Abstract
Background:
Adrenocortical carcinoma (ACC) is an aggressive malignancy that frequently metastasizes to the liver. Given the limitations of systemic therapy in this setting, we sought to determine characteristics associated with a two-fold increase in survival with surgical management compared to what has been reported with chemotherapy alone (∼12 months).
Methods:
Patients who underwent resection/ablation at our institutions for ACC liver metastases were identified. Those who survived between 12–24 months after metastasectomy were excluded, as the aim was to characterize patients who most clearly benefited from these procedures. Clinicopathologic and treatment characteristics were assessed for associations with survival.
Results:
Sixty-two patients met inclusion criteria, of whom 44 survived greater than 24 months and 18 survived less than 12 months. Patients with extended survival were less likely to have functioning tumors (p=0.047), had fewer liver metastases (p=0.047), and a longer disease-free interval (DFI) (median 17.6 vs 2.3 months, p<0.0001). On multivariable analysis, DFI (OR=1.33, 95%CI=1.12–1.58, p=0.0012) and non-functioning tumor (OR=0.13, 95%CI=0.13–0.56, p=0.0056) were independently associated with prolonged survival.
Discussion:
Metastasectomy or ablation should be considered for patients with ACC liver metastases. Prolonged DFI and non-functioning tumors are associated with extended survival and may be useful in selecting optimal candidates for these procedures.
INTRODUCTION
Adrenocortical carcinoma (ACC) is an aggressive malignancy with a high propensity for metastasis and limited systemic treatment options. Nearly a third of ACC patients present with stage IV disease,(1–3) and more than half of those who undergo resection of localized disease develop distant recurrences.(2–4) Although used routinely in its treatment, mitotane and cytotoxic chemotherapy are of limited benefit in advanced ACC, and overall survival is approximately one year on the most effective multi-drug regimens available.(5, 6) Furthermore, the discovery of potential molecular targets such as insulin-like growth factor 2 (IGF-2) have not produced any efficacious targeted agents to date.(7, 8) Given the current limitations of systemic therapy, complete resection remains the only potentially curative treatment for metastatic ACC.(2, 9, 10)
The liver is one of the most common sites of ACC metastasis.(3, 4) Several retrospective series have suggested that local treatment with hepatic metastasectomy or radiofrequency ablation (RFA) confers a substantial survival benefit to select ACC patients. However, the extent of this benefit and the factors most predictive of improved survival are inconsistent between studies.(2, 11–14) Two outcomes that are consistent in these series include a high post-procedural recurrence rate, ranging from 80–100%, and morbidity rates ranging from 47–55%.(2, 13, 14) Clearly, careful patient selection is paramount to maximize survival and quality of life while minimizing morbidity from these procedures. In this multi-institutional study, we sought to determine which patients most clearly benefit from metastasectomy or RFA for ACC liver metastases. Specifically, we compared patients with survival less than or equal to that reported for the most effective systemic therapies to those with a two-fold or greater increase in survival in order to aid clinicians in selecting patients for these invasive procedures, and better delineate expectations for patients.
METHODS
Data Collection
This study was approved by the Institutional Review Board at each institution prior to initiation. A retrospective chart review identified patients with metastatic ACC who underwent hepatic resection or RFA at the National Cancer Institute (Bethesda, MD), Memorial Sloan Kettering Cancer Center (New York, NY), or Erasmus University Medical Center (Rotterdam, Netherlands), from 1977 until 2017. Inclusion criteria included pathologically confirmed ACC from a resected liver lesion or from a biopsy prior to RFA. Complete staging was performed with history and physical examination, biochemical assessment, and computed tomography of the chest, abdomen, and pelvis. Pertinent clinical data on patient, disease, and treatment variables were collected retrospectively utilizing institutional electronic medical records.
Stage was established based on the American Joint Committee on Cancer (AJCC) or the European Network for the Study of Adrenal Tumors (ENSAT) TNM staging system for ACC. Tumors associated with supraphysiologic levels of aldosterone, cortisol, feminizing hormones, or virializing hormones were considered functioning tumors. Major hepatectomy was defined as the removal of three or more liver segments. The disease-free interval (DFI) was defined as the elapsed time between primary tumor resection and recurrence at any site. Overall survival (OS) was defined as the time from hepatic metastasectomy or RFA to death or last follow-up. Patients still living at the time of last follow-up were censored in the survival analysis. In the interest of identifying factors associated with an unambiguous survival benefit from hepatic metastasectomy or RFA, patients were stratified into two groups: those who survived greater than 24 months after their procedure versus those that lived less than 12 months after their procedure. Patients who survived between 12–24 months or had less than 12 months of follow-up were excluded (Figure 1). This stratification was determined prior to any statistical analysis and was established based on existing literature citing approximately 12 months of survival for patients with metastatic ACC treated with the most effective systemic therapy.(6) 24 months was chosen as a threshold for extended survivorship because it represented a two-fold improvement in survival.
