Abstract
Our objective was to estimate the US national cost burden of hospital‐acquired pressure injury (HAPI) using economic simulation methods. We created a Markov simulation to estimate costs for staged pressure injuries acquired during hospitalisation from the hospital perspective. The model analysed outcomes of hospitalised adults with acute illness in 1‐day cycles until all patients were terminated at the point of discharge or death. Simulations that developed a staged pressure injury after 4 days could advance from Stages 1 to 4 and accrue additional costs for Stages 3 and 4. We measured costs in 2016 US dollars representing the total cost of acute care attributable to HAPI incidence at the patient level and for the entire United States based on the previously reported epidemiology of pressure injury. US HAPI costs could exceed $26.8 billion. About 59% of these costs are disproportionately attributable to a small rate of Stages 3 and 4 full‐thickness wounds, which occupy clinician time and hospital resources. HAPIs remain a concern with regard to hospital quality in addition to being a major source of economic burden on the US health care system. Hospitals should invest more in quality improvement of early detection and care for pressure injury to avoid higher costs.
Keywords: cost, economic evaluation, medicare, pressure injury, pressure ulcer
1. INTRODUCTION
Although most hospital‐acquired pressure injuries (HAPIs) are reasonably preventable,1, 2, 3, 4 approximately 2.5 million individuals in the United States develop a pressure injury in acute care facilities every year.5 Many of those who develop pressure injuries during their acute care episode are elderly, malnourished, and have remained hospitalised for longer periods of time.6 These pressure injuries can result in extensive harm, including chronic wounds, and as many as 60 000 deaths annually.7 In contrast, about 63 600 deaths were related to drug overdose in 2016,8 44 000 people committed suicide in 2015,9 and nearly 56 000 died of influenza between 2015 and 2016.10 Yet despite accounting for similar, if not greater, numbers of deaths, pressure injuries have received much less attention as a public health crisis.
HAPI care represents a substantial financial burden on health care systems. Previous estimates of the national cost of treating HAPIs ranged from $3.3 billion11 to $11 billion12 annually. In comparison, recent claims by Medicare beneficiaries showed that chronic pressure injury care accounted about $22 billion.13 As the US Centers for Medicare and Medicaid Services (CMS) reduced reimbursement related to hospital‐acquired conditions including HAPIs, hospitals have faced the full financial burden of these harms12, 14; a single HAPI episode could cost hospitals anywhere from $500 to more than $70 000.15
Several studies have provided estimates of patient‐level costs attributable to HAPIs, yet none have recently quantified the annual national cost of HAPI treatment in hospitals. Doing so presents a challenge as HAPIs are not reliably coded in billing claims, leaving the epidemiology and economics of HAPIs incomplete. In 2010, the Society of Actuaries reported the national cost of medical errors but aggregated both the inpatient and post‐discharge costs associated with HAPIs.16 Several economic evaluations estimated the value of new modalities, technologies, and nursing care to prevent HAPIs from the hospital perspective.4, 17, 18 These latter studies were performed primarily using simulation modelling methods in order to overcome limitations presented in hospital claims. Therefore, the objective of this analysis was to provide a complete estimate of HAPI costs to US hospitals using simulation methods combined with best available data from existing literature.
2. METHODS
2.1. Study design
We used a Markov model to simulate the daily accumulation of costs attributed to treating patients with HAPIs based on state transitions between different HAPI stages and death within acute care.17 The model represented the hospital perspective as it only considered costs delivered during inpatient care. The model was built using TreeAge Pro Healthcare (TreeAge Software, Williamstown, MA).19 Patients were recruited to the model at hospital admission and either remained in treatment for the index diagnosis, exited through death or discharge, or developed a HAPI post‐admission. Furthermore, a patient developing a HAPI experienced continuous progression of Stages 1, 2, 3, and 4; remained in their current pressure injury stage; or exited because of death or discharge.20, 21 Patients cycled until reaching a terminal node. Costs accumulated in 1‐day cycles during this time span.
