Abstract
Breast cancer is a serious disease in women. We estimated the global technical success rate and complication rates of percutaneous vacuum‐assisted breast biopsy (VABB). PubMed, Embase, Web of Science, and Scopus databases were retrieved up to July 2018 to find studies in which technical success rate and complication rates of VABB were available. Pooled rates were calculated according to location mode (ultrasonography [US] or mammography) and needle type (8‐ or 11‐gauge Mammotome probes). Of the 36 articles with 20 868 cases, we found the pooled technical success rate 0.9999(0.9997, 1.0000) (I 2 = 17.1%, P = .187) and low complication risks including haematoma 0.1092(0.0748, 0.1437) (I 2 = 98.3%, P < .001), pain 0.0738(0.0334, 0.1141) (I 2 = 95.9%, P < .001), vasovagal reflex 0.0281(0.0035, 0.0527) (I 2 = 92.5%, P < .001), and infection 0.0027(−0.0019, 0.0073) (I 2 = 49.8%, P = .113). In this systematic review and meta‐analysis, the pooled data suggested that VABB with US or mammography could be promising for diagnosis and treatment of breast disease. Further studies were necessary to identify strategies for these findings.
Keywords: breast, mammography, meta, ultrasound, vacuum‐assisted breast biopsy
Abbreviations
- CI
confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis
- VABB
vacuum‐assisted breast biopsy
1. INTRODUCTION
Breast cancer is the most common malignancy worldwide. A total of about 1.7 million people were diagnosed with this disease in 2012 worldwide, and 521 900 patients died from it.1, 2, 3 Breast cancer incidences were high in Western (0.960 ‰) and Northern (0.916 ‰) Europe, Northern America (0.894 ‰), and Australia/New Zealand (0.858 ‰); mortality rates were high in Western (0.201 ‰) and Northern (0.174 ‰) Africa, melanesia (0.197 ‰), and Central and Eastern Europe (0.165 ‰).3 Before the treatment for breast masses, traditional staging examinations included chest radiograph, abdominal ultrasound, and computed tomography (CT) scans. And its diagnosis was usually identified using core biopsy. Early finding is important to decrease the number of cancer deaths and to improve prognosis.
In the recent decades, percutaneous vacuum‐assisted breast biopsy (VABB) has been the minimally invasive technique for preoperative histopathologic diagnosis of breast masses. VABB is usually performed under ultrasonography (US) or mammography guidance. Regarding complication rates for haematoma, bleeding, skin ecchymosis, pain, vasovagal reflex, and infection using VABB, data available through the literature were insufficient. Therefore, we conduct this meta‐analysis to quantify the complication rates in order to confirm the safety and efficacy of these procedures.
2. METHODS
2.1. Search strategy
PubMed, Embase, Web of Science, and Scopus databases were searched by two individuals from database inception to July 2018. We used both MeSH terms and keywords for breast, mammotome, puncture, biopsy, complication, and related and exploded terms. No limitations were ruled for language or publication year.
2.2. Study selection
We included cohort and cross‐sectional studies for this meta‐analysis, in which technical success rate was checked or at least one defined complications of VABB were reported, such as hematoma, bleeding, skin ecchymosis, pain, vasovagal reflex, and infection. Articles failing to fulfil the inclusion criteria (did not report a definable outcome measure of interest) were excluded from next screening. We used Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) to guide this meta‐analysis.4 In addition, because this is the meta‐analysis, patient informed consent were not available by the Institutional Review Board.
2.3. Data extraction and statistical analysis
Following the initial screening for studies, more detailed information for each study was extracted by other two investigators based on the established form, which mainly included basic characteristics such as author, patient recruitment years, needle type, location mode, design, number of patients, age, and quality of the evidence. In this study, the technical success rate was calculated and its 95% credible confidence (CI). In order to estimate the risk of VABB complications, we included articles that presented incidence in haematoma, pain, vasovagal reflex, and infection, and then estimated their pooled values. When zero events encountered in one or two groups in the included studies, we add 0.5 into each group. Between‐study heterogeneity was explored by the I 2 statistic5 and appraised as low (25%‐50%), moderate (50%‐75%), and high (75%‐100%).6 Subsequently, we conducted a subgroup analysis for each complication according to location mode (US or mammography) and needle type (8‐ or 11‐gauge Mammotome probes).
