Key Points
Eculizumab discontinuation with close monitoring is safe in most patients with aHUS.
CFH and MCP variants may be associated with higher risk of relapse, which needs to be evaluated in larger, multicenter studies.
Abstract
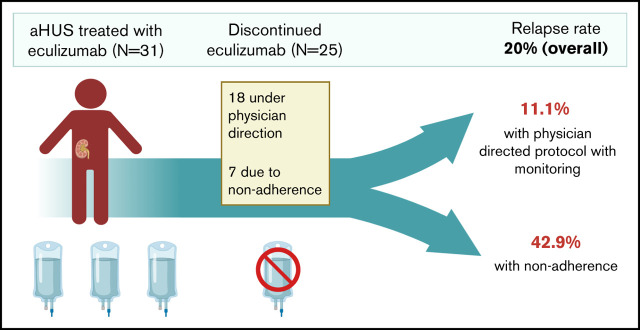
Terminal complement inhibition is the standard of care for atypical hemolytic uremic syndrome (aHUS). The optimal duration of complement inhibition is unknown, although indefinite therapy is common. Here, we present the outcomes of a physician-directed eculizumab discontinuation and monitoring protocol in a prospective cohort of 31 patients that started eculizumab for acute aHUS (and without a history of renal transplant). Twenty-five (80.6%) discontinued eculizumab therapy after a median duration on therapy of 2.37 (interquartile range: 1.06, 9.70) months. Eighteen patients discontinued per protocol and 7 because of nonadherence. Of these, 5 (20%) relapsed; however, relapse rate was higher in the case of nonadherence (42.8%) vs clinician-directed discontinuation and monitoring (11.1%). Four of 5 patients who relapsed were successfully retreated without a decline in renal function. One patient died because of recurrent aHUS and hypertensive emergency in the setting of nonadherence. Nonadherence to therapy (odds ratio, 8.25; 95% confidence interval, 1.02-66.19; P = .047) was associated with relapse, whereas the presence of complement gene variants (odds ratio, 1.39; 95% confidence interval, 0.39-4.87; P = .598) was not significantly associated with relapse. Relapse occurred in 40% (2 of 5) with a CFH or MCP variant, 33.3% (2 of 6) with other complement variants, and 0% (0 of 6) with no variants (P = .217). There was no decline in mean glomerular filtration rate from the date of stopping eculizumab until end of follow-up. In summary, eculizumab discontinuation with close monitoring is safe in most patients, with low rates of aHUS relapse and effective salvage with eculizumab retreatment in the event of recurrence.
Visual Abstract
Introduction
Atypical hemolytic uremic syndrome (aHUS) is an acute, life-threatening disorder caused by complement dysregulation leading to a thrombotic microangiopathy (TMA) and end organ dysfunction, predominantly affecting the renal circulation. Up to 50% to 70% of patients have mutations in complement regulation genes.1,2 In the pre-eculizumab era, 56% of patients with aHUS progressed to end-stage renal disease within the first year.3 Eculizumab, an anti-C5 monoclonal antibody, is highly effective in treating aHUS and leads to rapid and sustained improvement in hematologic parameters and renal function.1,4,5 Indefinite therapy with eculizumab is currently the standard of care for patients with aHUS. Recently, a longer-acting C5 inhibitor ravulizumab was also approved for aHUS.6 However, long-term complement inhibition therapy comes with an immense financial burden,7 in addition to subjecting patients to a small but real risk of meningococcal infection8 and the inconvenience of needing infusions every 2 weeks (eculizumab) or every 2 months (ravulizumab).
Advances in our understanding of aHUS also provide rationale for limited duration of complement inhibition therapy in aHUS. Penetrance of aHUS-associated complement mutations is low, and a strong complement amplifying trigger may be required for clinically apparent TMA to manifest.2,3 Reports suggest that many patients with aHUS may safely discontinue eculizumab therapy.9-16 Although a risk of relapse remains, most patients in these reports were successfully salvaged with timely retreatment without significant worsening of renal function. Our group has also previously described excellent results in 15 patients with aHUS who discontinued eculizumab therapy.17 Here, we present a physician-directed eculizumab discontinuation and monitoring protocol and report an updated analysis of outcomes of eculizumab discontinuation in a prospective cohort of 32 patients with aHUS enrolled in the Johns Hopkins Complement Associated Disease Registry. We also examined clinical and genetic risk factors for relapse after discontinuing eculizumab therapy.
