Abstract
The U.S. Food and Drug Administration (FDA) has recently issued an Emergency Use Authorization (EUA) for 2 highly effective coronavirus disease 2019 (COVID-19) vaccines from Pfizer-BioNTech and Moderna. This has brought hope to millions of Americans in the midst of an ongoing global pandemic. The FDA EUA guidance for both vaccines is to not administer the vaccine to individuals with a known history of a severe allergic reaction (eg, anaphylaxis) to any component of the COVID-19 vaccine. The Centers for Disease Control and Prevention (CDC) additionally advises individuals with a history of an immediate allergic reaction to a vaccine or injectable or any history of anaphylaxis be observed for 30 minutes after COVID-19 vaccination. All other individuals should be observed for 15 minutes after COVID-19 vaccination. Staff at vaccine clinics must be able to identify and manage anaphylaxis. Post–FDA EUA, despite very strong safety signals in both phase 3 trials, reports of possible allergic reactions have raised public concern. To provide reassurance and support during widespread global vaccination, allergists must offer clear guidance to individuals based on the best information available, but also in accordance with the broader recommendations of regulatory agencies. This review summarizes vaccine allergy epidemiology and proposes drug and vaccine allergy expert opinion informed risk stratification for Allergy specialist use in conjunction with guidance of public health and regulatory authorities. The risk stratification schema guide care for (1) individuals with different allergy histories to safely receive their first mRNA COVID-19 vaccine and (2) individuals who develop a reaction to their first dose of mRNA COVID-19 vaccine.
Key words: mRNA, COVID-19, Vaccine, Allergy, Allergic reactions, Anaphylaxis, Guidelines, Risk stratification, Polyethylene glycol, Polysorbate
Abbreviations used: CDC, Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; PEG, Polyethylene glycol; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VAERS, Vaccine adverse event reporting System
Introduction
Vaccination, one of the most effective public health interventions modern medicine can offer, has become increasingly relevant as the global pandemic from coronavirus disease 2019 (COVID-19) continues to worsen throughout the world. In the United States, the pandemic has risen to crisis levels in every state, setting records with tens of thousands of new cases reported daily and deaths mounting. As of January 9, 2021, more than 89 million people globally have had confirmed infections and almost 2 million have died of COVID-19.1 Medical necessities are often in short supply, hospitals are overwhelmed, and health care workers are exhausted after months of fighting an uphill battle. Given the importance of the vaccine in fighting this public health crisis, understanding the allergic reactions with the approved mRNA COVID-19 vaccines is crucial. At the time of writing this review, immediate reactions clinically compatible with anaphylaxis have occurred at a rate of 11.1 per million doses of the Pfizer-BioNTech mRNA COVID-19 vaccine.2 Anaphylaxis has also been reported from the Moderna mRNA COVID-19 vaccine. Currently, the specific mechanism of allergy and the inciting antigen have not been identified. This review will summarize the current state of knowledge of immediate allergic reactions associated with the mRNA vaccines and potential mechanisms of hypersensitivity. We provide recommendations based on expert opinion using the currently available evidence to facilitate safe vaccination based on updated epidemiology and guidance provided by the Centers for Disease Control and Prevention (CDC), US Food and Drug Administration (FDA) and other regulatory agencies. The information provided in this manuscript is intended as guidance and a framework for the practicing Allergist based on the best available evidence at the time. Although it is expected to have global relevance it does not replace or override any guidance provided by public health or regulatory agencies.3
Clinical Trial Data
The US FDA's Emergency Use Authorization (EUA) of 2 highly effective COVID-19 vaccines in December 2020 was a landmark in the pandemic response for Americans. The Pfizer-BioNTech 2-dose COVID-19 vaccine regimen given on day 0 and 21 showed a 95% efficacy at preventing symptomatic COVID-19 infection, measured forward from 7 days after the second dose was administered.4 The vaccine appeared equally protective across age groups as well as racial and ethnic groups.4 The Moderna 2-dose COVID-19 vaccine regimen given on day 0 and 28 was 94% effective at preventing symptomatic COVID-19, measured from 14 days after the second dose onward.5 The efficacy of the Moderna vaccine appeared to be slightly lower in people 65 years and older, but it was equally effective across different racial and ethnic groups.5 The safety of both vaccines over a median of 2 months was similar to that of other viral vaccines. Severe systemic events were reported in less than 2% of Pfizer-BioNTech vaccine recipients after either dose, except for 3.8% reporting fatigue and 2.0% reporting headache after the second dose. Most adverse events reported after receiving the Moderna vaccine were mild or moderate in severity. A small number of participants reported systemic reactions lasting longer than 7 days, but there was no difference between vaccine and placebo groups. The Pfizer-BioNTech EUA is for individuals 16 years and older, whereas Moderna is for individuals 18 years and older. There are currently insufficient data for efficacy, safety, and effectiveness of these vaccines in children under 16 years of age but studies are ongoing.
Before FDA EUA of a COVID-19 vaccine, the United Kingdom Commission on Human Medicines initiated its COVID-19 vaccination program using the Pfizer-BioNTech vaccine. Within 48 hours, there were 2 reports of severe allergic reactions in the United Kingdom that prompted treatment with epinephrine. These reported allergic reactions led to a closer review of the Pfizer-BioNTech clinical trial data by the FDA. The Pfizer-BioNTech trial excluded patients with a previous history of severe adverse reactions associated with any vaccine, and both the Pfizer-BioNTech and Moderna trials further excluded severe allergic reaction (eg, anaphylaxis) to any component of their COVID-19 vaccine. Hypersensitivity-related adverse events were observed in 0.63% of Pfizer-BioNTech and 1.5% of Moderna vaccine clinical trial participants who received the vaccine, compared with 0.51% and 1.1%, respectively, in the placebo groups. One case of anaphylaxis and 1 drug hypersensitivity reaction were reported in the Pfizer-BioNTech trial,6 and 2 cases of delayed hypersensitivity reactions were reported in the Moderna trial.5 Only 1 of the Moderna delayed allergic reactions was in the vaccine group, and, because it occurred several months after vaccination, it was unlikely to be related to the vaccine. Also, in the Moderna trial, there were 3 events of lip/face swelling 1 to 2 days after vaccination but exclusively in patients with a history of dermal fillers but unclear from which manufacturer. There were no anaphylactic or severe hypersensitivity reactions reported with close temporal relation to administration of the Moderna vaccine.
US Regulatory Approval and Guidance
The FDA EUA guidance for the Pfizer-BioNTech COVID-19 vaccine specifies “do not administer Pfizer-BioNTech COVID-19 vaccine to individuals with known history of a severe allergic reaction (eg, anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 vaccine.” Given the reported reactions in health care workers, the US CDC subsequently advised that all patients—regardless of allergy history— should be observed for 15 minutes after COVID-19 vaccination and that vaccination staff must be trained to manage anaphylaxis. The CDC provided further recommendations “that persons who have had an immediate allergic reaction of any severity to any vaccine or injectable therapy (intramuscular, intravenous, or subcutaneous) discuss the risk of receiving the vaccine with their doctors and be monitored for 30 minutes afterward.” In addition, patients who have an immediate (within 4 hours) or severe allergic reaction (eg, anaphylaxis) to an mRNA COVID-19 vaccine should not receive a second dose. CDC precautions and contraindications for the Moderna vaccine under EUA were the same as for the Pfizer-BioNTech vaccine for the purpose of “harmonization.”3
US Postmarketing Experience
Similar to the UK experience, within a few days of widespread vaccination of health care workers in the United States, several reports of presumed allergic reactions to the COVID-19 vaccine emerged. At the time of publication, there have been 21 confirmed anaphylactic reactions to the Pfizer-BioNTech vaccine across almost 1.9 million doses administered in the United States.2 Of note, while 175 severe allergic reactions to the Pfizer-BioNTech were identified, upon further review, only 21 were consistent with anaphylaxis. This highlights the critically important role for allergists in clinical phenotyping of these reactions with subsequent risk stratification rather than vaccine avoidance. The majority of CDC-confirmed anaphylactic reactions (86%, 18/21) occurred within a 30-minute observation window, and patients were treated immediately with complete resolution of symptoms. While there are more epidemiologic data currently available for the Pfizer-BioNTech vaccine, the authors have personal knowledge through clinical care of at least 3 possible anaphylactic reactions to the Moderna COVID-19 vaccine. To date, there are no fatalities associated with reported allergic reactions to any COVID-19 mRNA vaccine.