Figure 1:
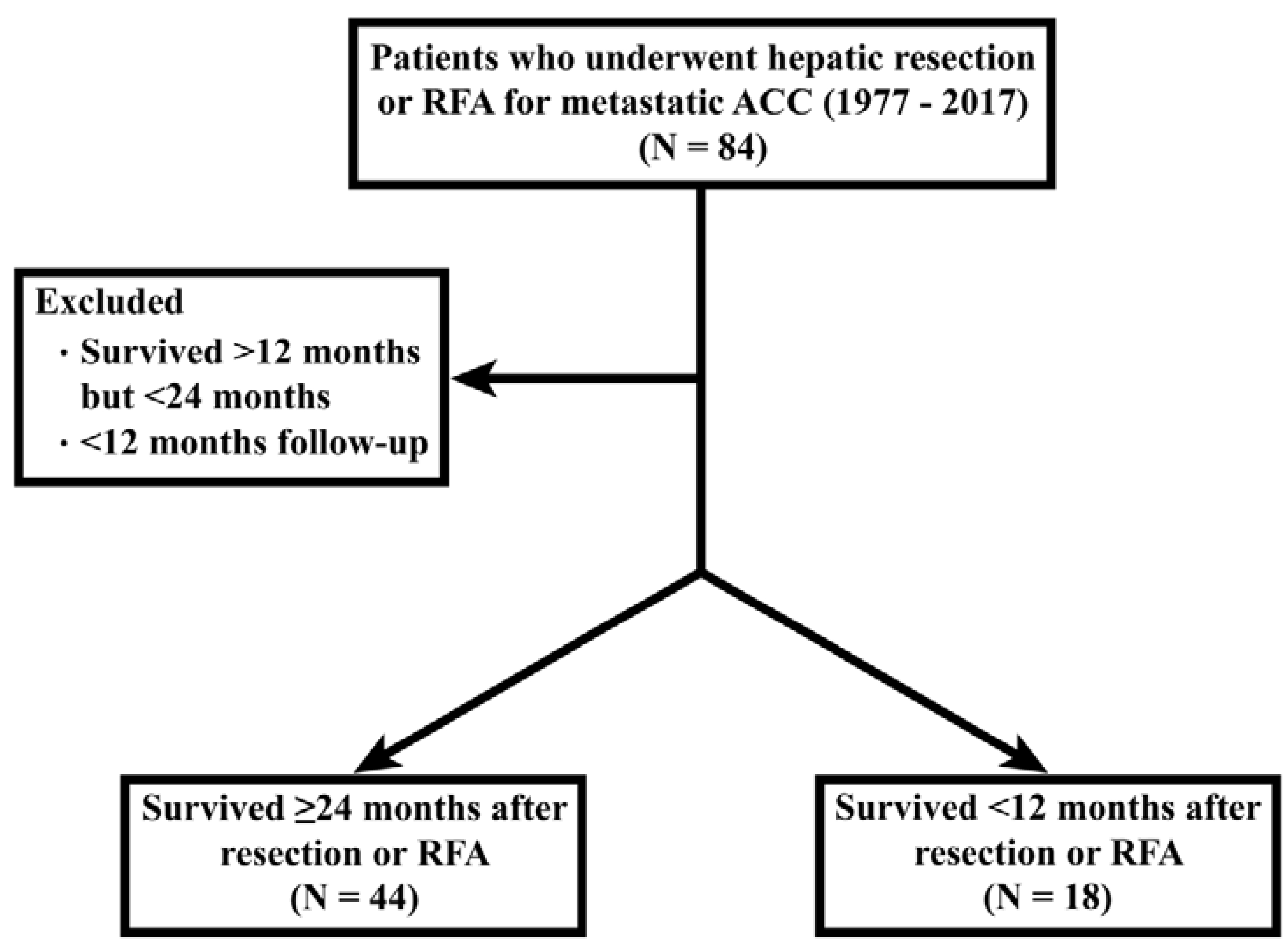
Consort diagram showing study cohort selection
Statistical Analysis
Patient characteristics were described overall and then stratified by survival groups. Differences in patient characteristics were compared with the Fisher’s Exact test and the Wilcoxon Rank Sum test where appropriate. Mehta’s modification to Fisher’s exact test was used for unordered categorical parameters and a Cochran-Armitage test was used for trend of ordered categorical parameters.