The probabilistic progression of HAPIs was modelled based on information available from the National Pressure Ulcer Advisory Panel (NPUAP) staging guidelines.22 Because of limited data on progression between Stages 3, 4, and unstageable, and to account for the substantial differences in cost because of the intensity of intervention (eg, debridement, excision, wound healing, radiology, associated operative and laboratory costs), these stages were collapsed into two groups: Stage 3/4 patients without significant additional costs (“Stage 3/4”) and Stage 3/4 patients who needed substantially more resources (“enhanced Stage 3/4”). In terms of progressions, “enhanced Stage 3/4” followed “Stage 3/4” (Figure 1).
Figure 1.
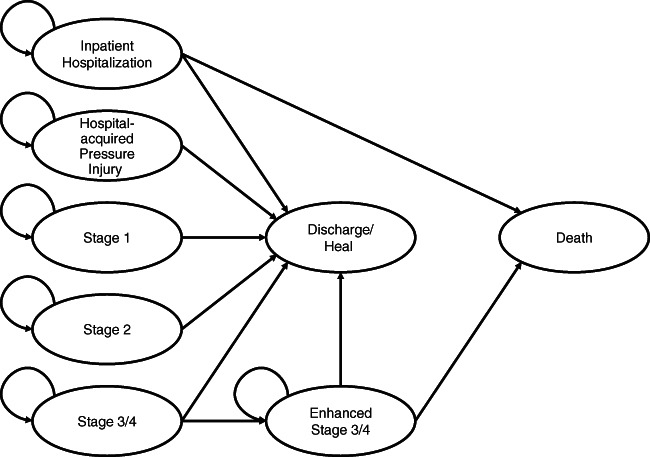
Markov model of transitioning health states between hospitalisation, discharge, death, or a staged pressure injury. Pressure injury staging begins with early Stage 1 symptoms and can then advance to ulcerated and full‐thickness wound stages (ie, Stages 2, 3, or 4). Some Stage 3 and 4 wounds may require extensive care in addition to standard nursing and monitoring
2.2. Transition probabilities and tunnel states
Transition probabilities were obtained from available literature. Probabilities were informed by a recent clinical trial on the effect of nursing quality improvement for the incidence of pressure injuries.23, 24 These trial results allowed for a calculation of HAPI cumulative incidence as well as the probability of remaining or advancing between HAPI stages (Table 1).
Table 1.
Model parameters
| 1a. Transition probabilities | Expected value | Range for sensitivity analysis | Source |
|---|---|---|---|
| Index hospitalisation to pressure injury | 0.045 | 0.038‐0.52 | 28 |
| Stage I to I | 0.12 | 0.088‐0.119 | 24 |
| Stage I to Stage II | 0.26 | 0.298‐0.403 | 24 |
| Stage I to heal | 0.54 | 0.46‐0.627 | 24 |
| Stage II to Stage II | 0.36 | 0.361‐0.489 | 24 |
| Stage II to Stage III/IV | 0.22 | 0.075‐0.1 | 24 |
| Stage II to heal | 0.42 | 0.414‐0.56 | 24 |
| Stage III/IV to heal | 0.8 | 0.829‐1.121 | 24 |
| Stage III/IV to repeat surgery | 0.2 | 0.17‐0.23 | 17 |
| Index discharge | 0.5 | 0.425‐0.575 | Assumed |
| Repeat debridement to heal | 0.25 | 0.21‐0.29 | 17 |
| Repeat debridement to repeat debridement | 0.4 | 0.31‐0.419 | 17 |
| Repeat debridement to death | 0.35 | 0.3‐0.4 | 17 |
| 1b. Daily costs for staged pressure injuries | Total cost ($) | Source | |
| Stage 1 | |||
| Basic pressure injury care | 334 | 17 | |
| Unforeseen costs (25%) | 84 | Assumed | |
| Base case—Stage 1 | 418 | ||
| Stage 2 | |||
| Days 1‐2 | |||
| Basic pressure injury care | 334 | 17 | |
| Unforeseen costs (25%) | 84 | Assumed | |
| Base case—Stage 2, Days 1‐2 | 418 | ||
| Days 3‐4 | |||
| Hospital accommodation | 2271 | 25 | |
| Basic pressure injury care | 334 | 17 | |
| Unforeseen costs (25%) | 651 | Assumed | |
| Base case—Stage 2, Days 3‐4 | 3256 | ||
| Stage 3/4 | |||
| Day 1 | |||
| Hospital accommodation | 2271 | 25 | |
| Basic pressure injury care | 334 | 17 | |
| Debridement | 63 | 31 | |
| Operative management | 1687 | 31 | |
| Wound dressing | 16 | 12 | |
| Unforeseen costs (25%) | 1093 | Assumed | |
| Base case—Stage 3/4, Day 1 | 5464 | ||
