Key Points
In Tanzania, the rate of molecular response to imatinib in patients with CML is low compared to elsewhere.
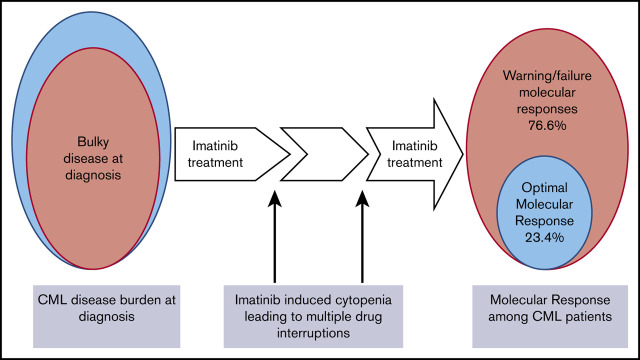
Advanced disease at presentation and multiple drug interruptions because of cytopenias are the main contributors to this low response.
Abstract
Imatinib is the mainstay of treatment of patients with chronic myeloid leukemia (CML) in Tanzania. Monitoring molecular response to therapy by real-time polymerase chain reaction at defined milestones is necessary for early detection of treatment failure. However, this assay is not routinely performed in Tanzania; therefore, the depth of molecular response among patients with CML is not known. A total of 158 patients with previously diagnosed CML who received imatinib treatment were recruited from January 2019 and followed up through October 2020 at Ocean Road Cancer Institute. Information was obtained at the time of diagnosis and follow-up. Blood samples were collected in EDTA tubes to measure the BCR/ABL ratio on the Gene Xpert system for molecular response determination. The median age of the 158 adult patients was 45 years (range, 18-86). By reference to established treatment milestones, only 37 (23.4%) achieved optimal molecular response. Signs of advanced-stage disease, in particular the need for red cell transfusions before diagnosis (adjusted odds ratio [AOR], 3.4; 95% CI, 1.32-9.17) and cytopenias (AOR, 2.26; 95% CI, 1.03-4.96) necessitating drug interruptions were statistically validated predictors of treatment failure on multivariate, multinomial logistic regression. Patient survival at the 22-month follow-up was lowest, with 78.6% (95% CI, 69.4-85.4) in the failure-to-respond category and highest in patients achieving optimal response 97.0% (95% CI, 80.9-99.6). In summary, the majority of patients with CML treated with imatinib in Tanzania do not obtain deep molecular response. This outcome can be attributed to late diagnosis, the development of cytopenias requiring multiple drug interruptions, and poor adherence to treatment.
Visual Abstract
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative disorder of pluripotent stem cells. It is characterized by a balanced reciprocal translocation t(9;22)(q34;q11) between the long arms of chromosomes 9 and 22, with consequent formation of the BCR-ABL chimeric fusion oncogene. BCR-ABL encodes an abnormal tyrosine kinase protein with constitutive activity that enhances proliferation of myeloid cells.1-3
The introduction of tyrosine kinase inhibitors (TKIs) such as imatinib has revolutionized CML treatment, and the once-deadly hematologic malignancy is currently a truly chronic disease with achievement of life expectancy similar to that of the general population.4,5 Given the effectiveness of TKIs in reducing the disease burden, increasingly sensitive techniques are needed to monitor the disease, once normalization of peripheral blood counts (hematologic response) and complete cytogenetic response (absence of Philadelphia-positive cells in marrow metaphase) have been achieved. Disease monitoring is performed today by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) assay of the BCR-ABL transcript, which can detect 1 CML cell in ∼100 thousand to 1 million cells.6 The dynamic of BCR-ABL transcript levels while patients are receiving treatment is a strong predictor of long-term outcome. In addition, it facilitates early detection of loss of response for early interventions, such as switching to second- or third-line TKIs.7 In practice, BCR-ABL quantification every 3 to 6 months by qRT-PCR is recommended for monitoring molecular response. Currently, the European Leukemia Net (ELN) has put forth different response milestones in relation to treatment duration, and BCR-ABL levels of <0.1% by real-time PCR (equivalent to a 3-log reduction) at 12 months of imatinib treatment is considered an optimal response.8
In Tanzania, patients with CML generally present late, with bulky disease including high rates of anemia, massive splenomegaly, and a very high white blood cell count (WBC). In a previous study, Tebuka et al reported that patients were symptomatic for at least a year before presenting to the tertiary hospital for diagnosis of CML.9 Usually, new patients presenting with CML-related symptoms are first seen at primary health care centers where they undergo routine assessment of anemia and presumptive treatment of iron and folate deficiency and worm infestation. By the time patients presented to the tertiary hospital, a majority had anemia (84%), splenomegaly (92%, massive in 62%), and a mean WBC count of ∼224 × 109/L.9
Imatinib is the mainstay TKI in the treatment of patients with CML in Tanzania. Since 2004, it has been available at no cost to all BCR-ABL+ patients with CML (regardless of their disease phase) under the sponsorship of Glivec International’s Patient Assistance Program (GIPAP). GIPAP is an international drug donation program established by Novartis Pharma AG that was implemented in partnership with the Max Foundation, a nonprofit, nongovernmental organization,10 with the aim of increasing access to cancer treatment in low- and middle-income countries. However, until 2018, there was only 1 center (Ocean Road Cancer Institute [ORCI] in Dar es Salaam) approved to receive donated imatinib through GIPAP. To date, only 1 additional center in Northern Tanzania (Moshi) has access to imatinib. Therefore, many patients have to incur traveling costs and other expenses to have access to imatinib; and often patients are unable to afford these expenses.