Epidemiology and Etiology of Allergic Reactions to Vaccines
Allergic reactions to vaccines are generally described as occurring at a rate of 1.31 (95% CI, 0.90-1.84) cases per million vaccine doses from a large population-based study, with no fatalities reported.7 Rates remain similar when stratified by age and sex, although slightly higher frequencies have been observed in females.7 The incidence of allergic reaction by specific vaccine, however, is difficult to quantify in epidemiologic studies, because often multiple vaccines are administered on the same day. The cases that followed the administration of a single vaccine involved predominantly trivalent influenza vaccine, for which the rate of reaction was estimated to be 1.35 (95% CI, 0.65-2.47) per million vaccine doses.7 Of concern is that, although numerically rare, vaccine reactions can cause substantial fear and anxiety in the general population and may contribute to decreased willingness to receive a COVID-19 vaccine. Additionally, not all immediate reactions that occur in association with vaccines are true allergic reactions (eg, flushing, transient dyspnea) and careful clinical phenotyping is necessary to prevent large-scale COVID-19 vaccine avoidance. This is apparent in the recent CDC report demonstrating that of 175 possible severe allergic reactions, 86 (49%) were nonanaphylactic allergic reactions.2
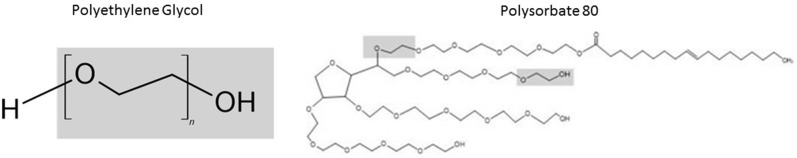
Confirmed allergic reactions to vaccines are not frequently attributed to the active ingredients, but rather to the inactive ingredients, or excipients, including egg protein, gelatin, formaldehyde, thimerosal, or neomycin. Excipients are necessary and added to a vaccine for specific purposes such as stimulating a stronger immune response, preventing contamination by bacteria, or stabilizing the potency of the vaccine during transportation and storage. Excipients represent the major contributor to specific IgE-mediated and immediate reactions associated with vaccines.8 Efforts to specifically decrease well-known excipients such as egg and gelatin in vaccines have been highly successful in reducing subsequent allergic reactions.9 , 10 Other excipients, such as polyethylene glycol (PEG) and polysorbate (Figure 1 ), are used to improve water solubility in drugs and vaccines. PEG itself has not previously been used in a vaccine, but polysorbate has been identified as a rare cause of allergic reactions to vaccines. First-dose reactions to vaccines containing polysorbates may have occurred because of previous sensitization from polysorbate 80.11 The recently approved Pfizer-BioNTech and Moderna COVID-19 mRNA vaccines are not formulated with any food, drugs, or latex, but both contain the excipient PEG (Tables I and II ) for the purpose of stabilizing the lipid nanoparticle containing the mRNA. The specific PEG in these vaccines is different from the PEG used most commonly in other health care products, both in molecular weight and due to its coformulation as a stabilizing portion of a liposome.11 , 13 The AstraZeneca and Johnson & Johnson COVID-19 vaccines currently under development do not contain PEG but instead contain the excipient polysorbate 80 (Table II).
Figure 1.
Chemical structure and similarities between PEG and polysorbate 80.
Table I.
Ingredients of the Pfizer-BioNTech and Moderna COVID-19 vaccines
| Ingredients | Pfizer-BioNTech | Moderna |
|---|---|---|
| Active | Nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2 | Nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2 |
| Inactive—lipids | (4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) | SM-102 (proprietary to Moderna) |
| 2[(PEG)-2000]-N,N-ditetradecylacetamide | PEG 2000 dimyristoyl glycerol | |
| 1,2-Distearoyl-sn-glycero-3-phosphocholine | 1,2-Distearoyl-sn-glycero-3-phosphocholine | |
| Cholesterol | Cholesterol | |
| Inactive—salts, sugars, buffers | Potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dehydrate | Tromethamin, Tromethamin hydrocholoride, acetid acid, sodium acetate |
| Sugar (sucrose) | Sugar (sucrose) | |
| Diluent (sodium chloride) | Diluent (none) |
Table II.
Polysorbate and PEG excipients in select vaccines12
| Excipient | Vaccine type | Vaccine | Amount per dose |
|---|---|---|---|
| Polysorbate 20 | Influenza | Flublok&Flublock quad | ≤27.5 μg (Tween20) |
| Polysorbate 20 | Hepatitis A | Havrix | 0.05 mg/mL |
| Polysorbate 20 | Hepatitis A&B | Twinrix | Unknown |
| Polysorbate 20∗ | SARS-CoV-2 (Sanofi) | ||
| Polysorbate 80 | Tdap | Boostrix | ≤100 μg (Tween 80) |
| Polysorbate 80 | Influenza | Fluad | 1.175 mg |
| Polysorbate 80 | Influenza | Fluarix quad | ≤0.055 mg (Tween 80) |
| Polysorbate 80 | Influenza | Flucelvax quad | ≤1500 μg (Tween 80) |
| Polysorbate 80 | Influenza | Flulaval Quad | ≤887 μg |
| Polysorbate 80 | HPV | Gardasil and Gardasil -9 | 50 μg |
| Polysorbate 80 | Hepatitis B | Heplisav-B | 0.1 mg/mL |
| Polysorbate 80 | DTaP | Infanrix | ≤100 μg (Tween 80) |
| Polysorbate 80 | Japanese encephalitis | JE-Vax | <0.0007% |
| Polysorbate 80 | DTaP + IPV | Kinrix | ≤100 μg (Tween 80) |
| Polysorbate 80 | DTaP + HepB + IPV | Pediarix | ≤100 μg (Tween 80) |
| Polysorbate 80 | Pneumococcal 13-valent | Prevnar 13 | 100 μg |
| Polysorbate 80 | DTaP + IPV | Quadracel | 10 ppm |
| Polysorbate 80 | Rotavirus | RotaTeq | ? |
| Polysorbate 80 | Zoster | Shingrix | 0.08 mg |
| Polysorbate 80 | Meningococcal group B | Trumenba | 0.018 mg |
| Polysorbate 80 | DTaP + IPV + HepB + Hib | Vaxelis | <0.0056% |
| Polysorbate 80∗ | SARS-CoV-2 (AstraZeneca) SARS-CoV-2 (Johnson & Johnson) |
||
| PEG2000 | SARS-CoV-2 (Moderna) SARS-CoV-2 (Pfizer) |
Not approved at the time of publication.
Numerous FDA-approved and over-the-counter products contain PEG, including medications, skin creams, and personal lubricants, as well as foods using PEG as an antifoaming agent (Table III ). In addition, PEG3350 is the active ingredient in several medications prescribed for treating constipation (eg, Miralax) and in bowel preps used before colonoscopy (eg, GoLytely). Although considered to be safe and biologically inert, several reports have shown that up to 70% of patients who have undergone treatment with PEGylated therapeutics will develop anti-PEG IgG antibodies.15 A more recent study in the general population showed that 5% to 9% of 1721 serum samples tested were positive for anti-PEG IgG, 3% to 6% of 948 such samples tested were positive for anti-PEG IgM, and 2 of 2091 (0.1%) samples tested were positive for anti-PEG IgE.16 Also, reactions to PEG-containing products on the first exposure suggest previous sensitization to PEG. However, a review of FDA voluntary reporting data from 2005 through 2017 identified an average of just 4 cases (range, 2-8 cases) per year of PEG-associated anaphylaxis during colonoscopy preparation or laxative use.11 Interestingly, more subtle PEG allergies are usually discovered during allergist evaluation of patients being evaluated for reactions to seemingly unrelated products, including injectable steroids, processed foods, cosmetics, drugs, and other substances that contain PEG.17 Specific IgE directed against PEG, currently a research tool, has recently been demonstrated in PEG-allergic patients who reacted both to PEGs and, in 1 case, to a PEGylated liposomal product used as an echocardiogram contrast, by 2 independent methods.11 , 13 , 16 In the earliest of these 3 reports, the binding of PEG-specific IgG from patients with PEG anaphylaxis showed increased avidity as the molecular weight of the PEG assayed increased from 1000 and above, with clinical tolerance of PEG300 upon challenge, suggesting that not all PEGs are equally risky to cause reactions.11 Although an exact threshold of reactivity based on the molecular weight of PEG is not known, tolerance of PEG with molecular weight less than 400 has been described in those who have documented anaphylaxis to PEG3350.11 It has been reported that those who lose reactivity to lower molecular weight PEG over time may still remain sensitized to very high molecular weight PEG.18 Additionally, there appears to be an increased incidence of allergic reactions in patients who receive intravenous PEG compared to the intramuscular route.19 , 20 Although anaphylaxis to oral PEG has been postulated to be due to an impaired epithelial barrier, as PEG is normally used as an osmotic laxative due to its lack of absorption, anaphylaxis with serum IgE detected to PEG has occurred in individuals without any apparent defect.21
Table III.
