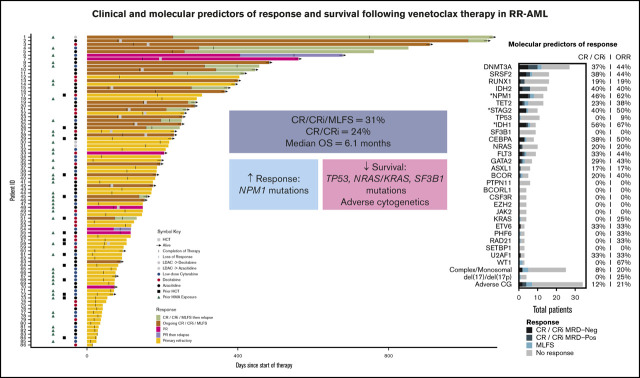
Key Points
In patients with RR-AML, venetoclax combination therapy resulted in responses in 31% of patients and a median OS of 6.1 months.
NPM1 mutations predicted higher response rates; adverse cytogenetics and mutations in TP53, KRAS/NRAS, and SF3B1 predicted worse OS.
Abstract
Azacitidine + venetoclax, decitabine + venetoclax, and low-dose cytarabine + venetoclax are now standard treatments for newly diagnosed older or unfit patients with acute myeloid leukemia (AML). Although these combinations are also commonly used in relapsed or refractory AML (RR-AML), clinical and molecular predictors of response and survival in RR-AML are incompletely understood. We retrospectively analyzed clinical and molecular characteristics and outcomes for 86 patients with RR-AML who were treated with venetoclax combinations. The complete remission (CR) or CR with incomplete hematologic recovery (CRi) rate was 24%, and the overall response rate was 31% with the inclusion of a morphologic leukemia-free state. Azacitidine + venetoclax resulted in higher response rates compared with low-dose cytarabine + venetoclax (49% vs 15%; P = .008). Median overall survival (OS) was 6.1 months, but it was significantly longer with azacitidine + venetoclax compared with low-dose cytarabine + venetoclax (25 vs 3.9 months; P = .003). This survival advantage of azacitidine + venetoclax over low-dose cytarabine + venetoclax persisted when patients were censored for subsequent allogeneic stem cell transplantation (8.1 vs 3.9 months; P = .035). Mutations in NPM1 were associated with higher response rates, whereas adverse cytogenetics and mutations in TP53, KRAS/NRAS, and SF3B1 were associated with worse OS. Relapse was driven by diverse mechanisms, including acquisition of novel mutations and an increase in cytogenetic complexity. Venetoclax combination therapy is effective in many patients with RR-AML, and pretreatment molecular characteristics may predict outcomes. Trials that evaluate novel agents in combination with venetoclax therapy in patients with RR-AML that have adverse risk genomic features are warranted.
Visual Abstract
Introduction
Venetoclax combination therapy is approved in the United States for treatment of newly diagnosed older or unfit adults with acute myeloid leukemia (AML). Venetoclax showed impressive response rates in combination with the hypomethylating agents (HMAs) azacitidine and decitabine, and with low-dose cytarabine in single-arm phase 1b/2 trials.1,2 Azacitidine-venetoclax was recently shown to improve survival compared with azacitidine alone in a large, phase 3 study.3 Venetoclax combination regimens have therefore been adopted as a new standard of care for initial treatment of older or unfit adults with AML.4
Given the success of these regimens in patients with newly diagnosed AML, venetoclax combination therapy is now frequently used in relapsed or refractory AML (RR-AML). Previous real-world studies have demonstrated variable responses in the RR population.5-14 One clinical trial has recently been published that reported the outcomes of patients with RR-AML treated with decitabine and venetoclax for 10 days, showing an encouraging overall response rate (ORR) of 62% but a short median overall survival (OS) of 7.8 months.15 Data are limited on clinical, molecular, and immunophenotypic predictors of outcomes of venetoclax-based therapy in RR-AML.
Here, we present the outcomes of 86 patients with RR-AML treated with venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine at Memorial Sloan Kettering Cancer Center (MSKCC). Our data represent the largest series of patients with RR-AML treated with both HMAs and low-dose cytarabine-venetoclax combinations reported to date. We also demonstrate the clinical and molecular characteristics associated with benefit from venetoclax-based therapy in the RR treatment setting.
Methods
Data source and eligibility
Data were retrospectively collected at MSKCC. All patients with AML per World Health Organization classification who received azacitidine + venetoclax, decitabine + venetoclax, or low-dose cytarabine + venetoclax for RR disease from 11 August 2016 to 5 February 2020 were included.16 None of the patients had received prior venetoclax therapy. Patients were treated according to a standardized protocol to adjust the dose of venetoclax for concomitant medications, which was developed in collaboration between the Leukemia Service and the Pharmacy Service at MSKCC and was based on recently published practice guidelines17 (supplemental Data). The study was approved by the MSKCC Institutional Review Board.
Patient characteristics
The number of lines of salvage therapy received before venetoclax therapy was recorded. A previous line of salvage therapy did not include intensive induction chemotherapy or a cycle of intensive reinduction therapy for patients who were refractory to the first cycle of induction therapy, nor did it include consolidation chemotherapy, previous allogeneic stem cell transplantation (allo-SCT), or maintenance therapy. Clinical and laboratory data were collected at initiation of venetoclax therapy. Cytogenetic, molecular, and immunophenotypic data were collected before venetoclax treatment started and at the time of relapse for patients who achieved a response and experienced a subsequent disease relapse (details are provided in the supplemental Data).
Response criteria and survival
Response to venetoclax therapy was determined by using the 2017 European LeukemiaNet (ELN) response criteria.18 Measurable residual disease (MRD) was assessed by multiparametric flow cytometric analysis of bone marrow aspirate samples, as described in the supplemental Data. Any level of residual disease was considered MRD positive. The ORR was defined as the combination of complete response (CR), CR with incomplete hematologic recovery (CRi), and morphologic leukemia-free state (MLFS). MRD-negative CR/CRi was defined as CR or CRi with no measurable disease by flow cytometry. OS was calculated from cycle 1 day 1 of therapy until death or time of last follow-up.
Analysis of immunophenotypic and genetic characteristics and statistical analysis
Multiparametric flow cytometry was performed on bone marrow aspirate samples at diagnosis and, where it occurred, relapse. We analyzed molecular predictors of response and patterns of disease evolution. Clinical, cytogenetic, and molecular data were extracted from the medical records using LeukNLP software (J.G.) and was manually curated for accuracy. The details of how we performed statistical analyses in provided in the supplemental Data.
Results
Study population
A total of 86 patients with RR-AML were treated with venetoclax-based combination therapy. Their baseline characteristics are provided in Table 1. Although 52% of patients were initially diagnosed with de novo AML, 48% of patients had secondary AML. More than half the patients (53%) received venetoclax-based therapy for primary treatment of refractory disease, and 47% of patients were treated for relapsed disease; 57% of patients had received previous treatment with HMAs, and 17% had undergone previous allo-SCT.
Table 1.
