Abstract
Pyoderma gangrenosum is a rare, neutrophil‐mediated, auto‐inflammatory dermatosis that wound care specialists must be prepared to recognise. This condition has clinical features analogous to infectious processes. There is no specific diagnostic test and the diagnosis is usually obtained from exclusion. Its early recognition and proper management with prompt initiation of immunosuppressive therapy are essential to improve the quality of life and the prognosis of patients.
Keywords: chronic ulcers, neutrophilic disease treatment, pain, pyoderma gangrenosum, wound care
1. INTRODUCTION
Pyoderma gangrenosum (PG) is a rare, chronic, rapidly evolving cutaneous ulcers, of unknown aetiology, diagnosed by exclusion of infection, neoplasm, thrombophilia, and other inflammatory conditions. 1 , 2 , 3 , 4 Is part of neutrophil‐mediated skin diseases, originally named neutrophilic dermatosis (NDs), where there is abundance of neutrophils in the absence of infection. 3 , 5
The typical clinical manifestations are single or multiple skin ulcers with undermined erythematous‐violaceous borders, accompanied by disproportionate pain, 6 loss of quality of life 7 and frequently associated with systemic conditions. 1 , 8 The diagnosis is based on symptomatology and compatible histopathological findings. 6 Patients need treatment with immunosuppressive drugs.
2. EPIDEMIOLOGY
PG is a rare inflammatory skin disease, with an estimated incidence of 3 to 10 cases per million per year. 3 , 9 It affects individuals of all ages, with an average of onset at 59 years old, 10 affecting predominantly female (68%), and Caucasian patients (78.5%). 2 , 9 , 11 Approximately 50% of patients have associated medical condition, as inflammatory bowel diseases (IBD), haematological malignancies (IgA monoclonal gammopathies, acute myelogenous leukaemia, myelodysplasia), solid malignancies and rheumatological disorders (Sjögren's disease, ankylosing spondylitis, lupus erythematosus). 3 , 8 , 9 , 11 , 12
Patients with PG who have an underlying hematologic cancer, dyscrasia, and vasculitis have worse hospital outcomes than do patients with inflammatory bowel disease or inflammatory arthritis. 13
3. PATHOLOGY
The pathogenesis of PG is multifactorial and involves neutrophilic dysfunction, inflammatory mediators, and genetic predisposition. 3 It belongs to the group of neutrophil‐mediated skin diseases, in which there is an altered neutrophil recruitment and activation in the skin, and is the hallmark of a heterogeneous group of polymorphic cutaneous manifestations. 8 , 14
There is an important role of upregulation of pro‐inflammatory cytokines and neutrophil chemotactic factors, that lead to a neutrophilic recruitment and activation. 2 , 8 There is also an increase in matrix metalloproteinase (MMP) expression, in particular MMP 9 and 10, that contribute to poor healing. 10
Genetics have been proven to play a role in described PG‐associated syndromes, with mutations in the PSTP1P1/CD2BP1 gene: PAPA syndrome (pyogenic sterile arthritis, PG, and cystic acne) PASH syndrome (PG with cystic acne and hidradenitis suppurativa) and PAPASH (PG with pyogenic arthritis, acne, and hidradenitis suppurativa) and autoinflammatory predisposition. 3 , 10 , 15 , 16
Increasing evidence suggests that oestrogen has pleomorphic effects on the immune system. 17 Oestrogen receptors are expressed on T cells, B cells, dendritic cells, neutrophils, macrophages, and NK cells. 11 , 18
4. CLINICAL FEATURES
There are five clinical subtypes of PG (Figures 1, 2, 3, 4, 5), which are summarised in Table 1. 2 , 8 , 9 , 10 , 19
FIGURE 1.
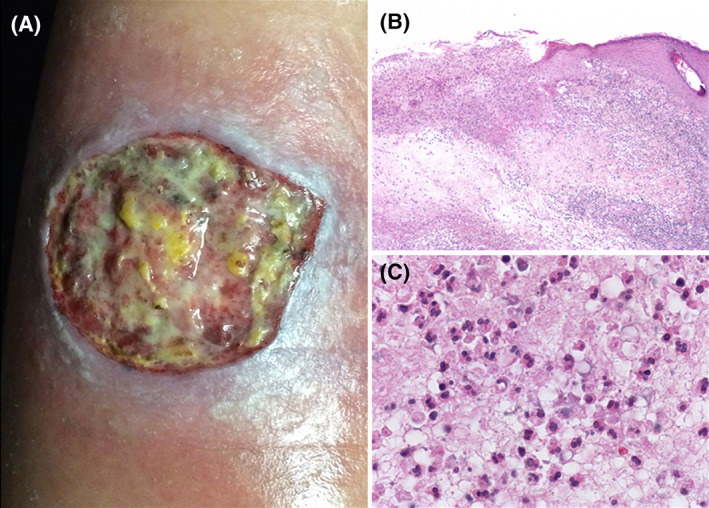
Classic ulcerative PG. A, Leg ulcer with raised violaceous edge and fibrinous wound bed. B, Biopsy from the edge of the ulcer, with a dense and diffuse intradermal inflammatory infiltrate, tissue necrosis and abscess formation. C, High‐power field showing numerous neutrophils within the dermal infiltrate
FIGURE 2.