Data on the response to imatinib are scarce in African countries, including Tanzania. Among the few studies published, lower response rates have been reported in comparison with those in more developed countries.11-13 Tebuka et al reported that complete hematologic response (CHR) was attained by more than 90% of patients in Tanzania at 3 months of imatinib treatment.9 The long-term outcomes of patients in Tanzania with CML treated with imatinib are largely unknown. Molecular monitoring is not routinely performed in Tanzania because of the limited accessibility and affordability of PCR testing for patients with CML. Herein, we report data on molecular response in these patients, after they have received imatinib for a minimum of 3 months.
Methods
Study population
Previously diagnosed patients with CML were recruited from January 2019 and followed up through October 2020 at the CML day clinic at ORCI in Tanzania. Diagnosis of CML was established by examination of peripheral blood and bone marrow and confirmed by PCR for the BCR-ABL fusion gene. The PCR test was carried out in all patients to fulfill a requirement for access to free imatinib under the GIPAP. Eligible patients were adults aged ≥18 years who had been receiving imatinib treatment for at least 3 months. Excluded were patients with newly diagnosed disease receiving imatinib treatment for <3 months, or receiving TKIs other than imatinib, or undergoing cytoreduction with hydroxyurea.
Data collection
Data from eligible consenting patients was collected at 3 time points: (1) at diagnosis traced retrospectively from the patient’s clinical files, (2) at a follow-up visit at which patients were recruited into this study, and (3) for 22 months after recruitment for assessment of survival. Retrospective data at the time of diagnosis from the patient’s clinical files included presenting symptoms, signs, and laboratory parameters, such as full blood cell count and blast count from bone marrow reports. The Sokal risk score at baseline was calculated according to each patient’s clinical and laboratory parameters. Baseline BCR-ABL was assumed to be 100% as per International Standard (IS) recommendations. Retrospective, systematic complete full blood count results at 3, 6, and 12 months were not available, primarily because patients’ clinic visits depend on the financial ability to travel to the prescribing center.
Data collected at the follow-up visit during the recruitment into the study included socio-demographic characteristics (age, sex, residence, marital status, living arrangement, education level, and employment status) and information on imatinib toxicity and adherence. A physical examination was performed for signs of imatinib toxicity or disease regression or progression, including spleen size (if palpable). After the interview, blood samples were collected in EDTA tubes to run a full blood panel on a hematology analyzer (Dymind DH 76) and PCR for BCR-ABL transcripts on the Gene Xpert diagnostic system, version 4.4a. Data collected during the 22-month follow-up included time and cause of death.
Variables and measurements
CML disease phases were assessed according to 2016 World Health Organization criteria as chronic phase: bone marrow blasts <10%; accelerated phase: blasts 10% to 19% or peripheral blood basophils ≥20%; and blast crisis: blasts ≥ 20%.14
CHR was defined as WBC <10 × 109/L with no immature granulocytes on peripheral blood, <5% basophils on differential, platelet count <450 × 109/L, and no palpable spleen.15
Molecular response was defined as magnitude of reduction of BCR-ABL transcripts from the original value of 100%. Molecular response was categorized into 3 groups as per ELN 2020 guidelines.15 (1) Optimal molecular response: at 3 months, BCR-ABL ≤10%; at 6 months, BCR-ABL ≤1%; and at ≥12 months, BCR-ABL ≤0.1%. (2) Warning: at 3 months, BCR-ABL >10%; at 6 months, BCR-ABL >1% to 10%; and at ≥12 months, BCR-ABL >0.1% to 1%. (3) Failure: at 3 months, BCR-ABL >10% if confirmed within 1 to 3 months; at 6 months, BCR-ABL >10%; and at 12 months and anytime BCR-ABL is >1%.
Adherence was defined as the extent to which a patient followed the clinician’s instructions on daily imatinib ingestion and was assessed with the 8-item Morisky Medication Adherence Scale (MMAS-8), as previously described.16,17 Adherence was categorized into 3 groups: low adherence, score 0 to <6; medium adherence, score 6 to <8; and high adherence, score 8.
Imatinib-induced myelotoxicity was defined per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.018: neutropenia grade 1, absolute neutrophils <2.0 × 109/L to 1.5 × 109/L; grade 2, <1.5 × 109/L to 1.0 × 109/L; grade 3, <1.0 × 109/L to 0.5 × 109/L; and grade 4, <0.5 × 109L. Thrombocytopenia: grade 1, <150 × 109/L to 75 × 109/L; grade 2, <75 × 109/L to 50 × 109/L; grade 3, <50 × 109/L to 25 × 109/L; and grade 4, <25 × 109/L. Anemia: grade 1, <12 to 10 g/dL; grade 2, <10 to 8 g/dL; and grade 3, <8 g/dL.
Statistical analysis
Descriptive statistics were used to summarize sociodemographic data and clinical and treatment-related characteristics. Bivariate multinomial logistic regression was conducted to determine the association of response to imatinib treatment with independent variables. All variables with P <0.25 in bivariate multinomial logistic regression were included in the multivariate, multinomial logistic regression. Survival probabilities were estimated using the Kaplan-Meier method and the log-rank test was used to estimate the statistical significance of survival functions among the 3 ELN treatment response categories: optimal, warning, and failure. Values with P < .05 were considered statistically significant.
Ethical considerations
The ethical clearance for the study was obtained from the Senate Research and Publication Committee of Muhimbili University of Health and Allied Sciences (MUHAS). Permission to conduct the study was obtained from the authorities and the administration of ORCI. Written informed consents were obtained from the patients before recruitment, in accordance with the Declaration of Helsinki.
Results
Of 191 patients attending the Ocean Road CML Clinic from January through May 2019, 33 were not eligible: 14 were <18 years of age, 15 had been receiving imatinib for <3 months, 3 were on the second line of TKIs, and 1 had undergone a hematopoietic stem cell transplant in India. Thus, 158 patients were recruited into the study after they provided informed written consent. Among those, 104 patients had data available for Sokal score calculation, and 132 had data available to determine the CML clinical phase.
