Abstract
To determine the effective indications of closed‐incisional negative‐pressure wound therapy (ciNPWT) following total hip or knee arthroplasty, this systematic review and meta‐analysis was conducted. The systematic search was performed on MEDLINE, Embase, and Cochrane Library, and 11 studies were included. The studies comparing between ciNPWT and conventional dressings were categorised into following subgroups based on patient risk and revision procedures: routine vs high‐risk patient; primary vs revision arthroplasty. Pooled estimates were calculated for wound complication and surgical site infection (SSI) rates in the subgroup analyses using Review Manager. In high‐risk patients, the overall rates of wound complication (odds ratio [OR] = 0.38; 95% confidence interval [CI] 0.15‐0.93; P = .030) and SSI (OR = 0.24; 95% CI = 0.09‐0.64; P = .005) were significantly lower in the ciNPWT; however, there were no differences in routine patients. In cases involving revision arthroplasties, the overall rates of wound complication (OR = 0.33; 95% CI = 0.18‐0.62; P < .001) and SSI (OR = 0.26; 95% CI = 0.11‐0.66; P = .004) were significantly lower in the ciNPWT; however, there were no differences in cases involving primary arthroplasties. In summary, ciNPWT showed a positive effect in decreasing the rates of wound complication and SSI in high‐risk patients and in revision arthroplasties.
Keywords: closed‐incisional negative‐pressure wound therapy, surgical site infection, total hip arthroplasty, total knee arthroplasty, wound complication
1. INTRODUCTION
Total hip arthroplasty (THA) and total knee arthroplasty (TKA) are the most common and successful operations in modern medicine.1, 2 However, persistent surgical site complications (SSCs), such as wound complications and surgical site infection (SSI) after THA and TKA, are major sources for periprosthetic joint infection (PJI) and remain concerns to orthopaedic surgeons.3, 4, 5, 6 Despite the low incidence, deep PJI has a devastating impact on not only the heath burden but also the distress or the economic burden to patients.3, 7, 8, 9
Given its considerable burden, great efforts to identify preoperative risk factors and to prevent SSCs and PJI in various ways have been made to date. Previous studies have demonstrated that the risk of SSCs and PJI can be significantly different based on the individual patient and the surgical risk factors.2, 6, 10, 11 Tan et al 2 demonstrated that a patient's comorbidities and the revision procedures should be considered as valid risk factors for PJI and the incidence of developing PJI can vary from 0.6% to 20.6% based on the risk factors. Because patients with certain risk factors are frequently associated with SSCs, a variety of dressing materials were applied to prevent SSCs.4, 12, 13 However, the proper indication and the best choice of dressing materials for wound management after THA and TKA still remains unclear.
Closed‐incisional negative‐pressure wound therapy (ciNPWT) has been recently developed and has shown better efficacy in decreasing SSCs than conventional dressings after THA or TKA.3, 4, 10, 14 Although a recent study showed the routine application of ciNPWT to all patients to be a cost‐effective intervention to reduce SSCs after primary THA and TKA, 15 the cost of ciNPWT application is substantially increased over that of conventional dressings.
Therefore, we designed a systematic review and meta‐analysis to determine the effective indication for ciNPWT in wound management following THA or TKA. We asked the following questions: Does the use of ciNPWT following THA or TKA compared with conventional dressings reduce the incidence of wound complication or SSI in (a) high‐risk patients compared with routine patients? and (b) revision arthroplasties compared with primary arthroplasties?
2. MATERIALS AND METHODS
2.1. Literature search
The present systematic review followed the recommendation of the Cochrane review methods. Based on the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines, 16 multiple comprehensive literature databases, including PubMed (MEDLINE), Embase, and the Cochrane Library were searched for studies that reported on the outcomes of ciNPWT in wound management following THA or TKA up to September 1st, 2019 using a prior search strategy. There were no restrictions on language or the year of publication. The search terms used in the title, abstract, Medical Subjects Heading, and keywords fields included the following search methodology: (“ciNPT” OR “ciNPWT” OR “closed incisional negative pressure therapy” OR “closed incisional negative wound therapy” OR “negative pressure wound therapy” OR “NPWT” OR “vacuum assisted closure” OR “VAC”) AND [(“TKA” OR “total knee arthroplasty” OR “total knee replacement” OR “arthroplasty, replacement, knee”) OR (“THA” OR “total hip arthroplasty” OR “total hip replacement” OR “arthroplasty, replacement, hip”)]. Manual searches were also performed for articles that could have been missed by the electronic search.
2.2. Study selection
Two reviewers independently evaluated titles and abstracts of the identified studies and selected eligible studies for a full review. If the abstract showed insufficient information for a decision, the full text of the article was reviewed. Articles that satisfied the following criteria were selected in this systematic review: (a) patients who underwent THA or TKA using ciNPWT for their surgical incisions; (b) studies that directly compared ciNPWT and conventional dressings in terms of wound complications and SSI; and (c) studies that fully reported the complete numbers of patients or enabled the calculation of the number and proportion of patients regarding wound complications and SSI. Studies not clearly reporting data regarding either wound complication or SSI, indicating vague definition of terms between wound complication and SSI, biomechanical and cadaveric studies, technical notes, letters to the editor, expert opinions, review articles, meta‐analyses, scientific conference abstracts, and case reports were excluded. A study of cohorts undergoing ciNPWT for periprosthetic fractures of THA and TKA was also excluded.
