Abstract

A novel series of copper-activatable drugs intended for use against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA) were synthesized, characterized, and tested against the MSSA strain Newman and the MRSA Lac strain (a USA300 strain), respectively. These drugs feature an NNSN structural motif, which enables the binding of copper. In the absence of copper, no activity against MSSA and MRSA at realistic drug concentrations was observed. Although none of the novel drug candidates exhibits a stereocenter, sub-micromolar activities against SA Newman and micromolar activities against SA Lac were observed in the presence, but not in the absence, of bioavailable copper. Copper influx is a component of cellular response to bacterial infections, which is often described as nutritional immunity.
Introduction
Although invasive methicillin-resistant Staphylococcus aureus (MRSA) infections have been declining in healthcare settings in the United States during recent years, 119,247 recognized MRSA bloodstream infections and 19,832 associated deaths were counted in 2017.1 According to the National Healthcare Safety Network (NHSN) and the Emerging Infections Program (EIP) surveillance system, the United States is “not on track to meet the 2020 goal of the Healthcare-Associated Infection National Action Plan of a 50% reduction in hospital-onset MRSA bloodstream infections from the 2015 baseline.”2
An assessment of all FDA-approved small-molecule drugs in 2015 revealed that natural products and their derivatives represent over one-third of all new molecular entities.3 One of the characteristics of natural products is that their stereochemistry is complex.4−6 Consequently, their efficient total synthesis is challenging. Therefore, plant-derived precursors are often utilized,7,8 which adds an element of uncertainty to numerous supply chains, especially in times of great demand, such as a pandemic. Virtually, all of the antibiotics in use today were discovered before the end of the 1970’s; daptomycin and linezolid were introduced in the 1980’s; Synercid in 1992; and so forth.9 Because of the ever-increasing bacterial resistance,9 there is a serious danger that soon we would reach the post-antibiotic era, which will be characterized by significantly increased mortality from bacterial infections.10 Only a few antibiotics have recently been discovered, by culturing fastidious bacterial species and analysis of metabolites (e.g., teixobactin).4 One of the major reasons for the lack of suitable drug candidates is that there is practically no academic discovery pipeline in the United States because of insufficient funding.9 Novel approaches to treat infectious diseases, for instance, the discovery and up-scaling of antibodies against bacterial pathogens,11,12 and identifying novel antibiotics through genomic mining13,14 are still in their infancy.
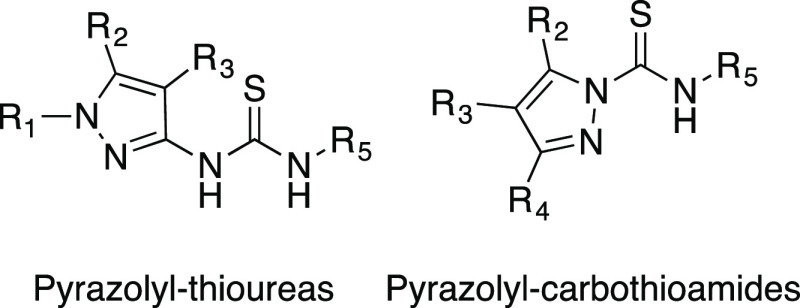
Recently, we have discovered the family of pyrazolyl thioureas that are highly active against Gram-positive bacteria. They feature a novel NNSN motif that was discovered by means of high-throughput screening.15,16 “NNSN” pertains to a structure derived from thiourea, which features a heterocylic aromatic component with at least one nitrogen in the ring (here, pyrazole).15,16 Our synthetic efforts toward pyrazolyl thioureas led to the discovery of pyrazolyl carbothioamides, which are structural isomers of pyrazolyl thioureas. Here, we report the synthesis of a total of 4 pyrazolyl ureas, 7 pyrazolyl thioureas, and 19 pyrazolyl carbothioamides with an NNSN motif and their activity against SA Newman and Lac. Both structurally related groups of antibiotics are copper-activated. Whereas SA Newman is a methicillin-sensitive S. aureus strain (MSSA), Lac is methicillin-resistant (MRSA). To the best of our knowledge, this is the first report detailing the synthesis of pyrazolyl thioureas and pyrazolyl carbothioamides with effective antibiotic properties. We report optimized conditions and yields for each synthetic step, as well as for the pyrazole derivatives, which were not commercially available (Scheme 1).
Scheme 1. Pyrazolyl Thioureas and Pyrazolyl Carbothioamides, Two Isomeric Classes of Effective, Copper (I)-Activated Antibiotics against Gram-Positive Bacteria.
Copper activation of NNSN compounds versus MSSA and MRSA is a concept that is synergetic with nutritional immunity [starving pathogens of trace minerals (iron and zinc) and increasing the concentration of copper in activated phagosomes] to fight bacterial infections.17,18 The dependence of drug activity on copper binding enables significant therapeutic indexes,19 which permit treating MRSA patients with appropriate doses. This approach will be able to avoid or at least greatly minimize systemic toxicity from antibiotics, which will have many advantages for the treated patients, including a better compliance with antibiotic regimens.
Results and Discussion
The convergent synthesis of both pyrazolyl thioureas and pyrazolyl carbothioamides comprises the addition of isothiocyanates20 with pyrazole or pyrazole derivatives. In some examples discussed here, this addition is competitive and can lead to both isomeric classes of antibiotics. The synthesis of inactive pyrazolyl ureas and pyrazolyl amides, which is reported here as well, involves isocyanates and a selection of pyrazole derivatives. In order to maximize the synthetic yields, we have developed three general methods. In all the cases, when a target molecule was synthesized according to more than one method, the experimental procedure which permitted higher yields is reported.
Method A consists of using the strong base NaH, which helps to deprotonate the hydrogen atoms on 1 NH of the pyrazole ring system (refer to 3ae, 3af, and 3ag). The acidity of this proton depends on the functional group(s) attached to the pyrazole ring. Electron-withdrawing groups, such as nitro or carbonyl, enhance the acidity, whereas electron-donating groups, such as amines and ethers, decrease 1 NH acidity as they increase the electron density of the pyrazole ring. Thus, using a strong base such as NaH can deprotonate that hydrogen atom effectively, which can increase the yield of the desired product. The only drawback of this method is the requirement of anhydrous reaction media.
In method B, there is no additional base used in the reaction because most of the compounds synthesized from this method contain free amines, which are basic enough to deprotonate the 1 NH proton of pyrazole. However, when having primary amines and secondary amines (pyrazole NH) in the reactive mixture, both amine groups may compete for the nucleophilic attack at thiosemicarbazone carbon. Column chromatography is performed to separate both the isomers. The absence of anhydrous reaction conditions proved to be an advantage for this method. The major drawback is that it requires more time to complete the reaction. Isometrically pure compounds (refer to 3be–3bi) can be recrystallized to obtain the desired products.
Method C is performed using the strong base potassium carbonate in distilled N,N-dimethylformamide (DMF).21 All the compounds synthesized in accordance with method C contain only one reactive NH center, except 3cg. It is noteworthy that whenever a competition between the 1 NH position of pyrazole and a primary aromatic amine ligand was possible during synthesis, higher yields for pyrazole addition were observed. This can be attributed to higher NH-acidity at this position (Tables 1 and 2).22
Table 1. Yield Percentages of Structural Isomers of Pyrazolyl Carbothioamides and Pyrazolyl Thioureas.
| compound | yield percentage % | compound | yield percentage % |
|---|---|---|---|
| 3ba | 65 | 3bb | 8 |
| 3bc | 55 | 3bd | 35 |
| 3bj | 66 | 3bk | 31 |
Table 2. Substrate Scope of Pyrazolyl Thioureas and Carbothioamidesa.
Reaction condition: 3aa–3ag (Method A); 1.0 equiv of pyrazole derivative and 1.0 equiv of p-substituted isocyanato/isothiocyanato benzene derivative, 1.0 equiv of NaH in dry THF stirred at room temperature overnight under Ar. 3ba–3bo (Method B); 1.0 equiv of pyrazole derivative and 1.0 equiv of p-substituted isothiocyanato benzene derivative in DCM refluxed overnight or stirred overnight at room temperature under Ar gas. 3ca–3ch (Method C); 1.0 equiv of pyrazole derivative and 1.0 equiv of p-substituted isothiocyanato benzene derivative, 1.0 equiv of K2CO3 in distilled DMF stirred at room temperature overnight under Ar gas.
Activity of the Newly Synthesized Pyrazolyl Thioureas and Carbothioamides against an MRSA Clinical Isolate
All compounds were tested against both S. aureus strain Newman, a clinical isolate,23 and S. aureus strain Lac, a USA300-type MRSA clinical isolate, as described previously.16,24 Briefly, compounds were resuspended in DMSO, aliquoted, and stored at −80 °C until use. For dose–response curves to determine the minimal inhibitory concentration (MIC), compounds were diluted in the Rosewell Park Memorial Institute medium (RPMI) with or without a final concentration of 50 μM copper sulfate. A logarithmic phase culture of S. aureus grown in Mueller Hinton broth was washed in RPMI, then diluted in fresh RPMI and added to plates at a final optical density of 0.005, and plates were incubated overnight at 37 °C. The MIC was determined by the addition of resazurin, a metabolic indicator, using 10% conversion of a blank subtracted and normalized value as the cutoff.