Common injectable medications containing PEG14
| Generic name (brand name) | Molecular weight | General description |
|---|---|---|
| Methylprednisolone acetate (Depo-Medrol) | PEG 3350 | An anti-inflammatory glucocorticoid for intramuscular, intra-articular, soft tissue or intralesional injection |
| Methoxy polyethylene glycol-epoetin beta (Micera) | 30-kD methoxy PEG butanoic acid | Used to treat anemia in adults with chronic kidney disease |
| Pegfilgrastim (Neulasta) | 20-kD monomethoxy PEG | Used to help reduce the chance of infection due to a low white blood cell count, in people with certain types of cancer (nonmyeloid), who receive anticancer medicines (chemotherapy) that can cause fever and low white blood cell count |
| Medroxyprogesterone acetate (Depo-Provera) | PEG 3350 | Contraceptive and adjunctive therapy and palliative treatment of inoperable, recurrent, and metastatic endometrial or renal carcinoma |
| Brilliant Blue G Ophthalmic Solution (TissueBlue) | PEG 3350 | Disclosing agent indicated to selectively stain the internal limiting membrane |
| Sulfur hexafluoride (Lumason) | PEG 4000 | Ultrasound contrast agent |
| Biomatoprost implant (Durysta) | PEG, unspecified | Reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension |
| Trastuzumab (Herceptin, Herzuma, Kanjinti, Ogivri, Ontruzant) | PEG 3350 | Adjuvant treatment of HER2 overexpressing node-positive or node-negative breast cancer |
| Rilonacept (Arcalyst) | PEG 3350 | IL-1 blocker for treatment of cryopyrin-associated periodic syndromes |
| Perflutren lipid microsphere (Definity) | PEG 5000 | Contrast agent used to brighten and clarify images of the heart during echocardiograms |
Polysorbate, structurally similar to PEG with polyether domains with observed clinical cross-reactivity (Figure 1), is also an excipient in a multitude of medical preparations (eg, vitamin oils, vaccines, and anticancer agents), creams, ointments, lotions, and medication tablets (Table IV ).22 For example, at least 70% of injectable biological agents and mAb treatments contain a polysorbate, most typically polysorbate 80.23 Unfortunately, polysorbate and its degradation products are known to be intrinsically anaphylactogenic, leading to a plausible explanation for multiple reports of anaphylaxis in patients receiving polysorbate-containing biologics, vaccines, steroids, and chemotherapeutics, although there is limited in vivo and in vitro evidence to support this, and isolated sensitization through polysorbates appears rare and less common than through higher molecular weight PEG.24, 25, 26, 27, 28, 29 Attempts have been made to address these issues by using safer alternatives to polysorbate, but the negative allergic outcomes are often outweighed by the clinical benefit of improved drug performance. In the context of evolving literature demonstrating PEG as an allergen, many allergists have hypothesized that any cases of anaphylaxis during the rollout of the Pfizer/BioNTech and Moderna severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, which use different liposomal delivery vehicles but contain PEG2000, could potentially be due to preexisting PEG allergy.30
Table IV.
Common injectable medications containing polysorbate
| Drug class | Generic name (brand name) | Polysorbate |
|---|---|---|
| Antiarrhythmic | Amiodarone hydrochloride (generics only) | Polysorbate 80 |
| Antidiabetic | Exenatide (Bydureon Bcise) | Polysorbate 20 |
| Insuline glargine (Lantus, Semglee) | Polysorbate 20 | |
| Insuline glulisine (Apidra) | Polysorbate 20 | |
| Dulaglutide (Trulicity) | Polysorbate 80 | |
| Antidote | Hyaluronidase (Hylenex Recombinant) | Polysorbate 80 |
| Antifungal | Anidulafungin (Eraxis) | Polysorbate 80 |
| Anti-inflammatory | Interferon beta 1a (Avonex, Plegridy) | Polysorbate 20 |
| Omalizumab (Xolair) | Polysorbate 20 | |
| Antineoplastic | Ofatumumab (Kesimpta) | Polysorbate 80 |
| Siltuximab (Sylvant) | Polysorbate 80 | |
| Antipsychotic | Paliperidone palmitate (Invega Trinza, Invega Sustenna) | Polysorbate 20 |
| Aripiprazole lauroxil (Aristada) | Polysorbate 20 | |
| Antiretroviral | Ibalizumab (Trogarzo) | Polysorbate 80 |
| Antipsoriatic | Adalimumab (Humira, Imraldi) | Polysorbate 20 (Imraldi)/Polysorbate 80 (Humira) |
| Golimumab (Simponi) | Polysorbate 80 | |
| Guselkumab (Tremfya) | Polysorbate 80 | |
| Infliximab - dyyb (Inflectra, Remicade, Renflexis) | Polysorbate 80 | |
| Ustekinumab (Stelara) | Polysorbate 80 | |
| Antiviral | Interferon alfa-2b (Intron A) | Polysorbate 80 |
| Biological response modifier | Interferon gamma-1b (Actimmune) | Polysorbate 20 |
| Cancer treatment | Ado-trastuzumab emtansine (Kadcyla) | Polysorbate 20 |
| Atezolizumab (Tecentriq) | Polysorbate 20 | |
| Avelumab (Bavencio) | Polysorbate 20 | |
| Bevacizumab (Avastin, Zirabev) | Polysorbate 20 | |
| Daratumumab/hyaluronidase (Darzalex Faspro) | Polysorbate 20 | |
| Denosumab (Prolia, Xgeva) | Polysorbate 20 | |
| Dinutuximab (Unituxin) | Polysorbate 20 | |
| Enfortumab (Padcev) | Polysorbate 20 | |
| Olaratumab (Lartruvo) | Polysorbate 20 | |
| Palifermin (Kepivance) | Polysorbate 20 | |
| Pertuzumab/trastuzumab/hyaluronidase (Phesgo) | Polysorbate 20 | |
| Polatuzumab vedotin (Polivy) | Polysorbate 20 | |
| Tafasitamab (Monjuvi) | Polysorbate 20 | |
| Trastuzumab (Herceptin, Herceptin Hylecta, Herzuma, Kanjinti, Ontruzant, Trazimera) | Polysorbate 20 | |
| Belantamab (Blenrep) | Polysorbate 80 | |
| Brentuximab vedotin (Adcetris) | Polysorbate 80 | |
| Cemiplimab (Libtayo) | Polysorbate 80 | |
| Docetaxel (Taxotere) | Polysorbate 80 | |
| Durvalumab (Imfinzi) | Polysorbate 80 | |
| Elotuzumab (Empliciti) | Polysorbate 80 | |
| Etoposide (Toposar, VePesid) | Polysorbate 80 | |
| Fam-trastuzumab deruxtecan (Enhertu) | Polysorbate 80 | |
| Fosaprepitant dimeglumine (EMEND, Fosaprepitant) | Polysorbate 80 | |
| Inotuzumab ozogamicin (Besponsa) | Polysorbate 80 | |
| Ipilimumab (Yervoy) | Polysorbate 80 | |
| Isatuximab (Sarclisa) | Polysorbate 80 | |
| Mogamulizumab (Poteligeo) | Polysorbate 80 | |
| Moxetumomab pasudotox (Lumoxiti) | Polysorbate 80 | |
| Nivolumab (Opdivo) | Polysorbate 80 | |
| Ofatumumab (Arzerra) | Polysorbate 80 | |
| Pembrolizumab (Keytruda) | Polysorbate 80 | |
| Ramucirumab (Cyramza) | Polysorbate 80 | |
| Rituximab (Truxima, Rituxan, Ruxience) | Polysorbate 80 | |
| Rituximab and hyaluronidase (Rituxan Hycela) | Polysorbate 80 | |
| Temsirolimus (Torisel) | Polysorbate 80 | |
| Temozolomide (Temodar) | Polysorbate 80 | |
| Contraceptive | Medroxyprogesterone acetate (Depo-Provera, Depo-Provera CI, Depo-subQ provera 104) | Polysorbate 80 |
| Corticosteroid | Methylprednisolone acetate (Depo-Medrol) | Polysorbate 80 |
| Triamcinolone acetonide (Aristocort Forte, Aristospan, Kenalog-40, Kenalog-10, Protherix, Triesence, Triloan Suik, Triloan II Suik, Zilretta) | Polysorbate 80 | |