Following a univariate logistic regression analysis to determine suitability for inclusion in a multivariable model (p<0.10), a multiple logistic regression model was constructed to analyze factors associated with extended survival time (>24 months). All p-values are two tailed and those less than 0.05 on multivariable analysis were considered statistically significant. All analyses were performed using SAS 9.3 (The SAS Institute, Cary, NC).
RESULTS
Characteristics of Cohort with Survival <12 months
There were 62 patients included in this study, 18 of whom survived less than 12 months after hepatic resection or RFA. Patients surviving less than 12 months were primarily female (N=11, 61%) with a mean age of 43 years. More than half the patients (N=10, 56%) had stage IV disease at the time of diagnosis. The majority of patients (N=14, 78%) had functioning tumors, including cortisol-secreting tumors (N=9, 50%), virilizing tumors (N=4, 22%), and aldosterone secreting tumors (N=1, 6%). At time of diagnosis of their primary adrenal tumor, 44% (N=8) of patients had synchronous liver metastases and 44% (N=8) had synchronous extrahepatic metastases (Table 1).
Table 1:
Patient Characteristics and Univariable Analysis.
| Survival <12 months N = 18 | Survival ≥24 months N = 44 | p value | |
|---|---|---|---|
| Age at time of liver procedure (mean ± standard error, years) | 43 ± 4.1 | 45 ± 2.4 | 0.77 |
| Gender | 0.78 | ||
| Male | 7 (39%) | 19 (43%) | |
| Female | 11 (61%) | 25 (57%) | |
| Variables at Time of Primary Tumor Diagnosis | |||
| Stage at diagnosis | 0.77 | ||
| I | 2 (11%) | 2 (5%) | |
| II | 0 (0%) | 9 (21%) | |
| III | 6 (33%) | 10 (23%) | |
| IV | 10 (56%) | 23 (52%) | |
| Functioning Primary Tumor | 0.047 | ||
| No | 4 (22%) | 23 (52%) | |
| Yes | 14 (78%) | 21 (48%) | |
| Hormone Secretion | |||
| Cortisol | 9 (50%) | 10 (23%) | |
| Virilizing | 4 (22%) | 5 (11%) | |
| Aldosterone | 1 (6%) | 4 (9%) | |
| Feminizing | 0 (0%) | 2 (5%) | |
| Synchronous liver metastases at time of primary diagnosis | 0.40 | ||
| No | 10 (56%) | 29 (67%) | |
| Yes | 8 (44%) | 14 (33%) | |
| Extra-hepatic disease at time of primary diagnosis | 0.38 | ||
| No | 10 (56%) | 30 (70%) | |
| Yes | 8 (44%) | 13 (30%) | |
| No evidence of disease after primary tumor resection | 12 (67%) | 37 (84%) | 0.17 |
| Variables Related to Liver Metastasis | |||
| Size of largest liver metastasis (mean ± standard error, cm) | 6.6 ± 1.3 | 5.4 ± 0.9 | 0.30 |
| Mean number of liver metastases (mean ± standard error) | 2.4 ± 0.2 | 1.8 ± 0.3 | 0.047 |
| Bilobar liver metastases present | 0.56 | ||
| No | 11 (61.1%) | 30 (69.8%) | |
| Yes | 7 (38.9%) | 13 (30.2%) | |
| Variables Related to First Liver Procedure | |||
| Extra-hepatic disease at time of liver procedure | 0.045 | ||
| No | 10 (56%) | 12 (27%) | |
| Yes | 8 (44%) | 32 (73%) | |
| Site of Extra-hepatic disease at time of liver procedure | 0.644 | ||
| Lung | 2 (11%) | 9 (20%) | |
| Ipsilateral adrenal bed | 0 (0%) | 5 (11%) | |
| Contralateral adrenal | 0 (0%) | 1 (2%) | |
| Diaphragm | 1 (6%) | 2 (5%) | |
| Inferior vena cava | 2 (11%) | 1 (2%) | |
| Retroperitoneum NOS | 2 (11%) | 9 (20%) | |
| Multiple sites | 1 (6%) | 5 (11%) | |
| Chemotherapy prior to liver procedure | 0.58 | ||
| No | 10 (56%) | 28 (64%) | |
| Yes | 8 (44%) | 16 (36%) | |
| Response to chemotherapy | 0.51 | ||
| Progressive disease | 7 (39%) | 14 (32%) | |
| Stable disease | 1 (6%) | 1 (2%) | |
| Partial response | 0 (0%) | 1 (2%) | |
| Underwent radiofrequency ablation | 0.66 | ||
| No | 17 (94%) | 38 (86%) | |
| Yes | 1 (6%) | 6 (14%) | |
| Underwent major hepatectomy (≥3 segments) | 0.57 | ||
| No | 12 (67%) | 25 (57%) | |
| Yes | 6 (33%) | 19 (43%) | |
| Resection status | 0.042 | ||
| Negative margins and no residual extra-hepatic disease | 14 (78%) | 24 (55%) | |
| R1: Positive microscopic margins | 2 (11%) | 6 (14%) | |
| R2: Positive macroscopic margins and no residual extra-hepatic disease | 2 (11%) | 3 (7%) | |
| Residual extra-hepatic disease only | 0 (0%) | 11 (25%) | |
| No evidence of disease after liver procedure | 14 (78%) | 24 (55%) | 0.083 |
| Variables Related to Treatment After First Liver Procedure | |||
| Additional liver resection or RFA performed after first liver procedure | 0.15 | ||
| No | 17 (94%) | 33 (75%) | |
| Yes | 1 (6%) | 11 (25%) | |
| Additional resection of any metastatic disease after first liver procedure | 0.025 | ||
| No | 13 (72%) | 17 (39%) | |
| Yes | 5 (28%) | 27 (61%) | |
| Disease Free Interval | |||
| Disease free interval (median, 25th – 75th percentile) | 2.3 (0.0–4.3) | 17.6 (5.3–27.2) | <0.0001 |
NOS: not otherwise specified.
