Abstract
The use of pressure‐offloading support surfaces is considered the standard of care for pressure ulcers (PUs) by most surgeons. The fluid immersion simulation system (FIS) has shown significant results in previous studies. We compared it, for the first time, with a representative air‐fluidised bed (AFB) for outcomes related to post‐surgical flap closures. This trial was performed over 25 months, in which 40 subjects between 18 and 85 years of age with ≤2 PUs and history of <3 surgical closures underwent reconstruction by one surgeon. Subjects were randomly assigned to either treatment group for 2 weeks after closure. The primary endpoint was success of closure after the study period. Secondary endpoints included incidence of complications and nursing and patient acceptability of the device. The FIS group included 19 subjects, and the AFB group included 21. Flap failure rate was similar between groups (15% vs 17%; P = .99). The Minor complications rate, particularly dehiscence, was higher in the FIS group (66.7% vs 15%; P = .02). Nurse and patient self‐reported acceptability had better mean numeric scores in the FIS compared with AFB (nurse: 1.5 vs 1.9; P = .12; patient: 1.9 vs 2.2; P = .14). Further analysis will be conducted to gain better insight on the FIS as an alternative treatment for PUs.
Keywords: air‐fluidised bed, complications, fluid immersion simulation system, pressure ulcers, surgical flap closure
1. INTRODUCTION
The National Pressure Ulcer Advisory Panel (NPUAP) defines pressure ulcers (PUs) as “localized injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure or pressure in combination with shear.”1 Incidence rates vary significantly by clinical care setting, reported to be up to 38%.2 In the US acute care facilities alone, an estimated 2.5 million PUs are treated yearly.3 The risk factors associated with PU development are broad, including older age, African descent, lower body weight, physical or cognitive impairment, malnutrition, incontinence, and specific medical comorbidities that affect circulation such as diabetes or peripheral vascular disease.4, 5, 6, 7, 8 Tissue changes related to spinal cord injury, such as muscular atrophy, intramuscular fat infiltration, and bone adaptation, also play a key role in the internal tissue mechanics, making a patient more vulnerable to PUs.9
PUs are the result of the sequential and gradual damage associated with direct deformation, inflammatory response, and ischaemia. Interactions between weight‐bearing tissues and support surfaces or tissues that are continuously distorted by medical devices determine the loading state of tissues and cells. These are affected by intrinsic factors such as tissue composition, stiffness, and internal anatomy, as well as extrinsic factors of the specific support surface. Injury thresholds for the damage build‐up are variable and dynamic in nature. PU development begins at the microscopic level, with cell deformation that damages the integrity of the cytoskeleton, which in turn will promote the release of chemokines. Meanwhile, reactive oxygen and nitrogen species are released to degrade the extracellular matrix, resulting in more tissue damage. The combination of deformation caused by bodyweight or other external forces, with the intensifying effects of oedema that impact the onset and progression of inflammatory damage, and the associated high interstitial pressure obstruct the vasculature and impair blood perfusion at the damage site, resulting in chronic inflammation and a continuous vicious cycle of more tissue damage.10
In addition, microclimate on the wound area has been described as an indirect risk factor for PU development. Microclimate affects skin humidity, temperature, and airflow, which in turn affect the degree of soft tissue deformation and responses to extrinsic factors.11, 12 PUs are often associated with worse overall prognosis, prolonged hospitalisations, and greater mortality risk.13, 14, 15, 16, 17
For types III and IV PU, surgical intervention, such as flap reconstruction, should be considered. Selection of flap type warrants consideration of several variables, including aetiology, anatomy, previous reconstruction attempts, and likelihood to regain functionality; therefore, it is a decision based on the surgeon's experience and the subject's characteristics.18, 19, 20 Predictors of the ultimate success of flap reconstruction are also related to the risk factors associated with the development of PUs, including bacterial inoculation,21, 22 ulcer location,16, 23 postoperative pressure relief,24, 25, 26, 27, 28 and microclimate.11 Recidivism rates because of recurrence or flap failure are currently unacceptably high, with overall rates as high as 70%,29, 30 while overall complication rates have been reported to be up to 58.7%.17
Support surfaces represent an intervention that addresses the extrinsic factors that contribute to the rise of PUs. Examples of these include low air loss, alternating pressure, or air fluidised systems. The air‐fluidised bed (AFB) is composed of small beads with air forced through to create a fluid‐like surface that redistributes pressure. According to a review by Allman et al,31 there is a statistically significant decrease in total wound surface when comparing the AFB system with alternatives. Findings from one of these trials also demonstrated significant benefits regarding wound healing and pain compared with conventional surfaces, even with a repositioning regimen of every 4 hours rather than every 2 hours in the conventional surface group. These are the same rationales used for the postoperative care of flap closure.