| Diagnostic | Sincalide (Kinevac) | Polysorbate 20 |
| Tuberculin purified protein derivative (Aplisol, Tubersol) | Polysorbate 80 | |
| Disease-modifying antirheumatic drug | Anakinra (Kineret) | Polysorbate 80 |
| Tocilizumab (Actemra) | Polysorbate 80 | |
| Enzyme | Velaglucerase alfa (Vpriv) | Polysorbate 20 |
| Imiglucerase (Cerezyme) | Polysorbate 80 | |
| Taliglucerase alfa (Elelyso) | Polysorbate 80 | |
| Erythoid maturation agent | Luspatercept (Reblozyl) | Polysorbate 80 |
| Factor Xa inhibitor antidote | Coagulation factor Xa (recombinant), inactivated-zhzo (Andexxa) | Polysorbate 80 |
| Gonadotropin | Follitropin (Menopur, Follistim) | Polysorbate 20 |
| Growth hormone analog | Somatropin (Nutropin AQ Nuspin 5) | Polysorbate 20 |
| Hematopoietic growth factor | Erythropoietin (Retacrit) | Polysorbate 20 |
| Pegfilgrastim (Fulphila, Neulasta, Nyvepria, Udenyca) | Polysorbate 20 | |
| Romiplostim (Nplate) | Polysorbate 20 | |
| Darbepoetin alfa (Aranesp) | Polysorbate 80 | |
| Filgrastim (Neupogen, Nivestym, Granix, Zarxio) | Polysorbate 80 | |
| Hepatitis B/Hepatitis C agent | Peginterferon (Pegasys Pegintron) | Polysorbate 80 |
| Hemostatic | Vitamin K (Phytonadione) | Polysorbate 80 |
| Immune globulin | Hepatitis B immune globulin (HepaGam B, Nabi-HB) | Polysorbate 80 |
| Rho (d) immune globulin (WinRho) | Polysorbate 80 | |
| Immunomodulator | Interferon beta-1a (Avonex, Avonex Pen) | Polysorbate 20 |
| Emapalumab (Gamifant) | Polysorbate 80 | |
| Immunosuppressant | Mycophenolate mofetil (Cellcept IV) | Polysorbate 80 |
| Inflammatory bowel disease agent | Vedolizumab (Entyvio) | Polysorbate 80 |
| Interleukin inhibitor | Sarilumab (Kevzara) | Polysorbate 20 |
| Dupilumab (Dupixent) | Polysorbate 80 | |
| Mepolizumab (Nucala) | Polysorbate 80 | |
| Secukinumab (Cosentyx) | Polysorbate 80 | |
| Tildrakizumab -asmn (Ilumya) | Polysorbate 80 | |
| Kallikrein inhibitor | Lanadelumab (Takhzyro) | Polysorbate 80 |
| Leptin analog | Metreleptin (Myalept) | Polysorbate 20 |
| Macular degeneration agent | Aflibercept (Eylea) | Polysorbate 20 |
| Ranibizumab (Lucentis) | Polysorbate 20 | |
| Brolucizumab (Beovu) | Polysorbate 80 | |
| mAb treatment | Ocrelizumab (Ocrevus) | Polysorbate 20 |
| Remdesivir (Veklury) Romosozumab (Evenity) |
Polysorbate 20 Polysorbate 20 |
|
| Teprotumumab (Tepezza) | Polysorbate 20 | |
| Atoltivimab/maftivimab/odesivimab-ebgn (Inmazeb) | Polysorbate 80 | |
| Bamlanivimab | Polysorbate 80 | |
| Burosumab (Crysvita) | Polysorbate 80 | |
| Canakinumab (Ilaris) | Polysorbate 80 | |
| Casirivimab/Imdevimab | Polysorbate 80 | |
| Eptinezumab (Vyepti) | Polysorbate 80 | |
| Fremanezumab (Ajovy) | Polysorbate 80 | |
| Inebilizumab (Uplizna) | Polysorbate 80 | |
| Raxibacumab | Polysorbate 80 | |
| Multiple sclerosis treatment | Natalizumab (Tysabri) | Polysorbate 80 |
| Muscle relaxant | Dantrolene sodium (Dantrium, Ryanodex) | Polysorbate 80 |
| P-selectin inhibitor | Crizanlizumab (Adakveo) | Polysorbate 80 |
| Proprotein convertase subtilisin kexin type 9 inhibitor | Alirocumab (Praluent) | Polysorbate 20 |
| Evolocumab (Repatha) | Polysorbate 80 | |
| Rheumatologic | Belimumab (Benlysta) | Polysorbate 80 |
| Thrombolytic | Tenecteplase (Tnkase) | Polysorbate 20 |
| Alteplase (Cathflo Activase) | Polysorbate 80 | |
| Reteplase (Retavase) | Polysorbate 80 | |
| Vitamin infusion | Calcitriol (Calcijex, Rocaltrol) | Polysorbate 20 |
| Doxercalciferol (Hectorol) | Polysorbate 20 | |
| Vitamins A, B1, B2, B6, C, D3, E, K (Infuvite) | Polysorbate 80 |
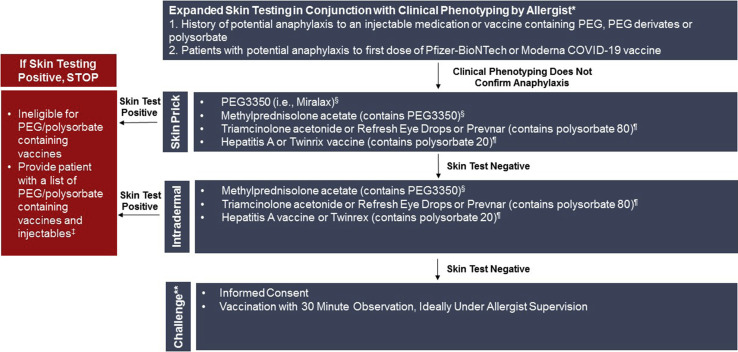
The positive and negative predictive values of skin testing to PEG in the evaluation of potential allergy to the mRNA COVID-19 vaccines are not clear but has shown utility in the evaluation of individuals with a history of anaphylaxis to PEG.11 , 18 Allergists have significant experience with skin testing, however drug skin testing relies on the use of nonirritating skin testing concentrations which are helpful when positive, but do not rule out allergy when negative. With the currently available mRNA COVID-19 vaccines, PEG is the only component that can be tested using skin prick and intradermal testing techniques. Thus, PEG skin testing could be considered in the evaluation of individuals with a history of IgE mediated allergy to a PEG containing injectable or a possible IgE mediated reaction to either of the currently available mRNA COVID-19 vaccines. We should remember, to date, that there is no confirmation IgE mediated reactions to PEG are responsible for reported reactions to the mRNA COVID-19 vaccines. Polysorbate, which is cross-reactive with PEG, is the excipient in both the AstraZeneca and Johnson & Johnson COVID-19 vaccines (currently not FDA approved). Therefore, PEG and polysorbate skin testing may be of value in shared decision making around future COVID-19 vaccination.31
In addition to considering excipients as the cause of IgE-mediated allergic reactions to the currently approved COVID-19 vaccines, alternative non-IgE pathways for activating mast cells and other inflammatory cells must be considered, because they can lead to a similar clinical presentation. For example, activation of the complement system leads to the generation of C3a, C4a, and C5a, which are potent activators of inflammation and are called anaphylatoxins due to their ability to cause non–IgE-mediated mast cell degranulation. One of the first reports of acute anaphylaxis associated with serum complement depletion was of a 45-year-old woman experiencing anaphylaxis after receiving lidocaine. She developed faintness, flushing, pallor, dyspnea, and hypotension, but she did not have urticaria or bronchospasm. Complement levels were markedly low (C1q, C3, C4, C5, and factor B).32 Depletion of complement levels and production of C3a and C5a have been seen in both mouse models of anaphylaxis and in clinical studies. C5a is the most potent of the anaphylatoxins and can contribute to vascular permeability as well as activation and chemotaxis of neutrophils, basophils, and mast cells. Infection and tissue injury can lead to activation of the complement system resulting in the generation of C3a and C5a, and these mediators can lead to anaphylaxis. PEG IgM and IgG can cause complement-activation–related pseudoallergy,33 a nonspecific immune response to PEGylated, nanoparticle-based medicines.34 This pathway may be responsible for reactions to medications such as liposomal doxorubicin35 and other drugs in clinical trials.34 Clearly, it is important to consider both IgE and alternative mechanisms for the current reactions. Measurement of serum tryptase and complement may help elucidate the mechanism of the drug-induced reactions in patients following COVID-19 vaccination.