Discussion
Copper (II/I) is present in limited quantity in the human body because it can produce reactive oxygen species, which are tissue damaging.25 Tight homeostatic regulation is mandatory, restricting the copper concentration in the blood and healthy tissue to about 1 μM or less.25 It is noteworthy that the copper (I) concentration is significantly increased in activated phagosomes in response to bacterial infections.19 Copper (I) accumulation is achieved by means of the high-affinity copper transporter CTR1 and by activating the copper ATPase ATP7A, which can directly pump copper into the phago(lyso)somes.26 Although the reported concentrations of copper in activated phagosomes vary in the literature,26 we can conservatively estimate at least 50 μmol (and beyond) in activated phagosomes.26,27 This represents a 50-fold enhancement compared to the maximal copper concentration in the human blood.26
A total of nine pyrazolyl carbothioamides and seven pyrazolyl thioureas with (sub)micromolar MICs against SA Newman (MSSA) were identified. The most active compounds against SA Newman were the pyrazolyl carbothioamides 3ch (MIC = 0.15 μM) and 3bh (MIC = 0.625 μM), as well as pyrazolyl thiourea 3bg (MIC = 0.625 μM). Only four of these pyrazolyl carbothioamides and five of these pyrazolyl thioureas showed micromolar activities against SA Lac. Two pyrazolyl carbothioamides displayed the highest efficacy against SA Lac [3bc and 3bh (MIC = 2.5 μM for both compounds)]. As summarized in Table 3, there is no simple relationship between the MIC of each compound against SA Newman and SA Lac. However, all pyrazolyl carbothioamides that are active against both strains are on average 2.3 times more active against N. Newman than against SA Lac. For the group of pyrazolyl thioureas, this ratio is approximately 2.2. To date, the lead compound with an NNSN motif against multi-resistant MRSA is 1-(1-(adamantyl)-(1H-pyrazol-3-yl)-3-(4-((1-methyl)-propyl)benzyl)thiourea (Scheme 2) with an MIC of 0.3 μM (0.123 μg/mL) against the S. aureus clinical isolate SA3 (resistant to ampicillin, clindamycin, erythromycin, penicillin, and tetracycline).16 Although a direct comparison is not warranted because of different SA strains and experimental conditions, the identified lead compounds 3bc and 3bh are about 10 times less active against SA Lac, whereas the activity of 3ch against SA Newman is twice as high.
Table 3. Antibacterial Activity of Compounds With an NNSN Motif Comprising pyrazolyl thioureas.
| compound | SA Newman |
SA Lac |
||
|---|---|---|---|---|
| MIC (μM) | MIC (μg/mL) | MIC (μM) | MIC (μg/mL) | |
| 3af | 10 | 2.66 | ND | ND |
| 3bb | 2.5 | 0.67 | 5 | 1.67 |
| 3bc | 1.25 | 0.32 | 2.5 | 0.63 |
| 3bd | 2.5 | 0.63 | 10 | 2.53 |
| 3be | 5 | 1.33 | ND | ND |
| 3bf | 20 | 5.61 | ND | ND |
| 3bg | 0.625 | 0.20 | 5 | 1.61 |
| 3bh | 0.625 | 0.16 | 2.5 | 0.63 |
| 3bk | 10 | 2.63 | 20 | 5.27 |
| 3bl | 10 | 2.48 | ND | ND |
| 3bm | 10 | 2.32 | 20 | 4.65 |
| 3bn | 1.25 | 0.37 | 5 | 1.48 |
| 3bo | 20 | 4.72 | ND | ND |
| 3ca | 5 | 1.54 | 5 | 1.54 |
| 3cg | 20 | 5.05 | ND | ND |
| 3ch | 0.15 | 0.05 | ND | ND |
In all cases, 50 μM copper sulfate was present. MIC: Minimum inhibitory concentration. Pyrazolyl thioureas: 3bb, 3bd, 3be, 3bf, 3bg, 3bk, and 3ca. Pyrazolyl carbothioamides: 3af, 3bc, 3bh, 3bl, 3bm, 3bn, 3bo, 3cg, and 3ch. None of the pyrazolyl ureas 3aa, 3ab, 3ac, and 3ad exhibited activity against SA Newman or SA Lac. None of the compounds showed activity against either SA Newman or SA Lac in the absence of copper (II) in the (micromolar) concentration range investigated. Details are provided in the Supporting Information section.
Scheme 2. Chemical Structures of the Lead Compound 1-(1-(Adamantyl)-(1H-pyrazol-3-yl)-3-(4-((1-methyl)-propyl)benzyl))thiourea16 with the Three Most Promising Pyrazolyl Carbothioamides that Were Newly Synthesized: 3-Amino-N-(4-chlorophenyl)-1H-pyrazole-1-carbothioamide (3bc), N-(4-Chlorophenyl)-3-methyl-1H-pyrazole-1-carbothioamide (3bh), and 3-(Benzyloxy)-N-(4-chloro-phenyl)-1H-pyrazole-1-carbothioamide (3ch).
It should be noted that both pyrazolyl carbothioamides and pyrazolyl thioureas discussed here feature only limited structural diversity, which does not yet enable meaningful structure–activity relationship studies. However, we were successful in demonstrating that pyrazolyl carbothioamides can be active against MSSA and MRSA. This establishes a paradigm that will be further explored in future studies. It should be noted that the synthesis of pyrazolyl carbothioamides is straightforward and carbon-effective. One of the requirements for the next generations of antibiotics is cost- and atom-effectiveness, because they must be globally available to make a difference in the 21st century. Therefore, effective antibiotics of simpler structures are highly desirable. For instance, drug candidates with only a few stereocenters, or even better, no stereocenters will enable inexpensive total synthesis, which equals world-wide accessibility.
Conclusions
Copper activation of toxicity against Gram-positive bacteria is an emerging concept. It takes advantage of “nutritional immunity” of infected cells. Compounds with an NNSN structural motif enhance the toxicity of copper cations that are available in activated phagosomes and enable huge therapeutic indexes. Both pyrazolyl carbothioamides and pyrazolyl thioureas with the NNSN motif exhibited sub-micromolar activity against SA Newman (MSSA) and micromolar activity against SA Lac (MRSA) in the presence of bioavailable copper. This study established the suitability of using pyrazolyl thioureas for the treatment of MRSA. Three lead compounds were identified, which will serve as the foundation for future studies.
Experimental Section
General Experimental Procedure
Unless otherwise noted, all commercial reagents and solvents were purchased with the highest purity grade from Fisher Scientific, Inc. Para-substituted isothiocyanato benzene derivatives, 4-chlorophenyl isothiocyanate, 4-chlorobenzyl isothiocyanate, 4-chlorobenzyl isocyanate, 1H-pyrazole, 1H-pyrazol-3-amine, 1-methyl-1H-pyrazol-3-amine, 3,4,5-trimethyl-1H-pyrazole, and 4-nitro-1H-pyrazole were purchased from Aldrich and used without further purification unless specified. Solvents mainly used, including DMF, dichloromethane (methylene dichloride; DCM), and tetrahydrofuran (THF), were distilled or purchased in high purity and anhydrous conditions. Silica gel (200–300 meshes) was used for column chromatography, and TLC investigation was done on Silica XHL TLC plates, w/UV254, glass-backed, 250 μm. All the reagents and products were stored under an Ar atmosphere. The synthesized compounds were stored in a −20 °C freezer. 1H (400, 600 MHz) and 13C (100, 151 MHz) spectra were obtained on Bruker-Ascend spectrometers. Infrared spectra were recorded with a Nicolet 380 FT-IR spectrometer. HRMS (+ESI-TOF) measurements were performed using an LCT Premier instrument.
1-(tert-Butyl)-1H-pyrazol-3-amine (2a)
1-(tert-Butyl)-1H-pyrazol-3-amine was prepared according to a method described in the literature.28 A dried 100 mL round-bottomed flask with a stir bar was loaded with tert-butylhydrazine (1 g, 21.71 mmol, 1.0 equiv), which was dissolved in 10 mL of distilled water (Scheme 3). To the resulting solution, the suspension of 2-chloroacrylonitrile (1.9 g, 21.71 mmol, 1.0 equiv, in distilled water) was added and stirred at room temperature for 20 h. The resulting mixture was extracted with ethyl acetate twice and the combined organic layers were washed with brine and dried over sodium sulfate, followed by concentration in vacuo. The crude was purified by flash column chromatography (DCM–methanol 20:1–2:1). The product was obtained as a dark reddish-yellow oil (71%). Only primary amine’s 2H signal considerably deviated from the original protocol because highly exchangeable amine protons can exist in a wide range. However, other proton signals are observed with similar chemical shifts. 1H NMR (400 MHz, chloroform-d): δ 7.23 (d, J = 2.4 Hz, 1H), 5.24 (d, J = 2.4 Hz, 1H), 3.63 (br s, 2H), 1.49 (s, 9H). 13C NMR (101 MHz, chloroform-d): δ 153.69, 126.82, 92.15, 57.39, 29.70. FTIR cm-1: 3421 (N–H stretch), 1617 (N–H bend), 1490, 1460, 1413 (−CH3). Melting point = 40–42 °C.
Scheme 3. Synthesis of 1-(tert-Butyl)-1H-pyrazol-3-amine (2a).