The average number of hepatic metastases was 2.4 tumors with a mean largest tumor size of 6.6 cm. Bilobar liver metastases were present in 39% (N=7) of this group. At the time of the liver procedure, 44% (N=8) had extra-hepatic metastases. The most common sites of extrahepatic disease were the lung (N=2, 11%), inferior vena cava (N=2, 11%), and retroperitoneum (N=2, 11%). Chemotherapy was administered to 44% (N=8) of patients prior to their liver procedure with no partial or complete responses reported. A major hepatectomy was performed in 33% (N=6) of patients. After the liver procedure, 78% (N=14) of patients had no evidence of disease, 11% (N=2) had positive microscopic margins, and 11% (N=2) had positive macroscopic margins without any residual extrahepatic disease (Table 1).
After their first hepatic procedure, one patient underwent a second liver RFA and four patients underwent an additional resection of extrahepatic disease. This cohort had a median DFI of 2.3 months (Table 1).
Characteristics of Cohort with Survival >24 months
Of the 62 patients included in this study, 44 survived more than 24 months. These patients were also primarily female (N=25, 57%) with a mean age of 45 years. More than half (N=23, 52%) had stage IV disease at the time of diagnosis. Forty-eight percent (N=21) of patients had functioning tumors, including cortisol secreting tumors (N=10, 23%), virilizing tumors (N=5, 11%), aldosterone secreting tumors (N=4, 9%), and feminizing tumors (N=2, 5%). Thirty-three percent (N=14) of patients had synchronous liver metastases and 30% (N=13) of patients had synchronous extrahepatic disease (Table 1).
The average number of hepatic metastases was 1.8 tumors with a mean largest tumor size of 5.4 cm. Bilobar liver metastases were present in 30% (N=13) of patients. At the time of the liver procedure, 73% (N=32) had extra-hepatic metastases. The most common sites of extrahepatic disease were the lung (N=9, 20%) and retroperitoneum (N=9, 20%). Thirty-six percent (N=16) of patients received chemotherapy prior to their liver procedures with only one partial response reported. Forty-three percent (N=19) of patients underwent a major hepatectomy. After the liver procedure, 55% (N=24) of patients had no evidence of disease, 14% (N=6) had positive microscopic margins, 7% (N=3) had positive macroscopic margins, and 25% (N=11) had residual extra-hepatic disease only.
After the first liver procedure, 25% (N=11) of patients underwent an additional liver resection or RFA and 36% (N=16) of patients underwent additional resection of extrahepatic disease. This cohort had a median DFI of 17.6 months (Table 1).
Survival Determinants
Patients with extended survival were less likely to have a functioning primary tumor (p=0.047) (Figure 2) and had a lower mean number of liver metastasis (p=0.047) with a longer DFI (median 17.6 vs 2.3 months, p<0.0001) (Table 1) (Figure 3). Interestingly, patients who survived ≥24 months were also more likely to have extra-hepatic disease (EHD) at time of their liver procedure (p=0.045). Resection status also differed significantly between groups (p=0.042), and the likelihood that patients were rendered no evidence of disease (NED) after the first liver procedure was sufficiently different (p=0.08) for inclusion in the multivariable analysis. Patients with extended survival were also more likely to undergo additional resection of metastatic disease after first liver procedure (p=0.025) (Table 1). However, this was not entered into the multivariable analysis as it is not a knowable parameter at the time of the first liver resection and would therefore not be available to guide patient selection.