It is also important to note that other nonimmunologic reactions may masquerade as allergic reactions including anaphylaxis. Vasovagal reactions are a well-known cause of hypotension and syncopal reactions associated with injections including vaccines. Panic or anxiety reactions can also present with symptoms masquerading as allergic reactions such as flushing, shortness of breath, tachycardia, and lightheadedness. Inducible laryngeal obstruction (ie, vocal cord dysfunction) may also masquerade as anaphylaxis, with prominent symptoms of shortness of breath and throat tightness but may also include flushing. Indeed, the most recent CDC report demonstrated that of 175 potential severe allergic reactions, 61 (35%) were determined to be nonallergic after case review.2
Evaluation of Patients With Severe Allergy Histories and Guidance for Initial Administration of COVID-19 Vaccine
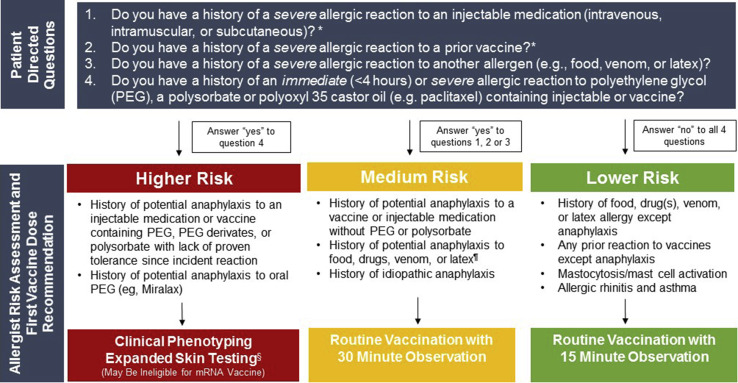
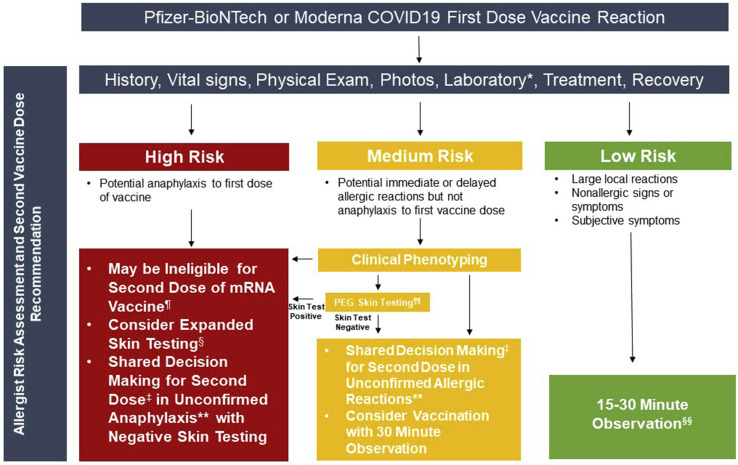
Massive vaccination programs were initiated within a few days after the FDA EUA of the COVID-19 vaccines. However, many questions surrounded the safety of giving these vaccines to individuals with a previous allergy history and further supported by recent data showing that the majority (81%, 17/21) of patients with confirmed anaphylaxis to the Pfizer-BioNTech mRNA vaccine had a prior allergy history and 33% (7/21) had a prior history of anaphylaxis.2 The CDC provides guidance with use of a recently developed pre-screening tool.36 A similar approach, commonly used in the allergy field, is to risk stratify patients based on a clinical assessment. The authors from Mass General Brigham (formerly Partners HealthCare; comprising 16 health care institutions in the New England area and the largest employer in Massachusetts with 80,000 employees) and Vanderbilt University Medical Center developed a plan of care to risk stratify employees and guide safe COVID-19 vaccination (Figure 2 ). To ensure vaccination in as many individuals as quickly as possible, our guidance, in line with FDA and CDC guidance, results in the rapid identification of high risk individuals needing Allergist assessment but does not preclude large groups of individuals with lower risk allergy histories from receiving the COVID-19 vaccine per usual protocol with either 15-minute or 30-minute observation periods.
Figure 2.
Risk stratification pathways with categories based on Mass General Brigham and Vanderbilt allergy expert consensus before initial COVID-19 vaccination. ∗If “yes” for questions 1 or 2, specific investigation as to the specific injectable products and vaccines should be pursued to determine whether these products could have contained high-molecular-weight PEG, polysorbate, or polyoxyl 35 castor oil (paclitaxel). See Tables II, III, and IV. ¶Current CDC guidance suggests 30 minutes of observation for patients with any history of anaphylaxis. §See Figures 3 and 4 for expanded skin testing procedures and non-irritating skin test concentrations. If skin testing to PEG is positive, as of December 28, 2020, Pfizer-BioNTech and Moderna are the only FDA approved vaccines and under EUA can not be given to an individual with a history of anaphylaxis to a component of the COVID-19 mRNA vaccine. Skin testing to polysorbate 20 and 80 become more important for patients with confirmed severe PEG allergy with regards to the safety of future vaccinations.
Four screening questions are presented to patients before the initial vaccination to assess risk:
-
1.
Do you have a history of a severe allergic reaction to an injectable medication (intravenous, intramuscular, or subcutaneous)?
-
2.
Do you have a history of a severe allergic reaction to a previous vaccine?
-
3.
Do you have a history of a severe allergic reaction to another allergen (eg, food, venom, or latex)?
-
4.
Do you have a history of an immediate or severe allergic reaction to PEG-, a polysorbate-, or polyoxyl 35 castor oil (eg, paclitaxel)-containing injectable or vaccine?
The screening questions address CDC guidelines and are accompanied by a “Frequently Asked Questions” document explaining medical terminology, including descriptions of PEG and polysorbates (Table V ).
Table V.
Frequently asked questions (patient handout)
| What is vaccine allergy? |
| Similar to medications or foods, people can be allergic to a vaccine. However, allergic reactions to vaccines are very rare (about 1 in 1 million people will have an allergic reaction to a vaccine). Some reactions are mild, such as hives as the only symptom, whereas others are more severe. A severe allergic reaction is called anaphylaxis. Symptoms start very quickly (usually within minutes) and almost always within 4 h of vaccination and typically include multiple parts of the body: hives on the skin; swelling of mouth, lips, tongue, or throat; shortness of breath, wheezing, or chest tightness; or low blood pressure or loss of consciousness. About half of allergic reactions to vaccines happen in the first 15 min after receiving the vaccination. Rarely delayed reactions such as fever and local arm swelling can occur up to 2-3 weeks later. These reactions should not preclude a second dose of the vaccine. |
| What about redness and swelling at the injection site—is that an allergic reaction? |
| Sometimes vaccines can cause large local reactions at the injection site, and these can begin hours after the vaccination or even the next day. The skin at the site of vaccination can become sore, swollen, red, and painful. Sometimes it can also become itchy. The symptoms can last several days. Although this type of reaction can be uncomfortable, if it does not include the symptoms of allergic reactions listed above, it is not an allergic reaction to the vaccine, there is no risk of an allergic reaction with the next vaccination, and an allergist consultation is not necessary. |
| What is a severe allergic reaction? |
| A severe allergic reaction is sometimes called anaphylaxis. Symptoms start very quickly (usually within minutes) and almost always within 4 h of vaccination and typically include hives; swelling of mouth, lips, tongue, or throat; shortness of breath, wheezing, or chest tightness; or low blood pressure or loss of consciousness. |
| What are the ingredients in the Pfizer-BioNTech COVID-19 vaccine? |
| 1. mRNA. The active ingredient is a nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2 |
| 2. Inactive ingredients: |
| • Lipids ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate), [(PEG)-2000]-N,N-ditetradecylacetamide, 1,2-distearoyl-sn-glycero-3-phosphocholine, and 0.2 mg cholesterol), |
| • Electrolytes potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dihydrate, and |
| • Sugar (sucrose) |
| • The diluent, added to the vaccine for administration, is saline (sodium chloride) |
| What are the ingredients in the Moderna COVID vaccine? |
| 1. mRNA |
| 2. Inactive ingredients: |
| • lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol, cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine), |
| • tromethamine, |
| • tromethamine hydrochloride, |
| • acetic acid, |
| • sodium acetate, |
| • Sugar (sucrose) |
| Which patients should speak to an allergist before receiving the vaccine? |
| In the vaccine trials, only patients with a history of severe allergic reaction associated with a vaccine and/or severe allergic reaction to any component of the vaccine were excluded. If you are unsure about your vaccine or PEG allergy history, an allergy consultation is recommended. In general, most patients allergic to one vaccine can receive other vaccinations safely. |
| What is PEG and what are common products that contain PEG? |
| PEG is a common, water-soluble ingredient in a wide variety of commercial products including some vaccines and more than 1000 FDA-approved medications. It is the primary ingredient in commonly used colonoscopy preparations (Golytely) or constipation treatment (Miralax) as well as in intraveous medications such as PEGylated medications. It is also used in ultrasound gel and injectable steroid injections such as methylprednisolone acetate. Reactions to PEG are exceedingly rare, but anaphylaxis has been reported. |
If the answer is “no” to all 4 questions, the individual would be deemed “lower risk” and receive the vaccine under usual protocol with a 15-minute observation period. If the answer to question 1, 2, or 3 is “yes,” the individual would be deemed “medium risk” and require a 30-minute observation period. In addition, if “yes” for 1 and 2, specific investigation as to the specific injectable products and vaccines should be pursued to determine whether these products could have contained high-molecular-weight PEG, polysorbate, or polyoxyl 35 (eg, paclitaxel). If the answer to question 4 is “yes,” the individual would be deemed “higher risk,” prompting evaluation with an allergist for clinical phenotyping and consideration of expanded skin testing if history is not consistent with confirmed anaphylaxis using nonirritating skin test concentrations (Figures 3 and 4 ).11 If skin testing result to PEG is positive, under EUA, the individual is not a candidate for the Pfizer-BioNTech or Moderna COVID-19 vaccines, and the skin test result to polysorbate 20 and 80 become important with regard to the safety of future SARS-CoV-2 vaccines in development. If skin testing to PEG is negative, vaccination with the Pfizer-BioNTech or Moderna COVID-19 vaccines could be considered in conjunction with shared decision making, informed consent, and would require 30 minutes of observation under Allergist supervision.