| Days 2‐6 | |||
| Hospital accommodation | 2271 | 25 | |
| Basic pressure injury care | 334 | 17 | |
| Wound dressing | 16 | 12 | |
| Unforeseen costs (25%) | 655 | Assumed | |
| Base case—Stage 3/4, Days 2‐6 | 3277 | ||
| 1c. Daily costs for Stages 3 and 4 HAPIs | Total cost ($) | Source | |
| Day 1 | 2271 | ||
| Hospital accommodation | 334 | 25 | |
| Basic pressure injury care | 1687 | 17 | |
| Operative management | 1528 | 31 | |
| Wound closure | 16 | 31 | |
| Wound dressing | 180 | 12 | |
| Laboratory | 4529 | 12 | |
| Radiology | 7180 | 12 | |
| Operating room fees | 130 | 12 | |
| Pathology | 11 592 | 12 | |
| Ancillary services | 1372 | 12 | |
| Consultation | 7705 | 12 | |
| Unforeseen costs (25%) | 38 526 | Assumed | |
| Base case—Day 1 | |||
| Days 2‐14 | |||
| Hospital accommodation | 2271 | 25 | |
| Basic pressure injury care | 334 | 17 | |
| Wound dressing | 16 | 12 | |
| Laboratory | 180 | 12 | |
| Pathology | 130 | 12 | |
| Unforeseen costs (25%) | 1093 | Assumed | |
| Base case—Days 2‐14 | 5464 | ||
Abbreviation: HAPI, hospital‐acquired pressure injury.
The transition probability from index hospitalisation to discharge was drawn from an exponential distribution function with a minimum of 0.50 probability of discharge, beginning on Day 4. This discharge transition reflected the total accumulated cost of an index hospitalisation without a pressure injury given a national mean length of stay (LOS) of 4.5 days.25 Once a HAPI developed, transition probabilities for remaining in the same stage, progressing to other stages, or termination were drawn from Dirichlet distributions using gross number input parameters from the literature. Patients could not regress in HAPI stages.
2.3. Costs
Patient's LOS factored into the calculation of inpatient costs. Patients with HAPIs tended to have higher acuity than average, making it difficult to attribute crude differences in LOS and gross costs to the presence of pressure injuries alone.16 Previous research showed that HAPIs led to increased mean LOS by 10.8 days.26 Other studies showed an average LOS between 2.1 and 2.6 days attributable to HAPIs.27, 28, 29 However, none of these studies stratified excess LOS by stage or by duration of pressure injury treatment. To calculate the excess cost resulting solely from increased LOS because of HAPIs, we assumed that Stage 1 would be treated for 2 days with no excess LOS costs (Table 1); Stage 2 would be treated for 4 days with 2 days excess LOS costs; Stage 3/4 would be treated for 7 days with 7 days excess LOS costs; and patients requiring additional care for Stage 3+ pressure injuries (eg, surgery) would be treated for 14 days with 14 days excess LOS costs (Table 1). Tunnel states, in which patients remained within a health state for a specified number of cycles before transitioning, were applied to reflect the assumed duration that patients remained in a particular stage. In addition to costs attributable to excess LOS from HAPI, we included direct costs of HAPI care, including materials, procedures, and staffing, according to NPUAP guidelines.2, 30 Procedural costs (eg, debridement) or other services were included in only one cycle of a tunnel state to avoid double‐counting costs.
