Abstract

Dengue and Zika are two mosquito-borne diseases of great impact on public health around the world in tropical and subtropical countries. DENV and ZIKV belong to the Flaviviridae family and the Flavivirus genus. Currently, there are no effective therapeutic agents to treat or prevent these pathologies. The main objective of this work was to evaluate potential inhibitors from active compounds obtained from Marcetia taxifolia by performing inverse molecular docking on ZIKV-NS3-helicase and ZIKV-NS5-RNA polymerase as targets. This computational strategy is based on renormalizing the binding scores of the compounds to these two proteins, allowing a direct comparison of the results across the proteins. The crystallographic structures of the ZIKV-NS3-helicase and ZIKV-NS5-RNA-polymerase proteins share a great similarity with DENV homologous proteins. The P-loop active site of the crystallographic structure of ZIKV-NS3-helicase presents a high percentage of homology with the four dengue serotypes. It was found that most ligands of the active compounds (5,3’-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (5DP); 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (5HH); myricetin-3-O-rhamnoside (M3OR)) from Marcetia taxifolia had a better affinity for ZIKV-NS3-helicase than for ZIKV-NS5-RNA polymerase, as indicated by the negative multiple active site correction (MASC) score, except for M3RG that showed a higher affinity for ZIKV-NS5-RNA polymerase. On the other hand, the AutoDock Vina scores showed that M3OR had the highest score value (−9.60 kcal/mol) and the highest normalized score (1.13) against ZIKV-NS3-helicase. These results in silico demonstrated that the nonstructural proteins NS3-helicase and NS5-RNA polymerase, which share similar molecular structures between the selected viruses, could become therapeutic targets for some bioactive compounds derived from Marcetia taxifolia.
Introduction
Four families belong to the arthropod-borne arbovirus group: the Togaviridae, Reoviridae, Flaviviridae, and Bunyaviridae families share one main characteristic, which is having a cycle where reservoir hosts are transmitted between vertebrates directly through arthropod vectors. They are responsible for the emerging and re-emerging diseases worldwide, with a remarkable incidence in recent decades.1,2
Vector-borne diseases represent more than 17% of all infectious diseases occasioning more than 700,000 deaths each year. This generates serious health issues and loss of quality of life in a large segment of our population leading to a strong negative impact at a social and economic level. The Pan American Health Organization classifies these diseases based on frequency and prevalence, leading to the following ranking: first dengue, spread in almost all of the countries of the Americas; second Chikungunya; third Zika followed by malaria, Chagas, leishmaniosis, and yellow fever (YFV).3
The dengue (DENV) and Zika (ZIKV) virus belong to the Flavivirus genus and share various characteristics. They have a length of 40–60 nm and are enveloped with an icosaedric nucleocapsid. These viruses present a single-strand positive-sense RNA of about 11,000 bases, with a unique open reading frame (ORF) that contains 3400 codons, which encode for a unique viral polyprotein presenting a type I cap in the 5′-terminal.4,5 Both viruses are closely related, with an amino acid (AA) sequence identity ranging from 55.1 to 56.3%.6 Consequently, the emerging literature shows similarities between these two viruses related to their interactions with the host innate and adaptive immune response. For DENV and ZIKV, the interferon plays a central role in inhibiting viral replication. For both viruses, the nonstructural proteins (NS) coordinate intracellular aspects of the viral cycle such as replication, assembly, proteolysis, maturation, and regulation of the host immune response.7
On the other hand, the treatment of these arboviruses is palliative since at the moment there are no medicines that show specific antiviral activity. Among the medicines usually prescribed are acetaminophen and nonsteroidal anti-inflammatories, which can lead to hemorrhages and internal bleeding.8 It is therefore necessary to develop effective therapeutic strategies combining high specificity and low costs that would contribute to the improvement of the quality of life of the patients. Natural plant extracts such as Marcetia taxifolia, a species from the Melastomataceae family, were evaluated as part of the effort to search for alternative therapies.
Melastomataceae is a diverse family of common plants, and these are abundant in tropical regions and mountain areas such as the ones found in Colombia, Venezuela, southeast Asia, and south of China.9 The family is composed of 166 genera and 3 subfamilies: Astronioideae, Melastomatoideae, and Memecyloideae, which are characterized as shrubs, epiphytic plants, vine, and annual and perennial herbs.10 Many of these plants have been used in treatments for skin diseases, dysentery, diarrhea, leukorrhea, or gum irritation, among others.11,12 While they are not extensively studied, compounds such as polyphenols, flavonoid, terpenes, and cyanogenic compounds could be extracted.13
Marcetia is a neotropical genus with more than 40 species described. Among them, the species M. taxifolia can be found in the Venezuelan Coastal Range, in the north of the Andes, and the Guiana Shield. Samples collected from this species contain flavonoids that are related to myrcetin, which was reported to have antiviral activity.14,15 However, few studies are related to the antiviral activity of the compounds present in this genus,13,16 and most of them were pioneered by our group headed by Dr. Suarez, who evaluated the antiviral effect of the flavonoids isolated from the aerial part of M. taxifolia against Hepatitis B virus (VHB), Herpes Simplex tipo 1 virus (HSV-1), and Poliovirus type 1 (PV-1). It was found that myricetin ramnoside (MyrG), myricetin3-α-O-ramnosil (1 → 6)-α-galactoside (MyrGG), 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (5DP), and 5-hydroxy-3,6,7,3′,4′-pentamethoxyflavone (PMF-OH) exhibited antiviral activities without cytotoxic effects. Methoxyflavones 5DP and PMF-OH were the most active of these compounds, showing an antiviral effect against all the tested virus.17 While recent studies showed that myrcetin inhibits the activity of the inverse transcriptase of HIV-1,13,18 studies about the antiviral activity of glycosylated flavonoids derived from M. taxifolia are still needed. As such, this work is part of a larger ongoing effort aiming at measuring experimentally the activities of these flavonoids against Zika and dengue virus.