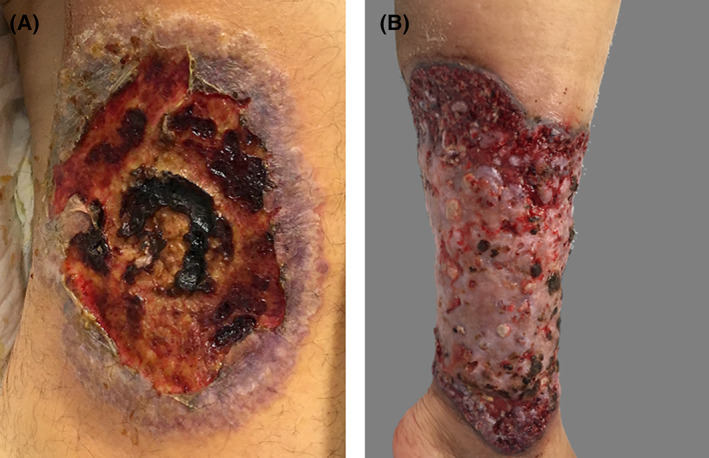
Classic ulcerative PG. A and B, Inferior leg ulcers with a violaceous edges and wound beds covered with areas of necrotic tissue
FIGURE 3.
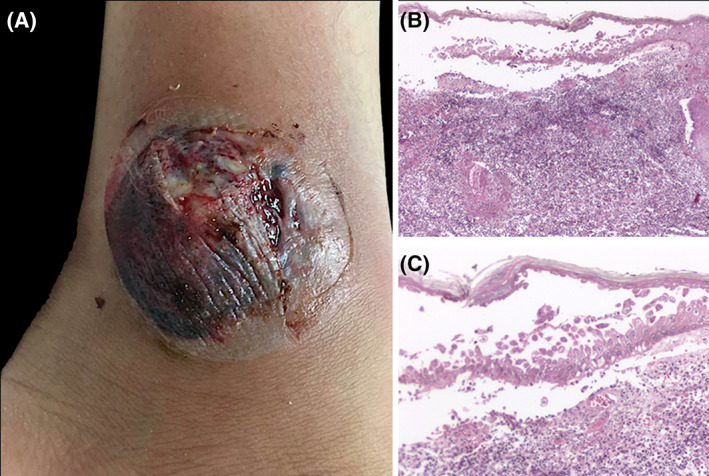
Bullous PG. A, Ankle with a tense hemorrhagic blister and adjacent ulceration with fibrin deposits. B, Biopsy shows intraepidermal bulla with acantholytic cells. C, Dense infiltrate of neutrophils with few eosinophils present in the dermis
FIGURE 4.
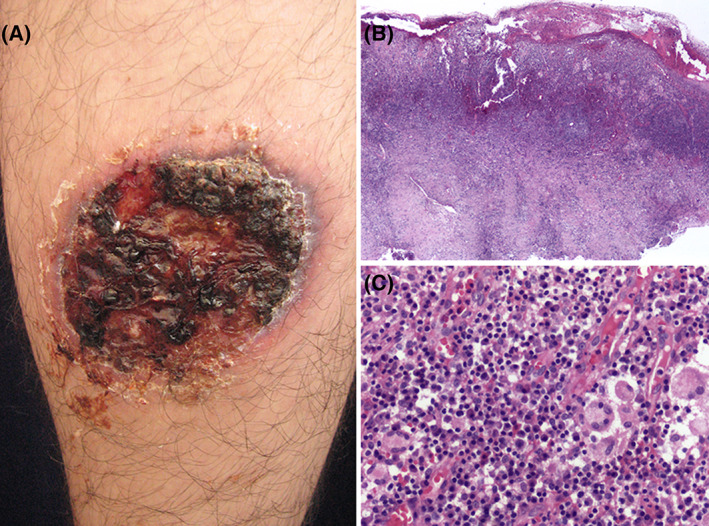
Vegetative PG. A, Leg ulcer with crusts and necrotic tissue in almost 90% of wound bed. B, Lowpower view microphotograph showing hyperplastic epidermis with intense inflammatory cell infiltrate in the dermis. C, High‐power magnification showing multinucleated giant cells intermingled with neutrophils. There are also conspicuous plasma cells
FIGURE 5.