Methods
Patients and data collection
Consecutive patients with aHUS enrolled in the Johns Hopkins Complement Associated Disease Registry between January 2014 and June 2020 who initiated eculizumab therapy during an acute episode of complement-mediated TMA and had at least 3 months of follow-up were included in the study. Inclusion criteria included age ≥18 years and a diagnosis of acute aHUS based on the following criteria: (1) platelet count <150 × 109/L, (2) serum creatinine >2.25 mg/dL, (3) schistocytes on peripheral blood smear and acute TMA on renal biopsy, if performed, (4) ADAMTS13 activity >10%, and (5) Shiga toxin negative. Criteria numbers 1 and 3 to 5 were required for diagnosis. Criteria 2 (serum creatinine >2.25 mg/dL) was considered supportive of the diagnosis but was not required, and patients with a lower serum creatinine could be included if otherwise consistent with aHUS. We included only patients who developed acute TMA with native kidneys and excluded those that started eculizumab in the peri-renal transplant setting for end-stage renal disease because of confirmed or presumed aHUS and those with a history of aHUS relapse, because discontinuation likely carries higher risk in these cases. We did not include patients that developed TMA associated with hematopoietic or solid organ transplantation. Patients were followed as clinically indicated, most commonly every 3 months, until death, or last clinical contact. Data regarding clinical presentation, laboratory studies, eculizumab therapy, and outcomes including death and renal function were collected as part of the registry. We collected results of clinical complement gene sequencing when available. We included any rare (minor allele frequency < 0.005) variants in C3, CFB. CFH, CFI, CFHR5, MCP, DGKE, THBD, and PLG. We did not include variants in ADAMTS13. We included homozygous (but not heterozygous) CFHR1 deletion, although this is not a rare variant, based on strong association with aHUS and presence of factor H autoantibodies.2,18-21 We also recorded the presence or absence of factor H autoantibodies. Pathogenic complement variants predispose to aHUS and can be considered risk factors for development of disease. Therefore, variants of undetermined significance were included in the analysis, especially because functional data are available for only a limited number of reported variants, and in silico prediction tools are not uniformly accurate. Data were collected and stored in RedCap, a secure, web-based, data management platform hosted at Johns Hopkins University. The institutional review board of the Johns Hopkins University School of Medicine approved this study. Study was conducted in accordance with the Declaration of Helsinki.
Hopkins protocol for eculizumab discontinuation and monitoring
Our eculizumab discontinuation and monitoring protocol is summarized in Figure 1. Guiding principles of this approach are that eculizumab discontinuation is considered if all of the following conditions are met: (1) no clinical evidence of ongoing thrombotic microangiopathy (platelets <150 × 109/L, lactate dehydrogenase > 1.5 times the upper limit of normal, schistocytes on blood smear), (2) renal function returned to normal or plateaued at a new baseline for at least 3 months, (3) no active trigger (for patients that have an identified trigger) and C reactive protein levels may be obtained when it is unclear whether the trigger has resolved, (4) no organ transplantation for aHUS, and (5) patient adherent with follow-up and able to make an informed decision regarding the risks vs benefits of discontinuation. Eculizumab may be discontinued if all the conditions mentioned above are met. Eculizumab was continued (or switched to ravulizumab) even if all conditions were met but patient was unwilling to stop therapy. Of note, we do not follow a prescribed minimum duration of therapy unlike other protocols that may require a minimum duration of therapy such as 6 months.16 Laboratory studies including complete blood count with peripheral smear, lactate dehydrogenase, serum creatinine, urinalysis, and urine protein/creatinine ratio are initially performed 2 weeks after the last eculizumab, then weekly for 1 month, every 2 weeks for 2 months, monthly for 3 months, and every 3 months thereafter. Patients