Patient baseline and treatment characteristics
| Characteristic | All patients (N = 86)* | Azacitidine + venetoclax (n = 35; 41%) | Decitabine + venetoclax (n = 20; 23%)† | Low-dose cytarabine + venetoclax (n = 27; 31%) | P |
|---|---|---|---|---|---|
| Sex | .26 | ||||
| Female | 32 (37) | 16 (46) | 7 (35) | 7 (26) | |
| Male | 54 (63) | 19 (54) | 13 (65) | 20 (74) | |
| Age, y | .006 | ||||
| Median (range) | 67 (29-86) | 65 (32-82) | 59 (29-79) | 74 (35-86) | |
| <65 | 37 (43) | 18 (51) | 12 (60) | 7 (35) | |
| 65-75 | 31 (36) | 15 (43) | 6 (30) | 9 (45) | |
| >75 | 18 (21) | 2 (6) | 2 (10) | 11 (55) | |
| AML type | .21 | ||||
| De novo | 45 (52) | 22 (63) | 11 (55) | 11 (41) | |
| Secondary | 41 (48) | 13 (37) | 9 (45) | 16 (59) | |
| Previous MDS/AML-MRC | 30 (35) | 9 (26) | 8 (40) | 12 (44) | |
| Previous MPN | 3 (3) | 1 (3) | 0 (0) | 2 (7) | |
| Previous MDS/MPN overlap | 2 (2) | 0 (0) | 0 (0) | 1 (4) | |
| Therapy related | 6 (7) | 3 (8) | 1 (5) | 1 (4) | |
| Treatment refractory | 46 (53) | 17 (49) | 11 (55) | 16 (59) | .72 |
| Relapsed | 40 (47) | 18 (51) | 9 (45) | 11 (41) | |
| Previous therapy | |||||
| HMA therapy | 49 (57) | 15 (43) | 7 (35) | 24 (88) | <.0001 |
| No. of HMA cycles | .0002 | ||||
| Naïve | 37 (44) | 20 (57) | 13 (65) | 3 (11) | |
| 1-4 | 22 (26) | 10 (29) | 4 (20) | 7 (26) | |
| >4 | 25 (30) | 5 (14) | 3 (15) | 15 (56) | |
| Median no. of previous lines of salvage therapy (range) | 0 (0-5) | 1 (0-4) | 0 (0-3) | 1 (0-5) | .29 |
| 0 | 39 (45) | 15 (43) | 11 (55) | 12 (44) | |
| 1 | 26 (30) | 10 (29) | 6 (30) | 9 (33) | |
| 2 | 13 (15) | 8 (23) | 2 (10) | 1 (4) | |
| ≥3 | 8 (10) | 2 (6) | 1 (5) | 5 (19) | |
| Previous allo-SCT | 15 (17) | 6 (17) | 3 (15) | 6 (22) | .82 |
| Previous FLT3 inhibitor therapy | 11 (13) | 7 (20) | 1 (5) | 3 (11) | .32 |
| Previous IDH inhibitor therapy | 12 (14) | 5 (14) | 3 (15) | 4 (15) | 1 |
| Cytogenetics | |||||
| Adverse | 34 (40) | 9 (26) | 15 (75) | 10 (37) | .001 |
| Complex/monosomal | 25 (29) | 7 (20) | 11 (55) | 7 (26) | .03 |
| del(17)/del(17p) | 4 (5) | 0 (0) | 3 (15) | 1 (4) | .03 |
| ELN risk | .005 | ||||
| Favorable | 12 (14) | 9 (26) | 1 (5) | 1 (4) | |
| Intermediate | 21 (24) | 6 (17) | 2 (10) | 12 (44) | |
| Adverse | 53 (62) | 20 (57) | 17 (85) | 14 (52) | |
| Median no. of cycles of venetoclax (range) |
2 (1-17) | 2 (1-15) | 2 (1-12) | 2 (1-13) | .51 |
| Allo-SCT after venetoclax combination therapy | 15 (17) | 10 (29) | 4 (20) | 1 (4) | .03 |
All data are presented as n (%), unless otherwise specified.
AML-MRC, AML with myelodysplasia-related changes; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm.
One patient received HMA + venetoclax → low-dose cytarabine + venetoclax, and 3 patients received low-dose cytarabine + venetoclax → HMA + venetoclax.
In all, 16 (80%) of 20 patients received decitabine for 5 days, 4 (20%) of 20 received decitabine for 10 days.
Treatment characteristics
Details of treatment characteristics are provided in Table 1 and Figure 1. Azacitidine + venetoclax was used more frequently than decitabine + venetoclax (41% vs 23%); low-dose cytarabine + venetoclax was given to almost one-third of patients. The majority of patients (80%) treated with decitabine + venetoclax received decitabine on a 5-day schedule, with 20% receiving decitabine on a 10-day schedule. Sequential therapy was given to only a small number of patients: 1 patient was initially treated with HMA + venetoclax and then transitioned to low-dose cytarabine + venetoclax, and 3 patients were given the reverse sequence. Patients received a median of 2 cycles of venetoclax therapy (range, 1-17 cycles), and 17% of patients underwent subsequent allo-SCT, with the highest allo-SCT rate in patients treated with azacitidine + venetoclax (29% azacitidine + venetoclax, 20% decitabine + venetoclax, 4% low-dose cytarabine + venetoclax). Patients treated with low-dose cytarabine + venetoclax were older (median age, 74 years) compared with those treated with azacitidine + venetoclax (median age, 65 years) or decitabine + venetoclax (median age, 59 years) (P = .006) and had more frequently received previous HMA therapy (88%, 43%, and 35%, respectively; P < .0001). Patients treated with decitabine + venetoclax had a higher percentage of adverse risk cytogenetics compared with patients treated with low-dose cytarabine + venetoclax or azacitidine + venetoclax (75%, 37%, and 26%, respectively; P = .001).
Figure 1.
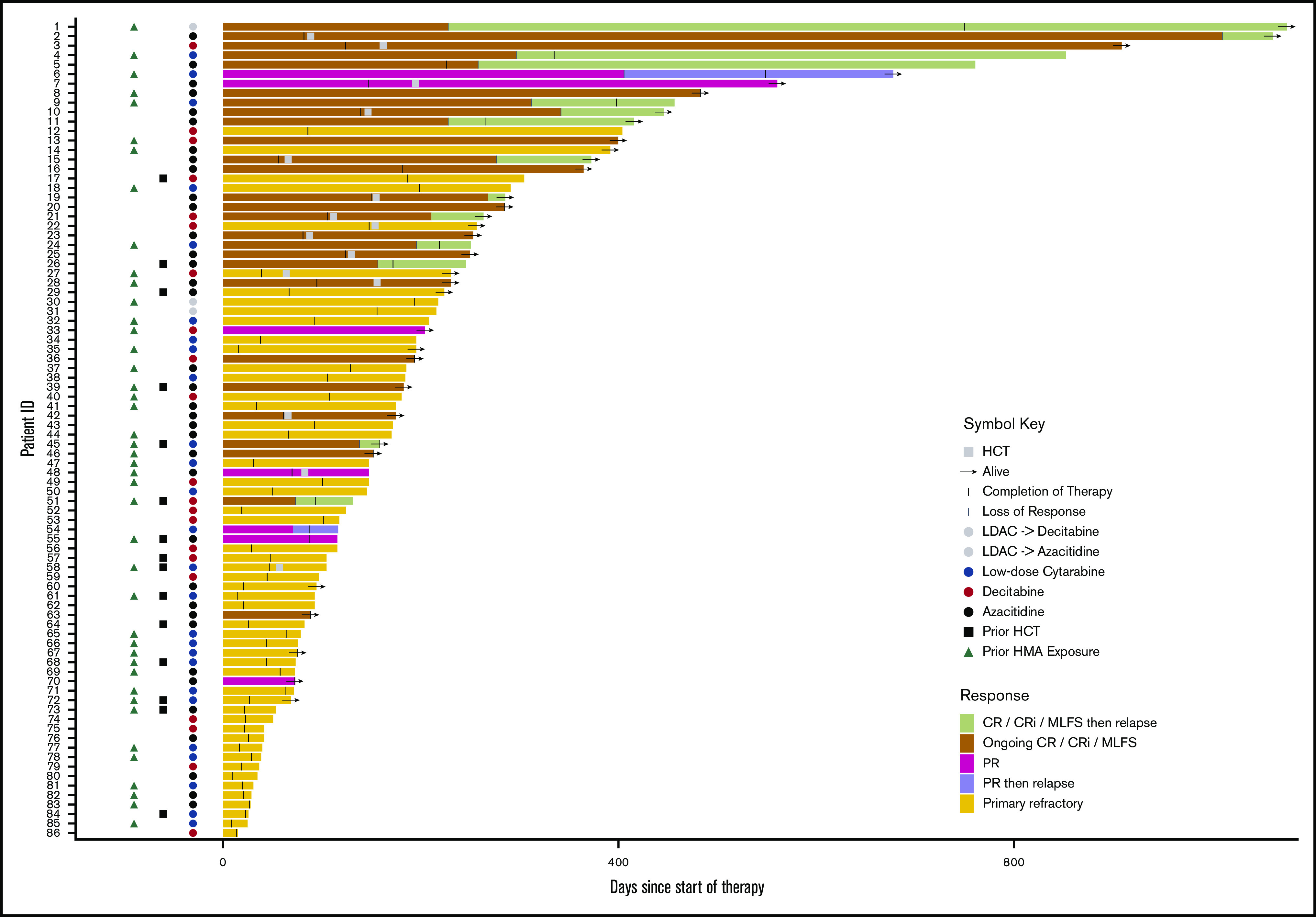
Treatment trajectories of all patients. Swimmers’ plot for all patients showing treatment duration, response, time of relapse, regimen used, previous hematopoietic cell transplantation (HCT), previous HMA, HCT status, and survival for all patients included in the RR treatment cohort. ID, identification; LDAC, low-dose cytarabine; PR, partial response.