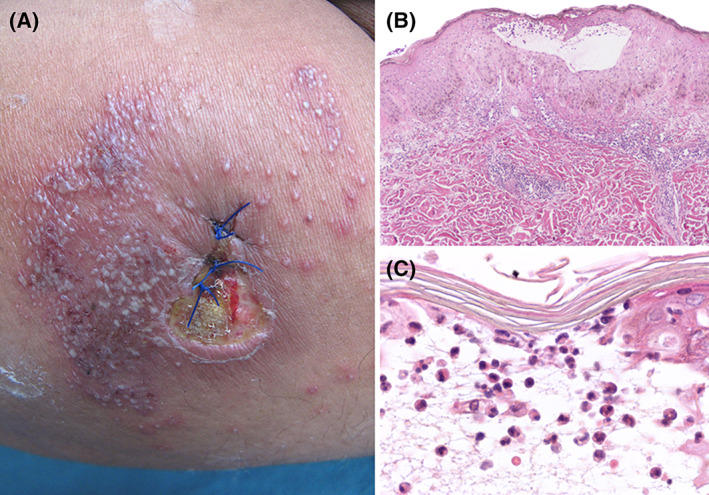
Pustular PG. A, Leg ulcer with fibrinous wound bed and raised erythematous border. Numerous pustules surrounding the ulcer, which appeared just after the biopsy was taken. B, Low‐power view of a biospy showing hyperplastic epidermis with an intraepidermal pustule. C, High‐power shows the blister cavity containing numerous neutrophils
TABLE 1.
Subtypes of Pyoderma Gangrenosum
| PG Subtype | Clinical Features | Histopathological Features |
|---|---|---|
|
Ulcerative |
Begins with a pustule, that rapidly expands into an ulcer, with violaceous edge. Associated with inflammatory bowel disease, rheumatoid arthritis and monoclonal gammopathies |
Neutrophilic infiltration. Derma edema |
|
Bullous (Figure 3) |
Rapid development of blue‐grey bullae, that coalesce and erode, leaving a superficial ulcer. More frequent on face, head and upper extremities. Associated with hematologic malignancies in 70% of patients |
Subepidermal bullae, with intraepidermal and dermal neutrophilic infiltrate |
|
Vegetative/granulomatous superficial (Figure 4) |
Progress more slowly Usually a single superficial ulcer. Lacks the violaceous border. No association with other medical condition |
Pseuoepitheliomatous hyperplasia and/or a palisading granulomatous reaction |
|
Pustular (Figure 5) |
Presents with multiple pustules with erythematous halo. Symmetric distribution. Strongly associated with inflammatory bowel disease |
Subcorneal pustules and/or perifollicular neutrophilic infiltrate |
| Peristomal | Painful papules they erode into ulcers, around a stoma | Mixed dermal inflammatory infiltrate with neutrophilic predilection. Granulation tissue |
Granulomatous superficial or vegetative PG, affects more frequently the trunk and characteristically begin as a single furunculoid purple abscess, nodule, or plaque that evolve more slowly into an ulcer in association with sinuses and cribriform scarring, most commonly on the trunk. 20 Superficial PG is the most uncommon and benign subtype and in contrast with classic PG, it is not routinely associated with underlying disorders. 9 During the regression of PG, the border of the lesion's collapses, erythema fades and granulation tissue appears on the ulcer. After healing, atrophic, cribriform scars persist. 8
PG can also be present on special anatomical sites (Figure 6).
FIGURE 6.