To explore potential ligands for drug discovery purposes, a ligand-protein inverse docking in silico technique called INVDOCK was previously developed that identifies proteins presenting bioactivities to the same molecular compound.19 In other words, this methodology maps a series of compounds to different protein binding pockets to find the best overall compound, which then acts as a common inhibitor to several proteins. This, in turn, allows us to study molecular recognition and to design new bioactive compounds.20 In this work, we propose to evaluate the susceptibility of the Zika virus to chemical compounds derived from the M. taxifolia plant due to its wide distribution in South America, particularly in Venezuela and Colombia. For this purpose, a series of compounds with affinity to NS proteins of ZIKV and DENV were identified (Figure 1). The synthetic compounds are well-known ligands taken from DENV4-NS3-helicase (2JLR), DENV2-NS5 (PDB id 5K5M), and DENV3-NS5 (PDB id 5HMY) polymerase, while the natural compounds come from extracts of the species M. taxifolia. We compared the results obtained from the inverse molecular docking of these compounds performed on ZIKV-NS3-helicase (5JMT) and of ZIKV-NS5-RNA (5U04) polymerase. These results should potentially be relevant by homology to the DENV and can be the focus of other studies.
Figure 1.
Structures of the extracted ligands from both crystallographic structures and M. taxifolia selected for the docking analysis.
Results and Discussion
Comparison of NS3-Helicase and NS5-RNA Polymerase Proteins of ZIKA Virus with Their Equivalents of the Dengue Virus
The helicase (PDB id 5JMT) and polymerase (PDB id 5U04) are pivotal enzymes in the replication process of this kind of virus; therefore, inhibition or interference in their activities will impact negatively the viral replication process.
While both proteins belong to ZIKV, they were strategically chosen to propose a multitarget active ligand that can act against both ZIKV and DENV. This is made possible by the high similitude of the active sites (priming loops also called the P-loop) of these two proteins in both DENV and ZIKV.
The P-loop of the NS3-helicase belonging to the ZIKV (PDB id 5JMT) presents high similitude (Figure 2A) to DENV4-NS3-helicase (PDB id 2JLR). The active sites of the NS3-helicase of both viruses were previously described by Tian el al.21 The active site of NS3-helicase from ZIKV is composed of K200, T201, R202, D285, E286, Q455, R459, and R462 amino acids, while the active site of the protein from DENV comprises K199, T200, K201, D284, Q456, and R463 amino acids.
Figure 2.
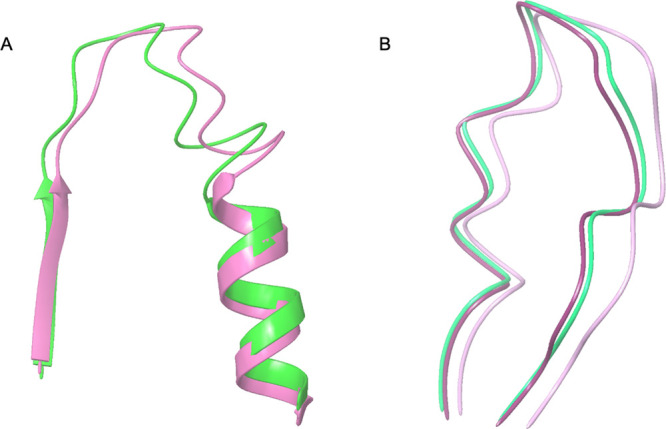
(A) Structural comparison between the P-loops of ZIKV-NS3-helicase (5JMT) in pink and DENV4-NS3-helicase (2JLR) in green. (B) Structural comparison between the P-loops of the ZIKV-NS5-polymerase (5U04) in light purple, DENV2-NS5-polymerase (5K5M) in purple, and DENV3-NS5-polymerase (5HMY) in blue green.
The complete P-loop active sites of ZIKV-NS3 and DENV4-NS3 can be observed in their crystallographic structure (Figure 2A) and in the BLAST sequence alignment (Figure 3) between amino acids D193 and R202. While the high percentage of homology (Table 1) between ZIKV-NS3-helicase (5JMT) and DENV1 (84%), DENV2 (84%), DENV3 (81%), DENV4 (82%), and DENV4-2JLR (82%) was expected, the high sequence identity (69.4%) is more remarkable and is in line with previous results in which NS3 shows approximately 65% sequence identity between DENV, ZIKV, and YFV.22 These findings allow us to establish NS3 as a pharmacological target against these viruses.
Figure 3.
BLAST sequence alignments of NS3-helicase of Zika (5JMT), dengue 4 (2JLR), and of four dengue serotypes DENV1 to DENV4. A total of 450 residues are aligned with the red rectangular markers corresponding to the active site P-loop.
Table 1. Percentage of Identity, Similarity, and Homology of NS3-Helicase and NS5-RNA Polymerase from Zika Virus with the Four Serotypes of Dengue Virusa.
| ZIKV | DENV1 | DENV2 | DENV2 (5K5M) | DENV3 | DENV3 (5HMY) | DENV4 | DENV4 (2JLR) | |
|---|---|---|---|---|---|---|---|---|
| identity | 100 (100) | 69 (51) | 71 (51) | (56) | 69 (51) | (54) | 69 (51) | 69 |
| similarity | 100 (100) | 83 (60) | 83 (60) | (65) | 81 (60) | (63) | 83 (61) | 83 |
| homology | 100 (100) | 84 (59) | 84 (59) | (65) | 81 (59) | (63) | 82 (59) | 82 |
Values for NS3 are in plain text, and the ones for NS5 are in parentheses.