2.3. Data extraction
Two investigators independently extracted data from each article using a predefined data extraction form. Any disagreements between two reviewers were solved by discussion. The extracted outcomes were SSCs including wound complications and SSI. Wound complications included wound discharge, wound dehiscence, hematoma, and seroma. The number of overall wound complications was reported in most included studies, if not, we added the number of specific wound complications. SSIs included both superficial and deep infection. Patient demographic, characteristic, and population data including sample size, mean age, sex, mean body mass index (BMI), and follow‐up period were recorded for each included study. If the follow‐up periods for wound complications and SSI were different, each follow‐up period was separately recorded. Details of wound management such as the specific material and duration of dressing changes were extracted from each included study. Details of study indications were also extracted from pooled studies such as whether routine patients were included or high‐risk patients having comorbidities were included, and whether primary and/or revision and THA and/or TKA was performed.
2.4. Assessment of methodological quality
Two investigators independently assessed the methodological quality of each study using the methodological index for non‐randomised studies (MINORS). 17 Using the MINORS checklist, the maximum score is 24 for a comparative study. Furthermore, MINORS has validity to assess the qualities of randomised controlled trials (RCTs) as well as non‐randomised studies. Any discrepancies in the scores between the two reviewers were resolved by discussion.
3. STATISTICS
Wound complication and SSI data recorded in the included studies were pooled. The main outcomes of the present study were mean differences in wound complication and SSI between ciNPWT and conventional dressings based on subgroups of patients as follows: routine patients vs high‐risk patients; primary arthroplastis vs revision arthroplastis. Thus, subgroup analyses of the studies were performed to determine the effective indication of ciNPWT after THA or TKA. Random‐effects meta‐analyses were performed to pool the outcomes across the included studies. Binary outcomes, such as the rates of wound complication and SSI were reported as odds ratios (ORs) and 95% confidence intervals (CIs). Heterogeneity was determined by estimating the proportion of between‐study inconsistencies because of actual differences between studies, rather than differences because of random error or chance, using the I 2 statistic, where 25% was considered low heterogeneity, 50% was considered moderate heterogeneity, and 75% was considered high heterogeneity. Forest plots were used to show the outcome, pooled estimate of effect, and overall summary effect of each study and constructed using the Review Manager software (RevMan version 5.3; Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration). A meta‐regression analysis was performed to assess the effects of age, sex, and follow‐up period on wound complication and SSI. Analyses were performed using RevMan version 5.3 and Open Meta‐Analyst (http://www.cebm.brown.edu/openmeta). Statistical significance was set at P < .05.
4. RESULTS
4.1. Identification of studies
Figure 1 shows the detail of the study identification, inclusion, and exclusion. An electronic search yielded 51 studies in PubMed (MEDLINE), 31 in Embase, and 35 in the Cochrane Library. An additional study was identified through manual searching. After removing 52 duplicate studies, 66 studies remained. After screening the titles and abstracts, and reading the full text, 55 studies were excluded. Thus, 11 studies were finally included in the present study, of which eight RCTs3, 4, 18, 19, 20, 21, 22, 23 and three cohort studies10, 14, 24 were eligible for data extraction and meta‐analysis.
FIGURE 1.
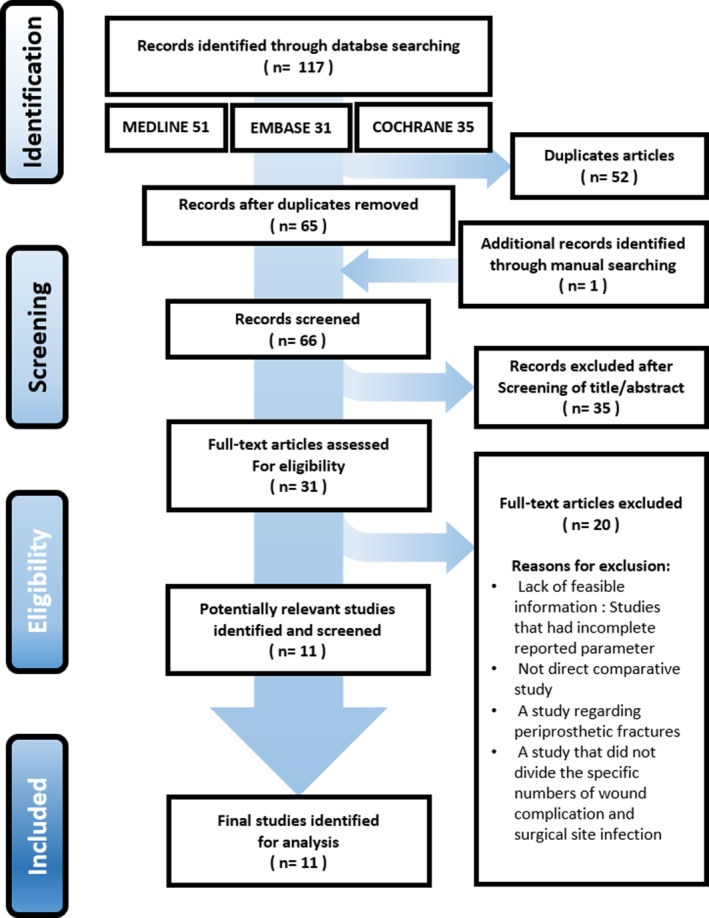
Flow diagram showing the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) methodology
4.2. Study characteristics and methodological quality assessment
A total of 1997 cases of THA or TKA were reported including 763 cases with ciNPWT management and 1234 cases with conventional wound management. The details of the study design and patient and population characteristics including age, percentage of female, mean BMI, follow‐up period, and MINORS quality score of each included study are summarised in Table 1. The median MINORS score of the included studies was 20 of 24 (range 16‐24). The details of wound management in ciNPWT and conventional wound dressings, the specific study indications for patients at risk, and the type of surgery are described in Table 2. Publication bias was not investigated for as it is not generally necessary when meta‐analyses include fewer than 10 studies. 25
TABLE 1.
