Key Points
The R-CEOP regimen is a valid alternative to R-CHOP for patients with de novo DLBCL and an absolute contraindication to anthracyclines.
With a median follow-up of >12 years, a plateau is apparent on the disease-specific survival curve, indicating curative potential.
Abstract
Doxorubicin plays an integral role in the treatment of patients with diffuse large B-cell lymphoma (DLBCL) but can be associated with significant toxicity. Treatment guidelines of British Columbia (BC) Cancer recommend the substitution of etoposide for doxorubicin in standard-dose R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (R-CEOP) for patients who have a contraindication to anthracyclines; however, it is unknown if this compromises treatment outcome. We identified all patients with newly diagnosed DLBCL who were treated in BC with curative intent with R-CEOP (n = 70) within the study period. Outcome in this population was compared with a 2:1 case-matched control group (n = 140) treated with R-CHOP and matched for age, clinical stage, and International Prognostic Index score. The 10-year time to progression and disease-specific survival were not significantly different for patients treated with R-CEOP compared with patients in the R-CHOP control group (53% vs 62% [P = .089] and 58% vs 67% [P = .251], respectively). The 10-year overall survival was lower in the R-CEOP group (30% vs 49%, P = .002), reflecting the impact of underlying comorbidities and frailty of this population. R-CEOP represents a useful treatment alternative for patients with DLBCL and an absolute contraindication to the use of anthracyclines, with curative potential.
Visual Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, representing ∼30% of all newly diagnosed cases.1 It is more common in the elderly, with a median age at diagnosis in the seventh decade and 30% of patients >75 years of age. The R-CHOP chemotherapy regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been the backbone of therapy for several decades, as attempts to improve efficacy with more intensive chemotherapy have failed to demonstrate further benefit.2 The addition of the chimeric monoclonal antibody rituximab to R-CHOP led to achievement of overall cure rates in the range of 60% to 70%.3,4
Anthracyclines are a crucial component for efficacy in the treatment of DLBCL5 but can be associated with cardiotoxicity, leading to cardiomyopathy and congestive heart failure. The risk of cardiotoxicity increases with age and the presence of cardiovascular risk factors such as a history of coronary artery disease, hypertension, diabetes, valvular heart disease, and obesity.6 Anthracycline-induced cardiotoxicity is cumulative and significantly increases above a lifetime doxorubicin dose of 450 to 550 mg/m2, leading to an empiric dose limit of ∼450 mg/m2 in clinical practice.7
Approximately 5% to 10% of patients diagnosed with DLBCL in the province of British Columbia (BC) have a contraindication to treatment with doxorubicin and are at risk of compromised outcomes. Most of these patients have a preexisting cardiac condition with a decreased left ventricular ejection fraction. Others have been previously treated with an anthracycline-containing regimen for an unrelated malignancy or present with additional comorbidities that would preclude its use. In an attempt to maintain treatment efficacy, the BC Cancer Lymphoma Tumor Group recommends substitution of etoposide for doxorubicin in standard-dose R-CHOP (R-CEOP) for patients for whom anthracyclines are contraindicated. The aim of this study was to evaluate the outcome in patients with de novo DLBCL and a contraindication to doxorubicin who were treated with R-CEOP over the decade from 2001 to 2009 to allow for mature follow-up and long-term assessment of the etoposide substitution.
