Abstract
Wound healing, especially diabetic ones, is a relevant clinical problem, so it is not surprising that surgical procedures are often needed. To overcome invasive procedures, several strategies with drugs or natural compound are used. Recently, in an experimental study, we described an increase in keratinocyte proliferation after their exposition to quercetin plus oleic acid. In the present clinical study, we evaluated both the clinical efficacy and the safety of nano‐hydrogel embedded with quercetin and oleic acid in the treatment of lower limb skin wound in patients with diabetes mellitus (DM). Fifty‐six DM patients (28 men and 28 women, mean age 61.7 ± 9.2 years) unsuccessfully treated with mechanical compression were enrolled and randomised to receive an add on treatment with hyaluronic acid (0.2%) or nano‐hydrogel embedded with quercetin and oleic acid. The treatment with nano‐hydrogel embedded with quercetin and oleic acid significantly (P < .01) reduced the wound healing time, in comparison to hyaluronic acid (0.2%) without developing of adverse drug reactions, suggesting that this formulation could be used in the management of wound healing even if other clinical trials must be performed in order to validate this observation.
Keywords: hyaluronic acid, nano‐hydrogel, oleic acid, quercetin, wound healing
1. INTRODUCTION
Wound healing is the physiological restorative and homeostatic response to tissue injury and consists of four overlapping classic phases (ie, haemostasis, inflammation, proliferation, and remodelling) where several cell populations and soluble mediators are involved.1 Pathological impairment of wound healing response causes persistent ulceration, as seen in diabetic patients or in subjects with vasculopathy, leading to a poor quality of life and a higher risk to develop infections.2 To date, the therapeutic approaches include surgical debridement (for local wound care), dressings promoting, a moist wound environment, wound off‐loading, vascular assessment, treatment of active infection, and glycemic control.3, 4, 5, 6, 7, 8, 9 Full thickness skin grafts are commonly used to cover the fingertip soft tissue defects and in some case the use of autologous growth factor blood derivatives is mandatory.1 However, new compounds from natural source as well as synthetic polymeric approaches based on hyaluronic acid, named nanohydrogel, represent an intense and promising field of research.10, 11 Previously, we documented the beneficial effects of flavonoids, in the treatment of chronic venous ulcerations4 and recently, it has been reported that, among the plethora of flavonoids, quercetin (Que) is able to promote wound healing process.12, 13, 14 On the other hand, oleic acid (OA), locally applied or took orally, is able to both modulate immune response and restore inflammatory acute phase in wound healing.14 Therefore, the aim of this pilot study was to evaluate both efficacy and safety of the nano‐hydrogel embedded with Que and OA as new topical formulation in the treatment of skin wound in diabetic patients.
2. METHODS
2.1. Drugs
Hyaluronic sodium salt (HA‐Na, Mw = 280 × 103) was provided by Contipro (Dolní Dobrouč, Czech Republic). Twelve men and 12 women, mean age 62 ± 9 years.
2.2. Patients
We performed a randomised, prospective, and parallel groups double blind study (patients and physicians did not know the group of treatment) from February 2017 to October 2019 in diabetes mellitus (DM) patients with foot skin wound admitted to the vascular division of the “Pugliese Ciaccio” Hospital of Catanzaro. The study protocol was approved by the Local Ethics Committee, and the work was conducted in agreement with Institutional Review Board/Human Subjects Research Committee requirements. Before the beginning of the study, all participants were informed about the aim, procedures, risks, and benefits of the study and they signed the informed consent.
2.2.1. Inclusion criteria
Patients of both sexes >18 year‐old and with chronic history of DM with foot skin wound.
2.2.2. Exclusion criteria
Hypersensitivity to flavonoids or to nutrients, severe clinical conditions (such as cancer, chronic hepatitis, human immunodeficiency virus, autoimmune diseases, platelet disorders, thrombocytopenia, bone marrow aplasia, uncompensated diabetes, sepsis, osteomyelitis, and loss of substance representing more than 50% of the segments); neuropsychiatric diseases (eg, psychosis and depression for the risk of low adherence to the treatment); local or systemic treatments (chemotherapy or radiotherapy, antiplatelets); non‐diabetic wound; patients that did not sign the informed consent.
2.3. End points
The primary efficacy end‐point was the statistically significant difference (P < .05) in the wound area between the two groups of treatment recorded during the follow‐ups.