Sociodemographic and clinical characteristics of patients with CML
The median age was 45 years (range, 18-86). Male patients were 55.7%, with a male/female ratio of 1.3. The majority of patients (n = 97; 61.4%) attending the CML clinic at ORCI resided outside Dar es Salaam and had to travel there from other regions to receive imatinib and for follow-up care (Table 1).
Table 1.
Patients demographics and clinical characteristics (N = 158)
| Characteristic | No. of patients, n (%) |
|---|---|
| Median age (range), y | 45 (18-86) |
| Sex | |
| Male | 88 (55.7) |
| Female | 70 (44.3) |
| Living Arrangement | |
| Lives alone | 24 (15.2) |
| Lives with someone | 134 (84.8) |
| Residence | |
| Dar es Salaam | 61 (38.6) |
| Outside Dar es Salaam | 97 (61.4) |
| History of CML at diagnosis | |
| Symptomatic at diagnosis | 155 (98.1) |
| Types of symptoms at diagnosis | |
| Anemia | 129 (81.7) |
| Requiring blood transfusion | 52 (32.9) |
| Number of blood transfusions | |
| 1-3 units | 36 (22.8) |
| 4-6 units | 14 (8.9) |
| >6 units | 2 (1.2) |
| Abdominal fullness | 136 (86.1) |
| Constitutional symptoms | 110 (69.6) |
| Bleeding tendencies | 34 (21.5) |
| Clinical signs at diagnosis | |
| Pallor | 139 (88.0) |
| Splenomegaly | 133 (84.2) |
| Massive splenomegaly (≥20cm) | 59 (44.2) |
| Hepatomegaly | 27 (17.1) |
| Skin nodules | 23 (14.6) |
| Lymph node enlargement | 9 (5.7) |
| Laboratory parameters at diagnosis, median (range) | |
| White blood cells, ×109/L | 241.0 (8.0-760.0) |
| Hemoglobin, g/dL | 7.8 (2.6-13.3) |
| Platelets, ×109/L | 318 (67-1832) |
| Disease phase at diagnosis, n = 132 | |
| Chronic phase | 123 (93.2) |
| Accelerated phase | 9 (6.8) |
| Blast crisis | 0 (0.0) |
| Sokal Risk groups, n = 104 | |
| Low | 10 (10.0) |
| Intermediate | 51 (49.0) |
| High | 43 (41.0) |
| Hydroxyurea use prior Imatinib | 101 (63.9) |
| Median hydroxyurea dose (range), mg | 2000 (500-3000) |
| Duration of imatinib treatment, median (range) | 32 (3-159) |
| <12 mo | 28 (17.7) |
| ≥12-24 mo | 37 (23.4) |
| >24 mo | 93 (58.9) |
| Imatinib starting dose | |
| 400 mg/d | 146 (92.4) |
| 600 mg/d | 9 (5.7) |
| 800 mg/d | 3 (1.9) |
| Adherence to imatinib | |
| Low (MMAS-8 score, <6) | 68 (43.0) |
| Medium (MMAS-8 score, 6 to <8) | 50 (31.7) |
| High (MMAS-8 score, 8) | 40 (25.3) |
Unless stated otherwise, data are number of patients (percentage of the total study group or the subgroup).
MMAS, Morisky Medication Adherence Scale.
Nearly all patients (98.1%) were symptomatic at diagnosis (Table 1) and had a high disease burden, as reflected by the severity of anemia (requiring blood transfusion in 32.9%), massive splenomegaly 44.2%, Sokal score groups, and a high median WBC count of 241 × 109/L (supplemental Figures 1 and 2).
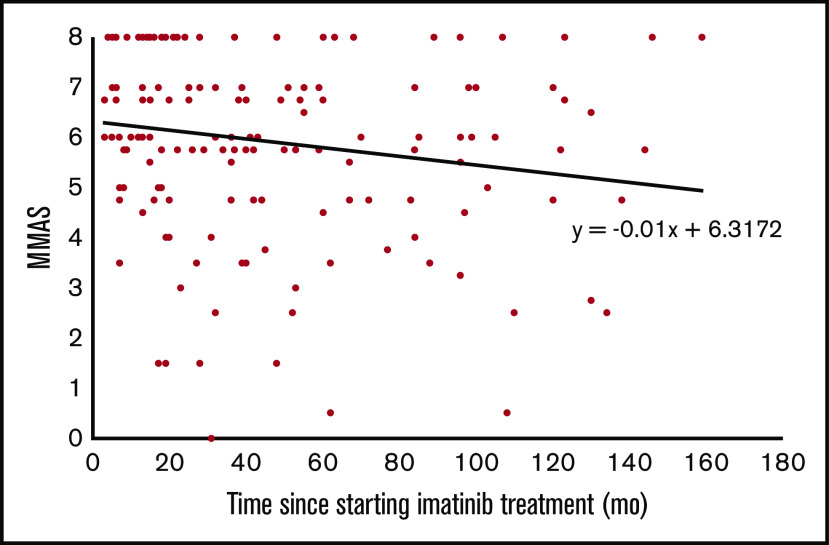
Nearly all patients (92.4%) were receiving imatinib 400 mg daily. The median duration of imatinib use was 32 months, and the majority of patients (82.3%) were beyond 12 months of treatment (Table 1). Adherence to imatinib, as assessed by MMAS-8, was low in almost one-half (43.0%) of all patients, and it decreased progressively with increasing time from when imatinib treatment started (Figure 1).
Figure 1.
Adherence to imatinib tends to decline with the duration of treatment. Only 25% of patients had perfect adherence. There was a modest but significant decline of MMAS score, by 0.01 point for each month on imatinib.