Despite not having solid evidence of superiority among support surfaces, those devices that allow immersion offer a greater surface for load transfer and fit to the body contour, therefore offering a great cushioning potential.9, 32 The fluid immersion simulation system (FIS) represents a novel technology that leverages an advanced 3D immersion simulation of a fluid environment, thus achieving a greater load transfer to the surface and maintaining near‐normal blood flow and optimising tissue oxygenation. The system is fully autonomous, with monitoring of the support surface of over 100 times per second, facilitating constant adjustments based on subject repositioning in contrast to the AFB, which requires various adjustments by health care staff. A recent study demonstrated statistically significant improvements in tissue blood flow compared with standard bed and gurney systems, with 87% retention of perfusion on the FIS mattress vs 16% on standard mattresses.33 Moreover, a 36‐bed long‐term acute care hospital replaced a 6‐week post‐myocutaneous flap protocol with an AFB system with a 6‐week protocol using the FIS‐based protocol. After intervention, subjects achieved equivalent flap outcomes.34 A comparison between AFB system and FIS system can be found in Table 1.
Table 1.
Feature comparison between fluid immersion simulation and air‐fluidised bed systems
| FIS | AFB | |
|---|---|---|
| Mechanism | Simulates immersion of the patient in a fluid medium | Combines air‐fluidised and low air loss therapies |
| Adjustment | Automatically adjusts to each patient's weight, surface, and repositioning | Manual adjustment of pressure, height, and head elevation |
| Risk reduction |
|
|
| Patient weight limit | 500 pounds (226.8 kg) | 350 pounds (159 kg) |
| Travel range | 7″ to 30″ | 21.5″ to 34.75″ |
| Standard sleep surfaces | 76″ or 80″/up to 84″ | 84″ |
| Microclimate | No interference. Requires additional component for management | Superior microclimate management |
| Patient's acceptability | Patients may find the immersion simulation uncomfortable. | May cause insensible water loss. In neuropathic patients, it may cause uncomfortable warmth. |
| Recommended use |
|
|
Abbreviations: AFB, air‐fluidised bed; FIS, fluid immersion simulation; PUs, pressure ulcers.
In this study, we performed the first randomised, prospective analysis of the outcomes in the acute postoperative period after flap reconstruction of a PU. We compared complication rates and acceptability by both the subject and the provider between this novel alternative and what many providers consider standard of care.
2. METHODS
The present study was a prospective, randomised, single‐centre, parallel‐group, human subject trial with a 1:1 allocation ratio comparing the FIS (Joerns Healthcare, LLC, Charlotte, North Carolina) with a representative AFB (Hill‐Rom, Chicago, Illinois). Subjects received assigned study therapy for 14 days following definitive PU closure, reflecting current standard practice for PU management. During this period, data regarding the success of closure and incidence of complications were recorded. In addition, nurse and patient acceptability was also recorded through a quantitative survey given at 7 and 14 days after definitive closure (Figure 1).
Figure 1.
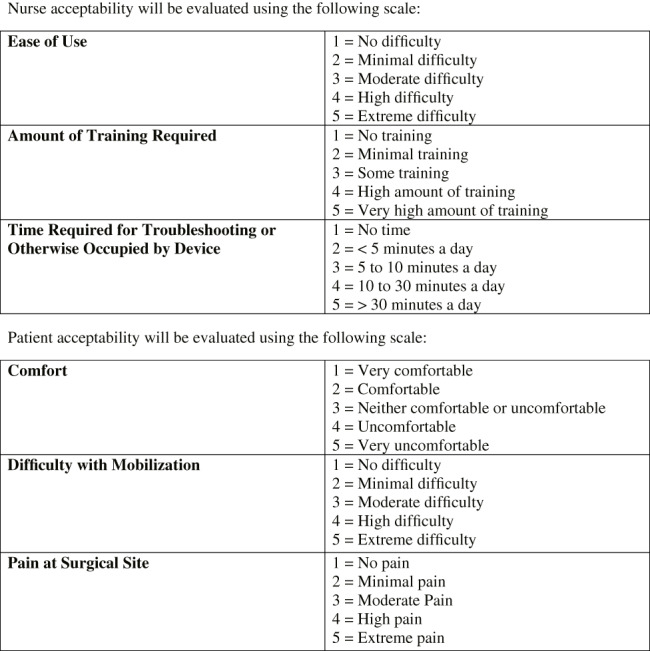
Nurse and patient acceptability survey form
Determination of a wound's appropriateness for definitive closure is often clinically subjective. Readiness for closure was established when the Principal Investigator (PI) perceived the wound to be adequately debrided and cleaned and determined that a definitive procedure could be performed. Subjects stayed hospitalised or were transferred to a step‐down facility for at least 14 days following their definitive surgical procedure according to their individual requirements. Subsequently, subjects were followed monthly for 1 year to evaluate the incidence of complications and the potential need for additional therapeutic interventions. The follow‐up period consisted of 365 (±20) days. Total duration of participation per subject could span up to 549 days. This is an ongoing study, and the present discussion is based on a midpoint analysis motivated by the observation of minor complications in both groups as detailed here.
Subjects who presented to Northwestern Memorial Hospital and were admitted as an inpatient for operative management of a stage III or IV PU were evaluated and approached to participate in the study.
During protocol development, an adaptive design was used to monitor the study to determine the target number of subjects required to achieve significance at the alpha = 0.05 level. A previous systematic review of complications following flap‐based surgery for PUs demonstrated a mean complication rate of 19.6%, with a SD of approximately 3% following the usage of perforator‐based flaps.24 This analysis determined that a difference in proportion of responders of at least 10% would be regarded as clinically meaningful. Assuming a 10% delta in proportion between support surfaces and a “confirmed” complication rate of approximately 20% with a SD of 5%, a sample size of 98 per group was needed to achieve a statistical power of 80% at the significance level of 0.05 (two‐sided). Therefore, a total of 200 subjects randomised, with an equal allocation ratio (1:1), to the FIS arm vs AFB arm were needed for the present study. This analysis was independently corroborated by our co‐author and statistician of the department, Irene B. Helenowski, who obtained similar results.