Design and methods
Patient selection and study design
The BC Cancer Centre for Lymphoid Cancer and Pharmacy databases were used to identify the study population. We included all patients with biopsy-proven de novo DLBCL, primary mediastinal B-cell lymphoma (PMBCL) and intermediate grade B-cell lymphoma not otherwise specified (NOS) treated with R-CEOP with curative intent prior to March 2009. The latter diagnosis was used when small biopsy samples did not allow a definitive diagnosis of DLBCL, although B lineage (CD20 positivity), large abnormal B cells, and a diffuse architecture were present. Patients who received partial treatment with anthracyclines were included in this study, provided that the etoposide substitution (R-CEOP) was made in >50% of the cycles delivered. Patients with transformed lymphoma and relapsed DLBCL were excluded from the analysis. The outcome of the study cohort was compared with a 2:1 ratio control group of randomly selected sequential patients with DLBCL treated with R-CHOP in the same time period after matching for age, clinical stage, and International Prognostic Index (IPI) score.8
Routine staging investigations included history and physical examination; laboratory analysis; computed tomography scanning of the neck, chest, abdomen, and pelvis; and bone marrow biopsy. Fluorodeoxyglucose positron emission tomography scanning was not used for staging in most patients. Patients with advanced-stage disease, defined as Ann Arbor stages III or IV or stages I and II with B-symptoms, bulky disease (≥10 cm), or disease that could not be encompassed within a single involved-field radiation port were intended to receive 6 to 8 cycles of chemotherapy. The use of consolidative radiation therapy was at the discretion of individual treating physicians. All other patients (limited stage) were eligible to receive 3 cycles of chemotherapy and involved-field radiation therapy unless they had a contraindication to radiation. The BC Cancer protocol for R-CHOP includes standard doses administered at 21-day intervals (http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Lymphoma-Myeloma/LYCHOPR_Protocol.pdf). Patients with a contraindication to anthracyclines receive the R-CEOP regimen, with etoposide substituted for doxorubicin, administered at a dose of 50 mg/m2 IV on day 1 and 100 mg/m2 orally on days 2 and 3. Growth factor use is not mandated and at the discretion of treating clinicians, unless clinically indicated as described in the protocol. This retrospective study was approved by the University of British Columbia-BC Cancer Research Ethics Board.
Statistical analysis
Clinical characteristics were compared between the study cohorts using the independent-samples t test for continuous variables and the χ2 test for categorical variables. Time to progression (TTP) was calculated from the date of diagnosis to the date of first progression, relapse, or death as a result of treatment toxicity or lymphoma; living patients without evidence of lymphoma and patients who died of unrelated causes were censored at the date of last follow-up or date of death, respectively. Disease-specific survival (DSS) was calculated from the date of diagnosis to the date of death from lymphoma or treatment-related toxicity; living patients or patients who died of unrelated causes were censored at the date of last follow-up or date of death, respectively. Overall survival (OS) was calculated from the date of diagnosis to the date of last follow-up or death from any cause. The Kaplan-Meier method was used to calculate TTP, DSS, and OS.9 Survival comparisons were performed using the log-rank test.10 All analyses were performed using SPSS version 14.0 (SPSS, Chicago, IL or GraphPad Prism 8.4.1).
Results
Clinical characteristics and treatment details
We identified 70 patients treated with R-CEOP who met the stated inclusion criteria. Median age was 73 years (range, 34 to 93 years). The majority of patients were male (69%) and had advanced-stage disease (83%). The control group was well matched for age, stage, and IPI risk score but had a slightly lower percentage of male patients. Clinical characteristics of the 2 study groups are listed in Table 1.
Table 1.
Baseline demographics and clinical characteristics at diagnosis
| Characteristic | R-CEOP | R-CHOP | P |
|---|---|---|---|
| Number of patients | 70 | 140 | |
| Age, y | |||
| Median | 73 | 73 | .997 |
| Range | 34-93 | 21-92 | |
| Sex | |||
| Male | 48 (69) | 75 (54) | .037 |
| Female | 22 (31) | 65 (46) | |
| Stage | |||
| Limited | 12(17) | 20 (14) | .587 |
| Advanced | 58 (83) | 120 (86) | |
| Diagnosis | |||
| DLBCL | 66 (94) | 132 (94) | 1.00 |
| PMBCL | 0 (0) | 8 (6) | |
| IGBCL NOS | 4 (6) | 0 | |
| B-symptoms* | |||
| Yes | 31 (44) | 52 (37) | .318 |
| IPI | |||
| 0, 1 | 14 (20) | 27 (19) | 1.0 |
| 2 | 27 (39) | 55 (39) | |
| 3 | 20 (28) | 30 (22) | |
| 4, 5 | 9 (13) | 28 (20) | |
| Radiation therapy | 17 (24) | 24 (17) | .218 |
Data are presented as n (%) of patients unless otherwise indicated.
IGBCL, intermediate grade B-cell lymphoma NOS.