Figure 2:
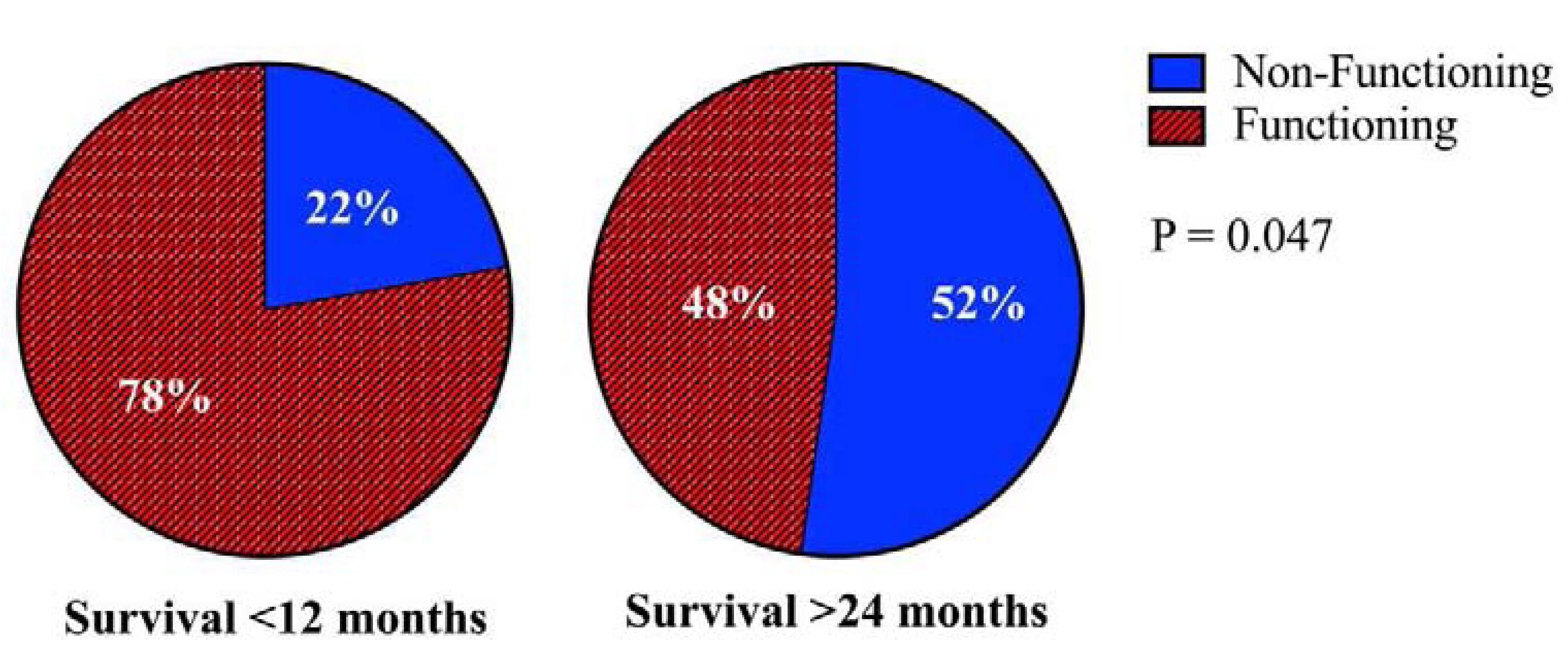
Non-functioning primary tumors are associated with improved survival.