3,5-Dimethyl-1H-pyrazole (2b)
This substituted pyrazole synthesis was completed with a solvent-less efficient method.29 2,4-Pentadiene (100 mg, 1.0 mmol) and hydrazine (35 mg, 1.1 mmol) were mixed together (Scheme 4). To this was added silicon dioxide (20 mg), which was then ground with a glass rod for 5 min. The reaction mixture was filtered to remove silicon dioxide, using ethyl acetate to rinse the product off the solid. The filtrate was concentrated to dryness to give the product as a white solid (62%). No characterization data were available in the original literature. 1H NMR (600 MHz, chloroform-d): δ 9.09 (br s, 1H), 5.83 (s, 1H), 2.28 (d, J = 0.6 Hz, 6H). 13C NMR (101 MHz, chloroform-d): δ 153.69, 126.82, 92.15, 57.39, 29.70. FTIR, cm-1: 3198 (N–H stretch), 1304 (aromatic C–N), 1483 and 1419 (−CH3). Melting point = 110–113 °C.
Scheme 4. Synthesis of 3,5-Dimethyl-1H-pyrazole (2b).
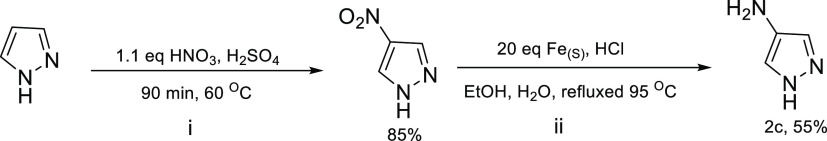
1H-Pyrazole-4-amine (2c)
Pyrazole (1.0 g, 14.69 mmol) was measured into a 100 mL round-bottomed flask and dissolved in 5 mL of H2SO4 (after 10 min, pyrazole was fully dissolved) (Scheme 5). Afterward, the solution was heated to 60 °C and 1.1 equiv of (1.46 mL) conc. HNO3 was added dropwise, followed by stirring for 90 min at 60 °C. After 90 min, the product solution was poured into 15 g of ice/water. The product was obtained as a white color solid precipitate (85%). To obtain the second crop, the mother liquor was extracted with ethyl acetate thrice, combined organic layers were washed with NaHCO3/brine and finally dried over sodium sulfate, followed by concentration in vacuo.
Scheme 5. Synthesis of 1H-Pyrazole-4-amine (2c).
4-Amino pyrazole was prepared according to the literature method, which showed the reduction of the aryl nitro group at the phenyl ring.30 4-Nitro-1H-pyrazole (100 mg, 0.88 mmol) was dissolved in 3 mL of EtOH/H2O and transferred to a 100 mL round-bottomed flask equipped with a stir bar. While the reaction stirred, 20 equiv (18.9 mmol, 1.056 g) of Fe powder (suspension in 10 mL of EtOH/H2O) was added and the system heated to 95 °C. At 95 °C, 200 μL of conc. HCl was added dropwise and refluxed at the same temperature for 90 min. The reaction was monitored by TLC (50% EtOAc/hexanes). Then, the reaction mixture was filtered hot by gravity filtration, followed by filtration through the Celite pad. The filtrate was purified by flash column chromatography (EtOAc/hexanes 10–40%) to afford 55% yield. 1H NMR (400 MHz, DMSO-d6): δ 6.85 (s, 1H), 6.67 (s, 1H), 4.35 (s, NH). 13C NMR (101 MHz, DMSO-d6): δ 128.47, 126.15, 122.87. FTIR, cm-1: 3367 (N–H stretch), 1620 (N–H bend). Melting point = 80–82 °C.
3-(Benzyloxy)-1H-pyrazole (2d)
This synthesis was performed according to a previously published protocol (Scheme 6).31 A suspension of 1H-pyrazol-3-ol (2 g, 23.8 mmol) in pyridine was heated to 95 °C with vigorous stirring. A mixture of acetic anhydride (2.55 g, 25 mmol, 1.05 equiv) in pyridine was added dropwise over 20 min. Then, the reaction mixture was stirred for 2 h at 95 °C. All the volatile components were removed in vacuo. 50 mL of diethyl ether was added to the residual solid mixture and the slurry was stirred overnight at room temperature. The resulting solid precipitate was vacuum-filtered, washed with cold diethyl ether, and dried in the drying cabinet. The product 1-(3-hydroxy-1H-pyrazol-1-yl)ethan-1-one was obtained as an off-white solid (87%). The suspension of previous product (1.0135 g, 8.04 mmol) and benzyl bromide (1.51 g, 8.844 mmol 1.1 equiv) in DMF was poured into a round-bottomed flask and K2CO3 (1.482 g, 10.72 mmol, 1.33 equiv) was added. The reaction mixture was stirred for 10 h at room temperature. DMF was removed with vacuum distillation and the resulting slurry was diluted with EtOAc and water. The organic phase was washed with brine (three times), followed by drying over sodium sulfate. EtOAc was evaporated under vacuum and the crude product was purified by flash column chromatography (5–10% EtOAC/hexane). TLC was done in 25% EtOAc/hexane. The purified product 1-[3-(phenylmethoxy)-1H-pyrazol-1-yl]ethenone was delivered as a white color solid (77%). It was dissolved in a mixture of MeOH/THF (2:3 v/v) and 10% NaOH (in H2O) was added. The reaction mixture was stirred for 5 h at room temperature. The reaction was monitored with TLC 25% (EtOAc/hexane). The volatile component was evaporated under vacuum and diluted with EtOAc and water. The aqueous phase was washed three times with EtOAc. The combined organic phase was washed with brine (three times) and dried over sodium sulfate. EtOAc was evaporated in vacuo to obtain an off-white solid 3-(benzyloxy)-1H-pyrazole (80%). Both the 1H NMR and 13C NMR characterization data are identical to the data given here. 1H NMR (600 MHz, DMSO-d6): δ 11.93 (s, 1H), 7.53 (s, 1H), 7.44 (d, J = 7.2 Hz, 2H), 7.38 (t, J = 7.5 Hz, 2H), 7.32 (dd, J = 8.4, 6.1 Hz, 1H), 5.72 (d, J = 2.4 Hz, 1H), 5.14 (s, 2H). 13C NMR (151 MHz, DMSO-d6): δ 163.30, 137.97, 130.38, 128.76, 128.21, 89.89, 70.34, 60.25. FTIR, cm-1: 3250 (N–H stretch), 1462 (−CH2−), and 1285 (C–O stretch). Melting point = 30–35 °C.
Scheme 6. Synthesis of 3-(Benzyloxy)-1H-pyrazole (2d).
General Procedure for the Synthesis of Pyrazolyl Ureas and Carbothioamides
Method A
To a 150 mL oven-dried single-neck round-bottomed flask containing a magnetic stirring bar was added pyrazolyl derivative (1 equiv) in dry THF, and the reaction mixture was placed in an ice bath while stirring. In a separate oven-dried single-neck round-bottom flask was dispersed NaH (1 equiv) in dry THF. This was cannulated to the pyrazolyl reaction mixture while maintaining it in the ice bath. Then, the reaction mixture was stirred at room temperature for 3 h under anhydrous conditions. Para-substituted isocyanato/isothiocyanato benzene derivatives (1 equiv) were dissolved in dry THF, then injected into the reaction mixture, and stirred overnight at room temperature. Then, the reaction mixture was filtered and concentrated in vacuo. The resulting solid was recrystallized using either hexane or diethyl ether or MeOH to obtain a pure solid product.
Method B
To a 150 mL oven-dried single-neck round-bottomed flask containing a magnetic stirring bar was added pyrazolyl derivative (1 equivalent) in DCM. Para-substituted isothiocyanato benzene derivatives (1 equiv) were dissolved in DCM and added to the reaction mixture and stirred (at room temperature) or refluxed overnight. The crude product was obtained and then purified by flash column chromatography (EtOAc/hexane or 100% DCM) or recrystallized with MeOH to give the required products.
Method C
A 150 mL oven-dried single-neck round-bottom flask containing a magnetic stirring bar was charged with anhydrous K2CO3 (1 equiv). Under argon, the pyrazolyl derivative (1 equiv) dissolved in anhydrous DMF was added and stirred for 30 min. Para-substituted isothiocyanato benzene derivatives (1 equiv) dissolved in anhydrous DMF were added slowly to the reaction mixture and stirred overnight at room temperature. K2CO3 was filtered, followed by distillation of DMF. The crude product was obtained and then purified by flash column chromatography (EtOAc/hexane or 100% DCM) or recrystallized with MeOH to give the required products.
Experimental Data
1-(1-(tert-Butyl)-1H-pyrazol-3-yl)-3-(4-chlorophenyl)urea (3aa)
1-(tert-Butyl)-1H-pyrazol-3-amine (250 mg, 2.6 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorophenyl isocyanate (395 mg, 2.6 mmol, 1 equiv) dissolved in DCM was added to the reaction mixture dropwise, stirred for 5 min at r.t. and refluxed at 60 °C for 16 h. The reaction was monitored by TLC (25% EtOAc/hexanes). Volatile components were removed under vacuum and the resulting solid precipitate was recrystallized with MeOH. The retrieved off-white color solid 3aa (68%) was oven-dried. 1H NMR (600 MHz, DMSO-d6): δ 9.15 (s, NH), 7.67 (d, J = 2.4 Hz, 1H), 7.48 (m, 2H), 7.33 (m, 2H), 6.17 (s, 1H), 1.49 (s, 9H). 13C NMR (151 MHz, DMSO-d6): δ 152.31, 147.36, 139.02, 129.14, 127.63, 125.84, 120.08, 94.82, 58.01, 29.72. FTIR, cm-1: 3245 (−NH−), 2974 (C–H aliphatic), 1538 (aromatic C=C), 1481 (aromatic C=N), 1686 (C=O), 734 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C14H17ClN4O, 292.1091; found, 292.1093.