Clinical outcomes
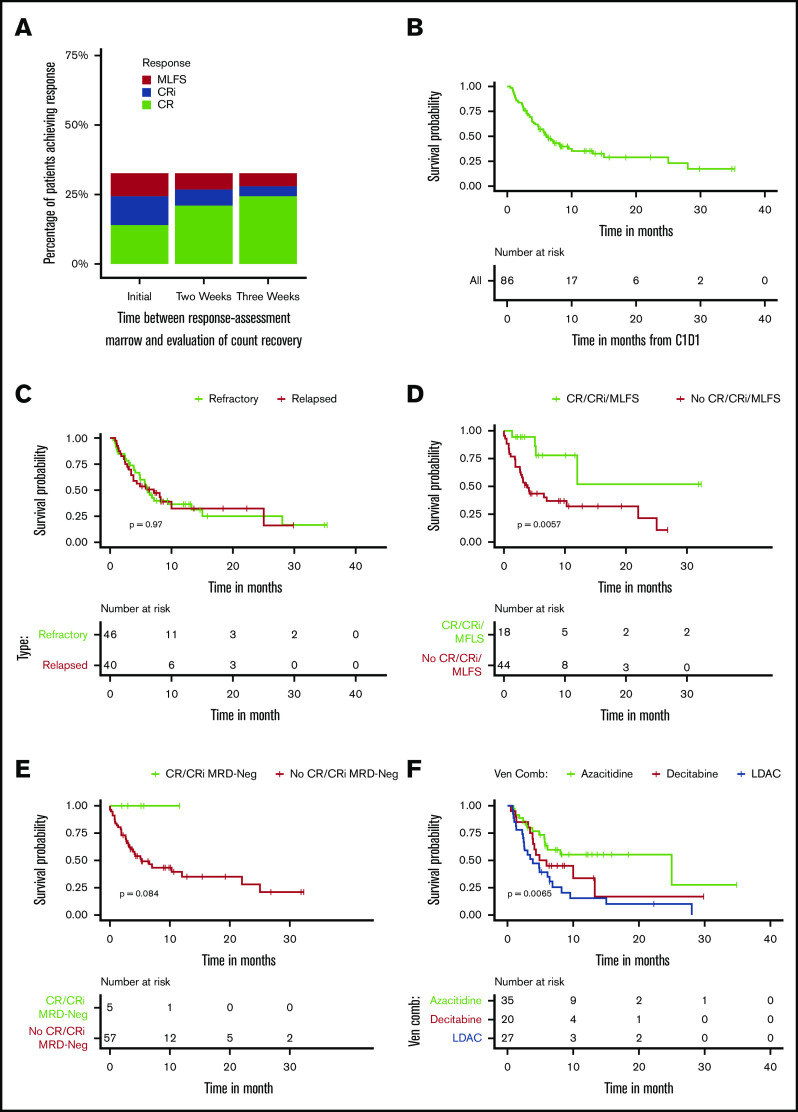
Treatment trajectories of all patients are shown in Figure 1. Response and survival outcomes are presented in Table 2 and Figure 2. The ORR was 31% with a CR/CRi rate of 24%. Using flow cytometry to assess MRD, 11 (40.7%) of the 27 patients who achieved a CR/CRi were MRD negative. Responses slightly improved when additional time for count recovery was allowed for adjudication of marrow response (Figure 2A). After 2 weeks of additional count recovery, the CR rate increased from 14% to 21% and the CR/CRi rate increased from 24% to 27%, whereas the MLFS rate decreased from 7% to 5%.
Table 2.
Clinical outcomes
| Clinical outcomes | All patients (N = 86*) | Azacitidine + venetoclax (n = 35) | Decitabine + venetoclax (n = 20) | Low-dose cytarabine + venetoclax (n = 27) | P |
|---|---|---|---|---|---|
| Response | |||||
| CR | 12/86 (14) | 9/35 (26) | 0/20 (0) | 2/27 (7) | .01 |
| CRi | 9/86 (10) | 4/35 (11) | 4/20 (20) | 1/27 (4) | .20 |
| MLFS | 6/86 (7) | 4/35 (11) | 1/20 (5) | 1/27 (4) | .56 |
| ORR (CR/CRi/MLFS) | 27/86 (31) | 17/35 (49) | 5/20 (25) | 4/27 (15) | .02 |
| CR/CRi | 21/86 (24) | 13/35 (37) | 4/20 (20) | 3/27 (11) | .07 |
| CR/CRi MRD-Neg | 11/86 (13) | 7/35 (20) | 2/20 (10) | 1/27 (4) | .16 |
| Partial remission | 7/86 (8) | 4/35 (16) | 1/20 (5) | 2/27 (7) | .78 |
| Persistent disease | 52/86 (60) | 14/35 (40) | 13/20 (65) | 21/27 (78) | .009 |
| ORR for patients with previous HMA therapy | 13/49 (27) | 6/15 (40) | 2/7 (29) | 4/24 (17) | .20 |
| ORR by no. of previous lines of salvage therapy | |||||
| 0 | 12/39 (31) | 7/15 (47) | 3/11 (27) | 2/11 (18) | .28 |
| 1 | 9/26 (35) | 5/10 (50) | 2/6 (33) | 1/9 (11) | .26 |
| 2 | 6/13 (46) | 5/8 (63) | 0/2 (0) | 1/1 (100) | .3 |
| ≥3 | 0/8 (0) | 0/2 (0) | 0/1 (0) | 0/5 (0) | 1 |
| Relapse | |||||
| Relapse rate after venetoclax therapy | 14/27 (52) | 7/17 (41) | 2/5 (40) | 0/4 (0) | .13 |
| Median duration of response, mo (95% CI) | 7.8 (6.00-NR) | 10.2 (6.00-NR) | NR (5.6-NR) | 6.2 (2.5-NR) | .2 |
| Survival | |||||
| Median OS, mo (95% CI) | 6.1 (4.9-10) | 25 (5.8-NR) | 5.4 (3.9-NR) | 3.9 (2.5-8.3) | .007 |
| OS censored for allo-SCT | 6.0 (4.8-8.1) | 8.1 (5.7-NR) | 4.9 (3.9-NR) | 3.9 (2.5-8.3) | .09 |
All data are presented as n (%), unless otherwise specified.
One patient received HMA + venetoclax → low-dose cytarabine + venetoclax, and 3 patients received low-dose cytarabine + venetoclax → HMA + venetoclax.
Figure 2.
Response and survival. (A) Percentage of patients achieving a response at time of bone marrow biopsy and with additional time for count recovery. (B) OS in all patients. (C) OS based on disease status (refractory vs relapsed). (D) OS based on response (CR/CRi/MLFS vs not) by 3 months after starting therapy. (E) OS based on response (CR/CRi MRD-Neg vs not) by 3 months after starting therapy. (F) OS based on treatment type (azacitidine + venetoclax vs decitabine + venetoclax vs low-dose cytarabine + venetoclax). C1D1, cycle 1,day 1; Ven Comb, venetoclax combination.