National costs were obtained by multiplying the average HAPI costs by the annual number of cases, estimated to be 2.5 million cases per year.31 All costs were inflation‐adjusted to 2016 USD. While the costs of deep tissue injuries (DTIs) were not explicitly represented in the costing model, DTIs are common precursors to Stages 3 and 4. The cost of DTI is implicitly modelled into the cost of Stage 2 injuries that represent the cost preceding the escalation of Stages 3 and 4.
2.4. Sensitivity analyses
Univariate sensitivity analyses were conducted by varying the cost parameters by ±15%. Two‐way sensitivity analyses were used to estimate the cost‐savings thresholds of increasing the cost of treating Stage 1 and Stage 2 HAPIs to reduce the probability of advancing beyond the respective stages by 50%. Probabilistic sensitivity analysis was conducted using 10 000 Monte Carlo simulations.
3. RESULTS
3.1. Incidence of HAPI
The cumulative incidence of HAPI among simulations was 8.3 HAPIs per 100 acutely ill patients. Among patients with a HAPI, 61.5% only had a Stage 1 injury, 25.2% progressed to Stage 2, 10.5% progressed to Stages 3 or 4, and 2.7% required enhanced Stage 3/4 care (eg, debridement, excision, and advanced wound care). Mortality was estimated to be 1.5% among all simulations. On average, patients with a HAPI had 2.2 days excess LOS in comparison with all hospitalised patients (6.7 days for patients with a HAPI against 4.5 days for all patients).
3.2. Expected cost
The average expected cost of an inpatient hospitalisation for all simulated patients was $11 887, with $867 (7.3%) being attributable to HAPIs (Table 2). The average cost of HAPI care was $10 708. This represented the incremental cost incurred because of excess LOS attributable only to HAPIs. In particular, only 8% ($893) of the incremental cost accumulated in Stage 1, 33% ($3560) accumulated in Stage 2, 31% ($2995) accumulated in uncomplicated Stage 3/4, and 28% ($3260) accumulated in more complicated Stage 3/4 injuries. Based on the distribution of HAPIs by stage in the United States, these costs could reflect a national cost burden of $26.8 billion.
Table 2.
Proportion of costs accumulated by HAPI stage
| A. All patients/hospitalisations | |||
|---|---|---|---|
| Maximum stage | Proportion of patients (%)a | Accumulated costs within stage ($)b | Percentage of incremental cost (%)c |
| No pressure injury | 91.7 | 10 980 | 92.69 |
| Stage 1 | 5.2 | 74 | 0.62 |
| Stage 2 | 2.1 | 293 | 2.47 |
| Stage 3/4 | 0.9 | 247 | 2.09 |
| Stage 3/4 (enhanced care) | 0.1 | 253 | 2.13 |
| Total | 100 | 11 847 | 100 |
| B. Patients with a pressure injury | |||
| Maximum stage | Proportion of patients (%) | Accumulated costs within stage ($) | Percentage of incremental cost (%) |
| Stage 1 | 61.5 | 893 | 8.32 |
| Stage 2 | 25.2 | 3560 | 33.25 |
| Stage 3/4 | 10.6 | 2995 | 27.97 |
| Stage 3/4 (enhanced care) | 2.7 | 3260 | 30.45 |
| Total | 100 | 10 708 | 100 |
Abbreviation: HAPI, hospital‐acquired pressure injury.
Indicates the proportion of simulated patients who exited at a given state because of discharge or death. For example, 91.7% of all patients did not have a HAPI, while 8.3% had at least a Stage 1 HAPI.
The average total costs accumulated within a stage.
The percentage of the total incremental cost accumulated because of a given state.