The details of demographic data and quality scores of the included studies a
| Author | Year | Study type | Sample size (n) | Mean age (years) | Percentage of female | BMI (kg/m2) | Follow‐up | MINORS score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ciNPWT | Control | ciNPWT | Control | ciNPWT | Control | ciNPWT | Control | |||||
| Main findings | ||||||||||||
| Cooper and Bas | 2016 | RCS | 30 | 108 | 71.7 | 70.9 | NR | 31.3 | 29.6 |
Wound: 4 weeks SSI: over 34 months |
19 | |
| ciNPWT may decrease wound complications and SSIs in high‐risk patients with multiple risk factors for SSI undergoing revision TKA or TKA. | ||||||||||||
| Curley et al | 2018 | RCS | 32 | 159 | 63.4 | 59.5 | NR | NR | Retrospective chart review | 16 | ||
| A lower infection rate was observed for the ciNPWT patients who had high‐risk factors for SSI undergoing primary TKA as opposed to the dry sterile dressing patients, although this difference was not statistically significant. | ||||||||||||
| Giannini et al | 2018 | RCT | 50 | 50 | 66 | 66.8 | 62.0 | 64.0 | 27.7 | 28.2 | 1 week | 20 |
| The results of this study do not support the routine use of ciNPWT following revisional THA or TKA. However, it could be beneficial for selected patients once high‐risk factors for wound complications have been determined. | ||||||||||||
| Gillespie et al | 2015 | RCT | 35 | 35 | 63.8 | 62.5 | 42.9 | 51.4 | 29.9 | 29.8 | 6 weeks | 21 |
| A reduction of 3% in SSI incidence suggests that a definitive trial requires approximately 900 patients per group. Yet, there is uncertainty around the benefit of NPWT after primary THA. | ||||||||||||
| Howell et al | 2011 | RCT | 24 | 36 | NR | NR | NR |
Wound: 1 week SSI: 12 months |
21 | |||
| ciNPWT did not appear to prove lower wound complications in high‐risk patients following primary TKA; however, it was associated with blisters. | ||||||||||||
| Karlakki et al | 2016 | RCT | 102 | 107 | 69 | 69.2 | 52.0 | 48.6 | 30.1 | 28.4 | 6 weeks | 23 |
| ciNPWT has a beneficial role in patients undergoing primary THA or TKA to minimise wound complications. | ||||||||||||
| Keeney et al | 2019 | RCT | 185 | 213 | 60.6 | 60.5 | 60.5 | 57.3 | 34.6 | 36.5 |
Wound: 12 weeks SSI: 24 months |
20 |
| ciNPWT improved wound complication rates compared with conventional dressings in patients following primary or revisional THA or TKA; however, SSI rate was not significant difference. Patients with a body mass index >35 kg/m2 showed to be more susceptible to wound complications. Specific study in this high‐risk patient group may be helpful to define the value of iNPWT. | ||||||||||||
| Manoharan et al | 2016 | RCT | 21 | 36 | 66 | 42.4 | 29.8 | 1.5 weeks | 17 | |||
| There was no benefit in wound healing or cost with NPWT post‐TKA. There was some benefit in ciNPWT on quality of life factors less wound leakage and better protection. | ||||||||||||
| Newman et al | 2019 | RCT | 79 | 80 | 65 | 65 | 49.4 | 43.8 | 33.4 | 33.4 | 12 weeks | 24 |
| ciNPWT may decrease the rate of postoperative wound complications in patients who are at an increased risk of such wound issues after revision THA or TKA. | ||||||||||||
| Pachowsky et al | 2012 | RCT | 9 | 10 | 66.2 | 70.5 | NR | NR | 1.5 weeks | 18 | ||
| There was a decreased development of postoperative seromas in the wound and improved wound healing in patients who used ciNPWT following primary THA. | ||||||||||||
| Redfern et al | 2017 | RCS | 196 | 400 | 66.9 | 66.8 | 65.8 | 54.0 | 30.5 | 30.9 |
Wound: 6 weeks SSI: 2 months |
21 |
| ciNPWT for THA and TKA in a comprehensive patient population reduced overall incidence of wound complication, but did not significantly impact the rate of SSI. | ||||||||||||
BMI, body mass index; ciNPWT, closed incision negative pressure therapy; MINORS, methodological items for non‐randomised studies; NR, not reported; RCS, retrospective comparison studies; RCT, randomised controlled trials; SSI, surgical site infection; THA, total hip arthroplasty; TKA, total knee arthroplasty.
TABLE 2.