The secondary efficacy end‐point was the statistically significant difference (P < .05) in the time of wound healing, between the two groups as well as the decrease in the indirect signs of inflammation (ie, oedema, redness, and pain). The primary safety end‐point was the difference (P < .05) in the development of skin adverse effects (eg, skin allergy or skin discomfort) during the topical administration of the compounds, resulting or not in a therapeutic intervention (eg, glucocorticoid administration). The relationship between adverse drug reactions (ADRs) and drug treatment was evaluated using the Naranjo adverse probability scale in agreement with our previous studies.15, 16 The secondary safety end‐point was defined as a statistically significant difference (P < .05) in the development of infections between the two groups. The development of infections was defined as the development of indirect signs of inflammation (ie, oedema, redness, and pain), resulting in a major therapeutic intervention (eg, surgery or antibiotic treatment).
2.4. Experimental protocol
Enrolled patients were randomised to receive an add on treatment with topical formulation of hyaluronic acid (2%) (Group A) or a topical formulation of nano‐hydrogel (0.2%) embedded with Qu and OA (equimolar doses) (Group B). In all patients, elastic (mechanical) compression was applied in order to avoid venous‐lymphatic interference with the regeneration process. To reduce the bias during the study, topical formulations were administered to patients, only for the first time, from the same nurse blinded to both treatment and experimental protocol. Before the hospital discharge, to each enrolled patient was given an anonymous formulation jar to continue the topical treatment at home. To exclude the loss of adherence at home, each patient and their caregiver (when older than 75 years) received a daily message on their mobile throughout the study period. During the study, a topical or systemic antimicrobial treatment was administered in accordance with clinical evaluation. Wound healing was assessed at the time of enrolment and after the discharge and during the follow‐ups using a direct ulcer tracing onto clear plastic sheet and subsequent computerised polarimetry, in agreement with our previous papers.17 In both groups, topical treatment was administered for 2 months, the follow‐ups were performed every week after the beginning of the treatment up to 12 months later (end of the study). The unresponsive patients were changed of group or made to undergo other treatments. In the presence of local infection detected during the follow‐up, the physician must add an antimicrobial treatment and in agreement with patient can choose to continue the study, to change the group, or to stop the study.
2.5. Statistical analysis
The statistical analysis was performed using the analysis of variance one‐way followed by Student–Newman–Keuls as post‐hoc test. The threshold of statistical significance was set at *P < .05. All statistical procedures were performed using the SPSS 21.0 software (SPSS Inc., Chicago, Illinois). In this experimental study, we did not determine a power calculation; therefore, this study must be evaluated as explorative.
3. RESULTS
After a detailed clinical history, 83 patients (43 men and 40 women, mean age 68.5 ± 9.6 years) with a history of diabetic foot ulcers (DFUs) unresponsive to the standard treatments were recruited, and 56 of these (28 men and 28 women, mean age 61.7 ± 9.2 years) were enclosed and randomised in two groups (Figure 1):
Figure 1.
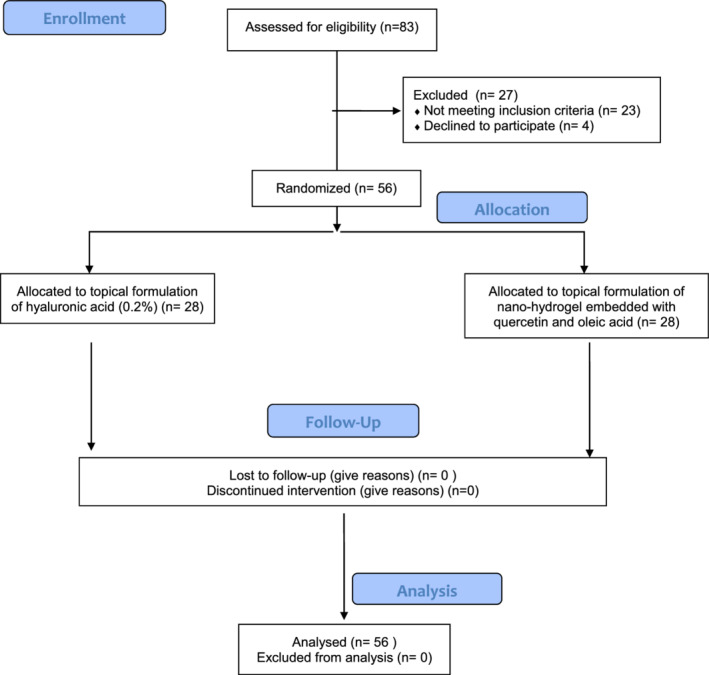
CONSORT 2010 flow diagram
Group A: hyaluronic acid (0.2%): 28 patients (14 men and 14 women; mean age 67.9 ± 8.4 years);
Group B: nano‐hydrogel embedded with Qu and OA: 28 patients (14 men and 14 women; mean age 69.3 ± 9.2 years).