Inclusion criteria comprised admission as an inpatient, ≥18 and ≤85 years of age at time of consent, able to provide informed consent, deemed by the investigators to be reasonably compliant, having a PU meeting criteria for stage III or IV, not participating in a clinical trial within 30 days prior to consent, and having a 30‐day wound history available if the wound was previously treated.
Exclusion criteria encompassed life expectancy of <12 months, not being healthy enough to undergo surgery for any reason, history of radiation therapy, being of the opinion of the PI, non‐compliant, history of >3 closures of PUs in the same site, history of a bleeding disorder, and/or severe faecal incontinence.
Those who met eligibility criteria had the study explained to them by the PI. Informed consent was obtained from all subjects prior to undergoing study procedures. Data were collected either at bedside during subjects' hospitalisation or through external facilities' staff, in addition to electronic medical records.
Subjects who consented to study participation were assigned a unique screening number. Only one wound per subject was included in the study. Subjects with multiple wounds were assessed by the PI, who selected the most appropriate wound to include in the study. For subjects with multiple PUs, any PUs not selected as the study wound were not followed but otherwise received institutional standard wound care at the direction of the treating clinician. At the conclusion of the initial surgical debridement, if all inclusion and no exclusion criteria continued to be met, the subject was randomised into a study group and assigned a unique randomization number; otherwise, they were documented as a screen failure. The reason for exclusion was noted on the screening log.
At the time of surgical closure, if all inclusion and exclusion criteria continued to be met, the randomisation number became a unique Subject ID number; otherwise, they were documented as a randomisation failure. The reason for exclusion was noted on the randomisation log.
A focused medical and surgical history, physical exam, and wound history were obtained and recorded. This included onset and chronicity of the wound and anatomic location, as well as prior wound‐related surgeries and treatments.
Wounds were measured consistently following NPUAP recommendations by length, width, and depth using a ruler. Wound length was measured along the surface of the body in the longest dimension of the wound. Width was determined from the widest point of the wound that is perpendicular to the length in the same plane of measurement. Depth was measured from the surface of the skin to the deepest part of the wound bed perpendicular to the plane of measurement for the length and width. After measurement, the wound was debrided. At the conclusion of the debridement, the wound was irrigated with 5 L of normal saline and measured again.
If the wound was determined to be ready for immediate closure, the closure procedure could be performed. Closure at the time of the initial surgical debridement was considered the initiation of the study period.
Digital photographs of the wound were taken at the pre‐debridement stage and following initial debridement. During the subject's hospital admission, interventions other than the support surface utilised were based on institutional standard of care practices. These included wound dressings, topical applications, and the use of adjunctive therapies such as vacuum‐assisted closure.
The PI determined if additional surgical debridement was required after the initial operating room (OR) visit. This decision is typically based on ulcer appearance and periodic post‐debridement culture results. Additional wound debridement followed the same procedures as the initial OR visit.
Definitive wound closure for this study was defined as complete approximation of the wound edges, coverage of the wound via tissue transfer or skin graft, or any combination of these definitive techniques that results in complete elimination of the wound bed. For this study, only tissue transfer has been performed as a definitive closure technique. Debridement was performed as necessary prior to surgical closure along with the respective digital photographs and measurements. If a flap failed during the immediate postoperative period, the subject was removed from the study and transitioned to a standard institutional support surface.
The primary objective was to compare the effects of the FIS with AFB in PUs undergoing operative closure by determining the status of the wound (open or closed) after 2 weeks of treatment. In addition, subjects were followed for 14 days after closure for the secondary endpoint of wound complications (moist, maceration, drainage, dehiscence, epidermolysis, necrosis, and demarcation) as reported by the medical records.
Nurses and subjects completed an acceptability survey at 7 and 14 days, establishing the end of the study period, at which time the final assessment was also performed. This survey consisted of three questions for the nurse caring for each subject, assessing ease of use, amount of training required, and time required for troubleshooting, and three questions for the subject, assessing comfort, difficulty for mobilisation, and pain at surgical site. Either set of questions had a numerical representation from 1 to 5, where the best acceptability was the lowest (Figure 1). These outcomes were analysed and compared between both treatment arms.
At the end of the initial debridement procedure, subjects who continued to meet all inclusion and met no exclusion criteria were randomised in a 1:1 ratio to be treated with either the FIS or the AFB system. A stratified randomisation was used for this study to prevent imbalance between treatment arms. Permuted blocks were used to achieve equal number of subjects assigned to the FIS or the AFB to generate a randomisation schedule including subject numbers and treatment assignments. Envelopes were prepared corresponding to each row in the randomisation schedule, and each subject number and treatment group was printed on labels.
Prior to study initiation, sealed pre‐numbered randomisation envelopes were provided to the research staff and were used to obtain a randomisation assignment. Opening of the randomisation envelope occurred within 2 to 4 days before the scheduled surgical closure of the wound, along with confirmation that all inclusion and no exclusion criteria were encountered. Study staff used the randomisation number labels contained in the envelope.