On the other hand, the ZIKV-NS5-RNA polymerase (5U04) also presents a high structural similitude (Figure 2B) with the homolog proteins DENV2-NS5 (NS5-dengue serotype 2, PDB id 5K5M) and DENV3-NS5 (NS5-dengue serotype 3, PDB id 5HMY)23 belonging to the DENV. The active site (P-loop) of the ZIKV-NS5-RNA was previously described by Godoy et al.23 and covers the G793, G803, E804, G801, and K802 amino acids, while the active sites of DENV2-NS5 and DENV3-NS5 involve the amino acids S791, H801, E802, A799, and K800, and S791, H801, Q802, A799, and H800, respectively. It should be noted that the sequences in P-loops, which are critical for NTP binding and catalysis of helicases and proteases from different Flavivirus, present a high percentage of conserved amino acids.21
Considering the BLAST alignment (Figure 4), we can locate the complete P-loop active sites of ZIKV-NS5 and DENV-NS5 in both crystallographic structures and BLAST sequence proteins as the residues between G793 and E804 considering ZIKV-NS5 as the query. Compared to the high percentage of homology observed for the NS3 proteins in the result above, sequences of the NS5 proteins showed lower values: DENV1 (59%), DENV2 (59%), DENV2-5K5M (65%), DENV3 (59%), DENV3-5HMY (63%), and DENV4 (59%). In previous studies, NS5 is considered to be a highly conserved protein among Flavivirus, with a homology of about 68% between DENV, ZIKV, and YFV,22,24 which is consistent with our results between DENV and ZIKV, albeit with a somewhat lower average homology percentage of 60.6%.
Figure 4.
BLAST sequence alignments of NS5-RNA polymerase of Zika (5U04), dengue 2 (5K5M), dengue 3 (5HMY), and of the 4 serotypes DENV1 to DENV4 of dengue virus. A total of 410 residues are aligned with the red rectangular markers corresponding to the active site P-loop.
According to the chemical compounds reported by Baptista et al., four methoxyflavone compounds myricetin-3-O-rhamnoside (M3OR), 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone (5DP), 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone (5HH), and myricetin 3-rhamnosyl(1 → 6)galactoside (M3RG) derived from the M. taxifolia plant were evaluated as ligands. We also studied the synthetic compounds phosphoaminophosphonicacid-adenilate ester (AMP-PNP), 5-[5-(3-hydroxyprop-1-yn-1-yl)thiophen-2-yl]-2,4-dimethoxy-N-[(3-methoxyphenyl)sulfonyl] benzamide (68T), and 2,20-(5-(5-(3-hydroxyprop-1-yn-1-yl)thiophen-2-yl)-1,3-phenylene)diacetic acid (LNY) taken from the crystallographic protein structures of the dengue virus previously described (DENV4-NS3-helicase, DENV2-NS5, and DENV3-NS5-polymerase). Abbreviations and codes of these compounds used for docking are indicated in Table 2. For the inverse docking, the structures of the synthetic ligands (AMP-PNP, 68T, and LNY) were taken from the crystallographic protein structures of the dengue virus, while the crystallographic structures of the protein active site were taken from the Zika virus.13
Table 2. Abbreviations and Codes of the Compounds Used for Docking.
| name | abbreviation | code/reference/source |
|---|---|---|
| myricetin-3-O-rhamnoside | M3OR | PubChem 5281673 |
| 5,3′-dihydroxy-3,6,7,8,4′-pentamethoxyflavone | 5DP | PubChem 369954 |
| 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone | 5HH | PubChem 136417 |
| myricetin 3-rhamnosyl(1→6)galactoside | M3RG | refs (25, 40) |
| phosphoaminophosphonicacid-adenilate ester | AMP | CAS 25612-73-1 |
| 5-[5-(3-hydroxyprop-1-yn-1-yl)thiophen-2-yl]-2,4-dimethoxy-N-[(3-methoxyphenyl)sulfonyl]benzamide | 68T | PubChem 121232415 |
| 2,20-(5-(5-(3-hydroxyprop-1-yn-1-yl)thiophen-2-yl)-1,3-phenylene)diacetic acid | LNY | PDB: www.rcsb.org/ligand/LNY |
Binding Modes and Molecular Interactions from the Docking Simulations
The root-mean-square deviation RMSD between the docked conformation of AMP-PNP in ZIKV-NS3 helicase and the crystal structure in the DENV4-NS3 helicase was 5.57 Å for the alpha carbons. The RMSD value and the pose obtained from the docking are consistent with the conformation of AMP-PNP in the DENV4-NS3 crystallographic structure (Figure 5). Importantly the phosphate groups and the aromatic rings in the docking pose were in the same direction as in the crystallographic structure. The interactions between AMP-PNP and ZIKV-NS3 are shown in Figure 5 where the AMP-PNP ligand fits snugly inside the P-loop with the phosphate group interacting with the R462 and the ribose group with R202 through the H-bond, which are maintained from the crystal structure. The purine ring also forms a π–cation interaction with R462.
Figure 5.

Crystal structure (2JLR) of AMP-PNP (blue) in DENV4-NS3-helicase (gold) superimposed to the docking poses of AMP (green) in the ZIKV-NS3-helicase (light blue) crystal structure (5JMT).
Figure 7A shows the docking result for the LNY ligand in ZIKV-NS5-RNA (5U04), where the ligand appears to be in a different pocket from the one it occupies in the crystal structure of DENV3-NS5-RNA (5HMY), which results in an RMSD of 11.6 Å between the two. The same is also observed with the ligand 68T (Figure 7B) for which the docking pose in ZIKV-NS5-RNA (5U04) is located in a pocket next to the P-loop compared to the ligand in the X-ray structure of DENV2-NS5-RNA (5HMY), resulting in an RMSD of 14.42 Å. An analysis of the surface charge distribution between DENV3-NS5, DENV2-NS5, and ZIKV-NS5-RNA polymerases shows a crucial difference in terms of the space available in the P-loop,23 with the binding site of ZIKV-NS5-RNA being noticeably smaller. As a consequence, the ligands extracted from the crystal structures of DENV3-NS5 (5HMY) and DENV2-NS5 (5K5M) were not able to fit in the smaller active site of ZIKV-NS5-RNA (5U04). The above is the reason for the difference observed between the X-ray conformation of the ligands and the conformations obtained from the docking simulation. Even though LNY and 68T predicted under our docking parameters were occupying the opposite position in the P-loop, both of them still presented interactions with the residues previously determined as the active ones;23 68T interacts with residues G801, G803, and W805, while LNY interacts with G801, L802, G803, and W805.
