Abstract
Venous leg ulcers (VLUs) result in substantial economic costs and reduced quality of life (QoL); however, there are few Australian cost estimates, especially using patient‐level data. We measured community‐setting VLU management costs and the impact on the QoL of affected individuals. VLU patients were recruited from a specialist wound clinic, an outpatient clinic, and two community care clinics in Queensland. Cost data were collected at the baseline visit. QoL (EQ‐5D‐5L) and wound status data were collected at baseline, 1, 3, and 6 months. Patients were classified into guideline‐based/optimal care and usual care groups. Average weekly costs per patient were statistically significantly different between the usual care and optimal care groups—$214.61 and $294.72, respectively (P = 0.04). Baseline average QoL score for an unhealed ulcer was significantly higher in the optimal care group compared with usual care (P = 0.025). Time to healing differed between the usual care group and the optimal care group (P = 0.04), with averages of 3.9 and 2.7 months, respectively. These findings increase the understanding of the costs, QoL, and healing outcomes of VLU care. Higher optimal care costs may be offset by faster time to healing. This study provides data to inform an economic evaluation of guideline‐based care for VLUs.
Keywords: compression therapy, cost, quality of life, venous leg ulcer
1. INTRODUCTION
There is a lack of nationally representative and recent data on the prevalence of venous leg ulcers (VLUs) in the Australian setting. In a 1991 study set in Perth, Western Australia, prevalence was estimated at 0.62 per 1000 in the general, metropolitan population.1 Prevalence of VLUs was the highest in older age groups, with a prevalence of 3.3 per 1000 in adults 60 years and older.1 As the ageing population in Australia is increasing,2 it follows that the prevalence and incidence of VLU has likely increased over time. Thus, prevalence estimates from the 1990s likely underestimate current prevalence.
Not only do VLUs represent a growing health burden, they also represent a condition that is expensive to treat for both patients and the health care system. Across the world, studies have estimated annual costs of up to €2585, €1994 (cost of treating an initial ulcer, per patient, limited to health system perspective), and €9569 (direct and indirect costs, per patient, societal perspective) in Sweden, the United Kingdom, and Germany respectively.3, 4 While these estimates illustrate the potential for high costs, because of differences in health care systems and services, these costs may not reflect the situation in Australia. Out‐of‐pocket costs of consumables alone have been estimated at $27.5 million per year for Australians over 60 years of age with VLUs, although this figure may be underestimated because of underlying data limitations.5
In Australia, the total, direct costs of treating VLUs (in public and private hospitals and residential care settings) was estimated to be US$802.55 million (±US$307.46 million).6 This estimate does not appear to include the costs in the non‐residential care community setting, such as general practitioner visits, of treating VLU. This presents a significant gap in our knowledge of VLU costs.
In addition to the economic burden, VLUs also negatively impact social functioning and reduce quality of life (QoL).7, 8 There is limited local data about the effect of VLUs on QoL, although it is expected to be significant. Elsewhere, a review described the impact of VLUs on QoL as “profound.”7 Iglesias et al describe an EQ‐5D score, measured on a scale between 0 (death) and 1 (perfect health), of 0.62 associated with VLUs.9 Greater understanding of the impact of VLUs on QoL would help to inform economic models.
Australian guidelines recommend compression therapy (CT) as the primary treatment for VLUs10, 11; however, evidence suggests that many people do not receive guideline‐based care.1, 12 Coyer et al13 identify three main evidence–practice gaps, relating to knowledge, costs, and systems, whereby care pathways are inconsistent. Yet, the implementation of guideline‐based, or optimal, care may represent improved health benefits and have important cost implications. For example, use of guidelines has been shown to reduce the costs of healing VLUs.14
No study in the Australian setting compares the resources used or time to healing of optimal (guideline‐based) care and usual (standard) care scenarios for VLUs. However, we do know that care pathways that increase access to specialist wound clinics and CT improve healing outcomes of leg and foot ulcers in the Australian setting,15 and evidence‐based practice has demonstrated improved healing outcomes in other settings.14 In addition, overall, we know little about the QoL benefits of optimal care compared with usual care.
The present study is part of a larger project. The overall project will perform an economic evaluation to capture the costs and benefits of providing optimal care to VLU patients. The project will bridge knowledge gaps regarding the implementation of guideline‐based/optimal care and contribute important knowledge of the costs, QoL, and healing outcomes associated with optimal care in the Queensland, Australia, setting. The present study describes costs of care for VLU patients and the impact on QoL associated with VLUs. Given the increasing population size affected by VLUs and the substantial economic burden and reduced QoL associated with VLUs, this research has significant implications for patients, health professionals, and health care policymakers.
2. METHODS
2.1. Participants and setting
Participants were recruited from four sites in Queensland, Australia: one private specialist wound clinic, one hospital outpatient clinic, and two community care clinics.