Information on B-symptoms is missing in 4 patients and 1 patient in the R-CEOP and R-CHOP group, respectively
The primary indication for initiating treatment with R-CEOP was the presence of cardiac dysfunction (n = 63; 90%). Five patients (7%) had been previously exposed to an anthracycline during therapy for a remote unrelated malignancy, while 2 patients (3%) were considered too frail for treatment with R-CHOP due to multiple comorbidities. The majority of patients (67%) did not receive any anthracycline during treatment of their lymphoma, whereas 33% of patients received partial treatment with doxorubicin, limited to less than half of the cycles delivered. Of the 23 patients in the R-CEOP group who received partial anthracyclines, 13 received only 1 cycle, 9 received 2 cycles, and 1 received 3 cycles with doxorubicin included. In these cases, patients were switched from R-CHOP to R-CEOP after reaching a lifetime dose for doxorubicin of 450 mg/m2 or when a contraindication to doxorubicin emerged (Table 2). In total, only 22 patients received 8 cycles of chemotherapy, 6 (9%) in the R-CEOP cohort and 16 (11%) in the R-CHOP cohort. In the R-CEOP cohort, 24% received radiation therapy as part of their primary treatment, which was similar to the 17% in R-CHOP control group. (Table 1)
Table 2.
Treatment details and toxicity
| R-CEOP | R-CHOP | |
|---|---|---|
| Anthracycline received | ||
| No anthracycline | 47 (67) | — |
| Partial anthracycline | 23 (33) | — |
| Reason for etoposide substitution | ||
| Cardiac contraindication | 63 (90) | — |
| Prior exposure | 5 (7) | — |