Figure 3.
Expanded skin testing procedure. ∗Recommended skin testing to evaluate only known potential IgE mechanism (PEG allergy). ¶Skin testing for consideration to evaluate PEG and polysorbate allergy. In patients with positive PEG skin testing, the result of polysorbate 20 and 80 skin testing becomes important with regards to the safety of future SARS-CoV-2 vaccines in development. Therefore, based on clinical history, skin testing to both PEG and polysorbate during 1 clinic visit may be appropriate. §Anaphylaxis with intradermal skin testing in PEG allergic patients has been described. We recommend staff have anaphylaxis training and anaphylaxis kit available in close proximity. ‡Tables II, III, and IV contain a list of PEG/polysorbate containing vaccines and injectables that can be shared with patients. ∗∗Recommended only after shared decision making between allergist and patient.
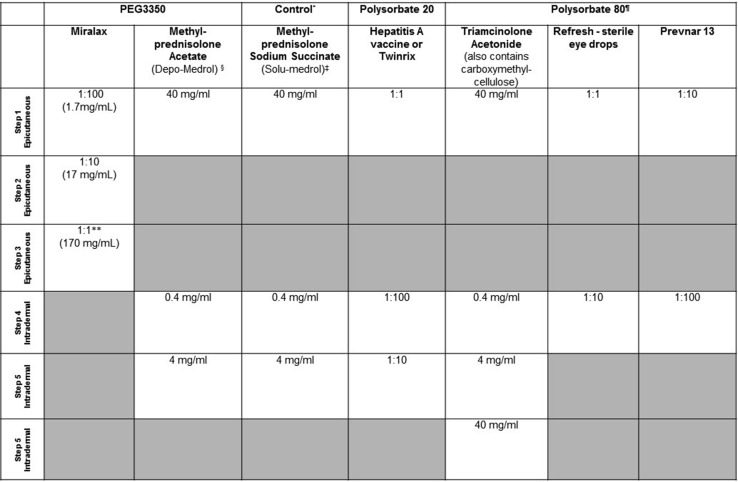
Figure 4.
Nonirritating skin testing concentrations for PEG3350 and polysorbate. ∗Methyl-prednisolone sodium succinate does not contain PEG or polysorbate 80 and can be used as an additional control. ¶Refresh Optive Advanced Lubricant eye drops and Prevnar are an alternate source for polysorbate 80 skin testing. §Some brands of methylprednisolone acetate contain polysorbate and PEG3350 while others only have PEG3350; use methylprednisolone acetate containing PEG3350 only. ‡Nonirritating skin testing concentrations for methyl-prednisolone sodium succinate and triamcinolone acetonide include a range of 10 to 40 mg/mL for initial skin prick testing with subsequent 10× dilutions.37 One author (E.P.) has extensive experience using 50 mg/mL as a non-irritating skin testing concentration for methyl-prednisolone sodium succinate skin prick testing with subsequent 10× dilutions. ∗∗Dissolve 17 gram miralax packet in 100mL of sterile water for 1:1 solution (170 mg/mL).
Evaluation and Management of Patients With Potential Reactions to the COVID-19 Vaccines
For patients presenting to the allergist with a possible reaction to the first dose of their COVID-19 vaccine, the primary concern is the ability to safely receive the second dose (Figure 5 ). Although the vaccines have some efficacy related to only 1 dose, both the Pfizer-BioNTech and Moderna vaccines received EUA approval for efficacy, which was evaluated with 2 doses. As such, a crucial determination will be establishing whether or not an allergic reaction occurred. Recent CDC data demonstrate that the vast majority, 84% (147/175) of reported severe “allergic” reactions, were unconfirmed after case review.2 Individuals with symptoms suggestive of a nonallergic reaction (eg, transient dyspnea, tachycardia alone, metallic taste, flushing, lip tingling) can proceed with a 15 or 30 minute observation for the second dose. For patients with a potential allergic reaction (eg, urticaria, angioedema) after their first dose that does not meet criteria for anaphylaxis, vaccination programs should prioritize follow-up with an allergist who can review the clinical history and consider PEG skin testing if an IgE-mediated reaction is suspected to risk stratify the patient prior to a second COVID-19 vaccine dose (Figure 5). Antihistamines do not prevent anaphylaxis and could mask cutaneous symptoms, leading to a delay in treatment. However, pretreatment with fexofenadine 180-360 mg or cetirizine 10-20 mg 1-2 hours prior to the second dose of COVID-19 vaccination can be considered in individuals with mild allergic symptoms (ie, pruritus or urticaria only), especially those that are delayed in onset. If a patient develops potential anaphylaxis to the first vaccine dose, shared decision making with an allergist including risk stratification and expanded skin testing should occur before any consideration of vaccine rechallenge (Figure 5). There are no data on the safety of the second vaccine after confirmed anaphylaxis to the first dose. For other vaccines for which there is much more allergy experience, split-dose challenges (eg, 10%-25% of the dose followed 30 minutes later by the remaining 75%-90% of the dose) have been used.38 Although some groups have indicated their intent to implement split dosing of the mRNA vaccines, there are no supportive efficacy or safety data. For both the Pfizer-BioNTech and Moderna vaccines, neither the stability of the vaccine diluted nor the safety and immunogenicity at altered doses or concentration have been studied. It should be remembered that these are not simple protein vaccines but instead are mRNA vaccines and subject to degradation. The Pfizer-BioNTech vaccine indeed is only 0.3 mL and there are no data, to date, for either mRNA vaccine showing split-dosing efficacy. We do not recommend vaccine skin testing at this time because of limited vaccine supply, lack of information on sensitivity or specificity, unclear safety of skin testing. At the time of publication, mRNA vaccines are under EUA and remain unlicensed for skin testing.
Figure 5.
Risk stratification pathways with categories based on Mass General Brigham and Vanderbilt allergy expert consensus after allergic reaction to first dose of COVID19 vaccine. ∗Ideal laboratory assessment includes reaction serum tryptase within 2 hours and complement activation by ELISA (C3a, C3b, C5a, C5b-9 ideally within 1 hour; send to National Jewish); follow-up baseline serum tryptase is also useful. ¶Follow CDC guidance.3 §See Figures 3 and 4 for expanded skin testing algorithm and nonirritating skin test concentrations. ‡Shared decision making with allergist considering eligibility for second dose or future challenge with other SARS-CoV-2 vaccines. There are no data on the safety of administering the second vaccine dose after potential anaphylaxis to the first dose and limited anecdotal evidence from the authors’ clinical experience suggesting that some patients can safely receive the second dose after more mild allergic type reactions to the first dose. If the decision is made to proceed with vaccination, staff should have anaphylaxis training and anaphylaxis kit needs to be available in close proximity. ∗∗84% (147/175) of potential anaphylaxis cases reported to the CDC were unconfirmed after their case review.2 ¶¶PEG skin testing can be considered to assist in the evaluation of a potential IgE mechanism but data confirming this mechanism is responsible for reported reactions to mRNA COVID-19 vaccines are lacking. §§Consider 15 or 30 minute observation based on clinical judgment.
