takemura Y.; Kato I.; Nohara S.; Sasayama T.; Yoshida T.; Enpuku K. Effects of Size and Anisotropy of Magnetic Nanoparticles Associated with Dynamics of Easy Axis for Magnetic Particle Imaging. J. Magn. Magn. Mater. 2019, 474, 311–318. 10.1016/j.jmmm.2018.11.043. [DOI] [Google Scholar]
- Wu K.; Liu J.; Su D.; Saha R.; Wang J.-P. Magnetic Nanoparticle Relaxation Dynamics-Based Magnetic Particle Spectroscopy for Rapid and Wash-Free Molecular Sensing. ACS Appl. Mater. Interfaces 2019, 11, 22979–22986. 10.1021/acsami.9b05233. [DOI] [PubMed] [Google Scholar]
- Rauwerdink A. M.; Weaver J. B. Measurement of Molecular Binding Using the Brownian Motion of Magnetic Nanoparticle Probes. Appl. Phys. Lett. 2010, 96, 033702. 10.1063/1.3291063. [DOI] [Google Scholar]
- Meyer M. H. F.; Hartmann M.; Krause H.-J.; Blankenstein G.; Mueller-Chorus B.; Oster J.; Miethe P.; Keusgen M. CRP Determination Based on a Novel Magnetic Biosensor. Biosens. Bioelectron. 2007, 22, 973–979. 10.1016/j.bios.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Meyer M. H. F.; Krause H.-J.; Hartmann M.; Miethe P.; Oster J.; Keusgen M. Francisella Tularensis Detection Using Magnetic Labels and a Magnetic Biosensor Based on Frequency Mixing. J. Magn. Magn. Mater. 2007, 311, 259–263. 10.1016/j.jmmm.2006.10.1175. [DOI] [Google Scholar]
- Tu L.; Jing Y.; Li Y.; Wang J.-P. Real-Time Measurement of Brownian Relaxation of Magnetic Nanoparticles by a Mixing-Frequency Method. Appl. Phys. Lett. 2011, 98, 213702. 10.1063/1.3595273. [DOI] [Google Scholar]
- Wu K.; Schliep K.; Zhang X.; Liu J.; Ma B.; Wang J.-P. Characterizing Physical Properties of Superparamagnetic Nanoparticles in Liquid Phase Using Brownian Relaxation. Small 2017, 13, 1604135. 10.1002/smll.201604135. [DOI] [PubMed] [Google Scholar]
- Wu K.; Su D.; Saha R.; Liu J.; Wang J.-P. Investigating the Effect of Magnetic Dipole-Dipole Interaction on Magnetic Particle Spectroscopy (MPS): Implications for Magnetic Nanoparticle-Based Bioassays and Magnetic Particle Imaging (MPI). J. Phys. D: Appl. Phys. 2019, 52, 335002. 10.1088/1361-6463/ab2580. [DOI] [Google Scholar]
- Sánchez F. H.; Zélis P. M.; Arciniegas M.; Pasquevich G. A.; Van Raap M. F. Dipolar Interaction and Demagnetizing Effects in Magnetic Nanoparticle Dispersions: Introducing the Mean-Field Interacting Superparamagnet Model. Phys. Rev. B: Condens. Matter Mater. Phys. 2017, 95, 134421. 10.1103/physrevb.95.134421. [DOI] [Google Scholar]
- Ivanov A. O.; Zverev V. S.; Kantorovich S. S. Revealing the Signature of Dipolar Interactions in Dynamic Spectra of Polydisperse Magnetic Nanoparticles. Soft Matter 2016, 12, 3507–3513. 10.1039/c5sm02679b. [DOI] [PubMed] [Google Scholar]
- Wu K.; Liu J.; Wang Y.; Ye C.; Feng Y.; Wang J.-P. Superparamagnetic Nanoparticle-Based Viscosity Test. Appl. Phys. Lett. 2015, 107, 053701. 10.1063/1.4928057. [DOI] [Google Scholar]
- Weaver J. B.; Harding M.; Rauwerdink A. M.; Hansen E. W.. The Effect of Viscosity on the Phase of the Nanoparticle Magnetization Induced by a Harmonic Applied Field; International Society for Optics and Photonics, 2010; pp 762627–762628. [Google Scholar]