The research staff noted treatment assignments and instructed the PI only after the closing procedure. Treatment therapy support surfaces were initiated following operative closure according to the manufacturer's recommendations.
Support surface therapy crossover prior to and during the study treatment period was not permitted.
Concealed therapy group assignments were stored in a cabinet and were opened only by research coordinators between 2 to 4 days before closing procedure for logistics purposes. Neither the subjects nor the surgeon were aware of the treatment group until the closing procedure was performed. After that, blinding was not possible.
Categorical variables were summarised by frequencies and percentages and assessed for differences between groups using Fisher's exact test. Continuous variables were summarised by means and SEs and assessed for differences between groups using the Wilcoxon rank‐sum test. Analyses were conducted in SAS v9.4 (SAS Institute, Cary, North Carolina).
3. RESULTS
From January 2016 to January 2018, 57 subjects were assessed for eligibility. Figure 2 shows the patients excluded and the reasons for their exclusion. After screening, 40 subjects were recruited; 1 subject was excluded after debridement, 2 subjects underwent a single‐stage flap closure, and 37 underwent a two‐stage flap closure. Study treatment started after the closure procedure except for two subjects. Both these subjects were removed from the study. Figure 2 shows in detail the transition of the subjects throughout the study period. The final sample included 12 subjects in the FIS group and 13 subjects in the AFB group.
Figure 2.
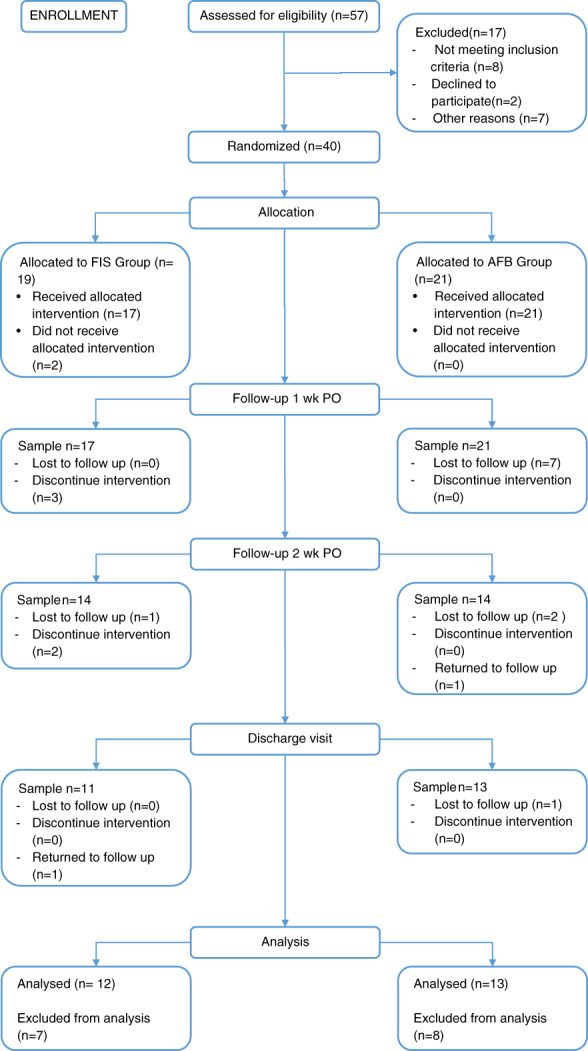
Participant flowchart. AFB, air‐fluidised bed; FIS, fluid immersion simulation; PO, postoperative
One surgeon, the PI, performed the debridement and closure procedures at the same site. Ultimately, 17 subjects completed the study period in our institution (FIS: 10 subjects; AFB: 7 subjects), and 8 subjects completed the study in an external facility (FIS: 2 subjects; AFB: 6 subjects).
There was no delay in randomisation; all subjects were randomised after the initial visit. The average interim time between the first intervention and closure was 8.1 days ± 5.13 (P = .52). Both support devices were started immediately after the closure procedure, except for the two subjects mentioned before. Treatment was uninterrupted during the 2‐week study period for both treatment arms, despite the length of stay as inpatients in our service. We shared institutional instructions and recommendations with the external facilities where subjects were discharged (Table 2).
Table 2.
Instructions for postoperative care for patients who underwent flap reconstruction (POD 1 ‐ POD 15)
|
Abbreviation: POD, postoperative day.
Clinical and epidemiological characteristics of the subjects and their distribution between therapy groups are summarised in Table 3.
Table 3.