Figure 7.
(A) Predicted binding pose of LNY (the green ligand on the left) in ZIKV-NS5-RNA polymerase (5U04, in red) compared to its crystal structure conformation (the green ligand on the right) in DENV3-NS5-RNA polymerase (5HMY, in white). (B) Predicted binding pose for 68T (the green ligand on the left) in ZIKV-NS5-RNA polymerase (5U04, in red) compared to its crystal conformation (the green ligand on the right) in DENV2-NS5-RNA polymerase (5K5M, in white).
While the binding modes were not adequately reproduced for ZIKV-NS5-RNA polymerase due to the size difference in the active sites of the DENV analog structures, they were well reproduced in the case of the ZIKV-NS3 helicase.
Docking Scores and Binding Affinities
The affinities of the ligands for the two Zika and dengue receptors were calculated using a normalized score devised by Lauro et al.26,27 and a multiple active site correction (MASC) score by Vigers and Rizzi,28 both of which are described in the Methodology section below (see Table 3).
Table 3. AutoDock Vina (Vo) and Normalized (V) Scores (kcal mol–1) for the Four Ligands from the Natural Extracts of the Species M. taxifolia and Three Known Ligands against Both Nonstructural Protein NS3-Helicasea.
| receptor
(ZIK) |
receptor
(DENV4) |
receptor
(DENV2) |
receptor
(DENV3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NS3-helicase |
NS5-RNA
polym. |
NS3-helicase
(2JLR) |
NS5-RNA
polym. (5K5M) |
NS5-RNA
polym. (5HMY) |
|||||||
| ligand | Vo | V | Vo | V | Vo | V | Vo | V | Vo | V | ML |
| 5DP | –8.17 | 1.08 | –6.20 | 0.91 | –5.90 | 0.88 | –6.80 | 0.97 | –6.90 | 0.97 | –6.79 |
| 5HH | –8.10 | 1.10 | –6.15 | 0.92 | –5.60 | 0.85 | –5.90 | 0.87 | –6.50 | 0.93 | s–6.45 |
| M3OR | –9.60 | 1.17 | –7.78 | 1.04 | –7.10 | 0.96 | –7.80 | 1.02 | –8.30 | 1.06 | –8.12 |
| M3RG | –7.81 | 0.97 | –7.97 | 1.09 | –7.50 | 1.04 | –7.50 | 1.00 | –8.30 | 1.08 | –7.82 |
| LNY | –7.95 | 1.05 | –6.72 | 0.98 | –5.80 | 0.86 | –6.90 | 0.98 | –7.10 | 0.99 | –6.90 |
| 68T | –7.44 | 0.94 | –6.84 | 0.95 | –6.80 | 0.95 | –8.50 | 1.15 | –8.40 | 1.11 | –7.60 |
| AMP | –9.02 | 1.15 | –6.37 | 0.89 | –8.00 | 1.14 | –6.70 | 0.92 | –7.00 | 0.94 | –7.42 |
| MR | –8.30 | –6.86 | –6.67 | –7.16 | –7.50 | ||||||
The interest in the normalized score is to allow the comparison of the results across the different proteins. Here, we find that the affinities of the synthetic ligands (LNY, AMP-PNP, and 68T) are higher for ZIKV-NS3-helicase than for ZIKV-NS5-RNA polymerase, as indicated by their higher V scores, except for 68T that shows the same V score for ZIKV-NS3-helicase (V = 0.96) and for ZIKV-NS5-RNA polymerase (V = 0.98). In the case of the ligands from M. taxifolia, 5DP, 5HH, and M3OR have a higher affinity for ZIKV-NS3-helicase than for ZIKV-NS5-RNA polymerase, while only M3RG has a higher affinity for ZIKV-NS5-RNA polymerase than for ZIKV-NS3-helicase. We can observe that NS5-RNA polymerases from Zika and Dengue show very consistent V scores for the different ligands, confirming at a structural level the similarity observed in the sequence of the P-loops, which are critical for NTP binding and catalysis of helicases and proteases.
Table 4 shows that a negative MASC score is obtained for most of the ligands (whether extracted from the crystallographic structure or the natural compounds) with ZIKV-NS3-helicase, indicating a higher affinity for this protein than for ZIKV-NS5-RNA. Only M3RG shows a higher affinity for ZIKV-NS5-RNA polymerase than for ZIKV-NS3-helicase. The results obtained for the MASC score are therefore consistent with the normalized V score, with the exception of 68T, which goes from showing the same affinity for both proteins to having a higher affinity for ZIKV-NS3-helicase. When looking at all the docking results (including those obtained for the DENV receptors), ZIK-NS3-helicase is the protein that shows the highest affinity overall when compared to ZIK-NS5-RNA polymerase, DEN-NS3-helicase, and DEN-NS5-RNA polymerase. These results indicate that ZIKV-NS3-helicase is the best target for these potential ligands.