| Other comorbidities | 2 (3) | — |
| Treatment tolerance | ||
| Percentage of deaths due to toxicity | 3 (4) | 5 (4) |
| Completed planned treatment | 54 (78) | 110 (79) |
Data are presented as n (%) of patients.
Outcome
With a median follow-up time for living patients of 12.4 years (range, 0.6-18.7 years), 59 out of 70 patients (84%) in the R-CEOP group have died, compared with 93 out of 140 patients (66%) in the R-CHOP control group. Overall, 27 out of 70 patients (39%) in the R-CEOP group, compared with 49 out of 140 patients (33%) in R-CHOP group, died due to lymphoma or treatment toxicity, with similar rates of death due to toxicity alone in each group (3/70 [4%] in the R-CEOP group vs 5/140 [4%] in the R-CHOP group). In total, 32 out of 70 patients (46%) receiving R-CEOP died of unrelated causes, compared with 47 out of 140 patients (33%) receiving R-CHOP, possibly reflecting the higher degree of comorbidities within the R-CEOP population. A higher proportion of patients treated with R-CEOP who died of unrelated causes eventually died of cardiac causes compared with those treated with R-CHOP (13/32 [41%] vs 13/47 [28%]; P = .23), although this was not statistically significant.
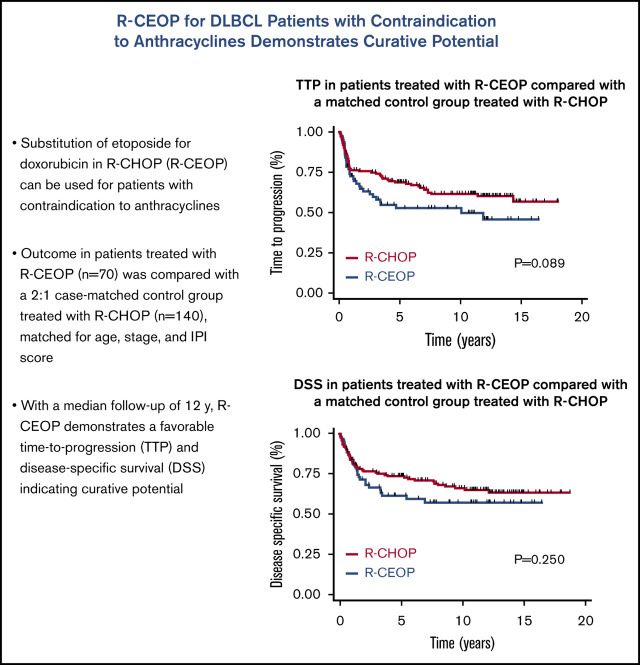
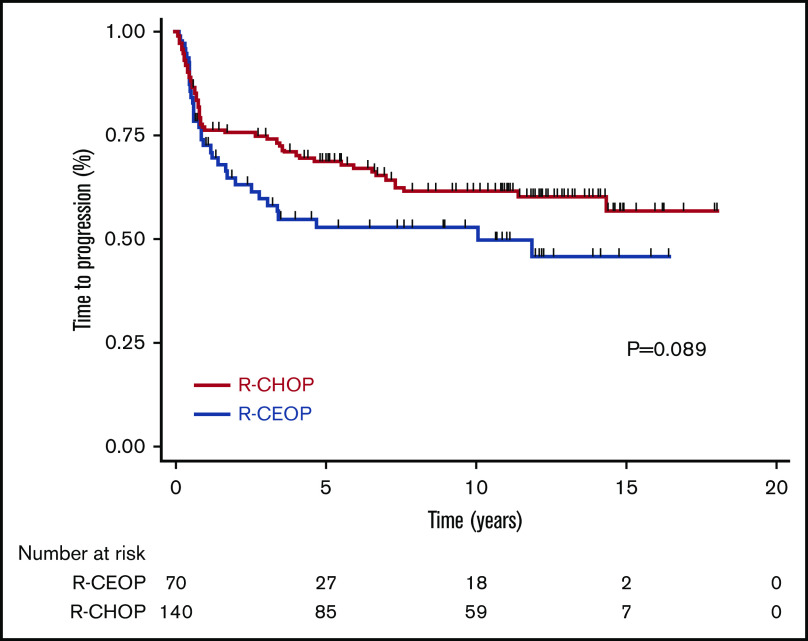
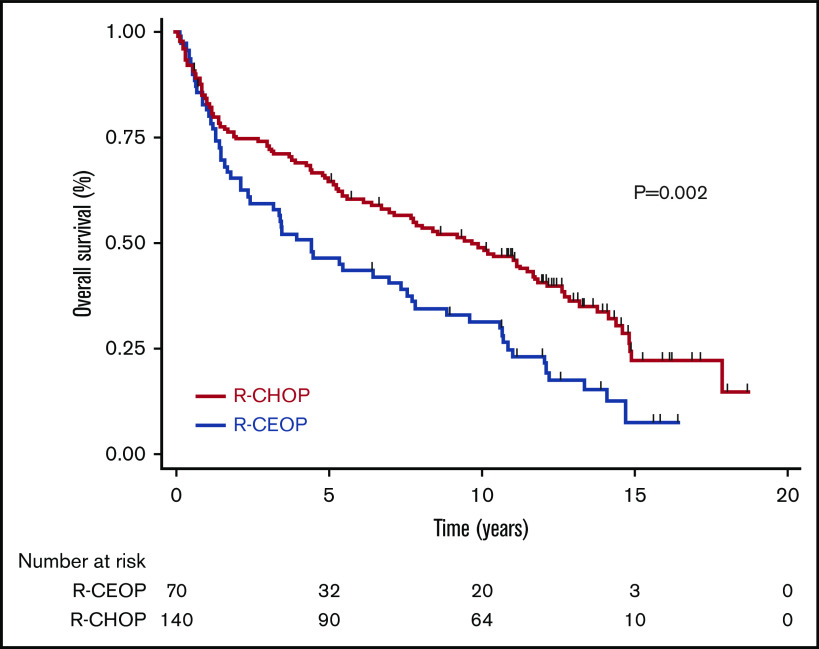
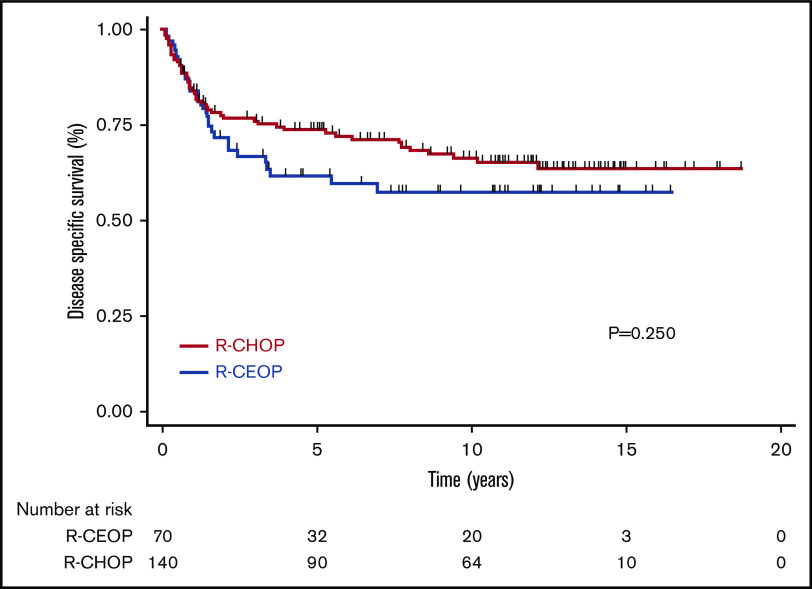