are also encouraged to perform home urine dipstick testing for proteinuria and to monitor their blood pressure at home. Additional laboratory tests may be performed in case of acute illness, surgery, or pregnancy. Eculizumab (or ravulizumab if long-term therapy is anticipated) is restarted if relapse is suspected. Temporarily restarting eculizumab is also considered in high-risk situations such as acute severe illness, pregnancy, or major surgery. For patients that are likely to be on long-term (indefinite) therapy with a C5 inhibitor, including those that are not candidates for discontinuing treatment or those that need to restart therapy because of TMA recurrence, we preferentially switch to ravulizumab because of less frequent infusions and lower cost.
Figure 1.
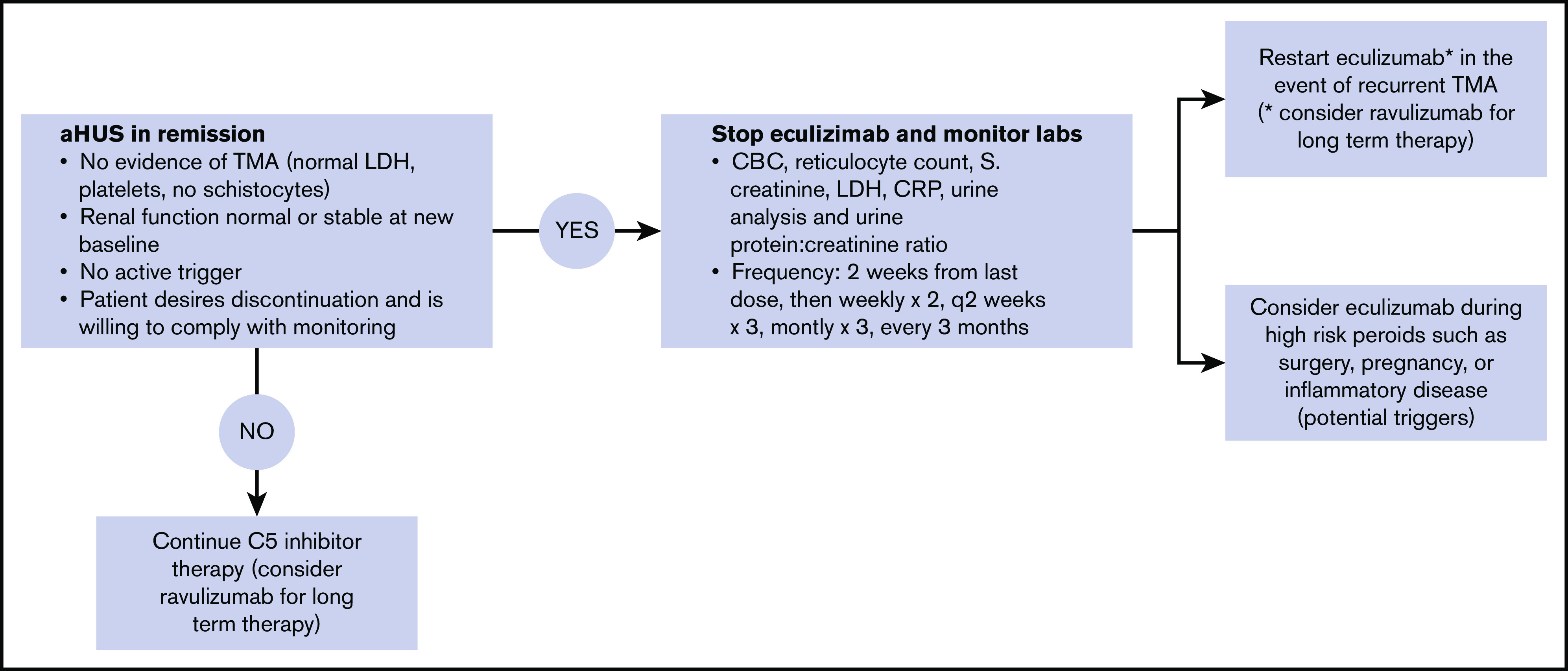
Protocol for eculizumab discontinuation and monitoring. All 4 of the following criteria must be met before we discontinue eculizumab: resolved TMA, renal function normal or stable at new baseline, no active trigger (in patients that had an identified trigger), and patients desire to stop therapy and agree to monitoring plan. Monitoring is conduced as outlined. Home urine dipstick monitoring may also be used as an adjunct. We restart a C5 inhibitor (eculizumab or ravulizumab) in the case of recurrent TMA, in which case therapy is continued indefinitely or possibly temporarily during high-risk periods such as pregnancy, surgery, or flare of inflammatory disease. When long-term therapy is anticipated, we suggest ravulizumab rather than eculizumab.
Data analysis
Data were summarized as counts and proportions for categorical variables and medians and interquartile ranges (IQR; or mean ± standard deviation) for continuous variables. The χ2 test was used to compare relapse rate in different groups. We attempted to evaluate clinical and genetic risk factors for relapse; however, the small number of relapses precluded multivariable analysis. We evaluated the mean ± standard deviation change in estimated glomerular filtration rate (eGFR) (using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation) from date of stopping eculizumab until last follow-up using the paired samples t test and separately analyzed outcomes of patients who stopped eculizumab on the physician-directed protocol vs nonadherence to therapy and relapsing vs nonrelapsing patients. P < .05 was considered significant. Stata version 15 (StataCorp.) was used for all analyses.
Results
Cohort characteristics
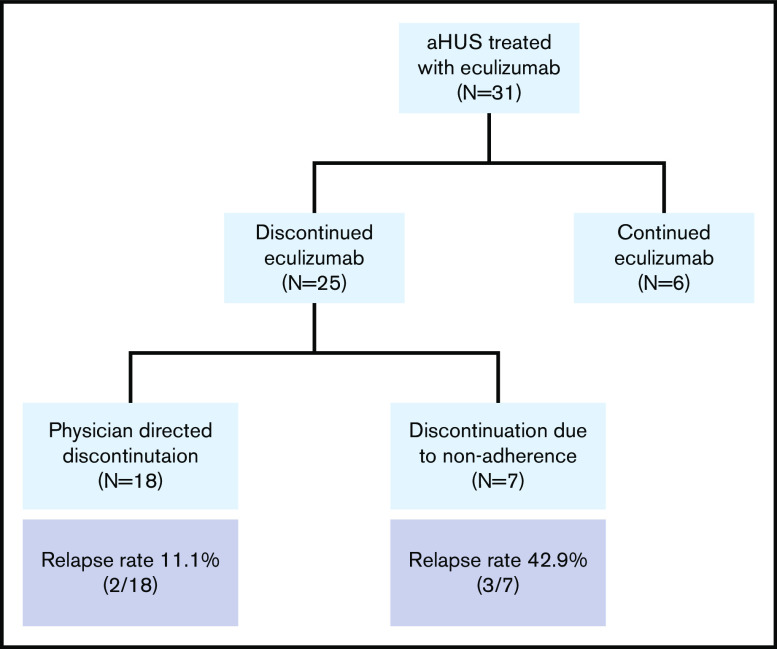
Between January 2011 and June 2020, 42 patients with aHUS received treatment with eculizumab at our institution during the study period. Of these, 10 received eculizumab in the perioperative setting for renal transplantation (because of end-stage renal disease attributed to prior aHUS) and were excluded from the analysis. Thirty-two patients received eculizumab for acute aHUS, of which 1 patient died during the acute aHUS episode. The 31 adult patients with aHUS who received treatment with eculizumab for acute TMA and had at least 3 months of follow-up are included in the analysis (Figure 2). Median age at initial diagnosis of aHUS was 44 (IQR: 25, 52) years, and 77.4% (24 of 31) were female. A putative trigger was identified for 67.7% (21 of 31) patients. Triggers included infections (N = 7), autoimmune disease flares (N = 4), pregnancy and cesarean section (N = 1), surgery (N = 1), cancer/chemotherapy (N = 4), and other inflammatory disorders (N = 4; 2 with pancreatitis that preceded onset of TMA and 1 each with nephrolithiasis and Stevens Johnson syndrome). Complement gene variants were present in 63.6% (14 of 22 patients with sequencing data available). The majority (59.3%) received plasma exchange before initiating eculizumab. Factor H autoantibody was detected in only 1 patient (of 22 tested). This patient (patient 57 in Figure 3) also had a compound heterozygous deletion of CFHR3-CFHR1 and CFHR1-CFHR4 (resulting in a homozygous CFHR1 deletion), as well as a variant of undetermined significance in C3. Median time from presentation to eculizumab therapy was 2 (IQR: 2, 5) days. Median follow-up of the cohort was 27 (IQR: 5, 50) months. Table 1 summarizes demographics and clinical characteristics of the cohort at initial presentation.