Patients attending the private specialist clinic and the community clinics were charged standard fees with no subsidy, and those attending the outpatient clinic were bulk‐billed (no out‐of‐pocket costs to patients). Patients attending the community care clinics pay a small out‐of‐pocket fee for each appointment and are charged separately for dressings used. The clinics arrange delivery of dressings to patients, and these dressings are then brought to and used at subsequent appointments. Patients attending the clinics are seen by registered nurses, podiatrists, dietitians, and occupational therapists.
The public hospital outpatient clinic provides a bulk‐billed service with all dressings and consultancy fees covered, with no out‐of‐pocket costs to the patient. Health care professionals in attendance include a vascular surgeon, registered nurses, and occupational therapists.
Patients attending the specialist wound clinic are charged a fee for each appointment, which covers the cost of consultancy and all dressings. Patients are seen by a multidisciplinary team comprised of a vascular specialist, a wound nurse practitioner candidate, a podiatrist, and a registered nurse. Patients receive tailored wound dressing plans, designed to be managed by the patient or primary carer outside the clinic, through further clinic visits or augmented through telehealth.
Patients with a VLU who met the inclusion criteria were invited to participate. Participants were eligible if they were over 18 years of age, the principal diagnosis was VLU (or mixed/arterial with predominantly venous origin), and if they could provide informed consent. Exclusion criteria included leg ulcers not of venous origin, inability to comply with the protocol of the study, and cognitive impairment. Recruitment occurred between December 2016 and September 2017. Data were collected at study admission (baseline) and at 1‐, 3‐, and 6‐month follow‐ups. Participation or non‐participation in the study did not impact the care received by patients.
2.2. Data collection
Information on patient demographic, medical history, comorbidities, and ulcer characteristics was collected on recruitment at baseline. Ulcer characteristics—including time of first onset, duration, number of wounds, current ulcer history, and clinical assessment (size, ankle brachial pressure index [ABPI] if available, whether new or recurrent, time to healing, time to recurrence, and exudate description)—were recorded. Body mass index (BMI) was calculated and categorised according to the World Health Organisation classification.16 Medical history and risk factors were sourced from patients and clinicians and were confirmed and supplemented by medical record review subject to availability. Data on the type of health services provided, investigations, types of dressings and bandages used, medication, travel, and product and service patient out‐of‐pocket costs were recorded. Data on QoL were collected using the EQ‐5D‐5L tool17 at baseline and again at 1, 3, and 6 months. Follow‐up data were collected during clinic visits or through telephone interviews.
2.3. Definitions
For data analysis, patients were classified into two groups based on collected data: those receiving guideline‐based prevention and treatment or “optimal care” and those receiving standard treatment or “usual care” at baseline. These classifications were made irrespective of the clinic of recruitment. Optimal care was defined as those patients who, during the study period and before healing, had at least one session of ABPI assessment to confirm venous origin (0.8 ≤ ABPI ≤ 1.2), or toe pressure, or had a reason as to why ABPI was not performed and who received CT (compression bandages, compression hosiery or tubular compression bandage applied in three layers18). All other participants not meeting the criteria for optimal care were classified as receiving usual care. The treatment recorded at baseline was assumed to continue for the study duration or until healed. It was not possible to ascertain patient compliance with treatment unless noted in the medical records. Time to healing was defined as the number of months that each ulcer took to heal from the time the patient was recruited into the study.
2.4. Costs
Data on costs of travel to receive wound care, consultancy with health professionals, and products used allowed the estimation of participants' average weekly costs. Patient out‐of‐pocket costs included costs to patients for travel and parking, private clinic consultation fees, and wound care products. Health system costs included the Medicare costs of medical services (doctors, specialists, nurses, and allied health professionals) and investigations as well as prescription costs for medications listed on the Pharmaceutical Benefits Scheme. Thus, this study takes a societal perspective. Costs are reported in Australian dollars unless otherwise indicated.
2.4.1. Travel and parking costs
Participant's travel and parking costs were estimated by capturing the mode of travel to the clinic, out‐of‐pocket costs, and the frequency of attendance. Those who travelled by public transport, community transport, or taxi provided exact travel costs. Car travel costs were calculated by multiplying the distance from clinic to participants’ homes in kilometres by $0.66.19 We assumed the same travel mode for each clinic visit.
2.4.2. Costs of medical services
The health services cost items included were all consultations with a community‐based nurse, podiatrist, and other allied health professional and medical specialists. Health services were valued in line with the Australian Federal Government reimbursements via Medicare or the Medicare Benefits Schedule (MBS). Nurse practitioner, vascular surgeon, podiatrist, and occupational therapist costs were based on MBS items 82215, 104, 10962, and 10958, respectively.20 Enrolled, registered, and student nurse time costs were calculated based on hourly salary,21 assuming a 30‐minute consultation in the absence of recorded appointment end time. The specialist wound clinic medical service was the patient out‐of‐pocket consultation fee, which included the cost of consumables.
In addition to their regular attendance at a clinic or through a home nursing service, many patients also used other health care services (eg, General Practitioner (GP) visits). These costs were calculated as a product of the cost of the visit to the service used and the frequency of service access.