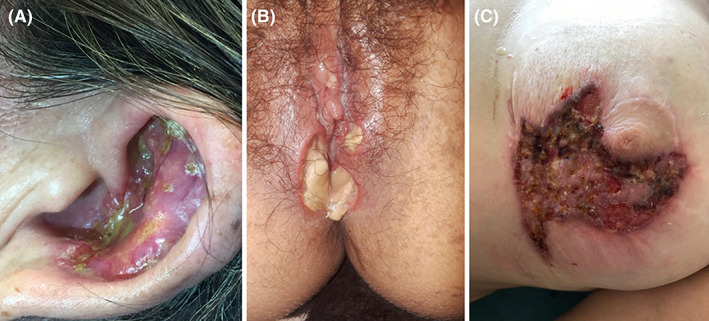
PG on special sites. A, PG on the ear of a young woman. B, PG affecting vulvar region. C, PG around the nipple, due to pathergy phenomenon after a mammoplasty surgery
5. DIAGNOSIS
PG is a diagnosis of exclusion. Clinical, histologic, and laboratory findings are nonspecific. It is important to think in PG as a differential diagnosis, because delays in diagnosis frequently expose patients to multiple disease‐exacerbating debridement, unnecessary antibiotics or other inappropriate therapies. 11 , 21
There are no universally accepted or validated criteria to diagnose PG.
Su et al have proposed a diagnostic criteria requiring two major and two minor criteria. 22
Major: (a) Rapid progression of a painful necrolytic cutaneous ulcer with an irregular, violaceous, and undermined border; (b) Other causes of cutaneous ulceration excluded. Minor: (a) History of pathergy or cribiform scarring clinically, (b) Associated systemic disease (IBD, IgA gammopathy or underlying malignancy), (c) Classic histopathological findings, (d) Treatment response: Rapid response to systemic steroid treatment.
More recently Maverakis et al, have proposed new criteria based on a consensus of international experts, requiring one major and four minor criteria. 23
Major: (a) Biopsy of ulcer edge demonstrating dense neutrophilic infiltrate.
Minor: (a) Exclusion of infection; (b) Pathergy; (c) History of inflammatory bowel disease or inflammatory arthritis. (d) History of papule, pustule, or vesicle ulcerating within 4 days of appearing; (e) Peripheral erythema, undermining border, and tenderness at ulceration site; (f) Multiple ulcerations, at least one on anterior lower leg; (g) Cribiform or “wrinkled paper” scar(s) at healed ulcer sites; (h) Decreased ulcer size within 1 month of initiating immunosuppressive medications.
Evaluation of a patient with suspected PG should include history, physical exam, sterile skin biopsy and laboratory tests. History and physical exam should look for signs and symptoms that suggest the possible co‐existence of an internal disease, such IBD, malignancies or conditions that can be confused as a differential diagnosis.
Laboratory evaluation should include a complete blood count (CBC), erythrocyte sediment rate, C‐reactive protein, liver and renal function test, protein electrophoresis, urinary Bence Jones protein, full hepatitis screen and targeted laboratory testing based on history and physical exam. 10 Gastrointestinal evaluation with a colonoscopy should be strongly considered given the strong association with IBD. Age appropriate cancer screening is recommended for all patients. 9
Skin biopsy can be supportive, to exclude other causes of chronic ulcers. 24 The biopsy should include an active border of the ulcer. 10 The histopathological findings are nonspecific and may vary depending on PG subtype and ulcer stage. The primary objective is to rule out other causes of ulceration. 22
Early lesions can show neutrophilic infiltrates around hair follicles and intradermal suppurative inflammation. Later lesions may evolve into ulcers due to epidermal and superficial dermal necrosis. Vascular changes suggestive of lymphocytic vasculitis or leukocytoclastic vasculitis may also be present. Bullous PG shows subepidermal bullae, subcorneal pustules characterise pustular PG and pseudoepitheliomatous hyperplasia and granulomatous infiltrates are features of vegetative PG. 25
5.1. Differential diagnosis
PG can mimic other cutaneous conditions that cause skin ulceration (Table 2). 9 , 10 , 22 , 26 , 27
TABLE 2.
Differential diagnosis of pyoderma gangrenosum
| Infections | Bacterial |
|---|---|
| Mycobacterial | |
| Fungal: Sporotrichosis, aspergillosis, cryptococcosis | |
| Parasites | |
| Viral: Herpes simplex | |
| Cutaneous primary tumours/metastasis | Skin lymphoma |
| Mycosis fungoides bullosa | |
| Langerhans cell histiocytosis | |
| Sickle cell disease | |
| Haematological causes | Cryoglobulinemia |
| Anti‐phospholipid syndrome | |
| Vascular Occlusive Disease | Livedoid vasculopathy |
| Venous stasis ulceration | |
| Small vessel occlusive arterial disease | |
| Type 1 cryoglobulinemia | |
| Anti‐phospholipid syndrome | |
| Autoimmune diseases with vasculitis | Systemic lupus erythematous |
| Behcet's disease | |
| Wegener granulomatosis | |
| Polyarteritis nodosa | |
| Pyoderma vegetans | |
| Munchausen syndrome and factitious disorder | |
| Drug‐induced and exogenous tissue injury | Hydroa‐induced ulceration |
| Bromoderma | |
| Drug‐induced lupus | |
5.2. Treatment
Before the beginning of treatment, other diseases or presence of infections should be discarded. 6
Only two randomised controlled trials in patients with neutrophilic diseases are reported in the literature 28 so management is challenging and treatment choice is based on severity and extent of PG (Figure 7). 10 Is it important to target the most three important areas: systemic therapies, topical therapies, and local wound care.
