Abstract
In recent years, the emergence of antibiotic resistant pathogens made increasingly difficult to establish appropriate empiric antimicrobial therapy protocols for acute diabetic foot infection (DFI) treatment. Early recognition of the population at‐risk for multidrug‐resistant (MDR) bacterial infection is of paramount importance in order to decrease large‐spectrum antibiotic overuse. This study used retrospective cohort study in a multidisciplinary tertiary diabetic foot unit. Patients with severe DFI were included and divided according to their infection resistance profile (susceptible vs MDR bacteria). Data regarding their comorbidities and length of hospital stay were collected. The primary endpoint was to determine the risk factors for MDR infections and to evaluate if these were associated with an increased length of stay (LOS). A total of 112 microbial isolates were included. Predominance of Gram‐positive bacteria was observed and 22.3% of isolated bacteria were MDR. Previous hospitalisation was associated with a higher likelihood of MDR infection. MDR bacterial infection was also associated with an increased LOS (P = .0296). Our study showed a high incidence of MDR bacteria in patients with a DFI, especially in those who had a recent hospitalisation. MDR infections were associated with a prolonged LOS and represent a global public health issue for which emergent measures are needed.
Keywords: bacterial infection, diabetic foot, drug resistance
1. INTRODUCTION
Diabetic foot infection (DFI) is the most common cause of nontraumatic amputation, hospitalisation, and reduction of quality of life in people with diabetes. 1
Most moderate and severe DFIs require systemic antibiotic therapy. Initial drug choice is usually empirical and based on the available clinical and epidemiologic data, 2 showing the importance of local population studies.
In recent years, the emergence of antibiotic resistant pathogens made increasingly difficult to select appropriate empirical antibiotic coverage for DFI treatment. 3 Based on our local findings, Neves et al 4 suggested piperacillin/tazobactam as the best first‐line empirical antibiotic option to treat moderate‐to‐severe DFI in our DF‐referral inpatient centre, with an additional Methicillin‐resistant Staphylococcus aureus (MRSA) coverage in high‐risk patients.
However, considering the dynamic environment of the microbiological flora in DFI, continuous monitoring of the bacterial prevalence in each diabetic foot centre is required. As such, this study aimed to analyse the microbiological profile of patients admitted to our diabetic foot centre during the entire year of 2018. Our purpose was also to identify the risk factors for the development of a multidrug‐resistant (MDR) infection and to determine if this type of infection was associated with an increase length of stay (LOS).
2. METHODS
A retrospective cohort study in a multidisciplinary tertiary diabetic foot unit was conducted. The electronic medical records of all patients hospitalised during a 1‐year period (2018) were retrieved and analysed for relevant epidemiological and clinical data.
The presence of an infected foot ulcer, localised below the malleolar process of a patient with diabetes mellitus, was the main inclusion criteria. DFIs are defined clinically (and not microbiologically) by the presence of at least two classic symptoms or signs of inflammation (erythema, warmth, tenderness, pain, or induration) or purulent exudate. 5 The assessment of infection severity was based on PEDIS scale (perfusion, extent, depth, infection, sensation), developed by the International Working Group on the Diabetic Foot. 5 Patients with moderate‐to‐severe disease (PEDIS grade 3 or 4) were included.
The first microbiological sample of all included patients was analysed. Superficial swabbed samples were collected by a trained physician after ulcer rinsing with saline water and gentle debridement of superficial debris, to avoid commensal flora isolates. In severe cases where emergent debridement was performed, deep incisional biopsies were also included in our study.
Standard processing methods for culture and antibiotic susceptibility test were used by our microbiology laboratory department. The anaerobic culture was not performed due to our lack of standard procedures for handling anaerobic samples. “Polymicrobial” cultures or cultures without isolation of a specific microorganism were excluded from the study. Isolates and respective in vitro antibiotic susceptibility and resistance profile were recorded. Patients were then divided into two groups, based on the presence (or absence) of an MDR infection.
The definition of MDR infection was based on international standards, published in 2011 by the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention joint initiative. 6
Statistical analysis for MDR risk factors assessment was performed using Pearson's chi‐square test. “Recent antibiotherapy” was defined by any registered antibiotic prescription during the last 6 months. “Hospitalisation during last year” included all the recorded inpatient admissions and not only those related to the current diabetic foot ulcer. A one‐sided t test was performed in order to access if MDR bacteria were associated with an increased LOS. A statistical significance of 0.05 with a confidence level of 95% was used. All analyses were performed with STATA 15, College Station, Texas: StataCorp.