2.4.3. Wound product costs
Product use data were collected through medical chart review, consultation with clinicians, or observation during clinic visits by research staff. Consumable item costs were based on a review of market prices and are available in a spreadsheet format from the authors on request. Product use recorded included: primary/secondary dressings (eg, absorbent dressings), compression systems (eg, short‐stretch bandages/hosiery), topical medications (eg, antimicrobial ointments), skin care items (eg, barrier creams), cleansers, and other disposables (eg, tape).
2.5. Quality of life
The EQ‐5D‐5L17 tool was used to capture QoL data. EQ‐5D‐5L is a generic, preference‐based tool that measures QoL according to five dimensions: mobility, self‐care, usual activities, pain/discomfort, and anxiety/depression.17 For each dimension, respondents were asked to choose from five levels to indicate whether they experienced any problems in each dimension of health. EQ‐5D‐5L survey answers were valued and transformed to a utility score using a scoring algorithm developed from a UK general population sample.22
2.6. Statistical analysis
Baseline characteristics and wound clinical outcomes at different time points for patients receiving optimal care and usual care were compared using a combination of t‐tests, Mann–Whitney tests, z tests, Fisher's exact tests, and median tests, as appropriate. Data were analysed using IBM SPSS Statistics 23, and z tests were performed using an online calculator.23 A significance level of 0.05 was used in the interpreting of the statistical test results. Median and mean time to healing was calculated in order to ensure comparability with previous studies.
Two patients had unknown costs related to travel, and three patients had unknown costs related to product use. The multiple imputation procedure in SPSS was used to impute 10 complete datasets to calculate the total cost estimate. Because of a small sample size, Fisher's exact test was deemed the most appropriate to test for differences in numbers of ulcers healed at 1, 2, 3, and 6 months.
2.7. Ethics
This study was approved by the Metro South Health Human Research Ethics Committee (HREC) and Queensland University of Technology (QUT) Human Research Ethics Committee (approval numbers HREC/16/QPAH/370 and 1 600 000 934, respectively). Written informed consent was obtained from all participants.
3. RESULTS
Participants' baseline information is summarised in Tables 1, 2, 3. For all tables, the available data were used; thus, not all figures displayed included patients with missing data. Eighty‐one participants were recruited; however, most baseline data for one participant was missing. This participant was lost to follow‐up thereafter, and was therefore excluded from analyses. Fifty‐four patients were classified into the usual care group and 26 patients into the optimal care group. At 3 months, 22.5% of participants were lost to follow up.
Table 1.
Participant baseline characteristics (continuous variables)
| Characteristic | All patients | Usual care | Optimal care | P‐value |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| n = 80 | n = 54 | n = 26 | ||
| Age (yrs) | 75.1 (13.9) | 73.3 (14.7) | 79 (11.3) | 0.053 |
| Height (cm) | 170.2 (11.6) | 171.7 (11.1) | 166 (12.3) | 0.094 |
| Weight (kg) | 93.3 (33.0) | 97.9 (34.3) | 80.6 (26.2) | 0.037 |
| BMI (kg/m2) | 33.1 (11.2) | 34.1 (11.8) | 30.0 (8.7) | 0.260 |
| Ankle circumference (per leg, cm) | 23.9 (2.5) | 24.2 (2.6) | 23.6 (2.5) | 0.450 |
| Calf circumference (per leg, cm) | 36.8 (4.9) | 37.6 (5.2) | 36.0 (4.5) | 0.289 |
| Left ABPI ratio | 1.01 (0.21) | 1.2 (0) | 0.98 (0.22) | — |
| Right ABPI ratio | 0.99 (0.25) | 1.09 (0.16) | 0.97 (0.27) | — |
| Toe pressure index | 0.69 (0.16) | 0.84 (0.3) | 0.64 (0.06) | — |
Abbreviations: ABPI, ankle brachial pressure index; BMI, body mass index. “—” designates samples with too few data collected or too few data for statistical testing.
Table 2.