Imatinib-related side effects
A total of 132 (83.5%) of 158 interviewed patients reported some form of side effects during treatment. The most common reported side effect was cytopenia (59.5%; Table 2). Interestingly, skin hypopigmentation was reported in 13.9% of patients and was one of the most common nonhematologic side effects. In almost one-half of patients, the side effects occurred within the first 12 months of treatment; and in general, the frequency of imatinib side effects decreased over time in the majority (75.8%) of patients. However, a significant number of patients (38.6%) reported side effects at various times beyond 12 months of treatment (supplemental Figure 3). A total of 93 (58.9%) patients required cessation of treatment for 1 to 2 weeks to allow recovery from imatinib-induced cytopenia, and 41 of the 93 patients had at least 3 treatment interruptions (Table 2). At the time of recruitment into the study, all-grade anemia in 65.1%, neutropenia in 41.1%, and thrombocytopenia in 21.5% were observed (Table 2).
Table 2.
Imatinib side effects (N = 158)
| Variable | No. of patients, n (%) |
|---|---|
| Reported side effects | 132 (83.5) |
| Types of side effects | |
| Cytopenia | 94 (59.5) |
| GI side effects | 42 (26.6) |
| Musculoskeletal pain | 23 (14.6) |
| Hypopigmentation | 22 (13.9) |
| Edema | 17 (10.8) |
| Skin rash | 12 (7.6) |
| Weight gain | 6 (3.8) |
| Hemorrhage | 4 (2.5) |
| Fever | 1 (0.6) |
| Imatinib interruption due to cytopenia | 93 (58.9) |
| Frequency of interruption | |
| 1 time | 35 (22.2) |
| 2 times | 17 (10.8) |
| ≥3 times | 41 (26.0) |
| Observed cytopenia at study recruitment | |
| Neutropenia, all grades | 65 (41.1) |
| Grade 1 | 35 (22.2) |
| Grade 2 | 17 (10.7) |
| Grade 3 | 11 (6.9) |
| Grade 4 | 2 (1.3) |
| Thrombocytopenia, all grades | 34 (21.5) |
| Grade 1 | 25 (15.8) |
| Grade 2 | 4 (2.5) |
| Grade 3 | 2 (1.3) |
| Grade 4 | 3 (1.9) |
| Anemia, all grades | 103 (65.1) |
| Grade 1 | 67 (42.4) |
| Grade 2 | 28 (17.7) |
| Grade 3 | 8 (5.0) |
Data are number of patients (percentage of total study group).
Response to imatinib treatment
At 3 months of imatinib use, 100% of patients had achieved a CHR. However, at 6 months, the rate of CHR was slightly lower (94.1%), and at ≥12 months, it was significantly lower (68.5%: Table 3). Overall, CHR was present in more than two-thirds (73.4%) of all patients.
Table 3.
Complete hematologic response to imatinib tends to fade with time
| Time since imatinib initiation | Complete hematologic response | P | Molecular response | P | |||
|---|---|---|---|---|---|---|---|
| Present | Absent | Optimal | Warning | Failure | |||
| 3 mo (n = 11) | 11 (100) | 0 (0) | .01 | 2 (18.2) | 3 (27.3) | 6 (54.5) | .45 |
| 6 mo (n = 17) | 16 (94.1) | 1 (5.9) | 5 (29.4) | 1 (5.9) | 11 (64.7) | ||
| ≥12 mo (n = 130) | 89 (68.5) | 41 (31.5) | 30 (23.1) | 13 (10.0) | 87 (66.9) | ||
| Total (N = 158) | 116 (73.4) | 42 (26.6) | 37 (23.4) | 17 (10.8) | 104 (65.8) | ||
Data are number of responses (percentage of subgroup responding at each time point).
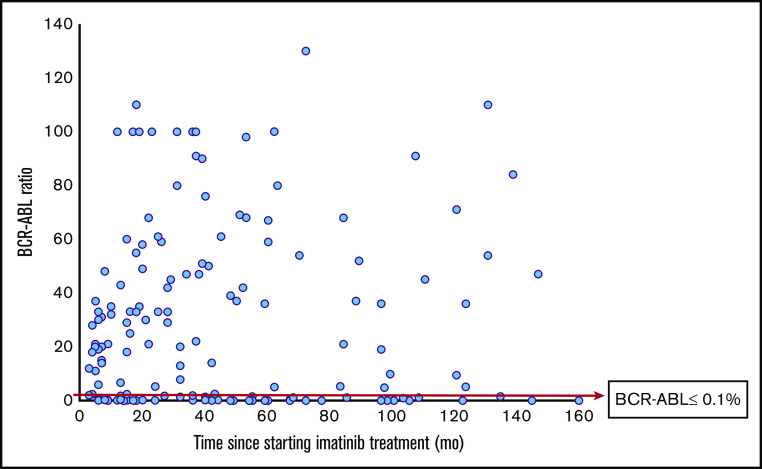
According to ELN criteria of optimal response (BCR-ABL ratio of ≤10% at 3 months, <1% at 6 months, and ≤0.1% at ≥12 months), only 37 of the 116 patients in CHR had an optimal molecular response at the time of testing. Among 130 patients at ≥12 months of imatinib use, a majority (66.9%; n = 87) fell in the ELN treatment category of failure (Table 3; Figure 2).
Figure 2.
Low rate of major molecular response in patients with CML in Tanzania. A total of 130 patients received imatinib for 12 months or more. Only 23.1% of patients reached a BCR/ABL ratio of ≤0.1%.
Clinical, laboratory, and treatment-related factors associated with molecular response to imatinib
The history of previous transfusions at diagnosis and development of cytopenia during imatinib treatment were statistically significant predictors of treatment failure (Table 4).
Table 4.