3. RESULTS
We studied 103 hospitalisation episodes from 96 patients. Seven patients were readmitted to our centre during the study period and each admission was analysed separately as a new case. Most patients were male (71.2%) and the mean age was 67.9 (40‐90) years.
The first microbiological sample of every patient was analysed. Contaminated samples (n = 12), negative microbial results (n = 5), and samples collected after antibiotherapy initiation (n = 3) were excluded. Thirty samples had more than one isolate.
A total of 112 bacteria isolates were included in our study (Table 1). A Gram‐positive bacteria predominance was observed (58.1% of microbial isolates; 1.38/1 Gram‐positive/Gram‐negative ratio). S. aureus (SA) was the most common Gram‐positive bacteria (n = 34), followed by Streptococcus spp (n = 17) and Enterococcus spp (n = 14). Gram‐negative isolates included Pseudomonas spp (n = 12), Enterobacter spp. (n = 8), and Klebsiella spp. (n = 8).
TABLE 1.
Microbiologic profile in our diabetic foot unit
| Pathogens | |
|---|---|
| Gram‐positive bacteria | 65 |
S. aureus
|
34 26 8 |
Streptococcus spp
|
17 11 5 1 |
Enterococcus spp
|
14 14 |
| Gram‐negative bacteria | 47 |
Pseudomonas spp
|
12 11 1 |
Enterobacter spp
|
8 5 2 1 |
Klebsiella spp
|
8 6 2 |
Proteus spp
|
7 4 2 1 |
| M. morganii | 5 |
| E. coli | 4 |
| C. braakii | 1 |
| S. maltophilia | 1 |
| S. marcescens | 1 |
Nearly a quarter (22.3%; n = 25) of the isolated pathogens were MDRs. MRSA (23.5% of all the SA isolates) was the most frequent MDR bacteria (n = 8), representing 32% of total MDRs. Klebsiella pneumoniae (n = 4; 16% of MDRs), Pseudomonas aeruginosa (n = 4; 16%), and Escherichia coli (n = 3; 12%) were other frequent MDRs.
We analysed several risk factors and associated comorbidities (Table 2), namely recent antibiotherapy; hospitalisation during last year; peripheral artery disease; chronic kidney disease; retinopathy; and arterial hypertension.
TABLE 2.
Proposed multidrug‐resistant (MDR)‐associated risk factors
| Proposed risk factors | MDR (n) | Non‐MDR (n) | P value |
|---|---|---|---|
| Recent antibiotherapy | 76% (19) | 67.8% (59) | .433 |
| Hospitalisation during last year | 52% (13) | 21.8% (19) | .003 |
| Peripheral artery disease | 84% (21) | 73.5% (64) | .282 |
| Chronic kidney disease | 24% (6) | 21.8% (19) | .819 |
| Retinopathy | 20% (5) | 22.9% (20) | .752 |
| Hypertension | 64% (16) | 63.2% (55) | .942 |
Patients with a hospitalisation during the last year had a higher likelihood of MDR infection (P = .03). None of the other proposed risk factors were considered to be statistically significant.
The mean LOS in patients infected by an MDR bacteria was 79.8 days and in non‐MDR patients was 57.6 days (Figure 1). Using a one‐sided t test, patients with an MDR infection had a prolonged LOS (P = .0296). Excluding MRSA infections (covered by a different empirical antibiotic scheme), the other MDR bacteria (n = 17) showed a 64.7% resistance to the proposed empirical antibiotherapy regimen with piperacillin‐tazobactam. In the non‐MDR group (excluding SA infections; n = 62), the piperacillin‐tazobactam resistance was 6.4% (n = 4). The overall resistance for piperacillin‐tazobactam in non‐SA infections (n = 79) was 26.5% (n = 21). Ertapenem resistance was 12% (n = 3) in the MDR group (excluding MRSA). Overall resistance to ertapenem was 36.7% in the non‐SA group (n = 29).
FIGURE 1.