Figure 2.
Cohort diagram.
Figure 3.
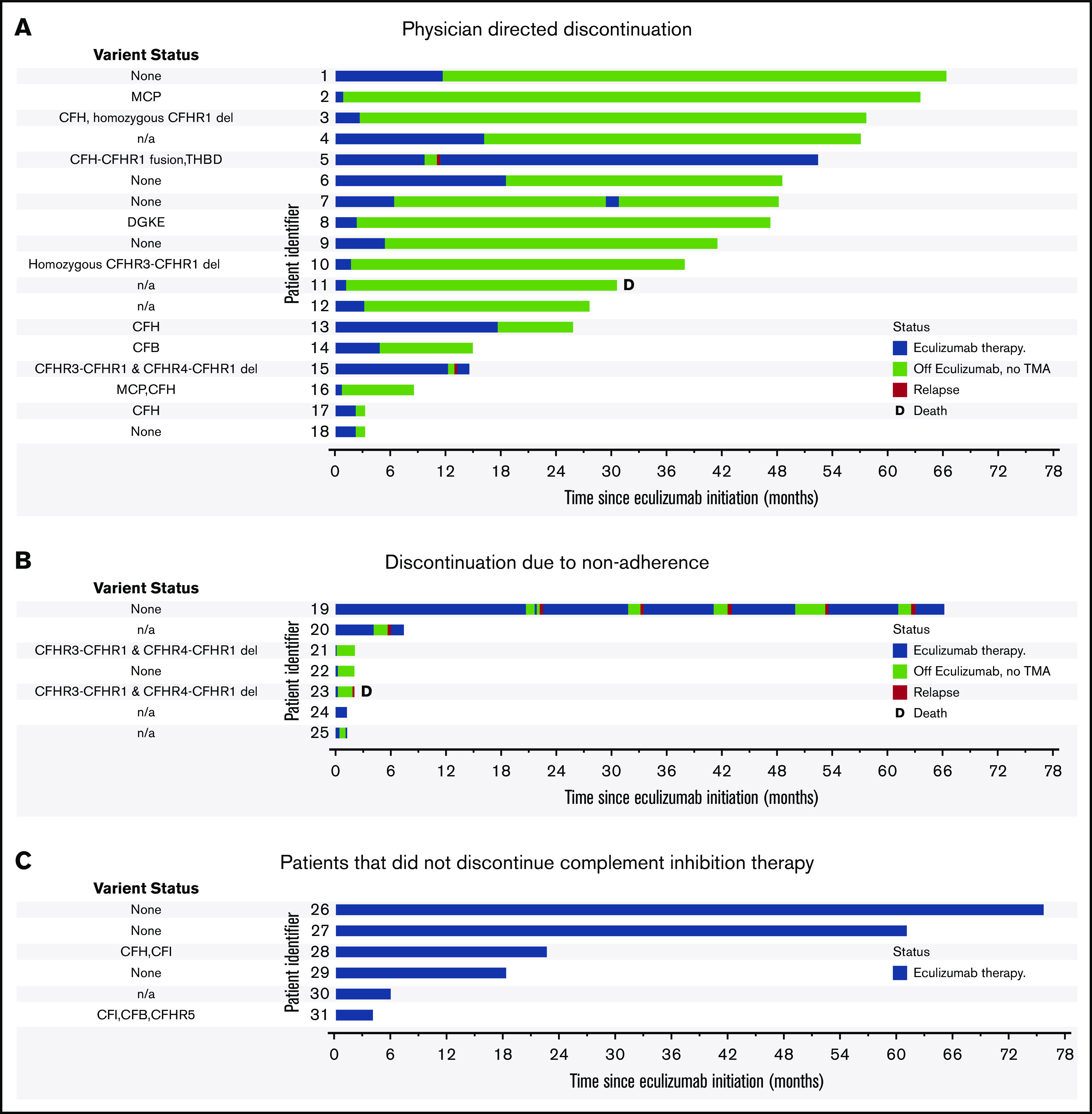
Clinical course after eculizumab cessation. (A) Patients that discontinued eculizumab on the physician-directed protocol. (B) Patients that discontinued or interrupted therapy due to nonadherence. (C) Patients that did not discontinue complement inhibition therapy. Origin of the x-axis is at initiation of eculizumab therapy for acute thrombotic microangiopathy. Duration of eculizumab therapy is indicated in blue, relapse is indicated in red, and relapse-free time off eculizumab therapy is indicated in green. Patient 23 (A) was briefly restarted on eculizumab in the setting of renal transplant. Patient 32 has switched to ravulizumab, and patients 51 and 55 are in the process of switching. D, death during follow-up.
Table 1.