Table 4. AutoDock Vina (Sij) and Modified* (Sij′) Scores (kcal mol–1) for the Four Ligands from the Natural Extracts of the Species M. taxifolia and for Three Known Ligands against Both Nonstructural Proteins NS3-Helicase and NS5-RNS Polymerase of the Zika and Dengue Virusa.
| receptor
(ZIK) |
receptor
(DEN) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS3-helicase |
NS5-RNA
polym. |
NS3-helicase |
NS5-RNA
polym. |
NS5-RNA
polym. |
||||||||
| Ligand | Sij | Sij′ | Sij | Sij′ | Sij | Sij′ | Sij | Sij′ | Sij | Sij′ | μi | σi |
| 5DP | –8.17 | –1.57 | –6.20 | 0.68 | –5.90 | 1.02 | –6.80 | –0.01 | –6.90 | –0.12 | –6.794 | 0.874 |
| 5HH | –8.10 | –1.68 | –6.15 | 0.31 | –5.60 | 0.87 | –5.90 | 0.56 | –6.50 | –0.05 | –6.450 | 0.980 |
| M3OR | –9.60 | –1.59 | –7.78 | 0.36 | –7.10 | 1.09 | –7.80 | 0.34 | –8.30 | –0.20 | –8.116 | 0.933 |
| M3RG | –7.81 | 0.02 | –7.97 | –0.46 | –7.50 | 0.93 | –7.50 | 0.93 | –8.30 | –1.43 | –7.816 | 0.338 |
| LNY | –7.95 | –1.37 | –6.72 | 0.23 | –5.80 | 1.42 | –6.90 | –0.01 | –7.10 | –0.27 | –6.894 | 0.772 |
| 68T | –7.44 | 0.19 | –6.84 | 0.92 | –6.80 | 0.97 | –8.50 | –1.10 | –8.40 | –0.98 | –7.596 | 0.821 |
| AMP | –9.02 | –1.48 | –6.37 | 0.97 | –8.00 | –0.54 | –6.70 | 0.66 | –7.00 | 0.39 | –7.418 | 1.083 |
When considering the best ligand, M3OR presents the highest normalized score (1.17) obtained when binding to ZIKV-NS3-helicase. It is followed closely by AMP also binding to ZIKV-NS3-helicase and 68T binding to DENV2-NS5-RNA polymerase (both with 1.15).
The interaction diagram of M3OR is shown in Figure 8. As was previously reported, there are interactions with the residues Q455, R459, R462, K200, T201, R202, and E286. This high affinity is explained by the hydrogen bonds between the residues A198, G199, K200, R462, and E231 with the oxygen groups of the molecule and the π–cation between the residue K200 and the aromatic ring of the molecule. Specifically, the π–cation interaction is between the R-group (NH3+) of the Lys residue and the aromatic ring of M3OR.
Figure 8.
Ligand interaction diagram for the poses (A) AMP-PNP in ZIKV-NS3-helicase and (B) M3OR with ZIKV-NS3-helicase (5JMT).
When considering the MASC score, the highest affinity is obtained between 5HH and ZIKV-NS3-helicase (−1.68) followed by M3OR (−1.59) and 5DP (−1.57) also with ZIKV-NS3-helicase. Whether using the Lauro V score or the MASC score, these results show that the natural ligands from M. taxifolia tend to have a similar or even a higher affinity than the known synthetic ligands.
Both NS3-helicase and NS5-RNA polymerase are pivotal enzymes in the replication process of these viruses, and the results of the docking simulations clearly indicate that these two enzymes are good candidates for a ligand that could target both viruses at the same time.
NS3 is responsible for cleaving the viral polyprotein at different cleavage sites and delivering mature NS proteins and a carboxyl-terminal domain that hold an RNA triphosphatase, an RNA helicase, and an RNA stimulating NTPase domain, all of which are essential for both virus replication and RNA synthesis.29 The serine protease domain of NS3 is essential in the life cycle of DENV.
On the other hand, the protein NS5 is the largest protein and is strongly conserved among the Flavivirus, with a homology of about 68% between DENV, ZIKV, and YFV.22,24 It comprises two domains: the methyltransferase domain CAPs of the viral RNA and the RNA-dependent RNA polymerase domain, which initiate viral RNA synthesis de novo. The NS5 protein is a potent IFN antagonist, which helps flaviviruses to evade the host innate immunity by either suppressing the JACK-STAT signaling pathway or modulating RNA splicing within the host cell.30 This protein may also be related to the modulation of cytokine gene expression since the NS5 protein has been mostly found in the nucleus in DENV-infected cells.31 It was because of its high conservation degree and its critical role during viral replication and evasion of the host immune system that NS5 was chosen as a target for the development of antivirals against Flavivirus infections.31,32
Conclusions
The NS3-helicase and NS5-RNA polymerase are pivotal enzymes in the replication process of the Zika and Dengue viruses, and as such, they were used as targets in an effort to determine potential inhibitors. Molecular docking simulations of a series of both synthetic and natural compounds were performed, the latter series coming from the M. taxifolia plant. We used two different scoring procedures as part of an inverse-docking protocol to determine which of the ZIKV-NS3-helicase or ZIKV-NS5-RNA-polymerase was the best target protein for each series of ligands. It was found that ZIKV-NS3-helicase presents a higher affinity than ZIKV-NS5-RNA polymerase for five of the seven ligands when using the Lauro renormalized scores and for six of the seven ligands when considering the MASC score. More specifically, in the case of the natural extracts, the ligands 5DP, 5HH, and M3OR have a better affinity for ZIKV-NS3-helicase than for ZIKV-NS5-RNA polymerase and only M3RG presents a higher affinity for ZIKV-NS5-RNA polymerase. Since ZIK-NS3-helicase is an enzyme that performs various functions critical to the replication of the virus, and given its higher affinity to most of the inhibitors studied in this work, the calculations performed in this study suggest that this enzyme could be a potential pharmacological target for the development of new bioactive compounds over ZIKV-NS5-RNA polymerase.
Methodology
Inverse Docking
The inverse docking technique can target several receptors with selected small molecules to obtain a multitarget mode.33 In our case, the method allows us to determine which of the two ZIKV receptors NS3-helicase and NS5-RNA polymerase is the best with respect to a specific ligand.