Summary of closed incision negative‐pressure therapy, conventional wound dressings, and details of study indication a
| Author | Year | ciNPWT | Conventional wound dressings | Indication | |||
|---|---|---|---|---|---|---|---|
| Material | Duration (day) | Material | Dressing changes | Surgery indication | Patients indication | ||
| Cooper and Bas | 2016 | Prevena (125 mm Hg, continuous) | 9.2 | AQUACEL Ag | Leave the dressing for a minimum of first 5 days | Revision THA or TKA | High‐risk factors for SSI: morbid obesity, multiple significant medical or social comorbidities, treatment of an infected joint arthroplasty, and wound closure under tension. |
| Curley et al | 2018 | Prevena (125 mm Hg, continuous) | 7 | Standard sterile gauze dressing | Depending on the wound leakage | Primary TKA | High‐risk factors for SSI: increased body mass index, smoking status, history of infection, and numerous comorbidities. |
| Giannini et al | 2018 | PICO (80 mm Hg, continuous) | 7 | Povidone‐iodine gauze dressing | Depending on the wound leakage | Revision THA or TKA | High‐risk factors for SSI (at least one risk factor): Age > 65 years, diabetes, smoking, obesity (BMI ≥30 kg/m2), hypertension, pulmonary disease, and vascular disease. |
| Gillespie et al | 2015 | PICO (80 mm Hg, continuous) | 5 | Hydrocolloid dressing | Depending on the wound leakage | Primary THA | Routine application to patients following arthroplasty. |
| Howell et al | 2011 | VAC (125 mm Hg, continuous) | 2 | Standard sterile gauze dressing | Leave the dressing on the second postoperative day | Primary TKA | High‐risk factors for SSI: Obesity (BMI >30 kg/m2) and enoxaparin sodium for deep venous thrombosis prophylaxis. |
| Karlakki et al | 2016 | PICO (80 mm Hg, continuous) | 7 | Mepore or Tegaderm | Mean days of dressing were 4.2 | Primary THA or TKA | Routine application to patients following arthroplasty. |
| Keeney et al | 2019 | PICO (80 ± 20 mm Hg, continuous) | 7 | Standard sterile gauze dressing | Subsequent dressing changes every 3 to 5 days | Primary or revision THA or TKA | Routine application to patients following arthroplasty. |
| Manoharan et al | 2016 | Prevena (125 mm Hg, continuous) | 8 | Standard sterile gauze dressing | Dressing changes on day 1 postoperatively | Primary TKA | Routine application to patients following arthroplasty. |
| Newman et al | 2019 | Prevena (125 mm Hg, continuous) | ≥2 | AQUACEL Ag | Dressing for 7 days | Revision THA or TKA | High‐risk factors for SSI: Obesity (BMI >35 kg/m2), use of anticoagulants other than aspirin, peripheral vascular disease, depression, diabetes mellitus, current smoker, history of a PJI in the limb undergoing revision surgery, on immunomodulators or corticosteroids, current history of cancer or haematological malignancy, inflammatory arthritis, renal failure or dialysis, malnutrition, liver disease, history of organ transplant, or HIV infection. |
| Pachowsky et al | 2012 | Prevena (125 mm Hg, continuous) | 5 | Standard sterile gauze dressing | Standard dressing changes | Primary THA | Routine application to patients following arthroplasty. |
| Redfern et al | 2017 | Prevena (125 mm Hg, continuous) | 7.1 | Standard sterile gauze dressing | Standard dressing changes | Primary THA or TKA | Routine application to patients following arthroplasty. |
BMI, body mass index; ciNPWT, closed‐incisional negative‐pressure wound therapy; HIV, human immunodeficiency virus; PJI, periprosthetic joint infection; SSI, surgical site infection; THA, total hip arthroplasty; TKA, total knee arthroplasty.
4.3. Routine patients vs high‐risk patients
In terms of wound complication, there were 6 studies with a total of 548 and 801 routine patients who received ciNPWT and conventional dressings, respectively. The overall rate of wound complication in routine patients was not significantly different between the ciNPWT and the conventional dressings (OR = 0.52; 95% CI = 0.21‐1.33; P = .17). Four studies included a total of 165 and 383 high‐risk patients for wound complication who received ciNPWT and conventional dressings, respectively. The overall rate of wound complication in high‐risk patients was significantly lower in the ciNPWT group than in the conventional dressings, and the summary OR was 0.38 (95% CI = 0.15‐0.93; P = .03) (Figure 2A). In terms of SSI, there were five studies with a total of 539 and 791 routine patients who received ciNPWT and conventional dressings, respectively. The overall rate of SSI in routine patients was not significantly different between the ciNPWT and the conventional dressings (OR = 0.49; 95% CI = 0.22‐1.11; P = .09). Five studies included a total of 215 and 433 high‐risk patients who received ciNPWT and conventional dressings, respectively. The overall rate of SSI in high‐risk patients was significantly lower in the ciNPWT group than in the conventional dressings, and the summary OR was 0.24 (95% CI = 0.09‐0.64; P = .005) (Figure 2B).
FIGURE 2.