Demographic and clinical characteristics at presentation by eculizumab discontinuation status
| Variable | Total cohort (N = 31) | Continued eculizumab (n = 6) | Stopped eculizumab (n = 25) | P |
|---|---|---|---|---|
| Age, median (IQR), y | 44 (25, 53.5) | 32 (25, 48) | 44 (32, 52) | .408 |
| Female sex | 80.6 | 66.6 | 80 | .483 |
| Race: White | 64.5 | 83.3 | 60 | .283 |
| Race: African American | 35.5 | 16.7 | 40 | |
| Clinical presentation | ||||
| Neurologic | ||||
| Seizure | 12.9 | 0 | 16.0 | .294 |
| Vision loss | 12.9 | 33.3 | 8.0 | .096 |
| Unconsciousness | 6.5 | 0 | 8.0 | .474 |
| Headache | 38.7 | 66.6 | 32.0 | .117 |
| Encephalopathy | 19.4 | 0 | 24.0 | .181 |
| Stroke | 9.7 | 0 | 12.0 | .372 |
| Gastrointestinal | ||||
| Hemorrhage | 3.2 | 0 | 4.0 | .618 |
| Epigastric pain | 12.9 | 33.3 | 8.0 | .096 |
| Pancreatitis | 9.7 | 0 | 12.0 | .372 |
| Transaminitis | 12.9 | 16.7 | 12.0 | .759 |
| Vomiting | 45.2 | 66.7 | 40.0 | .239 |
| Ischemic hepatitis | 3.2 | 0 | 4.0 | .618 |
| Abdominal pain | 38.7 | 33.3 | 40.0 | .763 |
| Respiratory | ||||
| Pulmonary edema | 9.7 | 0 | 12.0 | .372 |
| Pleural effusion | 6.5 | 16.7 | 4.0 | .257 |
| Other respiratory failure | 12.9 | 0 | 16.0 | .294 |
| New or worsening hypertension | 41.9 | 50 | 40 | .655 |
| Laboratory studies at presentation | ||||
| Hemoglobin, median (IQR), mg/dL | 8.8 (7.6, 9.8) | 9.1(8.3, 9.5) | 8.8 (7.4, 10.1) | .673 |
| Platelet count, median (IQR), ×109/L | 42 (18, 73) | 33 (13, 41) | 45 (19, 84) | .267 |
| Serum creatinine, median (IQR), mg/dL | 3.5 (2.8, 6.2) | 2.7 (5.5, 9.0) | 3.5 (2.9, 6.1) | .610 |
| Lactate dehydrogenase, median (IQR), U/L | 1329 (858, 2545) | 5571 (3240, 6870) | 1282 (800, 2501) | .036 |
| Trigger identified* | 67.7 | 66.6 | 68.0 | .950 |
| Complement variant present (n = 22 sequenced), % (n/N) | 63.6 (14/22) | 60 (3/5) | 64.7 (11/17) | .743 |
| Treatments | ||||
| Plasma exchange received | 58.1 | 83.3 | 52.0 | .162 |
| Time to starting eculizumab, median (IQR), d | 2 (2, 5) | 2.5 (2, 5) | 4 (2,5) | .487 |
Data are percentages unless otherwise noted.
Triggers included infections (n = 7), autoimmune disease flares (n = 4), pregnancy and cesarean section (n = 1), surgery (n = 1), cancer/chemotherapy (n = 4), and other inflammatory disorders (n = 4).
Outcomes of eculizumab discontinuation
Of the 31 patients followed after receiving eculizumab for an acute episode of aHUS, 25 (80.6%) discontinued complement inhibition therapy (Figure 2) after a median duration on therapy of 2.37 (IQR: 1.06, 9.70) months. Six (19.4%) patients continued eculizumab. Of the 25 patients that discontinued eculizumab, 18 (72%) discontinued under physician direction (17 per protocol because of TMA remission including 2 with no renal recovery and end-stage renal disease and 1 because of nonresponse and a metabolic TMA thought more likely). Seven (28%) patients interrupted or discontinued therapy because of nonadherence. Reasons for continuing eculizumab therapy included patient preference (N = 3), ongoing or recurrent trigger/inflammation (N = 2), and ongoing evaluation of underlying etiology (N = 1). Three patients have switched or are in the process of switching to ravulizumab (patients 32, 51, and 55 in Figure 2). No significant differences were noted in patients who discontinued vs continued complement inhibition therapy (Table 1).
Relapse occurred in 5 patients after stopping eculizumab, of which 3 were nonadherent with therapy and 2 had stopped under the treating physician’s direction and monitoring. Thus, the overall relapse rate was 20.0% (5 of 25); however, the relapse rate was higher in the case of nonadherence vs a physician-directed cessation protocol (42.9% vs 11.1%, P = .074), although this did not reach statistical significance. One patient (patient 23 in Figure 3) who did not have a relapse reinitiated eculizumab in the peritransplant setting. Of the 5 patients that relapsed, 4 were re-treated and successfully salvaged with eculizumab and achieved remission without a decline in renal function (compared with renal function on the date of stopping eculizumab). One patient (patient 43) died after presenting to the emergency room at another institution with a diagnosis of recurrent TMA and hypertensive emergency in the setting of nonadherence to eculizumab and antihypertensive therapy. Another patient (patient 30) presented with altered mental status, cardiogenic shock, and multiorgan failure along with thrombocytopenia in the setting of refractory B-cell lymphoma 64 months after stopping eculizumab. He was treated with plasma exchange, followed by eculizumab for a possible TMA recurrence, but the family pursued comfort measures because of the poor prognosis of his malignancy and neurologic injury.