1-(4-Chlorophenyl)-3-(1-methyl-1H-pyrazol-3-yl) (3ab)
1-Methyl-1H-pyrazol-3-amine (250 mg, 2.6 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorophenyl isocyanate (395 mg, 2.6 mmol, 1 equiv) dissolved in DCM was added to the reaction mixture dropwise, stirred for 5 min at r.t. and refluxed at 60 °C for 16 h. The reaction was monitored by TLC (25% EtOAc/hexanes). Volatile components were removed under vacuum and the resulting solid precipitate was recrystallized with MeOH. The retrieved white color solid 3ab (61%) was oven-dried. 1H NMR (600 MHz, DMSO-d6): δ 8.78 (s, NH), 7.79 (d, J = 2.3 Hz, 1H), 7.48 (d, J = 8.9 Hz, 2H), 7.33 (d, J = 8.8 Hz, 2H), 6.27 (d, J = 2.3 Hz, 1H), 3.88 (s, 3H). 13C NMR (151 MHz, DMSO-d6): δ 154.81, 150.21, 139.02, 133.25, 129.11, 125.94, 120.42, 95.27, CH3 overlapped with DMSO-d6. FTIR, cm-1: 3286 (−NH−), 1555 (aromatic C=C), 1486 (aromatic C=N), 1593 (C=O), 744 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11ClN4O, 250.0621; found, 250.0620.
1-(4-Chlorobenzyl)-3-(1-methyl-1H-pyrazol-3-yl)urea (3ac)
1-Methyl-1H-pyrazol-3-amine (92 mg, 0.95 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorobenzyl isocyanate (159 mg, 0.95 mmol, 1 equiv) dissolved in DCM was added to the reaction mixture dropwise, stirred for 5 min at r.t. and refluxed at 60 °C for 16 h. The reaction was monitored by TLC (25% EtOAc/hexanes). Volatile components were removed under vacuum and the resulting solid precipitate was recrystallized with MeOH. The retrieved white color solid 3ac (60%) was oven-dried. 1H NMR (600 MHz, DMSO-d6): δ 7.46 (s, 1H), 7.38 (m, 2H), 7.26 (m, 2H), 6.55 (t, J = 6.1 Hz, 1H), 4.21 (d, J = 6.1 Hz, 2H), 3.88 (s, 3H). 13C NMR (151 MHz, DMSO-d6): δ 158.18, 149.27, 140.52, 131.49, 130.35, 129.29, 128.61, 96.40, 42.74, CH3 overlapped with DMSO-d6. FTIR, cm–1: 3225 (−NH−), 2911 (C–H aliphatic), 1563 (aromatic C=C), 1495 (aromatic C=N), 1608 (C=O), 757 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C12H13ClN4O, 264.0778; found, 264.0777.
1-(1-(tert-Butyl)-1H-pyrazol-3-yl)-3-(4-chlorobenzyl)urea (3ad)
1-(tert-Butyl)-1H-pyrazol-3-amine (168 mg, 1.21 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorobenzyl isocyanate (203 mg, 1.21 mmol, 1 equiv) dissolved in DCM was added to the reaction mixture dropwise, stirred for 5 min at r.t. and refluxed at 60 °C for 16 h. The reaction was monitored by TLC (25% EtOAc/hexanes). Volatile components were removed under vacuum and the resulting solid precipitate was recrystallized with MeOH. The retrieved white color solid 3ad (68%) was oven-dried. 1H NMR (400 MHz, DMSO-d6): δ 9.58 (s, 1H), 7.21–7.37 (d, J 4H), 6.51 (s, 1H), 4.32 (s, 1H), 1.70 (s, 9H). 13C NMR (151 MHz, DMSO-d6): δ 158.23, 140.42, 135.37, 131.33, 129.41, 128.34, 126.14, 96.34, 55.10, 42.57, 29.47. FTIR, cm–1: 3317 (−NH−), 2926 (C–H aliphatic), 1560 (aromatic C=C), 1491 (aromatic C=N), 1614 (C=O), 750 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C15H19ClN4O, 306.1247; found, 306.1249.
N-(4-Chlorophenyl)-4-nitro-1H-pyrazole-1-carbothioamide (3ae)
4-Nitro-1H-pyrazole (75.4 mg, 0.66 mmol) was injected into a suspension of sodium hydride (26.4 mg, 0.66 mmol, 1 equiv) in 10 mL of THF (under Ar gas). Afterward, the solution was allowed to stir at room temperature under argon for 3 h. 4-Chlorophenyl isothiocyanate (112 mg, 0.66 mmol) was then added and the solution was kept stirred overnight. The reaction mixture was concentrated to dryness and recrystallized using hexane, yielding the product 3ae as an off-white solid (55%). 1H NMR (400 MHz, DMSO-d6): δ 9.46 (d, J = 3.6 Hz, N–H), 8.24 (s, J = 3.7 Hz, 1H), 7.98 (s, J = 3.7 Hz, 1H), 7.25 (dd, J = 8.8, 3.6 Hz, 2H), 7.12 (dd, J = 8.7, 3.6 Hz, 2H). 13C NMR (151 MHz, DMSO-d6): δ 166.68, 151.46, 136.64, 135.49, 129.15, 128.18, 125.56, 124.68. FTIR, cm-1: 3388 (−NH−), 3100 (C–H aromatic), 1652 (aromatic C=C), (aromatic C=N), 1254 (C=S), 760 (aromatic C–Cl), 1479 and 1275 (N=O). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H7ClN4O2S, 281.9978; found, 281.9980.
N-(4-Chlorophenyl)-3,5-dimethyl-1H-pyrazole-1-carbothioamide (3af)
A suspension of sodium hydride (38 mg, 1.56 mmol) in THF (10 mL) was cannulated under argon at 0 °C into a round-bottomed flask containing 3,5-dimethyl-1H-pyrazole 12 (100 mg, 1.042 mmol) in 10 mL of THF. Afterward, the solution was allowed to stir at room temperature under argon for 3 h. 4-Chlorophenyl isothiocyanate (197 mg, 1.15 mmol) was then added and the solution was kept stirred overnight. The reaction mixture was concentrated to dryness and recrystallized using diethyl ether, yielding the product 3af as a pale yellow solid (25%). 1H NMR (400 MHz, DMSO-d6): δ 7.19 (d, J = 8.2 Hz, 2H), 7.03 (d, J = 8.4 Hz, 2H), 5.77 (s, 1H), 2.39 (s, 3H), 2.10 (s, 3H)·13C NMR (101 MHz, DMSO-d6): δ 171.34, 152.67, 145.00, 138.76, 128.22, 124.93, 124.87, 106.32, 14.77, 14.10. FTIR, cm-1: 3275 (−NH−), 3130 (C–H aromatic), 2917 (aliphatic C–H), 1595 (aromatic C=C), 1572 (aromatic C=N), 1206 (C=S), 796 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C12H12ClN3S, 265.0440; found, 265.0436.
N-(4-Chlorophenyl)-3,4,5-trimethyl-1H-pyrazole-1-carbothioamide (3ag)
A suspension of sodium hydride (33 mg, 1.36 mmol) in THF (10 mL) was cannulated under argon at 0 °C into a round-bottomed flask containing 3,4,5-trimethyl-1H-pyrazole 13 (100 mg, 0.91 mmol) in 10 mL of THF. Afterward, the solution was allowed to stir at room temperature under argon for 3 h. 4-Chlorophenyl isothiocyanate (154 mg, 0.91 mmol) was then added and the solution was kept stirred overnight. The reaction mixture was concentrated to dryness and recrystallized using diethyl ether, yielding the product 3ag as a white solid (32%). 1H NMR (400 MHz, DMSO-d6): δ 7.20–7.16 (m, 2H), 7.02 (d, J = 8.5 Hz, 2H), 2.31 (s, 3H), 2.05 (s, 3H), 1.83 (s, 3H)·13C NMR (101 MHz, DMSO-d6): δ 171.60, 152.79, 144.21, 135.33, 128.17, 124.89, 124.78, 111.58, 56.71, 19.26, 13.06, 12.44, 8.51. FTIR, cm-1: 3250 (−NH−), 3180 (C–H aromatic), 2922 (aliphatic C–H), 1616 (aromatic C=C), 1574 (aromatic C=N), 1277 (C=S), 752 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C13H14ClN3S, 279.0597; found, 279.0600.