Participant baseline characteristics (categorical variables)
| Characteristic | All patients | Usual care patients | Optimal care patients | P‐value |
|---|---|---|---|---|
| n (%)a | n (%)a | n (%)a | ||
| n = 80 | n = 54 | n = 26 | ||
| BMI category | ||||
| Underweight | 2 (3.9) | 2 (5.3) | 0 (0) | 0.31 |
| Normal | 14 (27.5) | 8 (21.1) | 6 (46.2) | |
| Overweight | 8 (15.7) | 6 (15.8) | 2 (15.4) | |
| Obese | 27 (52.9) | 22 (57.9) | 5 (38.5) | |
| Gender (male) | 32 (40) | 25 (46.3) | 7 (26.9) | 0.1 |
| Venous insufficiency | 65 (81.3) | 46 (85.2) | 19 (73.1) | 0.19 |
| Reduced mobility | 70 (87.5) | 47 (87) | 23 (88.5) | 0.86 |
| Age > 70 | 56 (70) | 38 (70.4) | 18 (69.2) | 0.91 |
| Clinical signs of infection (at admission) | 18 (22.5) | 11 (20.4) | 7 (26.9) | 0.52 |
| Hypercholesterolemia | 10 (12.5) | 5 (9.3) | 5 (19.2) | 0.21 |
| Lymphedema/oedema (history) | 40 (49.4) | 27 (50) | 13 (50) | 1 |
| Oedema (at admission) | 51 (63.8) | 33 (61.1) | 18 (69.2) | 0.48 |
| Eczema (at admission) | 15 (18.8) | 10 (18.5) | 5 (19.2) | 0.94 |
| Hypertension | 31 (38.8) | 17 (31.5) | 14 (53.8) | 0.055 |
| Peripheral arterial disease | 4 (5) | 3 (5.6) | 1 (3.85) | 0.73 |
| Smoker | 5 (6.25) | 3 (5.6) | 2 (7.7) | 0.72 |
| Deep vein thrombosis | 2 (2.5) | 1 (1.9) | 1 (3.85) | 0.60 |
Abbreviation: BMI, body mass index. “—” designates samples with too few data or too few data for statistical testing. BMI category was tested using χ 2 analysis, and all other tests were completed using z test.
Please note that variables may not add to 100% as each variable had different numbers of available data.
Table 3.
Wound characteristics at baseline
| Wound measurement (cm [per wound]) | All patients (n = 80) | Usual care patients (n = 54) | Optimal care patients (n = 26) | P‐value |
|---|---|---|---|---|
| Wound length (mean [SD]) | 2.9 (2.3) | 3.4 (2.6) | 2.6 (2.2) | 0.426 |
| Wound width (mean [SD]) | 2.2 (1.5) | 2.3 (1.6) | 2.1 (1.4) | 0.983 |
| Wound depth (mean [SD]) | 0.6 (0.5) | — | 0.4 (0.3) | — |
| Ulcer duration (mean [SD], in mo) | 46.9 (91.3) | 46.1 (86.8) | 48.5 (100.7) | 0.885 |
| Ulcer duration (median [IQR]a, in mo) | 10 (42) | 12 (45) | 6 (29.75) | 0.309 |
Abbreviation: IQR, interquartile range. “—” designates samples with too few data collected or too few data for statistical testing.
For analysis, <1 was assumed to be 1, and > 360 months was assumed to be 360 months.
The majority (68.6%) of participants were overweight or obese and were over 70 years of age (n = 56, 70%) (Table 2). At baseline, the majority (81.3%) had venous insufficiency and reduced mobility (87.5%) recorded on their medical records (Table 2). Median ulcer duration at baseline was 10 months (IQR = 42) for all patients (Table 3). By definition, all optimal care participants received CT. The majority (n = 33, 61.1%) of usual care patients received CT as part of their care.
Average weekly costs of managing ulcers in the community are presented in Table 4. Average weekly cost was estimated to be $214.61 for those in the usual care group and $294.72 in the optimal care group. This difference was statistically significant (P = 0.04). Total patient out‐of‐pocket costs per week were significantly higher for patients receiving optimal care (P = 0.016). At baseline, the mean utility score of optimal and usual care patients were 0.75 (±0.16) and 0.64 (±0.26) respectively (Table 5). The difference in these scores reached statistical significance (p=0.025).
Table 4.
Average weekly costs per patient at baseline (AUD$)
| Usual care (mean) | Optimal care (mean) | P‐value | |
|---|---|---|---|
| Transport cost | $13.95 | $17.69 | |
| Consultancy cost (out‐of‐pocket) | $16.37 | $67.10 | |
| Consultancy cost (health care system) | $71.41 | $78.13 | |
| Costs of using other medical services (out‐of‐pocket) | $9.94 | $8.27 | |
| Costs of using other medical services (health care system) | $38.95 | $37.60 | |
| Product cost | $62.87 | $85.93 | |
| Total weekly costs (health system) | $110.36 | $115.73 | 0.736 |
| Total weekly costs (out‐of‐pocket) | $104.25 | $178.99 | 0.016 |
| Total weekly costs | $214.61 | $294.72 | 0.04 |
The “Totals” rows display the pooled results from the use of multiple imputation to handle missing data. Only the total weekly costs (by payer perspective and overall) were statistically tested.
Table 5.
Baseline and 3‐month EQ‐5D‐5L scores
| Time point | All patients (mean [SD]) | Usual care group (mean [SD]) | Optimal care group (mean [SD]) | Difference between optimal and usual care groups (P‐value [95% CI]) |
|---|---|---|---|---|
| Baseline | 0.67 (±0.24) | 0.64 (±0.26) | 0.75 (±0.16) | 0.025 (0.014 to 0.206) |
| 3 mo | 0.80 (±0.18) | 0.78 (±0.19) | 0.83 (±0.15) | 0.414 (−0.061 to 0.146) |
CI, Confidence interval. At baseline, the mean utility score of optimal and usual care patients were 0.75 (±0.16) and 0.64 (±0.26), respectively. The difference in these scores reached statistical significance (P = 0.025).