We assessed the risk of bias for each study according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria. A total of four quality dimensions (high quality, moderate quality, low quality, and very low quality) were useful for each study.7 All included studies were all individually cross‐checked and assessed to minimise subjectivity and bias. Sensitivity analysis was performed by excluding each study. Publication bias was estimated using Egger's test.8 All statistical analyses were performed using the Stata 12.0 software.
3. RESULTS
3.1. Study selection and description of studies
The detailed flow chart of study selection is displayed in Figure 1, in which 20 528 articles were potentially relevant after initial searching. Table 1 shows the characteristics of all included studies. Among the final 36 eligible studies, 31 were cohort studies (20 142 cases),9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 1 was randomised controlled trial (RCT) (74 cases),40 and 4 non‐randomised controlled trials (NRCTs) (574 cases),41, 42, 43, 44 describing a total of 20 868 patients (mean age, 36–56 years), derived from China (7), United States (6), Italy (5), Germany (5), Korea (2), Japan (2), Spain (1), Poland (1), France (1), United Arab Emirates (1), Britain (1), Switzerland (1), Israel (1), New Zealand (1), and Canada (1). VABB was performed using US (24) and mammography (12) location modes. In this study, it mainly used five techniques: VABB of 8‐(14), 9‐(1), 10‐(1), 11‐(24), or 14‐(5) gauge Mammotome probes. Through GRADE guidelines, we assessed the quality of evidence for each study as high (4), moderate (19), low (13), and very low (1) (Table 1).
Figure 1.
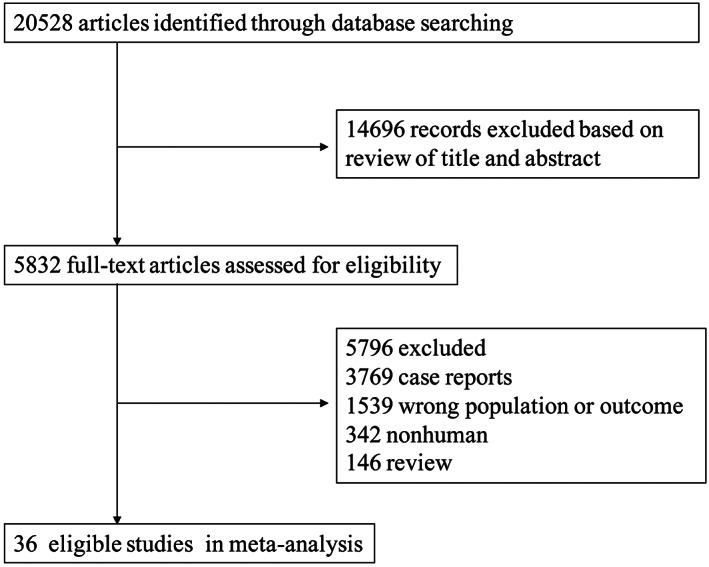
Study identification and selection
Table 1.
Characteristics of the 36 studies of image‐guided vacuum‐assisted breast biopsy in this meta‐analysis
| Author | Patient recruitment years | Needle type | Location mode | Design | Number of patients | Age (years) |
|---|---|---|---|---|---|---|
| Park et al9 | 2003.1‐2015.12 | 8 | US | Retrospective cohort | 8748 | 37.8 |
| Liu et al10 | 2009.1‐2014.1 | 8 | US | Retrospective cohort | 1267 | 39.1 ± 12.36 |
| Berná‐Serna et al11 | NA(18 months) | 10 | US | Retrospective cohort | 118 | 34.5 ± 12.7 |
| Pinkney et al40 | 2014.5‐2015.2 | 9 | X‐ray mammography | RCT | 74 | 57.9 ± 12.4 |
| Choi et al12 | 2013.3‐2014.12 | 13 | US | Retrospective cohort | 114 | 50 |
| Pagni et al13 | 2009.12‐2013.12 | 8/11/14 | US | Retrospective cohort | 712 | 55 |
| Ohsumi et al14 | 1999.5‐2007.2 | 11/14 | X‐ray mammography | Retrospective cohort | 488 | 51 |
| Yi et al15 | 2005.12‐2011.12 | 8 | US | Retrospective cohort | 136 | 48.4 |
| Kibil et al16 | 2000‐2011 | 11 | US | Retrospective cohort | 76 | 51.5 |
| Jiang et al17 | 2008.1‐2012.12 | 8 | US | Retrospective cohort | 3681 | 37.8 |