- Möddel M.; Meins C.; Dieckhoff J.; Knopp T. Viscosity Quantification Using Multi-Contrast Magnetic Particle Imaging. New J. Phys. 2018, 20, 083001. 10.1088/1367-2630/aad44b. [DOI] [Google Scholar]
- Utkur M.; Muslu Y.; Saritas E. U. Relaxation-Based Viscosity Mapping for Magnetic Particle Imaging. Phys. Med. Biol. 2017, 62, 3422. 10.1088/1361-6560/62/9/3422. [DOI] [PubMed] [Google Scholar]
- Wu K.; Ye C.; Liu J.; Wang Y.; Feng Y.; Wang J.-P. In Vitro Viscosity Measurement on Superparamagnetic Nanoparticle Suspensions. IEEE Trans. Magn. 2016, 52, 1. 10.1109/tmag.2016.2529426. [DOI] [Google Scholar]
- Rauwerdink A. M.; Hansen E. W.; Weaver J. B. Nanoparticle Temperature Estimation in Combined Ac and Dc Magnetic Fields. Phys. Med. Biol. 2009, 54, L51. 10.1088/0031-9155/54/19/l01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J. B.; Rauwerdink A. M.; Hansen E. W. Magnetic Nanoparticle Temperature Estimation. Med. Phys. 2009, 36, 1822–1829. 10.1118/1.3106342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J.; Paysen H.; Kosch O.; Trahms L.; Wiekhorst F. Temperature Dependence in Magnetic Particle Imaging. AIP Adv. 2018, 8, 056703. 10.1063/1.5004506. [DOI] [Google Scholar]
- Tu L.; Wu K.; Klein T.; Wang J.-P. Magnetic Nanoparticles Colourization by a Mixing-Frequency Method. J. Phys. D: Appl. Phys. 2014, 47, 155001. 10.1088/0022-3727/47/15/155001. [DOI] [Google Scholar]
- Kötitz R.; Weitschies W.; Trahms L.; Brewer W.; Semmler W. Determination of the Binding Reaction between Avidin and Biotin by Relaxation Measurements of Magnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 194, 62–68. 10.1016/s0304-8853(98)00580-0. [DOI] [Google Scholar]
- Torres-Diaz I.; Cortes A.; Cedeño-Mattei Y.; Perales-Perez O.; Rinaldi C. Flows and Torques in Brownian Ferrofluids Subjected to Rotating Uniform Magnetic Fields in a Cylindrical and Annular Geometry. Phys. Fluids 2014, 26, 012004. 10.1063/1.4863201. [DOI] [Google Scholar]
- Krause H.-J.; Wolters N.; Zhang Y.; Offenhäusser A.; Miethe P.; Meyer M. H. F.; Hartmann M.; Keusgen M. Magnetic Particle Detection by Frequency Mixing for Immunoassay Applications. J. Magn. Magn. Mater. 2007, 311, 436–444. 10.1016/j.jmmm.2006.10.1164. [DOI] [Google Scholar]
- Nikitin P. I.; Vetoshko P. M.; Ksenevich T. I. New Type of Biosensor Based on Magnetic Nanoparticle Detection. J. Magn. Magn. Mater. 2007, 311, 445–449. 10.1016/j.jmmm.2006.10.1180. [DOI] [Google Scholar]
- Achtsnicht S.; Pourshahidi A. M.; Offenhäusser A.; Krause H.-J. Multiplex Detection of Different Magnetic Beads Using Frequency Scanning in Magnetic Frequency Mixing Technique. Sensors 2019, 19, 2599. 10.3390/s19112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-F.; Wang Y.-F.; Yan X.-P. Amine-Functionalized Magnetic Nanoparticles for Rapid Capture and Removal of Bacterial Pathogens. Environ. Sci. Technol. 2010, 44, 7908–7913. 10.1021/es102285n. [DOI] [PubMed] [Google Scholar]
- Wu K.; Liu J.; Saha R.; Su D.; Krishna V. D.; Cheeran M. C.-J.; Wang J.-P. Magnetic Particle Spectroscopy for Detection of Influenza A Virus Subtype H1N1. ACS Appl. Mater. Interfaces 2020, 12, 13686–13697. 10.1021/acsami.0c00815. [DOI] [PubMed] [Google Scholar]