Clinical baseline distribution between FIS and AFB groups
| FIS | AFB | P‐value | |||
|---|---|---|---|---|---|
| Mean age | 47.31 ± 12.26 | 46.14 ± 11.40 | 0.81 | ||
| n | 19 | 48% | 21 | 53% | |
| CI 95% | 5.51 | 4.88 | |||
| Gender | 0.54 | ||||
| Male | 12 | 63% | 11 | 52% | |
| Female | 7 | 37% | 10 | 48% | |
| Race/ethnicity | 0.76 | ||||
| Non‐Hispanic White | 12 | 63% | 11 | 52% | |
| African American | 6 | 32 | 6 | 29% | |
| Hispanic | 1 | 5% | 3 | 14% | |
| Other | 0 | 0% | 1 | 5% | |
| Tobacco use | 0.26 | ||||
| Current | 4 | 21% | 3 | 14% | |
| Past | 9 | 47% | 6 | 29% | |
| Never | 6 | 32% | 12 | 57% | |
| Diabetes status | 0.99 | ||||
| No | 16 | 84% | 16 | 76% | |
| Type 1 | 0 | 0% | 1 | 5% | |
| Type 2 | 3 | 16% | 4 | 19% | |
| Wound location | 0.66 | ||||
| Sacrum | 8 | 42% | 9 | 43% | |
| Ischium | 8 | 42% | 10 | 48% | |
| Trochanteric | 3 | 16% | 1 | 5% | |
| Other | 0 | 0% | 1 | 5% | |
| Number of wounds | 0.99 | ||||
| Multiple | 7 | 37% | 8 | 38% | |
| Single | 12 | 63% | 13 | 62% | |
| Pre‐closure measurements | |||||
| Length | 10.92 ± 4.51 | 9.05 ± 2.88 | 0.12 | ||
| Width | 8.33 ± 3.61 | 6.19 ± 2.17 | 0.08 | ||
| Depth | 4.03 ± 1.20 | 4.15 ± 1.90 | 0.56 | ||
| History of wound | |||||
| Recurrent | 4 | 21% | 4 | 19% | 0.99 |
| Non‐recurrent | 15 | 79% | 17 | 81% | |
| No previous Tx | 12 | 67% | 11 | 58% | 0.74 |
| Previous Tx | 6 | 33% | 8 | 42% | |
| NPWT | 3 | 60% | 4 | 67% | 0.45 |
| AMWT | 2 | 40% | 0 | 0% | |
| Hyperbaric | 0 | 0% | 1 | 17% | |
| Biologics | 0 | 0% | 1 | 17% | |
| Previous debrid. | 12 | 67% | 9 | 45% | 0.21 |
| No debrid. | 6 | 33% | 11 | 55% | |
| Prior closure | 2 | 11% | 5 | 25% | 0.41 |
| No closure | 17 | 89% | 15 | 75% | |
Abbreviations: AFB, air‐fluidised bed; AMWT, advanced moist wound therapy; CI, confidence interval; FIS, fluid immersion simulation; NPWT, negative pressure wound therapy.
Only 15 subjects from the FIS group and 20 subjects from the AFB group were considered for the complication analysis. In the FIS group, four subjects exited the study: one before closure, one after closure and two within the first 24 hours after closure. In the AFB group, two subjects were lost to follow up, but a complication was recorded in one of them before the loss. Complications were present in both groups, but they were significantly higher in the FIS group, accounting for 10 of 15 subjects (66.7%), while in the AFB group, they were present in 3 of 20 subjects (15%) (P = .004) (Figure 3).
Figure 3.
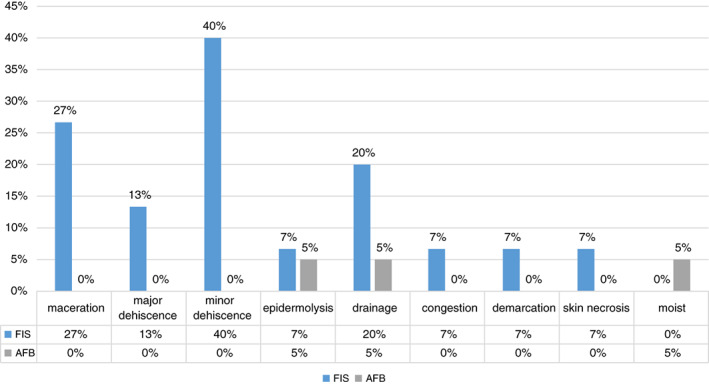
Complication rate distribution between treatment groups. AFB, air‐fluidised bed; FIS, fluid immersion simulation
Dehiscence was the most common complication, present in eight subjects from the FIS group and not present in the AFB group. However, when further analysing those subjects, we found that two of them (13%) were clinically significant: one of them required a re‐intervention, and the other was discontinued from the study and placed in prone position because of his large wounds (24 cm x 15 cm x 5 cm) doubling the mean length in our sample. The other six (40%) subjects' dehiscence represented a minor wound problem as they self‐resolved by postoperative day (POD) 14 in four of the subjects, by the first monthly follow up in one subject, and by the third monthly follow‐up in another subject (Table 4).
Table 4.
Outcomes of subjects presenting dehiscence as a complication
| Wound status at POD14 | Number of subjects | Outcome |
|---|---|---|
| Open | 2 | 1 self‐resolved (3 mo) |
| 1 required re‐intervention | ||
| Closed | 4 | 2 stayed closed |
| 1 reopen (1 mo) self‐resolved(2 mo) | ||
| 1 LTFU | ||
| Study exit | 2 | 1 self‐resolved |
| 1 open until 2 mo, LTFU |
Abbreviations: LTFU, lost to follow up; POD, postoperative day.
Other wound complications were maceration, present in four subjects (27%; P = .03) in the FIS group, and drainage, present in three subjects (20%) in the FIS and one (5%) in the AFB groups (P = .29). As expected, some subjects presented more than one complication. Our analysis at this point could not identify a correlation between risk factors and complications development).