At admission, enrolled patients showed similar comorbidity (ie, hypertension and chronic obstructive pulmonary disease [COPD]) and similar characteristic of ulcers (Table 1). All patients successfully completed the protocol and no one was lost during the follow‐ups.
Table 1.
Characteristics of patients enrolled in Group A (hyaluronic acid 2%) and in Group B (nano‐hydrogel embedded with quercetin plus oleic acid)
| Characteristics | Group A (n) | Group B (n) |
|---|---|---|
| Mean age | 67.9 ± 8.4 | 69.3 ± 9.2 |
| Male | 14 | 14 |
| Female | 14 | 14 |
| Diabetes type II | 28 | 28 |
| Blood hypertension | 21 | 20 |
| COPD | 15 | 14 |
| Malleolar wound | 16 | 17 |
| Hell wound | 8 | 8 |
| Finger wound | 4 | 3 |
3.1. EFFICACY OUTCOMES
Discomfort pain was more frequent in Group A (pain score measure through the visual analogical scale [VAS]: 4) than in Group B (VAS: 2), 5 days after procedure (P < .05). After 1 month of treatment, we documented a complete healing in nine patients of Group A (32.2%; VAS: 2) and in 19 of Group B (67.8%; VAS: 1) (P < .01), with a shorter time of healing (P < .01) in Group B (10 ± 5 days) with respect to the Group A (25 ± 4 days), depending on the wound size, wound depth, and general patient condition. Within 60 days, we documented a total re‐epithelization in 16 patients in Group A (57.1%) and in 26 patients enrolled in Group B (92.8%) (P < .01). The unresponsive patients enrolled in Group A (N:3 2 women and 1 man) were changed to Group B (nano‐hydrogel embedded with Qu and OA) with a complete wound healing in 1 month. Only two patients of 56 (3.6%) were unresponsive to the treatment after 3 months. In particular, one of these enrolled in Group B underwent surgery, the second patient changed from Group A to Group B with an initial healing 40 days later. No local recurrence was observed during the follow‐up period.
3.2. SECURITY OUTCOMES
Sixteen of 28 patients (57.2%) enrolled in Group A, developed a local infection, the patients chose to continue the study and to start an antimicrobial drug that induced the healing of infection. This event was not recorded in Group B. Moreover, during the study we did not record the development of other ADRs (eg, topical skin allergy, skin discoloration, or keloid scars) in any group.
4. DISCUSSION
DFUs are a prevalent complication of DM and the International Diabetes Federation reports that 9.1 to 26.1 million patients develop DFUs annually18 Walsh et al,19 described that patients with DFU have a 5% mortality in the first 12 months, 42% within 5 years, and a 2.5‐fold increased risk of death compared with diabetic patients without foot ulcers. Although the principles that guide the standard of care are rigorous, there is still a significant gap between our current and desired wound healing outcomes.20, 21 In this study, we evaluated the effects of a new compound (nano‐hydrogel embedded with Qu and OA) in the management of DFU.
We decide to use Qu and OA in equimolar concentration because recently we documented both in vivo and in vitro studies that an ester synthetic, derivative of those molecules, is able to restore skin lesions acting as a GPR40 agonist.22 It is worth noting that keratinocytes contain the enzymatic set capable to form esters. In the present observational blinded study, we recognised that this new topical formulation induced a rapid wound healing compared to topical administration of hyaluronic acid (2%).20 Currently, Qu and related flavonoids are well‐known phosphatidylinositol 3 kinase (PI3‐kinase), xanthine oxidase, cyclooxygenase inhibitors, and GPR40 agonist; therefore has anti‐inflammatory anti‐plateles and antiedematous effects. Similarly, OA modulates immune response in wound healing and restores inflammatory acute phase supporting the synergic effect of Qu and OA in the management of DFU. In order to improve these effects, we embedded these molecules in a nano‐hydrogel containing hyaluronic acid that is able both to reduce pain and improve tissue viscoelasticity. Moreover, several authors reported that hyaluronic acid hydrates and modulates the cellular microenvironment, while its cell surface receptor bindings induce cell‐to‐cell adhesions, cell‐substrate adhesions, proliferations, and cell migrations. Therefore, hyaluronic acid facilitates the entry of a large number of cells to the wound site and also contributes to the orientation of the extracellular matrix and fibrous component.23, 24, 25 Considering these effects, we reported an improvement with both formulations even if we reached the primary end‐point in nine patients treated with the new compound (nano‐hydrogel embedded with Qu and OA) respect to four patients treated with hyaluronic acid alone, supporting a synergistic affects between hyaluronic acid, quercetin, and OA. Interestingly, this formulation also induced a significant decrease in pain that could facilitate the topical treatment in chronic conditions. Only two patients did not reach the primary end‐point efficacy, but this could be related to the clinical condition of DFU. Furthermore, patients living with DFUs suffer great morbidity, lower health‐related quality of life, and poorer psychosocial adjustment26 and have a high risk of ADRs or local infections.