TTP was not significantly different for patients receiving R-CEOP compared with R-CHOP (log-rank P = .089), with 5-year and 10-year TTP estimates of 53% and 53% vs 69% and 62%, respectively (Table 3 and Figure 1). This was also true for TTP according to clinical stage at presentation, with no significant differences observed between the subgroups. (Figure 2) Interestingly, TTP was also similar in patients who received R-CEOP and partial therapy with doxorubicin compared with those who did not receive any anthracycline (supplemental Appendix and supplemental Figure 1) Five-year and 10-year OS were lower for patients receiving R-CEOP compared with R-CHOP (47% and 30% vs 65% and 49%, respectively; log-rank P = .002) due to a higher number of deaths from unrelated causes likely due to the frailty of this population (Table 3 and Figure 3). However, 5-year and 10-year DSSs were not significantly different (62% and 58% vs 74% and 67%, respectively; P = .250) (Table 3 and Figure 4). Overall results were unchanged when the analysis was limited to patients with a histologic diagnosis of DLBCL, excluding those with intermediate grade lymphoma, NOS, and PMBCL (data not shown). As this was a historical cohort, cell-of-origin (COO) data (assessed by immunohistochemistry using the Hans algorithm) was available on only 12 patients in the R-CEOP group (8 germinal center B cell (GCB) and 4 non-GCB). Only 2 of 8 GCB and 1 of 4 non-GCB R-CEOP patients died due to lymphoma.
Figure 1.
TTP in patients treated with R-CEOP compared with a matched control group treated with R-CHOP.
Figure 2.
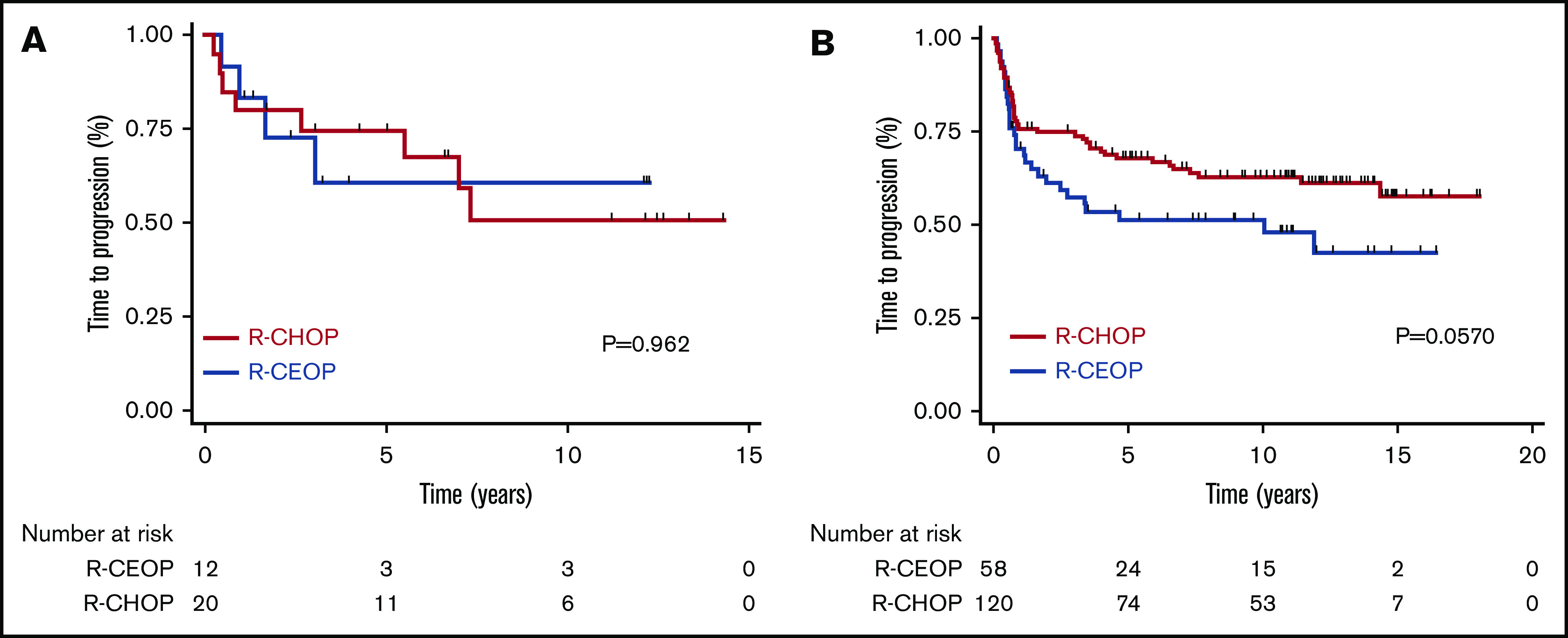
TTP according to the clinical stage at presentation in patients treated with R-CEOP compared with a matched control group treated with R-CHOP. (A) Limited stage. (B) Advanced stage.