The data from the acceptability survey on the first week was obtained from all of the eligible subjects and their nurses. The mean combined scores are presented in Table 5, where we can appreciate a slightly better acceptability (lower score) of the subjects in the FIS group, but this difference was not statistically significant (patient acceptability P = .14, nurse acceptability P = .12).
Table 5.
Nurse and patient acceptability
| Assessment | Ease of use | Required training | Time required | Overall nurse acceptability | Comfort | Difficulty moving | Pain | Overall patient acceptability |
|---|---|---|---|---|---|---|---|---|
| FIS | 1.3 (0.1) | 1.8 (0.2) | 1.6 (0.2) | 1.6 (0.1) | 2.0 (0.2) | 2.2 (0.3) | 1.5 (0.1) | 1.9 (0.2) |
| AFB | 1.5 (0.2) | 2.3 (0.2) | 2.0 (0.2) | 1.9 (0.2) | 2.2 (0.2) | 2.3 (0.2) | 1.9 (0.2) | 2.2 (0.1) |
Note: Mean scores obtained from acceptability survey. Standard error in parenthesis.
Abbreviations: AFB, air‐fluidised bed; FIS, fluid immersion simulation.
At the end of the study period, 25 subjects were eligible for assessment, 12 from the FIS group and 13 from the AFB group (Figure 4). At this point, 2 subjects per group, representing 15% and 17%, respectively, presented an open wound, while 10 (85%) and 11 (83%) subjects in each group presented a closed wound (P = .99).
Figure 4.
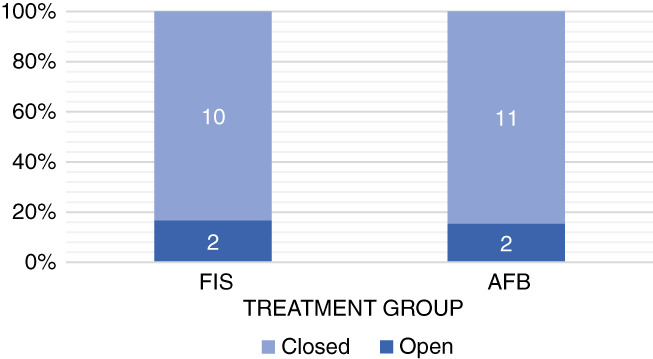
Wound status at POD14
The analysis of the two subjects in the FIS group who had open wounds by POD 14 was already mentioned in the complications section. Regarding the two subjects in the AFB group with open wounds, it is worth noting that one of them was not compliant with the care of the wound and was therefore excluded from the study, and the other one was lost to follow up before completing the study period.
4. DISCUSSION
Assessment of underlying conditions predisposing to PU, as well as the presence of urostomy and/or colostomy, was not the focus of this study, and therefore, no stratification for those factors was made. In addition, our institutional protocol is to discharge patients as early as possible, among other reasons, to avoid further exposure to pathogens. Only those who required to be hospitalised for complications related or unrelated to the intervention spent most or all of their postoperative period in our institution. Subjects being transferred to external facilities presented a challenge for the investigators that, in some cases, was not possible to overcome, resulting in loss to follow up.
There is general agreement on the standards of care with respect to the types of treatment used; nevertheless, evidence regarding superiority among support surfaces is still inconclusive and comparing postoperative care still sparse.32, 35 The inclusion of new technologies, both in the fabrication and the assessment of support surfaces, appears to contribute significantly to the biomechanics understanding and the application of this knowledge into developing these surfaces. Finite element modelling, although limited, has been useful for analysing multiple combinations of intrinsic and extrinsic factors that play a role in the development of PUs. Therefore, it could be helpful in the future to analyse and compare the FIS system with other support surfaces in terms of prevention and postoperative care.
Previous studies have not compared the FIS system with other standard‐of‐care support surfaces in terms of the success of operative closure and flap complications. The present study examines the potential benefits of the FIS compared with an AFB in order to offer a novel, suitable, and cost‐effective technology to prevent and manage PUs. We decided to perform a midpoint analysis of this study because of the presence of dehiscence in more subjects from the FIS group than from the AFB group to assess the possible benefits and disadvantages of the FIS system.
Despite the randomisation and similar clinical baseline data between treatment groups, we found a difference in the length of stay for all subjects participating in the study, which was statistically significant (FIS: 18.28 days ± 7.17 vs AFB: 10.05 days ± 5.38; P = .0003). This difference decreased but was still significant when we considered only the subjects who finished the study period (FIS: 16.17 days ± 4.11 vs AFB: 12.14 days ± 5.35; P = .04). This difference can be interpreted as patients who were randomly assigned to the FIS group being in worse general conditions than those in the AFB group.
Although our primary endpoint showed equivalence between both treatment groups, it is important to note that, during the 2‐week postoperative period, in the FIS group, five patients were discontinued from the study because of associated complications, mainly dehiscence, deeming it necessary to stop the treatment. Standard of care was delivered to these patients; therefore, the status of the wound at the end of the study period for these patients was not evaluated. This was different for the AFB group, where, despite the presence of minor complications, it was not necessary for them to the exit the study.
However, eight patients using AFB were lost to follow up, and the status of the wound at the end of the study period could not be assessed. Measures to decrease the number of losses have been taken, including calendar reminders for each patient, more than one contact number, detailed information from the external facilities, and making staff in these facilities aware of the ongoing investigation.