3-Amino-N-(4-Chlorobenzyl)-1H-Pyrazole-1-carbothioamide (3ba) and 1-(4-chlorobenzyl)-3-(1H-pyrazol-3-yl)thiourea (3bb)
1H-Pyrazol-3-amine (1 g, 12.04 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorobenzyl isothiocyanate (2.2 g, 12.04 mmol) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C. The reaction was monitored by TLC (50% EtOAc/hexanes). 3ba shows Rf = 0.75 and 3bb shows Rf = 0.45. All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% hexanes to 25% EtOAc/hexanes) to afford 65% yield of 3ba and 8% yield of 3bb as white color solids. 3ba: 1H NMR (400 MHz, DMSO-d6): δ 10.55 (s, 1H), 7.38 (d, J = 2.0 Hz, 1H), 7.36 (s, 2H), 7.35 (s, 2H), 7.33 (d, J = 2.4 Hz, 2H), 5.40 (d, J = 2.0 Hz, 1H), 4.77 (d, J = 4.6 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 176.76, 152.41, 142.01, 137.33, 132.27, 130.12, 128.91, 89.21, 46.44. FTIR, cm-1: 3406, 3297 (−NH2), 3247 (−NH−), 3047 (C–H aromatic), 2960, 2919 (CH pyrazole), 1605 (aromatic C=C), 1517 (aromatic C=N), 1215 (C=S), 731 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11ClN4S, 266.039; found, 266.038. 3bb:1H NMR (400 MHz, DMSO-d6): δ 9.86 (t, J = 6.3 Hz, 1H), 8.29 (d, J = 2.9 Hz, 1H), 7.37 (m, 2H), 7.35 (s, 2H), 5.87 (d, J = 2.9 Hz, 1H), 5.57 (s, 1H), 4.79 (d, J = 6.3 Hz, 2H). 13C NMR (101 MHz, DMSO-d6): δ 175.11, 159.26, 137.76, 133.18, 132.26, 130.13, 128.90, 101.56, 47.48. FTIR, cm-1: 3278 (−NH−), 3112 (C–H aromatic), 1564 (aromatic C=C), 1524 (aromatic C=N), 1268 (C=S), 724 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11ClN4S, 266.039; found, 266.036.
3-Amino-N-(4-chlorophenyl)-1H-pyrazole-1-carbothioamide (3bc) and 1-(4-Chlorophenyl)-3-(1H-pyrazol-3-yl)thiourea (3bd)
1H-Pyrazol-3-amine (1 g, 12.04 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorophenyl isothiocyanate (2.04 g, 12.04 mmol) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then stirred for 20 h at room temperature. The reaction was monitored by TLC (50% EtOAc/hexanes). 3bc shows Rf = 0.75 and 3bd shows Rf = 0.45. All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% hexanes to 25% EtOAc/hexanes) to afford 55% yield of 3bc as a white color solid and 35% yield of 3bd as an off-white color solid. 3bc:1H NMR (400 MHz, DMSO-d6): δ 10.99 (s, J = 4.5 Hz, 1H), 8.41 (s, 1H), 7.67 (d, 2H), 7.45 (d, 2H), 6.98 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 176.30, 149.26, 137.93, 129.68, 129.08, 128.35, 125.75, 94.31. FTIR, cm-1: 3471, 3460 (−NH2), 3305 (−NH−), 3060–3005 (C–H aromatic), 2960, 2920 (CH pyrazole), 1610, 1588 (aromatic C=C), 1516 (aromatic C=N), 1242 (C=S), 640 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9ClN4S, 252.0236; found, 252.0234.
3bd: 1H NMR (600 MHz, DMSO-d6): δ 12.70 (s, 1H), 11.78 (s, 1H), 10.83 (s, 1H), 7.76 (d, J = 2.4 Hz, 1H), 7.76 (m, 2H), 7.44 (m, 2H), 6.04 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 176.74, 149.74, 138.41, 130.20, 129.57, 128.86, 126.27, 94.81. FTIR, cm-1: 3361 (−NH−), 3060 (C–H aromatic), 1577 (aromatic C=C), 1524 (aromatic C=N), 1258 (C=S), 754 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9ClN4S, 252.0236; found, 252.0236.
1-(4-Chlorophenyl)-3-(1-methyl-1H-pyrazol-3-yl)thiourea (3be)
1-Methyl-1H-pyrazol-3-amine (460 mg, 4.73 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorophenyl isothiocyanate (800 mg, 4.73 mmol) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then stirred for 20 h at room temperature. The reaction was monitored by TLC (50% EtOAc/hexanes). All the volatile components were removed under vacuum and the resulting off-white solid was recrystallized with MeOH. The retrieved off-white solid 3be (69%) was oven-dried for NMR analysis. 1H NMR (600 MHz, DMSO-d6): δ 7.70 (d, J = 2.3 Hz, 2H), 7.67 (d, J = 2.3 Hz, 2H), 7.43 (d, 1H), 6.01 (s, 1H), 3.82 (s, 3H). 13C NMR (151 MHz, DMSO-d6): δ 176.63, 149.12, 138.45, 132.38, 129.59, 129.17–128.40 (m), 126.22, 95.32, 39.02. FTIR, cm-1: 3186 (−NH−), 3013 (C–H aromatic), 1573 (aromatic C=C), 1526 (aromatic C=N), 1266 (C=S), 659 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11ClN4S, 266.0393; found, 266.0394.
1-(4-Chlorobenzyl)-3-(1-methyl-1H-pyrazol-3-yl)thiourea (3bf)
1-Methyl-1H-pyrazol-3-amine (47 mg, 0.48 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorobenzyl isothiocyanate (88 mg, 0.48 mmol) was dissolved in DCM separately and added to the stirring reaction mixture. The reaction mixture was then stirred for 20 h at room temperature. The reaction was monitored by TLC (30% EtOAc/hexanes). All the volatile components were removed under vacuum and the resulting off-white solid was recrystallized with MeOH. An off-white solid 3bf (85%) was retrieved. 1H NMR (400 MHz, chloroform-d): δ 9.77 (s, 1H), 8.00 (s, 1H), 6.99 (m, 4H), 6.87 (d, J = 2.3 Hz, 1H), 5.42 (d, J = 2.3 Hz, 0H), 4.61 (dd, 2H), 3.41 (d, J = 1.3 Hz, 3H). 13C NMR (101 MHz, chloroform-d): δ 178.71, 154.57, 148.93, 136.57, 131.32, 129.05, 94.14, 92.95, 49.02, 38.63. FTIR, cm-1: 3199 (−NH−), 3061 (C–H aromatic), 1584 (aromatic C=C), 1530 (aromatic C=N), 1301 (C=S), 744 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C12H13ClN4S, 280.0549; found, 280.0550.
1-(1-(tert-Butyl)-1H-pyrazol-3-yl)-3-(4-chlorobenzyl)thiourea (3bg)
1-(tert-Butyl)-1H-pyrazol-3-amine (75 mg, 0.54 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorobenzyl isothiocyanate (108 mg, 0.58 mmol) was dissolved in DCM separately and added to the stirring reaction mixture. The reaction mixture was then stirred for 20 h at room temperature. The reaction was monitored by TLC (30% EtOAc/hexanes). All the volatile components were removed under vacuum and the resulting off-white solid was recrystallized with MeOH. An off-white solid 3bg (58%) was retrieved. 1H NMR (400 MHz, chloroform-d): δ 10.31 (s, 1H), 8.59 (s, 1H), 7.35 (s, 1H), 7.32 (m, 4H), 5.76 (s, 1H), 4.88 (s, 2H), 1.40 (s, 9H). 13C NMR (101 MHz, chloroform-d): δ 178.17, 148.53, 136.35, 133.59, 129.45, 129.05, 126.69, 93.38, 58.71, 49.34, 29.54. FTIR, cm-1: 3202 (−NH−), 3075 (C–H aromatic), 2974 (aliphatic C–H), 1579 (aromatic C=C), 1528 (aromatic C=N), 1295 (C=S), 734 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C15H19ClN4S, 322.1019; found, 322.1016.
N-(4-Chlorophenyl)-3-methyl-1H-pyrazole-1-carbothioamide (3bh)
3-Methyl-1H-pyrazole (213 mg, 2.59 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Chlorophenyl isothiocyanate (440 mg, 2.6 mmol, 1 equiv) dissolved in DCM was added to the reaction mixture dropwise and stirred for 20 h at room temperature. The reaction mixture was monitored by TLC (25% EtOAc/hexanes). There was a solid precipitate in a slightly orange color solution. The precipitate was isolated by gravity filtration and washed several times with cold DCM. The resulting solid precipitate was recrystallized with MeOH. The retrieved off-white color solid 3bh (76.1%) was oven-dried. 1H NMR (400 MHz, DMSO-d6): δ 11.70 (s, J = 4.5 Hz, 1H), 8.63 (s, J = 2.8 Hz, 1H), 7.66 (d, J = 6.1 Hz, 2H), 7.48 (d, J = 6.1 Hz, 2H), 6.46 (s, J = 2.8 Hz, 1H), 2.33 (s, J = 5.1 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 174.28, 152.93, 137.11, 132.51, 129.87, 128.37, 127.74, 127.46, 110.72, 103.59, 13.61. FTIR, cm-1: 3278 (−NH−), 3050 (C–H aromatic), 2921 (aliphatic C–H), 1587 (aromatic C=C), 1534 (aromatic C=N), 1189 (C=S), 694 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H10ClN3S, 251.0284; found, 251.0283.