| Wang et al18 | 2005.3‐2009.5 | 8 | US | Retrospective cohort | 143 | 40.1 ± 21.2 |
| Schaefer et al41 | 2008.1‐2009.12 | 8/11 | US | NRCT | 115 | 52 |
| Luo et al19 | 2007.6‐2009.5 | 8 | US | Retrospective cohort | 1119 | 36.6 |
| He et al20 | 2006.1‐2010.1 | 8 | US | Retrospective cohort | 20 | 24.7 |
| Abbate et al21 | 2010 | 11 | US | Retrospective cohort | 141 | 48 |
| Nakamura et al22 | 2005.6‐2007.3 | 11 | X‐ray mammography | Retrospective cohort | 124 | 52.5 |
| Salem et al23 | 2001.6‐2005.5 | 8/11 | X‐ray mammography | Retrospective cohort | 967 | 53.6 ± 10.3 |
| Hertl et al24 | 2005.10‐2006.2 | 11 | US | Prospective cohort | 45 | 50 |
| He et al25 | 2006.6‐2007.7 | 8 | US | Retrospective cohort | 86 | 36 |
| Faour et al26 | 2003.1‐2006.8 | 11 | X‐ray mammography | Retrospective cohort | 101 | 50 |
| Govindarajulu et al27 | NA | 11 | US | Prospective cohort | 77 | NA |
| Weber et al42 | 1997.4‐2003.8 | 11 | X‐ray mammography | NRCT | 228 | 56 |
| Kettritz et al28 | 2000.1‐2003.8 | 11 | X‐ray mammography | Retrospective cohort | 485 | 56 |
| Diebold et al29 | 2002 | 8 | X‐ray mammography | Prospective cohort | 58 | NA |
| Costantini et al30 | 1998.3‐2002.7 | 11/14 | US | Retrospective cohort | 305 | 54 |
| Sperber et al31 | 1999.5‐2001.5 | 11 | US | Prospective cohort | 52 | 19–68 |
| Mariotti et al32 | 1999.6‐2001.12 | 11/14 | X‐ray mammography | Retrospective cohort | 282 | 51 |
| Greenberg et al33 | NA | 11 | X‐ray mammography | Retrospective cohort | 39 | NA |
| Fine et al34 | NA | 8/11 | US | Retrospective cohort | 216 | 36 ± 11 |
| Baez et al35 | NA | 11 | US | Retrospective cohort | 20 | 39.2 |
| Johnson et al36 | 2000.4‐2002.1 | 8/11 | US | Retrospective cohort | 81 | 46.8 ± 15.4 |
| Parker et al37 | 2000.5‐2000.7 | 11 | US | Retrospective cohort | 113 | NA |
| Meloni et al43 | 1999.6‐2000.4 | 11 | US | NRCT | 73 | 51.4 ± 8.6 |
| Dennis et al38 | 1996.1‐1999.6 | 11 | US | Retrospective cohort | 49 | 52 |
| Klem et al39 | 1996.11‐1997.12 | 11 | X‐ray mammography | Retrospective cohort | 279 | 52.1 ± 11.4 |
| Heywang‐Köbrunner et al44 | NA | 11/14 | X‐ray mammography | NRCT | 236 | NA |
Abbreviations: NA, not available; NRCT: non‐randomised controlled trial; RCT, randomised controlled trial; US, ultrasonography.
3.2. Subgroup analysis
To assess the complication risk in VABB for diagnosis of breast masses, we pooled results from all included studies and found pooled high technical success rate 0.9999 (0.9997, 1.0000) (I 2 = 17.1%, P = .187), and low complication risks including haematoma 0.1092 (0.0748, 0.1437) (I 2 = 98.3%, P < .001), pain 0.0738 (0.0334, 0.1141) (I 2 = 95.9%, P < .001), vasovagal reflex 0.03 (0–0.05) (I 2 = 92.5%, P < .001), and infection 0.0027 (−0.0019, 0.0073) (I 2 = 49.8%, P = .113) (Figure 2).
Figure 2.