There was an overall difference in the time to healing per ulcer (P = 0.04). The average ulcer healing time in the usual care group (3.9 months) was longer compared with the optimal care group (2.7 months). While the medians of the two care groups were identical, the median test results rejected the null hypothesis that the medians in both groups were identical as there was a larger proportion of patients in the usual care group with time to healing greater than the overall median compared with the optimal care group (Table 6).
Table 6.
Time to healing
| Number of ulcers healed after 1, 2, 3, and 6 mo | Usual care (n = 28) | Optimal care (n = 19) | P‐value |
|---|---|---|---|
| 1‐mo healing (n [%]) | 6 (21.4) | 5 (26.3) | 0.04 |
| 2‐mo healing (n [%]) | 1 (3.6) | 2 (10.5) | |
| 3‐mo healing (n [%]) | 8 (28.6) | 10 (52.6) | |
| 6‐mo healing (n [%]) | 13 (46.4) | 2 (10.5) |
| Time to healing | Usual care (n = 54) | Optimal care (n = 26) | P‐value |
|---|---|---|---|
| Time to healing (mo) (mean, per ulcer) | 3.9 | 2.7 | — |
| Time to healing (mo) (median [IQR] per ulcer) | 3 (3) | 3 (1.5) | 0.012 |
| Total number of ulcers during the first 3 mo | 94 | 75 | |
| Ulcers healed at 3‐mo data collection (n, %) | 15 (16%) | 17 (22.7%) | 0.27 |
| Patients healed at 3‐mo data collection (n, %) | 12/42 (28.6%)* | 6/20 (30%)* | 0.912 |
IQR, interquartile range. “—” designates samples with too few data collected or too few data for statistical testing.
At 3 months, 18 (22.5%) participants were lost to follow‐up (12 from the usual care and 6 from the optimal care group). Patients were considered healed if all their ulcers had healed. Hence, total ulcers healed at 3 months does not equal the patients healed at 3 month data collection.
3.1. Service provider
Most patients (85%) received care from a registered nurse (tabulated data not shown). Similar proportions of optimal and usual care participants accessed registered nurses for care (Figure 1). A larger proportion (37%) of usual care participants received care from a vascular surgeon compared with optimal care participants (19.2%), while a larger proportion of optimal care participants received care from enrolled nurses (11.5%) and podiatrists (23.1%) than usual care (3.7% and 14.8%, respectively) patients. Most participants (61.5% of optimal care and 53.7% of usual care participants) accessed two or more service providers at baseline. A larger proportion of usual care participants accessed three service providers at baseline, 20.4% compared with 7.7% of optimal care participants.
Figure 1.
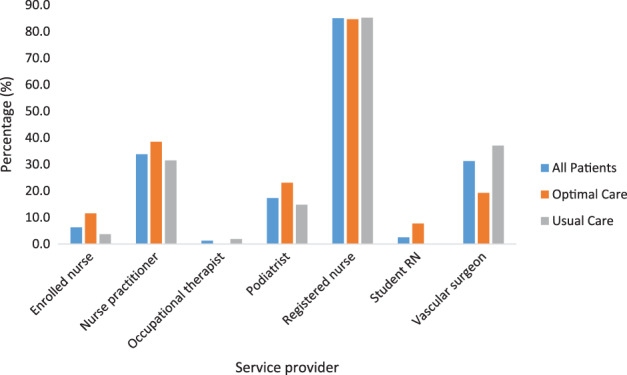
Proportions of participants receiving care from different service providers
3.2. Additional services use
At baseline, 52 participants (n = 18, (69.2%) optimal care, and n = 34, (63%) usual care) used services additional to their main clinic attendance for ulcer wound care (Figure 2).
Figure 2.
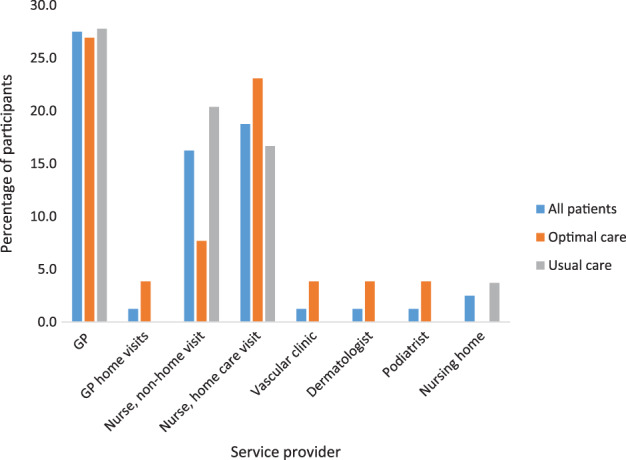
Use of additional services at baseline
3.3. Total wound management costs by clinic
The total weekly cost of care provision by the specialist wound clinic was $445.13, of which $123.00 was the health system cost, and $322.13 was the patient out‐of‐pocket cost (Figure 3). The costs associated with primary consultations appear to be the main contributor to high overall costs of care at the specialist wound clinic. Total weekly costs of care by the community wound clinics were $214.36, comprising $108.52 in health system costs and $105.84 in patient out‐of‐pocket costs. The cost of “other” medical services appears to be similar for all categories, perhaps because the care is mostly from community services and therefore invite fees of a similar magnitude. Product costs fell almost exclusively onto patients attending the specialist wound clinic and the community clinics. Patients who attended the specialist wound clinic paid $105.34, while those attending community clinics paid $68.87. The hospital outpatient clinic incurred health system product costs of $58.00, with no out‐of‐pocket costs to patients (Figure 3).