3.3. Sensitivity analyses
Results did not change significantly based on one‐way sensitivity analyses. Reducing transition probabilities between stages by 50% decreased the estimated incremental costs. Two‐way threshold analyses demonstrated that it was cost‐saving to pay up to five times the average cost of Stage I treatment to reduce the probability of transitioning from Stage I to Stage II by 50%, and spending 1.8 times the amount of Stage 2 care was cost‐saving if it led to a 50% reduction in advancing to Stage 3. Probabilistic Monte Carlo simulations produced a mean of $11 863 (95% confidence interval [CI]: $11 704‐$12 021) for inpatient hospitalisations and $11 007 (95% CI: $10 485‐$11 529) for incremental costs because of HAPIs.
4. DISCUSSION
Our analysis suggests that a HAPI could cost $10 708 per patient on average, exceeding a total of approximately $26.8 billion in the United States annually based on 2.5 million reported cases. This analysis also highlights that Stage 3/4 HAPIs accounted for 58% of all HAPI costs despite being a rare outcome. Based on the CMS policy of reduced reimbursements for HAPIs, this tremendous potential financial burden should provide an impetus to hospitals to strengthen their harm reduction strategies aimed at preventing the development of HAPIs.
Previous studies dating back over a decade estimated the annual cost of HAPIs at $11 billion.16, 28 These new results suggest that the true national cost of HAPI care to hospitals has grown to $10 billion, likely as a function of quantity and price. On the one hand, wound care is more costly as the field has introduced more expensive, life‐saving technology to grapple with the complexity of pressure injuries. On the other hand, the United States is experiencing a spike in HAPI rates that counters the purpose of CMS reimbursements – one study reports nearly a 30% increase in pressure injuries in academic medical centres between 2015 and 2017.29 These dynamics could result in the total cost increase exhibited by this simulation. Even so, these costs represents a lower‐bound estimate of the societal impact of HAPI, which also includes loss in labour productivity to the patient and uncompensated informal care provided by family caregivers.
The solution to a rapidly growing concern is complex. The 2008 CMS non‐payment model for hospital‐acquired conditions appear to work at reducing HAPI rates in the short term. The growing rates and costs of HAPIs in the late 2010s could be linked with a lack of incentives to invest solely in HAPI prevention when other hospital‐acquired conditions under the PSI‐90 payment penalty model are easier to prevent. If penalties are not working, then CMS and other payers should consider equal‐sided risk models. That is, by penalising hospitals that perform poorly with respect to HAPIs, CMS could generate revenue to reward exemplary performers of HAPI prevention. A “carrot and stick” approach would motivate hospitals performing poorly to achieve better outcomes to gain financial rewards.
This study has several limitations. First, the transition probabilities may not represent current US practice standards and population trends as these were obtained from a randomized controlled trial (RCT) conducted in Australia; we assume that the treatment effect of HAPI care is consistent between the United States and Australia. Second, the cost parameters were derived from older literature and might not reflect current trends in the cost of actual clinical practice, especially for the most severe HAPIs. New NPUAP guidelines on pressure injury treatment have been released recently that may have influenced the treatment of HAPIs.15 Third, well‐characterised and validated data on the overall duration of HAPI treatment and the associated excess LOS were not available; expert opinion guided the choices for tunnel states and hospital accommodation costs. Fourth, data on the variation between the costs associated with the treatment of Stages 3 and 4 HAPIs are limited. Thus, we assumed dichotomy between Stage 3/4 patients who are low‐cost or require more extensive, high‐cost care over a longer timeframe. Finally, DTIs and unstageable cases were not included in the model because of a scarcity of data on these categories. We assumed that these stages were subsumed by the model.
While this analysis relied on limited data and several assumptions about the cost and epidemiological structure of HAPI disease progression, the ancillary results support the reliability of the estimated total incremental costs of HAPIs. In particular, the results from our simulation of excess LOS are consistent with previous empirical estimates.27, 28, 30 Furthermore, the cumulative HAPI incidence of 8.3 per 100 patients and the incidence across stages were also consistent with prior research.31, 32, 33
In conclusion, incremental costs to hospitals regarding treating HAPIs could be about $10 708 per patient. These costs amount to $26.8 billion in the United States, most of which is represented by the extensive cost of treating Stages 3 and 4 HAPIs. Decreasing the probability of HAPI progression across stages has been demonstrated to have the greatest effect on lowering costs. Prevention efforts and early interventions may be the most cost‐effective for hospitals.34
ACKNOWLEDGEMENTS
We thank Matthew Lucci, MPH, for his help in conducting this analysis early in its conceptualisation.