For those who achieved a response to therapy, 45% of patients relapsed and had a median duration of response of 7.8 months with a median follow-up of 12 months (95% confidence interval [CI], 8.5-15.9 months) (Figure 1). The median OS was 6.1 months (95% CI, 4.9-10 months) (Figure 2B) with no statistically significant difference in OS between patients who were primary treatment refractory vs relapsed (Figure 2C). After cessation of venetoclax therapy, the median OS was only 2.5 months (95% CI, 1.9-3.8 months).
Clinical characteristics associated with response
Univariable analysis of clinical predictors of response are shown in supplemental Table 2. The backbone used for combination therapy was associated with the likelihood of response. Patients treated with azacitidine + venetoclax had significantly higher odds of responding to therapy than those treated with low-dose cytarabine + venetoclax (49% vs 15%; odds ratio [OR], 5.43; 95% CI, 1.55-19; P = .008). Similarly, the response rate to decitabine + venetoclax was higher than that for low-dose cytarabine + venetoclax in patients with RR-AML but it did not reach statistical significance (25% vs 15%; OR, 1.92; 95% CI, 0.44-8.31; P = .38). Even in the setting of previous HMA therapy, the response rate to azacitidine + venetoclax was higher than that for low-dose cytarabine + venetoclax (40% vs 17%; OR, 3.3; 95% CI, 0.75-14.9; P = .14) without reaching statistical significance. The likelihood of response was not affected by previous HMA use in the overall cohort, but there was a differential response to venetoclax combination therapy based on DNMT3A mutational status and previous HMA treatment. Patients who had received ≥3 previous lines of salvage therapy had decreased odds of response to venetoclax therapy compared with patients who had received <3 previous lines of salvage therapy (P = .04).
Clinical characteristics associated with survival
Details of the univariable analysis of clinical predictors of OS are provided in supplemental Table 2. The achievement of a response (CR/CRi/MLFS) was associated with significantly improved OS (hazard ratio [HR], 0.1; 95% CI, 0.04-0.3; P < .001), including in a 3-month landmark analysis (median OS not achieved vs 3.9 months; P = .0057) (Figure 2D). Patients with MRD-negative responses had a longer OS compared with those who did not achieve MRD-negative responses. This was demonstrated in a 3-month landmark analysis; however, MRD-negative responses were uncommon, and the difference did not reach statistical significance (median OS, 5.3 months vs not reached [NR]; P = .084) (Figure 2E). Previous allo-SCT had a negative impact on survival (HR, 2.0; 95% CI, 1.06-3.96; P = .03), whereas consolidative allo-SCT after venetoclax therapy was associated with improved OS (HR, 0.17; 95% CI, 0.04-0.74; P = .02). Receipt of ≥3 previous lines of salvage therapy was associated with a negative impact on OS (HR, 3.12; 95% CI, 1.45-6.7; P = .004).
The type of venetoclax combination treatment was associated with differences in OS. Patients who were treated with azacitidine + venetoclax had a median OS of 25 months (95% CI, 5.8 months to NR) compared with 5.4 months (95% CI, 3.9 months to NR; P = .13) for patients treated with decitabine + venetoclax and 3.9 months (95% CI, 2.5-8.3 months; P = .003) for patients treated with low-dose cytarabine + venetoclax (Figure 2F). The survival benefit achieved with azacitidine + venetoclax over low-dose cytarabine + venetoclax persisted when patients were censored for subsequent allo-SCT. Median OS was 8.1 months (95% CI, 5.7 months to NR) for patients treated with azacitidine + venetoclax compared with 4.9 months (95% CI, 3.9 months to NR; P = .16) for patients treated with decitabine + venetoclax and 3.9 months (95% CI, 2.5-8.3 months; P = .035) for patients treated with low-dose cytarabine + venetoclax. Even in patients who had received previous HMA therapy, low-dose cytarabine + venetoclax was not superior to azacitidine + venetoclax or decitabine + venetoclax (median OS, 3.9 vs 25.0 vs 6.4 months; P = .007).
Molecular predictors of response
Molecular predictors of response are shown in the oncoprint (Figure 3) and univariable analysis (supplemental Table 3). Analysis of co-occurrence patterns of mutations is shown in supplemental Table 4. The presence of an NPM1 (CR/CRi, 46%; ORR, 62%; OR, 4.53; 95% CI, 1.31-15.66; P = .02) or an IDH1 (CR/CRi, 56%; ORR, 67%; OR, 5.3; 95% CI, 1.21-23.24; P = .03) but not an IDH2 mutation (CR/CRi, 40%; ORR, 40%; OR, 1.57; 95% CI, 0.49-4.99; P = .54) was associated with a statistically significant increased response rate. When adjusting for co-occurrence of favorable prognostic mutations, only NPM1 mutations (OR, 3.95; 95% CI, 1.01-15.39; P = .048) remained associated with improved response to venetoclax therapy (supplemental Table 5).
Figure 3.
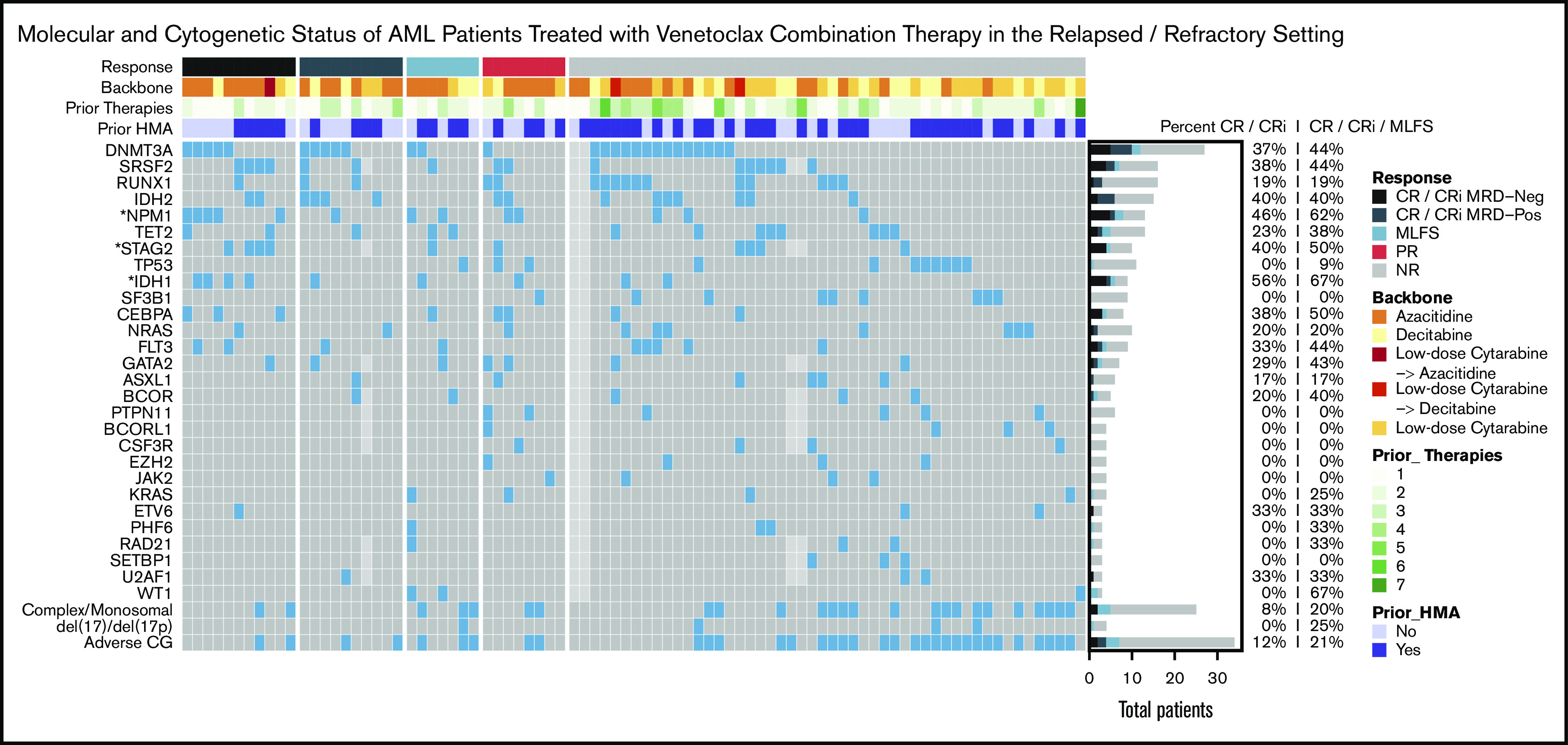
Molecular predictors of response. Oncoprint showing mutational and cytogenetic characteristics at diagnosis for 86 patients treated in the RR setting. Light gray boxes represent missing data. Patients are grouped by best response, annotated with colored bars above the grid. The type of backbone used in combination with venetoclax, the number of previous lines of therapy, and previous exposure to HMA are also annotated at the top. The filled bar plot on the right shows the number of patients with each mutation who achieved CR, CRi, and MLFS, with percentages to the right. Asterisks indicate genes with P < .05 for either percent CR/CRi MRD negative, CR/CRi, or ORR.