FIGURE 7.
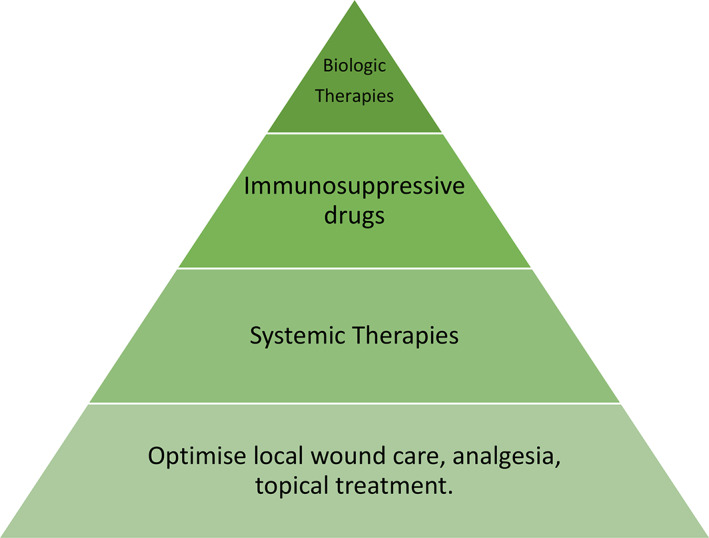
Treatment election based on severity and extension of pyoderma gangrenosum
5.2.1. Systemic treatment
Systemic corticosteroids, like oral prednisone, at the dose of 0.5 to 1 mg/kg/day, are the mainstay and first‐line therapy in all neutrophilic diseases. 8 , 9
Immunosuppressants, like cyclosporin (3‐4 mg/kg/day) or immunomodulating agents like dapsone (1‐1.5 mg/kg/day), can be used like steroid‐sparing drugs.
Dapsone, which is a great and inexpensive medicine, has anti‐inflammatory and anti‐neutrophilic effects, likely through blockade of myeloperoxidase, which have been shown to be effective in managing PG. 8 , 29 There are some authors that priorities the use of Dapsone besides any other steroid‐sparing drugs. 6
The use of antibiotics, like doxycycline, are also used for their anti‐inflammatory and antimicrobial effect. 6
Targeted therapies have been used in the management of PG, and there is a growing body of evidence to support biologic therapies. 6 , 14 Such as tumour necrosis factor alpha antagonist, infliximab, etanercept, and adalimumab; Anti IL‐1, anakinra, rilonacepto, gevokizumab, and canakinumab; Anti IL‐17, secukinumab.
Another important aspect of treatment is the pain, which should be addressed depending on the intensity of pain, initially with oral administration of acetaminophen and nonsteroidal anti‐inflammatory drugs for nociceptive pain (gnawing, aching, tender, and/or throbbing pain), then as necessary mild opioids such as codeine, then strong opioids such as morphine, until the patient is free of pain. 30 The use of opioids for the treatment of noncancer pain remains controversial, thus, clinicians need to be careful to minimise abuse and to treat pain effectively 31 Initial treatment of neuropathic pain (burning, stinging, shooting, or stabbing) can be managed with gabapentin and pregabalin, along with nortriptyline at night to facilitate sleep. 32
5.2.2. Local treatment
Local wound care can be potentiated with local therapies to help control the inflammation. Those with the most evidence are topical corticosteroids and topical calcineurin inhibitors. 9 Local treatments could be used as first line therapy in patients with localised disease, in which the size of the ulcer is small. 6
Local wound care includes cleansing and preventing secondary bacterial infections and the appropriate utilisation of antibacterial agents in the presence of localised infection. 33
Maintaining a moist wound environment is a basic principle of wound therapy. 8
At the beginning PG may present a paradoxical condition in which exudate is abundant, 34 so it is recommended the use absorbent dressing as hidrofiber, foam, or alginate. As the inflammation decreases, the exudate also does, so management can be changed to non‐absorbent dressings, such as, vaseline gauze.