In our study, patients treated with hyaluronic acid alone developed a local infection that required an antimicrobial treatment, while the association of Qu plus OA embedded in nano‐hydrogel did not induce the development of topical or systemic side effects suggesting that it can be safely used in diabetic patients. This issue is very relevant and represents a strong opportunity considering that diabetic foot infection (eg, related to Staphylococcus aureus or Pseudomonas aeruginosa infection) is a frequent and severe complication in those patients27 in which antimicrobial/antibiotic treatment is mandatory, despite the presence of drug resistance.2, 28 This latter issue represents both, clinical and social problem and it is actually under consideration by the world health organisation. The clinical evidence herein highlighted by this formulation is in agreement with the action of each compound used alone. In fact, Qu by itself has strong antimicrobial and antioxidant activity,29, 30, 31 while AO increases the expression of vascular endothelial growth factor‐alpha (VEGF‐α) and interleukin‐1beta (IL‐1β).32 Finally, several authors documented that the treatment of DFUs accounts for approximately one‐third the total cost of diabetic care,33 but only few patients, about 20%, have unhealed DFUs at 1 year34, 35 and about 40% have a recurrence in 1 year.18 Even if in our study we do not performed an economic evaluation, we can affirm that the topical treatment was performed at home and did not require other treatment or hospitalisation, and also no recurrence occured, suggesting that this treatment could be cost/saving. The little number of enrolled patients represents the limitation of this study, but it has the merit to evaluate for the first time the effect of a new compound that could play a role in the management of this chronic disease. In conclusion, this pilot study documented that this new topical pharmacological formulation may be effective and safe for skin repair in diabetic patients. Furthermore, other clinical trials should be performed in order to validate these data in a large group of patients.
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
Gallelli G, Cione E, Serra R, et al. Nano‐hydrogel embedded with quercetin and oleic acid as a new formulation in the treatment of diabetic foot ulcer: A pilot study. Int Wound J. 2020;17:485–490. 10.1111/iwj.13299
REFERENCES
- 1. Romano F, Rizzo BA, Sacco L, et al. A novel use of growth factors, CD34 positive cells, and fibrin for fingertip injury: description of a case. J Dermatol Dermatol Surg. 2016;20:62‐64. [Google Scholar]
- 2. Serra R, Grande R, Butrico L, et al. Chronic wound infections: the role of Pseudomonas aeruginosa and Staphylococcus aureus . Expert Rev Anti Infect Ther. 2015;13(5):605‐613. [DOI] [PubMed] [Google Scholar]
- 3. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112‐126. [DOI] [PubMed] [Google Scholar]
- 4. Serra R, Gallelli L, Conti A, et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug des Devel Ther. 2014;8:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013;103(1):2‐7. [DOI] [PubMed] [Google Scholar]
- 6. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132‐e173. [DOI] [PubMed] [Google Scholar]
- 7. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence‐based global consensus. Diabetes Metab Res Rev. 2016;32(suppl 1):2‐6. [DOI] [PubMed] [Google Scholar]
- 8. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Metab Res Rev 2016;32 suppl 1: 7–15. [DOI] [PubMed] [Google Scholar]
- 9. Schaper NC, Van Netten JJ, Apelqvist J, Lipsky BA, Bakker K. Prevention and management of foot problems in diabetes: a summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Res Clin Pract. 2017;124:84‐92. [DOI] [PubMed] [Google Scholar]
- 10. Di Meo C, Martinez‐Martinez M, Coviello T, et al. Long‐circulating Hyaluronan‐based Nanohydrogels as carriers of hydrophobic drugs. Pharmaceutics. 2018;10(4):E213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manconi M, Manca ML, Caddeo C, et al. Preparation of gellan‐cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur J Pharm Biopharm. 2018;127:244‐249. [DOI] [PubMed] [Google Scholar]
- 12. Gopalakrishnan A, Ram M, Kumawat S, Tandan S, Kumar D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of VEGF and TGF‐beta1. Indian J Exp Biol. 2016;54(3):187‐195. [PubMed] [Google Scholar]