All patients with potential allergic reactions should be reported through formal processes, which include the Vaccine Adverse Event Reporting System (VAERS; https://vaers.hhs.gov); Patients should be encouraged to use V-Safe (https://vsafe.cdc.gov), a CDC application for second-dose reminders and to enter reaction information.39 , 40
Supporting Safe Vaccination and Addressing Public Concern: A Role for the Allergist
Allergists' expertise in the diagnosis and treatment of allergic reactions is invaluable for the screening of high-risk individuals, the training of clinic staff conducting vaccinations, and the management of patients who experience potentially allergic reactions to a COVID-19 vaccine. To date, Mass General Brigham and Vanderbilt have instituted screening methods before and after vaccination which consist of self-reported answers to a questionnaire followed by telemedicine visits with an allergy clinician for high-risk patients prior to the first vaccine dose or after potential allergic reactions to the first dose. This allows for clinical phenotyping, risk stratification, and reassurance to patients deemed at lower risk to safely receive the vaccine. For reactions that happen onsite, vaccination clinics are reliant on staff that do not regularly diagnose and treat anaphylaxis, but CDC guidance emphasized a minimum of 15 minutes of observation after all doses and ready access to appropriate medical treatment for allergic reactions. Those staffing the vaccination clinic should have education around anaphylaxis treatment guidelines. Anaphylaxis is a life-threatening hypersensitivity reaction where rapid, early administration of epinephrine is vital for recovery. Along with the CDC educational and training videos,41 allergists can help reassure patients and educate providers by addressing a few key issues:
-
1.
Education on the diagnosis of anaphylaxis.42 Allergists should include differentiating vasovagal and anxiety reactions from anaphylaxis and defining anaphylaxis with infographics (Table VI and Figure 6 ).
-
2.
Education on the treatment of anaphylaxis. Allergists should review epinephrine use and anaphylaxis-kit contents. For example, before the rollout of Mass General Brigham employee vaccination, allergists provided education and replaced epinephrine vials with epinephrine autoinjectors. A nonsedating antihistamine was added to the anaphylaxis kits. It is necessary to recognize that some medications in anaphylaxis kits contain PEG, and as such it is important for clinics to use epinephrine for suspected anaphylaxis cases.
-
3.
Providing at-the-elbow support to vaccination programs. Allergists may need to be on-site or on-call during higher risk vaccination. This will ensure that vaccinated individuals with possible reactions receive the best diagnostic evaluation and treatment plan, while linking them to appropriate follow-up care before the second vaccine dose.
-
4.
Providing support to individuals with benign symptoms after discharge. Up to 80% of individuals in the vaccine clinical trials had local symptoms after vaccination. Large local reactions with symptoms of pain, itching, burning, or swelling at the site of injection do not preclude an individual from getting the vaccine again. Delayed local hypersensitivity reactions, with onset after Day 8, have been observed specifically with Moderna's vaccine. Nonsteroidal anti-inflammatory drugs used to treat fever or myalgias may precipitate urticaria that could be misattributed to the vaccine. Allergists can provide assessments and reassurance and encourage completion of vaccination.
Table VI.
Anaphylaxis compared with vasovagal reaction43
| Signs and symptoms | Vasovagal reaction | Anaphylaxis |
|---|---|---|
| Interval (after injection) | Sometimes before, usually after a few seconds to a few minutes after the injection | Within 30 min after injection; the most severe reactions begin within the first 15 min |
| Consciousness | Fainting sensation, dizziness, loss of consciousness in some cases | Anxiety, which may progress into unconsciousness in severe cases |
| Breathing | Slow, with a few seconds of apnea in some cases | Respiratory difficulties; coughing, sneezing, wheezing, stridor |
| Pulse | Slow and weak, but regular | Rapid, weak and irregular |
| Skin | Diaphoresis, clammy skin, pallor |
|
| Blood pressure | Transient hypotension | Hypotension (systolic pressure <90 mm Hg), which may progress to cardiovascular collapse |
| Gastrointestinal system | Nausea, vomiting | Nausea, vomiting, abdominal pains, diarrhea |
| Treatment |
|
See Manitoba Health Protocol for Management of Suspected Anaphylaxis |
| Prevention | Do not vaccinate a standing person. Before vaccinating, ask if the person tends to faint; if so, ask patient to lie down | Before vaccinating, ask if the person has ever had an anaphylactic reaction to any product; if yes, ask for the name of the product and decide accordingly |
Figure 6.
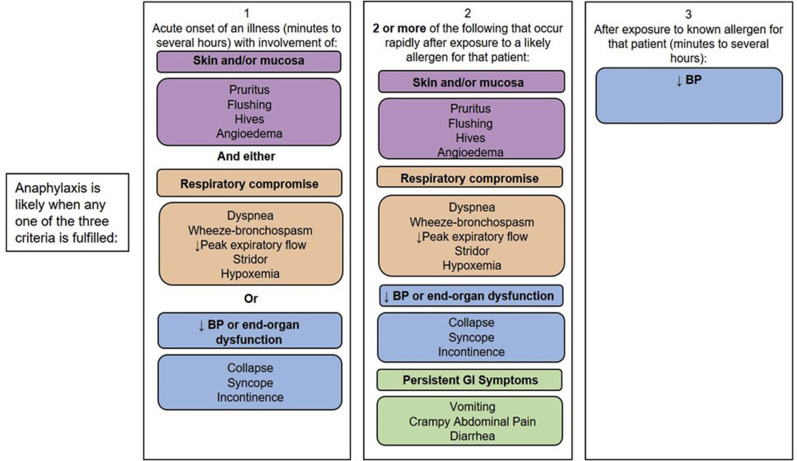
Graphic to assist in the recognition of anaphylaxis.44BP, Blood pressure; GI, gastrointestinal.
Summary
To date, allergic reactions to vaccines have been rare and often attributed to various vaccine components. Current reports from the CDC suggest that anaphylactic reactions to the Pfizer-BioNTech mRNA vaccine may occur more frequently than seen with other vaccines. Therefore, to support large-scale COVID-19 vaccine rollout programs, allergists can offer clinical phenotyping, risk stratification, and clear recommendations based on the best available information to date. At this point in time, the etiology of reactions to the Pfizer-BioNTech and Moderna mRNA vaccines is not clear. Avoidance of both mRNA COVID-19 vaccines in individuals with a history of anaphylaxis to PEG, PEG derivatives or polysorbate is recommended by the CDC. However, understanding the negative and positive predictive value of skin testing to PEG and polysorbate may play an important role in future risk stratification as many vaccines contain these excipients. Although these challenges require attention immediately during the current vaccination process, it is of equal importance that we must also design and conduct adequately powered studies to investigate the potential mechanistic etiology of these reactions. We need to understand the safety issues surrounding these vaccines, because the success of this mRNA vaccine platform is foundational to the flexibility of the COVID-19 response and our response to other viruses with similar vaccines in phase I and II trials. We must also remain vigilant to the rare potential for PEG sensitization to occur as individuals are receiving 2 doses of vaccine with PEG2000 in close succession and the mechanism for PEG sensitization leading to PEG allergy has not been determined.
Alongside guidance from public health and regulatory agencies, our goal was to provide a framework and guidance to practicing Allergists. As the US prepares for massive COVID-19 vaccination, allergists must prepare for 2 main population health challenges: (1) ensuring that highly allergic individuals feel appropriately informed and supported to receive the COVID-19 vaccines and (2) ensuring that patients who suffer from a potentially allergic reaction to the first dose of a SARS-CoV-2 vaccine have a careful evaluation to determine if a true allergic reaction occurred along with the requisite information and support needed to decide whether and how to receive the second dose. Allergists are well versed in applying risk-benefit assessments in relative data and evidence free zones by using shared decision models to achieve safe and effective outcomes. The potential life-saving benefit of vaccination in the setting of a global pandemic makes it essential that we carefully evaluate every patient with a possible allergic reaction to prevent denying access to the vaccine unnecessarily. Our knowledge is rapidly evolving and future recommendations may change with additional data. Our intent is to provide updates on a regular basis as more information becomes available.
Acknowledgments
We thank the Mass General Brigham health system faculty and staff, including Dean Hashimoto, MD, Tanya Laidlaw, MD, David Hong, MD, Anna Feldweg, MD, Karen Ferreira, Kenisha Lewis, Barbara Schmidt, Nahal Beik, Adam Landman, MD, Erica S. Shenoy, MD, PhD, and Rajesh Patel, MD, MPH. We thank Upeka Samarakoon, PhD, MPH, Allen Judd, Christian Mancini, Amelia Cogan, and Aubree McMahon for their research assistance.
Footnotes
No funding was received for this work.