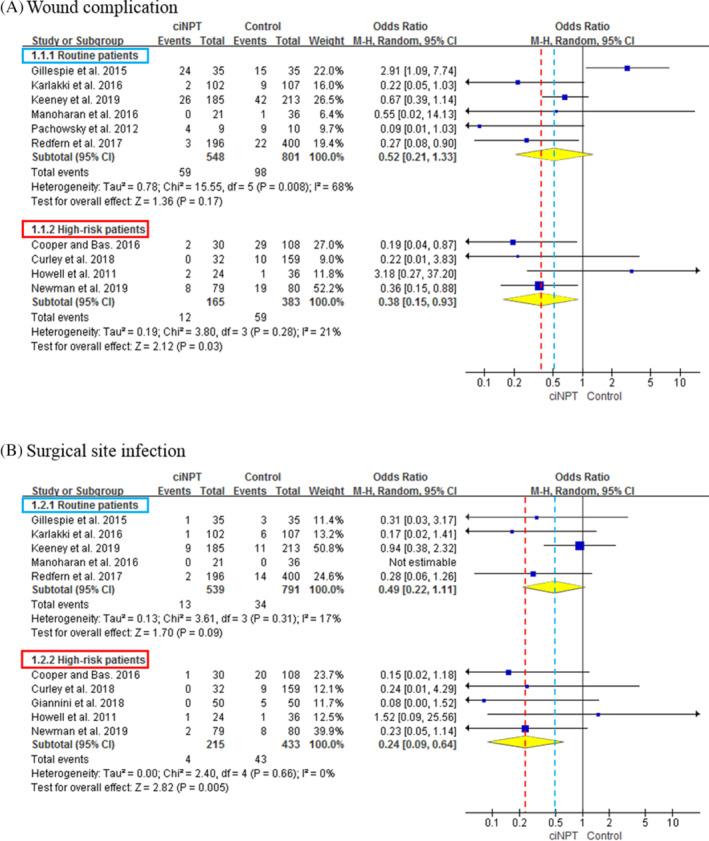
Forest plots showing the overall rates of wound complication, A, and surgical site infection (SSI), B, between the ciNPWT and control groups in routine patients and high‐risk patients. In routine patients, there were no differences in the rates of wound complication and SSI between the two groups. However, in high‐risk patients, the overall rates of wound complication (odds ratio [OR] = 0.38; 95% CI = 0.15‐0.93; P = .03) and SSI (OR = 0.24; 95% CI = 0.09‐0.64; P = .005) were significantly lower in the ciNPWT group. ciNPWT, closed‐incisional negative‐pressure wound therapy
4.4. Primary arthroplasty vs revision arthroplasty
In terms of wound complication, there were 8 studies with a total of 561 and 955 patients who received ciNPWT and conventional dressings, respectively, following primary arthroplasty. The overall wound complication rate in primary arthroplasty was not significantly different between the ciNPWT and the conventional dressings (OR = 0.59; 95% CI = 0.25‐1.40; P = 0.23). Three studies included a total of 152 and 229 patients for wound complication who received ciNPWT and conventional dressings, respectively, after revision arthroplasty. The overall wound complication rate in revision arthroplasty was significantly lower in the ciNPWT group than in the conventional dressings, and the summary OR was 0.33 (95% CI = 0.18‐0.62; P < .001) (Figure 3A). In terms of SSI, there were 7 studies with a total of 552 and 945 patients who received ciNPWT and conventional dressings, respectively, following primary arthroplasty. The overall rate of SSI in primary arthroplasty was not significantly different between the ciNPWT and the conventional dressings (OR = 0.54; 95% CI = 0.26‐1.12; P = .10). Four studies included a total of 202 and 279 patients who received ciNPWT and conventional dressings, respectively, following revision arthroplasty. The overall rate of SSI in revision arthroplasty was significantly lower in the ciNPWT group than in the conventional dressings, and the summary OR was 0.26 (95% CI = 0.11‐0.66; P = .004) (Figure 3B).
FIGURE 3.
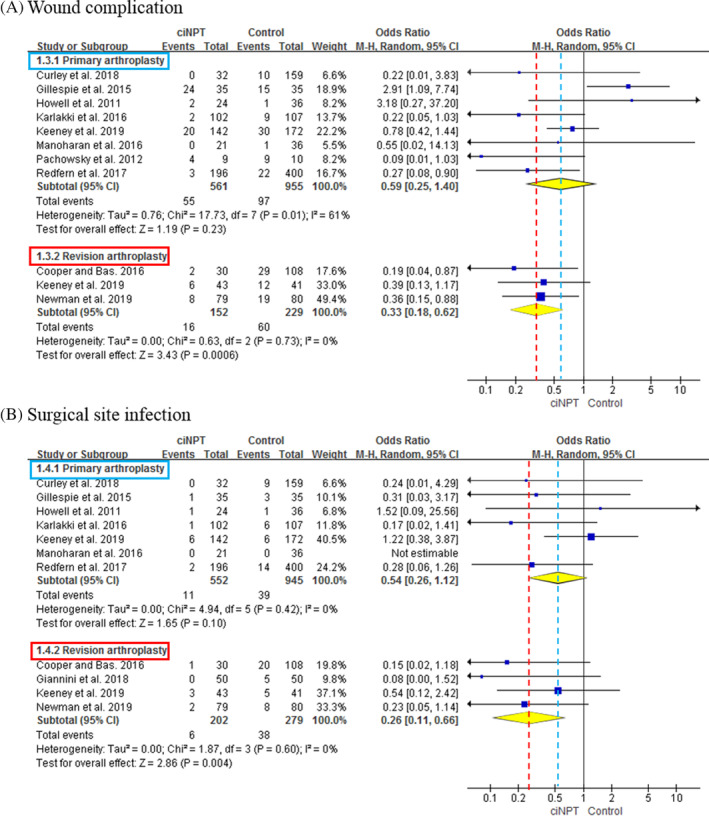
Forest plots showing the overall rates of wound complication, A, and surgical site infection (SSI), B, between the ciNPWT and control groups in primary arthroplasty and revision arthroplasty. In primary arthroplasty, there were no differences in the rates of wound complication and SSI between the two groups. However, in revision arthroplasty; the overall rates of wound complication (odd ratio [OR] = 0.33; 95% CI = 0.18‐0.62; P < .001) and SSI (OR = 0.26; 95% CI = 0.11‐0.66; P = .004) were significantly lower in the ciNPWT group. ciNPWT, closed‐incisional negative‐pressure wound therapy
4.5. Meta‐regression analysis
The results of the meta‐regression analyses are shown in Table 3. Patient characteristics including age, sex, and follow‐up were not significantly associated with the rates of wound complication and SSI.
TABLE 3.