We examined factors associated with relapse. On univariate analysis, nonadherence with therapy (odds ratio [OR], 8.25; 95% confidence interval [CI], 1.02-66.19; P = .047) was associated with relapse, whereas underlying complement gene variant (OR, 1.39; 95% CI, 0.39-4.87; P = .598) and the presence/absence of a trigger for aHUS (OR, 0.66; 95% CI, 0.09-4.79; P = .687) were not significantly associated with relapse. These analyses are limited by the small sample size and limited number of events (5 relapses), which also precluded multivariable regression analysis. Based on previous studies that found that CFH and MCP mutations were associated with relapse on discontinuing therapy,15,22 we examined relapse rates in patients with CFH or MCP variants (40%, 2 of 5), other variants (33.3%, 2 of 6), no variants (0%, 0 of 6), and sequencing not completed (11.1%, 1 of 9; P = .217). Of note, 2 patients with CFH variants, 1 with other variants (CFI, CFB, and CFHR5), 2 without variants, and 1 who had not undergone sequencing continued on eculizumab and were excluded from the population at risk for relapse.
Renal outcomes after eculizumab discontinuation
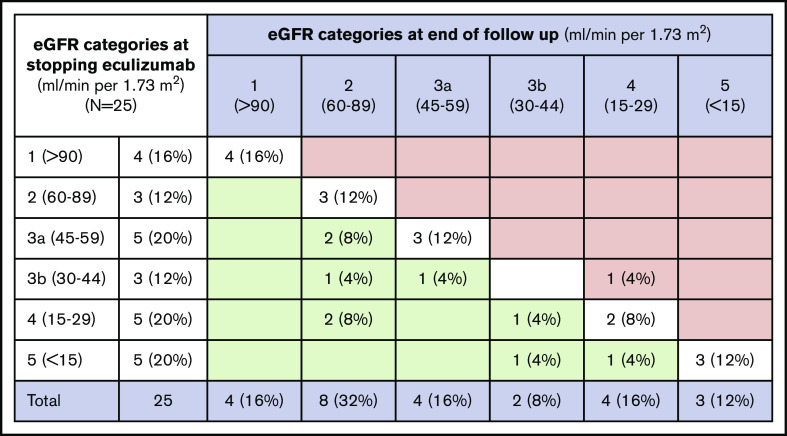
Among the patients that discontinued eculizumab, there was no significant decline in mean eGFR from the date of stopping eculizumab (47.1 ± 28.2 mL/min per 1.73 m2) until most recent follow-up (57.0 ± 28.0 mL/min per 1.73 m2; 1-sided t test, P = .987). As shown in Figure 4, improvement in eGFR (defined by change to lesser stage of chronic kidney disease between stopping eculizumab and end of follow-up) was seen in 9 of 25 patients (36%), and stable eGFR (no change in stage of chronic kidney disease) was seen in 15 of 25 patients (60%). One patient (4%) had a decline in eGFR at the end of follow-up. The mean change in eGFR at the end of follow-up was not significantly different for patients that relapsed vs those that did not relapse (5.5 ± 12.0 vs 10.8 ± 20.5 mL/min per 1.73 m2, P = .625) or between patients that stopped eculizumab because of nonadherence (with or without subsequent relapse) vs those that stopped eculizumab on the clinician-directed protocol (16.8 ± 23.0 vs 7.3 ± 17.5 mL/min per 1.73 m2, P = .305).
Figure 4.
Change in eGFR categories from date of stopping eculizumab until end of follow-up. Data from 25 patients that discontinued eculizumab presented as n (%). eGFR categories are shown in milliliters per minute per 1.73 m2. Green cells represent improvement from baseline to day 183, red cells represent worsening, and white cells represent no change.
Discussion
Our single-center experience demonstrates eculizumab discontinuation with close monitoring is safe in most patients with aHUS (with native kidneys), with low rates of TMA recurrence and effective salvage with eculizumab retreatment in the event of a recurrence. We also propose a protocol for discontinuation of complement inhibitory therapy and monitoring for recurrence (Figure 1).
Other studies have reported outcomes of eculizumab discontinuation in small cohorts of patients with aHUS, with median time on treatment before discontinuation ranging from 3 to 17.5 months (Table 2).9-16 Recurrent TMA occurred in 20% to 30%, and retreatment with eculizumab led to remission, without progressive loss in renal function in most patients, although a recent observational study including patients from 5 parent eculizumab trials reported a decline in renal function in 40% of patients that stopped eculizumab.13 The rate of recurrent TMA in our study was slightly lower (20%) than that reported previously. One reason for this may be the fewer number of patients in our cohort with CFH variants, which are associated with higher risk of relapse. Another possible explanation is most patients in our cohort stopped eculizumab while in remission from TMA and were monitored closely, whereas other cohorts have variable numbers of patients that discontinued or interrupted therapy because of nonadherence. In fact, nonadherence with therapy and monitoring were associated with a nearly fourfold higher rate of relapse in our cohort and were associated with the only aHUS relapse that led to worsening renal function and death.
Table 2.