Figure 3.
OS in patients treated with R-CEOP compared with a matched control group treated with R-CHOP.
Figure 4.
DSS in patients treated with R-CEOP compared with a matched control group treated with R-CHOP.
Toxicity and tolerance
As this was a retrospective population-based review, details regarding specific toxicities during therapy were not retrievable. However, deaths due to toxicity were similar between the groups (4% in each cohort) (Table 2). Causes of toxic deaths were pneumonia (n = 2) and myocardial infarction (n = 1) in the R-CEOP group and pneumonia (n = 2), sepsis (n = 1), myocardial infarction (n = 1), and fulminant liver failure (n = 1) in the R-CHOP group. The percentage of patients who completed all planned therapy was comparable between the groups (78% vs 79%, respectively). Despite the presence of significant comorbidities in patients receiving R-CEOP, treatment was generally well tolerated, with a high completion rate.
Table 3.
Survival outcomes at 5 and 10 years for R-CEOP vs R-CHOP
| R-CEOP | R-CHOP | ||||
|---|---|---|---|---|---|
| 5-y % | 10-y % | 5-y % | 10-y % | P | |
| TTP | 53 | 53 | 69 | 62 | .089 |
| DSS | 62 | 58 | 74 | 67 | .251 |
| OS | 47 | 30 | 65 | 49 | .002 |
Discussion
Anthracyclines remain a key component of therapy for the treatment of aggressive lymphoma, despite a toxicity profile that includes a risk of cardiotoxicity.11 As the majority of patients with DLBCL are older, a substantial number of patients are not candidates for anthracycline-based therapy due to preexisting cardiac abnormalities and additional comorbidities. These patients require treatment modification, with the goal of optimizing outcomes while minimizing toxicity.
Several strategies have been employed to minimize the cardiotoxicity associated with doxorubicin. Dexrazoxane, a free-radical scavenger, has been shown to be cardioprotective in patients treated for a variety of cancer types12 but has not been extensively studied in hematologic malignancies. Moreover, concerns have been raised regarding the possibility of a reduction in therapeutic efficacy if dexrazoxane is administered coincidently with standard chemotherapy. Modification of the anthracycline dosing schedule using a continuous infusion strategy to lower the peak dose delivered failed to reduce long-term cardiotoxicity.13 Similarly, structural analogs, such as epirubucin and mitoxantrone, did not appear to be less cardiotoxic when used at doses equipotent to doxorubicin.14,15 The role of nonpegylated liposomal doxorubicin in place of conventional doxorubicin in standard R-CHOP (R-COMP) has also been assessed.16-18 However, a randomized phase 3 trial comparing R-COMP to standard R-CHOP in 88 patients with newly diagnosed DLBCL and normal cardiac function failed to demonstrate a significant reduction in cardiotoxicity.17
In the current study, we examined outcomes in patients with DLBCL and a contraindication to anthracyclines who were treated with R-CEOP in which etoposide is substituted for doxorubicin in otherwise standard-dose R-CHOP. Etoposide has proven efficacy in non-Hodgkin lymphoma and importantly does not induce cardiac toxicity.19,20 This regimen is universally used in BC, and therefore, results should be representative of population-based expectations. Using the BC Cancer Lymphoid Cancer and Pharmacy databases, we identified all patients who were treated with curative intent with R-CEOP over an entire decade prior to March 2009 in the province of BC. The most common reason for doxorubicin omission was a cardiac contraindication (90%), while a minority of patients had been previously exposed to doxorubicin or had significant other comorbidities. Despite the frailty of this population, R-CEOP was generally well tolerated, with a low rate of treatment-associated toxic deaths (4%) and a high rate of therapy completion (78%).