Meta‐regression analysis for the assessment of the influence of age, percentage of female, body mass index, and follow‐up period on the wound complication and surgical site infection a
| Variable | Coefficient | SE | P value | 95% CI |
|---|---|---|---|---|
| Wound complication | ||||
| Age | −0.254 | 0.158 | .107 | −0.564 to 0.055 |
| Percentage of female | −0.112 | 0.122 | .360 | −0.352 to 0.128 |
| Follow‐up (week) | −0.091 | 0.265 | .731 | −0.611 to 0.429 |
| Surgical site infection | ||||
| Age | −0.197 | 0.174 | .257 | −0.539 to 0.144 |
| Percentage of female | −0.084 | 0.103 | .412 | −0.285 to 0.117 |
| Follow‐up (month) | 0.089 | 0.095 | .349 | −0.097 to 0.274 |
BMI, body mass index; CI, confidence interval.
5. DISCUSSION
Recent studies, differing in indications of surgical or patient risk factor, have reported outcomes of ciNPWT after THA or TKA. Although most outcomes were shown to be effective, the application of ciNPWT to all postoperative wounds would lead to considerable economic burden. Therefore, we performed this systematic review and meta‐analysis to assess the effective indications for ciNPWT in wound management following THA or TKA. The most important findings of this study are that wound complication and SSI were significantly less likely to occur in high‐risk patients or in revision arthroplasties using ciNPWT compared with conventional dressings. Conversely, there were no significant differences of wound complication and SSI in routine patients or in primary arthroplasties between ciNPWT and conventional dressings.
The number of the arthroplasties is expected to increase dramatically with the ageing population of late, 26 and the ageing population is likely to have several comorbidities. Patients with comorbidities have been shown to increase the risk of both SSC and PJI2, 6, 27; thus, accurate risk stratification of patients for SSC and PJI following THA or TKA is essential. Bozic et al 28 identified specific patient comorbidities such as rheumatoid disease, obesity, coagulopathy, and preoperative anaemia that were independently associated with an increased risk of PJI following THA. Namba et al 27 analysed 56 216 TKAs and demonstrated obesity, diabetes mellitus, male sex, American Society of Anesthesiologists score of ≥3, and posttraumatic arthritis are patient factors associated with PJI. Furthermore, several studies have similarly shown that comorbidities associated with immune deficiency, such as renal, rheumatologic, and liver disease, are related with SSC and PJI.2, 29, 30, 31 Although many studies have sought to identify risk factors for SSC and PJI, there are relatively little studies that have suggested a method to reduce SSC and PJI in patients having those risk factors, except for controlling or compensating for the comorbidities. Thus, the results of our study indicate that ciNPWT could be a new solution for reducing the wound complication and SSI risk in high‐risk patients with comorbidity after THA or TKA.
The prevalence of revision THA and TKA have been increasing with time as primary arthroplasties that have been performed in the past decades require revision and the surgical indications for primary arthroplasties had been broadened recently.10, 14, 26, 32 Compared with primary arthroplasties, the revision procedure of THA or TKA requires a longer surgical time, has a longer surgical incision and results in more difficult wound healing because of the previous scar, which frequently causes SSC and consequently increases the risk of PJI.2, 4, 10, 18, 33 Many studies have identified that the revision procedure is one of the most crucial risk factors for SSC and PJI after THA or TKA, resulting in poorer clinical outcomes, longer hospital stay, and greater economic burden.1, 2, 28, 34, 35, 36 Although several attempts to decrease the infection risk during the revision procedure have been shown such as using antibiotic‐laden cement, an irrigation solution of antibiotics, and prophylactic antibiotics, the efficacy of those attempts remains unclear.27, 37, 38 Given wound‐related complications are a great concern for revision arthroplasty, our results identified that ciNPWT significantly reduced wound complication and SSI in revision THA or TKA compared with conventional dressings.
Increasingly, ciNPWT systems have been applied to high‐risk wounds in various fields and showed a notable efficacy of reducing SSC.39, 40, 41, 42 Specific to patients following THA or TKA in our study, the use of ciNPWT similarly showed significantly lower rates of wound complication and SSI in high‐risk patients and in revision arthroplasties than with conventional dressings. Clearly, ciNPWT offers several potential benefits to improve wound healing and prevent SSI of closed surgical incisions. One important explanation may be a reduction of the relative motion on incisional edges by mechanical stabilisation.43, 44 Another explanation includes reducing dead space, subcutaneous hematoma, and seroma, and improving perfusion and lymphatic flow, all of which contribute to a better environment for wound healing.23, 44, 45 Other explanations may include that ciNPWT keeps surgical wounds sterile in the role of a mechanical barrier and requires fewer dressing changes and a longer duration from the initial application.10, 46, 47, 48
We acknowledge several limitations of this study. The heterogeneity of the demographic data among included studies, including differences in age, sex distribution, as well as differences in follow‐up duration, may be potential confounding factors. However, our meta‐regression analysis showed that age, sex distribution, BMI, and follow‐up were not significantly associated with rates of wound complication and SSI. Although surveillance for the first 12 months after THA and TKA is recommended,49, 50 only three included studies3, 10, 20 had more than 12 months of follow‐up. However, wound‐related SSI is likely to occur in the acute setting 51 and a follow‐up duration of less than 12 months might be acceptable to evaluate the efficacy of the ciNPWT system on wound management. Second, studies differing in the indication of patient comorbidities might include selection bias. Although a small difference in the specific indication of comorbidities might be a potential selection bias, the details of the indication had a similarity among the included studies of high‐risk patients according to Table 2. Third, included studies were fewer in subgroups of high‐risk patients and revision arthroplasty than in the subgroups of routine patients and primary arthroplasty, which might include confounding factors. However, the present study has a strength as the first systematic review and meta‐analysis regarding this topic because ciNPWT has recently started to be administered to patients undergoing THA or TKA. Finally, we could not perform a cost‐effectiveness analysis of the ciNPWT system because of a lack of published studies.