Figure 3:
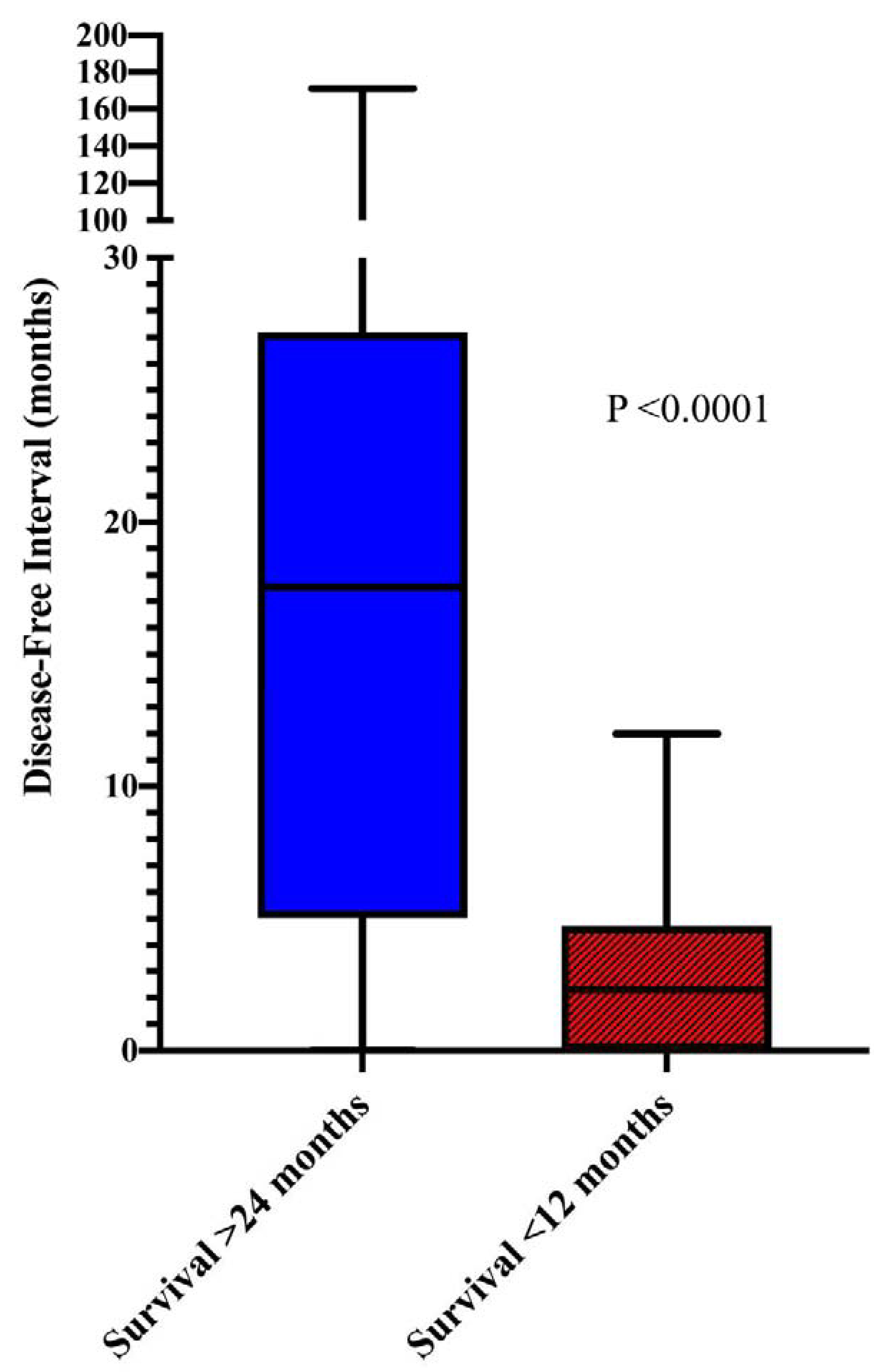
Prolonged disease-free interval is associated with improved survival.
DFI, mean number of liver metastases, resection status, tumor hormonal status, EHD at the time of first liver procedure, and NED after first liver procedure were entered into a multivariable logistic regression (Supplemental Table 1). After backward selection, only DFI (OR 1.33, CI 1.12 – 1.58, p=0.0012) and nonfunctioning primary tumor (OR 0.13, CI 0.03 – 0.56, p=0.0056) were independently associated with extended survival (Table 2). A classification rule constructed using these parameters was able to correctly predict 73% (32/44) of those who survived ≥24 months and 89% (16/18) of those who died within 12 months of surgery (Supplemental Figure 1).
Table 2:
Multivariable analysis showing factors independently associated with survival.
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Disease-free interval | 1.33 | 1.12 – 1.58 | 0.0012 |
| Functioning primary tumor | 0.13 | 0.03 – 0.56 | 0.0056 |
DISCUSSION
Adrenocortical carcinoma is an aggressive malignancy that frequently metastasizes to the liver. Although current guidelines recommend the resection or ablation of metastatic ACC when feasible, reports of outcomes after these procedures have heretofore been limited by small sample sizes and have yielded inconsistent results with regard to optimal patient selection.(2, 14, 15) In this multi-institutional study, we undertook the largest analysis to date of patients undergoing resection or RFA for ACC liver metastases. We demonstrate that an extended DFI and non-functioning primary tumor are predictive of a clear survival benefit from these procedures.