Figure 3.
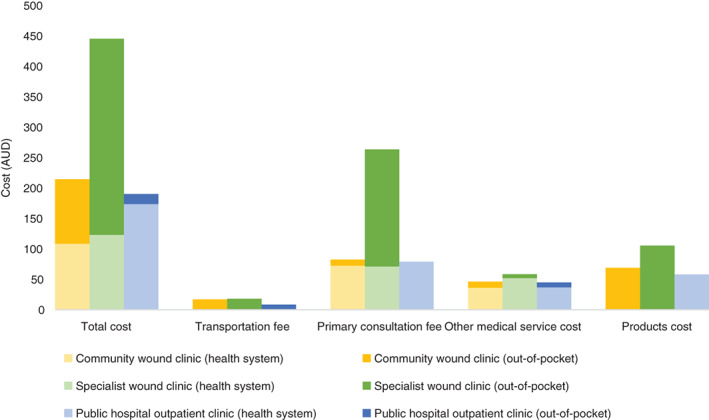
Weekly cost of venous leg ulcer (VLU) management by clinic
4. DISCUSSION
4.1. Costs
We present detailed findings of the cost of treating VLUs in the community setting in Australia. To our knowledge, this is the first study to present such detailed costs for this setting. Understanding costs is the first step to assessing cost‐effectiveness and to the better understanding of the burden this chronic health condition poses. These results have significant implications for VLU patients, policymakers, and health professionals.
We estimated an average total weekly cost of $214.61 and $294.72 for those in usual and optimal care groups, respectively. The optimal care group had higher costs overall, appearing to be driven by a higher cost of consultancy and product costs. Given that the majority of patients in the optimal care group attended the specialist wound clinic, it is likely the out‐of‐pocket payment to the clinic influenced these results. However, the extra cost of attending the specialist wound clinic may be offset by factors such as faster time to healing.
A 2010 study estimated out‐of‐pocket costs to patients for the management of a VLU, finding a cost of $114 ($157.6, 2018 dollars) per month,24, 25 while the present study estimated out‐of‐pocket costs of between $104.25 and $178.99 per week, which appears to be substantially different. One point of difference for the study was that participants self‐reported travel and consultation costs and, as the authors suggest, may have underestimated them for the use of private vehicles,24 while in the present study, costs of using a car for travel was calculated by the research team, and consultation costs were collected prospectively by the research team.
Another report5 assessed the cost‐effectiveness of VLU treatment in the Australian context. However, the report was unable to include out‐of‐pocket costs to patients for travel to wound clinics in economic models because of inadequate data.5
A third study measured costs for people living in the community, in Australia and Wales, with chronic wounds generally.26 This study found that participants spent AU$2475.00 (2017 dollars), on average, on wound dressing products since the wound started (with an average wound duration of 109 weeks).26 It also reported that people spent AU$121.82 on their wound in the most recent 28 days, which represented 10% of their disposable income.26 Although this study was not specific to VLUs, it supports our finding that the cost of wound care is significant.
4.2. Quality of life
The baseline utility score, 0.67 (SD ± 0.24), for all patients at baseline (ie, in the unhealed ulcer state) was similar to that found by Iglesias et al,9 who reported a score of 0.62 (standard error 0.02) based on the EQ‐5D tool. However, when comparing the type of care received, while usual care participants scores were similar to those reported by Iglesias et al,9 optimal care patients had a 0.75 (SD ± 0.16) average utility score at baseline. As participants were not matched by characteristics, it is not necessarily surprising that the groups differed. After 3 months, the utility scores estimated in the present study were slightly higher than those reported by Iglesias et al9 for the same time point.
4.3. Healing outcomes
Previous work found a median time to ulcer healing of 12 weeks (3 months),15 which compares similarly to our median findings of 3 months (IQR = 3 for usual care; IQR = 1.5 for optimal care) for both optimal and usual care groups. Time to healing was significantly different between usual care and optimal care groups. It is expected that decreased time to healing may reduce the overall costs for treating VLU, despite the continued recommendation for CT, which incurs additional costs. Apart from costs, considering the negative social functioning and QoL burden that ulcers can place on patients,7, 8 reduced time to healing may have implications for improved QoL and social function.