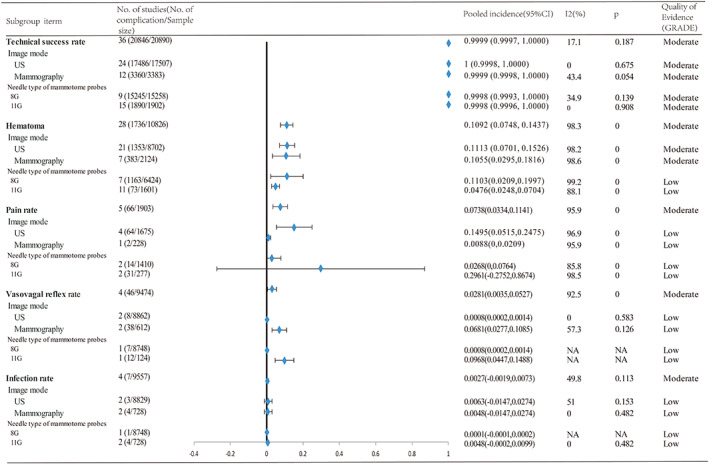
Results of subgroup analysis for the pooled complication incidences of VABB for breast masses. VABB, vacuum‐assisted breast biopsy
Subgroup analysis indicated that the technical success rate in US and mammography was 1 (0.9998, 1.0000) and 0.9999 (0.9998, 1.0000), and complication rates in US and mammography were as the following: haematoma 0.1113 (0.0701, 0.1526) and 0.1055 (0.0295, 0.1816); pain 0.1495 (0.0515, 0.2475) and 0.0088 (0,0.0209); vasovagal reflex 0.0008 (0.0002, 0.0014) and 0.0681 (0.0277, 0.1085); infection 0.0063 (−0.0147, 0.0274) and 0.0048 (−0.0147, 0.0274). Subgroup analysis suggested that the technical success rate in 8‐ and 11‐gauge Mammotome probes were 0.9998 (0.9993, 1.0000) and 0.9998 (0.9996, 1.0000), and complication rates in US and mammography were as the following: haematoma 0.1103 (0.0209, 0.1997) and 0.0476 (0.0248, 0.0704); pain 0.0268 (0, 0.0764) and 0.2961 (−0.2752, 0.8674); vasovagal reflex 0.0008 (0.0002, 0.0014) and 0.0968(0.0447, 0.1488); infection 0.0001 (−0.0001, 0.0002) and 0.0048 (−0.0002, 0.0099) (Figure 2).
3.3. Sensitivity analysis and publication bias
Sensitivity analysis indicated that no individual study has an impact on the overall pooled results. There were evidences of publication bias using the Egger test in technical success rate, bleeding, skin ecchymosis, infection (t = −8.05, P < .01; t = 2.28, P = .035; t = 2.29, P = .037; t = 605.02, P < .01).
4. DISCUSSION
In recent decades, more breast lesions have been diagnosed by US and many other tests. In 1982, it was reported that mammotome began to be clinically used.45 In our meta‐analysis, we assessed the pooled technical success rate and complication rate of VABB in terms of haematoma, bleeding, skin ecchymosis, pain, vasovagal reflex, and infection. Haematoma is the most common postoperative complications after VABB. Most breast masses are centrally distributed with an affluent blood supply. Kettritz et al indicated that 6 patients developed haematomas at least 4 cm in diameter after VABB in 500 women with microcalcifications.28 Similarly, haematoma occurred in 12 (8.82%) patients using the 8‐gauge probe, of which size ranged from 3 to 6 cm.15 Pagni et al reported the haematoma rate 9% (62/712) with 8‐, 11‐, or 14‐gauge Mammotome probes.13 Furthermore, considering the different needle sizes of VABB, a previous study indicated that bleedings and haematomas for 8‐gauge Mammotome system were significantly more than that following 11‐gauge Mammotome system (41.9% vs 8.4%, P < .001; 35.5% vs 16.7%, P = .029).41 Most haematomas recorded were minimal, and they could be gradually resolved without special management. During ultrasound‐guided VABB for 8748 patients, only one case experienced massive bleeding requiring a blood transfusion of about 1000 mL.9 It was reported that bloody nipple discharge was observed in 5.40% (9/136) patients using the 8‐gauge probe.15 Lidocaine and puncture needle were used in the targeting region in the procedure. Most haematomas and bleeding may result from inadequate compression or fixation, and they gradually resolved in short time.