In conclusion, the current study showed that the application of ciNPWT reduced the incidence of wound complication and SSI in high‐risk patients and in revision procedures after THA or TKA compared with conventional dressings. Our findings suggest that ciNPWT should be considered for high‐risk patients and in revision procedures for wound management following THA or TKA.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
ACKNOWLEDGEMENTS
We thank to the library team in Samsung Medical Center for gathering the data. The authors did not receive any outside funding or grants in support of their research for or preparation of this work.
Kim J‐H, Lee D‐H. Are high‐risk patient and revision arthroplasty effective indications for closed‐incisional negative‐pressure wound therapy after total hip or knee arthroplasty? A systematic review and meta‐analysis. Int Wound J. 2020;17:1310–1322. 10.1111/iwj.13393
REFERENCES
- 1. Lavernia CJ, Alcerro JC. Quality of life and cost‐effectiveness 1 year after total hip arthroplasty. J Arthroplasty. 2011;26(5):705‐709. [DOI] [PubMed] [Google Scholar]
- 2. Tan TL, Maltenfort MG, Chen AF, et al. Development and evaluation of a preoperative risk calculator for periprosthetic joint infection following Total joint arthroplasty. J Bone Joint Surg Am. 2018;100(9):777‐785. [DOI] [PubMed] [Google Scholar]
- 3. Keeney JA, Cook JL, Clawson SW, Aggarwal A, Stannard JP. Incisional negative pressure wound therapy devices improve short‐term wound complications, but not long‐term infection rate following hip and knee arthroplasty. J Arthroplasty. 2019;34(4):723‐728. [DOI] [PubMed] [Google Scholar]
- 4. Newman JM, Siqueira MBP, Klika AK, Molloy RM, Barsoum WK, Higuera CA. Use of closed incisional negative pressure wound therapy after revision total hip and knee arthroplasty in patients at high risk for infection: a prospective, randomized clinical trial. J Arthroplasty. 2019;34(3):554‐559.e551. [DOI] [PubMed] [Google Scholar]
- 5. Weiss AP, Krackow KA. Persistent wound drainage after primary total knee arthroplasty. J Arthroplasty. 1993;8(3):285‐289. [DOI] [PubMed] [Google Scholar]
- 6. Patel VP, Walsh M, Sehgal B, Preston C, DeWal H, Di Cesare PE. Factors associated with prolonged wound drainage after primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2007;89(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 7. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984‐991. [DOI] [PubMed] [Google Scholar]
- 8. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61‐65. e61. [DOI] [PubMed] [Google Scholar]
- 9. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22‐28. [DOI] [PubMed] [Google Scholar]
- 10. Cooper HJ, Bas MA. Closed‐incision negative‐pressure therapy versus antimicrobial dressings after revision hip and knee surgery: a comparative study. J Arthroplasty. 2016;31(5):1047‐1052. [DOI] [PubMed] [Google Scholar]
- 11. Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient‐related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470(1):130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poultsides LA, Ma Y, Della Valle AG, Chiu YL, Sculco TP, Memtsoudis SG. In‐hospital surgical site infections after primary hip and knee arthroplasty—incidence and risk factors. J Arthroplasty. 2013;28(3):385‐389. [DOI] [PubMed] [Google Scholar]
- 13. Cai J, Karam JA, Parvizi J, Smith EB, Sharkey PF. Aquacel surgical dressing reduces the rate of acute PJI following total joint arthroplasty: a case‐control study. J Arthroplasty. 2014;29(6):1098‐1100. [DOI] [PubMed] [Google Scholar]
- 14. Redfern RE, Cameron‐Ruetz C, O'Drobinak SK, Chen JT, Beer KJ. Closed incision negative pressure therapy effects on postoperative infection and surgical site complication after total hip and knee arthroplasty. J Arthroplasty. 2017;32(11):3333‐3339. [DOI] [PubMed] [Google Scholar]
- 15. Nherera LM, Trueman P, Karlakki SL. Cost‐effectiveness analysis of single‐use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regen. 2017;25(3):474‐482. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. [DOI] [PubMed] [Google Scholar]
- 18. Giannini S, Mazzotti A, Luciani D, et al. Postoperative wound management with negative pressure wound therapy in knee and hip surgery: a randomised control trial. J Wound Care. 2018;27(8):520‐525. 10.12968/jowc.2018.27.8.520. [DOI] [PubMed] [Google Scholar]
- 19. Gillespie B, Rickard C, Thalib L, et al. Use of negative‐pressure wound dressings to prevent surgical site complications after primary hip arthroplasty: a pilot RCT. Surg Innov. 2015;22(5):488‐495. 10.1177/1553350615573583. [DOI] [PubMed] [Google Scholar]
- 20. Howell R, Hadley S, Strauss E, Pelham F. Blister formation with negative pressure dressings after total knee arthroplasty. Curr Orthop Prac. 2011;22(2):176‐179. 10.1097/BCO.0b013e31820b3e21. [DOI] [Google Scholar]
- 21. Karlakki S, Hamad A, Whittall C, Graham N, Banerjee R, Kuiper J. Incisional negative pressure wound therapy dressings (inpWTd) in routine primary hip and knee arthroplasties: a randomised controlled trial. Bone J Res. 2016;5(8):328‐337. 10.1302/2046-3758.58.BJR-2016-0022.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Manoharan V, Grant AL, Harris AC, Hazratwala K, Wilkinson MP, McEwen PJ. Closed incision negative pressure wound therapy vs conventional dry dressings after primary knee arthroplasty: a randomized controlled study. J Arthroplasty. 2016;31(11):2487‐2494. [DOI] [PubMed] [Google Scholar]