The finding that 61.5% of optimal care patients and 53.7% of usual care participants accessed two or more service providers is consistent with the literature, which suggests that patients often have to seek multiple pathways/providers to receive care.15
We described a 61.1% rate of CT in the usual care group for VLU care. With CT being a significant factor in guideline‐based care, this low rate is concerning, particularly as these patients continue to pay out‐of‐pocket costs for care but are not receiving guideline‐based treatment. A comparison could also be drawn between the lower costs of usual care and the lower rates of CT usage—compression bandaging systems and hosiery significantly contribute to upfront costs, particularly with the lack of reimbursement in place for this therapy.27
4.4. Limitations
The type of wound care (eg, compression/no compression) provided was recorded at baseline and was assumed to continue throughout the study. Recall bias may have influenced the estimation of the costs for health professional visits outside of the main treatment visit as this relied on patient self‐reported data. We assumed that patients used the same transport mode for all visits as travel costs were calculated from baseline data.
Participants who were recruited to the study had an existing ulcer and may have been receiving treatment for this ulcer for several months prior to recruitment. Hence, time to healing may have been underestimated and proportion of patients healed at 3 months in each treatment group should be interpreted with caution. In addition, the 18 patients lost to follow‐up may have included healed patients.
Participants were classified according to the available data. As such, some patients may have been misclassified. For example, they were receiving CT, but ABPI data were unavailable, and so, were classified into usual care. Some patient follow ups were conducted via telephone and relied on self‐reported data, without inspection of the wound by a health care professional.
We did not compare the compression systems used or the level of compression used by participants. However, the Australian and New Zealand Clinical Practice Guideline for the Prevention and Management of Venous Leg Ulcers recommends that some compression is better than no compression and, furthermore, do not recommend the use of a specific type of compression system but, rather, a range that is acceptable depending on the range of patient characteristics, including patient tolerance.10 Data were not collected on patient compliance with recommended therapy. Other studies have found that patient compliance with CT may be limited, and this could be the subject of further enquiry.28
5. CONCLUSION
Patients receiving guideline‐based optimal care incur higher costs initially, but these costs are expected to be outweighed by the long‐term gains through fast healing times and improved QoL. The higher costs of optimal care could be a contributing factor to the lower rates of patients receiving it, particularly as the majority of the costs are incurred by patients through product and service costs. If we are to begin closing the gap between the rates of optimal and usual care for patients, consideration should be given to providing reimbursement for wound‐related costs such as dressings, CT, and health care provision.
In addition, higher costs for optimal care could be outweighed by long‐term savings through faster healing times, reduced recurrence, and hospitalisation avoidance. This will be explored in an economic evaluation of optimal and usual care for VLUs using the data provided in this paper.
ACKNOWLEDGEMENTS
We thank Dr John Bingley and Alison Vallejo for their assistance with patient recruitment, data collection, and for providing clinical expertise. This research was conducted with support from the Wound Management Innovation Cooperative Research Centre (WMI CRC). We also acknowledge the support of the Australian Government's Cooperative Research Centres Program. The Wound Management Innovation Cooperative Research Centre (WMI CRC) received funding from the Australian Government, Curtin University of Technology, Queensland University of Technology, Smith & Nephew Pty Limited, Southern Cross University, University of South Australia, Wounds Australia, Blue Care, the Department of Health South Australia, the Department of Health Victoria, Ego Pharmaceuticals Pty Ltd, Metropolitan Health Service/Wounds West, Department of Health, Queensland, Royal District Nursing Service Limited, Royal Melbourne Institute of Technology, and Silver Chain Group. The funding sources played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the article; or in the decision to submit the article for publication.
Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. Int Wound J. 2019;16:112–121. 10.1111/iwj.13000
Funding information Wound Management Innovation Cooperative Research Centre (WMI CRC)
REFERENCES
- 1. Baker SR, Stacey MC, Jopp‐McKay AG, Hoskin SE, Thompson PJ. Epidemiology of chronic venous ulcers. Br J Surg. 1991;78(7):864‐867. [DOI] [PubMed] [Google Scholar]
- 2. Australian Bureau of Statistics . Population by age and sex, Australia, states and territories. Canberra, ACT, Australia. 2017. http://www.abs.gov.au/ausstats/abs@.nsf/0/1CD2B1952AFC5E7ACA257298000F2E76?OpenDocument. Updated December 13, 2017. Accessed March 5, 2018.
- 3. Ragnarson Tennvall G, Hjelmgren J. Annual costs of treatment for venous leg ulcers in Sweden and the United Kingdom. Wound Repair Regen. 2005;13(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 4. Purwins S, Herberger K, Debus ES, et al. Cost‐of‐illness of chronic leg ulcers in Germany. Int Wound J. 2010;7(2):97‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. KPMG . An economic evaluation of compression therapy for venous leg ulcers. Australian Wound Management Association; 2013. http://www.woundsaustralia.com.au/publications/kpmg_report_brief_2013.pdf. Accessed March 5, 2018.