Conflicts of interest: E. Phillips reports grants from the National Institutes of Health (grant nos. P50GM115305, R01HG010863, R01AI152183, R21AI139021, and U01AI154659) and from the National Health and Medical Research Council of Australia; receives Royalties from Uptodate and consulting fees from Janssen, Vertex, Regeneron, and Biocryst; is codirector of IIID Pty Ltd, which holds a patent for HLA-B∗57:01 testing for abacavir hypersensitivity, and has a patent pending for Detection of Human Leukocyte Antigen-A∗32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. Funders played no role in any aspect of this review. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Adeline S., Jin C.H., Hurt A., Wilburn T., Wood D., Talbot R. Coronavirus by the numbers: coronavirus is surging: how severe is your state’s outbreak? National Public Radio. December 24, 2020. https://www.npr.org/sections/health-shots/2020/09/01/816707182/map-tracking-the-spread-of-the-coronavirus-in-the-u-s Available from:
- 2.Centers for Disease Control and Prevention COVID-19 Response Team Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14 – 23, 2020. MMWR. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States—Appendix B. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#Appendix-B Available from:
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Moderna COVID-19 vaccine [FDA briefing document]. Silver Spring, MD: U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee; 2020. https://www.fda.gov/media/144434/download Available from:
- 6.U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine (BNT162, PF-07302048) [FDA briefing document]. Silver Spring, MD: U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee; 2020. https://www.fda.gov/media/144246/download Available from:
- 7.McNeil M.M., Weintraub E.S., Duffy J., Sukumaran L., Jacobsen S.J., Klein N.P., et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone C.A., Jr., Rukasin C.R.F., Beachkofsky T.M., Phillips E.J. Immune-mediated adverse reactions to vaccines. Br J Clin Pharmacol. 2019;85:2694–2706. doi: 10.1111/bcp.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakayama T., Aizawa C. Change in gelatin content of vaccines associated with reduction in reports of allergic reactions. J Allergy Clin Immunol. 2000;106:591–592. doi: 10.1067/mai.2000.108433. [DOI] [PubMed] [Google Scholar]
- 10.Andersen D.V., Jørgensen I.M. MMR vaccination of children with egg allergy is safe. Dan Med J. 2013;60:A4573. [PubMed] [Google Scholar]
- 11.Stone C.A., Jr., Liu Y., Relling M.V., Krantz M.S., Pratt A.L., Abreo A., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7:1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute for Vaccine Safety Excipients in vaccines per 0.5 mL dose. https://vaccinesafety.edu/components-excipients.htm Available from:
- 13.Krantz M.S., Liu Y., Phillips E.J., Stone C.A., Jr. Anaphylaxis to PEGylated liposomal echocardiogram contrast in a patient with IgE-mediated macrogol allergy. J Allergy Clin Immunol Pract. 2020;8:1416–1419.e3. doi: 10.1016/j.jaip.2019.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. National Library of Medicine DailyMed Advanced Search. https://dailymed.nlm.nih.gov/dailymed/advanced-search.cfm Available from:
- 15.Yang Q., Lai S.K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Z.H., Stone C.A., Jr., Jakubovic B., Phillips E.J., Sussman G., Park J., et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021;9:1718–1720. doi: 10.1016/j.jaip.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 18.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9:670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Pidaparti M., Bostrom B. Comparison of allergic reactions to pegasparaginase given intravenously versus intramuscularly. Pediatr Blood Cancer. 2012;59:436–439. doi: 10.1002/pbc.23380. [DOI] [PubMed] [Google Scholar]
- 20.Hasan H., Shaikh O.M., Rassekh S.R., Howard A.F., Goddard K. Comparison of hypersensitivity rates to intravenous and intramuscular PEG-asparaginase in children with acute lymphoblastic leukemia: A meta-analysis and systematic review. Pediatr Blood Cancer. 2017;64:81–88. doi: 10.1002/pbc.26200. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.H., Hwang S.H., Park J.S., Park H.S., Shin Y.S. Anaphylaxis to polyethylene glycol (Colyte®) in a patient with diverticulitis. J Korean Med Sci. 2016;31:1662–1663. doi: 10.3346/jkms.2016.31.10.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coors E.A., Seybold H., Merk H.F., Mahler V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol. 2005;95:593–599. doi: 10.1016/S1081-1206(10)61024-1. [DOI] [PubMed] [Google Scholar]
- 23.Hawe A., Filipe V., Jiskoot W. Fluorescent molecular rotors as dyes to characterize polysorbate-containing IgG formulations. Pharm Res. 2010;27:314–326. doi: 10.1007/s11095-009-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price K.S., Hamilton R.G. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. 2007;28:313–319. doi: 10.2500/aap.2007.28.3003. [DOI] [PubMed] [Google Scholar]
- 25.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. bcr0220125797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steele R.H., Limaye S., Cleland B., Chow J., Suranyi M.G. Hypersensitivity reactions to the polysorbate contained in recombinant erythropoietin and darbepoietin. Nephrology (Carlton) 2005;10:317–320. doi: 10.1111/j.1440-1797.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- 27.Limaye S., Steele R.H., Quin J., Cleland B. An allergic reaction to erythropoietin secondary to polysorbate hypersensitivity. J Allergy Clin Immunol. 2002;110:530. doi: 10.1067/mai.2002.126460. [DOI] [PubMed] [Google Scholar]
- 28.Bibera M.A.T., Lo K.M.K., Steele A. Potential cross-reactivity of polysorbate 80 and cremophor: a case report. J Oncol Pharm Pract. 2020;26:1279–1281. doi: 10.1177/1078155219896848. [DOI] [PubMed] [Google Scholar]
- 29.Palacios Castaño M.I., Venturini Díaz M., Lobera Labairu T., González Mahave I., Del Pozo Gil M.D., Blasco Sarramián A. Anaphylaxis due to the excipient polysorbate 80. J Investig Allergol Clin Immunol. 2016;26:394–396. doi: 10.18176/jiaci.0109. [DOI] [PubMed] [Google Scholar]
- 30.Cabanillas B., Akdis C., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? [published online ahead of print December 15, 2020]. Allergy. [DOI] [PubMed]
- 31.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tannenbaum H., Ruddy S., Schur P.H. Acute anaphylaxis associated with serum complement depletion. J Allergy Clin Immunol. 1975;56:226–234. doi: 10.1016/0091-6749(75)90094-9. [DOI] [PubMed] [Google Scholar]
- 33.Szebeni J., Fontana J.L., Wassef N.M., Mongan P.D., Morse D.S., Dobbins D.E., et al. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: a model for pseudoallergic cardiopulmonary reactions to liposomes: role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation. 1999;99:2302–2309. doi: 10.1161/01.cir.99.17.2302. [DOI] [PubMed] [Google Scholar]
- 34.de Vrieze J. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science Magazine. December 21, 2020. https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions Available from:
- 35.Neun B.W., Barenholz Y., Szebeni J., Dobrovolskaia M.A. Understanding the role of anti-PEG antibodies in the complement activation by doxil in vitro. Molecules. 2018;23:1700. doi: 10.3390/molecules23071700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Prevaccination checklist for COVID-19 vaccines. https://www.cdc.gov/vaccines/covid-19/downloads/pre-vaccination-screening-form.pdf Available from: Accessed January 9, 2021. [PubMed]
- 37.Broyles A.D., Banerji A., Barmettler S., Biggs C.M., Blumenthal K., Brennan P.J., et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. 2020;8:S16–S116. doi: 10.1016/j.jaip.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention, U.S. Food and Drug Administration, U.S. Department of Health and Human Services. Vaccine Adverse Event Reporting System (VAERS). Washington, DC: United States Department of Health and Human Services. https://vaers.hhs.gov Available from:
- 40.Centers for Disease Control and Prevention. V-safe after vaccination health checker. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html Available from: Accessed January 9, 2021.
- 41.Centers for Disease Control and Prevention. Interim considerations: preparing for the potential management of anaphylaxis after COVID-19 vaccination. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/managing-anaphylaxis.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fpfizer%2Fanaphylaxis-management.html Available from: Accessed January 9, 2021.
- 42.Shaker M.S., Wallace D.V., Golden D.B.K., Oppenheimer J., Bernstein J.A., Campbell R.L., et al. Anaphylaxis—a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020;145 doi: 10.1016/j.jaci.2020.01.017. 1082-123. [DOI] [PubMed] [Google Scholar]
- 43.Winnipeg Regional Health Authority Anaphylaxis vs vasovagal reactions. https://professionals.wrha.mb.ca/old/professionals/immunization/files/AnaphyvsVasReactionTable.pdf Available from:
- 44.Manivannan V., Decker W.W., Stead L.G., Li J.T.C., Campbell R.L. Visual representation of National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network criteria for anaphylaxis. Int J Emerg Med. 2009;2:3–5. doi: 10.1007/s12245-009-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]