In addition, in this study, the scar formation rate was 0.0738(0.0334, 0.1141). Wang et al retrospectively showed that pains appeared in 5.6% (8/143) cases with 8‐gauge Mammotome system,18 consistent with 9.7% reported by Fine et al.34 Previous studies revealed that in a total of 8748 patients, vasovagal reactions existed in seven cases following 1% lidocaine injection during VABB, such as bradycardia, dyspnea, nausea, and hypotension.9
Mammotome has a single insertion of the puncture needle with the repeated incision, avoiding repeated puncture and reducing the incidence of needle‐tract implantation metastases. Sufficient biopsy acquisitions led to a reduction in false‐negative rates and underestimation of histology. For small benign breast tumours, therapeutic resection could be performed without the permanent scar for minimising the cosmetic injury. Particularly within US guidance, US could display where the puncture needle was in real time and enable the accurate location according to the different angles and depths. Comparing US, the mammography guidance of breast is relatively fixed. When the three‐dimensional positioning is performed at the puncture point, the position and depth of the puncture needle are fixed. All these advantages guarantee the safety of VABB.
There were several limitations in the present meta‐analysis. First, most included studies were retrospective in study design, which could result in the patient selection bias. Second, the number of included studies was small in different complications, particularly in vasovagal reflex and infection, which may contribute to heterogeneity in the results. Third, we used random‐effect models to pool studies and could cause overly narrow credible intervals, particularly when there were a few studies.46 Fourth, different sizes of needles may cause different levels of complications. Because some literature studies reported several types of needles, we performed subgroup analysis according to 8‐ and 11‐gauge needles here.
Due to the high prevalence of benign and malignant breast masses in women, finding a quick and safe way to facilitate diagnosing breast disease became an urgent problem. In this meta‐analysis, we appraised the risk of bias for each study using GRADE guidelines. To minimise potential heterogeneity, we estimated the reliability and safety of VABB using the random‐effects model through technical success rate and possible complications.
5. CONCLUSIONS
In a word, the combination of VABB with US or mammography is minimally invasive, safe, and accurate in view of the low complication rate and effectiveness, which could be regarded as a promising candidate of the standard biopsy methods for detecting nonpalpable lesions. Further research studies will be continually needed to identify these findings.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
M.F. contributed to the study concept and design, the drafting of the manuscript, and study supervision. M.F., G.Li., G.Lu., and T.W. contributed to the acquisition of data. M.F. and G.Li. contributed to the analysis and interpretation of data. G.Li. and G.Lu. did the critical revision of the manuscript for important intellectual content and did the statistical analysis. G.Lu. and T.W. contributed to technical or material support.
Fang M, Liu G, Luo G, Wu T. Feasibility and safety of image‐guided vacuum‐assisted breast biopsy: A PRISMA‐compliant systematic review and meta‐analysis of 20 000 population from 36 longitudinal studies. Int Wound J. 2019;16:1506–1512. 10.1111/iwj.13224
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134‐1150. [DOI] [PubMed] [Google Scholar]
- 3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006‐1012. [DOI] [PubMed] [Google Scholar]
- 5. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Naunyn‐Schmiedebergs Archiv für experimentelle . Pathologie und Pharmakologie. 2011;5:S38. [Google Scholar]
- 6. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE working group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ. 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- 8. McShane BB, Bockenholt U, Hansen KT. Adjusting for publication bias in meta‐analysis: an evaluation of selection methods and some cautionary notes. Perspect Psychol Sci. 2016;11:730‐749. [DOI] [PubMed] [Google Scholar]