The importance of surgical resection or locally ablative therapy for metastatic ACC is underscored by the shortcomings of systemic therapy in treating this disease. Guidelines from the European Society for Medical Oncology (ESMO) and the European Network for the Study of Adrenal Tumors (ENSAT) recommend mitotane-based therapy for advanced ACC.(16, 17) Although mitotane has been the backbone of ACC systemic therapy for over three decades, its efficacy as a monotherapy is limited, with partial response rates of 10–30% at best.(5, 18–20) Moreover, the effectiveness of mitotane is contingent upon a high serum drug level that is poorly tolerated by a significant number of patients.(19, 20) The addition of etoposide, doxorubicin, and cisplatin to mitotane (EDP-M) is the only systemic treatment for ACC that has been validated in a randomized trial. The FIRM-ACT study group compared EDP-M to mitotane plus streptozocin for metastatic ACC. While this study demonstrated a higher response rate in patients treated with EDP-M (23.2% vs 9.2%, p<0.001), there was no difference in OS between the groups (14.8 months vs 12.0 months, p=0.07), suggesting that this response was not a durable one. The only other randomized trial of systemic treatment for advanced ACC compared Linsitinib, an inhibitor of IGF-2 signaling, to placebo. Despite the fact that IGF-2 overexpression is present in over 90% of ACC, the inhibition of IGF-2 in this trial failed to produce an improvement in survival. The limited efficacy of systemic therapy for ACC was corroborated in our cohort of patients, wherein the vast majority of patients progressed through systemic therapy. Although systemic therapy may be of value in the adjuvant setting and for patients with inoperable disease, surgical resection remains a cornerstone of ACC treatment.
The selection of patients for hepatic resection or RFA for metastatic ACC has been examined in several retrospective studies with inconsistent results. One of the first series to specifically address this topic included 27 patients who underwent resection or RFA of ACC liver metastasis at the NCI. The authors reported that a DFI greater than 9 months was the only factor predictive of prolonged survival on multivariable analysis.(13) Shortly thereafter, a series of 28 patients who underwent hepatic metastasectomy for ACC at MSKCC was published, showing that surgical resection of recurrent disease (after index hepatectomy) and nonfunctional hormone status were predictive of prolonged survival. In this study, a prolonged DFI was not associated with improved survival.(2) The largest investigation prior to the current study was a retrospective review of the German Adrenocortical Carcinoma Registry including 43 patients who underwent hepatic metastasectomy and 34 patients who were treated nonoperatively. A survival analysis of the operative cohort suggested that increased time to recurrence or metastasis and solitary liver metastases were associated with prolonged survival, but neither of these associations were significant on multivariable analysis.(14) By combining data from the NCI, MSKCC, and Erasmus University, we were able to describe a significantly larger cohort than any of these individual studies, and overcome to some degree the limitations presented by very small sample sizes and institutional biases. In doing so, we established DFI as a clear predictor of survival benefit after resection or RFA of ACC liver metastases.
In addition to DFI, we identified non-functional primary tumor as a predictor of extended survival. The aforementioned MSKCC series is the only other study to identify hormone status as a prognostic factor in patients undergoing hepatic metastasectomy for ACC. However, hormone secretion has been reported as an indicator of poor prognosis for ACC in multiple prior investigations. In a recent meta-analysis of studies including 19 studies and 3,814 patients, any hormone secretion (weighted RR=1.26, 95%CI=1.03–1.54), and cortisol secretion in particular (weighted RR=1.71; 95%CI=1.18–2.47), were associated with decreased survival. Interestingly, cortisol secretion was also associated with an increased risk of recurrence (RR 1.43, 95%CI 1.18–1.73). The association between hormone secretion and decreased survival may be related to the physiologic derangements accompanying states of hormonal excess such as the metabolic syndrome seen with hypercortisolemia and hypertension seen with hyperaldosteronism. However, the increased risk of tumor recurrence associated with cortisol secretion cannot be explained by the physiologic effects of hormones alone and suggests an undetermined relationship between cortisol secretion and a more aggressive tumor biology. In our study, the majority of patients with functional primary tumors in both long- and short-survival groups had cortisol secreting tumors. Due to limited numbers of other functioning tumors, a survival analysis based on type of hormone secreted was not possible.
We believe the findings described here will be useful in guiding the selection of patients with ACC liver metastases for hepatic metastasectomy or RFA. However, this study is not without significant limitations. Firstly, the retrospective nature of this investigation and the use of data from three highly specialized cancer centers are obvious sources of selection bias. However, the rarity of ACC makes randomized surgical trials for this disease prohibitively challenging, and the absence of good metastasectomy data in large national databases makes a more generalizable analysis extremely difficult. Secondly, the patients described in this study received treatment over four decades, during which time advancements in systemic therapy, surgical techniques, and perioperative care have occurred that may lead to differences in survival. Thirdly, a true DFI, while a useful surrogate for disease biology, is often unavailable to clinicians treating patients with metastatic ACC. This is evidenced by the fact that a large number of metastases, including those in this cohort, are diagnosed synchronously. Finally, we acknowledge that while there is precedent in the published literature, the <12-month and >24-month survival thresholds used here are somewhat arbitrary, and results may have differed had different cutoff values been used.