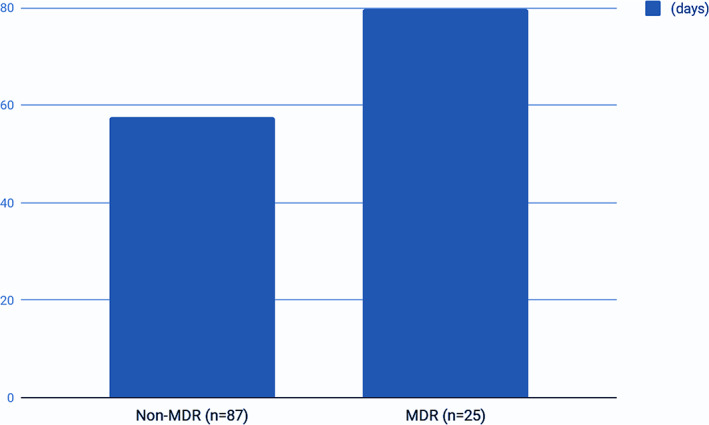
Length of hospital stay (LOS) in multidrug‐resistant (MDR) and non‐MDR patients
4. DISCUSSION
DF infectious process causes a vicious cycle of extensive decompensation in glucose metabolism, as hyperglycemia further increases the severity of the infection itself. 7 As such, proper antibiotic coverage is a key element in early stabilisation of these patients and should be based on known potential etiologic agents.
In Portugal, few studies have been performed to characterise the local causative pathogens of DFIs. An apparent Gram‐positive predominance (especially of Staphylococcus) has been initially suggested 4 , 8 , 9 , 10 but some authors had recently described a shift towards Gram‐negative isolates, possibly in relation with the rising prevalence of neuroischemic infected ulcers. 11 Our results do not corroborate these latest findings but we acknowledge that they should be properly confirmed by further larger studies.
Regarding MDR infections, a high incidence has been ubiquitously described 4 , 8 , 9 , 10 , 11 and our study also highlights that these infections correlate with an increased LOS. Despite controversy regarding the association of MDR infections with a worse outcome in DFIs, 12 , 13 , 14 our data are a surrogate marker for a poor overall prognosis.
Some authors suggested that previous hospitalisation, 14 , 15 long‐standing diabetes, 16 recent antibiotherapy, 15 retinopathy, 17 ulcer size, 15 , 16 and the presence of osteomyelitis 14 , 15 were associated with an increased risk of an MDR infection. In our study, we were able to show that hospitalisation during the previous year is a statistically significant risk factor for the occurrence of DFI by MDR bacteria. However, given our limited sample and the retrospective nature of our study, we were unable to find any other factors associated with a statistically significant increased risk for an MDR infection.
The absence of well‐defined, evidence‐based policy of broad spectrum antibiotherapy has led to overuse and to a strikingly increase in the number of MDR bacteria. 18 Our study shows an alarming resistance of these agents to a recently optimised empirical antibiotherapy regimen, suggesting that we are running off of proper antibiotics for DFI. 18 , 19 , 20 , 21 Currently in our unit we are facing a huge dilemma: should we use piperacillin‐tazobactam, prioritising a better overall coverage but knowing that we are delaying proper care for the severe MDR infections, linked to poorer outcomes? Or must we be focused on these MDR agents, favouring ertapenem usage and acknowledging that we are allowing more non‐MDR to be mistreated?