The different energy scores obtained from AutoDock Vina for the ligands using both protein crystallographic structures (5JMT for NS3-helicase and 5U04 for NS5-RNA polymerase) were normalized using the methodology previously devised by Lauro et al.26,27 and also described by Eriksson et al.33 considering eq 1
| 1 |
where V is the normalized score of each ligand for a given receptor, V0 is the original docking score obtained by the molecular docking calculations in AutoDock Vina, ML is the average binding energy score of each ligand across both targets, and MR is the average binding energy of each receptor with all ligands. Each term is in kcal mol–1. V is a mathematical term that allows us to determine how promising the interaction between a ligand is with its target with higher V indicating a more promising interaction.
The second correction used in the methodology is the MASC term, which is composed of the three equations (eqs 2–4). This term is used to determine how far apart a value is from the average, with a more negative value being indicative of a better interaction score between the ligands and the protein.
| 2 |
| 3 |
| 4 |
where Sij is the same value of V0, the original docking score calculated for the ith ligand and jth protein (in kcal mol–1) and Sij′ is the MASC score for compound i in active site j. μi and σi are, respectively, the averages and the standard deviation of the binding energies for the ligand i across the proteins j.
Sequence Alignment
In order to compare the active sites (P-loops) of the crystallographic structures of ZIKV-NS3 and DENV4-NS3 helicases, we aligned and superimposed them using the Schrödinger graphical interface Maestro.34 FASTA sequences of the four dengue serotypes (DENV1, DENV2, DENV3, and DENV4) were obtained from the NCBI database.35 NS3-proteins were extracted for each serotype. DENV1-NS3-protein (AMN88557.1) is located in the AA range of 1476–2094, DENV2-NS3-protein (AII99332.1) is in the range of 1476–2093, DENV3-NS3-protein (ABV03585.1) is in the range of 1474–2092, and DENV4-NS3-protein (AEX09561.1) is in the range of 1475–2092.
We also performed the sequence alignment of ZIKV-NS5-RNA (5U04), DENV2-NS5 (5K5M), and DENV3-NS5 (5HMY) polymerases with DENV1–4 using BLAST. DENV1-NS5-protein (AMN88557.1) is located in the range of 2494–3392, DENV2-NS5-protein (AII99332.1) is in the range of 2492–3391, DENV3-NS3-protein (ABV03585.1) is in the range of 2491–3390, and DENV4-NS3-protein (AEX09561.1) in the range of 2488–3387.
Molecular Docking
The Flavivirus genome is formed by three structural proteins (the C protein of the nucleocapsid, a glycoprotein precursor of the prM membrane, and a glycosylated envelope protein E) and seven nonstructural proteins (NS1, NS2A/B, NS3, NS4A, NS4B, and NS5),36 from which we selected the NS5-RNA polymerase and NS3 helicase nonstructural proteins of the Zika virus.
NS5-RNA polymerase (PDB id 5U04) and NS3 helicase (PDB id 5JMT) both share some residues and structural conformation of the active site similar to the counterpart proteins of the dengue virus.
The AMP-PNP ligand used for the standardization of ZIKV-NS3-helicase was also docked against ZIKV-NS5-RNA polymerase, and the ligands LNY and 68T used for the standardization of ZIKV-NS5-RNA polymerase were docked against ZIKV-NS3-helicase to compare the affinities among them.
For NS3-helicase, a grid box of dimensions 20x, 22y, 20z (Å) with a spacing of 1 Å was defined. The 5JMT crystallographic y structure had a resolution of 1.8 Å and an adequate metric validation. In the case of 5U04 NS5-RNA polymerase, the grid box dimensions for the protein structure has a size of 20x, 20y, 26z (Å) and the same spacing. This protein presents a resolution of 1.9 Å and adequate metric validation. The above benchmarking calculations with known crystal ligand structures of the analog complexes defined these grid box dimensions as the most accurate docking parameters.
The preselected ligands correspond to the natural extracts of the species M. taxifolia originally described by Baptista et al.13 The 3D structures of the natural extract compounds (5DP, 5HH, M3OR, and M3RG) were obtained using the PubChem database,37 and its charges were added using the Maestro 2018 program.34 Known ligands were retrieved from the crystal structures of their corresponding analogs proteins: 68T (PDB id 5K5M), LNY (PDB id 5HMY), and AMP-PNP (PDB code 2JLR) (Table 1 and Figure 6).
Figure 6.
ZIKV-NS5-RNA-polymerase (5U04) and ZIKV-NS3-helicase (5JMT) represented by secondary structures. (A) Selected grid box corresponding to ZIKV-NS5-RNA-polymerase (5U04) with dimensions 20x, 20y, 26z (Å) and spacing of 1 Å. (B) Selected grid box corresponding to ZIKV-NS3-helicase(5JMT) with dimensions 20x, 22y, 20z (Å) and spacing of 1 Å.
In order to optimize the docking parameters for proper ligand docking interactions with both proteins, we used the known ligands from the crystallographic structures previously mentioned. Because ZIKV-NS3-helicase is similar to DENV4-NS3-helicase, which is complexed with AMP-PNP (PDB code 2JLR) (Table 1), we extracted AMP-PNP and used it to predict an accurate docking pose using the crystal structure of ZIKV-NS3-helicase. The final grid was designed to cover enough space with a box of dimension of 20x, 22y, 20z (Å) and spacing of 1 Å (Figure 6) in which the known ligand for the DENV4-NS3-helicase is located. Here, it is important to notice that this benchmark does not approximate a perfect match because we carried out the docking analysis with a different crystallographic structure, ZIKV-NS3-helicase.