Literature reports that >30% of individuals with wounds experience dressing‐related pain most or all of the time, and 60% reported that the pain took longer than 1 hour to resolve. 35 Interventions such as dressing removal, debridement, and inappropriate dressing selection contribute to increase pain. Wound margin maceration and skin damage facilitate the increased pain even before dressing changes. 36 Is recommended that wound care practitioners choose nontraumatic dressing that reduces discomfort with dressing changes and wear, soak a dressing before removal, and allow patient control to minimise the pain. Use warm isotonic solution during wound irrigation and protect the periwound skin from excoriation with an appropriate barrier after the irrigation. 37 If this is not enough, local anaesthetics can be used to further reduce the pain of the patient with PG.
Non‐pharmacological approaches, such as diversional therapy, breathing and relaxation exercises, and music may also help to level down anxiety, particularly during dressing changes. The power of talking to patients prior to dressing changes cannot be underestimated as well as explaining the procedures to be performed and the measures that will be taken to minimise pain. Communication prior to action will reduce the feelings of fear and anxiety. 38 Anxiety generates an autonomic response such as muscle tension and increased heart rate, which along with past experiences may cause patients to perceive greater pain. Thus, care should be provided in a warm and calm environment that will allow the patient to relax. 31
Wound debridement often presents a challenge in PG because of its feature of pathergy, or exacerbation in response to incidental or iatrogenic trauma. 39
For this reason, surgical debridement is not generally recommended. Di Xia et al, describe that the 15.1% of patients experienced postsurgical recurrence or exacerbation of PG; risk increased with more invasive procedures and chronic PG at the time of the procedure, with a small but clinically meaningful risk for postsurgical recurrence or exacerbation of PG in patients with a known history of PG. 40
Conservative debridement (enzymatic, autolytic) to remove nonviable tissue should be performed with caution. 41
Maggot debridement therapy (MDT), shows considerable promise for debridement in chronic PG ulcers because of the less risk of presenting pathergy, 42 and because enzymatic secretions of the larvae that have been found to disinfect wounds, reduce biofilm formation, regulate MMPs production, and improve oxygenation. 43
Negative pressure wound therapy (NPWT) is a good option for wound bed preparation in the management of complex wounds and can be use in PG with adequate immunosuppression. 44
Surgical reconstruction of PG is indeed challenging as the treatment itself has the potential to induce pathergy, though, adequate immunosuppression split‐thickness skin graft, secured by NPWT is a potential treatment option for PG that may help achieve rapid wound closure and a favourable cosmetic appearance. 45
5.3. Prognosis
PG is a chronic, relapsing disease, possible lasting for months to years if untreated. Patients with PG experience considerable health‐related reductions in quality of life due to severe pain, sleep disturbances, and poor appetite. 26
PG is associated with high morbidity and mortality. Even when patients respond well to therapy, relapses can occur in up to 70% of cases. 46 The long‐term outcome of PG remains unpredictable, with mortality rates reported as high as 30%.
Mortality risk varied substantially among patients with small vessel vasculitis and hematologic malignancy/dyscrasia in comparison with that in patients with IBD and IA. 13
6. CONCLUSION
Clinicians should consider PG as one of the differential diagnoses in chronic wounds, especially if they present skin ulcers with undermined erythematous‐violaceous borders accompanied by disproportionate pain. Careful clinical assessment could establish an early diagnosis and formulate an effective management plan, improving the quality of life and the prognosis of patients.