Bivariate and multivariate, multinomial logistic regression of clinical and treatment-related factors associated with molecular response
| Variable | Molecular response, n (%) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Failure, n = 104 (65.8) | Warning, n = 17 (10.8) | Optimal, n = 37 (23.4) | Warning vs optimal | Failure vs optimal | Warning vs optimal | Failure vs optimal | |
| Abdominal fullness | |||||||
| Present | 92 (67.6) | 16 (11.8) | 28 (20.6) | 5.14 (0.60- 44.4) | 2.46 (0.94-6.45) | — | — |
| Not present | 12 (54.6) | 1 (4.5) | 9 (40.9) | 1.00 | 1.00 | — | — |
| Previous BT | |||||||
| Yes | 41 (78.9) | 5 (9.6) | 6 (11.5) | 2.11 (0.55- 8.40) | 3.36 (1.29-8.77) | 2.11 (0.54- 8.25) | 3.48 (1.32-9.17) |
| No | 63 (59.4) | 12 (11.3) | 31 (29.3) | 1.00 | 1.00 | 1.00 | 1.00 |
| Sokal risk groups | |||||||
| High | 32 (74.4) | 3 (7.0) | 8 (18.6) | 9.2 × 105 (0,–) | 2.67 (0.60-11.7) | — | — |
| Intermediate | 35 (68.6) | 6 (11.8) | 10 (19.6) | 1.4 × 106 (0,–) | 2.33 (0.55-9.92) | — | — |
| Low | 6 (60.0) | 0 (0.0) | 4 (40.0) | 1.00 | 1.00 | — | — |
| Cytopenia from imatinib | |||||||
| Present | 70 (74.5) | 6 (6.4) | 18 (19.1) | 0.58 (0.18-1.88) | 2.17 (1.01-4.66) | 0.59 (0.18-1.93) | 2.26 (1.03-4.96) |
| Not present | 34 (53.1) | 11 (17.2) | 19 (29.7) | 1.00 | 1.00 | 1.00 | 1.00 |
| Adherence | |||||||
| High | 24 (60.0) | 5 (12.5) | 11 (27.5) | 0.84 (0.21-3.43) | 0.59 (0.23-1.51) | — | — |
| Medium | 32 (64.0) | 5 (10.0) | 13 (26.0) | 0.71 (0.18-2.84) | 0.67 (0.27-1.62) | — | — |
| Low | 48 (70.6) | 7 (10.3) | 13 (19.1) | 1.00 | 1.00 | — | — |
Data in column headings are the percentages of the total group of 158 patients. Data in the table body are number of patients (percentage of the response category). Bold statistics have P < .05.
BT, blood transfusion.
We also found patients with a higher mean WBC count (277.6 ± 142.7) × 109/L at diagnosis were less likely to have an optimal response than those with a slightly lower mean WBC count (230.2 ± 110.7) ×109/L. This difference was not statistically significant in the 3 ELN categories; however, when the warning and treatment failure categories were collapsed to form 1 category termed suboptimal response, the difference in the mean WBC count together with the presence of abdominal fullness at diagnosis were found to be statistically significant determinants of suboptimal molecular response (supplemental Tables 1 and 2).
Interestingly, Sokal score risk groups did not influence molecular response in this study. In particular, of 10 patients with low Sokal risk at diagnosis, less than half (4 of 10) achieved optimal molecular response.
Survival rate and molecular response
After 22 months (January 2019 through October 2020), the overall survival rate was 81.7% (95% CI, 74.7-86.9). A total of 29 patients died during the study period. Of those, 24 died because of progression of CML. The causes of death for the remaining 5 patients were 1 prostate carcinoma, 1 cervical carcinoma, 1 motor vehicle accident, and 2 unknown causes unrelated to CML.
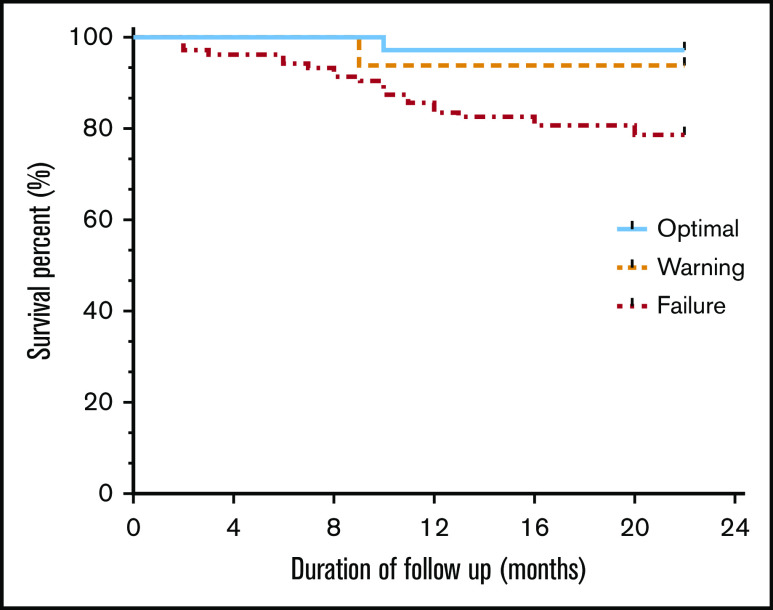
After adjustment for CML-unrelated deaths, the survival rate was highest in the optimal response category (97.0%; 95% CI, 80.9-99.6), followed by the warning response category (93.8%; 95% CI, 63.2-99.1) and the failure response category (78.6%; 95% CI, 69.4-85.4; Figure 3). This observed difference was statistically significant by log-rank test (P = .024).
Figure 3.
Kaplan-Meier estimated survival rates according to ELN molecular response categories. Overall survival of patients with CML receiving imatinib correlates strongly with the molecular response. Note that the observed failure curve did not reach a plateau.