Padula WV, Delarmente BA. The national cost of hospital‐acquired pressure injuries in the United States. Int Wound J. 2019;16:634–640. 10.1111/iwj.13071
REFERENCES
- 1. Duncan KD. Preventing pressure ulcers: the goal is zero. Jt Comm J Qual Patient Saf. 2007;33(10):605‐610. [DOI] [PubMed] [Google Scholar]
- 2. Berlowitz D, VanDeusen LC, Parker V, et al. Preventing Pressure Ulcers in Hospitals: A Toolkit for Improving Quality of Care. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 3. Edsberg LE, Langemo D, Baharestani MM, Posthauer ME, Goldberg M. Unavoidable pressure injury: state of the science and consensus outcomes. J Wound Ostomy Continence Nurs. 2014;41(4):313‐334. [DOI] [PubMed] [Google Scholar]
- 4. Pham B, Stern A, Chen W, et al. Preventing pressure ulcers in long‐term care: a cost‐effectiveness analysis. Arch Intern Med. 2011;171(20):1839‐1847. [DOI] [PubMed] [Google Scholar]
- 5. Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Treatment of pressure ulcers: a systematic review. JAMA. 2008;300(22):2647‐2662. [DOI] [PubMed] [Google Scholar]
- 6. VanGilder C, Lachenbruch C, Algrim‐Boyle C, Meyer S. The international pressure ulcer prevalence survey: 2006‐2015: a 10‐year pressure injury prevalence and demographic trend analysis by care setting. J Wound Ostomy Continence Nurs. 2017;44(1):20‐28. [DOI] [PubMed] [Google Scholar]
- 7. Bauer K, Rock K, Nazzal M, Jones O, Qu W. Pressure ulcers in the United States' inpatient population from 2008 to 2012: results of a retrospective nationwide study. Ostomy Wound Manage. 2016;62(11):30‐38. [PubMed] [Google Scholar]
- 8. Hedegaard H, Warner M, Miniño AM. Drug Overdose Deaths in the United States, 199–2016. NHCS Data Brief. 2017;294:1‐8. [PubMed] [Google Scholar]
- 9. US Centers for Disease Control and Prevention . National violent death reporting system. https://www.cdc.gov/violenceprevention/nvdrs/index.html. 2017. Accessed February 22, 2018.
- 10. US Centers for Disease Control and Prevention . Estimating seasonal influenza‐associated deaths in the United States. https://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm. 2018. Accessed February 22, 2018.
- 11. Van Den Bos J, Rustagi K, Gray T, et al. The $17.1 billion problem: the annual cost of measurable medical errors. Health Aff (Millwood). 2011;30(4):596‐603. [DOI] [PubMed] [Google Scholar]
- 12. Brem H, Maggi J, Nierman D, et al. High cost of stage IV pressure ulcers. Am J Surg. 2010;200(4):473‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Medicare and Medicaid Services . Eliminating serious, preventable, and costly medical errors ‐ never events. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-Sheets/2006-Fact-Sheets-Items/2006-05-18.html. 2006. Accessed February 22, 2018.