N-(4-Nitrophenyl)-1H-pyrazole-1-carbothioamide (3bi)
Pyrazole (189 mg, 2.78 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Nitrophenyl isothiocyanate (500 mg, 2.78 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then stirred vigorously for 20 h at room temperature. The reaction was monitored by TLC (DCM/methanol 20:1). All the volatile components were removed under vacuum and the resulting yellow solid was recrystallized with MeOH. The retrieved yellow color solid 3bi (65%) was oven-dried. 1H NMR (400 MHz, DMSO-d6): δ 8.77 (d, J = 2.9 Hz, 1H), 8.29 (t, 2H), 8.04 (d, 2H), 7.66 (d, 1H), 6.68 (t, 1H). 13C NMR (101 MHz, DMSO-d6): δ 144.43, 132.77, 127.95, 126.51, 126.01, 124.71, 111.28, 104.87. FTIR, cm-1: 3254 (−NH−), 3125 (C–H aromatic), 1605 (aromatic C=C), 1593 (aromatic C=N), 1238 (C=S), 1501 and 1322 (N=O). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H8N4O2S, 248.0368; found, 248.0366.
3-Amino-N-(4-nitrophenyl)-1H-pyrazole-1-carbothioamide (3bj) and 1-(4-Nitrophenyl)-3-(1H-pyrazol-3-yl)thiourea (3bk)
1H-Pyrazol-3-amine (230 mg, 2.77 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Nitrophenyl isothiocyanate (500 mg, 2.77 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C. The reaction was monitored by TLC (DCM/methanol 50:2). All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (DCM/methanol 1–10%) to afford 66% yield of 3bj, a yellow color solid, and 31% yield of 3bk, a pale yellow color solid. 3bj: 1H NMR (400 MHz, DMSO-d6): δ 11.35 (s, N–H), 8.44 (d, J = 3.0 Hz, 1H), 8.26 (d, 2H), 8.05 (d, 2H), 6.04 (d, J = 3.1 Hz, 1H), 5.85 (s, NH2). 13C NMR (151 MHz, DMSO-d6): δ 172.90, 159.33, 144.98, 144.54, 133.27, 125.10, 124.43, 103.37. FTIR, cm-1: 3492 and 3380 (−NH2), 3228 (−NH−), 3140 (C–H aromatic), 1610 (aromatic C=C), 1593 (aromatic C=N), 1251 (C=S), 1502 and 1329 (N=O). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9N5O2S, 263.0477; found, 263.0475. 3bk: 1H NMR (400 MHz, DMSO-d6): δ 12.76 (NH, 1H), 11.03 (NH, 1H), 8.24 (d, J = 8.5 Hz, 2H), 8.05 (d, J = 8.3 Hz, 2H), 7.75 (d, J = 7.4 Hz, 1H), 6.10 (d, J = 7.4 Hz, 1H). 13C NMR (101 MHz, DMSO-d6): δ 176.55, 149.63, 146.04, 143.91, 130.54, 125.05, 123.21, 95.57. FTIR, cm–1: 3303 (−NH−), 3060 (C–H aromatic), 1645 (aromatic C=C), 1593 (aromatic C=N), 1272 (C=S), 1483 and 1316 (N=O). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9N5O2S, 263.0477; found, 263.0478.
3-Amino-N-(4-methoxyphenyl)-1H-pyrazole-1-carbothioamide (3bl)
1H-Pyrazol-3-amine (251 mg, 3.03 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Methoxyphenyl isothiocyanate (450 mg, 3.03 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C. The reaction was monitored by TLC (100% DCM). All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% DCM) to afford 75% yield of 3bl, an off-white solid. 1H NMR (400 MHz, DMSO-d6): δ 7.73 (d, J = 2.4 Hz, 1H), 7.44 (d, J = 8.5 Hz, 2H), 6.93 (d, 2H), 6.01 (s, 1H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO-d6): δ 177.36, 157.56, 150.18, 132.52, 130.29, 126.85, 114.33, 94.83, 55.94. FTIR, cm-1: 3413 (−NH2), 3184 (−NH−), 3051 (C–H aromatic), 2929 (C–H), 1583 (aromatic C=C), 1535 (aromatic C=N), 1250 (C=S), 1232 (C–O). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H12N4OS, 248.0730; found, 248.0731.
3-Amino-N-(p-tolyl)-1H-pyrazole-1-carbothioamide (3bm)
1H-Pyrazol-3-amine (278 mg, 3.35 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Tolyl isothiocyanate (500 mg, 3.35 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C. The reaction was monitored by TLC (DCM/methanol, 50:2). All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% DCM) to afford 62% yield of 3bm, an off-white solid. 1H NMR (400 MHz, DMSO-d6): δ 7.73 (d, J = 2.0 Hz, 1H), 7.47 (dd, J = 8.3, 2.0 Hz, 2H), 7.17 (d, 2H), 6.01 (s, 1H), 2.29 (d, J = 1.9 Hz, 3H). 13C NMR (101 MHz, DMSO-d6): δ 176.97, 150.15, 137.11, 135.13, 129.60, 124.88, 102.22, 94.87, 21.23. FTIR, cm-1: 3374 (−NH2), 3185 (−NH−), 3046 (C–H aromatic), 2980 (aliphatic C–H), 1573 (aromatic C=C), 1537 (aromatic C=N), 1250 (C=S), 1520 (C=C–C). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H12N4S, 232.0783; found, 232.0781.
3-Amino-N-(4-bromophenyl)-1H-pyrazole-1-carbothioamide (3bn)
1H-Pyrazol-3-amine (194 mg, 2.34 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Bromophenyl isothiocyanate (500 mg, 2.34 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C. The reaction was monitored by TLC (DCM/methanol, 50:2). All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% DCM) to afford 50% yield of 3bn, a pale yellow color solid. 1H NMR (400 MHz, DMSO-d6): δ 7.73 (d, J = 3.1 Hz, 1H), 7.60 (d, 2H), 7.54 (d, 2H), 6.02 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 176.66, 149.72, 138.84, 131.78, 130.20, 126.53, 117.75, 94.80. FTIR, cm-1: 3361, 3338 (−NH2), 3176 (−NH−), 3047 (C–H aromatic), 2960, 2920 (CH pyrazole), 1581, (aromatic C=C), 1533 (aromatic C=N), 1264 (C=S), 701 (aromatic C–Br). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9BrN4S, 295.9731; found, 295.9734.
3-Amino-N-(4-fluorophenyl)-1H-pyrazole-1-carbothioamide (3bo)
1H-Pyrazol-3-amine (271 mg, 3.26 mmol) was dissolved in DCM and stirred in a 100 mL round-bottomed flask. 4-Fluorophenyl isothiocyanate (500 mg, 3.26 mmol, 1 equiv) was dissolved in DCM separately and added to the stirring reaction mixture dropwise. The reaction mixture was then refluxed for 20 h at 60 °C and solid sedimentation was observed during refluxing. The reaction was monitored by TLC (DCM/methanol 50:2). All volatile components were evaporated under vacuum and the resulting crude product was purified by flash column chromatography (100% DCM) to afford 55% yield of 3bo, an off-white to pale yellow color solid. 1H NMR (400 MHz, DMSO-d6): δ 7.74 (s, 1H), 7.60 (dt, J = 9.4, 4.8 Hz, 2H), 7.21 (td, J = 8.9, 4.9 Hz, 2H), 6.02 (s, 1H). 13C NMR (151 MHz, DMSO-d6): δ 177.22, 160.81, 149.81, 135.73 (d, J = 2.9 Hz), 130.15, 127.18 (d, J = 8.3 Hz), 115.61 (d, J = 22.4 Hz), 94.70. FTIR, cm-1: 3332 (−NH2), 3166 (−NH−), 3062 (C–H aromatic), 1608 (aromatic C=C), 1579 (aromatic C=N), 1273 (C=S), 1184 (aromatic C–F). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9FN4S, 236.0532; found, 236.0530.
1-(1-(tert-Butyl)-1H-pyrazol-3-yl)-3-(4-chlorophenyl)thiourea (3ca)
A solution was prepared by combining 1-(tert-butyl)-1H-pyrazol-3-amine 2 (359 mg, 2.58 mmol), 4-chlorophenyl isothiocyanate (438 mg, 2.58 mmol), and 25 mL of distilled THF. This solution was kept stirred under argon at room temperature overnight. The reaction mixture was concentrated to dryness and then recrystallized using diethyl ether to give the product 3ca as an off-white solid (45%). 1H NMR (400 MHz, DMSO-d6): δ 11.85 (s, 1H), 10.81 (s, 1H), 7.79 (d, J = 2.5 Hz, 1H), 7.74 (d, 1H), 7.43 (d, 1H), 5.98 (m, 1H), 1.50 (s, 9H). 13C NMR (101 MHz, DMSO-d6): δ 176.42, 148.88, 138.65, 129.48, 129.16, 128.63, 125.49, 95.17, 58.99, 29.79. FTIR, cm-1: 3193 (−NH−), 3011 (C–H aromatic), 2971 (aliphatic C–H), 1567 (aromatic C=C), 1524 (aromatic C=N), 1258 (C=S), 744 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C14H17ClN4S, 308.0862; found, 308.0865.