- 9. Park HL, Kim KY, Park JS, et al. Clinicopathological analysis of ultrasound‐guided vacuum‐assisted breast biopsy for the diagnosis and treatment of breast disease. Anticancer Res. 2018;38:2455‐2462. [DOI] [PubMed] [Google Scholar]
- 10. Liu S, Zou JL, Zhou FL, Fang YM. Efficacy of ultrasound‐guided vacuum‐assisted Mammotome excision for management of benign breast diseases: analysis of 1267 cases. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37:1121‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berna‐Serna JD, Guzman‐Aroca F, Berna‐Mestre JD, et al. A new method for the prevention of skin laceration during vacuum‐assisted breast biopsy. Br J Radiol. 2017;90:20160866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi ER, Han BK, Ko ES, et al. Initial experience with a wireless ultrasound‐guided vacuum‐assisted breast biopsy device. PLoS One. 2015;10:e0144046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pagni P, Spunticchia F, Barberi S, Caprio G, Paglicci C. Use of core needle biopsy rather than fine‐needle aspiration cytology in the diagnostic approach of breast cancer. Case Rep Oncol. 2014;7:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohsumi S, Taira N, Takabatake D, et al. Breast biopsy for mammographically detected nonpalpable lesions using a vacuum‐assisted biopsy device (Mammotome) and upright‐type stereotactic mammography unit without a digital imaging system: experience of 500 biopsies. Breast Cancer. 2014;21:123‐127. [DOI] [PubMed] [Google Scholar]
- 15. Yi W, Xu F, Zou Q, Tang Z. Completely removing solitary intraductal papillomas using the Mammotome system guided by ultrasonography is feasible and safe. World J Surg. 2013;37:2613‐2617. [DOI] [PubMed] [Google Scholar]
- 16. Kibil W, Hodorowicz‐Zaniewska D, Popiela TJ, Szpor J, Kulig J. Mammotome biopsy in diagnosing and treatment of intraductal papilloma of the breast. Pol Przegl Chir. 2013;85:210‐215. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Y, Lan H, Ye Q, et al. Mammotome biopsy system for the resection of breast lesions: clinical experience in two high‐volume teaching hospitals. Exp Ther Med. 2013;6:759‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang ZL, Liu G, Huang Y, Wan WB, Li JL. Percutaneous excisional biopsy of clinically benign breast lesions with vacuum‐assisted system: comparison of three devices. Eur J Radiol. 2012;81:725‐730. [DOI] [PubMed] [Google Scholar]
- 19. Luo HJ, Chen X, Tu G, Wang J, Wu CY, Yang GL. Therapeutic application of ultrasound‐guided 8‐gauge Mammotome system in presumed benign breast lesions. Breast J. 2011;17:490‐497. [DOI] [PubMed] [Google Scholar]
- 20. He Q, Zheng L, Zhuang D, Fan Z, Xi C, Zhou P. Surgical treatment of gynecomastia by vacuum‐assisted biopsy device. J Laparoendosc Adv Surg Tech A. 2011;21:431‐434. [DOI] [PubMed] [Google Scholar]
- 21. Abbate F, Cassano E, Menna S, Viale G. Ultrasound‐guided vacuum‐assisted breast biopsy: use at the European Institute of Oncology in 2010. J Ultrasound. 2011;14:177‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakamura Y, Urashima M, Matsuura A, et al. Stereotactic directional vacuum‐assisted breast biopsy using lateral approach. Breast Cancer. 2010;17:286‐289. [DOI] [PubMed] [Google Scholar]
- 23. Salem C, Sakr R, Chopier J, Antoine M, Uzan S, Daraï E. Pain and complications of directional vacuum‐assisted stereotactic biopsy: comparison of the Mammotome and Vacora techniques. Eur J Radiol. 2009;72:295‐299. [DOI] [PubMed] [Google Scholar]
- 24. Hertl K, Marolt‐Music M, Kocijancic I, Prevodnik‐Kloboves V, Zgajnar J. Haematomas after percutaneus vacuum‐assisted breast biopsy. Ultraschall Med. 2009;30:33‐36. [DOI] [PubMed] [Google Scholar]
- 25. He Q, Fan X, Guan Y, Tian J, Fan Z, Zheng L. Percutaneous excisional biopsy of impalpable breast lesions under ultrasound visualization. Breast. 2008;17:666‐670. [DOI] [PubMed] [Google Scholar]
- 26. Faour I, Al‐Salam S, El‐Terifi H, et al. The use of a vacuum‐assisted biopsy device (Mammotome) in the early detection of breast cancer in The United Arab Emirates. Ann N Y Acad Sci. 2008;1138:108‐113. [DOI] [PubMed] [Google Scholar]