- 6.Graves N, Zheng H. Modelling the direct health care costs of chronic wounds in Australia. Wound Pract Res. 2014;22(1):20‐24. 6–33. [Google Scholar]
- 7. Gonzalez‐Consuegra RV, Verdu J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011;67(5):926‐944. [DOI] [PubMed] [Google Scholar]
- 8. Green J, Jester R, McKinley R, Pooler A. The impact of chronic venous leg ulcers: a systematic review. J Wound Care. 2014;23(12):601‐612. [DOI] [PubMed] [Google Scholar]
- 9. Iglesias CP, Birks Y, Nelson EA, Scanlon E, Cullum NA. Quality of life of people with venous leg ulcers: a comparison of the discriminative and responsive characteristics of two generic and a disease specific instruments. Qual Life Res. 2005;14(7):1705‐1718. [DOI] [PubMed] [Google Scholar]
- 10. Australian Wound Management Association Inc. and New Zealand Wound Care Society . Australian and New Zealand Clinical Practice Guidelines for Prevention and Management of Venous Leg Ulcers. Watson, Australian Capital Territory: Cambridge Publishing; 2011. http://www.woundsaustralia.com.au/publications/2011_awma_vlug.pdf. Accessed March 5, 2018. [Google Scholar]
- 11. Australian Wound Management Association Inc. and New Zealand Wound Care Society . Flow chart for assessment of venous leg uclers 2011. http://www.woundsaustralia.com.au/publications/2011_assessment_flowchart_vlu.pdf. Accessed March 5, 2018.
- 12. Kruger AJ, Raptis S, Fitridge RA. Management practices of Australian surgeons in the treatment of venous ulcers. ANZ J Surg. 2003;73(9):687‐691. [DOI] [PubMed] [Google Scholar]
- 13. Coyer FM, Edwards HE, Finalayson KJ. National Institute for Clinical Studies Report for Phase 1, Evidence Uptake Network : Best Practice Community Care for Clients with Chronic Venous Leg Ulcers. Queensland University of Technology; 2005. https://eprints.qut.edu.au/54240/2/54240.pdf. Accessed March 5, 2018.
- 14. McGuckin M, Waterman R, Brooks J, et al. Validation of venous leg ulcer guidelines in the United States and United Kingdom. Am J Surg. 2002;183(2):132‐137. [DOI] [PubMed] [Google Scholar]
- 15. Edwards H, Finlayson K, Courtney M, Graves N, Gibb M, Parker C. Health service pathways for patients with chronic leg ulcers: identifying effective pathways for facilitation of evidence based wound care. BMC Health Serv Res. 2013;13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organisation . BMI Classification. 2016. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Updated March 6, 2018. Accessed March 6, 2018.
- 17. EuroQol Research Foundation . EQ‐5D‐5L—About. 2017. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/. Updated April 18, 2017. Accessed March 5, 2018.
- 18. Weller CD, Evans SM, Staples MP, Aldons P, McNeil JJ. Randomized clinical trial of three‐layer tubular bandaging system for venous leg ulcers. Wound Repair Regen. 2012;20(6):822‐829. [DOI] [PubMed] [Google Scholar]
- 19. Australian Taxation Office . Car expenses. 2017. https://www.ato.gov.au/Individuals/Income‐and‐deductions/Deductions‐you‐can‐claim/Vehicle‐and‐travel‐expenses/Car‐expenses/. Updated September 1, 2017. Accessed March 6, 2018.
- 20. Australian Government . MBS online. 2018. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home. Updated January 11, 2018. Accessed March 5, 2018.
- 21. Queensland Government . Wage rates—nursing stream. 2017. https://www.health.qld.gov.au/hrpolicies/wage_rates/nursing. Updated September 4, 2017. Accessed March 5, 2018.
- 22. Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health‐related quality of life: an EQ‐5D‐5L value set for England. Health Econ. 2018;27(1):7‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stangroom J. Social science statistics. [place unknown]. 2018. http://www.socscistatistics.com/tests/ztest/Default2.aspx. Accessed March 22, 2018.
- 24. Smith E, McGuiness W. Managing venous leg ulcers in the community: personal financial cost to sufferers. Wound Pract Res. 2010;18(3):134‐139. [Google Scholar]
- 25. Australian Bureau of Statistics . 6401.0—Consumer Price Index, Australia, Mar 2018, TABLE 7. CPI: Group, Sub‐group and Expenditure Class, Weighted Average of Eight Capital Cities. Canberra, Australia. 2018. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/6401.0Mar%202018?OpenDocument. Updated April 23, 2018. Accessed May 21, 2018.
- 26. Kapp S, Santamaria N. The financial and quality‐of‐life cost to patients living with a chronic wound in the community. Int Wound J. 2017;14(6):1108‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Norman RE, Gibb M, Dyer A, et al. Improved wound management at lower cost: a sensible goal for Australia. Int Wound J. 2016;13(3):303‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Ann Vasc Surg. 2007;21(6):790‐795. [DOI] [PubMed] [Google Scholar]