Published series of eculizumab discontinuation in aHUS
| Series | n | Median duration of therapy (range or IQR), mo | Median follow-up (since cessation) (mo) | Relapse rate, n (%) | Outcome of relapses |
|---|---|---|---|---|---|
| Ardissino et al10 (Italy) | 16 | 4.3 (0.5-14.4) | 13.1 (1.2-28.2) | 5 (31.3) | Retreated with eculizumab, no progressive renal injury |
| Sheerin et al9 (UK) | 14 | — | — | 3 (21.4) | Retreated without chronic sequelae |
| Wijnsma et al15 (Netherlands) | 17 | 3.8 (2.8-5.8) | 27.4 (7.8-42) | 5 (29.4) | Retreated with eculizumab, no progressive renal injury |
| Fakhouri et al22 (France) | 38 | 17.5 (2-50) | 22 (5-43) | 12 (31.6) | Eculizumab resumed with no significant change in GFR |
| Menne et al 13 | 42 | 19.6 (0.2-86.9) | — | 11 (26.1)* | 40% had a decline in renal function after discontinuing but eGFR remained >60 mL/min/1.73 m2 |
| Fakhouri et al16 (France) | 55 | Mean 16.5 (0.95, 59) | 24 | 13 (23) | 11 of 13 regained baseline renal function |
| Current study | 25 | 2.4 (IQR 1.1, 9.7) | 27 (IQR 5, 50) | 5 (20) | Four salvaged with prompt eculizumab therapy. One patient died during recurrent TMA (nonadherent with therapy) |
—, not reported.
In this study, 21 (50%) restarted therapy for TMA relapse/renal impairment (n = 11), preparation for a kidney transplant (n = 5), short discontinuation period because of change in dose or missed doses (n = 2), administrative reasons (n = 2), and multiple serious adverse effects and a change in dosing (n = 1).
Limited experience suggests that pathogenic variants in CFH may be associated with increased risk of aHUS recurrence off therapy.9-11 In a retrospective analysis of 38 patients from the French aHUS registry, recurrence rates after eculizumab discontinuation were higher in patients with rare variants in CFH (72%) or MCP (50%) genes vs those without complement gene variants (0%).22 In our cohort, the 6 patients without complement gene variants did not relapse, although the presence of rare complement gene variants was not associated with a statistically significantly higher risk of recurrent aHUS. Although not statistically significant, 40% of patients with CFH or MCP variants relapsed after discontinuing therapy compared with 33.3% of patients with variants other than CFH and MCP. These findings, however, may be because of the relatively small number of patients and relapses. Despite these concerns, it is reassuring that relapsing patients with underlying variants were successfully retreated without a loss in renal function. Large prospective multicenter studies are needed to definitively establish the relationship between complement gene variants and risk of recurrent TMA after discontinuing complement inhibitory therapy. A major limitation is that robust functional data are available for few variants and in silico predictions are not uniformly reliable.12,23,24 For the purpose of this study, we included all rare variants (minor allele frequency <0.005) regardless of functional assessment. There is increasing interest in identifying biomarkers of aHUS recurrence that would allow individualization of therapy. Unfortunately, serum complement assays such as C3, C4, C5b-9, AP50, or CH50 have not proved to be reliable biomarkers of disease activity for the diagnosis of aHUS25-27 and are unlikely to be useful in monitoring for relapse off therapy. Functional assays such as the modified Ham assay28 and assays looking at deposition of complement products on the endothelium have been developed and perform well in distinguishing aHUS from non–complement-mediated TMA.29,30 These, however, are not available clinically, and their utility in monitoring still needs to be established. Finally, our cohort includes only adult patients with aHUS, and the applicability of our eculizumab discontinuation protocol should not be extrapolated to children with aHUS without evaluation in that population.
Other than relapse, long-term renal outcomes are a critical end point in studies evaluating restrictive eculizumab (or ravulizumab) strategies in complement-mediated TMA. At least 1 series reported a trend toward decreasing renal function over time from discontinuation.13 Our relatively small cohort did not show a clinically significant decline in renal function after stopping eculizumab, which is concordant with previous reports. However, this needs to be established in large multicenter prospective studies. Finally, there is an established role of complement activation in atherosclerosis and coronary artery disease,31 and variants in complement system genes C3 and MBL are associated with cardiac disease.32,33 Cardiovascular disease affecting the large- and medium-sized arteries has been reported even late in the course of aHUS.34 It is plausible that ongoing subclinical complement activation, even if insufficient to cause overt TMA, may contribute to adverse cardiovascular outcomes. Long-term vascular outcomes of eculizumab cessation also need to be evaluated.
In summary, we show that for most aHUS patients, discontinuation of C5 inhibitor (eculizumab) therapy is safe and acceptable without loss in renal function when done under close monitoring to allow early detection and treatment of relapses. Multicenter, prospective studies are needed to establish the renal and vascular safety of limited duration eculizumab therapy for aHUS, which has the potential to reduce the financial burden, the inconvenience of frequent infusions, and the small but real risk of meningococcal infection associated with indefinite therapy with terminal complement inhibitors.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants K99HL150594 (S.C.) and R01HL133113 (R.A.B.).
Footnotes
We did not generate large scale genomic data. However, all sequencing data of patient samples will be shared by contacting schatur3@jhmi.edu for original data pertaining for this manuscript. No identifiable data will be shared. Requests for de-identified data from internal or external investigators will be evaluated on an individual basis.
Authorship
Contribution: S.C. collected data, designed and performed the analyses, interpreted the data, and wrote the first draft of the manuscript; N.D. and H.U. collected data; S.H. collected data and prepared figures; E.M.B., X.Y., C.J.S., A.R.M., and R.A.B. interpreted the data and critically reviewed the manuscript; and all authors critically reviewed the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shruti Chaturvedi, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Building, Room 1025, Baltimore, MD 21205; e-mail: schatur3@jhmi.edu.