The level of acceptability by nurses and patients was slightly better, albeit insignificantly different, in the FIS group than in the AFB group. Despite this resemblance, the primary outcome should be taken into consideration when trying to determine non‐inferiority of the FIS over the AFB.
One of our hypotheses behind the higher frequency of minor complications, particularly dehiscence, in the FIS group is the mechanism of action. The AFB forces air through the beads inside, which also exits the mattress and achieves contact with the patient, thus actively affecting microclimate management, while the FIS has no active role on the microclimate. Complications like maceration and perhaps dehiscence could be related to this difference in mechanisms. Therefore, interventions towards avoiding extreme changes in the subjects' microclimate should be addressed.
In conclusion, despite more complications in the FIS than the AFB group, the status of the wound at the end of the study period was similar in both groups. We will have to further analyse the recurrence rate during a 1‐year period once we have concluded the study.
The results obtained in this midpoint analysis show that the FIS can be an adequate alternative to the traditional AFB. Taking into consideration that, despite the higher number of wound complications, subjects presented in the FIS group, there was a low percentage of those who were actually clinically significant as pointed out in our outcomes analysis.
Despite the demographic and clinical baseline data being similar in both treatment groups, and sample sizes maintaining balance throughout the study period, the overall sample size may be too small for our results to be generalisable at this point. We believe that, once we have reached our target sample size, we will not only reach a generalisable set of results but also incorporate additional observations on recurrence, microbiology, and other ancillary analysis, as well as examine whether the addition of interventions towards moisture management exert a direct measurable effect on complications.
CONFLICT OF INTEREST
The Principal Investigator, RDG, has been a consultant for Hill Rom, manufacturer of the AFB used as a comparator in this study.
ACKNOWLEDGEMENTS
The authors thank Kindred Healthcare staff for their collaboration and Northwestern University Plastic Surgery residents and staff for their help throughout the study. Special thanks to Salva Balbale, MS, for her guidance on writing this manuscript. This work was supported by Joerns Healthcare, LLC, Charlotte, NC.
Mendoza RA, Lorusso GA, Ferrer DA, et al. A prospective, randomised controlled trial evaluating the effectiveness of the fluid immersion simulation system vs an air‐fluidised bed system in the acute postoperative management of pressure ulcers: A midpoint study analysis. Int Wound J. 2019;16:989–999. 10.1111/iwj.13133
Funding information Joerns Healthcare, LLC
REFERENCES
- 1. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance . In: Haesler E, ed. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Perth, Australia: Cambridge Media; 2014. [Google Scholar]
- 2. Allman RM. Pressure ulcers among the elderly. N Engl J Med. 1989;320(13):850‐853. [DOI] [PubMed] [Google Scholar]
- 3. Staas WE Jr, Cioschi HM. Pressure sores‐‐a multifaceted approach to prevention and treatment. West J Med. 1991;154(5):539‐544. [PMC free article] [PubMed] [Google Scholar]
- 4. VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: an analysis of the 2006‐2007 international pressure ulcer prevalence surveys. J Nurs Care Qual. 2009;24(2):127‐135. [DOI] [PubMed] [Google Scholar]
- 5. Kottner J, Gefen A, Lahmann N. Weight and pressure ulcer occurrence: a secondary data analysis. Int J Nurs Stud. 2011;48(11):1339‐1348. [DOI] [PubMed] [Google Scholar]
- 6. Johansen E, Moore Z, Van Etten M, Strapp H. Pressure ulcer risk assessment and prevention: what difference does a risk scale make? A comparison between Norway and Ireland. J Wound Care. 2014;23(7):369‐378. [DOI] [PubMed] [Google Scholar]
- 7. Beeckman D, Van Lancker A, Van Hecke A, Verhaeghe S. A systematic review and meta‐analysis of incontinence‐associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health. 2014;37(3):204‐218. [DOI] [PubMed] [Google Scholar]
- 8. Garcia‐Fernandez FP, Agreda JJ, Verdu J, Pancorbo‐Hidalgo PL. A new theoretical model for the development of pressure ulcers and other dependence‐related lesions. J Nurs Scholarsh. 2014;46(1):28‐38. [DOI] [PubMed] [Google Scholar]
- 9. Levy A, Shoham N, Kopplin K, Gefen A. The critical characteristics of a good wheelchair cushion. In: Romanelli M, Clark M, Gefen A, Ciprandi G, eds. Science and Practice of Pressure Ulcer Management. 2nd ed. United Kingdom: Springer; 2018. pp. 17–31. [Google Scholar]
- 10. Gefen A. The future of pressure ulcer prevention is here: detecting and targeting inflammation early. EWMA J. 2018;19:7‐13. [Google Scholar]
- 11. Kottner J, Black J, Call E, Gefen A, Santamaria N. Microclimate: a critical review in the context of pressure ulcer prevention. Clin Biomech (Bristol, Avon). 2018;59:62‐70. [DOI] [PubMed] [Google Scholar]