In contrast, adverse cytogenetics predicted lower odds of response (CR/CRi, 12%; ORR, 21%; OR, 0.32; 95% CI, 0.11-0.9; P = .03). The low CR/CRi rates associated with TP53 (0%), NRAS/KRAS (20%/0%), SF3B1 (0%), ASXL1 (17%), and EZH2 (0%) mutations (Figure 3) were not significant in our sample, possibly because of sample size. Although the impact of the 2017 ELN molecular risk classification18 on the response to venetoclax therapy was not verified for RR-AML, we analyzed it and found that adverse molecular risk was associated with decreased odds of responding to venetoclax compared with favorable and intermediate risk (ORR, 21%, 67%, and 38%, respectively; P = .006; supplemental Table 6).
Interestingly, the likelihood of achieving a response to venetoclax-based therapy in patients carrying a DNMT3A mutation correlated with previous exposure to HMA therapy. Patients carrying a DNMT3A mutation who had received previous HMA therapy had significantly lower odds of achieving a response to venetoclax therapy (ORR, 17%; 2 of 12 patients) compared with patients carrying a DNMT3A mutation who had not received previous HMA therapy (ORR, 67%; 10 of 15 patients; OR, 0.1; 95% CI, 0.02-0.65; P = .02; Figure 3). We found that the association between previous HMA and lower odds of response among patients with DNMT3A mutations persisted, even after accounting for the number of lines of therapy.
Molecular predictors of survival
Molecular predictors of OS are shown in Kaplan-Meier survival curves stratified by mutational status (Figure 4) and univariable analysis (supplemental Table 3). Adverse-risk cytogenetics (HR, 1.95; 95% CI, 1.13-3.35; P = .01; Figure 4A), a complex/monosomal karyotype (HR, 1.98; 95% CI, 1.13-3.5; P = .02), mutations in KRAS/NRAS (HR, 2.1; 95% CI, 1.04-4.22; P = .03; Figure 4B), TP53 (HR, 2.24; 95% CI, 1.07-4.69; P = .03; Figure 4C), SF3B1 (HR, 2.5; 95% CI, 1.1-5.65; P = .02; Figure 4D), and EZH2 (HR, 4.13; 95% CI, 1.43-11.96; P = .01; Figure 4E) were associated with decreased OS. We observed a trend toward an improved OS for patients carrying either an IDH1/2 or NPM1 mutation compared with patients who had neither of these mutations; however, this did not reach statistical significance (Figure 4F). Patients with STAG2 mutations had improved OS (HR, 0.32; 95% CI, 0.1-1.02; P = .04; Figure 4G). Although the impact of the 2017 ELN molecular risk classification18 on OS was not verified for RR-AML, we analyzed it and found that adverse molecular risk was associated with worse OS compared with favorable and intermediate risk (median OS, 5.62 vs 15.02 months; P = .034; supplemental Table 6; supplemental Figure 1).
Figure 4.
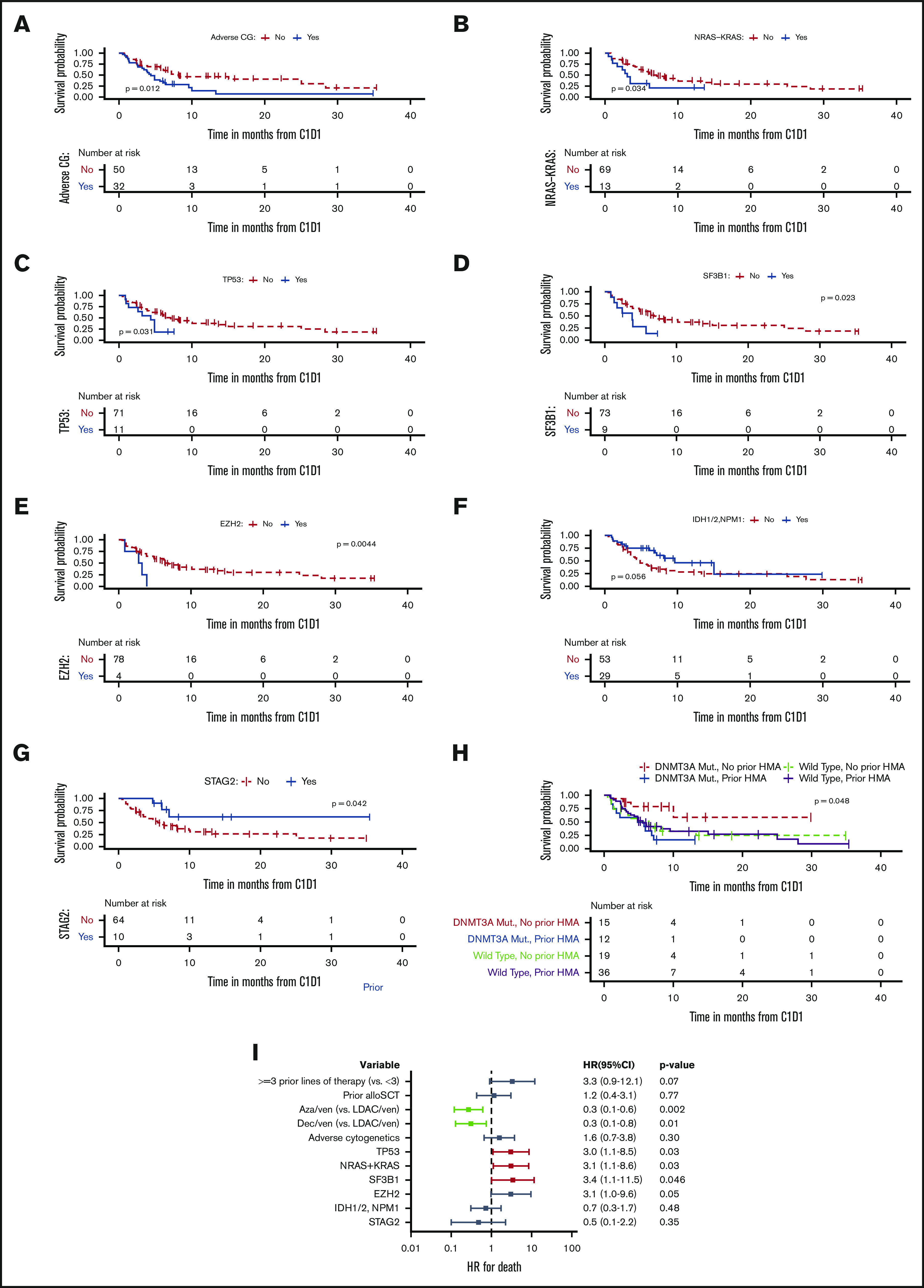
Molecular predictors of OS. Kaplan-Meier survival blots stratified by adverse cytogenetics (CG) vs not (A), NRAS/KRAS mutation vs not (B), TP53 mutation vs not (C), SF3B1 mutation present vs not (D), EZH2 mutation present vs not (E), IDH1/2 or NPM1 mutation present vs not (F), STAG2 mutation present vs not (G), and DNMT3A mutation present vs not and previous HMA received vs no previous HMA received (H). (I) Multivariable analysis of clinical and genetic predictors of OS. Aza, azacytidine; Dec, decitabine; LDAC, low-dose cytrabine; Mut, mutation; ven, venetoclax.