- 27. Govindarajulu S, Narreddy SR, Shere MH, et al. Sonographically guided mammotome excision of ducts in the diagnosis and management of single duct nipple discharge. Eur J Surg Oncol. 2006;32:725‐728. [DOI] [PubMed] [Google Scholar]
- 28. Kettritz U, Morack G, Decker T. Stereotactic vacuum‐assisted breast biopsies in 500 women with microcalcifications: radiological and pathological correlations. Eur J Radiol. 2005;55:270‐276. [DOI] [PubMed] [Google Scholar]
- 29. Diebold T, Hahn T, Solbach C, et al. Evaluation of the stereotactic 8G vacuum‐assisted breast biopsy in the histologic evaluation of suspicious mammography findings (BI‐RADS IV). Invest Radiol. 2005;40:465‐471. [DOI] [PubMed] [Google Scholar]
- 30. Costantini R, Sardellone A, Marino C, Giamberardino MA, Innocenti P, Napolitano AM. Vacuum‐assisted core biopsy (Mammotome) for the diagnosis of non‐palpable breast lesions: four‐year experience in an Italian center. Tumori. 2005;91:351‐354. [DOI] [PubMed] [Google Scholar]
- 31. Sperber F, Blank A, Metser U, Flusser G, Klausner JM, Lev‐Chelouche D. Diagnosis and treatment of breast fibroadenomas by ultrasound‐guided vacuum‐assisted biopsy. Arch Surg. 2003;138:796‐800. [DOI] [PubMed] [Google Scholar]
- 32. Mariotti C, Feliciotti F, Baldarelli M, et al. Digital stereotactic biopsies for nonpalpable breast lesion. Surg Endosc. 2003;17:911‐917. [DOI] [PubMed] [Google Scholar]
- 33. Greenberg D, Johnston J, Hart R, Weston M, Benson‐Cooper D. Stereotactic breast biopsy: an audit of 18 months at BreastScreen Auckland. Australas Radiol. 2003;47:261‐267. [DOI] [PubMed] [Google Scholar]
- 34. Fine RE, Whitworth PW, Kim JA, Harness JK, Boyd BA, Burak WE Jr. Low‐risk palpable breast masses removed using a vacuum‐assisted hand‐held device. Am J Surg. 2003;186:362‐367. [DOI] [PubMed] [Google Scholar]
- 35. Baez E, Huber A, Vetter M, et al. Minimal invasive complete excision of benign breast tumors using a three‐dimensional ultrasound‐guided mammotome vacuum device. Ultrasound Obstet Gynecol. 2003;21:267‐272. [DOI] [PubMed] [Google Scholar]
- 36. Johnson AT, Henry‐Tillman RS, Smith LF, et al. Percutaneous excisional breast biopsy. Am J Surg. 2002;184:550‐554. discussion 554. [DOI] [PubMed] [Google Scholar]
- 37. Parker SH, Klaus AJ, McWey PJ, et al. Sonographically guided directional vacuum‐assisted breast biopsy using a handheld device. AJR Am J Roentgenol. 2001;177:405‐408. [DOI] [PubMed] [Google Scholar]
- 38. Dennis MA, Parker S, Kaske TI, Stavros AT, Camp J. Incidental treatment of nipple discharge caused by benign intraductal papilloma through diagnostic Mammotome biopsy. AJR Am J Roentgenol. 2000;174:1263‐1268. [DOI] [PubMed] [Google Scholar]
- 39. Klem D, Jacobs HK, Jorgensen R et al. Stereotactic breast biopsy in a community hospital setting. Am Surg. 1999; 65:737–740; discussion 740–731. [PubMed] [Google Scholar]
- 40. Pinkney DM, Mychajlowycz M, Shah BA. A prospective comparative study to evaluate the displacement of four commercially available breast biopsy markers. Br J Radiol. 2016;89:20160149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schaefer FK, Order BM, Eckmann‐Scholz C, et al. Interventional bleeding, hematoma and scar‐formation after vacuum‐biopsy under stereotactic guidance: Mammotome‐system 11 g/8 g vs. ATEC‐system 12 g/9 g. Eur J Radiol. 2012;81:e739‐e745. [DOI] [PubMed] [Google Scholar]
- 42. Weber WP, Zanetti R, Langer I, et al. Mammotome: less invasive than ABBI with similar accuracy for early breast cancer detection. World J Surg. 2005;29:495‐499. [DOI] [PubMed] [Google Scholar]
- 43. Meloni GB, Dessole S, Becchere MP, et al. Ultrasound‐guided mammotome vacuum biopsy for the diagnosis of impalpable breast lesions. Ultrasound Obstet Gynecol. 2001;18:520‐524. [DOI] [PubMed] [Google Scholar]
- 44. Heywang‐Kobrunner SH, Schaumloffel U, Viehweg P, et al. Minimally invasive stereotaxic vacuum core breast biopsy. Eur Radiol. 1998;8:377‐385. [DOI] [PubMed] [Google Scholar]
- 45. Sanchez AE. The "mammotome"‐‐a new surgical blade. Ann Plast Surg. 1982;9:513‐515. [DOI] [PubMed] [Google Scholar]
- 46. Cornell JE, Mulrow CD, Localio R, et al. Random‐effects meta‐analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160:267‐270. [DOI] [PubMed] [Google Scholar]