Discussion
Monitoring of the molecular response of patients with CML who are receiving imatinib; has not been a routine practice in Tanzania thus far, as RT-PCR testing for the BCR-ABL gene transcript is not readily available and affordable. To the best of our knowledge, this is the first study performed in this country to provide data on both hematologic and molecular responses in a large cohort of patients.
Hematologic response
After 3 months of treatment with imatinib, it was gratifying to observe CHR in 100% of patients. This outcome was in accordance with a previous report by Tebuka et al.9 However, it was disappointing to find that after 12 months of imatinib treatment, CHR was considerably lower (68.8%), in contrast to that in studies in other populations in high-income settings where patients with durable CHR, even beyond 24 months of treatment, ranged from 89% to 93%.19-22 Because blood counts at 3, 6, and 12 months were unfortunately not available in this study, we do not know the time point at which CHR was lost. One possible explanation of this loss of CHR over time is decreased adherence to medication, which was also observed to decrease with time since imatinib initiation. Loss of response has major clinical implications; indeed, a persisting BCR-ABL+ cell population increases the risk of blast transformation of CML.20,23
Molecular response
Also in this respect, we found significant differences compared with what is reflected in abundant literature from other countries. Only a quarter (23.4%) of patients had an optimal molecular response to imatinib: a much lower proportion than in Europe or the United States.7,22,24 In a 5-year follow-up of the IRIS study, levels of BCR-ABL transcripts after 12 months of treatment had fallen by at least 3 logs in 57% of patients, and they had a 100% probability of remaining progression-free at 24 months of treatment.25 In our study we had only a single follow-up PCR value; therefore, we cannot say whether, in individual patients, we have uncovered loss of a previously attained response or a failure to achieve the ELN-defined milestone.
We have identified 2 factors that significantly influence molecular response: (1) late presentation and (2) cytopenia during treatment. With respect to factor 1, Liu and colleagues reported from China that patients with CML-related anemia at diagnosis had poor prognoses compared with patients with anemia unrelated to CML.26 CML-related anemia correlated in turn with splenomegaly, high WBC count, and higher risk scores at diagnosis,26,27 similar to what we observed. Severe anemia requiring blood transfusion is not a common feature in the CML literature. Although not all other causes of anemia were systematically excluded in this study, we believe low hemoglobin would be accounted for in most cases by the bulky disease at presentation. Surprisingly, Sokal score did not influence the molecular response in our study. Rather, parameters of disease burden (other than assessed by the Sokal score) seemed to determine the patients’ molecular response. Particularly, a high WBC count at diagnosis is a strong predictor of the depth of molecular response.28 In our study, patients with a higher mean WBC count and severe anemia requiring transfusion were less likely to reach optimal molecular response. We must also consider that, even though 93.2% of our patients were categorized in the chronic phase, based on the unfavorable Sokal scores and the observed warning and failure responses, they were at high risk of disease progression.
With respect to factor 2, the rate of imatinib toxicity (83.5%), particularly cytopenias (59.5%), was strikingly high in our cohort. Cytopenia occurred at the standard dose of 400 mg and at different times during treatment, not just within the first months of treatment, as reported in other studies. The cytopenia that develops during therapy has been recognized to be associated with poorer outcome because (1) the withholding of doses on account of cytopenia decreases overall exposure to imatinib, and (2) cytopenia may result from a low residual normal stem cell pool, with consequent lower chance of recovery of normal hematopoiesis after suppression of the malignant clone.26,27,29 The combination of severe myelosuppression and inadequate response to treatment in the chronic phase has been associated with a high risk of transformation to the accelerated or blastic phase and consequently reduced survival.30,31 Sadly, this is what we observed in our study. We lack exact data on the timing, duration, and depth of the worst cytopenias, but myelosuppression may have been severe enough to demand dose reductions or dose interruptions. During recruitment, a significant number of patients had anemia (65.1%), neutropenia (41.1%), or thrombocytopenia (21.5%), but in most cases, they were grade 1 or 2. This rate of imatinib toxicity is comparable to that reported by Francis et al in India, where 68% of patients experienced various adverse events, predominantly hematologic toxicity-thrombocytopenia, occurring in 21% of patients.32 It is not clear to what extent the lower normal value for neutrophils in African populations (benign ethnic neutropenia)33,34 may have contributed to dose interruptions that were triggered by neutrophil counts of less than 1.0 × 109/L. It is conceivable that, on account of this threshold value, some patients may have been unintentionally undertreated.
Nonhematologic toxicity
Fluid retention and gastrointestinal side effects were not as frequent in this study as reported by studies from Europe or the United States.24,35-37 Instead, skin hypopigmentation was the third most common (13.9%) after gastrointestinal and musculoskeletal side effects. Skin hypopigmentation has not been widely reported in previous studies, perhaps because in most studies so far, most patients were of European descent. Thus, the imatinib toxicity profile in our Tanzanian cohort differs from that seen elsewhere. Genetic differences in drug metabolism and individual responses (pharmacogenetics) may be a plausible explanation and may have contributed to the low rate of molecular response that we observed. In the future, imatinib dose titration to minimum effective dose should be performed on the basis of cutoff values for neutropenia derived from our normal population group. This approach may avoid unnecessary drug interruptions, and it may decrease the rate of disappointing levels of molecular response.