Consistent with what we observed with the response to venetoclax therapy, we saw a significant interaction between DNMT3A mutations and previous exposure to HMA therapy with regard to OS in patients with RR-AML (P = .048; Figure 4H). Specifically, patients carrying a DNMT3A mutation without previous exposure to HMA therapy had favorable survival (median OS, NR; 95% CI, 10 months to NR), and patients carrying a DNMT3A mutation who were previously exposed to HMA had inferior survival (median OS, 5.3 months; 95% CI, 1.8 months to NR).
Multivariable model of clinical characteristics and molecular predictors associated with survival
A multivariable analysis was performed using significant predictors from the univariable analyses (Figure 4I). Azacitidine + venetoclax and decitabine + venetoclax (compared with low-dose cytarabine + venetoclax) were associated with improved OS, whereas mutations in TP53, NRAS/KRAS, and SF3B1 remained associated with worse OS. Previous treatment with ≥3 previous lines of therapy and mutations in EZH2 were associated with worse OS reaching borderline statistical significance. Importantly, even after adjusting for previous HMA therapy, DNMT3A mutational status, age, and receipt of subsequent allo-SCT, azacitidine + venetoclax (compared with low-dose cytarabine + venetoclax) remained associated with statistically significantly improved OS (supplemental Table 7).
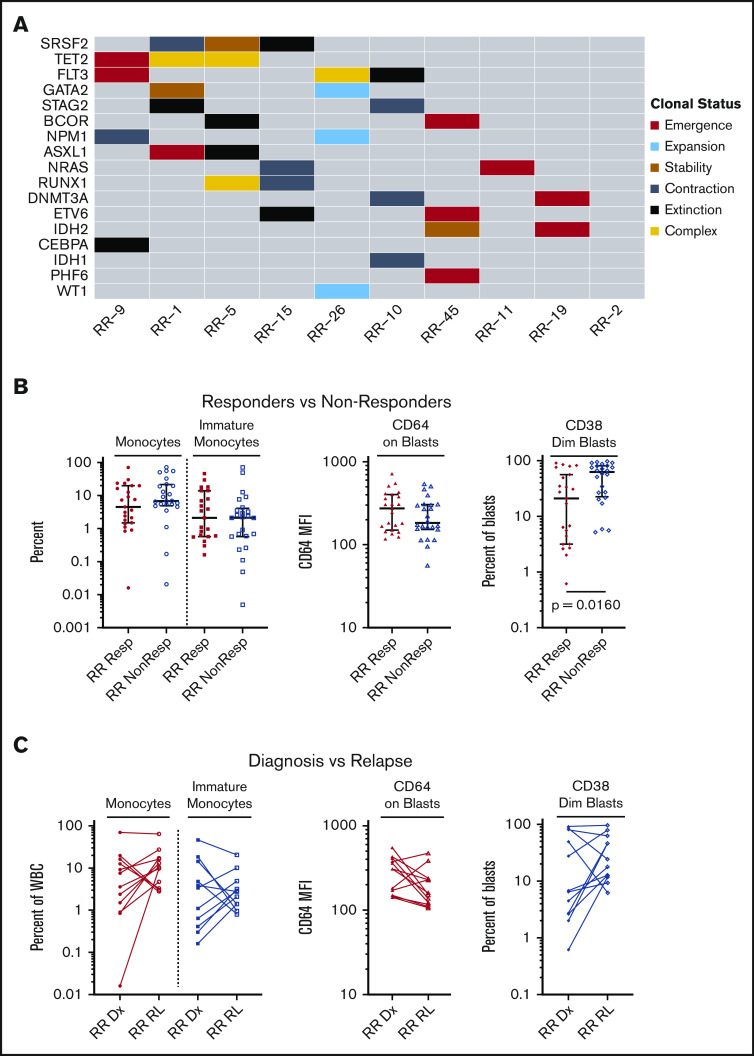
Molecular characteristics at the time of disease relapse
Paired molecular data were available for 10 patients (71%) who achieved an initial response to venetoclax-combination treatment but subsequently relapsed. We compared the molecular profile of patients at the time of treatment initiation with the molecular profile at the time of disease relapse (Figure 5A; supplemental Figure 1). In half of these patients, the molecular profile at the time of relapse was characterized by the emergence of a novel mutation which was not present before the use of venetoclax therapy, including mutations in NRAS, FLT3, BCOR, TET2, DNMT3A, and ASXL1. A novel IDH2 (R172K) mutation was seen in 1 patient. In 3 of 5 patients with an emergence pattern, more than 1 novel mutation was detected at relapse, reflecting the complex polyclonal nature of AML. Cytogenetic changes at the time of relapse were also evaluated (supplemental Table 4) for patients who had data available at both time points. Eight of 10 patients had signs of increasing complexity or a new abnormality at the time of relapse.
Figure 5.
Molecular and immunophenotypic characteristics at time of disease relapse. (A) Changes in molecular architecture seen between time of therapy initiation and relapse for 10 patients. Patient identifiers are labeled along the x-axis and correspond to patients in the swimmer plot (Figure 1). Changes in variant allele frequency for each variant are rendered on a per-patient basis in supplemental Figure 2. (B) Immunophenotypic differences comparing responding patients (Resp) with nonresponding patients (NonResp). Resp indicates patients who achieved a CR, CRi, or MLFS. NonResp indicates patients who did not achieve a CR, CRi, or MLFS. From left to right: (1) Monocytes as a percentage of total bone marrow white blood cells (WBCs). Monocytes were identified by moderate side scatter, variable expression of CD14, CD45, and HLA-DR, and relatively bright expression of CD64. (2) Immature monocytes as a percentage of total bone marrow WBCs. Immature monocytes were identified as a monocyte population with CD11bdim, CD14dim, and HLA-DRbright. (3) Expression of CD64 on blasts, expressed as mean fluorescence intensity (MFI). Blasts were identified by CD34 or CD117 expression. (4) CD38dim blasts as a percentage of total bone marrow blasts. CD38dim blasts were identified as a blast population expressing CD38dim. (C) Changes in the immunophenotypic characteristics comparing time of treatment start (diagnosis [Dx]) to time of relapse (RL). From left to right: (1) Monocytes as a percentage of total bone marrow WBCs. Monocytes were identified by moderate side scatter, variable expression of CD14, CD45, and HLA-DR, and relatively bright expression of CD64. (2) Immature monocytes as a percentage of total bone marrow WBCs. Immature monocytes were identified as a monocyte population with CD11bdim, CD14dim, and HLA-DRbright. (3) Expression of CD64 on blasts, expressed as MFI. Blasts were identified by CD34 or CD117 expression. (4) CD38dim blasts as a percentage of total bone marrow blasts. CD38dim blasts were identified as a blast population expressing CD38dim.
Immunophenotypic predictors of response and characteristics at relapse
There were no significant differences between responders and nonresponders in the percentage of monocytes or immature monocytes or in the mean fluorescent intensity of CD64 on blasts, a marker of monocytic differentiation (Figure 5B). Interestingly, the percentage of immature CD38dim blasts was higher in nonresponding patients (mean, 54.75%; standard deviation [SD], 31.73%) compared with responders (mean, 31.5%; SD, 31.96%; P = .016). In a paired comparison between available diagnostic and relapse samples, there were no differences in the percentage of immature monocytes, CD38dim blasts, or CD64 mean fluorescent intensity on blasts at diagnosis vs relapse, indicating that there was no evidence of selection for monocytic lineage at the time of relapse (Figure 5C).