N-(4-Chlorophenyl)-1H-pyrazole-1-carbothioamide (3cb)
1H-Pyrazole (200 mg, 2.94 mmol) was dissolved in THF and stirred in a 100 mL round-bottomed flask. Sodium carbonate (311 mg, 2.94 mmol, 1 equiv) was placed in the reaction mixture and stirred vigorously for 1 h at 50 °C. 4-Chlorophenyl isothiocyanate (500 mg, 3.25 mmol, 1 equiv) was added and refluxed at 70 °C for 15 h. The reaction mixture was monitored by TLC (25% EtOAc/hexanes). The sodium carbonate solid was filtered off by gravity filtration. THF was evaporated in vacuo. The resulting pale yellow solid was recrystallized with minimum MeOH. The retrieved off-white-solid 3cb (62%) was oven-dried. 1H NMR (400 MHz, DMSO-d6): δ 8.77 (s, J = 2.6 Hz, 1H), 7.95 (d, 1H), 7.48 (d, 1H), 7.21 (d, 2H), 7.04 (d, 2H), 6.26 (s, J = 1.9 Hz, 1H). 13C NMR (151 MHz, DMSO-d6): δ 169.00, 162.79, 152.47, 140.03, 130.88, 127.91, 125.19, 105.34. FTIR, cm-1: 3453 (−NH−), 3250 (C–H aromatic), 1645 (aromatic C=C), 1538 (aromatic C=N), 1236 (C=S), 762 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H8ClN3S, 237.0127; found, 237.0124.
N-(4-Methoxyphenyl)-1H-pyrazole-1-carbothioamide (3cc)
A mixture of 1H-pyrazole (206 mg, 3.03 mmol) and 4-methoxyphenyl isothiocyanate (500 mg, 3.03 mmol) was mixed in DMF. Anhydrous potassium carbonate (321 mg, 3.03 mmol) was added to the reaction mixture and stirred at room temperature for 20 h. The reaction mixture was monitored by TLC (33% EtOAc/hexanes). Na2CO3 was filtered off by gravity filtration. DMF was removed from the filtrate by vacuum. The resulting crude mixture was gluey and retained on the glass wall. The product was recrystallized with methanol to retrieve a pale yellowish solid product 3cc (65%). 1H NMR (400 MHz, DMSO-d6): δ 8.76 (s, 1H), 7.45 (s, 1H), 7.05 (d, J = 8.0 Hz, 2H), 6.78 (d, J = 8.8 Hz, 2H), 6.22 (s, 1H), 3.70 (m, 3H). 13C NMR (101 MHz, DMSO-d6): δ 173.45, 156.67, 146.35, 133.82, 128.15, 123.66, 114.84, 109.64, 79.93. FTIR, cm-1: 3272 (−NH−), 3125 (C–H aromatic), 2840 (C–H), 1651 (aromatic C=C), 1490 (aromatic C=N), 1250 (C=S), 1234 (C–O). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11N3OS, 233.0623; found, 233.0623.
N-(4-Fluorophenyl)-1H-pyrazole-1-carbothioamide (3cd)
1H-Pyrazole (221 mg, 3.25 mmol) was dissolved in THF and stirred in a 100 mL round-bottomed flask. Sodium carbonate (344 mg, 3.25 mmol, 1 equiv) was placed in the reaction mixture and stirred vigorously for 1 h at 50 °C. 4-Fluorophenyl isothiocyanate (500 mg, 3.25 mmol, 1 equiv) was added and refluxed at 70 °C for 12 h. The reaction was monitored by TLC (33% ethyl acetate/hexane). The sodium carbonate solid was filtered off by gravity filtration. THF was evaporated in vacuo. The resulting pale yellow solid was recrystallized with minimum MeOH. The retrieved pale yellow color solid 3cd (55%) was oven-dried. 1H NMR (400 MHz, DMSO-d6): δ 11.85 (s, 1H), 8.74 (s, 1H), 7.98 (s, 1H), 7.63 (d, 2H), 7.28 (d, J = 9.2 Hz, 2H), 6.63 (s, 1H). 13C NMR (101 MHz, DMSO-d6): δ 175.46, 144.06, 132.66, 128.91, 117.70, 116.09, 110.72, 104.89. FTIR, cm-1: 3245 (−NH−), 3150 (C–H aromatic), 1608 (aromatic C=C), 1548 (aromatic C=N), 1291 (C=S), 1215 (aromatic C–F). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H8FN3S, 221.0423; found, 221.0421.
N-(p-Tolyl)-1H-pyrazole-1-carbothioamide (3ce)
A mixture of 1H-pyrazole (228 mg, 3.34 mmol) and 4-tolyl isothiocyanate (500 mg, 3.34 mmol) was mixed in DMF. Anhydrous sodium carbonate (355 mg, 3.34 mmol) was added to the reaction mixture and stirred at room temperature for 20 h. The reaction was monitored by TLC (33% EtOAc/hexanes). Na2CO3 was filtered off by gravity filtration. DMF was removed in vacuo. The resulting crude mixture was of orange color, gluey, and retained on the glass wall. The product was recrystallized with methanol washed with cold DCM to retrieve an off-white solid product 3ce (68%). 1H NMR (600 MHz, DMSO-d6): δ 8.75 (d, J = 2.8 Hz, 1H), 7.97 (d, J = 1.6 Hz, 1H), 7.50 (m, 2H), 7.25 (m, 2H), 6.63 (dd, J = 2.8, 1.7 Hz, 1H), 2.33 (s, 3H). 13C NMR (151 MHz, DMSO-d6): δ 174.62, 143.66, 132.32, 130.81, 129.42, 125.91, 110.32, 104.67, 21.17. FTIR, cm-1: 3277 (−NH−), 3119 (C–H aromatic), 2914 (aliphatic C–H), 1658 (aromatic C=C), 1584 (aromatic C=N), 1240 (C=S), 1516 (C=C–C). HRMS (+ESI-TOF) m/z: [M+] calcd for C11H11N3S, 217.0674; found, 217.0671.
N-(4-Bromophenyl)-1H-pyrazole-1-carbothioamide (3cf)
A mixture of 1H-pyrazole (159 mg, 2.33 mmol) and 4-bromophenyl isothiocyanate (500 mg, 2.33 mmol) was mixed in DMF. Anhydrous sodium carbonate (248 mg, 2.33 mmol) was added to the reaction mixture and stirred at room temperature for 20 h. The reaction was monitored by TLC (33% EtOAc/hexanes). Na2CO3 was filtered off by gravity filtration. DMF was distilled off under reduced pressure. The crude product was recrystallized with methanol and washed with cold DCM to retrieve an off-white solid product 3cf (60%). 1H NMR (400 MHz, DMSO-d6): δ 8.73 (d, 1H), 7.93 (d, J = 14.6 Hz, 1H), 7.71–7.48 (m, 2H) over lapped, 7.40 (d, 2H), 6.59 (d, 1H). 13C NMR (101 MHz, DMSO-d6): δ 170.15, 143.69, 133.50, 131.99, 128.70, 121.33, 110.26, 104.87. FTIR, cm-1: 3263 (−NH−), 3152 (C–H aromatic), 1630 (aromatic C=C), 1584 (aromatic C=N), 12360 (C=S), 668 (aromatic C–Br). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H8BrN3S, 280.9622; found, 280.9620.
4-Amino-N-(4-chlorophenyl)-1H-pyrazole-1-carbothioamide (3cg)
1H-pyrazol-4-amine, 10 (325 mg, 3.92 mmol) was dissolved in THF and stirred in a 100 mL round-bottomed flask. Sodium carbonate (416 mg, 3.92 mmol, 1 equiv) was placed in the reaction mixture and stirred vigorously for 1 h at 50 °C. 4-Chlorophenyl isothiocyanate (665 mg, 3.92 mmol) was added and refluxed at 70 °C for 18 h. The reaction was monitored by TLC (25% EtOAc/hexanes). The sodium carbonate solid was filtered off by gravity filtration. THF was evaporated in vacuo. The resulting crude product was purified by flash column chromatography (100% hexane to 10% ethyl acetate/hexane) to afford 55% yield of 3cg, as an off-white to pale yellow color solid. 1H NMR (600 MHz, Chloroform-d): δ 8.81 (m, NH), 7.34 (d, J = 8.2 Hz, 2H), 7.29 (s, 1H), 7.26 (m, 2H), 4.17 (d, J = 9.9 Hz, 1H). 13C NMR (151 MHz, chloroform-d): δ 189.45, 139.87, 135.41, 130.83, 129.19, 124.52 122.93, 117.47. FTIR, cm-1: 3234, 3170 (−NH2), 3104 (−NH−), 3055 (C–H aromatic), 2919 (CH pyrazole), 1590, (aromatic C=C), 1544 (aromatic C=N), 1200 (C=S), 650 (aromatic C–Cl). HRMS (+ESI-TOF) m/z: [M+] calcd for C10H9ClN4S, 252.0236; found, 252.0232.