- 15. Haesler E. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Cambridge, UK: Cambridge Media; 2014. [DOI] [PubMed] [Google Scholar]
- 16. Shreve J, Van Den Bos J, Gray T, et al. The Economic Measurement of Medical Errors sponsored by the society of actuaries' health section. Seattle, WA: Milliman Inc; 2010. [Google Scholar]
- 17. Padula WV, Mishra MK, Makic MB, Sullivan PW. Improving the quality of pressure ulcer care with prevention: a cost‐effectiveness analysis. Med Care. 2011;49(4):385‐392. [DOI] [PubMed] [Google Scholar]
- 18. Trueman P, Whitehead SJ. The economics of pressure relieving surfaces: an illustrative case study of the impact of high‐specification surfaces on hospital finances. Int Wound J. 2010;7(1):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. TreeAge Software . TreeAge Pro Healthcare 2018 user's manual. Williamstown, MA: TreeAge Software Inc.; 2018. [Google Scholar]
- 20. Lyder CH. Pressure ulcer prevention and management. JAMA. 2003;289(2):223‐226. [DOI] [PubMed] [Google Scholar]
- 21. Briggs A, Sculpher M. An introduction to markov modelling for economic evaluation. Pharmacoeconomics. 1998;13(4):397‐409. [DOI] [PubMed] [Google Scholar]
- 22. Edsberg LE, Black JM, Goldberg M, et al. Revised national pressure ulcer advisory panel pressure injury staging system: revised pressure injury staging system. J Wound Ostomy Continence Nurs. 2016;43(6):585‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chaboyer W, Bucknall T, Webster J, et al. The effect of a patient centred care bundle intervention on pressure ulcer incidence (INTACT): a cluster randomised trial. Int J Nurs Stud. 2016;64:63‐71. [DOI] [PubMed] [Google Scholar]
- 24. Webster J, Bucknall T, Wallis M, et al. Does participating in a clinical trial affect subsequent nursing management? Post‐trial care for participants recruited to the INTACT pressure ulcer prevention trial: a follow‐up study. Int J Nurs Stud. 2017;71:34‐38. [DOI] [PubMed] [Google Scholar]
- 25. Kaiser Family Foundation . Hospital adjusted expenses per inpatient day. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. 2017. Accessed February 22, 2018.
- 26. Scott JR, Gibran NS, Engrav LH, Mack CD, Rivara FP. Incidence and characteristics of hospitalized patients with pressure ulcers: state of Washington, 1987 to 2000. Plast Reconstr Surg. 2006;117(2):630‐634. [DOI] [PubMed] [Google Scholar]
- 27. Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol. 2005;26(3):293‐297. [DOI] [PubMed] [Google Scholar]
- 28. Lyder CH, Wang Y, Metersky M, et al. Hospital‐acquired pressure ulcers: results from the national medicare patient safety monitoring system study. J Am Geriatr Soc. 2012;60(9):1603‐1608. [DOI] [PubMed] [Google Scholar]
- 29. Padula WV, Black JM, Davidson PM, Kang SY, Pronovost PJ. Adverse effects of the Medicare PSI‐90 hospital penalty system on revenue‐neutral hospital‐acquired conditions. J Patient Saf. 2018; 10.1097/PTS.0000000000000517. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30. Theisen S, Drabik A, Stock S. Pressure ulcers in older hospitalised patients and its impact on length of stay: a retrospective observational study. J Clin Nurs. 2012;21(3–4):380‐387. 10.1111/j.1365-2702.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 31. Mallah Z, Nassar N, Kurdahi BL. The effectiveness of a pressure ulcer intervention program on the prevalence of hospital acquired pressure ulcers: controlled before and after study. Appl Nurs Res. 2015;28(2):106‐113. [DOI] [PubMed] [Google Scholar]
- 32. Santamaria N, Liu W, Gerdtz M, et al. The cost‐benefit of using soft silicone multilayered foam dressings to prevent sacral and heel pressure ulcers in trauma and critically ill patients: a within‐trial analysis of the border trial. Int Wound J. 2015;12(3):344‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swafford K, Culpepper R, Dunn C. Use of a comprehensive program to reduce the incidence of hospital‐acquired pressure ulcers in an intensive care unit. Am J Crit Care. 2016;25(2):152‐155. [DOI] [PubMed] [Google Scholar]
- 34. Padula WV, Pronovost PJ, Makic MBF, et al. Value of hospital resources for effective pressure injury prevention: a cost‐effectiveness analysis. BMJ Qual Saf. 2018. 10.1136/bmjqs-2017-007505. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