In our view, the solution relies on multiple empiric antibiotic regimens that are guided by the patient risk factors for MDR infections. As such, we believe that future studies should be focused on the development of a reliable risk stratification tool, allowing proper antibiotic selection for both high and low‐risk patients.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Pessoa e Costa T, Duarte B, João AL, et al. Multidrug‐resistant bacteria in diabetic foot infections: Experience from a portuguese tertiary centre. Int Wound J. 2020;17:1835–1839. 10.1111/iwj.13473
REFERENCES
- 1. Boulton AJM, Vileikyte L, Ragnarson‐Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005. Nov;366(9498):1719‐1724. [DOI] [PubMed] [Google Scholar]
- 2. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013. Jan;103(1):2‐7. [DOI] [PubMed] [Google Scholar]
- 3. Olid AS, Sola I, Barajas‐Nava LA, Gianneo OD, Cosp XB, Lipsky BA. Systemic antibiotics for treating diabetic foot infections. Cochrane Database Syst Rev. 2015;(9):CD009061. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009061.pub2/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Neves JM, Duarte B, Pinto M, Formiga A, Neves J. Diabetic foot infection: causative pathogens and empiric antibiotherapy considerations—the experience of a tertiary center. Int J Low Extrem Wounds. 2019;18(2):122‐128. [DOI] [PubMed] [Google Scholar]
- 5. Lipsky BA, Aragón‐Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016. Jan;32(suppl 1):45‐74. [DOI] [PubMed] [Google Scholar]
- 6. Magiorakos A‐P, Srinivasan A, Carey RB, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012. Mar;18(3):268‐281. [DOI] [PubMed] [Google Scholar]
- 7. Butranova OI, Razdrogina TN. Antibiotics for skin and soft tissues infections in type 2 diabetes mellitus. Int J Risk Saf Med. 2015;27(suppl 1):S57‐S58. [DOI] [PubMed] [Google Scholar]
- 8. Cabete J, Martins de Carvalho F, Moniz L, Pinto M, Neves J, Pereira Alves C. Microbiological profile and antibiotic susceptibility patterns of organisms isolated from diabetic foot ulcers in a Portuguese hospital. Rev Port Cir Cardiotorac Vasc. 2011. Jan;18(1):53‐60. [PubMed] [Google Scholar]
- 9. Mendes JJ, Marques‐Costa A, Vilela C, et al. Clinical and bacteriological survey of diabetic foot infections in Lisbon. Diabetes Res Clin Pract. 2012. Jan;95(1):153‐161. [DOI] [PubMed] [Google Scholar]
- 10. Barbosa A, Carvalho R, Carvalho A. Evaluation of the efficacy of a diabetic foot infection empirical antibiotic therapy protocol: a retrospective inpatient study. Revista Portuguesa de Diabetes. 2016. Mar 1;11:14‐17. [Google Scholar]
- 11. Machado C, Teixeira S, Fonseca L. Evolutionary trends in bacteria isolated from moderate and severe diabetic foot infections in a Portuguese tertiary center. Diabetes Metab Syndr Clin Res Rev. 2020;14(3):205‐209. [DOI] [PubMed] [Google Scholar]
- 12. Richard J‐L, Lavigne J‐P, Got I, et al. Management of patients hospitalized for diabetic foot infection: results of the French OPIDIA study. Diabetes Metab. 2011. Jun;37(3):208‐215. [DOI] [PubMed] [Google Scholar]
- 13. Zenelaj B, Bouvet C, Lipsky BA, Uçkay I. Do diabetic foot infections with methicillin‐resistant Staphylococcus aureus differ from those with other pathogens? Int J Low Extrem Wounds. 2014;13(4):263‐272. [DOI] [PubMed] [Google Scholar]
- 14. Hartemann‐Heurtier A, Robert J, Jacqueminet S, et al. Diabetic foot ulcer and multidrug‐resistant organisms: risk factors and impact. Diabet Med. 2004. Jul;21(7):710‐715. [DOI] [PubMed] [Google Scholar]
- 15. Ji X, Jin P, Chu Y, Feng S, Wang P. Clinical characteristics and risk factors of diabetic foot ulcer with multidrug‐resistant organism infection. Int J Low Extrem Wounds. 2014;13:64‐71. 10.1177/1534734614521236. [DOI] [PubMed] [Google Scholar]
- 16. Mohi G, Datta P, Chander J, Gupta V, Attri A. Evaluation of various risk factors associated with multidrug‐resistant organisms isolated from diabetic foot ulcer patients. Journal of Laboratory Physicians. 2019;11:58. 10.4103/jlp.jlp_106_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richard J‐L, Sotto A, Jourdan N, et al. Risk factors and healing impact of multidrug‐resistant bacteria in diabetic foot ulcers. Diabetes Metab. 2008. Sep;34(4) Pt 1:363‐369. [DOI] [PubMed] [Google Scholar]
- 18. Lipsky BA. Diabetic foot infections: current treatment and delaying the “post‐antibiotic era”. Diabetes Metab Res Rev. 2016. Jan;32(suppl 1):246‐253. [DOI] [PubMed] [Google Scholar]
- 19. Neu HC. The crisis in antibiotic resistance. Science. 1992;257(5073):1064‐1073. [DOI] [PubMed] [Google Scholar]
- 20. Alanis AJ. Resistance to antibiotics: are we in the post‐antibiotic era? Arch Med Res. 2005. Nov;36(6):697‐705. [DOI] [PubMed] [Google Scholar]
- 21. Blair JMA, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015. Jan;13(1):42‐51. [DOI] [PubMed] [Google Scholar]