In this multi-center retrospective study, we demonstrate that prolonged disease-free interval and non-functioning primary tumor are associated with a clear survival benefit after hepatic resection for metastatic ACC. For clinicians who manage this challenging disease, this suggests that patients with longer DFI and hormonally inactive tumors may benefit more from surgical management, whereas those with foreshortened DFI or functional tumors may be better served with a non-operative approach. Further study is needed to define the relationship between hormone positivity and aggressive ACC biology and to identify genetic and molecular prognostic markers that are more readily available than DFI in the treatment of this challenging malignancy.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Tella SH, Kommalapati A, Yaturu S, Kebebew E. Predictors of survival in Adrenocortical Carcinoma: An analysis from the National Cancer Database (NCDB). J Clin Endocrinol Metab. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Gaujoux S, Al-Ahmadie H, Allen PJ, Gonen M, Shia J, D’Angelica M, et al. Resection of adrenocortical carcinoma liver metastasis: is it justified? Ann Surg Oncol. 2012;19(8):2643–51. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Shen WT, Elaraj D, Bentrem DJ, Winchester DJ, Kebebew E, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113(11):3130–6. [DOI] [PubMed] [Google Scholar]
- 4.Abiven G, Coste J, Groussin L, Anract P, Tissier F, Legmann P, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91(7):2650–5. [DOI] [PubMed] [Google Scholar]
- 5.Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–80. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–97. [DOI] [PubMed] [Google Scholar]
- 7.Stratakis CA. Adrenal cancer in 2013: Time to individualize treatment for adrenocortical cancer? Nat Rev Endocrinol. 2014;10(2):76–8. [DOI] [PubMed] [Google Scholar]
- 8.Fassnacht M, Berruti A, Baudin E, Demeure MJ, Gilbert J, Haak H, et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–35. [DOI] [PubMed] [Google Scholar]
- 9.Datrice NM, Langan RC, Ripley RT, Kemp CD, Steinberg SM, Wood BJ, et al. Operative management for recurrent and metastatic adrenocortical carcinoma. J Surg Oncol. 2012;105(7):709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran TB, Postlewait LM, Maithel SK, Prescott JD, Wang TS, Glenn J, et al. Actual 10-year survivors following resection of adrenocortical carcinoma. J Surg Oncol. 2016;114(8):971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood BJ, Abraham J, Hvizda JL, Alexander HR, Fojo T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer. 2003;97(3):554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Carlo I, Toro A, Sparatore F, Cordio S. Liver resection for hepatic metastases from adrenocortical carcinoma. HPB (Oxford). 2006;8(2):106–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ripley RT, Kemp CD, Davis JL, Langan RC, Royal RE, Libutti SK, et al. Liver resection and ablation for metastatic adrenocortical carcinoma. Ann Surg Oncol. 2011;18(7):1972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baur J, Buntemeyer TO, Megerle F, Deutschbein T, Spitzweg C, Quinkler M, et al. Outcome after resection of Adrenocortical Carcinoma liver metastases: a retrospective study. BMC Cancer. 2017;17(1):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaujoux S, Mihai R, joint working group of E, Ensat. European Society of Endocrine Surgeons (ESES) and European Network for the Study of Adrenal Tumours (ENSAT) recommendations for the surgical management of adrenocortical carcinoma. Br J Surg. 2017;104(4):358–76. [DOI] [PubMed] [Google Scholar]
- 16.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, deKrijger R, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46. [DOI] [PubMed] [Google Scholar]
- 17.Berruti A, Baudin E, Gelderblom H, Haak HR, Porpiglia F, Fassnacht M, et al. Adrenal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii131–8. [DOI] [PubMed] [Google Scholar]
- 18.Luton JP, Cerdas S, Billaud L, Thomas G, Guilhaume B, Bertagna X, et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N Engl J Med. 1990;322(17):1195–201. [DOI] [PubMed] [Google Scholar]
- 19.Haak HR, Hermans J, van de Velde CJ, Lentjes EG, Goslings BM, Fleuren GJ, et al. Optimal treatment of adrenocortical carcinoma with mitotane: results in a consecutive series of 96 patients. Br J Cancer. 1994;69(5):947–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veytsman I, Nieman L, Fojo T. Management of endocrine manifestations and the use of mitotane as a chemotherapeutic agent for adrenocortical carcinoma. J Clin Oncol. 2009;27(27):4619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