The ZIKV-NS5-RNA polymerase is structurally similar to DENV2-NS5 (PDB id 5K5M) and DENV3-NS5 (PDB id 5HMY), which are in complex with the ligands 68T and LNY38 (Table 1), respectively. We used both ligands to parametrize the docking parameters for this protein. The ligands 68T and LNY were extracted from the DENV3 and DENV2 crystallographic structures in order to get an accuracy posed into the crystal structure of ZIKV-NS5-RNA-polymerase. A grid box was defined with the coordinates 20x, 20y, 26z (Å) and spacing of 1 Å (Figure 6) in which both known ligands for the DENV3-NS5 and DENV2-NS5 polymerases are located.
A total of four natural compounds of M. taxifolia species were evaluated using the software AutoDock Vina (ADV).40 We used a maximum energy difference of 5 kcal mol–1 between the best and the worst poses and comprehensiveness of 8, which is the number of evaluations that take place in the local optimization of a conformer.
In this way, a large conformational sampling was carried out, and an in-house script was used to obtain a maximum of 100 molecular docking orientations. The best 10 positions (Top-10) according to the ADV scoring function were taken for each ligand. An average structure of the Top-10 and a ligand interaction diagram were determined through each ligand against both structures. All structures were prepared using the Maestro 2018 software suite.34 The corresponding docking box was created using AutoDock Tools39,40 considering the previous active site described for each protein.
Acknowledgments
The authors would like to thank Colciencias (Research Grant No. 124380864546 - contract CT. FP 80740- 152-2019).
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
References
- Hubálek Z.; Rudolf I.; Nowotny N. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 2014, 89, 201–275. 10.1016/B978-0-12-800172-1.00005-7. [DOI] [PubMed] [Google Scholar]
- Weaver S. C.; Reisen W. K. Present and future arboviral threats. Antiviral Res. 2010, 85, 328–345. 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vector Borned Diseases (VBD) in the Region of the Americas. http://ais.paho.org/phip/viz/cha_cd_vectorborndiseases.asp. accessed 12 February 2019
- Chen S.; Wu Z.; Wang M.; Cheng A. Innate immune evasion mediated by flaviviridae non-structural proteins. Viruses. 2017, 9, 291. 10.3390/v9100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton M. A.; Basu M. Functions of the 3′ and 5′ genome RNA regions of members of the genus flavivirus. Virus Res. 2015, 206, 108–119. 10.1016/j.virusres.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elong Ngono A.; Shresta S. Immune response to dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. 10.1146/annurev-immunol-042617-053142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi M.; Sharma N.; Singh S. K. Flavivirus NS1: a multifaceted enigmatic viral protein. Virol. J. 2016, 13, 1–10. 10.1186/s12985-016-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal A.; Khandia R.; Dhama K.; Sachan S.; Karthik K.; Tiwari R.; Malik Y. S.; Kumar D.; Singh R. K.; Iqbal H. M. N.; Joshi S. K. Advances in developing therapies to combat Zika virus: current knowledge and future perspectives. Front. Microbiol. 2017, 8, 1469. 10.3389/fmicb.2017.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelangeli F. A.; Guimaraes P. J. F.; Penneys D. S.; Almeda F.; Kriebel R. Phylogenetic relationships and distribution of new world melastomeae (melastomataceae). Bot. J. Linn. Soc. 2013, 171, 38–60. 10.1111/j.1095-8339.2012.01295.x. [DOI] [Google Scholar]
- Clausing G.; Renner S. S. Molecular phylogenetics of melastomataceae and memecylaceae: implications for character evolution. Am. J. Bot. 2001, 88, 486–498. 10.2307/2657114. [DOI] [PubMed] [Google Scholar]
- Srinivasan R.; Natarajan D.; Shivakumar M. S. Spectral characterization and antibacterial activity of an isolated compound from memecylon edule leaves. J. Photochem. Photobiol. B Biol. 2017, 168, 20–24. 10.1016/j.jphotobiol.2017.01.019. [DOI] [PubMed] [Google Scholar]
- Afagnigni A. D.; Nyegue M. A.; Ndoye Foe C. F.; Njankouo Ndam Y.; Njayou F. N.; Fonkoua M. C.; Etoa F. X. Antidiarrheal activity of dissotis multiflora (sm) triana (melastomataceae) leaf extract in wistar rats and subacute toxicity evaluation. Evidence Based Complement. Altern. Med. 2017, 2017, 1–9. 10.1155/2017/4038371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista J.; Chávez K.; Torrico F.; Trejo E.; Garcia C.; Urbina J.; Carrasco J.; Taddei A.; Tillett S.; Suárez A. I. Constituyentes químicos y actividad antiinflamatoria de Marcetia taxifolia. CIENCIA. 2016, 24, 81–94. [Google Scholar]
- Isaza J. H.; Ito H.; Yoshida T. Oligomeric hydrolyzable tannins from monochaetum multiflorum. Phytochemistry 2004, 65, 359–367. 10.1016/j.phytochem.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Ono K.; Nakane H.; Fukushima M.; Chermann J.-C.; Barré-Sinoussi F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular dna and rna polymerases. Eur. J. Biochem. 1990, 190, 469–476. 10.1111/j.1432-1033.1990.tb15597.x. [DOI] [PubMed] [Google Scholar]