Adherence to medication
This is a well-recognized challenge in chronic conditions, with adherence rates ranging from 50% to 60%.38,39 Studies from Brazil and the Congo showed that adherence rates in patients with CML on imatinib ranged between 20% and 50%.17,40,41 A similar situation was observed in our cohort, where almost one-half (43%) of patients had low adherence. Poor adherence may result from multiple social, financial, and treatment factors. Among those investigated in this study, the total time after imatinib initiation was found to be a strong predictor of adherence in both simple and multiple linear regression analysis. Adherence progressively decreased with increased time since imatinib initiation. This finding is similar to what has been reported by Dos Reis et al, Marin et al, and Gater et al.41-43 It appears that with prolonged drug use, disappearance of symptoms, and normalization of blood counts, patients simply get tired of taking medications on a daily basis and start missing some of the doses. This is made worse when an objective assessment of minimum residual disease is lacking. Regular RT-PCR molecular monitoring provides an incentive for adherence, thus lowering the risk of disease progression and mortality.44 Despite the availability of imatinib at no direct cost, the patients still need to incur traveling costs to the centers where imatinib is available. The majority of patients (60%) in this study lived outside the region where imatinib is available. Although distance was not a statistically significant factor in the observed low adherence, it is likely that financial constraints played a role.
Impact of imatinib on survival
With the introduction of imatinib, the overall survival of patients with CML now approaches, for the first time, that of the general population in high-income countries.45,46 The IRIS trial reported median 5- and 10-year overall survival of 89% and 83%, respectively.20,47 In our study, the overall survival within the observation period of 22 months was only 81.7%, clearly a reflection of the low number of patients with optimal molecular response (23.4%), given that its depth influences the survival rate, which may be 90% or more in those with a major molecular response or better.47 In our study, patients who had an optimal response had a survival rate at 2 years of 97%; but it was only 78.6% in the response failure category. Careful management of dose adjustments, better adherence, combined with close monitoring of response, are clearly necessary to improve our patients’ rate of attaining desirable molecular milestones.
Conclusion
To our knowledge, this is the first large study in Africa to determine the risk factors that underlie a low rate of molecular response to imatinib in patients with CML. Although imatinib is clearly highly effective in our patient population, late presentation with massive disease bulk is probably a major factor in compromising treatment response. Efforts aimed at earlier diagnosis, better assessment and management of imatinib toxicity, and increased adherence, are all needed to improve overall survival of our patients with CML.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients with CML for their cooperation during the study and Muhimbili National Hospital (MNH) for providing the Gene Xpert machine.
This work was supported by Muhimbili University of Health and Allied Sciences (MUHAS), Health Education Fund of Council of England (HEFCE) and the United Kingdom’s Global Challenge Research Fund (GCRF) through the MUHAS-Oxford partnership. The research was funded/supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (A.S.).
The views expressed are those of the author(s) and are not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Footnotes
For original data, please e-mail Ahlam Nasser (anasser@muhas.ac.tz).
Authorship
Contribution: A.N., C.C., M.Y., A.S., and L.L. designed the study; A.N., A.H., and R.M., collected, assembled, analyzed, and interpreted the data; and all authors wrote and reviewed the manuscript and provided final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ahlam Mohamed Nasser, Department of Haematology and Blood Transfusion, Muhimbili University of Health and Allied Sciences, PO Box 65001, Upanga, Dar es Salaam, Tanzania; e-mail: dr.ahlamnasser@gmail.com.
References
- 1.Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341(3):164-172. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining [letter]. Nature. 1973;243(5405):290-293. [DOI] [PubMed] [Google Scholar]
- 3.Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113(8):1619-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Höglund M, Sandin F, Hellström K, et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood. 2013;122(7):1284-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851-2857. [DOI] [PubMed] [Google Scholar]
- 6.Cross NC, Melo JV, Feng L, Goldman JM. An optimized multiplex polymerase chain reaction (PCR) for detection of BCR-ABL fusion mRNAs in haematological disorders. Leukemia. 1994;8(1):186-189. [PubMed] [Google Scholar]
- 7.Hughes T, Branford S. Molecular monitoring of BCR-ABL as a guide to clinical management in chronic myeloid leukaemia. Blood Rev. 2006;20(1):29-41. [DOI] [PubMed] [Google Scholar]
- 8.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tebuka E, Makubi A, Maunda K. Complete haematological response to imatinib in chronic myeloid leukaemia patients attending the Ocean Road Cancer Institute in Tanzania. Tanzan J Health Res. 2016;18(4):doi:10.4314/thrb.v18i4 1. [Google Scholar]
- 10.Garcia-Gonzalez P, Boultbee P, Epstein D. Novel Humanitarian Aid Program: The Glivec International Patient Assistance Program-Lessons Learned From Providing Access to Breakthrough Targeted Oncology Treatment in Low- and Middle-Income Countries. J Glob Oncol. 2015;1(1):37-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koffi KG, Nanho DC, N’Dathz E, et al. The effect of imatinib mesylate for newly diagnosed Philadelphia chromosome-positive, chronic-phase myeloid leukemia in sub-Saharan African patients: The experience of Côte d’Ivoire. Adv Hematol. 2010;2010:268921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Entasoltan B, Bekadja MA, Touhami H, et al. Outcome of frontline treatment with “generic” imatinib in adult patients with chronic myeloid leukemia in Algerian population: a multicenter study. Mediterr J Hematol Infect Dis. 2017;9(1):e2017062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faye BF, Dieng N, Seck M, et al. Pattern of chronic myeloid leukemia in the imatinib era in a Sub-Saharan African setting. Ann Hematol. 2016;95(10):1603-1610. [DOI] [PubMed] [Google Scholar]