- 12. Zeevi T, Levy A, Brauner N, Gefen A. Effects of ambient conditions on the risk of pressure injuries in bedridden patients‐multi‐physics modelling of microclimate. Int Wound J. 2018;15(3):402‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Control Hosp Epidemiol. 2005;26(3):293‐297. [DOI] [PubMed] [Google Scholar]
- 14. Russo CA, Steiner C, Spector W. Hospitalizations Related to Pressure Ulcers Among Adults 18 Years and Older, 2006: Statistical Brief #64. Rockville, MD: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 15. Cushing CA, Phillips LG. Evidence‐based medicine: pressure sores. Plast Reconstr Surg. 2013;132(6):1720‐1732. [DOI] [PubMed] [Google Scholar]
- 16. Schryvers OI, Stranc MF, Nance PW. Surgical treatment of pressure ulcers: 20‐year experience. Arch Phys Med Rehabil. 2000;81(12):1556‐1562. [DOI] [PubMed] [Google Scholar]
- 17. Bamba R, Madden JJ, Hoffman AN, et al. Flap reconstruction for pressure ulcers: an outcomes analysis. Plast Reconstr Surg Glob Open. 2017;5(1):e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurteau JE, Bostwick J, Nahai F, Hester R, Jurkiewicz MJ. V‐Y advancement of hamstring musculocuataneous flap for coverage of ischial pressure sores. Plast Reconstr Surg. 1981;68(4):539‐542. [DOI] [PubMed] [Google Scholar]
- 19. Tobin GR, Sanders BP, Man D, Weiner LJ. The biceps femoris myocutaneous advancement flap: a useful modification for ischial pressure ulcer reconstruction. Ann Plast Surg. 1981;6(5):396‐401. [DOI] [PubMed] [Google Scholar]
- 20. Bhattacharya S, Mishra RK. Pressure ulcers: current understanding and newer modalities of treatment. Indian J Plast Surg. 2015;48(1):4‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calderon W, Chang N, Mathes SJ. Comparison of the effect of bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1986;77(5):785‐794. [DOI] [PubMed] [Google Scholar]
- 22. Gosain A, Chang N, Mathes S, Hunt TK, Vasconez L. A study of the relationship between blood flow and bacterial inoculation in musculocutaneous and fasciocutaneous flaps. Plast Reconstr Surg. 1990;86(6):1152‐1162. [PubMed] [Google Scholar]
- 23. Biglari B, Buchler A, Reitzel T, et al. A retrospective study on flap complications after pressure ulcer surgery in spinal cord‐injured patients. Spinal Cord. 2014;52(1):80‐83. [DOI] [PubMed] [Google Scholar]
- 24. Bauer J, Phillips LG. MOC‐PSSM CME article: pressure sores. Plast Reconstr Surg. 2008;121(1 suppl):1‐10. [DOI] [PubMed] [Google Scholar]
- 25. Sameem M, Au M, Wood T, Farrokhyar F, Mahoney J. A systematic review of complication and recurrence rates of musculocutaneous, fasciocutaneous, and perforator‐based flaps for treatment of pressure sores. Plast Reconstr Surg. 2012;130(1):67e‐77e. [DOI] [PubMed] [Google Scholar]
- 26. McInnes E, Jammali‐Blasi A, Bell‐Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev. 2015;(9);CD001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tchanque‐Fossuo CN, Kuzon WM Jr. An evidence‐based approach to pressure sores. Plast Reconstr Surg. 2011;127(2):932‐939. [DOI] [PubMed] [Google Scholar]
- 28. Qaseem A, Humphrey LL, Forciea MA, Starkey M, Denberg TD. Clinical guidelines Committee of the American College of P. treatment of pressure ulcers: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2015;162(5):370‐379. [DOI] [PubMed] [Google Scholar]
- 29. Keys KA, Daniali LN, Warner KJ, Mathes DW. Multivariate predictors of failure after flap coverage of pressure ulcers. Plast Reconstr Surg. 2010;125(6):1725‐1734. [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto Y, Tsutsumida A, Murazumi M, Sugihara T. Long‐term outcome of pressure sores treated with flap coverage. Plast Reconstr Surg. 1997;100(5):1212‐1217. [DOI] [PubMed] [Google Scholar]
- 31. Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air‐fluidized beds or conventional therapy for pressure sores. A randomized trial. Ann Intern Med. 1987;107(5):641‐648. [DOI] [PubMed] [Google Scholar]
- 32. Levy A, Kopplin K, Gefen A. An air‐cell‐based cushion for pressure ulcer protection remarkably reduces tissue stresses in the seated buttocks with respect to foams: finite element studies. J Tissue Viability. 2014;23(1):13‐23. [DOI] [PubMed] [Google Scholar]
- 33. Kohanzadeh S, Breithaupt A, Bondarchulk A, et al. Effectiveness of the Biologics™ Dolphin bed as a tool to improve tissue perfusion in points of compression. Paper presented at: Symposium on Advanced Wound Care; 2009; San Diego, CA.
- 34. Mayes KL, Melendez J. Cost effective care without clinical compromise: incorporating the dolphin fluid immersion simulation mattress system into the postoperative care of patients undergoing myocutaneous flaps. Paper presented at: Wild on Wounds National Conference; September 12‐15, 2012; Las Vegas, NV.
- 35. Levine SM, Sinno S, Levine JP, Saadeh PB. An evidence‐based approach to the surgical management of pressure ulcers. Ann Plast Surg. 2012;69(4):482‐484. [DOI] [PubMed] [Google Scholar]