- 13. Hatahet T, Morille M, Hommoss A, Devoisselle JM, Muller RH, Begu S. Quercetin topical application, from conventional dosage forms to nanodosage forms. Eur J Pharm Biopharm. 2016;108:41‐53. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues HG, Vinolo MA, Magdalon J, et al. Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J Invest Dermatol. 2012;132(1):208‐215. [DOI] [PubMed] [Google Scholar]
- 15. Gallelli L, Ferreri G, Colosimo M, et al. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol Res. 2003;47(6):493‐499. [DOI] [PubMed] [Google Scholar]
- 16. Gallelli L, Staltari O, Palleria C, De Sarro G, Ferraro M. Hepatotoxicity induced by methimazole in a previously healthy patient. Curr Drug Saf. 2009;4(3):204‐206. [DOI] [PubMed] [Google Scholar]
- 17. Serra R, Gallelli L, Buffone G, et al. Doxycycline speeds up healing of chronic venous ulcers. Int Wound J. 2015;12(2):179‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367‐2375. [DOI] [PubMed] [Google Scholar]
- 19. Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabet Med. 2016;33(11):1493‐1498. [DOI] [PubMed] [Google Scholar]
- 20. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levy N, Gillibrand W. Management of diabetic foot ulcers in the community: an update. Br J Community Nurs. 2019;24(suppl 3):S14‐S19. [DOI] [PubMed] [Google Scholar]
- 22. Carullo G, Governa P, Leo A, et al. Quercetin‐3‐oleate contributes to skin wound healing targeting GPR40. ChemistrySelect. 2019;4:1‐6. [Google Scholar]
- 23. Cervelli V, De Angelis B, Lucarini L, et al. Tissue regeneration in loss of substance on the lower limbs through use of platelet‐rich plasma, stem cells from adipose tissue, and hyaluronic acid. Adv Skin Wound Care. 2010;23(6):262‐272. [DOI] [PubMed] [Google Scholar]
- 24. Costagliola M, Agrosi M. Second‐degree burns: a comparative, multicenter, randomized trial of hyaluronic acid plus silver sulfadiazine vs. silver sulfadiazine alone. Curr Med Res Opin. 2005;21(8):1235‐1240. [DOI] [PubMed] [Google Scholar]
- 25. Prosdocimi M, Bevilacqua C. Impaired wound healing in diabetes: the rationale for clinical use of hyaluronic acid plus silver sulfadiazine. Minerva Med. 2012;103(6):533‐539. [PubMed] [Google Scholar]
- 26. Goodridge D, Trepman E, Embil JM. Health‐related quality of life in diabetic patients with foot ulcers: literature review. J Wound Ostomy Continence Nurs. 2005;32(6):368‐377. [DOI] [PubMed] [Google Scholar]
- 27. Ambrosch A, Haefner S, Jude E, Lobmann R. Diabetic foot infections: microbiological aspects, current and future antibiotic therapy focusing on methicillin‐resistant Staphylococcus aureus . Int Wound J. 2011;8(6):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallelli L, Gallelli A, Vero G, et al. Acute renal failure probably induced by prulifloxacin in an elderly woman: a first case report. Clin Drug Investig. 2006;26(1):49‐53. [DOI] [PubMed] [Google Scholar]
- 29. Carvalho D, Paulino M, Polticelli F, Arredondo F, Williams RJ, Abin‐Carriquiry JA. Structural evidence of quercetin multi‐target bioactivity: a reverse virtual screening strategy. Eur J Pharm Sci. 2017;106:393‐403. [DOI] [PubMed] [Google Scholar]
- 30. Vu TT, Kim H, Tran VK, et al. Antibacterial activity of tannins isolated from Sapium baccatum extract and use for control of tomato bacterial wilt. PLoS One. 2017;12(7):e0181499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widsten P, Cruz CD, Fletcher GC, Pajak MA, McGhie TK. Tannins and extracts of fruit byproducts: antibacterial activity against foodborne bacteria and antioxidant capacity. J Agric Food Chem. 2014;62(46):11146‐11156. [DOI] [PubMed] [Google Scholar]
- 32. Pereira LM, Hatanaka E, Martins EF, et al. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem Funct. 2008;26(2):197‐204. [DOI] [PubMed] [Google Scholar]
- 33. de Smet GHJ, Kroese LF, Menon AG, et al. Oxygen therapies and their effects on wound healing. Wound Repair Regen. 2017;25(4):591‐608. [DOI] [PubMed] [Google Scholar]
- 34. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE study. Diabetologia. 2008;51(5):747‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vella L, Formosa C. Characteristics predicting the outcome in individuals with diabetic foot ulcerations. J Am Podiatr Med Assoc. 2017;107(3):180‐191. [DOI] [PubMed] [Google Scholar]