Discussion
The approval of venetoclax combination therapy in newly diagnosed AML has led to the use of these combinations in patients with RR disease, a population with a poor prognosis and in great need of novel therapeutics.1-3 Here, we demonstrate that venetoclax combination therapies can be effective in the RR treatment setting. Importantly, other studies have reported outcomes of patients with RR-AML treated with these therapies, but our study represents the largest cohort of real-world patients treated with both HMA and low-dose cytarabine + venetoclax combinations published to date.5-14 Leveraging our well annotated data, we provide a detailed analysis of the clinical, molecular, and immunophenotypic predictors of response, survival, and relapse for patients with RR-AML who are receiving venetoclax.
We show that in RR-AML, azacitidine + venetoclax was associated with significantly superior responses and survival compared with low-dose cytarabine + venetoclax. Because the higher rate of subsequent allo-SCT in patients treated with azacitidine + venetoclax compared with patients treated with low-dose cytarabine + venetoclax (29% vs 4%) might have confounded the much longer median OS achieved with azacitidine + venetoclax (25 vs 3.9 months), we censored patients at the time of allo-SCT. Even after censoring for allo-SCT, azacitidine + venetoclax was associated with a significantly longer OS compared with low-dose cytarabine + venetoclax (8.1 vs 3.9 months; P = .035). Azacitidine + venetoclax remained associated with improved survival compared with low-dose cytarabine + venetoclax, even when adjusting for age, previous HMA use, and subsequent allo-SCT, suggesting that HMA + venetoclax might be preferred over low-dose cytarabine + venetoclax in the RR treatment setting. However, this finding requires confirmation by either a propensity score matched analysis in a larger retrospective cohort of patients or ideally by a randomized prospective study comparing HMA + venetoclax with low-dose cytarabine + venetoclax in the RR treatment setting.
Importantly, a CR/CRi rate of 31% and median OS of 10 months (95% CI, 5.65 months to NR) achieved with HMA + venetoclax in our study compares favorably to what was observed in a historic control group of 655 RR-AML patients treated with HMA monotherapy.19 In that study, HMA monotherapy led to a CR/CRi rate of 16% and a median OS of 6.7 months (95% CI, 6.1-7.3 months).
Interestingly, we found that decitabine + venetoclax was associated with a lower response rate (25% vs 49%) and shorter survival compared with azacitidine + venetoclax (median OS, 4.9 vs 8.1 months after censoring for allo-SCT; P = .16), although these differences were not statistically significant. In a recently published trial that examined a 10-day schedule of decitabine + venetoclax, 10-day decitabine + venetoclax was associated with a relatively high ORR (62%) in RR-AML but limited median OS (7.8 months),15 which was similar to what was seen in our data set, in which most patients received decitabine on a 5-day schedule. Future trials are needed to directly compare clinical outcomes achieved with azacitidine + venetoclax on a 5-day treatment schedule compared with a 10-day treatment schedule of decitabine + venetoclax in RR-AML.
We found that outcomes differed dramatically depending on the specific molecular profile of the patient’s disease. Similar to what was reported previously for the first-line setting, mutations in NPM1 (CR/CRi, 46%; ORR, 62%) and IDH1 (CR/CRi, 56%; ORR, 67%) were associated with high response rates to venetoclax therapy in the RR setting.1,20 We also observed a novel association, that STAG2 mutations were associated with improved survival. STAG2 mutations had previously been reported to predict response to HMA monotherapy; however, no OS advantage has been reported despite an increased response to HMAs.21 In contrast, adverse risk cytogenetics and KRAS/NRAS and TP53 mutations were associated with worse OS, suggesting that the greatest benefit of current venetoclax combinations may be in patients with RR-AML without these specific adverse features. We also found that SF3B1 and EZH2 mutations predicted shorter survival, findings which have not been reported in newly diagnosed patients with AML treated with venetoclax combinations. Concordantly, in a large data set from the BEAT AML study group, samples from patients carrying an SF3B1 mutation were recently found to be highly resistant to venetoclax as indicated by higher area under the curve in an ex vivo venetoclax drug screen.22
Our molecular analysis argues for future clinical trials in RR-AML that focus on testing rationally designed combination therapies to improve the outcomes for patients carrying the most adverse risk mutations, including TP53 and SF3B1. Combining azacitidine with the anti-CD47 antibody magrolimab in TP53-mutated AML has shown promising activity in early-phase clinical trials.23 In addition, the splicing modulator E7107 was demonstrated to sensitize chronic lymphocytic leukemia cells to venetoclax by increasing BCL2 dependence and by inducing mis-splicing of other BCL2 family proteins such as MCL1 and BCL2A1, whose upregulation represents a well-known resistance mechanism to venetoclax therapy.24,25
Interestingly, previous HMA therapy had a significant impact on response and survival in patients with RR-AML carrying a DNMT3A mutation. Previous reports of DNMT3A mutations predicting high response rates to single-agent HMA therapy found this trend only among patients treated in the first-line setting. Once RR patients were included in the cohort, DNMT3A was no longer predictive of response to HMAs.26 In our study, DNMT3A-mutated RR patients who were naïve to HMAs achieved high response rates and prolonged survival with venetoclax combination therapy. Conversely, for DNMT3A-mutated patients who had previously received HMA therapy, response to HMA + venetoclax was low and survival was poor.
This may suggest that HMA-naïve DNMT3A-mutated patients are uniquely sensitive to epigenetic modification, and that this response in the RR setting is rescued when epigenetic modifiers are given in combination with venetoclax. Many studies to date have focused on the metabolic and apoptotic effects of these combinations, but our findings highlight the need for further study into how venetoclax therapy may act synergistically with HMAs to modify epigenetic targets in these patients. For patients who did not have a DNMT3A mutation, survival was similar regardless of whether they had received previous HMA therapy. Overall, our results argue for incorporating DNMT3A mutational status and previous HMA treatment history into predicting response to HMA + venetoclax in RR-AML.
Apart from noting new mutations in ASXL1, DNMT3A, FLT3, NRAS, and TET2 at relapse, we also found that, in 1 patient, a new IDH2 mutation emerged under the selective pressures of venetoclax combination therapy despite the high response rates to venetoclax-based treatment in IDH-mutated AML. We also found increased disease complexity at relapse, with several patients developing a more complex karyotype or having ≥1 new mutations emerging simultaneously. Because our data suggest that relapse may occur through a variety of molecular mechanisms, similar to what has been reported with induction chemotherapy, patients treated with venetoclax combination therapy may benefit from sequential therapy or consolidation with a cytarabine-containing regimen.27 Contrary to a previous report in the first-line treatment setting, we did not observe an association between treatment resistance and monocytic differentiation in the RR setting.28 Patients with RR-AML who did not respond to treatment had a more immature immunophenotype, similar to what has been described at relapse after standard induction therapy.29
Our data are retrospective, with small numbers of patients in some subgroups, and require confirmation in a prospective setting. In particular, for some mutations that occurred infrequently, no conclusive statement can be made regarding their impact on response and survival. Nevertheless, our results suggest that azacitidine + venetoclax may provide a highly effective treatment option for some patients with RR-AML and that patients with specific clinical and molecular profiles are more likely to derive benefit from current venetoclax combinations. In contrast, patients with RR-AML that have high-risk genetic features such as adverse cytogenetics and mutations in TP53, KRAS/NRAS, SF3B1, and EZH2 continue to have poor outcomes, even with current venetoclax combinations. Such high-risk patients may benefit from clinical trial protocols that combine azacitidine + venetoclax with novel targeted or immunotherapeutic agents.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Cancer Institute (P30 CA016359), and by a Cancer Center Support Grant/Core Grant (P30 CA008748) (Memorial Sloan Kettering Cancer Center [MSKCC]).