- Ortega J. T.; Suárez A. I.; Serrano M. L.; Baptista J.; Pujol F. H.; Rangel H. R. The role of the glycosyl moiety of myricetin derivatives in anti-hiv-1 activity in vitro. Aids res. Ther. 2017, 14, 57. 10.1186/s12981-017-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J. T.; Serrano M. L.; Suaěrez A. I.; Baptista J.; Pujol F. H.; Cavallaro L. V.; Campos H. R.; Rangel H. R. Antiviral Activity of Flavonoids Present in Aerial Parts of Marcetia Taxifolia Against Hepatitis b Virus, Poliovirus, and Herpes Simplex Virus in Vitro. EXCLI Journal 2019, 18, 1037–1048. 10.17179/excli2019-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasetto S.; Pardi V.; Murata R. M. Anti-hiv-1 activity of flavonoid myricetin on hiv-1 infection in a dual-chamber in vitro model. PLoS One 2014, 9, e115323 10.1371/journal.pone.0115323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Z.; Zhi D. G. Ligand - protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins 2001, 43, 217–226. . [DOI] [PubMed] [Google Scholar]
- Vasseur R.; Baud S.; Steffenel L. A.; Vigouroux X.; Martiny L.; Krajecki M.; Dauchez M. Inverse Docking Method for New Proteins Targets Identification: A Parallel Approach. Parallel Comput. 2015, 42, 48–59. 10.1016/j.parco.2014.09.008. [DOI] [Google Scholar]
- Tian H.; Ji X.; Yang X.; Xie W.; Yang K.; Chen C.; Wu C.; Chi H.; Mu Z.; Wang Z.; Yang H. The Crystal Structure of Zika Virus Helicase: Basis for Antiviral Drug Design. Protein Cell. 2016, 7, 450–454. 10.1007/s13238-016-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C.; Bisaillon M.; Geiss B. J. Organization of the flavivirus RNA replicase complex. WIREs RNA 2017, 8, e1437 10.1002/wrna.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy A. S.; Lima G. M.; Oliveira K. I.; Torres N. U.; Maluf F. V.; Guido R. V.; Oliva G. Crystal Structure of Zika Virus NS5 RNA-Dependent RNA Polymerase. Nat. Commun. 2017, 1–6. 10.1038/ncomms14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potisopon S.; Priet S.; Collet A.; Decroly E.; Canard B.; Selisko B. The methyltransferase domain of dengue virus protein NS5 ensures efficient RNA synthesis initiation and elongation by the polymerase domain. Nucleic Acids Res. 2014, 42, 11642–11656. 10.1093/nar/gku666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossen T.; Frøystein N. Å.; Andersen Ø. M. Myricetin 3-Rhamnosyl(1 → 6)Galactoside from Nymphaea X Marliacea. Phytochemistry 1998, 49, 1997–2000. 10.1016/S0031-9422(98)00420-8. [DOI] [Google Scholar]
- Lauro G.; Romano A.; Riccio R.; Bifulco G. Inverse virtual screening of antitumor targets: pilot study on a small database of natural bioactive compounds. J. Nat. Prod. 2011, 74, 1401–1407. 10.1021/np100935s. [DOI] [PubMed] [Google Scholar]
- Lauro G.; Masullo M.; Piacente S.; Riccio R.; Bifulco G. Inverse Virtual Screening allows the discovery of the biological activity of natural compounds. Bioorg. Med. Chem. 2012, 20, 3596–3602. 10.1016/j.bmc.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Vigers G. P. A.; Rizzi J. P. Multiple active site corrections for docking and virtual screening. J. Med. Chem. 2004, 47, 80–89. 10.1021/jm030161o. [DOI] [PubMed] [Google Scholar]
- Natarajan S. NS3 protease from flavivirus as a target for designing antiviral inhibitors against dengue virus. Genet. Mol. Biol. 2010, 33, 214–219. 10.1590/S1415-47572010000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. L.; Jones C. T.; Rice C. M. Architects of assembly: roles of flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Microbiol. 2008, 6, 699–708. 10.1038/nrmicro1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sahili A.; Lescar J. Dengue virus non-structural protein 5. Viruses. 2017, 9, 91. 10.3390/v9040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Gao G. F. Structural biology of the Zika virus. Trends Biochem. Sci. 2017, 42, 443–456. 10.1016/j.tibs.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Saenz-Méndez P.; Eriksson M.; Eriksson L. A. Ligand Selectivity between the ADP-Ribosylating Toxins: An Inverse-Docking Study for Multitarget Drug Discovery. ACS Omega 2017, 2, 1710–1719. 10.1021/acsomega.7b00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger Release 2018-1: Maestro; Schrödinger, LLC, New York, 2018.
- Sayers E. W.; Agarwala R.; Bolton E. E.; Brister J. R.; Canese K.; Clark K.; Connor R.; Fiorini N.; Funk K.; Hefferon T.; Holmes J. B.; Kim S.; Kimchi A.; Kitts P. A.; Lathrop S.; Lu Z.; Madden T. L.; Marchler-Bauer A.; Phan L.; Schneider V. A.; Schoch C. L.; Pruitt K. D.; Ostell J. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2019, 47, D23–D28. 10.1093/nar/gky1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati M.; Alvarez K.; Assenberg R.; Baronti C.; Canard B.; Cook S.; Coutard B.; Decroly E.; de Lamballerie X.; Gould E. A.; Grand G.; Grimes J. M.; Hilgenfeld R.; Jansson A. M.; Malet H.; Mancini E. J.; Mastrangelo E.; Mattevi A.; Milani M.; Moureau G.; Neyts J.; Owens R. J.; Ren J.; Selisko B.; Speroni S.; Steuber H.; Stuart D. I.; Unge T.; Bolognesi M. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antiviral Res. 2010, 87, 125–148. 10.1016/j.antiviral.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.; Thiessen P. A.; Bolton E. E.; Chen J.; Fu G.; Gindulyte A.; Han L.; He J.; He S.; Shoemaker B. A.; Wang J.; Yu B.; Zhang J.; Bryant S. H. PubChem Substance and Compound Databases. Nucleic Acids Res. 2016, 44, D1202–D1213. 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D.; Xu T.; Watson R. P.; Scherer-Becker D.; Sampath A.; Jahnke W.; Yeong S. S.; Wang C. H.; Lim S. P.; Strongin A.; Vasudevan S. G.; Lescar J. Insights into RNA unwinding and ATP hydrolysis by the flavivirus NS3 protein. The EMBO Journal 2008, 27, 3209–3219. 10.1038/emboj.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. M.; Huey R.; Lindstrom W.; Sanner M. F.; Belew R. K.; Goodsell D. S.; Olson A. J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]