- 23. Pachowsky M, Gusinde J, Klein A, Lehrl S, Schulz‐Drost S, Schlechtweg P. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. International orthopaedics. 2011;36(4):719‐722. 10.1007/s00264-00011-01321-00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curley AJ, Terhune EB, Velott AT, Argintar EH. Outcomes of prophylactic negative pressure wound therapy in knee arthroplasty. Orthopedics. 2018;41(6):e837‐e840. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://handbook.cochrane.org/
- 26. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780‐785. [DOI] [PubMed] [Google Scholar]
- 27. Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95(9):775‐782. [DOI] [PubMed] [Google Scholar]
- 28. Bozic KJ, Lau E, Kurtz S, et al. Patient‐related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am. 2012;94(9):794‐800. [DOI] [PubMed] [Google Scholar]
- 29. Bozic KJ, Ong K, Lau E, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res. 2013;471(2):574‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCleery MA, Leach WJ, Norwood T. Rates of infection and revision in patients with renal disease undergoing total knee replacement in Scotland. J Bone Joint Surg Br. 2010;92(11):1535‐1539. [DOI] [PubMed] [Google Scholar]
- 31. Jiang SL, Schairer WW, Bozic KJ. Increased rates of periprosthetic joint infection in patients with cirrhosis undergoing total joint arthroplasty. Clin Orthop Relat Res. 2014;472(8):2483‐2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ricciardi BF, Liu AY, Qiu B, Myers TG, Thirukumaran CP. What is the association between hospital volume and complications after revision total joint arthroplasty: a large‐database study. Clin Orthop Relat Res. 2019;477(5):1221‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keswani A, Lovy AJ, Robinson J, Levy R, Chen D, Moucha CS. Risk factors predict increased length of stay and readmission rates in revision joint arthroplasty. J Arthroplasty. 2016;31(3):603‐608. [DOI] [PubMed] [Google Scholar]
- 34. Goltz DE, Baumgartner BT, Politzer CS, DiLallo M, Bolognesi MP, Seyler TM. The American College of Surgeons National Surgical Quality Improvement Program Surgical Risk Calculator has a role in predicting discharge to post‐acute care in total joint arthroplasty. J Arthroplasty. 2018;33(1):25‐29. [DOI] [PubMed] [Google Scholar]
- 35. Schairer WW, Sing DC, Vail TP, Bozic KJ. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Surin VV, Sundholm K, Backman L. Infection after total hip replacement. With special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65(4):412‐418. [DOI] [PubMed] [Google Scholar]
- 37. Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register‐based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 38. AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008;90(7):915‐919. [DOI] [PubMed] [Google Scholar]
- 39. Hyldig N, Birke‐Sorensen H, Kruse M, et al. Meta‐analysis of negative‐pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Semsarzadeh NN, Tadisina KK, Maddox J, Chopra K, Singh DP. Closed incision negative‐pressure therapy is associated with decreased surgical‐site infections: a meta‐analysis. Plast Reconstr Surg. 2015;136(3):592‐602. [DOI] [PubMed] [Google Scholar]
- 41. Stannard JP, Volgas DA, McGwin G 3rd, et al. Incisional negative pressure wound therapy after high‐risk lower extremity fractures. J Orthop Trauma. 2012;26(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 42. Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high‐risk patients with laparotomy incisions using negative‐pressure therapy. Am J Surg. 2013;205(6):647‐654. [DOI] [PubMed] [Google Scholar]
- 43. Wilkes RP, Kilpad DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov. 2012;19(1):67‐75. [DOI] [PubMed] [Google Scholar]
- 44. Kilpadi DV, Lessing C, Derrick K. Healed porcine incisions previously treated with a surgical incision management system: mechanical, histomorphometric, and gene expression properties. Aesthetic Plast Surg. 2014;38(4):767‐778. [DOI] [PubMed] [Google Scholar]
- 45. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen. 2011;19(5):588‐596. [DOI] [PubMed] [Google Scholar]
- 46. Liu D, Zhang L, Li T, et al. Negative‐pressure wound therapy enhances local inflammatory responses in acute infected soft‐tissue wound. Cell Biochem Biophys. 2014;70(1):539‐547. [DOI] [PubMed] [Google Scholar]
- 47. Morykwas MJ, Argenta LC, Shelton‐Brown EI, McGuirt W. Vacuum‐assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38(6):553‐562. [DOI] [PubMed] [Google Scholar]
- 48. Blum ML, Esser M, Richardson M, Paul E, Rosenfeldt FL. Negative pressure wound therapy reduces deep infection rate in open tibial fractures. J Orthop Trauma. 2012;26(9):499‐505. [DOI] [PubMed] [Google Scholar]
- 49. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250‐278. [DOI] [PubMed] [Google Scholar]
- 50. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97‐132. [PubMed] [Google Scholar]
- 51. Scalise A, Calamita R, Tartaglione C, et al. Improving wound healing and preventing surgical site complications of closed surgical incisions: a possible role of incisional negative pressure wound therapy. A systematic review of the literature. Int Wound J. 2016;13(6):1260‐1281. [DOI] [PMC free article] [PubMed] [Google Scholar]