- 14.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geissler J, Sharf G, Bombaci F, et al. Factors influencing adherence in CML and ways to improvement: Results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143(7):1167-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okouango N, Elira D, Ngolet L. Adherence to imatinib in patients with chronic myeloid leukemia in the Congo. Austin Hematol. 2017;2(1):1-5. [Google Scholar]
- 18.National Cancer Institute (US) . Common terminology criteria for adverse events (CTCAE) v5. 0. The Cancer Therapy Evaluation Program. Bethesda MD: NCI Office of Cancer Genomics; 2017. [Google Scholar]
- 19.Roy L, Guilhot J, Krahnke T, et al. Survival advantage from imatinib compared with the combination interferon-α plus cytarabine in chronic-phase chronic myelogenous leukemia: historical comparison between two phase 3 trials. Blood. 2006;108(5):1478-1484. [DOI] [PubMed] [Google Scholar]
- 20.Druker BJ, Guilhot F, O’Brien SG, et al. ; IRIS Investigators . Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Sawyers C, Hochhaus A, et al. ; International STI571 CML Study Group . Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645-652. [DOI] [PubMed] [Google Scholar]
- 22.Hochhaus A, O’Brien SG, Guilhot F, et al. ; IRIS Investigators . Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia [published correction appears in Leukemia. 2010;24(5):1102]. Leukemia. 2009;23(6):1054-1061. [DOI] [PubMed] [Google Scholar]
- 23.Gaiger A, Henn T, Hörth E, et al. Increase of bcr-abl chimeric mRNA expression in tumor cells of patients with chronic myeloid leukemia precedes disease progression. Blood. 1995;86(6):2371-2378. [PubMed] [Google Scholar]
- 24.O’Brien SG, Guilhot F, Larson RA, et al. ; IRIS Investigators . Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994-1004. [DOI] [PubMed] [Google Scholar]
- 25.Hughes TP, Kaeda J, Branford S, et al. ; International Randomised Study of Interferon versus STI571 (IRIS) Study Group . Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423-1432. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Shi Y, Yan Z, et al. Impact of anemia on the outcomes of chronic phase chronic myeloid leukemia in TKI era. Hematology. 2020;25(1):181-185. [DOI] [PubMed] [Google Scholar]
- 27.Sneed TB, Kantarjian HM, Talpaz M, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100(1):116-121. [DOI] [PubMed] [Google Scholar]
- 28.Qin YZ, Jiang Q, Jiang H, et al. Combination of white blood cell count at presentation with molecular response at 3 months better predicts deep molecular responses to imatinib in newly diagnosed chronic-phase chronic myeloid leukemia patients. Medicine. 2016;95(2):e2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park M, Park CJ, Cho YW, et al. Alterations in the bone marrow microenvironment may elicit defective hematopoiesis: a comparison of aplastic anemia, chronic myeloid leukemia, and normal bone marrow. Exp Hematol. 2017;45:56-63. [DOI] [PubMed] [Google Scholar]
- 30.Ashutosh AKT, Anant K. Total Leukocyte Counts and the Requirement of Dose Reduction due to Cytopenias as Prognostic Indicators Affecting Response to Imatinib in Chronic Myeloid Leukemia. Indian J Hematol Blood Transfus. 2011;27(1):7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin D, Marktel S, Bua M, et al. Prognostic factors for patients with chronic myeloid leukaemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia. 2003;17(8):1448-1453. [DOI] [PubMed] [Google Scholar]
- 32.Francis J, Palaniappan M, Dubashi B, Pradhan SC, Chandrasekaran A. Adverse drug reactions of imatinib in patients with chronic myeloid leukemia: A single-center surveillance study. J Pharmacol Pharmacother. 2015;6(1):30-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reich D, Nalls MA, Kao WH, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh MM, Tisdale JF, Rodgers GP, Young NS, Trimble EL, Little RF. Neutrophil count in African Americans: lowering the target cutoff to initiate or resume chemotherapy? J Clin Oncol. 2010;28(10):1633-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauro MJ, Deininger MW. Management of drug toxicities in chronic myeloid leukaemia. Best Pract Res Clin Haematol. 2009;22(3):409-429. [DOI] [PubMed] [Google Scholar]
- 36.Thanopoulou E, Judson I. The safety profile of imatinib in CML and GIST: long-term considerations. Arch Toxicol. 2012;86(1):1-12. [DOI] [PubMed] [Google Scholar]
- 37.Gambacorti-Passerini C, Antolini L, Mahon F-X, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. J Natl Cancer Inst. 2011;103(7):553-561. [DOI] [PubMed] [Google Scholar]
- 38.Kleinsinger F. The unmet challenge of medication nonadherence. Perm J. 2018;22:18-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oung AB, Kosirog E, Chavez B, Brunner J, Saseen JJ. Evaluation of medication adherence in chronic disease at a federally qualified health center. Ther Adv Chronic Dis. 2017;8(8-9):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves AR, Lima WG, Nagai MM, et al. Adherence and/or discontinuation of imatinib mesylate in patients with chronic myeloid leukemia. Braz J Pharm Sci. 2016;52(4):581-589. [Google Scholar]
- 41.Dos Reis SR, Quixadá AT, Nunes ST, et al. Adherence to treatment with imatinib in chronic myeloid leukemia: a study of the first decade of responses obtained at a Brazilian hospital. Rev Bras Hematol Hemoter. 2013;35(3):174-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gater A, Heron L, Abetz-Webb L, et al. Adherence to oral tyrosine kinase inhibitor therapies in chronic myeloid leukemia. Leuk Res. 2012;36(7):817-825. [DOI] [PubMed] [Google Scholar]
- 44.Haque R, Shi J, Chung J, et al. Medication adherence, molecular monitoring, and clinical outcomes in patients with chronic myelogenous leukemia in a large HMO. J Am Pharm Assoc (2003). 2017;57(3):303-310.e2. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Wang H, Kantarjian H, Cortes J. Trends in chronic myeloid leukemia incidence and survival in the United States from 1975 to 2009. Leuk Lymphoma. 2013;54(7):1411-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantarjian H, O’Brien S, Jabbour E, et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single-institution historical experience. Blood. 2012;119(9):1981-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochhaus A, Larson RA, Guilhot F, et al. ; IRIS Investigators . Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376(10):917-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.