3-(Benzyloxy)-N-(4-chlorophenyl)-1H-pyrazole-1-carbothioamide (3ch)
3-(Benzyloxy)-1H-pyrazole (17) (400 mg, 2.30 mmol) was dissolved in dry THF and transferred into a round-bottomed flask, which consisted of K2CO3 (317.8 mg, 2.30 mmol). The reaction mixture was stirred vigorously for 1 h at 50 °C. Then, 4-chlorophenyl isothiocyanate (390 mg, 2.30 mmol) was added and refluxed at 70 °C for 18 h. The reaction was monitored by TLC (25% EtOAc/hexanes). The potassium carbonate solid was filtered off by gravity filtration. THF was evaporated in vacuo. The resulting crude product was purified by flash column chromatography (100% hexane to 5% ethyl acetate/hexane) to afford 49% yield of 3ch, an off-white color solid. 1H NMR (600 MHz, chloroform-d): δ 10.24 (s, N–H), 8.61 (t, J = 3.3 Hz, 1H), 7.73 (dd, J = 8.7, 3.2 Hz, 2H), 7.49 (dd, J = 7.9, 2.9 Hz, 2H), 7.42 (dddd, J = 15.0, 13.0, 8.4, 4.5 Hz, 5H), 6.07 (t, J = 3.3 Hz, 1H), 5.33 (d, J = 3.2 Hz, 2H). 13C NMR (151 MHz, Chloroform-d): δ 173.04, 164.78, 135.83 (d, J = 8.7 Hz), 133.02, 132.08, 129.12, 128.71, 128.54, 127.99, 125.25, 99.46, 71.37. FTIR, cm-1: 3294 (−NH−), 3010 (C–H aromatic), 2929 (aliphatic C–H), 1558 (aromatic C=C), 1502 (aromatic C=N), 1246 (C=S), 762 (aromatic C–Cl), 1342 and 1002 (alkyl aryl C–O–C). HRMS (+ESI-TOF) m/z: [M+] calcd for C17H14ClN3OS, 343.0546; found, 343.0548.
Acknowledgments
The authors acknowledge financial support from NIH/DHHS 1R01Al121364-01A1; PI: O.K. at UAB and the Johnson Cancer Research Center at Kansas State University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c04513.
1H NMR and 13C NMR spectra of all newly synthesized compounds and a selection of typical UPLC-MS chromatograms (PDF)
Author Present Address
∥ East Tennessee State University, Bill Gatton College of Pharmacy, Johnson City, TN 37614, United States.
Author Present Address
⊥ Department of Chemical and Biomolecular Engineering, Vanderbilt University, Nashville, TN 37235, United States.
Author Present Address
# Department of Chemistry, Southwestern College, 100 College Street, Winfield, KS 67156, United States.
The authors declare no competing financial interest.
Supplementary Material
References
- https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e1.htm (accessed on October 28, 2020).
- https://health.gov/hcq/prevent-hai-action-plan.asp (accessed on October 28, 2020).
- Patridge E.; Gareiss P.; Kinch M. S.; Hoyer D. An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discovery Today 2016, 21, 204–207. 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Durand G. A.; Raoult D.; Dubourg G. Antibiotic discovery: history, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. 10.1016/j.ijantimicag.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Igarashi M. New natural products to meet the antibiotic crisis: a personal journey. J. Antibiot. 2019, 72, 890–898. 10.1038/s41429-019-0224-6. [DOI] [PubMed] [Google Scholar]
- Rossiter S. E.; Fletcher M. H.; Wuest W. M. Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G.; Berni R.; Muñoz-Sanchez J.; Apone F.; Abdel-Salam E.; Qahtan A.; Alatar A.; Cantini C.; Cai G.; Hausman J.-F.; Siddiqui K.; Hernández-Sotomayor S.; Faisal M. Production of plant secondary metabolites: examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. 10.3390/genes9060309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H. A. Harnessing evolutionary diversification of primary metabolism for plant synthetic biology. J. Biol. Chem. 2019, 294, 16549–16566. 10.1074/jbc.rev119.006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. Where have All the Antibiotics Gone?. Can. J. Infect. Dis. Med. Microbiol. 2006, 17, 287–290. 10.1155/2006/707296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie K. R. Antimicrobial resistance: the good, the bad, and the ugly. Emerging Top. Life Sci. 2020, 4, 129–136. 10.1042/etls20190194. [DOI] [PubMed] [Google Scholar]
- Macario A. J. L.; De Macario E. C. Monoclonal antibodies against bacteria. Biotechnol. Adv. 1988, 6, 135–150. 10.1016/0734-9750(88)90001-8. [DOI] [PubMed] [Google Scholar]
- Wu C.; Nakka S.; Mansouri S.; Bengtsson T.; Nayeri T.; Nayeri F. In vitro model of production of antibodies; a new approach to reveal the presence of key bacteria in polymicrobial environments. BMC Microbiol. 2016, 16, 209. 10.1186/s12866-016-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanjary M.; Medema M. H. Mining bacterial genomes to reveal secret synergy. J. Biol. Chem. 2018, 293, 19996–19997. 10.1074/jbc.h118.006669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings M. I.; Truman A. W.; Wilkinson B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Haeili M.; Moore C.; Davis C. J. C.; Cochran J. B.; Shah S.; Shrestha T. B.; Zhang Y.; Bossmann S. H.; Benjamin W. H.; Kutsch O.; Wolschendorf F. Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 3727–3736. 10.1128/aac.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalecki A. G.; Malalasekera A. P.; Schaaf K.; Kutsch O.; Bossmann S. H.; Wolschendorf F. Combinatorial phenotypic screen uncovers unrecognized family of extended thiourea inhibitors with copper-dependent anti-staphylococcal activity. Metallomics 2016, 8, 412–421. 10.1039/c6mt00003g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold A. N.; Culbertson E. M.; Culotta V. C. The Yin and Yang of copper during infection. JBIC, J. Biol. Inorg. Chem. 2016, 21, 137–144. 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl-Fie T. E.; Skaar E. P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. 10.1016/j.cbpa.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer A.; Shrestha T. B.; Bossmann S. H.; Basaraba R. J.; Harber G. J.; Michalek S. M.; Niederweis M.; Kutsch O.; Wolschendorf F. Copper-boosting compounds: a novel concept for antimycobacterial drug discovery. Antimicrob. Agents Chemother. 2013, 57, 1089–1091. 10.1128/aac.01781-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschliman K.; Bossmann S. Synthesis of Isothiocyanates: An Update. Synthesis 2019, 51, 1746–1752. 10.1055/s-0037-1612303. [DOI] [Google Scholar]
- Forryan C. L.; Klymenko O. V.; Brennan C. M.; Compton R. G. Heterogeneous kinetics of the dissolution of an inorganic salt, potassium carbonate, in an organic solvent, dimethylformamide. J. Phys. Chem. B 2005, 109, 8263–8269. 10.1021/jp0407573. [DOI] [PubMed] [Google Scholar]
- Ganguly S.; Jacob S. K. Therapeutic Outlook of Pyrazole Analogs: A Mini Review. Mini. Rev. Med. Chem. 2017, 17, 959–983. 10.2174/1389557516666151120115302. [DOI] [PubMed] [Google Scholar]
- Baba T.; Bae T.; Schneewind O.; Takeuchi F.; Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 2008, 190, 300–310. 10.1128/jb.01000-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalecki A. G.; Zorn K. M.; Clark A. M.; Ekins S.; Narmore W. T.; Tower N.; Rasmussen L.; Bostwick R.; Kutsch O.; Wolschendorf F. High-throughput screening and Bayesian machine learning for copper-dependent inhibitors of Staphylococcus aureus. Metallomics 2019, 11, 696–706. 10.1039/c8mt00342d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiero H.; Townsend D. M.; Tew K. D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besold A. N.; Culbertson E. M.; Culotta V. C. The Yin and Yang of copper during infection. JBIC, J. Biol. Inorg. Chem. 2016, 21, 137–144. 10.1007/s00775-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D.; Maser J.; Lai B.; Cai Z.; Barry C. E. III; Höner zu Bentrup K.; Russell D. G.; Bermudez L. E. Elemental Analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-Containing Phagosomes Indicates Pathogen-Induced Microenvironments within the Host Cell’s Endosomal System. J. Immunol. 2005, 174, 1491–1500. 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- Ji N.; Meredith E.; Liu D.; Adams C. M.; Artman G. D.; Jendza K. C.; Ma F.; Mainolfi N.; Powers J. J.; Zhang C. Syntheses of 1-substituted-3-aminopyrazoles. Tetrahedron Lett. 2010, 51, 6799–6801. 10.1016/j.tetlet.2010.10.073. [DOI] [Google Scholar]
- Chen X.; She J.; Shang Z.-C.; Wu J.; Zhang P. Room-temperature synthesis of pyrazoles, diazepines, β-enaminones, and β-enamino esters using silica-supported sulfuric acid as a reusable catalyst under solvent-free conditions. Synth. Commun. 2009, 39, 947–957. 10.1080/00397910802441551. [DOI] [Google Scholar]
- Merlic C. A.; Motamed S.; Quinn B. Structure Determination and Synthesis of Fluoro Nissl Green: An RNA-Binding Fluorochrome. J. Org. Chem. 1995, 60, 3365–3369. 10.1021/jo00116a020. [DOI] [Google Scholar]
- Huang H.; Si P.; Wang L.; Xu Y.; Xu X.; Zhu J.; Jiang H.; Li W.; Chen L.; Li J. Design, Synthesis, and Biological Evaluation of Novel Nonsteroidal Farnesoid X Receptor (FXR) Antagonists: Molecular Basis of FXR Antagonism. ChemMedChem 2015, 10, 1184–1199. 10.1002/cmdc.201500136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.