- Gordon-Wylie S. W.; Ness D. B.; Shi Y.; Mirza S. K.; Paulsen K. D.; Weaver J. B. Measuring Protein Biomarker Concentrations Using Antibody Tagged Magnetic Nanoparticles. Biomed. Phys. Eng. Express 2020, 6, 065025. 10.1088/2057-1976/abc45b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Jyoti D.; Gordon-Wylie S. W.; Weaver J. B. Quantification of Magnetic Nanoparticles by Compensating for Multiple Environment Changes Simultaneously. Nanoscale 2020, 12, 195–200. 10.1039/c9nr08258a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtsnicht S.; Neuendorf C.; Faßbender T.; Nölke G.; Offenhäusser A.; Krause H.-J.; Schröper F. Sensitive and Rapid Detection of Cholera Toxin Subunit B Using Magnetic Frequency Mixing Detection. PLoS One 2019, 14, e0219356 10.1371/journal.pone.0219356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabehi A.; Garlan B.; Achtsnicht S.; Krause H.-J.; Offenhäusser A.; Ngo K.; Neveu S.; Graff-Dubois S.; Kokabi H. Magnetic Detection Structure for Lab-on-Chip Applications Based on the Frequency Mixing Technique. Sensors 2018, 18, 1747. 10.3390/s18061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J.; Lucht N.; Hankiewicz B.; Schilling M.; Ludwig F. Magnetic Field Orientation Dependent Dynamic Susceptibility and Brownian Relaxation Time of Magnetic Nanoparticles. Appl. Phys. Lett. 2019, 115, 133102. 10.1063/1.5120609. [DOI] [Google Scholar]
- Maldonado-Camargo L.; Torres-Díaz I.; Chiu-Lam A.; Hernández M.; Rinaldi C. Estimating the Contribution of Brownian and Néel Relaxation in a Magnetic Fluid through Dynamic Magnetic Susceptibility Measurements. J. Magn. Magn. Mater. 2016, 412, 223–233. 10.1016/j.jmmm.2016.03.087. [DOI] [Google Scholar]
- Shi Y.; Weaver J. B. Concurrent Quantification of Magnetic Nanoparticles Temperature and Relaxation Time. Med. Phys. 2019, 46, 4070–4076. 10.1002/mp.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu I.; Natividad E.; Solozábal L.; Roubeau O. Nano-Objects for Addressing the Control of Nanoparticle Arrangement and Performance in Magnetic Hyperthermia. ACS Nano 2015, 9, 1408–1419. 10.1021/nn505781f. [DOI] [PubMed] [Google Scholar]
- Bañobre-López M.; Teijeiro A.; Rivas J. Magnetic Nanoparticle-Based Hyperthermia for Cancer Treatment. Rep. Practical Oncol. Radiother. 2013, 18, 397–400. 10.1016/j.rpor.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Andujar C.; Teran F.; Ortega D.. Current Outlook and Perspectives on Nanoparticle-Mediated Magnetic Hyperthermia. Iron Oxide Nanoparticles for Biomedical Applications; Elsevier, 2018; pp 197–245. [Google Scholar]
- Martinez-Boubeta C.; Simeonidis K.; Makridis A.; Angelakeris M.; Iglesias O.; Guardia P.; Cabot A.; Yedra L.; Estradé S.; Peiró F. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. 10.1038/srep01652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdaoui B.; Meffre A.; Carrey J.; Lachaize S.; Lacroix L.-M.; Gougeon M.; Chaudret B.; Respaud M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. 10.1002/adfm.201101243. [DOI] [Google Scholar]
- Sun C.; Lee J. S. H.; Zhang M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Delivery Rev. 2008, 60, 1252–1265. 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.