Alonso‐León T, Hernández‐Ramírez HH, Fonte‐Avalos V, Toussaint‐Caire S, E. Vega‐Memije M, Lozano‐Platonoff A. The great imitator with no diagnostic test: pyoderma gangrenosum. Int Wound J. 2020;17:1774–1782. 10.1111/iwj.13466
REFERENCES
- 1. Moen BH, Nystad TW, Barrett TM, Sandvik LF. A boy in his teens with large ulcerations of the head and neck. Tidsskr nor Laegeforen [Internet]. 2019;139(7):1–7. http://www.ncbi.nlm.nih.gov/pubmed/30969044. [DOI] [PubMed] [Google Scholar]
- 2. Almukhtar R, Armenta AM, Martin J, et al. Delayed diagnosis of post‐surgical pyoderma gangrenosum: a multicenter case series and review of literature. Int J Surg Case Rep. 2018;44:152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braswell SF, Kostopoulos TC, Ortega‐Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol [Internet]. 2015;73(4):691‐698. Available from: 10.1016/j.jaad.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 4. Salcido R. Pyoderma gangrenosum: the great impostor. Adv Ski Wound Care. 2017;30(12):533. [DOI] [PubMed] [Google Scholar]
- 5. Yu YM, Lai FJ, Feng C, Chen BL, Cao YS. Pyoderma gangrenosum around an ileostoma: a case report. Med (United States). 2018;97(48):2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soto Vilches F, Vera‐Kellet C. Pyoderma gangrenosum: classic and emerging therapies. Med Clin (Barc). 2017;149(6):256‐260. [DOI] [PubMed] [Google Scholar]
- 7. Gerard AJ, Feldman SR, Strowd L. Quality of life of patients with pyoderma gangrenosum and hidradenitis suppurativa. J Cutan Med Surg. 2015;19(4):391‐396. [DOI] [PubMed] [Google Scholar]
- 8. Marzano AV, Borghi A, Wallach D, Cugno M. A comprehensive review of neutrophilic diseases. Clin Rev Allergy Immunol. 2018;54(1):114‐130. [DOI] [PubMed] [Google Scholar]
- 9. Ashchyan HJ, Nelson CA, Stephen S, et al. Neutorphilic dermatoses. Part II. J Am Acad Dermatol. 2018;79(6):1009‐1022. [DOI] [PubMed] [Google Scholar]
- 10. George C, Deroide F, Rustin M. Pyoderma gangrenosum ‐ a guide to diagnosis and management. Clin Med J R Coll Phys Lond. 2019;19(3):224‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu A, Balgobind A, Strunk A, Garg A, Alloo A. Prevalence estimates for pyoderma gangrenosum in the United States: an age‐ and sex‐adjusted population analysis. J Am Acad Dermatol. 2019;83(2):425–429. [DOI] [PubMed] [Google Scholar]
- 12. Jain AG, Sharbatji M, Afzal A, Afridi SM, Gordon D. Pyoderma Gangrenosum in the absence of any underlying predisposing condition: a diagnostic dilemma. Cureus. 2019;11(3):e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaffenberger BH, Hinton A, Krishna SG. The impact of underlying disease state on outcomes in patients with pyoderma gangrenosum: a national survey. J Am Acad Dermatol. 2018;79(4):659‐663.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marzano AV, Ortega‐Loayza AG, Heath M, Morse D, Genovese G, Cugno M. Mechanisms of inflammation in neutrophil‐mediated skin diseases. Front Immunol. 2019;10(MAY):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Niv D, Ramirez JA, Fivenson DP. Pyoderma gangrenosum, acne, and hidradenitis suppurativa (PASH) syndrome with recurrent vasculitis. JAAD Case Reports [Internet]. 2017;3(1):70‐73. Available from: 10.1016/j.jdcr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Join‐Lambert O, Duchatelet S, Delage M, et al. Remission of refractory pyoderma gangrenosum, severe acne, and hidradenitis suppurativa (PASH) syndrome using targeted antibiotic therapy in 4 patients. J Am Acad Dermatol. 2015;73(5):S66‐S69. [DOI] [PubMed] [Google Scholar]
- 17. Khan D, Ansar Ahmed S. The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol. 2016;6(JAN):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dai R, Cowan C, Heid B, et al. Neutrophils & neutrophil serine proteases are increased in the spleens of estrogentreated C57BL/6 mice & several strains of spontaneous lupus‐prone mice. PLoS One. 2017;12(2):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244‐1250. [DOI] [PubMed] [Google Scholar]
- 20. Jin Y, Qu C, Shi T, Wang C, Yu H, Zhang F. A case of vegetative pyoderma gangrenosum. Dermatologica Sin [Internet]. 2015;33(3):170‐172. Available from: 10.1016/j.dsi.2014.12.009. [DOI] [Google Scholar]
- 21. Tolkachjov SN, Fahy AS, Cerci FB, Wetter DA, Cha SS, Camilleri MJ. Postoperative Pyoderma Gangrenosum. Mayo Clin Proc. 2016;91(9):1267‐1279. [DOI] [PubMed] [Google Scholar]