M.S. received funding from the MSKCC Clinical Scholars T32 Program (T32 CA009512-31) and from the Conquer Cancer Foundation of the American Society of Clinical Oncology (GC241610). A.D.G. received funding from an American Society of Hematology (ASH) Fellow Scholar Award in Clinical Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
To request data, please e-mail Maximilian Stahl (stahlm@mskcc.org) and Aaron D. Goldberg (goldbera@mskcc.org).
Authorship
Contribution: M.S., K.M., A.D., and A.D.G. designed and conducted the research and wrote and reviewed the paper; and A.C., W.X., J.G., A.C.K., A.F.D., C.F., B.M.C., T.Z.H., O.A.-W., R.L.L., A.D.V., E.M.S., S.F.C., M.R., M.S.T., and A.D.G. helped conduct the research and wrote and reviewed the paper.
Conflict-of-interest disclosure: W.X. received research funding from Stemline Therapeutics. J.G. served as a consultant for the Gerson Lehman Group. A.C.K. served on an advisory board for AbbVie. A.D. has a patent licensed in part by Caribou Biosciences. O.A.-W. served as a consultant for Janssen and Merck, served as a consultant and received research funding from H3 Biomedicine, and is a current equity holder in privately held Envisagenics. R.L.L. received research funding from Prelude Therapeutics; served as a consultant for Janssen, Astellas, Morphosys, and Novartis; served as a consultant for and received honoraria from Eli Lilly; served as a consultant for and received honoraria and research funding from Celgene and Roche; served as a member of the Board of Directors or advisory committee for and is a current equity holder for publicly traded company Qiagen; served as a member of the Board of Directors or advisory committee and is a current equity holder for privately held Loxo, Imago, C4 Therapeutics, and Isoplexis; and received honoraria from Amgen and Gilead. E.M.S. received research funding from Bayer; was a consultant for Amgen, AbbVie, Seattle Genetics, and Biotheryx; served as a consultant and received research funding from Syndax; was a member of the Board of Directors or advisory committee for PTC Therapeutics and Syros; served as a consultant and was member of the Board of Directors or advisory committee for Astellas Pharmaceutical, Agios Pharmaceuticals, and Genentech; served as a consultant, received research funding, and was a member of the Board of Directors or advisory committee for Daiichi-Sankyo, Celgene Pharmaceuticals, and Novartis; and is a current equity holder in privately held Auron Therapeutics. S.F.C. has consulted for and holds equity interest in Imago Biosciences. M.S.T. received research funding from AbbVie, Cellerant, Orsenix, ADC Therapeutics, Glycomimetics, Rafael, and Amgen; was a member of the Board of Directors or advisory committee for Bioline rx, Daiichi-Sankyo, KAHR, Rigel, Delta Fly Pharma, Oncolyze, Jazz Pharma, Roche, and Novartis; received research funding from and was a member of the Board of Directors or advisory committee for BioSight; has served on advisory boards for Innate Pharaceuticals, Kura Oncology, and Syros Pharmaceuticals; and has a patent and received royalties from UpToDate. A.D.G. received research funding from Celularity, ADC Therapeutics, Aprea, AROG, Pfizer, and Prelude; received research funding from and served as a consultant for Aptose and Daiichi Sankyo; served as a consultant and member of the Board of Directors or advisory committee for Astellas, Celgene, and Genentech; received research funding from, served as a consultant for, and was a member of the Board of Directors or advisory committee for AbbVie; and received honoraria from Dava Oncology. The remaining authors declare no competing financial interests.
Correspondence: Maximilian Stahl, Leukemia Service, Division of Hematologic Malignancies, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: stahlm@mskcc.org; and Aaron D. Goldberg, Leukemia Service, Division of Hematologic Malignancies, Department of Medicine, Memorial Sloan Kettering Cancer Center, 530 East 74th St, New York, NY 10021; e-mail: goldbera@mskcc.org.
References
- 1.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei AH, Strickland SA Jr., Hou JZ, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: Results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network, Inc. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia, version 3.2020. Plymouth Meeting, PA: National Comprehensive Cancer Network, Inc. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 5.DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol. 2018;93(3):401-407. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg AD, Horvat TZ, Hsu M, et al. Venetoclax combined with either a hypomethylating agent or low-dose cytarabine shows activity in relapsed and refractory myeloid malignancies [abstract]. Blood. 2017;130(suppl 1). Abstract 1353. [Google Scholar]
- 7.Shahswar R, Hamwi I, Lueck C, et al. Registry for the off-label use of venetoclax in patients with relapsed or refractory acute myeloid leukemia [abstract]. European Hematology Association (EHA). 2018. Abstract PB1735. https://library.ehaweb.org/eha/2018/stockholm/216245/rabia.shahswar.registry.for.the.off-label.use.of.venetoclax.in.patients.with.html?f=menu=6*ce_id=1346*ot_id=19045*media=3*marker=168
- 8.Ram R, Amit O, Zuckerman T, et al. Venetoclax in patients with acute myeloid leukemia refractory to hypomethylating agents-a multicenter historical prospective study. Ann Hematol. 2019;98(8):1927-1932. [DOI] [PubMed] [Google Scholar]
- 9.Aldoss I, Yang D, Pillai R, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253-E255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morsia E, McCullough K, Joshi M, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: Mayo Clinic series on 86 patients. Am J Hematol. 2020;95(12):1511-1521. [DOI] [PubMed] [Google Scholar]
- 11.Ganzel C, Ram R, Gural A, et al. Venetoclax is safe and efficacious in relapsed/refractory AML. Leuk Lymphoma. 2020;61(9):2221-2225. [DOI] [PubMed] [Google Scholar]
- 12.Wang YW, Tsai CH, Lin CC, et al. Cytogenetics and mutations could predict outcome in relapsed and refractory acute myeloid leukemia patients receiving BCL-2 inhibitor venetoclax. Ann Hematol. 2020;99(3):501-511. [DOI] [PubMed] [Google Scholar]
- 13.Aldoss I, Zhang J, Mei M, et al. Venetoclax and hypomethylating agents in FLT3-mutated acute myeloid leukemia. Am J Hematol. 2020;95(10):1193-1199. [DOI] [PubMed] [Google Scholar]
- 14.Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404-e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiNardo CD, Maiti A, Rausch CR, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7(10):e724-e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 17.Jonas BA, Pollyea DA. How we use venetoclax with hypomethylating agents for the treatment of newly diagnosed patients with acute myeloid leukemia. Leukemia. 2019;33(12):2795-2804. [DOI] [PubMed] [Google Scholar]
- 18.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl M, DeVeaux M, Montesinos P, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thota S, Viny AD, Makishima H, et al. Genetic alterations of the cohesin complex genes in myeloid malignancies. Blood. 2014;124(11):1790-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Nakauchi Y, Köhnke T, et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer. 2020;1(8):826-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sallman DA, Al Malki M, Asch AS, et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: Phase Ib results [abstract]. J Clin Oncol. 2020;38(15_suppl). Abstract 7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ten Hacken E, Valentin R, Regis FFD, et al. Splicing modulation sensitizes chronic lymphocytic leukemia cells to venetoclax by remodeling mitochondrial apoptotic dependencies. JCI Insight. 2018;3(19):e121438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aird D, Teng T, Huang CL, et al. Sensitivity to splicing modulation of BCL2 family genes defines cancer therapeutic strategies for splicing modulators. Nat Commun. 2019;10(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coombs CC, Sallman DA, Devlin SM, et al. Mutational correlates of response to hypomethylating agent therapy in acute myeloid leukemia. Haematologica. 2016;101(11):e457-e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ball BJ, Hsu M, Devlin SM, et al. RAS mutations are independently associated with decreased overall survival and event-free survival in patients with AML receiving induction chemotherapy [abstract]. Blood. 2019;134(suppl 1). Abstract 18. [Google Scholar]
- 28.Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10(4):536-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas X, Campos L, Archimbaud E, et al. Surface marker expression in acute myeloid leukaemia at first relapse. Br J Haematol. 1992;81(1):40-44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.