We compared outcomes in patients treated with R-CEOP to those of a control group of patients treated with R-CHOP who were matched for age, stage, and IPI score. Patients in the R-CEOP cohort had a poorer OS, partly due to a higher rate of death from unrelated causes, reflecting their underlying comorbidities. TTP and DSS of the R-CEOP cohort was not significantly different from that observed in the R-CHOP control group, although due to modest cohort size, statistical power was limited. Interestingly, R-CEOP patients who received partial treatment with anthracyclines appeared to have a similar outcome compared with those who received no anthracycline therapy at all. With a median follow-up of >12 years, there appears to be a plateau on the R-CEOP DSS curve, suggesting that a majority of patients may be cured with this non-anthracycline-based regimen.
A number of clinical trials have addressed the management of elderly patients with DLBCL. Dose-reduced R-CHOP (R-miniCHOP) has become a popular regimen for patients who are too frail for R-CHOP, but it remains inappropriate in patients with a contraindication to anthracyclines.21 Several trials have investigated the role of bendamustine and rituximab in the frontline setting for patients unfit to be treated with R-CHOP.22,23 While bendamustine and rituximab can induce high response rates, the OS rates are generally low, making this a palliative regimen. In the majority of trials evaluating elderly patients with DLBCL, those with significant cardiac dysfunction have been excluded. More recently, Fields et al reported a phase 2 multicenter trial showing encouraging results with the combination of rituximab, gemcitabine, cyclophosphamide, vincristine, and prednisolone (R-GCVP) in patients considered unfit for anthracycline-containing chemotherapy due to cardiac comorbidity.24 With a median follow-up of 25 months, in 61 patients treated, the 2-year progression-free survival (PFS) and OS were 50% and 56%, respectively.
Our study represents the largest experience describing the safety and efficacy of R-CEOP in patients with DLBCL and a contraindication to anthracyclines. A recent study by Rashidi et al reported the outcome of 26 patients treated at a single institution with R-CEOP.25 After a short median follow-up of 19 months, 2-year PFS and OS of this very frail population were 49% and 59%, respectively. Interestingly, only COO was significantly associated with PFS and OS, with a notably better outcome seen in patients with GCB subtype. Unfortunately, in our analysis, COO data were only available for a small number of patients, although no obvious difference in outcome by COO was observed.
Our study demonstrates that the substitution of etoposide for doxorubicin in otherwise standard-dose R-CHOP is feasible and well tolerated in patients with significant comorbidities. It should be noted that the intent of this trial was not to definitively compare the efficacy of R-CEOP with R-CHOP, as the modest patient number limits the power to demonstrate noninferiority. In addition, while this matched comparison was controlled for age, stage, and IPI score, imbalances in other relevant factors, such as COO, cannot be excluded. However, with a median follow-up of >12 years, long-term disease control with R-CEOP was relatively high, suggesting that a significant proportion of patients may be cured by this combination. The standard management of patients with DLBCL continues to require the use of an anthracycline when feasible. Alteration of doxorubicin in the R-CHOP regimen should only be considered within clinical trials or if doxorubicin cannot be safely administered. However, treatment intent for these patients should not necessarily be palliative. For patients with de novo DLBCL and an absolute contraindication to anthracyclines, the R-CEOP regimen represents a valid alternative to R-CHOP with curative potential.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This study was supported by the Fondazione San Salvatore, the British Columbia Cancer Foundation, the Turner Family Outcomes Fund, and the Mary Toye Memorial Fund.
Footnotes
For original data, please contact lsehn@bccancer.bc.ca.
Authorship
Contribution: A.A.M. was the principal investigator and takes primary responsibility for the paper; A.A.M., L.H.S., C.F., and J.M.C. analyzed results, made the figures, and designed the research; and A.A.M., K.S., C.F., P.J.H., R.J.K., K.J.S., T.N.S., R.D.G., J.M.C., and L.H.S. wrote the paper.
Conflict-of-interest disclosure: A.A.M. has served on the advisory board of Roche, Janssen, and Takeda. C.F. received research funding from Roche and Teva and honoraria from Seattle Genetics, Janssen, Amgen, Celgene, AbbVie, and Sanofi. T.N.S. has served on the advisory board of Eli Lilly, Roche, Novartis, and Purdue. L.H.S. discloses honoraria from Abbvie, Celgene, Janssen, and Lundbeck; honoraria from and consultancy for Acerta, Amgen, apobiologix, AstraZeneca, Gilead, Karyopharm, Kite, Merck, Morphosys, Takeda, Teva, and TG Therapeutics; and consultancy for and honoraria and research funding from Roche/Genetech. The remaining authors declare no competing financial interests.