- 22. Su WPD, Davis MDP, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43(11):790‐800. [DOI] [PubMed] [Google Scholar]
- 23. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum a delphi consensus of international experts. JAMA Dermatol. 2018;154(4):461‐466. [DOI] [PubMed] [Google Scholar]
- 24. Khoobyari S, Miller TI, Shinohara MM. Utility of skin biopsy and culture in the diagnosis and classification of chronic ulcers: a single‐institution, retrospective study. Am J Dermatopathol. 2019;41(5):343‐346. [DOI] [PubMed] [Google Scholar]
- 25. Powell FC, Su D, Perry H. Pyoderma gangrenosum: classification and management. JAAD. 1996;34(3):395‐409. [DOI] [PubMed] [Google Scholar]
- 26. Gameiro A, Pereira N, Cardoso JC, Gonçalo M. Pyoderma gangrenosum: challenges and solutions. Clin Cosmet Investig Dermatol. 2015;8:285‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aksu Çerman A, Aktaş E, Kivanç Altunay I, Demirkesen C. Pyoderma vegetans misdiagnosed as verrucous carcinoma. Am J Dermatopathol. 2016;38(2):144‐147. [DOI] [PubMed] [Google Scholar]
- 28. Brooklyn TN, Dunnill MGS, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55(4):505‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown RE, Lay L, Graham D. Bilateral pyoderma gangrenosum of the hand: treatment with dapsone. J Hand Surg Am. 1993;18(1):119‐121. [DOI] [PubMed] [Google Scholar]
- 30. Sibbald RG, Goodman L, Woo KY, et al. Special considerations in wound bed preparation 2011: an update©. Adv Skin Wound Care. 2011;24(9):415‐436. [DOI] [PubMed] [Google Scholar]
- 31. Serena T, Yaakov R, Aslam S, Aslam R. Preventing, minimizing, and managing pain in patients with chronic wounds: challenges and solutions. Chronic Wound Care Manag Res. 2016;3:85‐90. [Google Scholar]
- 32. Woo KY. Pra AMA. E X Tra. 2012;25(1):38‐44. [Google Scholar]
- 33. Alavi A, French LE, Davis MD, Brassard A, Kirsner RS. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18(3):355‐372. [DOI] [PubMed] [Google Scholar]
- 34. Lozano‐Platonoff A, Mejía‐Mendoza MDF, Ibáñez‐Doria M, Contreras‐Ruiz J. Assessment: cornerstone in wound management. J Am Coll Surg. 2015;221(2):611‐620. [DOI] [PubMed] [Google Scholar]
- 35. Price PE, Fagervik‐Morton H, Mudge EJ, et al. Dressing‐related pain in patients with chronic wounds: an international patient perspective. Int Wound J. 2008;5(2):159‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woo KY, Coutts PM, Price P, Harding K, Sibbald RG. A randomized crossover investigation of pain at dressing change comparing 2 foam dressings. Adv Skin Wound Care. 2009;22(7):304‐310. [DOI] [PubMed] [Google Scholar]
- 37. Bishop SM, Walker M, Rogers AA, Chen WY. Importance of moisture balance at the wound‐dressing interface. J Wound Care. 2003;12(4):125‐128. [DOI] [PubMed] [Google Scholar]
- 38. Bechert K, Abraham SE. Pain management and wound care. J Am Col Certif Wound Spec. 2009;1(2):65‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ashchyan HJ, Butler DC, Nelson CA, et al. JAMA dermatology original investigation the association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154(4):409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Di Xia F, Liu K, Lockwood S, et al. Risk of developing pyoderma gangrenosum after procedures in patients with a known history of pyoderma gangrenosum—a retrospective analysis. J Am Acad Dermatol. 2018;78(2):310‐314.e1. [DOI] [PubMed] [Google Scholar]
- 41. Tolkachjov SN, Fahy AS, Wetter DA, et al. Postoperative pyoderma gangrenosum (PG): the Mayo Clinic experience of 20 years from 1994 through 2014. J Am Acad Dermatol. 2015;73(4):615‐622. [DOI] [PubMed] [Google Scholar]
- 42. Din RS, Tsiaras WG, Mostaghimi A. Two cases of maggot debridement therapy in pyoderma gangrenosum. JAAD Case Reports. 2018;4(10):1027‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abela G. Clinical focus. Horm Res Paediatr. 2006;65(4):29‐34. [Google Scholar]
- 44. Kostaras EK, Tansarli GS, Falagas ME. Use of negative‐pressure wound therapy in breast tissues: evaluation of the literature. Surg Infect (Larchmt). 2014;15(6):679‐685. [DOI] [PubMed] [Google Scholar]
- 45. Powers JG, Higham C, Broussard K, Phillips TJ. Wound healing and treating wounds chronic wound care and management. J Am Acad Dermatol. 2016;74(4):607‐625. [DOI] [PubMed] [Google Scholar]
- 46. Saracino A, Kelly R, Liew D, Chong A. Pyoderma gangrenosum requiring inpatient management: a report of 26 cases with follow up. Australas J Dermatol. 2011;52(3):218‐221. [DOI] [PubMed] [Google Scholar]