References
- 1.Fakhouri F, Zuber J, Frémeaux-Bacchi V, Loirat C. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681-696. [DOI] [PubMed] [Google Scholar]
- 2.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169-2181. [DOI] [PubMed] [Google Scholar]
- 5.Fakhouri F, Hourmant M, Campistol JM, et al. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68(1):84-93. [DOI] [PubMed] [Google Scholar]
- 6.Rondeau E, Scully M, Ariceta G, et al. ; 311 Study Group . The long-acting C5 inhibitor, Ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287-1296. [DOI] [PubMed] [Google Scholar]
- 7.Khedraki R, Noor Z, Rick J. The most expensive drug in the world: to continue or discontinue, that is the question. Fed Pract. 2016;33(7):22-28. [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara LATN, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High risk for invasive meningococcal disease among patients receiving eculizumab (soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66(27):734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheerin NS, Kavanagh D, Goodship TH, Johnson S. A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM. 2016;109(1):27-33. [DOI] [PubMed] [Google Scholar]
- 10.Ardissino G, Testa S, Possenti I, et al. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64(4):633-637. [DOI] [PubMed] [Google Scholar]
- 11.Wetzels JF, van de Kar NC. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65(2):342. [DOI] [PubMed] [Google Scholar]
- 12.Feitz WJC, van de Kar NCAJ, Orth-Höller D, van den Heuvel LPJW, Licht C. The genetics of atypical hemolytic uremic syndrome. Med Genetik. 2018;30(4):400-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menne J, Delmas Y, Fakhouri F, et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study [correction published in Pediatr Nephrol. 2019;34:741-742]. BMC Nephrol. 2019;20(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wijnsma KL, Duineveld C, Wetzels JFM, van de Kar NCAJ. Eculizumab in atypical hemolytic uremic syndrome: strategies toward restrictive use. Pediatr Nephrol. 2019;34(11):2261-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijnsma KL, Duineveld C, Volokhina EB, van den Heuvel LP, van de Kar NCAJ, Wetzels JFM. Safety and effectiveness of restrictive eculizumab treatment in atypical haemolytic uremic syndrome. Nephrol Dial Transplant. 2018;33(4):635-645. [DOI] [PubMed] [Google Scholar]
- 16.Fakhouri F, Fila M, Hummel A, et al. Eculizumab discontinuation in children and adults with atypical haemolytic uremic syndrome: a prospective multicentric study. Blood. 2020;blood.2020009280. [DOI] [PubMed] [Google Scholar]
- 17.Merrill SA, Brittingham ZD, Yuan X, Moliterno AR, Sperati CJ, Brodsky RA. Eculizumab cessation in atypical hemolytic uremic syndrome. Blood. 2017;130(3):368-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abarrategui-Garrido C, Martínez-Barricarte R, López-Trascasa M, de Córdoba SR, Sánchez-Corral P. Characterization of complement factor H-related (CFHR) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114(19):4261-4271. [DOI] [PubMed] [Google Scholar]
- 19.Dragon-Durey MA, Blanc C, Marliot F, et al. The high frequency of complement factor H related CFHR1 gene deletion is restricted to specific subgroups of patients with atypical haemolytic uraemic syndrome. J Med Genet. 2009;46(7):447-450. [DOI] [PubMed] [Google Scholar]
- 20.Józsi M, Licht C, Strobel S, et al. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with CFHR1/CFHR3 deficiency. Blood. 2008;111(3):1512-1514. [DOI] [PubMed] [Google Scholar]
- 21.Zipfel PF, Edey M, Heinen S, et al. Deletion of complement factor H-related genes CFHR1 and CFHR3 is associated with atypical hemolytic uremic syndrome. PLoS Genet. 2007;3(3):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhouri F, Fila M, Provôt F, et al. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez de Córdoba S, Harris CL, Morgan BP, Llorca O. Lessons from functional and structural analyses of disease-associated genetic variants in the complement alternative pathway. Biochim Biophys Acta. 2011;1812(1):12-22. [DOI] [PubMed] [Google Scholar]
- 24.Marinozzi MC, Vergoz L, Rybkine T, et al. Complement factor B mutations in atypical hemolytic uremic syndrome-disease-relevant or benign? J Am Soc Nephrol. 2014;25(9):2053-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips EH, Westwood JP, Brocklebank V, et al. The role of ADAMTS-13 activity and complement mutational analysis in differentiating acute thrombotic microangiopathies. J Thromb Haemost. 2016;14(1):175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123(24):3733-3738. [DOI] [PubMed] [Google Scholar]
- 27.Mannucci PM, Cugno M. The complex differential diagnosis between thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: Laboratory weapons and their impact on treatment choice and monitoring. Thromb Res. 2015;136(5):851-854. [DOI] [PubMed] [Google Scholar]
- 28.Gavriilaki E, Yuan X, Ye Z, et al. Modified Ham test for atypical hemolytic uremic syndrome. Blood. 2015;125(23):3637-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palomo M, Blasco M, Molina P, et al. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol. 2019;14(12):1719-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation. 1990;81(1):156-163. [DOI] [PubMed] [Google Scholar]
- 32.Császár A, Duba J, Melegh B, et al. Increased frequency of the C3*F allele and the Leiden mutation of coagulation factor V in patients with severe coronary heart disease who survived myocardial infarction. Exp Clin Immunogenet. 2001;18(4):206-212. [DOI] [PubMed] [Google Scholar]
- 33.Vengen IT, Madsen HO, Garred P, Platou C, Vatten L, Videm V. Mannose-binding lectin deficiency is associated with myocardial infarction: the HUNT2 study in Norway. PLoS One. 2012;7(7):e42113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noris M, Remuzzi G. Cardiovascular complications in atypical haemolytic uraemic syndrome. Nat Rev Nephrol. 2014;10(3):174-180. [DOI] [PubMed] [Google Scholar]