Correspondence: Laurie H. Sehn, BC Cancer, Vancouver Clinic, 600 West 10th Ave, Vancouver, BC V5Z 4E6, Canada; e-mail: lsehn@bccancer.bc.ca.
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780-2795. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328(14):1002-1006. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. [DOI] [PubMed] [Google Scholar]
- 4.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. [DOI] [PubMed] [Google Scholar]
- 5.McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976;38(4):1484-1493. [DOI] [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996-1002. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717. [DOI] [PubMed] [Google Scholar]
- 8.International Non-Hodgkin’s Lymphoma Prognostic Factors Project . A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987-994. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. [Google Scholar]
- 10.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 11.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(12):2629-2636. [DOI] [PubMed] [Google Scholar]
- 12.Venturini M, Michelotti A, Del Mastro L, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. J Clin Oncol. 1996;14(12):3112-3120. [DOI] [PubMed] [Google Scholar]
- 13.Lipshultz SE. Exposure to anthracyclines during childhood causes cardiac injury. Semin Oncol. 2006;33(3Suppl 8):S8-S14. [DOI] [PubMed] [Google Scholar]
- 14.Ryberg M, Nielsen D, Cortese G, Nielsen G, Skovsgaard T, Andersen PK. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100(15):1058-1067. [DOI] [PubMed] [Google Scholar]
- 15.Posner LE, Dukart G, Goldberg J, Bernstein T, Cartwright K. Mitoxantrone: an overview of safety and toxicity. Invest New Drugs. 1985;3(2):123-132. [DOI] [PubMed] [Google Scholar]
- 16.Luminari S, Montanini A, Caballero D, et al. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): results from the phase II EUR018 trial. Ann Oncol. 2010;21(7):1492-1499. [DOI] [PubMed] [Google Scholar]
- 17.Fridrik MA, Jaeger U, Petzer A, et al. Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: a randomised phase-III study from the Austrian Cancer Drug Therapy Working Group [Arbeitsgemeinschaft Medikamentöse Tumortherapie AGMT](NHL-14). Eur J Cancer. 2016;58:112-121. [DOI] [PubMed] [Google Scholar]
- 18.Rigacci L, Mappa S, Nassi L, et al. Liposome-encapsulated doxorubicin in combination with cyclophosphamide, vincristine, prednisone and rituximab in patients with lymphoma and concurrent cardiac diseases or pre-treated with anthracyclines. Hematol Oncol. 2007;25(4):198-203. [DOI] [PubMed] [Google Scholar]
- 19.Köppler H, Pflüger KH, Eschenbach I, et al. CHOP-VP16 chemotherapy and involved field irradiation for high grade non-Hodgkin’s lymphomas: a phase II multicentre study. Br J Cancer. 1989;60(1):79-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hainsworth JD, Johnson DH, Frazier SR, Greco FA. Chronic daily administration of oral etoposide in refractory lymphoma. Eur J Cancer. 1990;26(7):818-821. [DOI] [PubMed] [Google Scholar]
- 21.Peyrade F, Jardin F, Thieblemont C, et al. ; Groupe d’Etude des Lymphomes de l’Adulte (GELA) investigators . Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468. [DOI] [PubMed] [Google Scholar]
- 22.Weidmann E, Neumann A, Fauth F, et al. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011;22(8):1839-1844. [DOI] [PubMed] [Google Scholar]
- 23.Park SI, Grover NS, Olajide O, et al. A phase II trial of bendamustine in combination with rituximab in older patients with previously untreated diffuse large B-cell lymphoma. Br J Haematol. 2016;175(2):281-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields PA, Townsend W, Webb A, et al. De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: a United Kingdom National Cancer Research Institute trial. J Clin Oncol. 2014;32(4):282-287. [DOI] [PubMed] [Google Scholar]
- 25.Rashidi A, Oak E, Carson KR, Wagner-Johnston ND, Kreisel F, Bartlett NL. Outcomes with R-CEOP for R-CHOP-ineligible patients with diffuse large B-cell lymphoma are highly dependent on cell of origin defined by Hans criteria. Leuk Lymphoma. 2016;57(5):1191-1193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.